Ultrasound-Assisted Synthesis of Microcrystalline Lanthanide Terephthalates: Insights into Morphology and Structural Properties
Abstract
1. Introduction
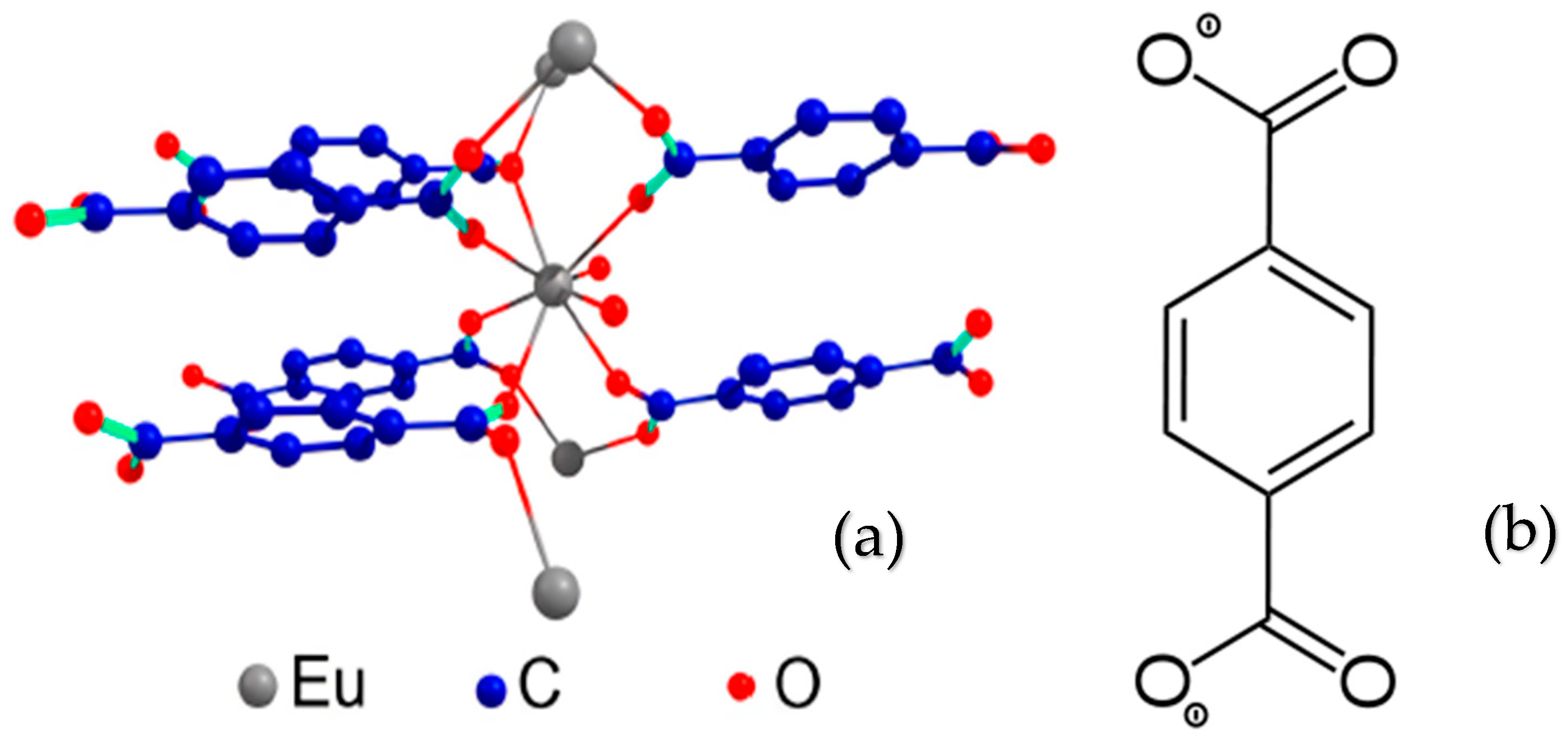
2. Materials and Methods
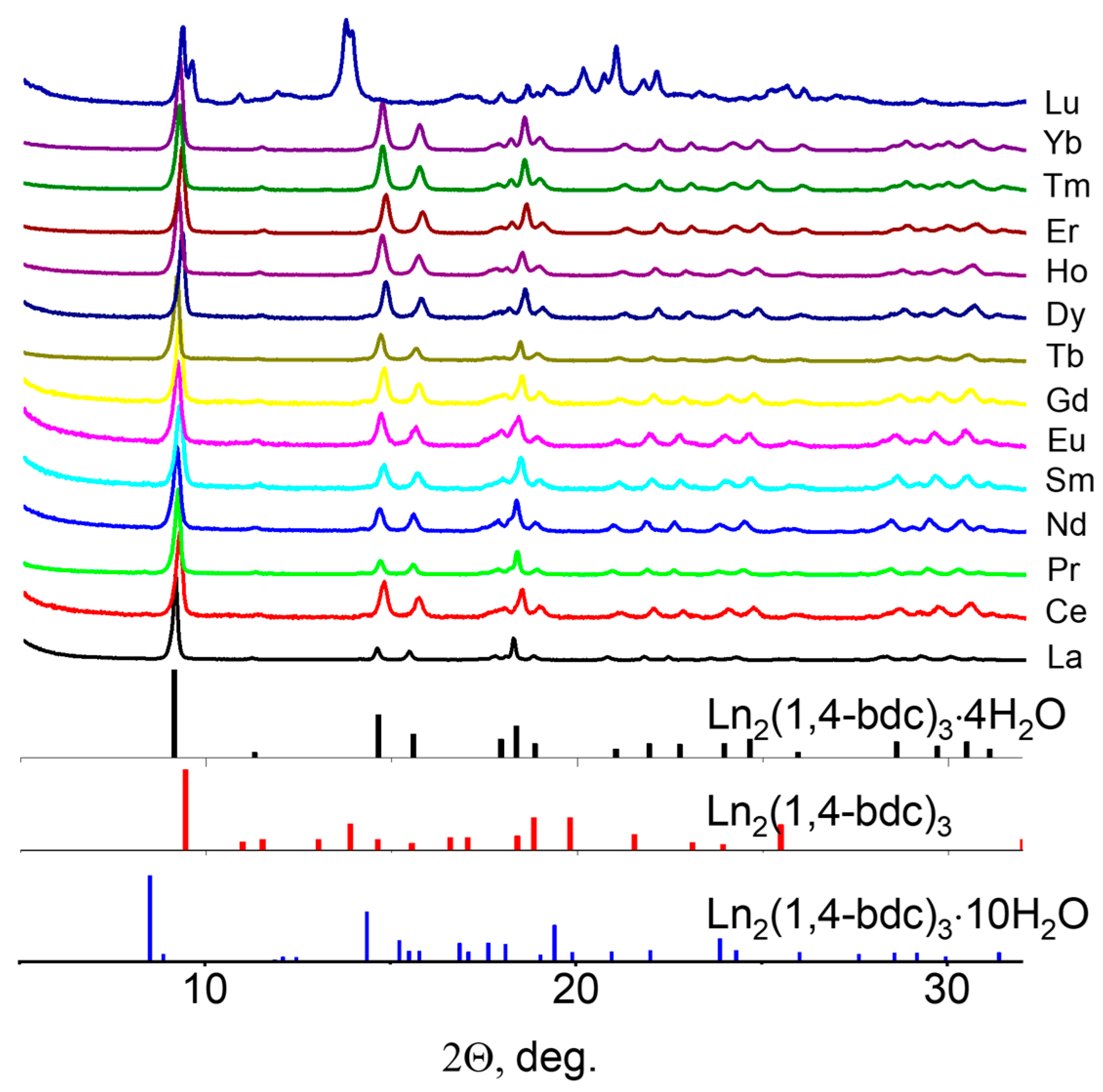
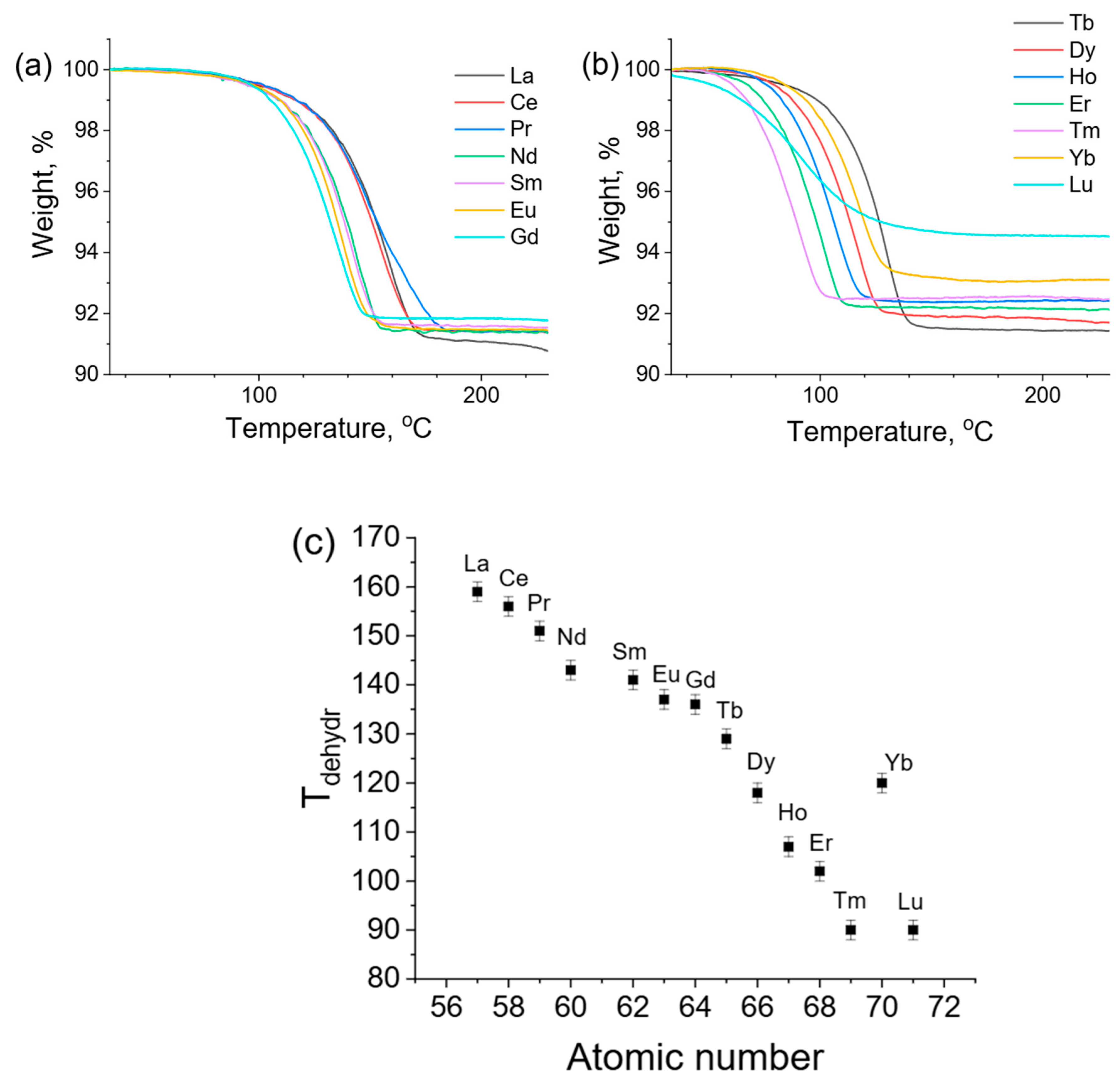
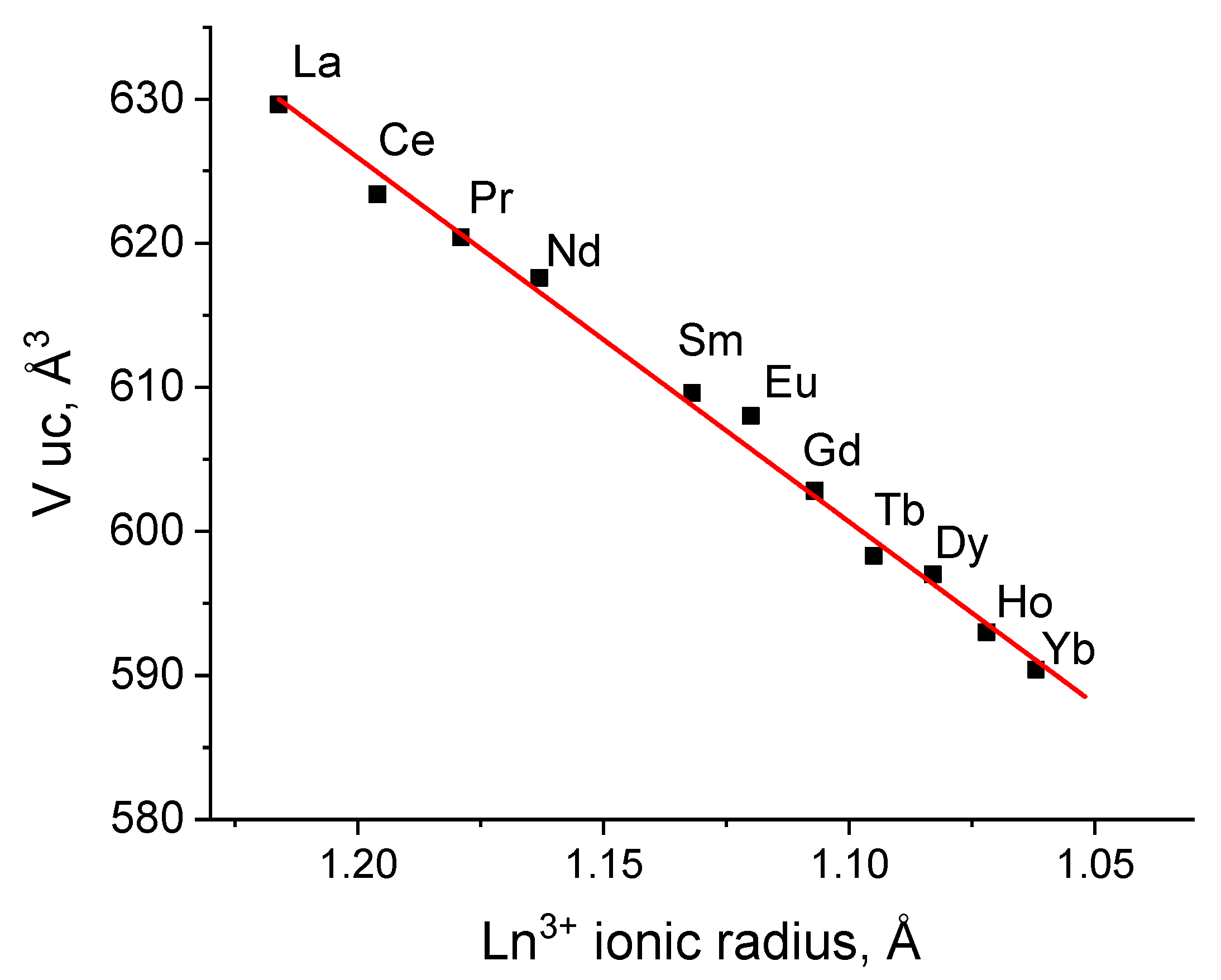
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Yue, Q.; Li, G.-D.; Cao, J.-J.; Li, G.-H.; Chen, J.-S. Structures, Photoluminescence, Up-Conversion, and Magnetism of 2D and 3D Rare-Earth Coordination Polymers with Multicarboxylate Linkages. Inorg. Chem. 2006, 45, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, C.-M.; Shao, T.; Li, Y.-Z.; Zhang, K.-L.; Zhang, H.-T.; You, X.-Z. Syntheses, Structures, and Luminescence Properties of a New Family of Three-Dimensional Open-Framework Lanthanide Coordination Polymers. Inorg. Chem. Commun. 2002, 5, 642–648. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Gu, W.; Li, B.; Liu, X.; Liao, D.-Z. {[Nd4(ox)4(NO3)2(OH)2(H2O)2] 5H2O} n: A Porous 3D Lanthanide-Based Coordination Polymer with a Special Luminescent Property. Inorg. Chem. 2007, 46, 622–624. [Google Scholar] [CrossRef]
- Yan, B.; Bai, Y.; Chen, Z. Synthesis, structure and luminescence of novel 1D chain coordination polymers [Ln(isophth)(Hisophth)(H2O)4·4H2O]n (Ln = Sm, Dy). J. Mol. Struct. 2005, 741, 141–147. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, D.S.; Rana, A.; Bera, M.; Zangrando, E.; Dalai, S. 3D Zinc(II) Coordination Polymers Built up by Triazole and Benzene-Polycarboxylate Anions: Synthesis, Crystal Structure, Thermal and Photoluminescence Characterization. Polyhedron 2009, 28, 2131–2136. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Rational Design of MOF-Based Materials for Next-Generation Rechargeable Batteries. Nanomicro Lett. 2021, 13, 203. [Google Scholar] [CrossRef]
- Annamalai, J.; Murugan, P.; Ganapathy, D.; Nallaswamy, D.; Atchudan, R.; Arya, S.; Khosla, A.; Barathi, S.; Sundramoorthy, A.K. Synthesis of Various Dimensional Metal Organic Frameworks (MOFs) and Their Hybrid Composites for Emerging Applications—A Review. Chemosphere 2022, 298, 134184. [Google Scholar] [CrossRef]
- Meng, Z.; Qiu, Z.; Shi, Y.; Wang, S.; Zhang, G.; Pi, Y.; Pang, H. Micro/Nano Metal–Organic Frameworks Meet Energy Chemistry: A Review of Materials Synthesis and Applications. eScience 2023, 3, 100092. [Google Scholar] [CrossRef]
- Xu, G.-R.; An, Z.-H.; Xu, K.; Liu, Q.; Das, R.; Zhao, H.-L. Metal Organic Framework (MOF)-Based Micro/Nanoscaled Materials for Heavy Metal Ions Removal: The Cutting-Edge Study on Designs, Synthesis, and Applications. Coord. Chem. Rev. 2021, 427, 213554. [Google Scholar] [CrossRef]
- Le, S.-K.; Jin, Q.-J.; Han, J.-A.; Zhou, H.-C.; Liu, Q.-S.; Yang, F.; Miao, J.; Liu, P.-P.; Zhu, C.-Z.; Xu, H.-T. Rare Earth Element-Modified MOF Materials: Synthesis and Photocatalytic Applications in Environmental Remediation. Rare Metals 2024, 43, 1390–1406. [Google Scholar] [CrossRef]
- Younis, S.A.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Rare Earth Metal–Organic Frameworks (RE-MOFs): Synthesis, Properties, and Biomedical Applications. Coord. Chem. Rev. 2021, 429, 213620. [Google Scholar] [CrossRef]
- Neufeld, M.J.; Winter, H.; Landry, M.R.; Goforth, A.M.; Khan, S.; Pratx, G.; Sun, C. Lanthanide Metal–Organic Frameworks for Multispectral Radioluminescent Imaging. ACS Appl. Mater. Interfaces 2020, 12, 26943–26954. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Chang, Y.; Zhou, Q.; Ding, S.; Gao, F. An Overview of the Design of Metal-Organic Frameworks-Based Fluorescent Chemosensors and Biosensors. Biosensors 2022, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kang, S.; Tai, M.; Wang, J.; Tian, Q.; Jin, D.; Wang, L. Synthesis, Modulation, and Characterization of Ln3+ Ions Doped Metal−organic Frameworks for WLED Applications. Dye. Pigment. 2023, 209, 110897. [Google Scholar] [CrossRef]
- Meng, S.; Li, G.; Wang, P.; He, M.; Sun, X.; Li, Z. Rare Earth-Based MOFs for Photo/Electrocatalysis. Mater. Chem. Front. 2023, 7, 806–827. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Y.; Wang, J.; Pei, R. Lanthanide-Based MOFs: Synthesis Approaches and Applications in Cancer Diagnosis and Therapy. J. Mater. Chem. B 2022, 10, 9535–9564. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; He, M.; Yuan, X.; Fang, Z.; Li, Z. Rare Earth-Based Nanomaterials in Electrocatalysis. Coord. Chem. Rev. 2023, 489, 215204. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ohodnicki, P.R.; Baltrus, J.P. Materials for the Photoluminescent Sensing of Rare Earth Elements: Challenges and Opportunities. J. Mater. Chem. C Mater. 2020, 8, 7975–8006. [Google Scholar] [CrossRef]
- Shen, G.; Zhong, L.; Liu, G.; Yang, L.; Wen, X.; Chen, G.; Zhao, J.; Hou, C.; Wang, X. Synthesis of Rare-Earth Metal-Organic Frameworks to Construct High-Resolution Sensing Array for Multiplex Anions Detection, Cell Imaging and Blood Phosphorus Monitoring. J. Colloid. Interface Sci. 2023, 652, 1925–1936. [Google Scholar] [CrossRef]
- Wu, N.; Bo, C.; Guo, S. Luminescent Ln-MOFs for Chemical Sensing Application on Biomolecules. ACS Sens. 2024, 9, 4402–4424. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Zheng, T.-T.; Jin, L.-P. Self-Assembly of Lanthanide Mixed-Carboxylates Coordination Polymers. J. Mol. Struct. 2005, 740, 31–35. [Google Scholar] [CrossRef]
- Rodríguez-Cortiñas, R.; Avecilla, F.; Platas-Iglesias, C.; Imbert, D.; Bünzli, J.-C.G.; de Blas, A.; Rodríguez-Blas, T. Structural and Photophysical Properties of Heterobimetallic 4f-Zn Iminophenolate Cryptates. Inorg. Chem. 2002, 41, 5336–5349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef]
- Dou, X.; Sun, K.; Chen, H.; Jiang, Y.; Wu, L.; Mei, J.; Ding, Z.; Xie, J. Nanoscale Metal-Organic Frameworks as Fluorescence Sensors for Food Safety. Antibiotics 2021, 10, 358. [Google Scholar] [CrossRef]
- Puglisi, R.; Pellegrino, A.L.; Fiorenza, R.; Scirè, S.; Malandrino, G. A Facile One-Pot Approach to the Synthesis of Gd-Eu Based Metal-Organic Frameworks and Applications to Sensing of Fe3+ and Cr2O72− Ions. Sensors 2021, 21, 1679. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, C.; Cen, P.; Jin, X.; Liang, C.; Yang, J.; Liu, X. Stable Ln-MOFs as Multi-Responsive Photoluminescence Sensors for the Sensitive Sensing of Fe3+, Cr2O72−, and Nitrofuran. CrystEngComm 2020, 22, 1695–1704. [Google Scholar] [CrossRef]
- Li, S.; Tan, L.; Meng, X. Nanoscale Metal-Organic Frameworks: Synthesis, Biocompatibility, Imaging Applications, and Thermal and Dynamic Therapy of Tumors. Adv. Funct. Mater. 2020, 30, 1908924. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.-C. Recent Progress in the Synthesis of Metal–Organic Frameworks. Sci. Technol. Adv. Mater. 2015, 16, 054202. [Google Scholar] [CrossRef]
- Reineke, T.M.; Eddaoudi, M.; Fehr, M.; Kelley, D.; Yaghi, O.M. From Condensed Lanthanide Coordination Solids to Microporous Frameworks Having Accessible Metal Sites. J. Am. Chem. Soc. 1999, 121, 1651–1657. [Google Scholar] [CrossRef]
- Butorlin, O.S.; Petrova, A.S.; Toikka, Y.N.; Kolesnikov, I.E.; Orlov, S.N.; Ryazantsev, M.N.; Bogachev, N.A.; Skripkin, M.Y.; Mereshchenko, A.S. The Structure and Optical Properties of Luminescent Europium Terephthalate Antenna Metal–Organic Frameworks Doped by Yttrium, Gadolinium, and Lanthanum Ions. Molecules 2024, 29, 3558. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Song, Y.; Wang, L. Nanoscale Luminescent Lanthanide-Based Metal–Organic Frameworks: Properties, Synthesis, and Applications. J. Nanopart. Res. 2015, 17, 310. [Google Scholar] [CrossRef]
- Kukkar, D.; Vellingiri, K.; Kim, K.-H.; Deep, A. Recent Progress in Biological and Chemical Sensing by Luminescent Metal-Organic Frameworks. Sens. Actuators B Chem. 2018, 273, 1346–1370. [Google Scholar] [CrossRef]
- Ren, X.-Y.; Lu, L.-H. Luminescent Nanoscale Metal–Organic Frameworks for Chemical Sensing. Chin. Chem. Lett. 2015, 26, 1439–1445. [Google Scholar] [CrossRef]
- Vetrivel, S.; Chen, C.-T.; Kao, H.-M. The Ultrafast Sonochemical Synthesis of Mesoporous Silica MCM-41. New J. Chem. 2010, 34, 2109. [Google Scholar] [CrossRef]
- Jabariyan, S.; Zanjanchi, M.A. A Simple and Fast Sonication Procedure to Remove Surfactant Templates from Mesoporous MCM-41. Ultrason. Sonochem. 2012, 19, 1087–1093. [Google Scholar] [CrossRef]
- Palani, A.; Wu, H.-Y.; Ting, C.-C.; Vetrivel, S.; Shanmugapriya, K.; Chiang, A.S.T.; Kao, H.-M. Rapid Temperature-Assisted Sonochemical Synthesis of Mesoporous Silica SBA-15. Microporous Mesoporous Mater. 2010, 131, 385–392. [Google Scholar] [CrossRef]
- Gnana Sundara Raj, B.; Asiri, A.M.; Qusti, A.H.; Wu, J.J.; Anandan, S. Sonochemically Synthesized MnO2 Nanoparticles as Electrode Material for Supercapacitors. Ultrason. Sonochem. 2014, 21, 1933–1938. [Google Scholar] [CrossRef]
- Sakthipandi, K.; Rajendran, V. On-Line Phase Transitions of Bulk and Nanocrystalline La1−xPbxMnO3 (X = 0.3, 0.4, and 0.5) Perovskite Manganite Materials Using Ultrasonic Measurements. Mater. Chem. Phys. 2013, 138, 581–592. [Google Scholar] [CrossRef]
- Sharifalhoseini, Z.; Entezari, M.H.; Jalal, R. Direct and Indirect Sonication Affect Differently the Microstructure and the Morphology of ZnO Nanoparticles: Optical Behavior and Its Antibacterial Activity. Ultrason. Sonochem. 2015, 27, 466–473. [Google Scholar] [CrossRef]
- Altaf, A.R.; Teng, H.; Gang, L.; Adewuyi, Y.G.; Zheng, M. Effect of Sonochemical Treatment on Thermal Stability, Elemental Mercury (Hg0) Removal, and Regenerable Performance of Magnetic Tea Biochar. ACS Omega 2021, 6, 23913–23923. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Xiao, Y.; Wang, T.; Wang, J.; Pan, W. Synthesis of O-Doped Coal-Based Carbon Electrode Materials by Ultrasound-Assisted Bimetallic Activation for Application in Supercapacitors. Appl. Surf. Sci. 2020, 529, 147074. [Google Scholar] [CrossRef]
- Teng, Z.; Han, K.; Li, J.; Gao, Y.; Li, M.; Ji, T. Ultrasonic-Assisted Preparation and Characterization of Hierarchical Porous Carbon Derived from Garlic Peel for High-Performance Supercapacitors. Ultrason. Sonochem. 2020, 60, 104756. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, Y.; Sun, Z.; Gao, S.; Ma, J.; Liu, L. A Simple Approach of Constructing Sulfur-Containing Porous Carbon Nanotubes for High-Performance Supercapacitors. Carbon. N. Y 2017, 115, 754–762. [Google Scholar] [CrossRef]
- Ho, P.H.; Salles, F.; Di Renzo, F.; Trens, P. One-Pot Synthesis of 5-FU@ZIF-8 and Ibuprofen@ZIF-8 Nanoparticles. Inorganica Chim. Acta 2020, 500, 119229. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A Review of the Features and Applications of ZIF-8 and Its Derivatives for Separating CO2 and Isomers of C3- and C4- Hydrocarbons. J. Nat. Gas. Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Ultrasonic-Assisted Linker Exchange (USALE): A Novel Post-Synthesis Method for Controlling the Functionality, Porosity, and Morphology of MOFs. Chem. A Eur. J. 2019, 25, 10876–10885. [Google Scholar] [CrossRef]
- Yu, K.; Lee, Y.-R.; Seo, J.Y.; Baek, K.-Y.; Chung, Y.-M.; Ahn, W.-S. Sonochemical Synthesis of Zr-Based Porphyrinic MOF-525 and MOF-545: Enhancement in Catalytic and Adsorption Properties. Microporous Mesoporous Mater. 2021, 316, 110985. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.-T.; Choi, S.B.; Sim, J.; Kim, J.; Ahn, W.-S. Control of Catenation in CuTATB-n Metal–Organic Frameworks by Sonochemical Synthesis and Its Effect on CO2 Adsorption. J. Mater. Chem. 2011, 21, 3070. [Google Scholar] [CrossRef]
- Jung, D.-W.; Yang, D.-A.; Kim, J.; Kim, J.; Ahn, W.-S. Facile Synthesis of MOF-177 by a Sonochemical Method Using 1-Methyl-2-Pyrrolidinone as a Solvent. Dalton Trans. 2010, 39, 2883. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Shao, Y.; Peng, L.; Zhang, Q.; Zhou, T.; Xiang, Y.; Ye, N. Ambient Temperature Fabrication of a Covalent Organic Framework from 1,3,5-Triformylphloroglucinol and 1,4-Phenylenediamine as a Coating for Use in Open-Tubular Capillary Electrochromatography of Drugs and Amino Acids. Microchim. Acta 2019, 186, 650. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, P.; Yang, H.; Bahri, M.; James, A.M.; Chen, H.; Liu, L.; Li, B.; Pang, Z.; Clowes, R.; et al. Using Sound to Synthesize Covalent Organic Frameworks in Water. Nat. Synth. 2022, 1, 87–95. [Google Scholar] [CrossRef]
- Yang, S.-T.; Kim, J.; Cho, H.-Y.; Kim, S.; Ahn, W.-S. Facile Synthesis of Covalent Organic Frameworks COF-1 and COF-5 by Sonochemical Method. RSC Adv. 2012, 2, 10179. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent Organic Frameworks (COFs) Functionalized Mixed Matrix Membrane for Effective CO2/N2 Separation. J. Memb. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Yusof, N.S.M.; Ashokkumar, M. Sonochemical Synthesis of Gold Nanoparticles by Using High Intensity Focused Ultrasound. Chemphyschem 2015, 16, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Khan, N.A.; Park, J.H.; Jhung, S.H. Synthesis of a Metal–Organic Framework Material, Iron Terephthalate, by Ultrasound, Microwave, and Conventional Electric Heating: A Kinetic Study. Chem. A Eur. J. 2010, 16, 1046–1052. [Google Scholar] [CrossRef]
- Hu, S.-M.; Niu, H.-L.; Qiu, L.-G.; Yuan, Y.-P.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Facile Synthesis of Highly Luminescent Nanowires of a Terbium-Based Metal–Organic Framework by an Ultrasonic-Assisted Method and Their Application as a Luminescent Probe for Selective Sensing of Organoamines. Inorg. Chem. Commun. 2012, 17, 147–150. [Google Scholar] [CrossRef]
- Kolesnik, S.S.; Nosov, V.G.; Kolesnikov, I.E.; Khairullina, E.M.; Tumkin, I.I.; Vidyakina, A.A.; Sysoeva, A.A.; Ryazantsev, M.N.; Panov, M.S.; Khripun, V.D.; et al. Ultrasound-Assisted Synthesis of Luminescent Micro- and Nanocrystalline Eu-Based MOFs as Luminescent Probes for Heavy Metal Ions. Nanomaterials 2021, 11, 2448. [Google Scholar] [CrossRef]
- Kolesnik, S.S.; Bogachev, N.A.; Kolesnikov, I.E.; Orlov, S.N.; Ryazantsev, M.N.; González, G.; Skripkin, M.Y.; Mereshchenko, A.S. Microcrystalline Luminescent (Eu1−XLnx)2bdc3·nH2O (Ln = La, Gd, Lu) Antenna MOFs: Effect of Dopant Content on Structure, Particle Morphology, and Luminescent Properties. Molecules 2024, 29, 532. [Google Scholar] [CrossRef]
- Daiguebonne, C.; Kerbellec, N.; Guillou, O.; Bünzli, J.-C.; Gumy, F.; Catala, L.; Mallah, T.; Audebrand, N.; Gérault, Y.; Bernot, K.; et al. Structural and Luminescent Properties of Micro- and Nanosized Particles of Lanthanide Terephthalate Coordination Polymers. Inorg. Chem. 2008, 47, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- Nosov, V.G.; Toikka, Y.N.; Petrova, A.S.; Butorlin, O.S.; Kolesnikov, I.E.; Orlov, S.N.; Ryazantsev, M.N.; Kolesnik, S.S.; Bogachev, N.A.; Skripkin, M.Y.; et al. Brightly Luminescent (TbxLu1−x)2bdc3·nH2O MOFs: Effect of Synthesis Conditions on Structure and Luminescent Properties. Molecules 2023, 28, 2378. [Google Scholar] [CrossRef] [PubMed]
- Toikka, Y.N.; Badikov, A.R.; Bogachev, N.A.; Kolesnikov, I.E.; Skripkin, M.Y.; Orlov, S.N.; Mereshchenko, A.S. Luminescent Properties and Thermal Stability of (Lu0.98Eu0.02)2bdc3·10H2O Metal–Organic Frameworks. Mendeleev Commun. 2024, 34, 634–636. [Google Scholar] [CrossRef]
- Zehnder, R.A.; Renn, R.A.; Pippin, E.; Zeller, M.; Wheeler, K.A.; Carr, J.A.; Fontaine, N.; McMullen, N.C. Network Dimensionality and Ligand Flexibility in Lanthanide Terephthalate Hydrates. J. Mol. Struct. 2011, 985, 109–119. [Google Scholar] [CrossRef]
- Schwarzenbach, G.; Flaschka, H.A. Complexometric Titrations; Methuen: London, UK, 1969; ISBN 9780389011439. [Google Scholar]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Cryst. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-Cell Refinement from Powder Diffraction Scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
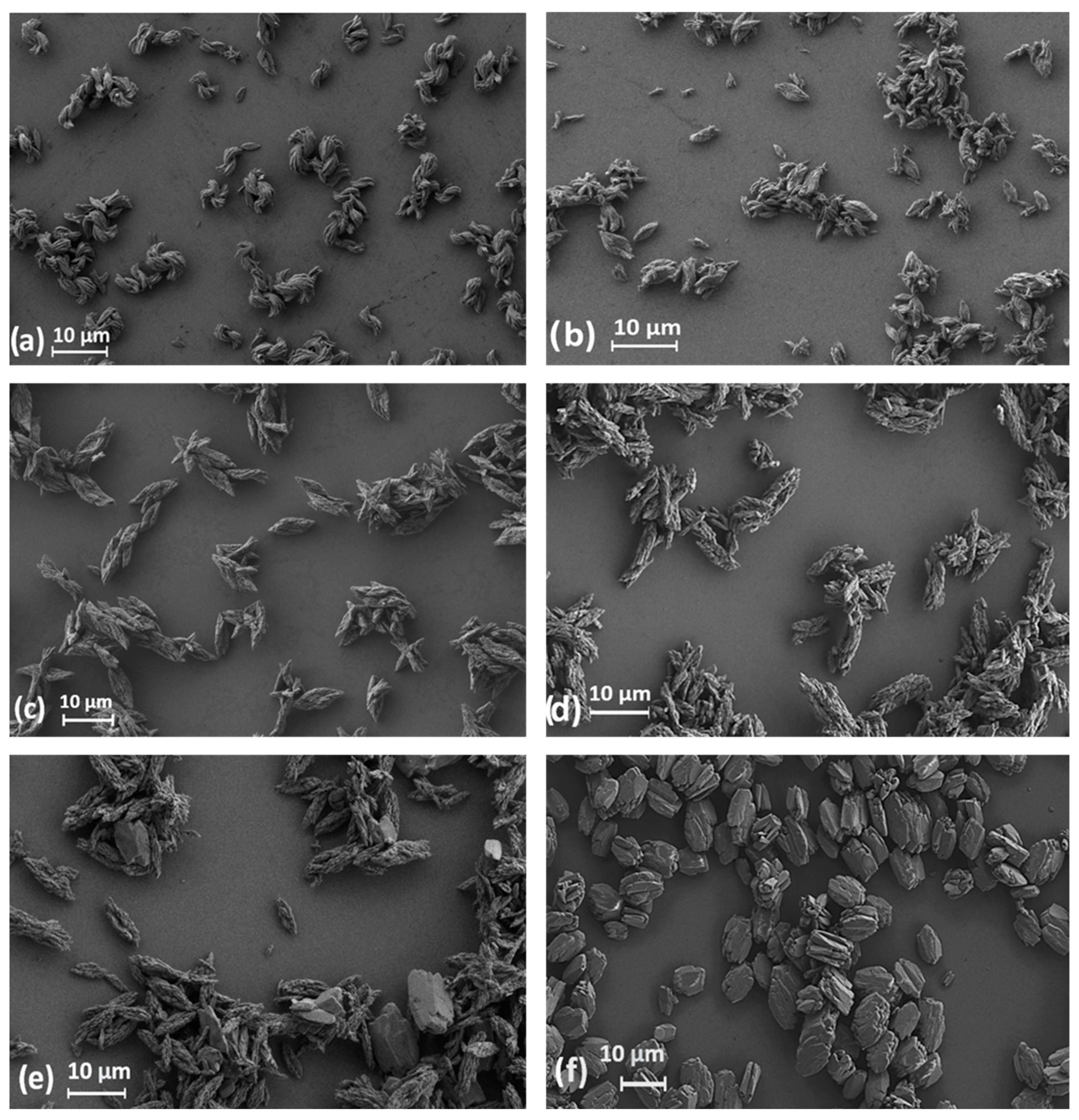
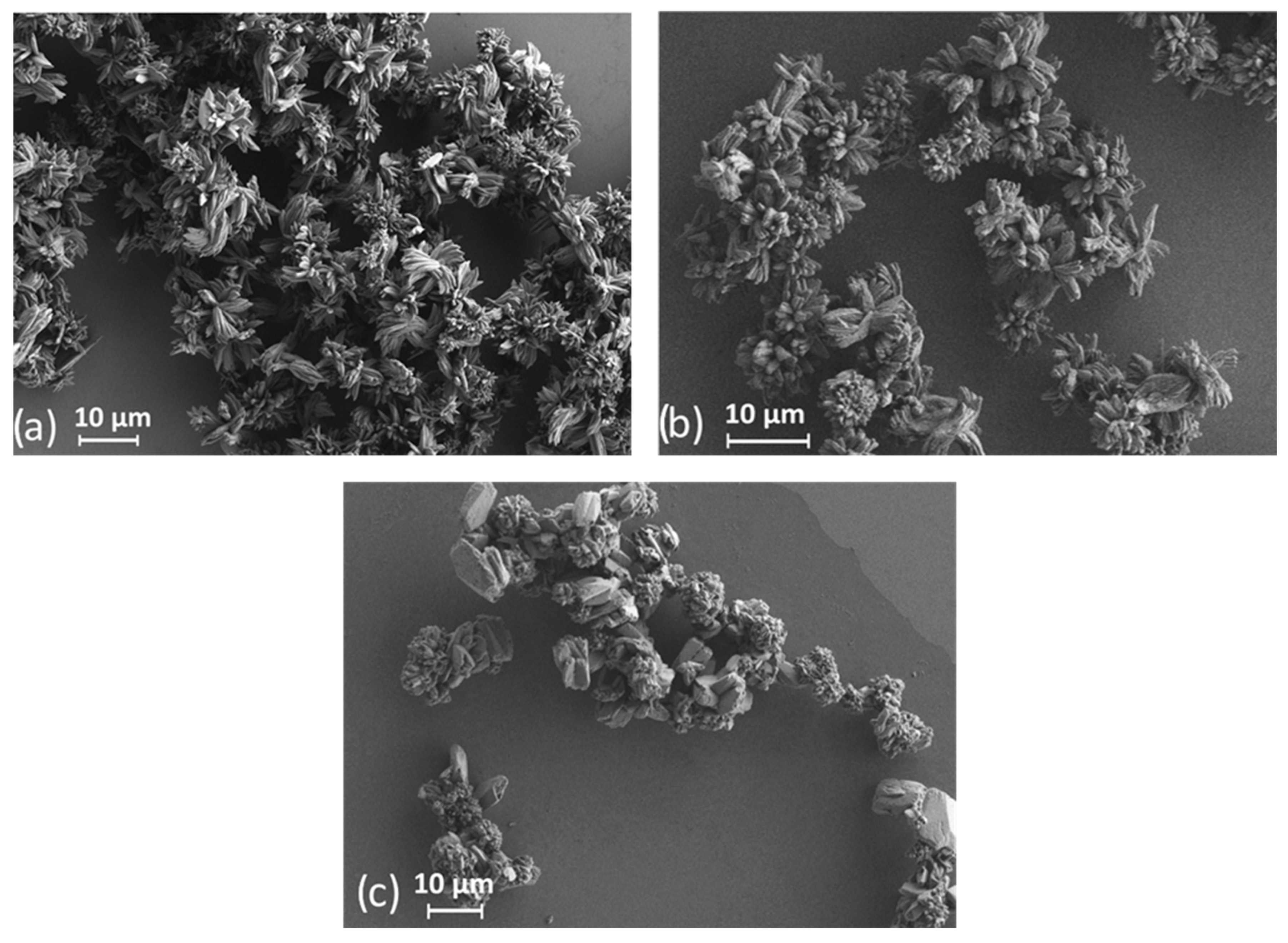




| Ln | Average Length, μm | Average Width, μm |
|---|---|---|
| La | 6.7 ± 1.3 | 2.8 ± 0.7 |
| Ce | 5.5 ± 1.1 | 2.6 ± 0.6 |
| Pr | 4.9 ± 0.9 | 2.1 ± 0.5 |
| Nd | 5.9 ± 1.1 | 2.6 ± 0.5 |
| Sm | 3.9 ± 0.6 | 1.6 ± 0.3 |
| Eu | 4.7 ± 0.9 | 1.9 ± 0.4 |
| Gd | 5.1 ± 1.1 | 2.2 ± 0.6 |
| Tb | 3.9 ± 1.0 | 1.5 ± 0.5 |
| Dy | 5.5 ± 2.0 | 2.2 ± 0.9 |
| Ho | 5.8 ± 1.3 | 2.3 ± 0.6 |
| Er | 9.3 ± 2.0 | 3.1 ± 0.6 |
| Tm | 7.1 ± 1.9 | 2.4 ± 0.6 |
| Yb | 8.9 ± 1.9 | 3.1 ± 0.8 |
| Lu | 7.7 ± 1.3 | 5.3 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toikka, Y.N.; Guseva, P.B.; Bogachev, N.A.; Kolesnik, S.S.; Glukhoedov, N.A.; Orlov, S.N.; Ryazantsev, M.N.; Skripkin, M.Y.; Mereshchenko, A.S. Ultrasound-Assisted Synthesis of Microcrystalline Lanthanide Terephthalates: Insights into Morphology and Structural Properties. Chemistry 2025, 7, 49. https://doi.org/10.3390/chemistry7020049
Toikka YN, Guseva PB, Bogachev NA, Kolesnik SS, Glukhoedov NA, Orlov SN, Ryazantsev MN, Skripkin MY, Mereshchenko AS. Ultrasound-Assisted Synthesis of Microcrystalline Lanthanide Terephthalates: Insights into Morphology and Structural Properties. Chemistry. 2025; 7(2):49. https://doi.org/10.3390/chemistry7020049
Chicago/Turabian StyleToikka, Yulia N., Polina B. Guseva, Nikita A. Bogachev, Stefaniia S. Kolesnik, Nikita A. Glukhoedov, Sergey N. Orlov, Mikhail N. Ryazantsev, Mikhail Yu. Skripkin, and Andrey S. Mereshchenko. 2025. "Ultrasound-Assisted Synthesis of Microcrystalline Lanthanide Terephthalates: Insights into Morphology and Structural Properties" Chemistry 7, no. 2: 49. https://doi.org/10.3390/chemistry7020049
APA StyleToikka, Y. N., Guseva, P. B., Bogachev, N. A., Kolesnik, S. S., Glukhoedov, N. A., Orlov, S. N., Ryazantsev, M. N., Skripkin, M. Y., & Mereshchenko, A. S. (2025). Ultrasound-Assisted Synthesis of Microcrystalline Lanthanide Terephthalates: Insights into Morphology and Structural Properties. Chemistry, 7(2), 49. https://doi.org/10.3390/chemistry7020049








