Carbon-Rich Selenide Monolayers as Metal-Free Catalysts for Oxygen Reduction Reactions: A First-Principles Investigation
Abstract
1. Introduction
2. Computational Methodology
3. Results and Discussion
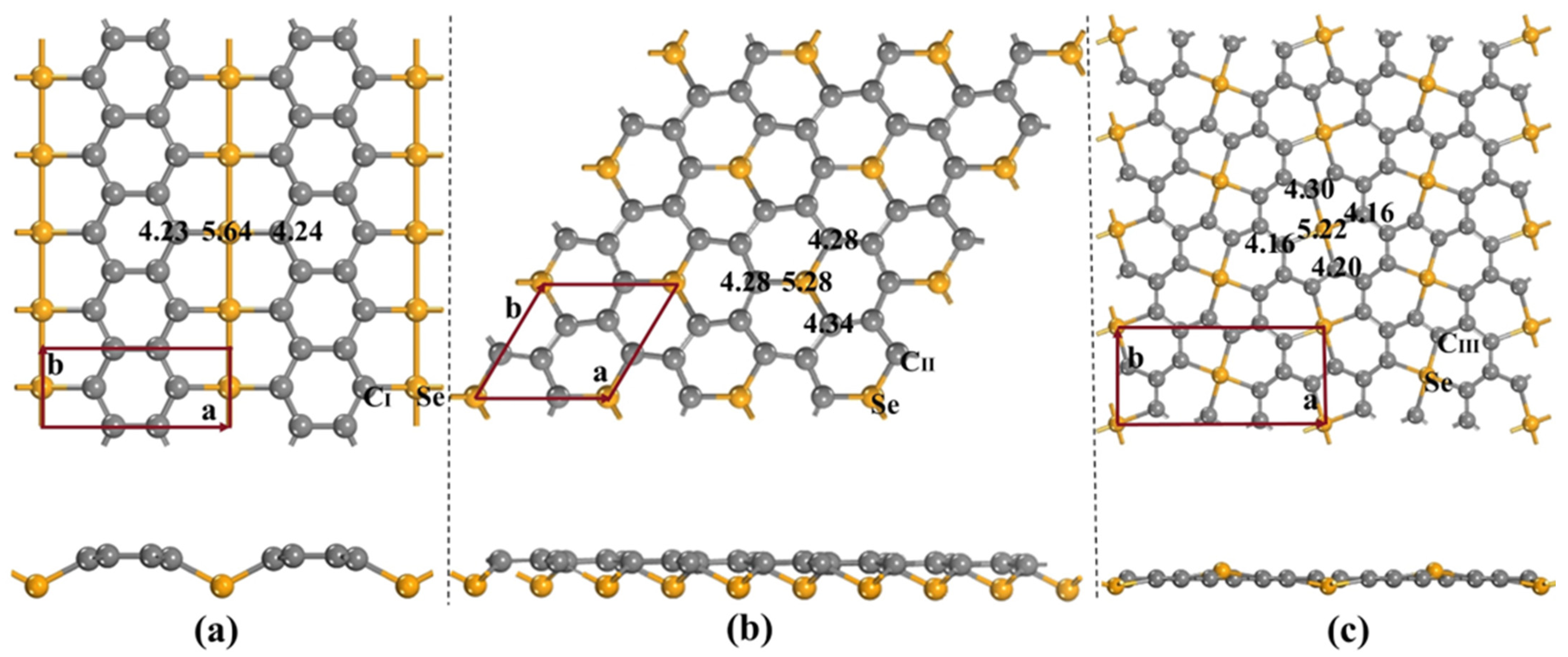
3.1. Stability
3.2. O2 Adsorption
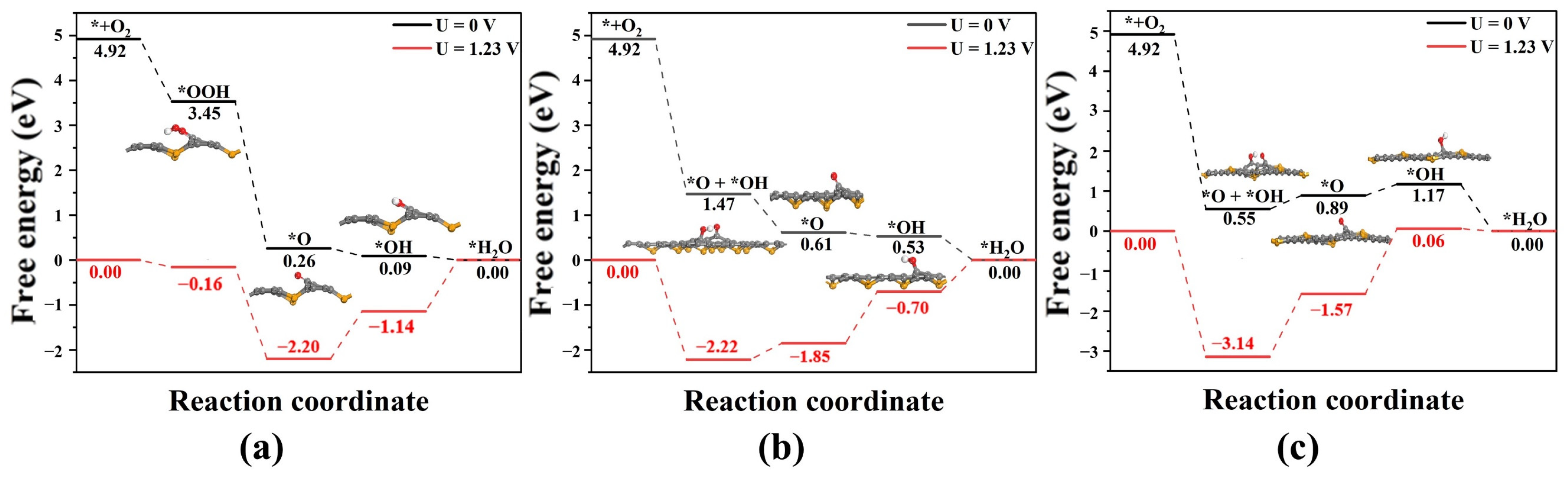
3.3. Gibbs Free Energy
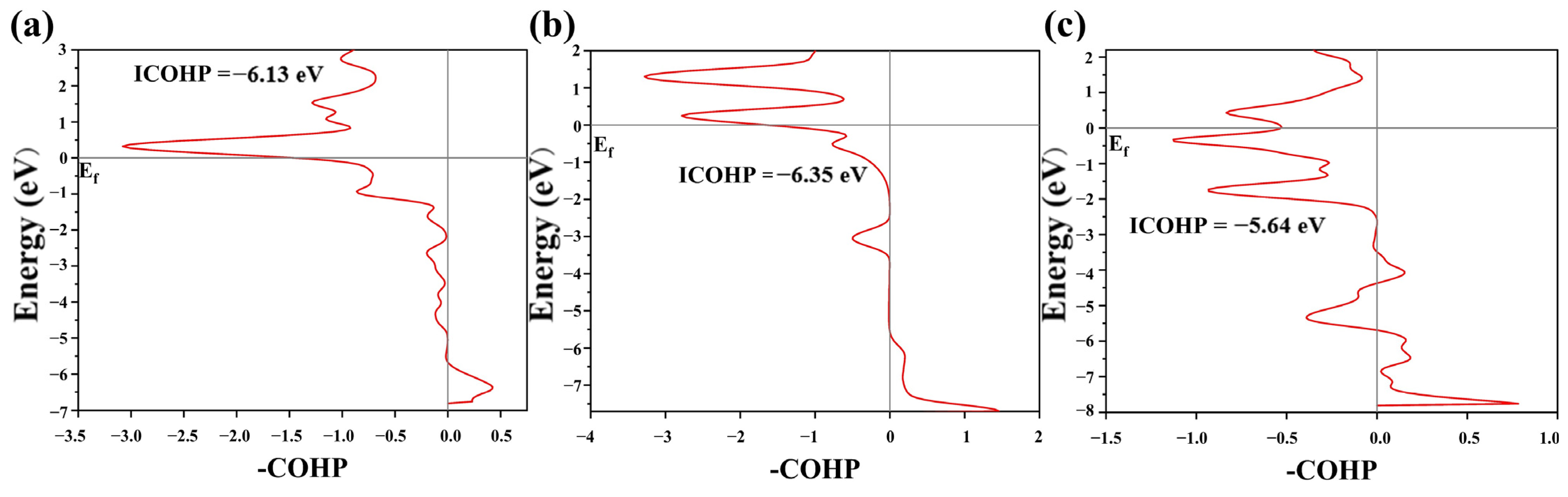
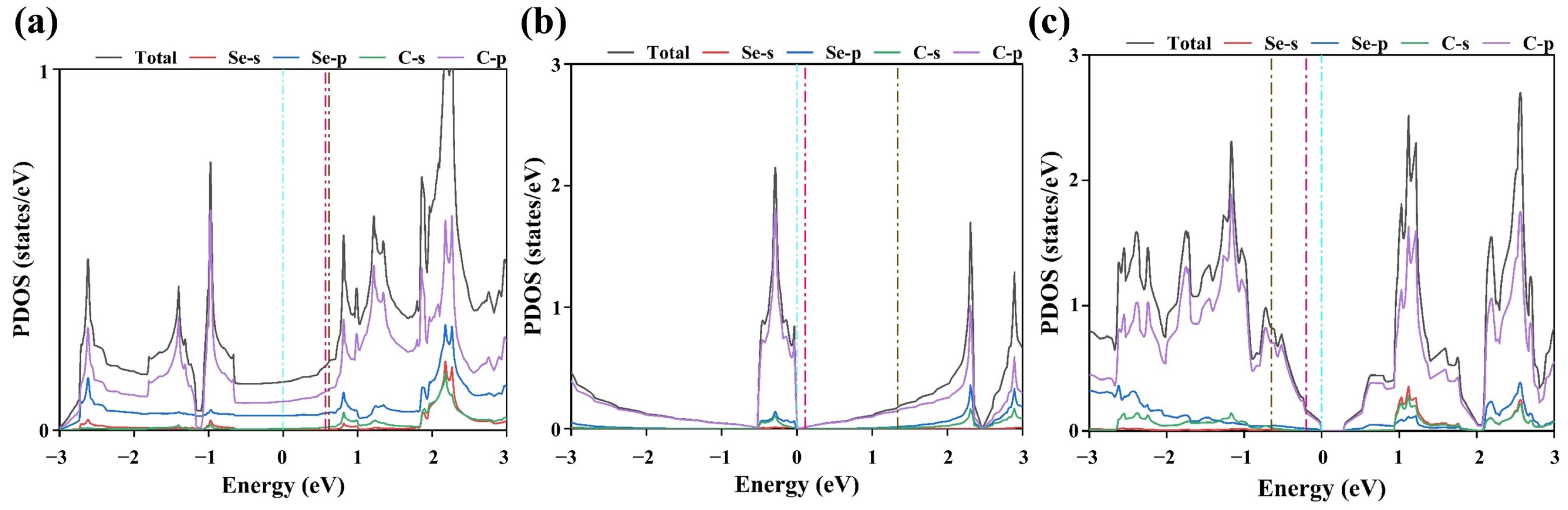
3.4. Origin of ORR Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, C.; Paul, R.; Dai, Q.; Dai, L. Carbon-based Metal-free Electrocatalysts: From Oxygen Reduction to Multifunctional Electrocatalysis. Chem. Soc. Rev. 2021, 50, 11785–11843. [Google Scholar] [CrossRef] [PubMed]
- Erable, B.; Féron, D.; Bergel, A. Microbial Catalysis of the Oxygen Reduction Reaction for Microbial Fuel Cells: A Review. ChemSusChem 2012, 5, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Lundin, A.; Pötting, K.; Quaino, P.; Schmickler, W. Hydrogen Evolution and Oxidation—A Prototype for an ElectroCatalytic Reaction. J. Solid State Electrochem. 2009, 13, 1101–1109. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, S.; Ai, H.; Lee, Y.J.; Kang, B. γ-Graphyne Nanotubes as Defect-free Catalysts of the Oxygen Reduction Reaction: A DFT Investigation. Phys. Chem. Chem. Phys. 2020, 28, 633–8638. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Dai, L. Carbon-based Electrocatalysts for Advanced Energy Conversion and Storage. Sci. Adv. 2015, 1, e1500564. [Google Scholar] [CrossRef]
- Singh, R.K.; Douglin, J.C.; Kumar, V.; Tereshchuk, P.; Santori, P.G.; Ferreira, E.B.; Jerkiewicz, G.; Ferreira, P.J.; Natan, A.; Jaouen, F.; et al. Metal-free advanced energy materials for the oxygen reduction reaction in anion-exchange membrane fuel cells. Appl. Catal. B Environ. 2024, 357, 124319. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Q.; Dai, L. Highly Efficient Metal-free Growth of Nitrogen-doped Single-walled Carbon Nanotubes on Plasma-etched Substrates for Oxygen Reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef]
- Ma, R.; Ren, X.; Bao, Y.X.; Zhou, Y.; Sun, C.; Liu, Q.; Liu, J.; Wang, J. Novel Synthesis of N-doped Graphene as an Efficient Electrocatalyst Towards Oxygen Reduction. Nano Res. 2016, 9, 808–819. [Google Scholar] [CrossRef]
- Gu, D.; Zhou, Y.; Ma, R.; Wang, F.; Liu, Q.; Wang, J. Facile Synthesis of N-doped Graphene-like Carbon Nanoflakes as Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction. Nano-Micro Lett. 2017, 10, 29. [Google Scholar] [CrossRef]
- Ly, A.; Murphy, E.; Wang, H.; Huang, Y.; Ferro, G.; Guo, S.; Asset, T.; Liu, Y.; Zenyuk, I.V.; Atanassov, P. Electrochemical Trends of a Hybrid Platinum and Metal-nitrogen-carbon Catalyst Library for the Oxygen Reduction Reaction. EES. Catal. 2024, 2, 624–637. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-doped Carbon Nanotubes as Metal-free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 123, 7270–7273. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Gao, H.L.; Bao, W.J.; Wang, F.B.; Xia, X.H. Synthesis of Boron Doped Graphene for Oxygen Reduction Reaction in Fuel Cells. J. Mater. Chem. 2012, 22, 390–395. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped Graphene as an Efficient Metal-free Cathode catalyst for oxygen reduction. ACS Nano 2011, 6, 205–211. [Google Scholar] [CrossRef]
- Liu, Z.W.; Peng, F.; Wang, H.J.; Yu, H.; Zheng, W.X.; Yang, J. Phosphorus-doped Graphite Layers with High Electrocatalytic Activity for the O2 Reduction in an Alkaline Medium. Angew. Chem. Int. Ed. 2011, 50, 3257–3261. [Google Scholar] [CrossRef]
- Yao, Z.; Nie, H.; Yang, Z.; Zhou, X.; Liu, Z.; Huang, S. Catalyst-free Synthesis of Iodine-doped Graphene via a Facile Thermal Annealing Process and its use for Electrocatalytic Oxygen Reduction in an Alkaline Medium. Chem. Commun. 2012, 48, 1027–1029. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Choi, H.J.; Choi, M.; Seo, J.M.; Jung, S.M.; Kim, M.J.; Zhang, S.; Zhang, L.; Xia, Z.; Dai, L.; et al. Facile, Scalable Synthesis of Edge-halogenated Graphene Nanoplatelets as Efficient Metal-free Eletrocatalysts for Oxygen Reduction Reaction. Sci. Rep. 2013, 3, 1810. [Google Scholar] [CrossRef]
- Dyjak, S.; Jankiewicz, J.B.; Kaniecki, S.; Kiciński, W. Selenium-doped Carbon Materials: Synthesis and Applications for Sustainable Technologies. Green Chem. 2024, 26, 2985–3020. [Google Scholar] [CrossRef]
- Kong, P.; Zhang, X.; Wang, J.; Tian, W.; Ni, Y.; Sun, B.; Wang, H.; Wang, H.; Feng, Y.P.; Chen, Y. Electron-phonon Coupling Superconductivity and Tunable Topological State in Carbon-rich Selenide Monolayers. Phys. Rev. B 2023, 107, 184115. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Z.; Liu, Y.; Li, F.; Shang, Y.; Liu, Y.; Cai, Q.; Zhao, J. A Metallic Cu2N Monolayer with Planar Tetracoordinated Nitrogen as a Promising Catalyst for CO2 Electroreduction. J. Mater. Chem. A 2022, 10, 1560–1568. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, R.W.; Ma, D.S.; Cai, Z.; Geng, D.; Sun, Z.; Zhao, Q.; Gao, J.; Cheng, P.; Chen, L.; et al. Realization of a Two-dimensional Checkerboard Lattice in Monolayer Cu2N. Nano Lett. 2023, 23, 5610–5616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, H.; Li, Y.; Chen, Z. Two-dimensional Iron-phthalocyanine (Fe-Pc) Monolayer as a Promising Single-atom-catalyst for Oxygen Reduction Reaction: A Computational Study. Nanoscale 2015, 7, 11633–11641. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Zhong, J.; Zhao, F.; Han, N.; Huang, W.; Zeng, M.; Fan, J.; Li, Y. Iron Polyphthalocyanine Sheathed Multiwalled Carbon Nanotubes: A High-performance Electrocatalyst for Oxygen Reduction Reaction. Nano Res. 2016, 9, 1497–1506. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Iinitio Total-energy Calculations Using a Plane-wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-wave Method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Chowdhury, C.; Datta, A. Silicon-doped Nitrogen-coordinated Graphene as Electrocatalyst for Oxygen Reduction Reaction. J. Phys. Chem. C 2018, 122, 27233–27240. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis as Projected from Plane-wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-wave Based DFT. J. Comput. Phys. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Gao, M.R.; Cao, X.; Gao, Q.; Xu, Y.F.; Zheng, Y.R.; Jiang, J.; Yu, S.H. Nitrogen-doped Graphene Supported CoSe2 Nanobelt Composite Catalyst for Efficient Water Oxidation. ACS Nano 2014, 8, 3970–3978. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, N.; Shao, Z.; Huang, K.; Li, Y.; He, F.; Wang, Q. A Facile Strategy to Construct Amorphous Spinel-based Electrocatalysts with Massive Oxygen Vacancies Using Ionic Liquid Dopant. Adv. Energy Mater. 2018, 8, 1800980. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, C.; Li, K.; Tang, H.; Wang, Y.; Wu, Z. Improved Oxygen Reduction Activity in Heteronuclear FeCo-codoped Graphene: A Theoretical Study. ACS Sustain. Chem. Eng. 2019, 7, 17273–17281. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; He, F.; Li, K.; Wu, Z. Haeckelite and N-doped Haeckelite as Catalysts for Oxygen Reduction Reaction: Theoretical Studies. J. Phys. Chem. C 2017, 121, 28339–28347. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, F. Carbon-Rich Selenide Monolayers as Metal-Free Catalysts for Oxygen Reduction Reactions: A First-Principles Investigation. Chemistry 2025, 7, 55. https://doi.org/10.3390/chemistry7020055
Xu Y, Li F. Carbon-Rich Selenide Monolayers as Metal-Free Catalysts for Oxygen Reduction Reactions: A First-Principles Investigation. Chemistry. 2025; 7(2):55. https://doi.org/10.3390/chemistry7020055
Chicago/Turabian StyleXu, Yao, and Fengyu Li. 2025. "Carbon-Rich Selenide Monolayers as Metal-Free Catalysts for Oxygen Reduction Reactions: A First-Principles Investigation" Chemistry 7, no. 2: 55. https://doi.org/10.3390/chemistry7020055
APA StyleXu, Y., & Li, F. (2025). Carbon-Rich Selenide Monolayers as Metal-Free Catalysts for Oxygen Reduction Reactions: A First-Principles Investigation. Chemistry, 7(2), 55. https://doi.org/10.3390/chemistry7020055






