Effect of Gamma Irradiation on Free Radicals and the Antioxidant Properties of Walnuts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Irradiation
2.3. EPR Investigations
2.3.1. Preparation of Extracts
2.3.2. Determination of DPPH Free Radical Scavenging Activity (FRSA) by EPR
2.3.3. Instrumentation
2.4. Determination of the Total Polyphenol and Total Flavonoid Contents, ORAC, and HORAC
2.4.1. Extract Preparation
2.4.2. Determination of the Total Polyphenol and Total Flavonoid Contents
2.4.3. Determination of Antioxidant Activity by ORAC and HORAC Assays
2.5. Statistical Analysis
3. Results and Discussion
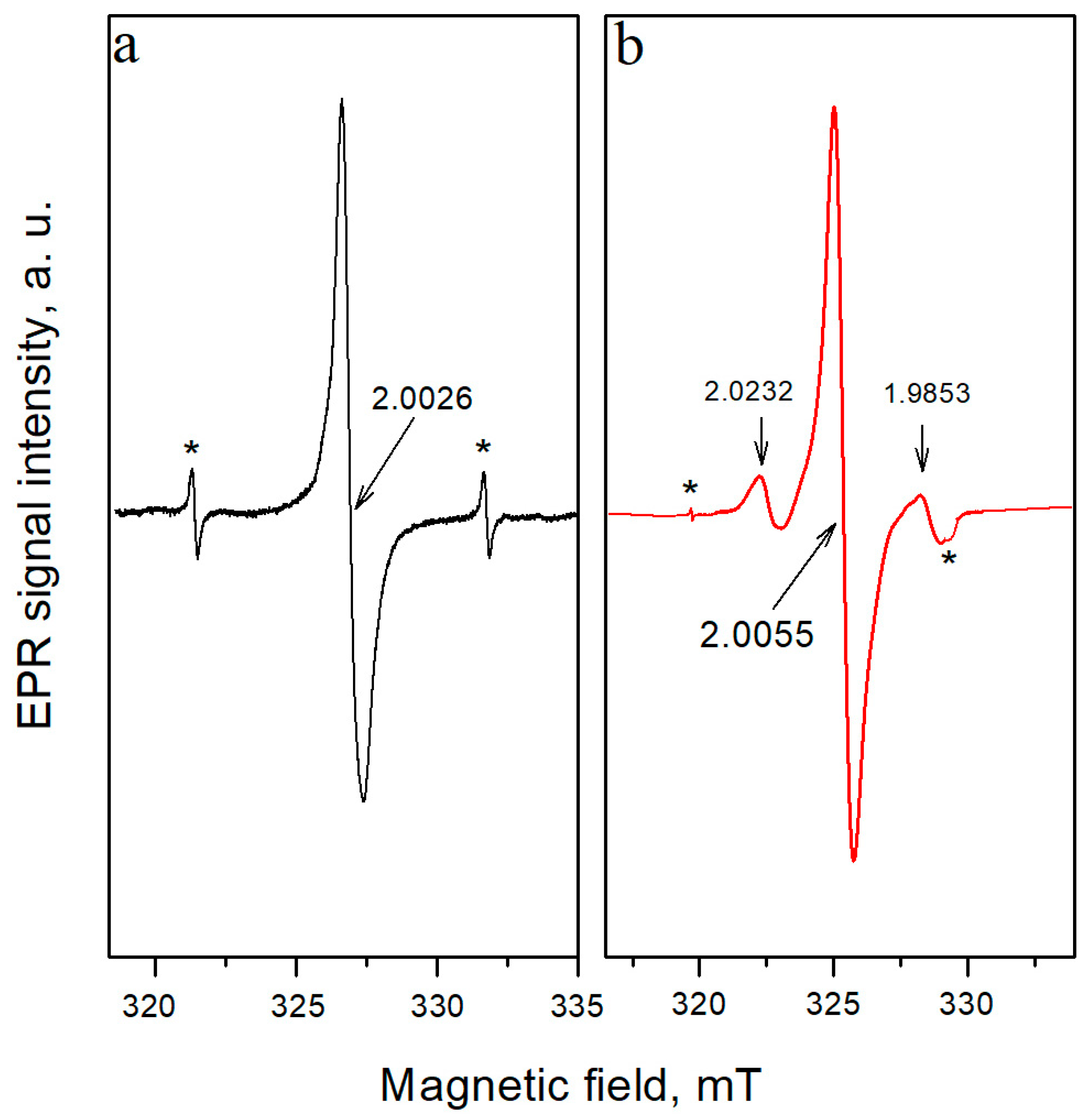
3.1. Characteristics of EPR Spectra
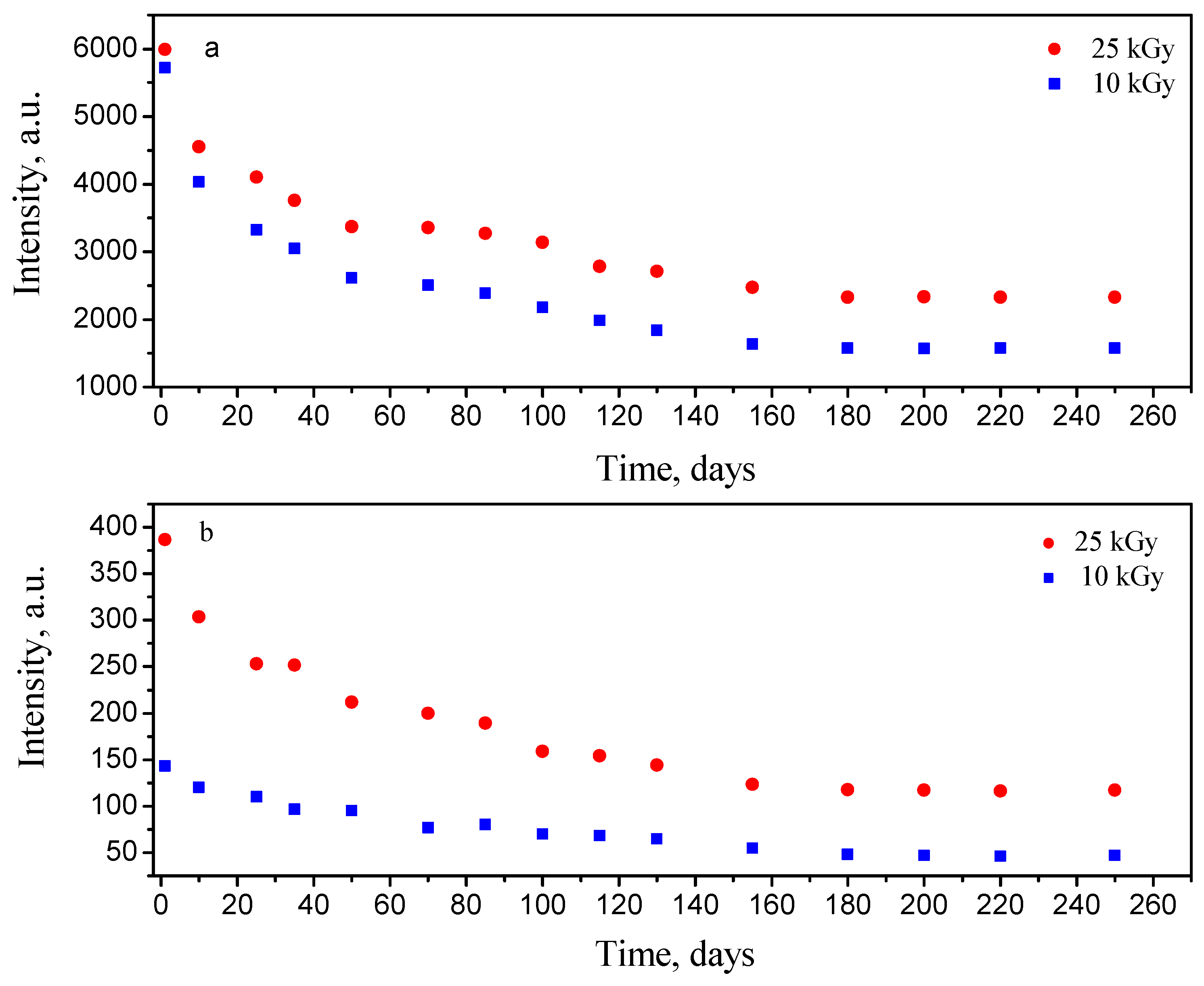
3.2. Kinetic Study
3.3. Total Polyphenol and Flavonoid Content
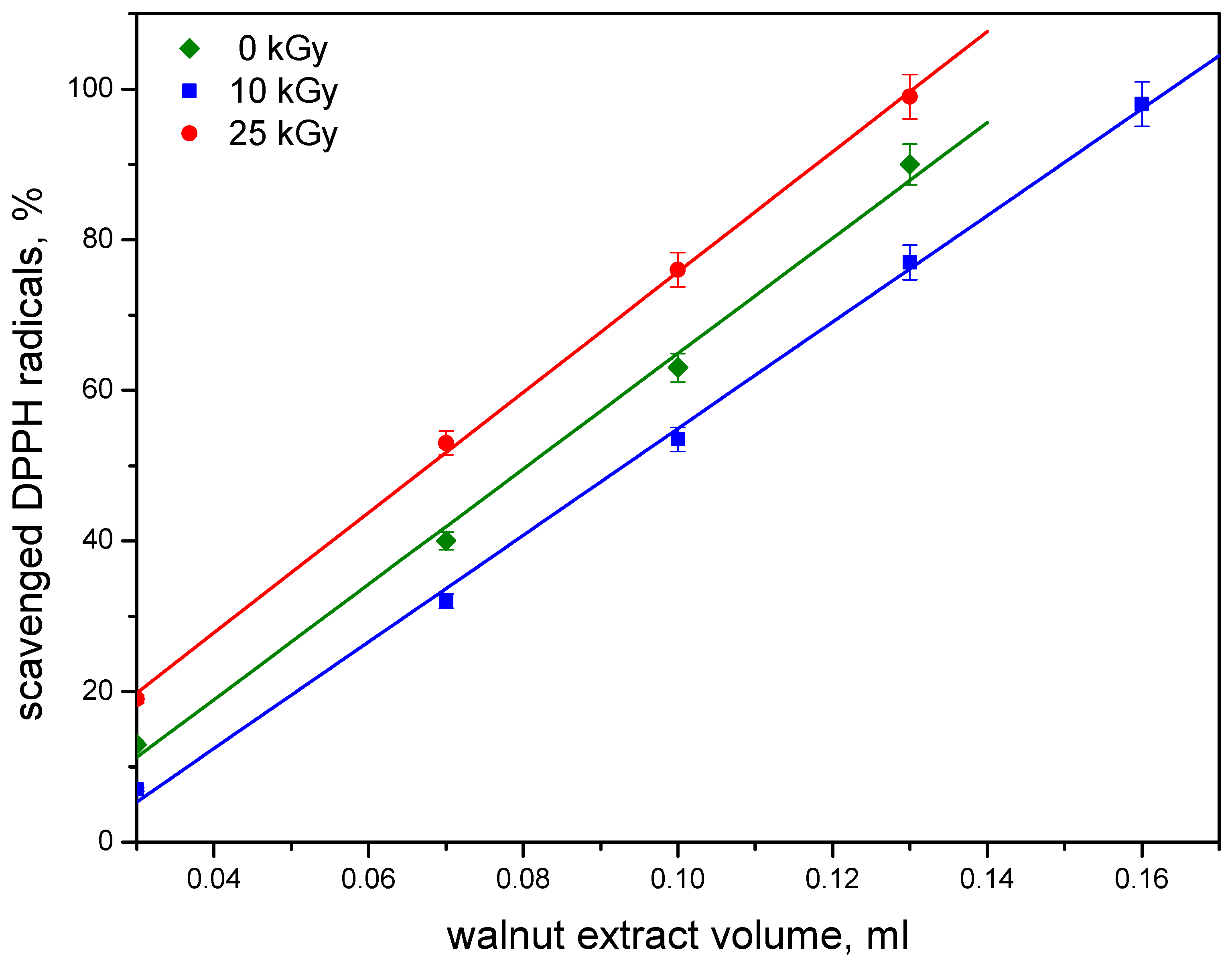
3.4. Free Radical Scavenging Activity (FRSA) Assay by EPR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPR | Electron Paramagnetic Resonance |
| ORAC | Oxygen Radical Absorbance Capacity |
| HORAC | Hydroxyl Radical Antioxidant Capacity |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| FRSA | Free radical scavenging activity |
| IC50 | the amount of antioxidant necessary to decrease the initial DPPH radical concentration by 50% |
| TEAC | Trolox equivalent antioxidant capacity |
| GAE | Gallic acid equivalents |
| SD | Standard deviation |
| QE | Quercetin equivalents |
References
- Aleksieva, K.I.; Yordanov, N.D. Various approaches in EPR identification of gamma-irradiated plant foodstuffs: A review. Food Res. Intern. 2018, 105, 1019–1028. [Google Scholar] [CrossRef]
- EN 1787; Foodstuffs-Detection of Irradiated Food Containing Cellulose—Method by ESR Spectroscopy. European Committee for Standardization: Brussels, Belgium, 2000.
- Aoude-Werner, D.; Straub, I.; Zumsteeg, V.; Kuntz, F. Identification of bleached and irradiated walnuts and hazelnuts by ESR spectroscopy. Rad. Phys. Chem. 2020, 173, 108882. [Google Scholar] [CrossRef]
- Bortolin, E.; Cardamone, C.; Chiaravalle, A.E.; Carratu, B.; Deiana, G.; Di Noto, A.; Di Schiavi, M.T.; D’Oca, M.C.; Gargiulo, R.; Mangiacotti, M.; et al. An inter-laboratory comparison to evaluate the suitability of EN 1787 standard to detect irradiation in plant-origin foods with health benefits. Food Control 2020, 117, 107326. [Google Scholar] [CrossRef]
- Maghraby, A.; Salama, E.; Sami, A.; Mansour, A.; El-Sayed, M. Identification and dosimetry of irradiated walnuts (Juglans regia) using EPR. Rad. Eff. Def. Solids 2012, 167, 170–178. [Google Scholar] [CrossRef]
- Raffi, J.; Yordanov, N.D.; Chabane, S.; Douifi, L.; Gancheva, V.; Ivanova, S. Identification of irradiation treatment of aromatic herbs, spices and fruits by electron paramagnetic resonance and thermoluminescence. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2000, 5, 409–416. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Mangiacotti, M.; Trotta, G.; Marchesani, G.; Chiappinelli, A.; Chiaravalle, A.E. Identification of X-ray irradiated walnuts by ESR spectroscopy. Rad. Phys. Chem. 2018, 150, 35–39. [Google Scholar] [CrossRef]
- D’Oca, M.C.; Bartolotta, A. Evaluation of the original dose in irradiated dried fruit by EPR spectroscopy. Rad. Meas. 2011, 46, 813–815. [Google Scholar] [CrossRef]
- Esteves, M.P.; Andrade, M.E.; Empis, J. Detection of prior irradiation of dried fruits by electron spin resonance (ESR). Rad. Phys. Chem. 1999, 55, 737–742. [Google Scholar] [CrossRef]
- Bayram, G.; Delincee, H. Identification of irradiated Turkish foodstuffs combining various physical detection methods. Food Control 2004, 15, 81–91. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Mathew, M.; Singh, A.; Simha, P. Influence of Microwave and Ultrasound pretreatment on Solvent Extraction of Bio-components from Walnut (Julgans regia L.) Shells. Period. Polytechn. Chem. Eng. 2016, 60, 40–48. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, S.-C.; Hu, C.-C.; Shyu, Y.-S.; Hsu, C.-Y.; Yang, D.-J. Effects of roasting temperature and duration on fatty acid composition, phenolic composition, Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, N.; Shahidi, F. Effect of Roasting on Phenolic Content and Antioxidant Activities of Whole Cashew Nuts, Kernels, and Testa. J. Agric. Food Chem. 2011, 59, 5006–5014. [Google Scholar] [CrossRef] [PubMed]
- Kita, A.; Figiel, A. Effect of roasting on properties of walnuts. Pol. J. Food Nutr. 2007, 57, 89–94. [Google Scholar]
- Momchilova, S.; Kazakova, A.; Taneva, S.; Aleksieva, K.; Mladenova, R.; Karakirova, Y.; Petkova, Z.; Kamenova-Nacheva, M.; Teneva, D.; Denev, P. Effect of Gamma Irradiation on Fat Content, Fatty Acids, Antioxidants and Oxidative Stability of Almonds, and Electron Paramagnetic Resonance (EPR) Study of Treated Nuts. Molecules 2023, 28, 1439. [Google Scholar] [CrossRef]
- Masoodi, L.; Masoodi, F.A.; Gull, A.; Gani, A.; Muzaffer, S.; Sidiq, M. Effect of γ-irradiation on the physicochemical and sensory properties of fresh walnut kernels (Juglans regia) during storage. Food Chem. Adv. 2023, 3, 100301. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) Official Methods of Analysis of AOAC International, 22nd ed.; 4.5.06 AOAC Official Method 2003.06 Crude Fat in Feeds, Cereal Grains, and Forages: Randall/Soxtec/Hexanes Extraction-Submersion Method; Oxford University Press: Oxford, UK, 2023; pp. C4-42–C4-43. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescence probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Polovka, M.; Suhaj, M. The effect of irradiation and heat treatment on composition and antioxidant properties of culinary herbs and spices—A review. Food Rev. Int. 2010, 26, 138–161. [Google Scholar] [CrossRef]
- Yordanov, N.D.; Aleskieva, K. X- and Q- band studies on fine powders of irradiated plants. New approach for detection of their radiation history by using Q-band EPR spectrometry. Radiat. Phys. Chem. 2004, 69, 59–64. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV-C illumination on antioxidant capacity and postharvest decay in blueberry fruit. J. Food Agric. Environ. 2013, 11, 434–436. [Google Scholar]
- Denev, P.; Klisurova, D.; Teneva, D.; Ognyanov, M.; Georgiev, Y.; Momchilova, S.; Kancheva, V. Effect of gamma-irradiation on the chemical composition and antioxidant activity of dried black chokeberry (Aronia melanocarpa) fruits. Bulg. Chem. Comm. 2019, 51, 270–275. [Google Scholar]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of Almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Variyar, P.S.; Limaye, A.; Sharma, A. Radiation-induced enhancement of antioxidant contents of soybean (Glycine max Merrill). J. Agric. Food Chem. 2004, 52, 3385–3388. [Google Scholar] [CrossRef]
- Aleksieva, K.I.; Mladenova, R.B.; Solakov, N.Y.; Loginovska, K.K. EPR analysis of free radical components and antioxidant activity of bee pollen before and after gamma-irradiation. J. Radioan. Nucl. Chem. 2021, 327, 713–719. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kim, J.-H.; Jeong, S.-M.; Kim, D.-R.; Ha, J.-U.; Nam, K.C.; Ahn, D.U. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J. Agric. Food Chem. 2003, 51, 4400–4403. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical profiles, and bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef]
| Total Polyphenols mg/100 g | Total Flavonoids mg/100 g | ORAC µmol TE/g | HORAC µmol GAE/g | |
|---|---|---|---|---|
| Control | 2915 ± 291 | 15.5 ± 0.7 | 235 ± 8 | 150 ± 7 |
| 10 kGy | 2948 ± 19 | 15.4 ± 0.6 | 254 ± 9 | 167 ± 9 |
| 25 kGy | 4210 * ± 12 | 26.0 * ± 1.1 | 320 * ± 6 | 178 * ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksieva, K.; Mladenova, R.; Taneva, S.; Denev, P.; Karakirova, Y. Effect of Gamma Irradiation on Free Radicals and the Antioxidant Properties of Walnuts. Chemistry 2025, 7, 52. https://doi.org/10.3390/chemistry7020052
Aleksieva K, Mladenova R, Taneva S, Denev P, Karakirova Y. Effect of Gamma Irradiation on Free Radicals and the Antioxidant Properties of Walnuts. Chemistry. 2025; 7(2):52. https://doi.org/10.3390/chemistry7020052
Chicago/Turabian StyleAleksieva, Katerina, Ralitsa Mladenova, Sabina Taneva, Petko Denev, and Yordanka Karakirova. 2025. "Effect of Gamma Irradiation on Free Radicals and the Antioxidant Properties of Walnuts" Chemistry 7, no. 2: 52. https://doi.org/10.3390/chemistry7020052
APA StyleAleksieva, K., Mladenova, R., Taneva, S., Denev, P., & Karakirova, Y. (2025). Effect of Gamma Irradiation on Free Radicals and the Antioxidant Properties of Walnuts. Chemistry, 7(2), 52. https://doi.org/10.3390/chemistry7020052









