Abstract
Microbiologically influenced corrosion (MIC) is one of the key causes of material failure in marine engineering, and sulfate-reducing bacteria (SRB) and iron-oxidizing bacteria (IOB) are typical representatives of anaerobic and aerobic microorganisms, respectively. These microorganisms are widely present in marine environments and can form synergistic communities on the surface of metal materials, posing a corrosion threat to them. At the same time, the presence of mixed bacteria may have an effect on cathodic protection, so this study investigates the growth metabolism of mixed SRB and IOB under different cathodic protection potentials in an impressed current cathodic protection (ICCP) system in a marine environment containing SRB and IOB. It also examines the attachment of these microorganisms to the anode and cathode, and the impact on cathodic protection efficiency. The results indicate that in a marine environment containing IOB and SRB, the cathodic protection efficiency of the ICCP system increases with the negative shift of the protection potential. A more positive cathodic protection potential promotes the adhesion of mixed bacteria on the electrode surface and the formation of a biofilm, which reduces cathodic protection efficiency. In contrast, at a cathodic protection potential of −1.05 V (SCE), bacterial growth is inhibited, and a dense crystalline corrosion film primarily composed of Fe2O3 and Fe(OH)3 forms on the cathode surface. This film effectively protects the cathodic metal, significantly mitigating MIC.
1. Introduction
The marine environment is complex and contains a wealth of usable resources. In order to efficiently and reasonably utilize these marine resources, many large-scale engineering facilities have been put into operation. With the development and utilization of marine resources, certain engineering facilities, such as oil and gas platforms, submarine cables, cross-sea bridges, underwater transportation pipelines, and ships, are facing significant corrosion threats [1,2,3]. Microbiologically influenced corrosion (MIC) is one of the main causes of steel structure failure in marine facilities [4]. The corrosion loss caused by MIC accounts for 20% of the total global corrosion loss [5]. As a result, the corrosion behavior of MIC on metal materials has attracted significant attention from researchers. MIC refers to the attachment of microorganisms to metal surfaces, forming biofilms that influence the kinetics of anodic or cathodic reactions [6]. Therefore, the occurrence of MIC is closely related to the colonization of microorganisms on metal surfaces [7,8].
Impressed current cathodic protection (ICCP) technology is particularly suited for protecting large structures and environments where operating conditions frequently change, due to its advantages such as providing a large protective current, high driving voltage, and the ability to automatically adjust the output of protective current in response to varying conditions [9,10]. The ICCP system typically consists of components such as a DC power supply, auxiliary anodes, and reference electrodes [11]. Although ICCP systems can adapt to the complex and dynamic marine environment, their protection efficiency can still be influenced by MIC [12,13,14]. In fact, MIC occurring in ICCP systems is a complex bioelectrochemical process that involves multiple disciplines, including physics, chemistry, electrochemistry, materials science, and microbiology [13,15]. The colonization, metabolism, and corrosion product generation by microorganisms on the metal surface alter the electrochemical properties of the metal–solution interface [16], which in turn impacts the cathodic protection efficiency. At the same time, the microbial corrosion behavior continually threatens the protected metal.
In actual marine environments, MIC is not caused by a single microorganism; it is often the result of the synergistic coexistence of multiple microorganisms [17]. This phenomenon of synergistic coexistence is most common at the aerobic and anaerobic interfaces, where multiple microorganisms grow synergistically on the metal surface, depend on each other, and form biofilms [18]. The production of extracellular polymeric substances (EPS) and the accumulation of metabolic by-products continuously influence the electrochemical properties of the metal–solution interface, which is a key factor leading to corrosion failure of metal materials [19]. In this synergistic coexistence, the interdependence between sulfate-reducing bacteria (SRB) and iron-oxidizing bacteria (IOB) is a typical example [19,20]. SRB, as anaerobic microorganisms, are considered one of the common microbes responsible for MIC [21,22,23]. Dong et al. [24] found that the colonization of SRB on metal surfaces and the formation of biofilms lead to the production of a large amount of corrosion products, which are unevenly attached to the metal surface, causing localized corrosion to intensify. Guan et al. [25] studied the corrosion behavior of SRB under different cathodic polarization potentials and found that the presence of SRB affects cathodic protection efficiency. At a cathodic protection potential of −0.85 V (SCE), the metabolic activity of SRB was even enhanced. Gu et al. [26] explained the mechanism of SRB-induced corrosion from the perspective of extracellular electron transfer (EET). In the EET-MIC process involving SRB, Fe releases electrons through the cell wall, which are ultimately used for sulfate reduction in the SRB cytoplasm, leading to corrosion of steel materials. IOB also plays an important role in the MIC process [6,27]. The aerobic IOB can use Fe2+ released from the metal surface as an electron donor for metabolism [28], thus contributing to metal corrosion. These two types of bacteria are closely connected. Specifically, IOB, as an aerobic microorganism, consumes oxygen through its metabolism, forming a nodular layer with gradually depleted oxygen content, thereby providing favorable conditions for the growth, metabolism, and reproduction of SRB. Their mutual cooperation poses a significant threat to the integrity of metals [29].
In an ICCP system, the cathodic protection potential determines the efficiency of protection. If the cathodic protection potential is too positive, it may be insufficient to protect the target metal, resulting in low cathodic protection efficiency. Conversely, if the cathodic protection potential is too negative, overprotection may occur, leading to issues such as hydrogen embrittlement [30]. Therefore, selecting a reasonable and effective cathodic protection potential is crucial. The choice of cathodic protection potential primarily depends on the environment in which the protected metal is located while also considering the impact of MIC. Currently, research on the interaction between different cathodic protection potentials and MIC in ICCP systems mainly focuses on single species. However, for complex systems like marine environments, it is essential to consider the synergistic effects between multiple species and the mutual influence between these species and the cathodic protection potential. Moreover, corrosion failures caused by the synergistic actions of IOB and SRB are common in the working environments of marine engineering facilities. Therefore, this study focuses on examining the impact of the synergistic effects of IOB and SRB on cathodic protection efficiency under different cathodic protection potentials in an ICCP system.
2. Materials and Methods
2.1. Electrode Material
2.1.1. Working Electrode
Offshore platform steel, commonly used in marine environments, was selected as the working electrode. This type of steel is widely utilized in offshore platforms due to its high strength, hardness, excellent corrosion resistance, and strong toughness. Its main composition and proportions are listed in Table 1. The presence of small amounts of Mn and Si can influence the steel’s microstructure, corrosion resistance, and electrical conductivity depending on the cooling rate during forging and the environmental conditions. A copper wire was soldered to the back of the electrode using tin and leaving a 1 cm2 surface area, while the rest was sealed with epoxy resin. The working surface was polished stepwise using SiC papers with grit sizes ranging from P120 to P3000 in a vertical direction [31], followed by ultrasonic cleaning with distilled water and anhydrous ethanol. Before use, the working electrode was exposed to UV light for at least 30 min to ensure no microbial contamination.

Table 1.
Composition of platform steel.
2.1.2. Auxiliary Anode
Industrial-grade pure titanium with an externally sprayed ruthenium coating mixed-metal oxide (MMO) was selected as the auxiliary anode. The surface was perforated for connection to a lead wire, with a 1 cm2 surface area, while the remaining portions were sealed with epoxy resin. Before the experiment, the anode was ultrasonically cleaned with anhydrous ethanol and exposed to UV light for 30 min to eliminate any potential microbial contamination. The prepared anode was stored in a sterile workspace until use.
2.2. Bacteria and Media
An SRB strain, Desulfovibrio caledoniensis, was isolated from marine sediment near the coast of Qingdao. Studies have shown that this bacterium can directly acquire electrons from an electrode [32,33,34]. An IOB, Pseudomonas sp., was isolated from the steel rust layer in the Qingdao marine area. SRB was inoculated into sterilized modified Postgate’s C (PGC) medium [25], with the following specific formulation: 0.5 g K2HPO4, 1.0 g NH4CL, 0.06 g CaCl2·6H2O, 0.06 g MgSO4·7H2O, 6 mL 70% sodium lactate, 0.3 g sodium citrate, 1 g yeast extract, and 1 L natural seawater from the Qingdao coastal area. High-purity N2 gas was bubbled through the medium for 20 min to expel O2. IOB was inoculated into sterilized modified Winogradsky medium [35], with the following specific formulation: 10 g ammonium iron citrate, 0.5 g MgSO4·7H2O, 0.5 g NaNO3, 0.5 gK2HPO4, 0.5 g (NH4)2SO4, 0.2 g CaCl2, and 1 L sterilized natural seawater. After culturing both microorganisms at 30 °C for 5 days until the exponential growth phase, they were harvested for the biological experiments. The IOB and SRB strains were mixed with the corresponding culture media and sterilized seawater in appropriate proportions to prepare the mixed-bacteria seawater experimental solution, the SRB seawater experimental solution, and the IOB seawater experimental solution, respectively. Details are provided in Table 2.

Table 2.
Volume Composition of Different Experimental Solutions.
All bacterial culture media were sterilized in an autoclave at 121 °C for 20 min after adjusting the pH to 7.2 ± 0.1 using 2 mol/L NaOH prior to inoculation. After cooling, the media were inoculated and stored in wide-mouth bottles. The bottles were sealed with rubber stoppers, and a hole was made in the stopper to insert a sterile syringe for sampling. The sterile syringe was sealed with a 0.22 μm filter membrane to ensure air circulation between the bottle and the outside environment during the experiment. The initial inoculum concentration of SRB was 4.6 × 108/mL, and the initial inoculum concentration of IOB was 2.6 × 108/mL. During the 15-day experiment, samples were taken daily through the sampling port. The SO42− concentration in the mixed bacterial experimental solution was measured using a DX-120 ion chromatograph (DX-120, Thermo Fisher Scientific, Waltham, MA, USA) with an Anion Separation Column (Dionex AS 9-HC, Thermo Fisher Scientific, Waltham, MA, USA). The optical density (OD) value of the mixed bacterial experimental solution was measured at 600 nm using a UV–visible spectrophotometer (Evolution 300, Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Electrochemical Tests
A multi-channel potentiostat (CHI1030C, Shanghai CH Instruments, Shanghai, China) was used to apply cathodic protection potentials of −0.85 V (SCE), −0.95 V (SCE), and −1.05 V (SCE) to the mixed-bacteria seawater experimental solution containing IOB and SRB under a constant temperature of 30 °C. The experiment was conducted for 15 days, with continuous monitoring of current variations. After the 15-day continuous experiment, electrochemical tests were performed on the platform steel. Electrochemical tests were conducted using a Princeton P4000 electrochemical workstation (P4000A, Princeton Applied Research, Oak Ridge, TN, USA) with a three-electrode system. A platinum plate with an exposed area of 1 cm2 was used as the counter electrode, a saturated calomel electrode (SCE) was used as the reference electrode, and a Luggin capillary was employed to minimize the IR drop. The platform steel, after 15 days of cathodic protection at different cathodic protection potentials in the mixed-bacteria seawater experimental solution containing IOB and SRB, was used as the working electrode. Open circuit potential (OCP) measurements, electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization tests were conducted in a 3.5% NaCl solution at room temperature (26 °C). Electrochemical impedance spectroscopy was performed under open circuit potential, with a sinusoidal voltage amplitude of 10 mV and a scanning frequency range from 10−2 Hz to 10−5 Hz. The test results were analyzed and fitted using the ZsimpWin Version 3.21 software. The potentiodynamic polarization curve was scanned from −0.5 V vs. OCP to +0.5 V vs. OCP, with a scan rate of 0.1 mV/s.
2.4. Weight Loss Tests
For each test condition, three cathodic protection potentials of −0.85 V (SCE), −0.95 V (SCE), and −1.05 V (SCE) were set, with three parallel samples for each potential to ensure the reproducibility of the experimental results. Acid washing solution was used to remove the biofilm and corrosion products from the surface of the platform steel. The samples were then washed sequentially with distilled water and anhydrous ethanol and finally dried with high-purity N2. Based on the weight loss of the samples, the uniform corrosion rate (Vcorr, mm/y) of the platform steel was calculated using Equation (1).
where Δm is the weight difference of the sample before and after immersion, ρ is the density of the sample (g/cm3), A is the exposed surface area of the sample (cm2), and t is the immersion time (h).
2.5. Surface Morphology Analysis
2.5.1. Scanning Electron Microscope (SEM) Observation
After the 15-day experiment, the working electrodes subjected to different cathodic protection potentials were removed from the corresponding experimental solutions and rinsed with sterilized PBS solution to remove any unadsorbed suspended particles from the surface. The specimens were then fixed in 2.5% glutaraldehyde for 2 h. Dehydration was carried out sequentially using 50%, 70%, 90%, and 100% ethanol for 15 min each, followed by vacuum critical-point drying and gold sputter coating. A scanning electron microscope (SEM, VEGA3, TESCAN, Brno, Czech Republic) was used for observation. Additionally, the fracture morphology of the electrodes was examined, and the corrosion products and biofilm thickness on the surface were measured. The elemental composition of the corrosion products was analyzed using energy dispersive spectroscopy (EDS, XFlash® 6-60, Bruker, Berlin, Germany). The chemical composition of the corrosion products was analyzed by X-ray diffraction (XRD, SmartLab, Rigaku, Tokyo, Japan).
2.5.2. Fluorescence Microscopy Observation
After 15 days of immersion, electrodes subjected to different cathodic protection potentials were removed from the medium to evaluate microbial adhesion on the MMO anode surface via fluorescence staining. The electrodes were first rinsed with sterile PBS to eliminate loosely attached suspensions. The working surface was then stained using a mixture of SYTO-9 and propidium iodide (PI) dyes, followed by a 15-min incubation in darkness to ensure a complete reaction. Subsequently, fluorescence microscopy (BX53, Olympus, Tokyo, Japan) was employed to examine the stained electrodes and assess the distribution and viability of surface-adhered microorganisms.
2.5.3. Confocal Laser Scanning Microscope (CLSM) Observation
After the 15-day experiment, the working electrodes were removed from the corresponding experimental solutions and acid-washed using a corrosion product acid washing solution. The samples were then rinsed with deionized water to remove the acid washing solution and quickly dried with nitrogen. Finally, the samples were observed under a confocal laser scanning microscope (CLSM, LSM 800, Carl Zeiss AG, Oberkochen, Germany) to measure the depth and width of the surface corrosion pits.
3. Results and Discussion
3.1. Effects of Different Cathodic Protection Potentials on the Growth Metabolism of IOBs in Synergistic Coexistence with Srbs
Optical density (OD) is commonly used in microbiological research to measure bacterial growth. It provides an objective indication of microbial population size, particularly for free-floating microorganisms in solution, allowing for a more intuitive assessment of their quantitative variations [36]. Figure 1 shows the variation in OD values in the IOB and SRB mixed seawater experimental solution. Under all three cathodic protection potentials, the OD values of the mixed bacteria showed an initial increase followed by a decrease. On one hand, this is because, as the experiment progresses, the mixed bacteria first gradually enter the logarithmic growth phase, then transition to the stationary phase, and eventually enter the decline phase [37]. On the other hand, the colonization of the mixed bacteria on the electrode surface, changing from a free-living to an attached state, also leads to a gradual decrease in the free bacteria. Furthermore, it can be observed that under the −0.85 V (SCE) cathodic protection potential, the OD value of the mixed bacteria is the highest, indicating that the more positive cathodic protection potential promotes the growth of the mixed bacteria. Under the −0.95 V (SCE) cathodic protection potential, the OD value decreased more rapidly, likely due to the weakening of the dominance of individual bacterial species or the colonization of mixed bacteria on the electrode surface. Under the −1.05 V (SCE) cathodic protection potential, the OD value of the mixed bacteria was the lowest, which can be attributed to the production of hypochlorous acid [38] (Equation (2)), which exerts an inhibitory effect on the mixed bacteria. Overall, with the negative shift of the cathodic protection potential, the number of free-living mixed bacteria gradually decreased.
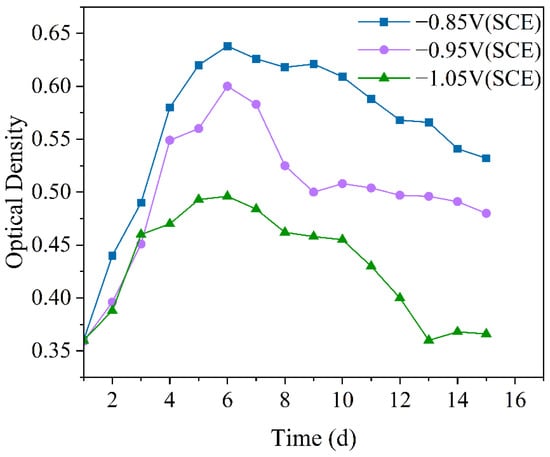
Figure 1.
Variation of optical density values over 15 days under different cathodic protection potentials in the mixed bacterial seawater experimental solution at a constant temperature of 30 °C.
SRB uses sulfate ions (SO42−) as the final electron acceptor, so the concentration of SO42− can indirectly reflect the metabolic activity of SRB [39]. Figure 2 shows the variation in SO42− ion concentration in the mixed bacterial experimental solution under different cathodic protection potentials. By examining the changes in SO42− concentration, the metabolic and reproductive activity of SRB in the mixed bacteria can be directly understood. Additionally, combining this with OD values allows for an inference about the growth and metabolism of IOB. From the figure, it can be seen that the ion concentration of SO42− under all three cathodic protection potentials shows a decreasing trend. Under the −0.85 V (SCE) cathodic protection potential, the concentration of SO42− decreased most significantly. From day 1 to day 2, the SO42− concentration decreased slowly, during which SRB began to metabolize, but their numbers were still low. From day 2 to day 6, the concentration of SO42− decreased rapidly, as SRB proliferated and metabolized in large numbers. From day 6 to day 15, the rate of decrease slowed down. Under the −0.95 V (SCE) cathodic protection potential, from day 1 to day 3, SRB metabolism was slow. From day 3 to day 6, the SO42− concentration decreased rapidly, indicating rapid growth and proliferation of SRB. From day 6 to day 15, SRB metabolism slowed down. Under the −1.05 V (SCE) cathodic protection potential, the SO42− concentration decreased slowly from day 1 to day 4, during which SRB began to grow and reproduce. From day 4 to day 10, the concentration of SO42− decreased rapidly, with SRB’s metabolism and reproduction reaching their peak. From day 10 to day 15, the decrease in SO42− concentration slowed down as SRB metabolism stabilized and entered the decline phase. Overall, as the cathodic protection potential shifted in the positive direction, the consumption rate of SO42− gradually increased. This is attributed to the varying degrees of SRB inhibition under different cathodic protection potentials. At more negative cathodic protection potentials, the metabolic activity of SRB is suppressed, weakening its sulfate-reducing capability and slowing the decline in sulfate concentration. As the cathodic protection potential becomes more positive, SRB inhibition decreases. At −0.85 V (SCE), the metabolic activity of SRB may even be promoted, leading to an accelerated sulfate-reduction rate with the positive shift of the cathodic protection potential.
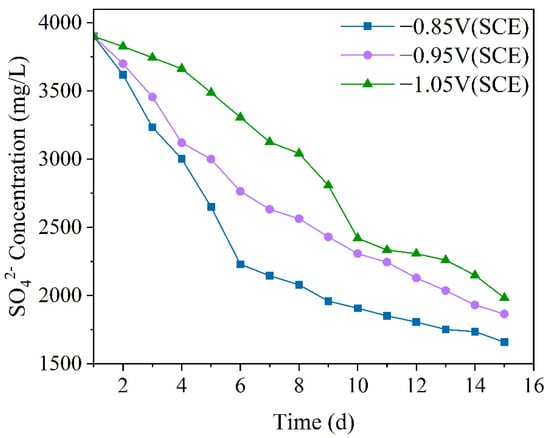
Figure 2.
The SO42− concentration variation over 15 days under different cathodic protection potentials in a 30 °C thermostatic mixed bacterial seawater solution.
By combining the changes in OD values and SO42− ion concentrations, the following analysis can be made: under the −0.85 V (SCE) cathodic protection potential, from days 1 to 2, the concentration of SO42− decreased slowly, while the OD value showed a rapid increase. This indicates that SRB metabolism is slow during this period, while IOB grows and proliferates rapidly. From days 2 to 6, the SO42− concentration decreased rapidly, as the rapid metabolism of IOB consumed large amounts of oxygen, creating favorable conditions for SRB growth and proliferation. As SRB gradually became the dominant species, IOB metabolism weakened, and the oxygen concentration in the solution rose [40]. From days 6 to 15, SRB’s metabolic intensity gradually weakened. Similarly, under the −0.95 V (SCE) cathodic protection potential, from days 1 to 3, the SO42− concentration decreased slowly, while the OD value rose rapidly. During this time, IOB proliferated in large numbers and began to consume the oxygen in the solution, creating an anaerobic environment for SRB. From days 3 to 6, SRB gradually became the dominant species and started to proliferate significantly. From days 6 to 15, as IOB metabolism weakened, SRB metabolism also slowed down. Under the −1.05 V (SCE) cathodic protection potential, from days 1 to 4, IOB proliferated rapidly, causing the OD value to rise quickly. From days 4 to 10, SRB began to proliferate in large numbers due to the anaerobic environment created by IOB. From days 10 to 15, as the anaerobic environment gradually diminished, SRB metabolism weakened. Comparing the abrupt changes in OD values and SO42− concentrations under the three cathodic protection potentials, it can be observed that the OD value decreased gradually after day 6, indicating a reduction in free microorganisms after this point. The phenomenon of the SO42− ion concentration decrease slowing down can be seen after day 10, suggesting that during the period from days 6 to 10, SRB metabolism and proliferation remained high, but free microorganisms decreased. Therefore, the number of microorganisms attached to the electrode surface tended to increase during this period.
Based on the changes in OD values and SO42− ion concentrations, it can be concluded that in the mixed culture of IOB and SRB, IOB rapidly proliferates and metabolizes in the early stage, consuming oxygen in the solution, reducing oxygen content, and creating a low-oxygen interface on the electrode surface. This provides favorable conditions for the anaerobic SRB to grow and metabolize. As time progresses, SRB gradually becomes the dominant species and begins to colonize the electrode surface [35]. Under the three cathodic protection potentials, at −0.85 V (SCE), the number of free mixed bacteria was higher, and SRB metabolism was the most rapid, indicating that at this cathodic protection potential, the growth and metabolism of mixed bacteria are promoted. At −1.05 V (SCE), there were fewer free mixed bacteria, and SRB metabolism was the slowest, suggesting that at this potential, the growth and metabolism of mixed bacteria are suppressed.
3.2. Effect of Synergistic Coexistence of IOB and SRB on the Corrosion Rate of Protected Metals at Different Cathodic Protection Potentials
The weight loss test can be used to determine the overall corrosion rate, providing insight into the effect of the synergistic interaction between mixed bacteria on the corrosion behavior of the protected metal. Figure 3 presents the variation in corrosion rate of the cathodic metal under different cathodic protection potentials after 15 days of cathodic protection in four environments: sterile, single-SRB, single-IOB, and mixed bacterial conditions. Compared to the control group without cathodic protection, the application of cathodic protection reduced the corrosion rate in all experimental groups. However, the protection efficiency was dependent on the applied cathodic protection potential. In the sterile and single-bacteria groups, the corrosion rate of the protected metal was minimized at a cathodic protection potential of −0.95 V (SCE), whereas at −0.85 V (SCE), the corrosion rate was relatively higher. In contrast, in the mixed bacterial group, the lowest corrosion rate was observed at −1.05 V (SCE), while at −0.85 V (SCE), the corrosion rate remained relatively high. These findings indicate that under the synergistic interaction of IOB and SRB, the effectiveness of cathodic protection at different potentials varies, leading to changes in the cathodic protection efficiency of the protected metal. Previous studies have shown that −0.95 V (SCE) is more suitable for ICCP systems in a single-species environment [25], but for mixed bacterial species, an effective cathodic protection potential requires a more negative shift.
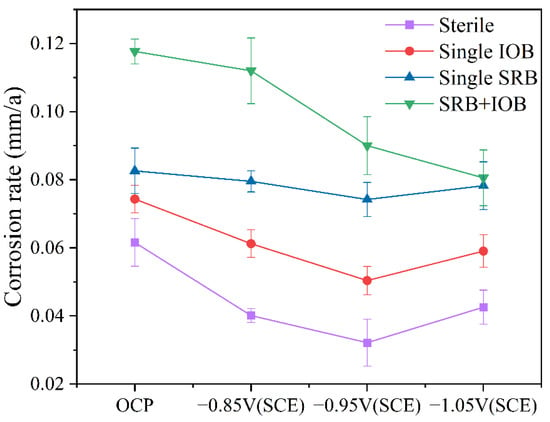
Figure 3.
Corrosion rate variation of cathodic metal with cathodic protection potential after 15 days of cathodic protection at 30 °C in sterile, single-SRB, single-IOB, and mixed bacterial environments.
3.3. Effect of Different Cathodic Protection Potentials on the Colonization of Mixed Strains on Cathodes and Anodes
The colonization of microorganisms on metal surfaces and the formation of biofilms are the main causes of MIC [41]. In an ICCP system, different cathodic protection potentials have varying effects on microorganisms, which results in different attachment behaviors on the electrodes. For auxiliary anodes, microbial colonization is often accompanied by the production of corrosion products [42]. The attachment of biofilms and corrosion product layers on the auxiliary anode alters the electrochemical activity of the anode surface, influencing the anode reaction and consequently affecting cathodic protection efficiency. For the protected metal, the formation of biofilms is associated with the occurrence of MIC, which means that in addition to the corrosion threat caused by environmental factors, the protected metal also faces the threat of MIC.
3.3.1. Mixed Bacterial Colonization on the Anode Surface at Different Cathodic Protection Potentials
Fluorescence staining and fluorescence microscopic observation of the MMO anode surface revealed the colonization of mixed bacteria, as shown in Figure 4. At a cathodic protection potential of −0.85 V (SCE) (Figure 4a), there was almost no colonization of mixed bacteria on the anode surface. This is because at this potential, mixed bacteria tend to colonize the cathode surface and form biofilms. The relatively positive cathodic protection potential does not suppress the mixed bacteria, while the colonization of IOB on the cathode metal surface provides a favorable anaerobic environment for SRB. At −0.95 V (SCE) (Figure 4b), mixed bacteria are more likely to colonize the anode surface, and a large number of microorganisms are attached to the MMO anode surface, mainly distributed in the grooves of the MMO anode. On the one hand, this is due to the hydrogen evolution reaction occurring at the cathode (Equation (3)), which makes the environment near the cathode more alkaline, unfavorable for the growth of IOB, and unable to create an anaerobic environment for SRB. However, the region near the anode is relatively neutral, which makes IOB more likely to survive near the anode [43,44]. At the same time, SRB’s growth and reproduction also migrate along with IOB. On the other hand, the rough surface of the MMO anode also provides favorable conditions for the colonization of mixed bacteria. At a cathodic protection potential of −1.05 V (SCE) (Figure 4c), there was a small amount of mixed bacteria colonizing the MMO anode surface, but they were widely scattered and showed no tendency to form biofilms. The higher cathodic protection potential leads to the production of Cl2 (Equation (4)) and the generation of HClO (Equation (2)) during the anode reaction. HClO has bactericidal effects [45,46], which prevents mixed bacteria from forming biofilms on the anode surface, even though they tend to colonize there.
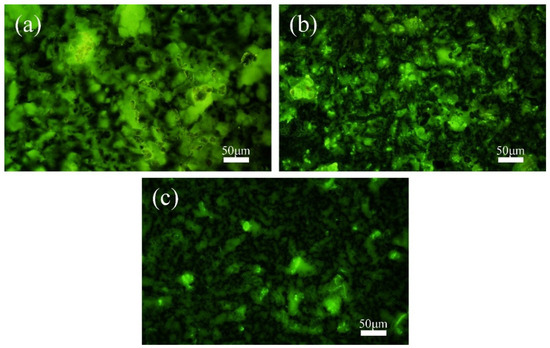
Figure 4.
Fluorescence microscopy images of the MMO anode surface after 15 days of cathodic protection at different cathodic protection potentials in a 30 °C mixed bacterial seawater environment: (a) −0.85 V (SCE); (b) −0.95 V (SCE); (c) −1.05 V (SCE).
3.3.2. Mixed Bacterial Colonization of Cathode Surface at Different Cathodic Protection Potentials
To further investigate the colonization of mixed bacteria on the anode and cathode, a scanning electron microscopy (SEM) was performed on the cathode metal, as shown in Figure 5. It is evident from the images that, at different cathodic protection potentials, the cathode metal surface shows adhesion of mixed bacteria or corrosion products, which can affect the electrochemical activity of the cathode metal, thereby impacting the cathodic protection efficiency of the ICCP system [47]. Specifically, at the cathodic protection potential of −0.85 V (SCE) (Figure 5a,a1), the cathode surface showed the most severe colonization of mixed bacteria, with a large amount of EPS matrix encapsulating the mixed bacteria and forming a dense biofilm. At this point, the relatively positive cathodic protection potential stimulated the growth of mixed bacteria [25]. In the early stages of the experiment, IOB began to colonize the cathode metal surface and rapidly metabolize, consuming oxygen and creating an anaerobic interface suitable for SRB growth. In the middle of the experiment, SRB began to colonize the cathode metal surface and formed a biofilm in cooperation with IOB. At the cathodic protection potential of −0.95 V (SCE) (Figure 5b,b1), EPS matrix and mixed bacteria can be observed on the cathode metal surface, but a complete biofilm had not yet formed, though there was a trend toward biofilm formation. At the cathodic protection potential of −1.05 V (SCE) (Figure 5c,c1), no obvious colonization of mixed bacteria could be observed on the cathode metal surface. However, a large number of crystalline corrosion products were attached to the cathode metal surface. At this cathodic protection potential, the colonization of mixed bacteria on the cathode metal surface was suppressed, but the corrosion products generated by the metabolism of free mixed bacteria still attached to the cathode metal surface. The dense corrosion products adhered to the cathode metal surface, which, on one hand, can alter the electrochemical activity of the cathode metal; on the other hand, their uneven distribution can lead to localized corrosion [48].
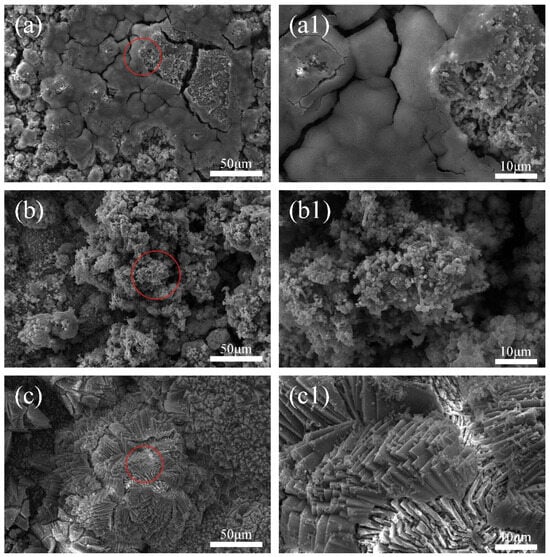
Figure 5.
Surface morphology of the cathodic metal without removal of corrosion products and surface microorganisms after 15 days of cathodic protection at different cathodic protection potentials in a 30 °C mixed bacterial seawater solution: (a,a1) −0.85 V (SCE); (b,b1) −0.95 V (SCE); (c,c1) −1.05 V (SCE).
3.3.3. Analysis of Cathodic Fracture Morphology and Corrosion Products at Different Cathodic Protection Potentials
To further investigate the adhesion of biofilms and corrosion products on the cathode metal surface under different cathodic protection potentials and to understand the composition of the corrosion products and biofilm, a cross-sectional analysis of the cathode metal surface was performed. The fracture morphology was observed using SEM, and the element distribution of the biofilm and corrosion product layers was analyzed. Figure 6 shows the fracture morphology and element distribution of the biofilm and corrosion product layers on the cathode metal surface under different cathodic protection potentials. From left to right, the substrate of the cathode metal and the corrosion film (corrosion products and biofilm) can be clearly distinguished. At the cathodic protection potential of −0.85 V (SCE) (Figure 6a), the corrosion film thickness was measured to be approximately 32.8 μm. The corrosion film was rich in O, S, Cl, and C; at the cathodic protection potential of −0.95 V (SCE) (Figure 6b), the corrosion film thickness was approximately 35.39 μm, similarly rich in O, S, Cl, and C; at the cathodic protection potential of −1.05 V (SCE) (Figure 6c), the corrosion film thickness was approximately 150 μm. The corrosion film primarily contained Fe, O, Cl, and C, with a lower amount of C compared to the −0.85 V (SCE) and −0.95 V (SCE) corrosion films. Based on the analysis of the cathode metal surface morphology, it can be concluded that, at the cathodic protection potentials of −0.85 V (SCE) and −0.95 V (SCE), the corrosion film was predominantly biofilm, while at −1.05 V (SCE), the corrosion film was primarily composed of corrosion products. Thus, it can be inferred that the presence of C in the corrosion film mainly originates from the biofilm. Additionally, the S element in the corrosion film primarily comes from the metabolic products of SRB involved in the MIC process. However, at the −1.05 V (SCE) cathodic protection potential, the corrosion film on the cathode metal surface contained very little S, indicating minimal SRB metabolism at this potential. The corrosion products at this potential are mainly due to IOB involvement in MIC. The distribution of O elements indicates the presence of IOB on the cathode metal surface. At the cathodic protection potentials of −0.85 V (SCE) and −0.95 V (SCE), the distribution of O elements gradually decreased from the outer to the inner layers of the corrosion film, indicating that IOB were primarily in the outer layers, while SRB were mainly in the inner layers. At the −1.05 V (SCE) cathodic protection potential, the O element distribution in the corrosion film was relatively uniform, suggesting that IOB were distributed throughout the entire corrosion layer. Furthermore, at all three cathodic protection potentials, the enrichment of Cl elements was relatively lower at −1.05 V (SCE). Huang et al. [49] found that sulfide films (such as FeS) covering the metal surface have an anion selectivity, meaning that Cl elements accumulate on the metal surface due to the selectivity of iron sulfides for anions. The accumulation of Cl elements through corrosion products on the metal surface can influence the corrosion of the metal substrate.
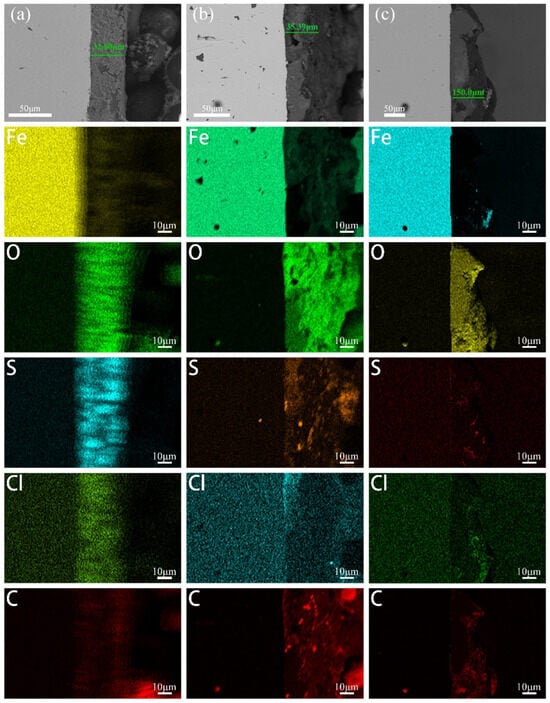
Figure 6.
Fracture morphology and elemental distribution of the corrosion film on the cathodic metal without removal of corrosion products and surface microorganisms after 15 days of cathodic protection at different cathodic protection potentials in a 30 °C mixed bacterial environment: (a) −0.85 V (SCE); (b) −0.95 V (SCE); (c) −1.05 V (SCE).
Figure 7 shows the X-ray diffraction (XRD) analysis results of the corrosion products on the cathodic metal after 15 days of cathodic protection at different cathodic protection potentials in a 30 °C mixed bacterial marine environment. The corrosion products on the cathode metal surface at the three different cathodic protection potentials all contained Fe2O3 and Fe(OH)3, which are the main products of IOB metabolism [50]. This indicates that IOB participated in the MIC process at all three cathodic protection potentials. At −0.85 V (SCE) and −0.95 V (SCE) cathodic protection potentials, the corrosion product film contained FeS, while at −1.05 V (SCE), the corrosion product film did not contain FeS. This result is consistent with the EDS analysis, which indicates that at the more negative cathodic protection potential, the MIC caused by mixed microbial species involved only a small amount of SRB. At the same time, based on the morphology of the characteristic peaks, it can be observed that the FeS characteristic peaks were more pronounced at the cathodic protection potential of −0.95 V (SCE). This is because, as the cathodic protection potential shifts negatively, the activity of SRB is gradually inhibited, leading to the stabilized formation of FeS. With sufficient time for crystallization, larger grains are formed. This indicates that as the cathodic protection potential shifts negatively, the formation of corrosion products becomes progressively more stable.
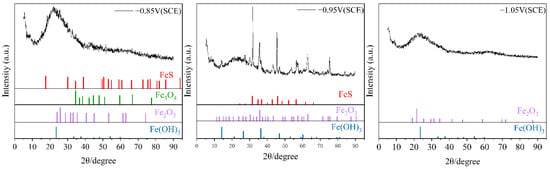
Figure 7.
XRD analysis of corrosion products on cathodic metal after 15 days of cathodic protection under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C.
In summary, there are significant differences in the colonization of mixed bacteria and the attachment of corrosion products on the cathodic and anodic surfaces at different cathodic protection potentials. At −0.85 V (SCE), the mixed bacteria attachment on the anode surface was not prominent, while a dense biofilm formed on the cathode surface. At this potential, the mixed bacteria were more likely to colonize the cathode. At −0.95 V (SCE), mixed bacteria were observed to colonize both the cathode and anode surfaces, with the most severe colonization occurring on the anode. At −1.05 V (SCE), mixed bacteria colonization on both the cathode and anode surfaces was not obvious, with a large number of crystalline corrosion products attached to the cathode surface and a small amount of mixed bacteria attached to the anode surface but with no biofilm formation. The different surface morphologies of the anode and cathode affect the electrochemical activity, and consequently, they influence the cathodic protection efficiency at the corresponding cathodic protection potentials [51]. Analysis of the OD value results revealed that at −0.85 V (SCE), the mixed bacteria OD value was the highest, with the most severe microbial attachment on both the anode and cathode surfaces. At this potential, the growth and reproduction of mixed bacteria were stimulated, whether in free or attached form. At −1.05 V (SCE), the number of free mixed bacteria was the lowest, and microbial attachment on the anode and cathode surfaces was minimal. At this potential, the growth and metabolism of mixed bacteria were inhibited, which is related to the suppression effect of the more negative potential on microorganisms and the production of HClO on the anode [52]. Analysis of the fracture morphology and elemental distribution of corrosion films on the cathode metal surfaces at the three cathodic protection potentials indicated that at −0.85 V (SCE) and −0.95 V (SCE), the corrosion film was primarily composed of biofilm, with IOB and SRB acting synergistically in the MIC process. The IOB were distributed in the outer layer of the corrosion film, while the SRB were located in the inner layer of the corrosion film. At −1.05 V (SCE), the corrosion layer was mainly composed of corrosion products, with dense corrosion products adhering to the metal substrate surface. The primary microorganism involved in the MIC process is IOB, while SRB plays a minimal role in the corrosion process. The analysis of corrosion products further supports this conclusion.
3.4. Effect of Synergistic IOB and SRB on Cathodic Protection Efficiency at Different Cathodic Protection Potentials
By applying different cathodic protection potentials in the ICCP system and measuring the current over 15 days in a marine environment containing mixed bacteria, the variation in cathodic protection efficiency at specific potentials can be analyzed. As shown in Figure 8, the calculated changes in current density over time for different cathodic protection potentials are presented. For all three cathodic protection potentials, the current density initially shifted in the positive direction before transitioning to a more negative value over time. This trend is attributed to the presence of an oxide film on the protected metal surface in the early stages of the experiment, which reduces its electrochemical activity, resulting in a relatively positive current density. As the immersion time increased, the mixed bacteria began to metabolize and proliferate, leading to the degradation of the oxide film and an increase in the electrochemical activity of the metal, which in turn caused the current density to shift in the negative direction. Among the three cathodic protection potentials, the current density at −0.85 V (SCE) was the least negative, while at −1.05 V (SCE), it was the most negative, indicating differences in cathodic protection efficiency across the tested potentials. Specifically, at the cathodic protection potential of −0.85 V (SCE), the relatively positive potential failed to provide sufficient protection for the cathodic metal [25]. Additionally, the adhesion of mixed bacteria and the formation of a biofilm on both the anode and cathode surfaces hindered electrochemical reactions, resulting in a less negative current density and lower cathodic protection efficiency. At −0.95 V (SCE), as immersion time increased, mixed bacteria began to colonize the cathode and anode surfaces. However, due to the low compactness of the biofilm and the absence of a fully developed structure, the influence on the electrochemical reactions remained limited. Meanwhile, the accumulation of microorganisms and corrosion products on the cathode alters the surface morphology, affecting its electrochemical activity. Consequently, the initial protective current density became insufficient to maintain the designated cathodic protection potential, leading to a continuous shift toward more negative current density over time. When the cathodic protection potential shifted negatively to −1.05 V (SCE), a more negative current density was required to sustain this potential, resulting in an initially more negative current density compared to the other two cathodic protection potentials. Notably, after three days of experimentation, a significant negative shift in current density was observed. At this stage, IOB proliferated extensively and generated corrosion products. Subsequently, a large amount of dense corrosion products adhered to the cathodic metal surface, forming crystalline structures that significantly altered its electrochemical activity. To maintain the highly negative cathodic protection potential, the current density underwent a pronounced negative shift.
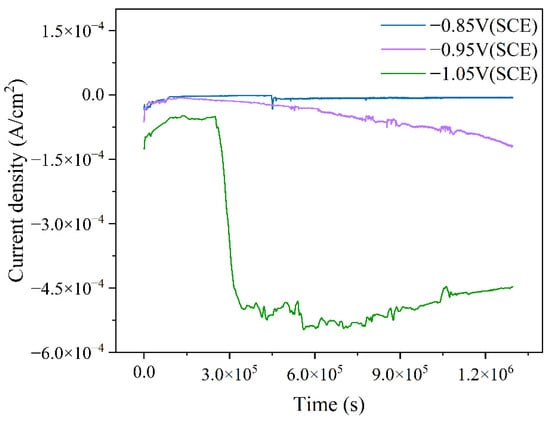
Figure 8.
Time-dependent variation of cathodic metal current density over 15 days under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C.
In a marine environment containing mixed IOB and SRB, the corrosion film formed under a more negative cathodic protection potential, which is primarily composed of corrosion products and has a greater impact on altering the electrochemical activity of the electrode surface compared to the biofilm-dominated corrosion film formed under a more positive cathodic protection potential. The dense corrosion products adhering to the protected metal surface reduce its electrochemical activity, leading to a negative shift in current density. At the same time, the corrosion product film acts as a protective barrier, shielding the cathodic metal from MIC, thereby enhancing cathodic protection efficiency [53].
Because the anodic dissolution process of the protected metal follows the Tafel law, it can be assumed that only one electrode reaction occurs in the strong polarization region of the Tafel polarization curve on the electrode surface. Assuming that the reaction’s kinetic mechanism remains unchanged from the corrosion potential to the measured polarization potential, the kinetics of this reaction can be analyzed using the following expression [25]. The relationship between the cathodic protection potential Epr and the anodic dissolution current Ia under Epr can be expressed as follows:
Ecorr represents the corrosion potential of platform steel under non-polarized conditions, and Icorr represents the corrosion current density of platform steel under non-polarized conditions.
In the strongly polarized region, log |Ia| is proportional to ∆E; ba and Icorr can be calculated from the polarization curves; therefore, Ia at the protection potential Epr can be calculated from Equation (6) [54].
Figure 9 shows the cathodic metal potentiodynamic polarization curves after 15 days of immersion in a marine environment containing IOB and SRB at different cathodic protection potentials. The results are shown in Table 3. The parameters are as follows:
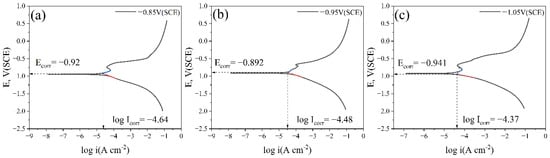
Figure 9.
Potentiodynamic polarization curves of cathodic metal without removal of corrosion products and surface microorganisms after 15 days of cathodic protection under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C: (a) potentiodynamic polarization curve after 15 days at a cathodic protection potential of −0.85 V (SCE); (b) potentiodynamic polarization curve after 15 days at a cathodic protection potential of −0.95 V (SCE); (c) potentiodynamic polarization curve after 15 days at a cathodic protection potential of −1.05 V (SCE).

Table 3.
Fitting parameters of potentiodynamic polarization curves of cathodic metal without removal of corrosion products and surface microorganisms after 15 days of cathodic protection under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C.
ba: anodic Tafel slope.
Ecorr: corrosion potential.
Icorr: corrosion current density at the corrosion potential.
Ia: anodic dissolution current density at the respective cathodic protection potential.
By comparing the potentiodynamic polarization curves of the cathodic metal at the three different cathodic protection potentials, it can be seen that at −0.85 V (SCE), the cathodic metal had a low corrosion current density (Icorr). Although the corrosion potential (Ecorr) was high, the anodic dissolution current density (Ia) was the lowest. At this potential, the cathodic protection efficiency was relatively high, but the positive protection potential was insufficient to provide enough protection for the cathodic metal. At −0.95 V (SCE), the corrosion current density and anodic dissolution current density of the cathodic metal were higher than at −0.85 V (SCE). At this potential, a large number of microorganisms attached to both the cathodic and anodic surfaces. Although no biofilm formed on the cathode, the abundant EPS matrix and corrosion products altered the electrochemical activity of the cathodic metal, causing interference with the cathodic reaction. Additionally, the formation of biofilms on the anodic surface also hindered the anodic reaction, leading to a decrease in cathodic protection efficiency [55]. At −1.05 V (SCE), both the corrosion current density and anodic dissolution current density of the cathodic metal were the highest. At this potential, a dense layer of corrosion products attached to the cathodic surface, greatly hindering the occurrence of cathodic reactions. As a result, the cathodic protection efficiency was reduced. However, it is worth noting that the dense corrosion product film also provided protection to the cathodic metal, isolating the metal surface from the external medium and thus exhibiting a corrosion-inhibiting effect.
To better understand the relationship between cathodic protection efficiency and cathodic protection potential in an ICCP system in marine environments containing SRB and IOB, electrochemical impedance spectroscopy tests were conducted on the cathodic metal at different cathodic protection potentials. Figure 10 shows the electrochemical impedance spectroscopy of the cathodic metal at different cathodic protection potentials in the marine environment containing SRB and IOB. The impedance spectra were fitted using the equivalent circuit shown in Figure 11, where Rs: solution resistance; Qf: constant phase element (CPE) representing the biofilm or corrosion product layer; Rpore.s: the resistance of the corrosion film (biofilm or corrosion product); Qdl: the constant phase element (CPE) for the double-layer capacitance; Rct: charge transfer resistance of the double layer. The fitted data are shown in Table 4.
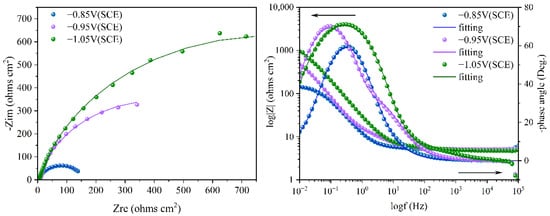
Figure 10.
Electrochemical impedance spectroscopy of cathodic metal after 15 days of cathodic protection under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C.
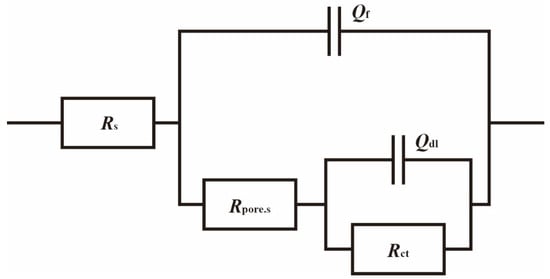
Figure 11.
Electrochemical impedance spectroscopy fitting circuit of cathodic metal after 15 days of cathodic protection under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C.

Table 4.
Electrochemical impedance spectroscopy fitting data of cathodic metal after 15 days of cathodic protection under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C.
The electrochemical impedance spectra indicate that after 15 days of immersion in a marine environment containing SRB and IOB, the cathodic metal at different cathodic protection potentials showed varying corrosion levels. Specifically, at the −0.85 V (SCE) cathodic protection potential, the corrosion of the cathodic metal was the most severe, while at −1.05 V (SCE), the corrosion was the least. To be specific, at the −0.85 V (SCE) cathodic protection potential, the relatively positive potential promoted the growth and proliferation of mixed bacteria. The metabolism of IOB created an anaerobic environment favorable for the growth of SRB, which then colonized the cathodic surface and formed a biofilm. This biofilm further facilitated the synergistic growth of the mixed bacteria [17]. Additionally, corrosion products such as FeS accumulated and promoted the diffusion of Cl− in the seawater environment. However, the relatively positive cathodic protection potential was insufficient to provide adequate protection to the cathodic metal. Therefore, the metal faced the dual threat of MIC and corrosion from the marine environment. At this time, the Rct value of the cathodic metal was low, and surface morphology analysis coupled with corrosion film resistance (Rpore.s) revealed that the corrosion film was primarily composed of a biofilm that was thicker than the biofilm-dominated corrosion film observed at −0.95 V (SCE). At −0.95 V (SCE), the impedance values of the cathodic metal increased with the more negative potential. Both the cathodic and anodic surfaces had microbial attachments, and although the cathodic surface did not form a complete biofilm, a significant amount of EPS matrix was produced. This resulted in inhibition of the cathodic reaction. The biofilm on the anodic surface also hindered anodic reactions. Although the −0.95 V (SCE) potential provided sufficient protection to the metal in the marine environment, the microbial attachment on the cathodic surface impeded the cathodic reaction and altered the electrochemical activity of the metal surface. This indicates that a more negative cathodic protection potential was needed to protect the cathodic metal, and the cathodic metal also faced the threat of MIC. Thus, although Rct increased, the cathodic protection effect was not ideal. At the −1.05 V (SCE) cathodic protection potential, the metabolic proliferation of mixed bacteria was inhibited. This led to a reduction in microbial attachment on both the cathodic and anodic surfaces. Almost no microorganisms attached to the cathodic surface, but corrosion products generated by free mixed bacteria still attached to the cathodic surface. The dense corrosion products made it necessary to apply higher current to maintain the cathodic protection potential, indirectly improving the cathodic protection efficiency. Surface morphology analysis indicated that the corrosion film was primarily composed of corrosion products, with a larger Rpore.s, confirming the thick and dense nature of the corrosion layer. This dense corrosion product layer provided a certain degree of corrosion inhibition [56], protecting the cathodic metal from corrosion threats posed by both the marine environment and MIC. As a result, the Rct value was the highest, and the cathodic protection effect was optimal.
Through confocal laser scanning microscopy (CLSM), the cathodic metal with corrosion products and surface microorganisms removed was observed. It was found that the surface of the cathodic metal under the three different cathodic protection potentials exhibited varying degrees of pitting corrosion. The pits were measured and counted, as shown in Figure 12. Among the three cathodic protection potentials, at −0.85 V (SCE), the number of pits on the cathodic metal surface was relatively low, but the depth was greater, showing a trend of development towards the metal interior. This was caused by the attachment of mixed bacteria to the metal surface. Simultaneously, uniform corrosion was the most severe, which also indicates that the less negative cathodic protection potential could not provide adequate protection for the cathodic metal. At −0.95 V (SCE), the cathodic metal surface exhibited the most pits, which were relatively wide but shallow. Under this cathodic protection potential, a complete biofilm was not formed on the metal surface, and the bacteria were distributed more sporadically, leading to localized pitting at multiple points on the metal. At −1.05 V (SCE), the cathodic metal surface exhibited the fewest and lightest pits, with only a small number of shallow pits present. These pits were caused by the MIC during the early stages of the experiment, before the dense accumulation of corrosion products. Later, the dense corrosion product film accumulated on the metal surface, alleviating the MIC caused by the mixed bacteria. Simultaneously, the more negative cathodic protection potential not only provided adequate protection to the metal but also inhibited the growth of mixed bacteria. Combined with the surface morphology and elemental analysis of the corrosion film, it can be concluded that a relatively positive cathodic protection potential suppresses the growth of SRB [57]. At this time, the MIC process involves only a small number of SRB; thus, the formation of pitting corrosion is primarily associated with the metabolism of SRB, which accelerates the corrosion of the metal through metabolites such as FeS [58].
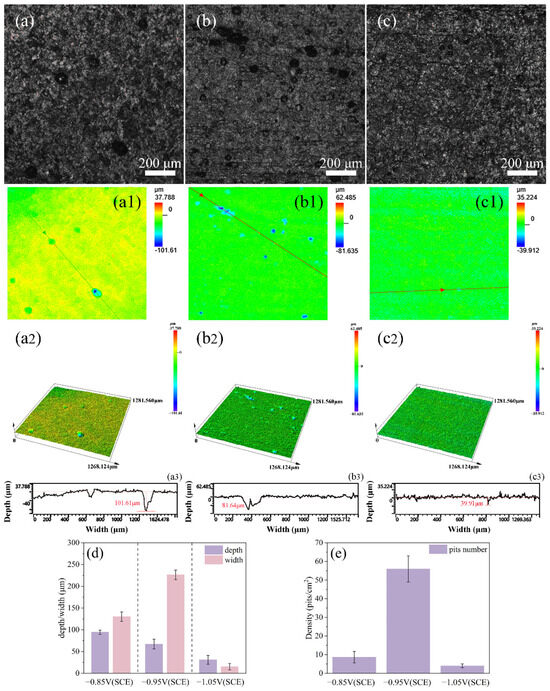
Figure 12.
CLSM images of cathodic metal after removing corrosion products and surface microorganisms following 15 days of cathodic protection under different cathodic protection potentials in a mixed bacterial marine environment at a constant temperature of 30 °C: (a–a3) −0.85 V (SCE); (b–b3) −0.95 V (SCE); (c–c3) −1.05 V (SCE). (d,e) Data on the number, depth, and width of corrosion pits after 15 days of cathodic protection under different cathodic protection potentials.
4. Conclusions
By studying the growth and metabolism of IOB and SRB mixed bacteria under different cathodic protection potentials in a marine environment as well as their attachment to the anode and cathode surfaces and the impact on cathodic protection efficiency, it was found that in marine environments containing IOB and SRB, the selection of protection potential for the ICCP system requires an appropriate negative shift. This shift helps improve cathodic protection efficiency, suppress bacterial growth, and alleviate microbial corrosion caused by the mixed bacteria. The specific findings are as follows:
- Among the three cathodic protection potentials used in the experiment, the −0.85 V (SCE) potential exhibited the weakest inhibitory effect on the mixed bacteria, even promoting their growth. Under this condition, the mixed bacteria tended to adhere to the cathode surface and form a biofilm. In contrast, the −1.05 V (SCE) cathodic protection potential effectively suppressed the growth of the mixed bacteria, resulting in minimal bacterial attachment on both the cathode and anode. Therefore, in a marine environment containing IOB and SRB, an appropriate negative shift in the cathodic protection potential can leverage the inhibitory effect of more negative potentials on microbial activity as well as the bacterial tendency to adhere under different protection potentials to effectively mitigate the threat of MIC;
- In a marine environment containing IOB and SRB, the corrosion products formed during cathodic protection of metals become increasingly stable as the cathodic protection potential shifts negatively. This phenomenon is closely related to the metabolic activity of the mixed bacteria. More positive cathodic protection potentials promote bacterial metabolic activity, leading to the formation of loose and compositionally diverse corrosion products. As the cathodic protection potential shifts negatively, bacterial metabolism is inhibited, resulting in the formation of denser and more stable corrosion products;
- At a more negative cathodic protection potential, a crystalline corrosion film primarily composed of corrosion products forms on the cathode surface, with Fe2O3 and Fe(OH)3 as its main components. Experimental results demonstrated that this corrosion film effectively isolates the metal surface from the external liquid environment, providing a protective effect on the metal. Additionally, the more negative cathodic protection potential inhibits SRB adhesion and metabolism on the metal surface, mitigating the corrosion threat caused by microbial attachment. Furthermore, due to the presence of the corrosion film, overprotection does not occur at this potential, thereby improving the efficiency of cathodic protection.
The experiment demonstrated that in a marine environment containing IOB and SRB, when impressed current cathodic protection is applied, a more negative cathodic protection potential can form a corrosion film predominantly composed of corrosion products on the cathode surface, thereby enhancing cathodic protection efficiency. In specific working environments, this characteristic can be utilized by appropriately shifting the cathodic protection potential to a more negative value, which not only improves cathodic protection efficiency but also mitigates MIC caused by mixed bacteria.
Author Contributions
Conceptualization, Z.Z. and J.Z.; methodology, X.C. and B.H.; software, J.D. and B.H.; validation, Z.Z.; formal analysis, Z.Z. and K.W.; investigation, Z.Z., J.D. and X.C.; resources, J.Z. and Q.H.; data curation, Z.Z.; writing—original draft, Z.Z.; writing—review & editing, J.Z.; visualization, X.C.; supervision, Q.H., C.Z., K.W., X.C. and B.H.; project administration, J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (No. 2024YFB4207000), the Major Basic Research Project of Natural Science Foundation of Shandong Province (No. ZR2023ZD31), and the National Natural Science Foundation of China (No. 42076043).
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.
Conflicts of Interest
Qingle Hou and Chengjun Zhang were employed by Wanhua Chemical Group Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rong, H.; Zhao, X.; Zhao, Z.; Sun, H.; Fu, Q.; Ding, R.; Yang, J.; Fan, W.; Xiao, F. Effect of Acinetobacter Sp. on Corrosion Behavior of 10MnNiCrCu Steel in Simulated Marine Environment. Int. J. Electrochem. Sci. 2021, 16, 210638. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Fan, Y.; Xu, D. Microbially Influenced Corrosion of Steel in Marine Environments: A Review from Mechanisms to Prevention. Microorganisms 2023, 11, 2299. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Liang, T.; Liu, Y.; Shi, Y.; Zhang, H.; Li, H.; Guo, S.; Pan, H.; Yang, K.; Zhao, Y. Microbiologically Influenced Corrosion Mechanism of Ferrous Alloys in Marine Environment. Metals 2022, 12, 1458. [Google Scholar] [CrossRef]
- Knisz, J.; Eckert, R.; Gieg, L.M.; Koerdt, A.; Lee, J.S.; Silva, E.R.; Skovhus, T.L.; Stepec, B.A.A.; Wade, S.A. Microbiologically influenced corrosion—More than just microorganisms. FEMS Microbiol. Rev. 2023, 47, fuad041. [Google Scholar] [CrossRef]
- Javaherdashti, R. Editorial: Microbiologically influenced corrosion (MIC): Its mechanisms, technological, economic, and environmental impacts. Front. Microbiol. 2023, 14, 1249565. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhang, J.; Mathivanan, K.; Wang, F.; Duan, J.; Hou, B. Advances in understanding biofilm-based marine microbial corrosion. Corros. Rev. 2024. [Google Scholar] [CrossRef]
- Lv, M.; Du, M. A review: Microbiologically influenced corrosion and the effect of cathodic polarization on typical bacteria. Rev. Env. Sci. Biotechnol. 2018, 17, 431–446. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Yao, G.; He, X.; Liu, J.; Guo, Z.; Chen, P. Test Study of the Bridge Cable Corrosion Protection Mechanism Based on Impressed Current Cathodic Protection. Lubricants 2023, 11, 30. [Google Scholar] [CrossRef]
- Dargahi, M.; Mahidashti, Z.; Rezaei, M. Corrosion prevention of storage tank bottom using impressed current cathodic protection—Experimental and simulation study. Eng. Fail. Anal. 2024, 158, 107982. [Google Scholar] [CrossRef]
- Gurrappa, I.; Yashwanth, I.V.S.; Mounika, I. Cathodic Protection Technology for Protection of Naval Structures Against Corrosion. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2015, 85, 1–18. [Google Scholar] [CrossRef]
- Erdogan, C.; Swain, G. The Effects of Biofouling and Corrosion Products on Impressed Current Cathodic Protection System Design for Offshore Monopile Foundations. J. Mar. Sci. Eng. 2022, 10, 1670. [Google Scholar] [CrossRef]
- Lv, M.; Li, X.; Du, M. The effect of cathodic polarization on the corrosion behavior of X65 steel in seawater containing sulfate-reducing bacteria. Mater. Corros. 2020, 71, 2038–2051. [Google Scholar] [CrossRef]
- Liduino, V.; Galvão, M.; Brasil, S.; Sérvulo, E. SRB-mediated corrosion of marine submerged AISI 1020 steel under impressed current cathodic protection. Colloids Surf. B Biointerfaces 2021, 202, 111701. [Google Scholar] [CrossRef]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Lv, M.; Du, M.; Li, Z. Investigation of mixed species biofilm on corrosion of X65 steel in seawater environment. Bioelectrochemistry 2022, 143, 107951. [Google Scholar] [CrossRef]
- Jogdeo, P.; Chai, R.; Shuyang, S.; Saballus, M.; Constancias, F.; Wijesinghe, S.L.; Thierry, D.; Blackwood, D.J.; McDougald, D.; Rice, S.A.; et al. Onset of Microbial Influenced Corrosion (MIC) in Stainless Steel Exposed to Mixed Species Biofilms from Equatorial Seawater. J. Electrochem. Soc. 2017, 164, C532–C538. [Google Scholar] [CrossRef]
- Lv, M.; Du, M.; Li, X.; Yue, Y.; Chen, X. Mechanism of microbiologically influenced corrosion of X65 steel in seawater containing sulfate-reducing bacteria and iron-oxidizing bacteria. J. Mater. Res. Technol. 2019, 8, 4066–4078. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Song, Y.; Liu, W.; Zhang, J.; Li, N.; Dong, K.; Cai, Y.; Han, E.-H. The respective roles of sulfate-reducing bacteria (SRB) and iron-oxidizing bacteria (IOB) in the mixed microbial corrosion process of carbon steel pipelines. Corros. Sci. 2024, 240, 112479. [Google Scholar] [CrossRef]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Anandkumar, B.; George, R.P.; Maruthamuthu, S.; Parvathavarthini, N.; Mudali, U.K. Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: An overview. Corros. Rev. 2016, 34, 41–63. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Huang, H.; Xiang, Y.; Yan, W. Symbiosis of Sulfate-Reducing Bacteria and Total General Bacteria Affects Microbiologically Influenced Corrosion of Carbon Steel. Coatings 2024, 14, 788. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Ruan, H.M.; Zhang, G.A. Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique. Corros. Sci. 2011, 53, 2978–2987. [Google Scholar] [CrossRef]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, M.; Hou, B. Influence of Sulfate-Reducing Bacteria on the Corrosion Behavior of High Strength Steel EQ70 under Cathodic Polarization. PLoS ONE 2016, 11, e0162315. [Google Scholar] [CrossRef]
- Xu, D.; Gu, T. Bioenergetics Explains When and Why More Severe MIC Pitting by SRB Can Occur, Corrosion/Paper. 2011. Available online: http://www.researchgate.net/publication/266345253_Bioenergetics_Explains_When_and_Why_More_Severe_MIC_Pitting_by_SRB_Can_Occur (accessed on 17 February 2025).
- Sachan, R.; Singh, A.K. Comparison of microbial influenced corrosion in presence of iron oxidizing bacteria (strains DASEWM1 and DASEWM2). Constr. Build. Mater. 2020, 256, 119438. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Haghighi, M.; Zarrini, G.; Javaherdashti, R. Corrosion behavior of carbon steel in the presence of two novel iron-oxidizing bacteria isolated from sewage treatment plants. Biodegradation 2012, 23, 69–79. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Kang, M.; Jia, S. Electrochemical mechanism of 317L stainless steel under biofilms with coexistence of iron-oxidizing bacteria and sulfatereducing bacteria. Int. J. Electrochem. Sci. 2019, 14, 10139–10152. [Google Scholar] [CrossRef]
- Junxi, Z.; Jinliang, L.; Licheng, Y.; Yu, F.; Lingsong, Z.; Yu, Z. The applicability of EIS to determine the optimal polarization potential for impressed current cathodic protection. Anti-Corros. Methods Mater. 2010, 57, 249–252. [Google Scholar] [CrossRef]
- ISO 6344-3; Coated Abrasives, Determination and Designation of Grain Size Distribution—Part 3: Microgrit Sizes P240 to P5000. International Organization for Standardization: Geneva, Switzerland, 2021.
- Liu, H.; Gu, T.; Zhang, G.; Wang, W.; Dong, S.; Cheng, Y.; Liu, H. Corrosion inhibition of carbon steel in CO2-containing oilfield produced water in the presence of iron-oxidizing bacteria and inhibitors. Corros. Sci. 2016, 105, 149–160. [Google Scholar] [CrossRef]
- Dinh, H.T.; Kuever, J.; Mußmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron corrosion by novel anaerobic microorganisms. Nature 2004, 427, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Duan, J.; Du, X.; Huang, Y.; Hou, B. Accelerated anaerobic corrosion of electroactive sulfate-reducing bacteria by electrochemical impedance spectroscopy and chronoamperometry. Electrochem. Commun. 2013, 26, 101–104. [Google Scholar] [CrossRef]
- Liu, H.; Fu, C.; Gu, T.; Zhang, G.; Lv, Y.; Wang, H.; Liu, H. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci. 2015, 100, 484–495. [Google Scholar] [CrossRef]
- Yoon, J.H.; Shin, J.-H.; Park, J.H.; Park, T.H. Effect of light intensity on the correlation between cell mass concentration and optical density in high density culture of a filamentous microorganism. Korean J. Chem. Eng. 2015, 32, 1842–1846. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chien, W.-S.; Duan, K.-J.; Chang, P.R. Effect of aeration timing and interval during very-high-gravity ethanol fermentation. Process Biochem. 2011, 46, 1025–1028. [Google Scholar] [CrossRef]
- Abe, H.; Kobayakawa, T.; Maruyama, H.; Wakabayashi, T.; Nakayama, M. Thin Film Coating of Mg-Intercalated Layered MnO2 to Suppress Chlorine Evolution at an IrO2 Anode in Cathodic Protection. Electrocatalysis 2019, 10, 195–202. [Google Scholar] [CrossRef]
- Chen, J.; Gan, L.; Han, Y.; Owens, G.; Chen, Z. Ferrous sulfide nanoparticles can be biosynthesized by sulfate-reducing bacteria: Synthesis, characterization and removal of heavy metals from acid mine drainage. J. Hazard. Mater. 2024, 466, 133622. [Google Scholar] [CrossRef]
- Xiaojing, D.; Haodan, P.; Maocheng, Y.; Udowo, V.M.; Xiaoxu, L. Microbial Corrosion of X60 Pipeline Steel in Groundwater Containing Sulfate-Reducing Bacteria/Iron-Oxidizing Bacteria Mixed Colonies. J. Mater. Eng. Perform. 2024, 33, 11852–11862. [Google Scholar] [CrossRef]
- Ogawa, A.; Hosaka, S.; Kanematsu, H.; Yoshitake, M. Marine Biofilm Model Comprising a Loop-Type Biofilm Reactor and a Halomonas Strain HIG FST4 1, an Active Biofilm-Forming Bacterium. Coatings 2022, 12, 1605. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Wan, J.; Zhao, P. Study of biofilm influenced corrosion on cast iron pipes in reclaimed water. Appl. Surf. Sci. 2015, 357, 236–247. [Google Scholar] [CrossRef]
- Liu, W.; Bi, W.; Hu, Y.; Lu, W.; Feng, W.; Wang, Y.; Li, Y.; Liu, J. Influence of initial pH and sulfate-reducing bacteria concentration on the microbiologically influenced corrosion of buried pipeline steel. Mater. Corros. 2024, 75, 1193–1203. [Google Scholar] [CrossRef]
- Little, B.J.; Wagner, P.A. The Interrelationship between Marine Biofouling and Cathodic Protection. Mater. Perform. 2007, 32, 8. [Google Scholar] [CrossRef]
- Fatemi, H.; Hadigheh, S.A.; Tao, Y.; Adam, G. Development of a novel and specialised cementitious matrix overlay for anode embedment in impressed current cathodic protection (ICCP) systems for reinforced concrete bridges. Case Stud. Constr. Mater. 2024, 20, e02908. [Google Scholar] [CrossRef]
- Castelli, F.; Delucchi, M.; Valenza, F.; Garaventa, F.; Faimali, M.; Turturro, T.; Benedetti, A. Behavior of biocide-free foul control paints for ships’ hulls in the immediate proximity of ICCP anodes. J. Coat. Technol. Res. 2024, 21, 383–399. [Google Scholar] [CrossRef]
- Dai, K.; Li, S.; Hu, P.; Jiang, N.; Wang, D. Effect of outer rust layer on cathodic protection and corrosion behavior of high-strength wire hangers with sheath crack in marine rainfall environment. Case Stud. Constr. Mater. 2023, 18, e02043. [Google Scholar] [CrossRef]
- Lu, J.C.; Wang, Z.B.; Hu, H.X.; Zheng, Y.G. Understanding localized corrosion mechanism of 90/10 copper-nickel alloy in flowing NaCl solution induced by partial coverage of corrosion products films. Corros. Sci. 2024, 227, 111716. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, P.; Zhao, X.Y.; Liu, J.; Hu, Q.; Cheng, Y.F. Effect of sulfide films formed on X65 steel surface on hydrogen permeation in H2S environments. Int. J. Hydrogen Energy 2017, 42, 4561–4570. [Google Scholar] [CrossRef]
- Yue, Y.; Lv, M.; Du, M. The corrosion behavior and mechanism of X65 steel induced by iron-oxidizing bacteria in the seawater environment. Mater. Corros. 2019, 70, 1852–1861. [Google Scholar] [CrossRef]
- Pal, A.; Thinaharan, C.; Krishna, N.G.; Shankar, A.R.; Philip, J. Studies on localized electrochemical activity of 304L SS-Zr-4 dissimilar joints using alternating current scanning electrochemical microscopy. Appl. Surf. Sci. 2022, 578, 151958. [Google Scholar] [CrossRef]
- Orozco-Cruz, R.; Bohórquez-Rico, J.; Marin, D.; Almeraya-Calderón, F.; Espinoza-Vázquez, A.; Carmona-Hernández, A.; Galván-Martínez, R. Effect of seawater pH variation on the growth of calcareous deposits and its effect on an impressed current cathodic protection system. J. Solid State Electrochem. 2023, 27, 3003–3016. [Google Scholar] [CrossRef]
- Hua, W.; Sun, R.; Wang, X.; Zhang, Y.; Li, J.; Qiu, R.; Gao, Y. Corrosion of Q235 carbon steel induced by sulfate-reducing bacteria in groundwater: Corrosion behavior, corrosion product, and microbial community structure. Environ. Sci. Pollut. Res. 2023, 31, 4269–4279. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, Y.-W. Cathodic protection criteria of thermally insulated pipeline buried in soil. Corros. Sci. 2001, 43, 2011–2021. [Google Scholar] [CrossRef]
- Qin, M.; Liao, K.; He, G.; Ye, N.; Zhao, S.; Zhang, S. Flow Influenced Initiation and Propagation of SRB Corrosion on L360N Carbon Steel. Arab. J. Sci. Eng. 2022, 47, 11469–11480. [Google Scholar] [CrossRef]
- Li, F.; An, M.; Duan, D. Corrosion inhibition of stainless steel by a sulfate-reducing bacteria biofilm in seawater. Int. J. Min. Met. Mater. 2012, 19, 717–725. [Google Scholar] [CrossRef]
- Wu, S.; Gao, Z.; Liu, Y.; Hu, W. Effect of cathodic protection potential on stress corrosion susceptibility of X80 steel. Corros. Sci. 2023, 218, 111184. [Google Scholar] [CrossRef]
- Zulkafli, R.; Othman, N.K.; Yaakob, N. Pencirian Permukaan Kakisan Keluli Karbon dengan Kehadiran Konsortium Bakteria Penurun Sulfat dalam Persekitaran Bergas CO2. JSM 2022, 51, 3113–3123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).