Abstract
Benzimidazolinones exhibit unique biological activities and serve as building blocks in synthesizing pharmaceutical compounds. Although multiple synthetic approaches involving intermolecular cyclization reactions have been reported, intramolecular cyclization reactions are scarce, and more rational synthetic methods are required. Hypervalent iodine-catalyzed oxidative C–N coupling is a potentially effective approach for synthesizing benzimidazolinones under metal-free conditions. In this study, we present a method utilizing hypervalent iodine catalysis for the oxidative cyclization of N’-aryl urea compounds, resulting in the first metal-free synthesis of various benzimidazolinones.
1. Introduction
Nitrogen-containing heterocyclic skeletons are commonly found in biologically active natural products [1], pharmaceuticals [2,3], and agrochemicals [4]. These skeletons are also present in the core structures of functional organic compounds and other molecules [5].
The benzimidazolinone scaffold is an important heterocyclic framework that exhibits various biological, pharmacological, and neurochemical activities, including anti-allergic activity [6], calcium channel inhibition [7], angiotensin II receptor antagonism [8], and antimicrobial activity [9] (Figure 1). Benzimidazolinones are utilized in the pharmaceutical and fine chemical industries as building blocks for the synthesis of drugs with diverse biological activities.
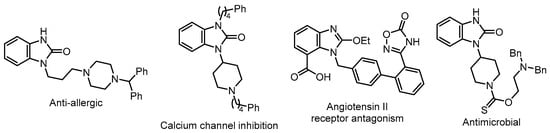
Figure 1.
Compounds containing a benzimidazolinone skeleton with biological activities.
The representative synthetic routes to benzimidazolinones are shown in Scheme 1. Reductive carbonylation is among the methods employed for the synthesis of benzimidazolinones (Scheme 1a, top) [10,11,12,13]. During this process, 2-nitroanilines undergo catalytic reductive activation in a CO atmosphere to produce isocyanates, which are used without isolation and subjected to subsequent intramolecular cyclization to yield benzimidazolinones. Although this method is highly useful for the synthesis of benzimidazolinones, the use of flammable and toxic CO gas is inevitable and requires special care and equipment. Another strategy for preparing benzimidazolinones is based on a two-step synthesis involving the reduction of 2-nitroanilines and cyclization of 1,2-diamines (Scheme 1a, bottom). Conventionally, highly toxic and corrosive phosgenes are used to accomplish cyclization reactions, during which a stoichiometric amount of hydrogen chloride is produced [14]. Eventually, phosgene was replaced with different reagents, including triphosgene, 1,1′-carbonyldiimidazole, and dimethyl carbonate [15,16,17]. An alternative technique for the synthesis of benzimidazolinones relies on an oxidative C–N coupling reaction that enables the direct conversion of aromatic C–H bonds into C–N bonds (Scheme 1b). An earlier study reported a pioneering oxidative C–N coupling reaction that produced various benzimidazolinones in good yields [18]. One major drawback of this protocol is that highly toxic lead tetraacetate is required to trigger the reaction, rendering its practical application challenging.
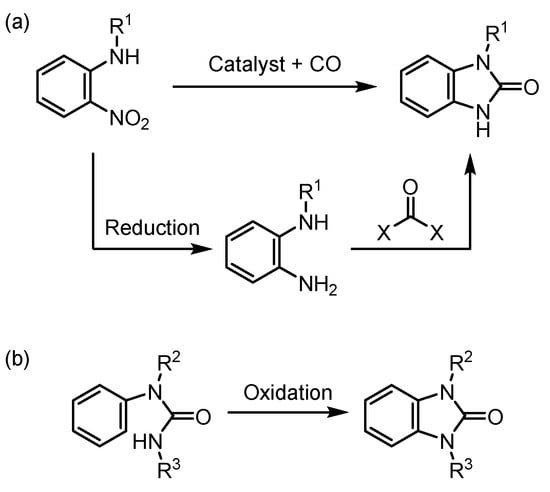
Scheme 1.
Representative synthetic routes to benzimidazolinones. (a) Reductive route (b) Oxidative Cyclization.
In our laboratory, hypervalent iodine reagents have long been developed as alternatives to heavy metal oxidants due to their low toxicity and ease of handling without the risk of explosions or other safety concerns. Recently, we demonstrated the efficient formation of aromatic C–N bond formation reactions using hypervalent iodine [19] and diiodoarene precursor catalysts [20,21,22,23]. Furthermore, our protocol for the iodonium-salt-mediated arylation of salicylamide derivatives provides access to coupling methods that do not require metal catalysts [24]. Here, we explore the formation of C–N bonds using an environmentally friendly catalyst that does not require transition-metal catalysts, special equipment, or a catalyst with a complex structure requiring multiple process adjustments. We constructed a benzimidazolinone framework through an intramolecular cyclization reaction, which was obtained by a hypervalent iodine catalytic reaction after the conversion of commercially available phenyl isocyanate to N-methoxy-N′-phenyl urea (Scheme 2).
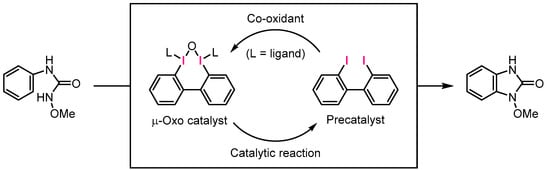
Scheme 2.
μ-Oxo hypervalent iodine-catalyzed oxidative C–N cyclization for benzimidazolinone synthesis.
2. Results and Discussion
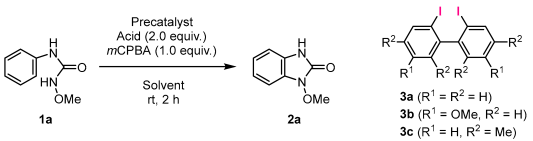
We investigated the synthesis of benzimidazolinones via intramolecular C–N coupling using oxygen-bridged hypervalent iodine catalysts with biaryl structures (Table 1). When urea derivative 1a was reacted with 5 mol% of the biaryl-type catalyst precursor 3a in the presence of trifluoroacetic acid and m-chloroperoxybenzoic acid (mCPBA) in hexafluoroisopropanol (HFIP), the formation of benzimidazolinone 2a was not observed (Entry 1). Similar results were obtained when dichloromethane or chloroform was selected as the solvent (Entries 2 and 3), and, when the reaction was performed with acetic acid instead of trifluoroacetic acid in HFIP, product 2a was not obtained (Entry 4). When dichloromethane was used as an alternative solvent, 2a was obtained in a moderate yield (Entry 5), and the yield increased when chloroform was used (Entry 6). When the reaction time was shortened, the yield of 2a decreased (Entry 7). The reaction proceeded effectively with other biaryl-type catalyst precursors 3b and 3c, giving 2a in good yields (Entries 8 and 9). Furthermore, when 10 mol% iodobenzene was used as the catalyst precursor, the reaction proceeded less effectively than with biaryl-type catalyst precursors (Entry 10).

Table 1.
Investigation of conditions for the construction of a benzimidazolinone skeleton using a hypervalent iodine catalyst a.
The versatility of the substrates was examined under the reaction conditions described in Entry 6 of Table 1 using catalyst precursor 3a (Scheme 3). Ureas 1b–e, with halogen or methyl groups in the para-position of the aromatic ring, gave the corresponding benzimidazolinones 2b–e in 56–75% yields, while substrates 1f and 1g, with methoxy or trifluoromethyl groups, gave cyclization products 2f and 2g in low yields. The reaction also proceeded when substrates 1h–i, with ortho substitution of methyl or phenyl groups, 1j with meta substitution of two methyl groups, and naphthalene-type urea 1k were used, resulting in products 2h–k with 32–57% yields. Ureas 1l–n substituted on the oxygen atom were also converted to 2l–n in good yields.
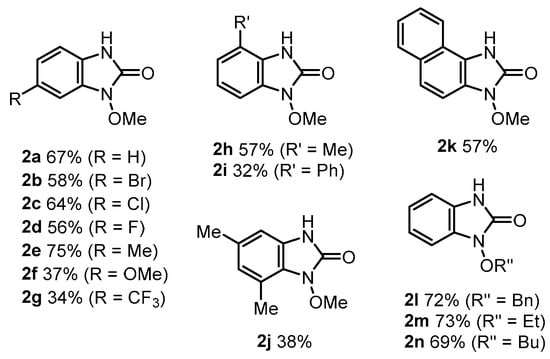
Scheme 3.
Substrate scope. Reaction conditions: 1a (0.30 mmol), 3a (5 mol%), AcOH (2.0 equiv.), wet mCPBA (contains ca. 30% water, 1.0 equiv.) in CHCl3 (1.5 mL, 0.2 M) at room temperature for 2 h.
Furthermore, the meta-substituted substrate 1o produced the regioisomeric cyclization products 2o and 2o’ in comparable yields (Scheme 4).
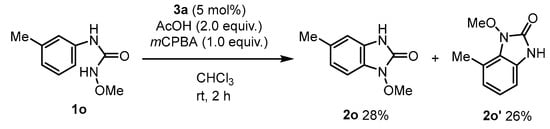
Scheme 4.
Reaction of the meta-substituted substrate.
Two additional experiments were conducted to better understand the C–N coupling (Scheme 5). When N-methoxy-N-methyl-N’-phenylurea 1p was used, it was recovered with an 89% yield, indicating a lower reactivity compared to 1a (Scheme 5a). This indicates that the N-methoxy amide moiety of 1a, rather than the anilide moiety, was involved in the C–N coupling process. The second experiment demonstrated that product 2a was intact under the catalytic conditions (Scheme 5b), implying that the overoxidation of 2a was negligible during the C–N coupling reaction of 1a.
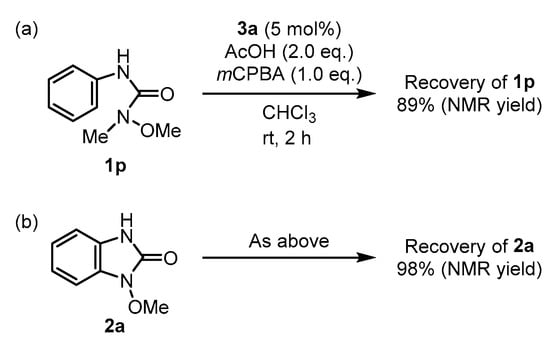
Scheme 5.
Additional experiments on the C–N coupling reaction. (a) N-substituted methoxyamide 1p (b) product stability under the catalytic conditions.
The proposed catalytic cycle is illustrated in Scheme 6. In the presence of mCPBA and AcOH, the iodine(I) precatalyst may undergo oxidation to form the reactive μ-oxo iodine(III) catalyst. Subsequently, ligand exchange can occur to replace the OAc ligand with the substrate, possibly yielding an electrophilic urea-I(III) intermediate, where the N-methoxy amide nitrogen of the substrate coordinates to iodine. According to DFT calculations by Houk and Xue, a dearomatizing spirolactonization reaction is facilitated by proton transfer from a carboxylic-acid-type substrate to a bridged oxygen of a μ-oxo catalyst [25]. Inspired by this computational study, we speculated that the urea-I(III) intermediate undergoes protonation, as depicted in Scheme 6, to form a reactive intermediate that can rapidly provide benzimidazolinone through oxidation of the urea ligand.
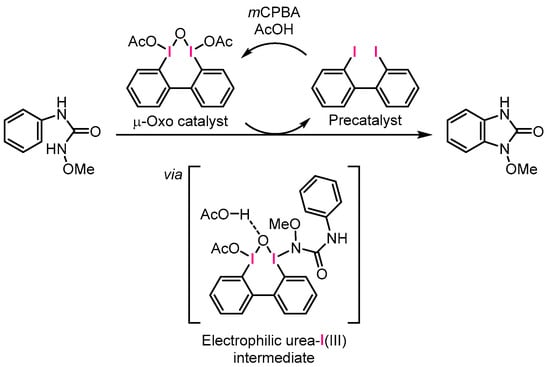
Scheme 6.
Proposed catalytic cycle and intermediate.
3. Materials and Methods
3.1. General Information
Infrared absorption (IR) spectra were measured using a Fourier transform infrared (FTIR) spectrophotometer (JASCO FT/IR-4200 type) by the KBr diffuse reflection method. Nuclear magnetic resonance (NMR; 1H NMR and 13C NMR) spectra were measured using a Fourier transform NMR instrument (JEOL JMN-EX 400 type; 400 MHz (1H NMR) and 100 MHz (13C NMR)) with chloroform (7.26 (1H NMR) and 77.0 ppm (13C NMR)) and dimethyl sulfoxide (2.50 (1H NMR) and 39.5 ppm (13C NMR)) used as internal standards. The multiplicity of each peak was abbreviated as s = singlet, d = doublet, t = triplet, q = quadruplet, or m = multiplet. High-resolution mass spectrometry (HRMS) spectra were measured by real-time direct analytical methods (DART). Merck Silica Gel 60 (230–400 mesh) was used as a sorbent for flash column chromatography. Thin-layer chromatography (TLC) was performed using Silica Gel 70 PF254 Plate-Wako, and spots and bands were determined by UV irradiation (254 nm) or by the respective TLC coloring reagents (p-anisaldehyde–ethanol solution, Phosphomolybdic acid–ethanol solution). The extracts were dried using anhydrous sodium sulfate, and various reaction reagents, additives, and organic solvents (Tokyo Kasei Corporation, Tokyo Japan, FUJIFILM Wako Pure Chemicals Corporation, Osaka, Japan, Sigma-Aldrich Japan LLC, Tokyo, Japan, Nacalai Tesque Corporation, Kyoto, Japan, BLD Pharmatech Ltd., Shanghai, China) were used without further purification using commercial products.
3.2. Synthesis of Urea Derivatives
General Procedure for Synthesis of Urea Substrate 1
Amine hydrochloride (3.0 mmol) was dissolved in a dichloromethane solution (3 mL), and N,N-diisopropylethylamine (DIPEA; 7.5 mmol, 1.3 mL, 2.5 equiv.) was subsequently added and stirred at room temperature for 5 min. Aryl isocyanate (3.0 mmol) was added and stirred at room temperature overnight, after which the product was concentrated under reduced pressure and purified by column chromatography to obtain the corresponding urea derivative 1.
3.3. Hypervalent Iodine Catalysis
3.3.1. General Procedure for Cyclization of Aryl Urea-1 Using Oxygen-Bridged Hypervalent Iodine Catalyst
The catalyst precursor 3a (6.1 mg, 0.015 mmol, 5 mol%) and acetic acid (34 µL, 0.60 mmol, 2.0 equiv.) were added to a chloroform solution (1.5 mL) containing urea 1 (0.30 mmol). Subsequently, wet mCPBA (74.0 mg, 70% purity contains ca. 30% water, 0.30 mmol, 1.0 equiv.) was added, and the mixture was stirred at room temperature for 2 h. Following completion of the reaction, ethyl acetate was added to the reaction solution, and the organic layer was washed with baking soda, a sodium thiosulfate solution, and saturated brine before drying over anhydrous sodium sulfate. The solution was filtered and concentrated under reduced pressure and purified by column chromatography to obtain the corresponding benzimidazolinone 2.
3.3.2. 1-Methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2a
According to the general procedure, the title compound was synthesized using N-methoxy-N′-phenylurea 1a in a 67% yield as a white solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.48 (s, 1H), 7.17–7.08 (m, 4H), 4.14 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 152.3, 126.8, 124.3, 122.2, 121.8, 110.5, 106.8, 64.7 ppm. IR (KBr): 3160 w, 3055 w, 2946 w, 1704 s, 1481 m, 1300 m, 1204 m cm−1. Melting Point: 157.2–157.8 °C (dec.). The spectra matched those previously reported [26].
3.3.3. 6-Bromo-1-methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2b
According to the general procedure, the title compound was synthesized using N-(4-bromophenyl)-N′-methoxyurea 1b in a 58% yield as a white solid. 1H NMR (400 MHz, DMSO-d6, δ/ppm): 11.22 (s, 1H), 7.36 (d, J = 2.0 Hz, 1H), 7.20 (dd, J = 8.3, 2.0 Hz, 1H), 6.98 (d, J = 8.3 Hz, 1H), 3.98 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6, δ/ppm): 150.3, 128.1, 124.1, 123.9, 112.8, 111.2, 109.0, 64.4 ppm. IR (KBr): 3276 w, 3200 w, 3157 w, 3052 w, 1701 s, 1483 m, 1376 m, 1281 m cm−1. Melting Point: 197.6–198.0 °C (dec.). The spectra matched those previously reported [26].
3.3.4. 6-Chloro-1-methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2c
According to the general procedure, the title compound was synthesized using N-(4-chlorophenyl)-N′-methoxyurea 1c in a 64% yield as a white solid. 1H NMR (400 MHz, DMSO-d6, δ/ppm): 11.21 (s, 1H), 7.25 (d, J = 2.0 Hz, 1H), 7.07 (dd, J = 8.3, 2.0 Hz, 1H), 7.02 (d, J = 8.3 Hz, 1H), 3.97 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6, δ/ppm): 150.5, 127.8, 125.4, 123.5, 121.3, 110.8, 106.5, 64.4 ppm. IR (KBr): 3254 w, 3222 w, 3168 w, 1747 s, 1479 m cm−1. Melting Point: 186.8–187.7 °C (dec.). The spectra matched those previously reported [26].
3.3.5. 6-Fluoro-1-methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2d
According to the general procedure, the title compound was synthesized using N-(4-fluorophenyl)-N′-methoxyurea 1d in a 56% yield as a white solid. 1H NMR (400 MHz, DMSO-d6, δ/ppm): 11.10 (s, 1H), 7.11 (dd, J = 8.3, 2.4 Hz, 1H), 7.01 (q, J = 4.4 Hz, 1H), 6.86 (td, J = 9.5, 2.4 Hz, 1H), 3.99 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6, δ/ppm): 157.9 (d, J = 235.8 Hz), 151.0, 127.4 (d, J = 13.2 Hz), 120.9, 110.2 (d, J = 9.1 Hz), 107.8 (d, J = 24.0 Hz), 94.9 (d, J = 29.0 Hz), 64.4 ppm. IR (KBr): 3257 w, 3214 w, 3188 w, 1772 s, 1689 s cm−1. Melting Point: 192.8–193.5 °C (dec.). The spectra matched those previously reported [26].
3.3.6. 1-Methoxy-6-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2e
According to the general procedure, the title compound was synthesized using N-methoxy-N′-(4-methylphenyl)urea 1e in a 75% yield as a white solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.44 (s, 1H), 7.03 (d, J = 8.3 Hz, 1H), 6.97 (s, 1H), 6.90 (d, J = 8.3 Hz, 1H), 4.12 (s, 3H), 2.41 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 152.5, 131.7, 126.9, 122.8, 122.1, 110.2, 107.3, 64.7, 21.4 ppm. IR (KBr): 3185 w, 3061 w, 2950 w, 2844 w, 1699 s, 1469 m, 1430 m, 1302 m cm−1. Melting Point: 159.6–160.1 °C (dec.). The spectra matched those previously reported [26].
3.3.7. 1,6-Dimethoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2f
According to the general procedure, the title compound was synthesized using N-methoxy-N′-(4-methoxyphenyl)urea 1f in a 37% yield as a brown solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.49 (s, 1H), 7.04 (d, J = 8.3 Hz, 1H), 6.74 (d, J = 2.0 Hz, 1H), 6.66 (dd, J = 8.3, 2.4 Hz, 1H), 4.12 (s, 3H), 3.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 155.7, 152.7, 127.5, 118.1, 111.0, 108.1, 93.5, 64.7, 55.9 ppm. IR (KBr): 3247 w, 3193 w, 3108 w, 2941 w, 1721 s, 1495 m, 1226 m cm−1. Melting Point: 118.2–119.4 °C (dec.). HRMS (DART) m/z: ([M + H]+) Calcd for C9H11N2O3+ 195.0764; Found 195.0763.
3.3.8. 1-Methoxy-6-(trifluoromethyl)-1,3-dihydro-2H-benzo[d]imidazol-2-one 2g
According to the general procedure, the title compound was synthesized using N-methoxy-N′-[4-(trifluoromethyl)phenyl]urea 1g in a 34% yield as a white solid. 1H NMR (400 MHz, DMSO-d6, δ/ppm): 11.53 (s, 1H), 7.48 (s, 1H), 7.41 (d, J = 8.3 Hz, 1H), 7.22 (d, J = 7.8 Hz, 1H), 4.05 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6, δ/ppm): 150.7, 127.8, 127.0, 124.7 (q, J = 271.4Hz), 121.9 (q, J = 31.7 Hz), 118.9 (q, J = 4.1 Hz), 109.8, 103.2 (q, J = 3.9 Hz), 64.6 ppm. IR (KBr): 3318 w, 3281 w, 3191 w, 3018 w, 1732 s, 1466 m, 1314 m cm−1. Melting Point: 157.1–157.9 °C (dec.). The spectra matched those previously reported [26].
3.3.9. 1-Methoxy-4-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2h
According to the general procedure, the title compound was synthesized using N-methoxy-N′-(2-methylphenyl)urea 1h in a 57% yield as a white solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.88 (s, 1H), 7.05 (t, J = 7.6 Hz, 1H), 6.99 (d, J = 7.8 Hz, 1H), 6.93 (d, J = 7.8 Hz, 1H), 4.11 (s, 3H), 2.44 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 152.4, 126.4, 123.4, 123.3, 121.7, 120.5, 104.4, 64.6, 15.9 ppm. IR (KBr): 3185 w, 3061 w, 2950 w, 1699 s, 1469 m, 1430 m, 1302 m, 966 m cm−1. Melting Point: 186.1–186.7 °C (dec.). The spectra matched those previously reported [26].
3.3.10. 1-Methoxy-4-phenyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2i
According to the general procedure, the title compound was synthesized using N-methoxy-N′-(2-methylphenyl)urea 1i in a 32% yield as an orange solid. 1H NMR (400 MHz, DMSO-d6, δ/ppm): 11.04 (s, 1H), 7.55–7.53 (m, 2H), 7.48 (t, J = 7.6 Hz, 2H), 7.40 (tt, J = 7.3, 1.5 Hz, 1H), 7.20–7.13 (m, 2H), 7.09 (dd, J = 6.8, 1.5 Hz, 1H), 4.00 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6, δ/ppm): 150.9, 136.6, 128.9, 128.2, 127.6, 127.4, 123.8, 122.0, 121.9, 121.7, 105.4, 64.2 ppm. IR (KBr): 3162 w, 3130 w, 3108 w, 3061 w, 3038 w, 1713 s, 1421 m, 1168 m cm−1. Melting Point: 184.5–185.6 °C (dec.). HRMS (DART) m/z: ([M + H]+) Calcd for C14H13N2O2+ 241.0972; Found 241.0970.
3.3.11. 1-Methoxy-5,7-dimethyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2j
According to the general procedure, the title compound was synthesized using N-methoxy-N′-(2,5-dimethylphenyl)urea 1j in a 38% yield as a white solid. 1H NMR (400 MHz, DMSO-d6, δ/ppm): 10.88 (s, 1H), 6.64 (s, 2H), 3.92 (s, 3H), 2.41 (s, 3H), 2.26 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6, δ/ppm): 151.7, 130.9, 125.3, 124.1, 123.0, 118.0, 107.7, 64.5, 20.8, 15.7 ppm. IR (KBr): 3138 w, 3055 w, 3018 w, 2050 w, 1722 s, 1456 m, 1427 m, 1377 m, 1168 m, 1002 m, 945 m cm−1. Melting Point: 159.5–160.4 °C (dec.). HRMS (DART) m/z: ([M + H]+) Calcd for C10H13N2O2+ 193.0972; Found 193.0970.
3.3.12. 3-Methoxy-1,3-dihydro-2H-naphtho[1,2-d]imidazol-2-one 2k
According to the general procedure, the title compound was synthesized using N-methoxy-N′-1-naphthalenylurea 1i in a 57% yield as a white solid. 1H NMR (400 MHz, DMSO-d6, δ/ppm): 11.92 (s, 1H), 8.11 (d, J = 8.3 Hz, 1H), 7.96 (d, J = 8.3 Hz, 1H), 7.70 (d, J = 8.3 Hz, 1H), 7.57 (t, J = 7.3 Hz, 1H), 7.50 (d, J = 8.8 Hz, 1H), 7.42 (t, J = 7.3 Hz, 1H), 4.07 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6, δ/ppm): 150.6, 129.1, 128.8, 126.3, 123.8, 122.4, 121.5, 120.3, 119.2, 118.1, 108.0, 64.5 ppm. IR (KBr): 3183 w, 3130 w, 3051 w, 2995 w, 2942 w, 1703 s, 1457 m, 1379 m, 1170 m cm−1. Melting Point: 163.9–165.8 °C (dec.). HRMS (DART) m/z: ([M + H]+) Calcd for C12H11N2O2+ 215.0815; Found 215.0813.
3.3.13. 1-(Benzyloxy)-1,3-dihydro-2H-benzo[d]imidazol-2-one 2l
According to the general procedure, the title compound was synthesized using N-phenyl-N′-(phenylmethoxy)urea 1l in a 72% yield as a brown solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.70 (s, 1H), 7.50 (q, J = 3.1 Hz, 2H), 7.36 (t, J = 3.2 Hz, 3H), 7.12 (d, J = 7.8 Hz, 1H), 7.04–6.97 (m, 2H), 6.82 (d, J = 7.3 Hz, 1H), 5.27 (s, 2H) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 152.8, 134.1, 129.9, 129.3, 128.6, 127.7, 124.2, 122.0, 121.6, 110.3, 107.2, 79.3 ppm. IR (KBr): 3193 w, 3154 w, 3130 w, 3107 w, 2960 w, 1713 s, 1477 m, 1396 m, 1297 m, 1199 m cm−1. Melting Point: 159.1–160.0 °C (dec.). The spectra matched those previously reported [26].
3.3.14. 1-Ethoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2m
According to the general procedure, the title compound was synthesized using N-ethoxy-N′-phenylurea 1m in a 73% yield as a beige solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.83 (s, 1H), 7.17–7.06 (m, 4H), 4.38 (q, J = 7.0 Hz, 2H), 1.46 (t, J = 7.1 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 152.8, 127.6, 124.3, 122.0, 121.6, 110.4, 106.9, 73.1, 13.7 ppm. IR (KBr): 3184 w, 3078 w, 3061 w, 1713 s, 1479 m, 1298 m, 1202 m, 1179 m, 1023 m cm−1. Melting Point: 137.7–138.3 °C (dec.). HRMS (DART) m/z: ([M + H]+) Calcd for C9H11N2O2+ 179.0815; Found 179.0813.
3.3.15. 1-Butoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2n
According to the general procedure, the title compound was synthesized using N-butoxy-N′-phenylurea 1n in a 69% yield as a white solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.79 (s, 1H), 7.17–7.06 (m, 4H), 4.30 (t, J = 6.8 Hz, 2H), 1.87–1.80 (m, 2H), 1.56 (m, 2H), 1.00 (t, J = 7.3 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 150.9, 127.6, 124.7, 121.4, 121.1, 109.5, 106.3, 76.2, 29.7, 18.6, 13.6 ppm. IR (KBr): 3194 w, 3140 w, 3130 w, 3097 w, 2955 w, 1699 s, 1474 m, 1398 m, 1301 m, 1202 m, 1177 m cm−1. Melting Point: 117.9–118.8 °C (dec.). HRMS (DART) m/z: ([M + H]+) Calcd for C11H15N2O2+ 207.1128; Found 207.1126.
3.3.16. 1-Methoxy-5-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one (2o) and 1-Methoxy-7-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one (2o’) (Mixture)
According to the general procedure, the title compounds were synthesized using N-methoxy-N′-(3-methylphenyl)urea 1o in a 28% yield (2o) and 26% yield (2o’) as an orange solid. 1H NMR (400 MHz, CDCl3, δ/ppm): 10.79 (s, 1H), 10.73 (s, 1H), 7.03–6.98 (m, 4H, 2o + 2o’), 6.94–6.86 (m, 2H, 2o + 2o’), 4.10 (s, 6H, 2o + 2o’), 2.56 (s, 3H, 2o’), 2.36 (s, 3H, 2o) ppm. 13C NMR (100 MHz, CDCl3, δ/ppm): 152.9, 152.5, 132.1, 125.1, 124.6, 124.6, 124.5, 124.2, 122.3, 122.2, 119.0, 111.1, 108.1, 106.5, 65.3, 64.6, 21.3, 16.2 ppm.
4. Conclusions
In summary, benzimidazolinones were obtained via the oxidative cyclization of N-aryl ureas under hypervalent iodine catalysis. This method was applied to the synthesis of various benzimidazolinone compounds. The benzimidazolinones were obtained in yields comparable to or higher than those observed in previous studies. With respect to the substrate range, our hypervalent iodine catalysis yielded the desired compounds after the transformation of various isocyanates into the urea forms. To the best of our knowledge, this is the first example of organocatalytic oxidative cyclization for the synthesis of NH-free benzimidazolinones.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemistry7020050/s1: 1H and 13C NMR spectroscopic charts for all the compounds 2a–o and 2o’.
Author Contributions
Conceptualization, T.D.; methodology, M.H. and S.H.; formal analysis, S.H.; investigation, M.H. and S.H.; resources, S.H., H.S. and M.H.; data curation, M.H., S.H. and N.T.; writing—original draft preparation, M.H. and S.H.; writing—review and editing, M.H., T.H. and T.D.; visualization, H.S. and T.D.; supervision, M.H. and T.D.; project administration, M.H. and T.D.; funding acquisition, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JST CREST grant number JPMJCR20R1. The authors also acknowledge support from the Ritsumeikan Global Innovation Research Organization (R-GIRO) project. M.H. gives thanks for the support from the Sasakawa Scientific Research Grant.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Devi, N.; Kumar, S.; Pandey, S.K.; Singh, V. 1(3)-Formyl-β-carbolines: Potential Aldo-X Precursors for the Synthesis of β-Carboline-Based Molecular Architectures. Asian J. Org. Chem. 2018, 7, 6–36. [Google Scholar]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [PubMed]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. [Google Scholar] [PubMed]
- Zakharychev, V.V.; Martsynkevich, A.M. Development of novel pyridine-based agrochemicals: A review. Adv. Agrochem. 2025, 4, 30–48. [Google Scholar]
- Bellotti, P.; Koy, M.; Hopkinson, M.N.; Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 2021, 5, 711–725. [Google Scholar]
- Awouters, F.; Niemegeers, C.J.E.; Van den Berk, J.; Van Nueten, J.M.; Lenaerts, F.M.; Borgers, M.; Schellekens, K.H.L.; Broeckaert, A.; De Cree, J.; Janssen, P.A.J. Oxatomide, a new orally active drug which inhibits both the release and the effects of allergic mediators. Experientia 1977, 33, 1657–1659. [Google Scholar]
- Kasanami, Y.; Ishikawa, C.; Kino, T.; Chonan, M.; Toyooka, N.; Takashima, Y.; Iba, Y.; Sekiguchi, F.; Tsubota, M.; Ohkubo, T.; et al. Discovery of pimozide derivatives as novel T-type calcium channel inhibitors with little binding affinity to dopamine D2 receptors for treatment of somatic and visceral pain. Eur. J. Med. Chem. 2022, 243, 114716. [Google Scholar]
- Perry, C.M. Azilsartan Medoxomil A Review of its Use in Hypertension. Clin. Drug Investig. 2012, 32, 621–639. [Google Scholar]
- Saïdani, N.; Botté, C.Y.; Deligny, M.; Bonneaud, A.-L.; Reader, J.; Lasselin, R.; Merer, G.; Niepceron, A.; Brossier, F.; Cintrat, J.-C.; et al. Discovery of Compounds Blocking the Proliferation of Toxoplasma gondii and Plasmodium falciparum in a Chemical Space Based on Piperidinyl-Benzimidazolone Analogs. Antimicrob. Agents Chemother. 2014, 58, 2586–2597. [Google Scholar]
- Wu, Q.; Chen, J.; Liu, Z.; Xu, Y. CO Activation Using Nitrogen-Doped Carbon Nanotubes for Reductive Carbonylation of Nitroaromatics to Benzimidazolinone and Phenyl Urea. ACS Appl. Mater. Interfaces 2020, 12, 48700–48711. [Google Scholar]
- Wang, X.; Ling, G.; Xue, Y.; Lu, S. Selenium-Catalyzed Reductive Carbonylation of 2-Nitrophenols to 2-Benzoxazolones. Eur. J. Org. Chem. 2005, 2005, 1675–1679. [Google Scholar]
- Qi, X.; Zhou, R.; Peng, J.-B.; Ying, J.; Wu, X.-F. Selenium-Catalyzed Carbonylative Synthesis of 2-Benzimidazolones from 2-Nitroanilines with TFBen as the CO Source. Eur. J. Org. Chem. 2019, 2019, 5161–5164. [Google Scholar]
- Tsuji, Y.; Takeuchi, R.; Watanabe, Y. Platinum complex catalyzed synthesis of urea derivatives from nitroarenes and amines under carbon monoxide. J. Organomet. Chem. 1985, 290, 249. [Google Scholar]
- English, J.P.; Clapp, R.C.; Cole, Q.P.; Halverstadt, I.F.; Lampen, J.O.; Roblin, R.O., Jr. Studies in Chemotherapy. IX. Ureylenebenzene and Cyclohexane Derivatives as Biotin Antagonist. J. Am. Chem. Soc. 1945, 67, 295–302. [Google Scholar]
- Purandare, A.V.; Gao, A.; Poss, M.A. Solid-phase synthesis of ‘diverse’ heterocycles. Tetrahedron Lett. 2002, 43, 3903–3906. [Google Scholar]
- Acharya, A.N.; Ostresh, J.M.; Houghten, R.A. Solid-phase parallel synthesis of substituted dihydroimidazolyl dihydrobenzimidazol-2-ones. Tetrahedron 2002, 58, 2095–2100. [Google Scholar]
- Nale, D.B.; Bhanage, B.M. Copper-catalyzed efficient synthesis of a 2-benzimidazolone scaffold from 2-nitroaniline and dimethyl carbonate via a hydrosilylation reaction. Green Chem. 2015, 17, 2480. [Google Scholar]
- Cooley, J.H.; Jacobs, P.T. Oxidative Ring Closure of 1-Benzyloxy-3-arylureas to 1-Benzyloxybenzimidazolones. J. Org. Chem. 1975, 40, 552–557. [Google Scholar]
- Dohi, T.; Maruyama, A.; Minamitsuji, Y.; Takenaga, N.; Kita, Y. First hypervalent iodine(III)-catalyzed C–N bond forming reaction: Catalytic spirocyclization of amides to N-fused spirolactams. Chem. Commun. 2007, 12, 1224–1226. [Google Scholar]
- Dohi, T.; Takenaga, N.; Fukushima, K.; Uchiyama, T.; Kato, D.; Shiro, M.; Fujioka, H.; Kita, Y. Designer μ-oxo-bridged hypervalent iodine(III) organocatalysts for greener oxidations. Chem. Commun. 2010, 46, 7697–7699. [Google Scholar]
- Dohi, T.; Sasa, H.; Dochi, M.; Yasui, C.; Kita, Y. Oxidative Coupling of N-Methoxyamides and Related Compounds toward Aromatic Hydrocarbons by Designer μ-Oxo Hypervalent Iodine Catalyst. Synthesis 2019, 51, 1185–1195. [Google Scholar] [CrossRef]
- Sasa, H.; Mori, K.; Kikushima, K.; Kita, Y.; Dohi, T. μ-Oxo-Hypervalent-Iodine-Catalyzed Oxidative C-H Amination for Synthesis of Benzolactam Derivatives. Chem. Pharm. Bull. 2022, 70, 106–110. [Google Scholar] [CrossRef]
- Sasa, H.; Hamatani, S.; Hirashima, M.; Takenaga, N.; Hanasaki, T.; Dohi, T. Efficient Metal-Free Oxidative C–H Amination for Accessing Dibenzoxazepinones via μ-Oxo Hypervalent Iodine Catalysis. Chemistry 2023, 5, 2155–2165. [Google Scholar] [CrossRef]
- Miyamoto, N.; Kikushima, K.; Sasa, H.; Katagiri, T.; Takenaga, N.; Kita, Y.; Dohi, T. Transition-metal-free dibenzoxazepinone synthesis by hypervalent iodine-mediated chemoselective arylocyclizations of N-functionalized salicylamides. Chem. Commun. 2025, 61, 1882–1885. [Google Scholar] [CrossRef]
- Zheng, H.; Sang, Y.; Houk, K.N.; Xue, X.-S.; Cheng, J.-P. Mechanism and Origins of Enantioselectivities in Spirobiindane-Based Hypervalent Iodine(III)-Induced Asymmetric Dearomatizing Spirolactonizations. J. Am. Chem. Soc. 2019, 141, 16046–16056. [Google Scholar] [CrossRef]
- Wang, Q.; An, J.; Alper, H.; Xiao, W.-J.; Beauchemin, A.M. Catalytic substitution/cyclization sequences of O-substituted Isocyanates: Synthesis of 1-alkoxybenzimidazolones and 1-alkoxy-3,4-dihydroquinazolin-2(1H)-ones. Chem. Commun. 2017, 53, 13055–13058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).