A Stable π-Expanded o-Quinodimethane via the Photochemical Dearomative Cycloaddition of Corannulene with an Isolable Dialkylsilylene
Abstract
1. Introduction
2. Results and Discussion
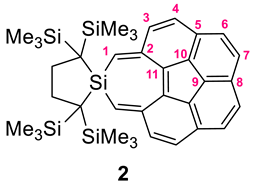
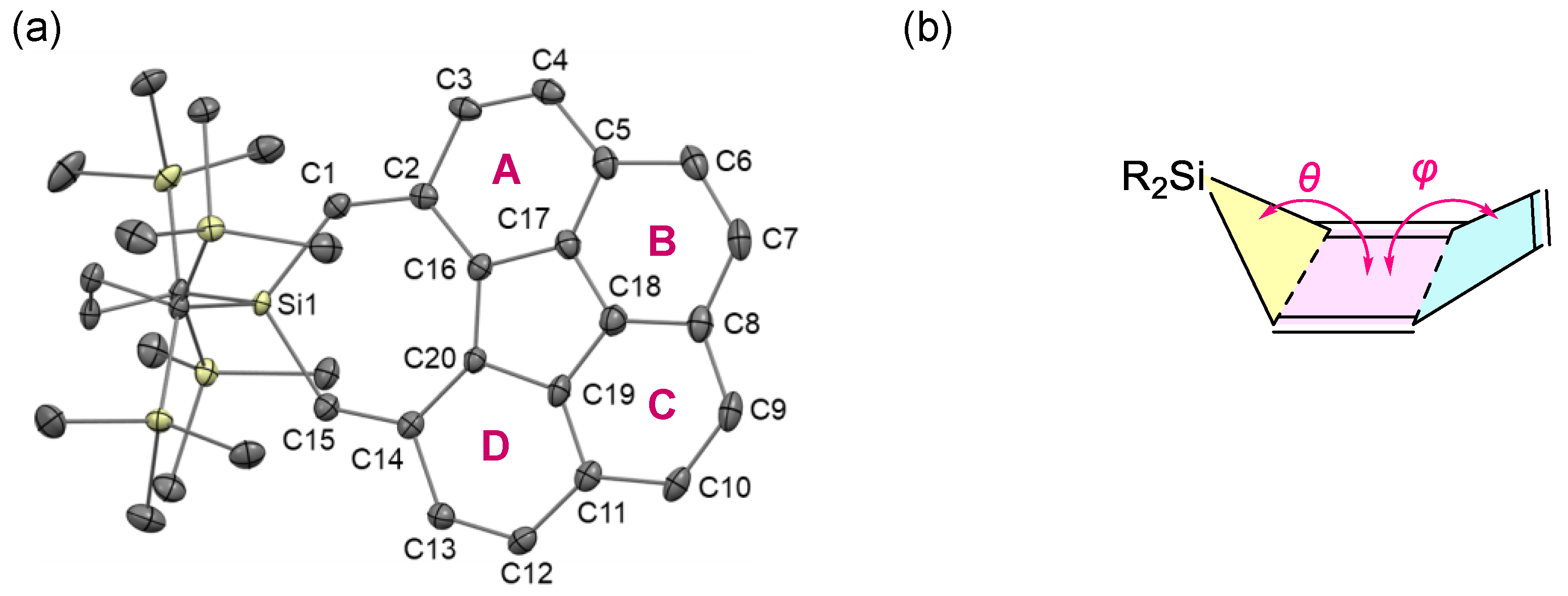
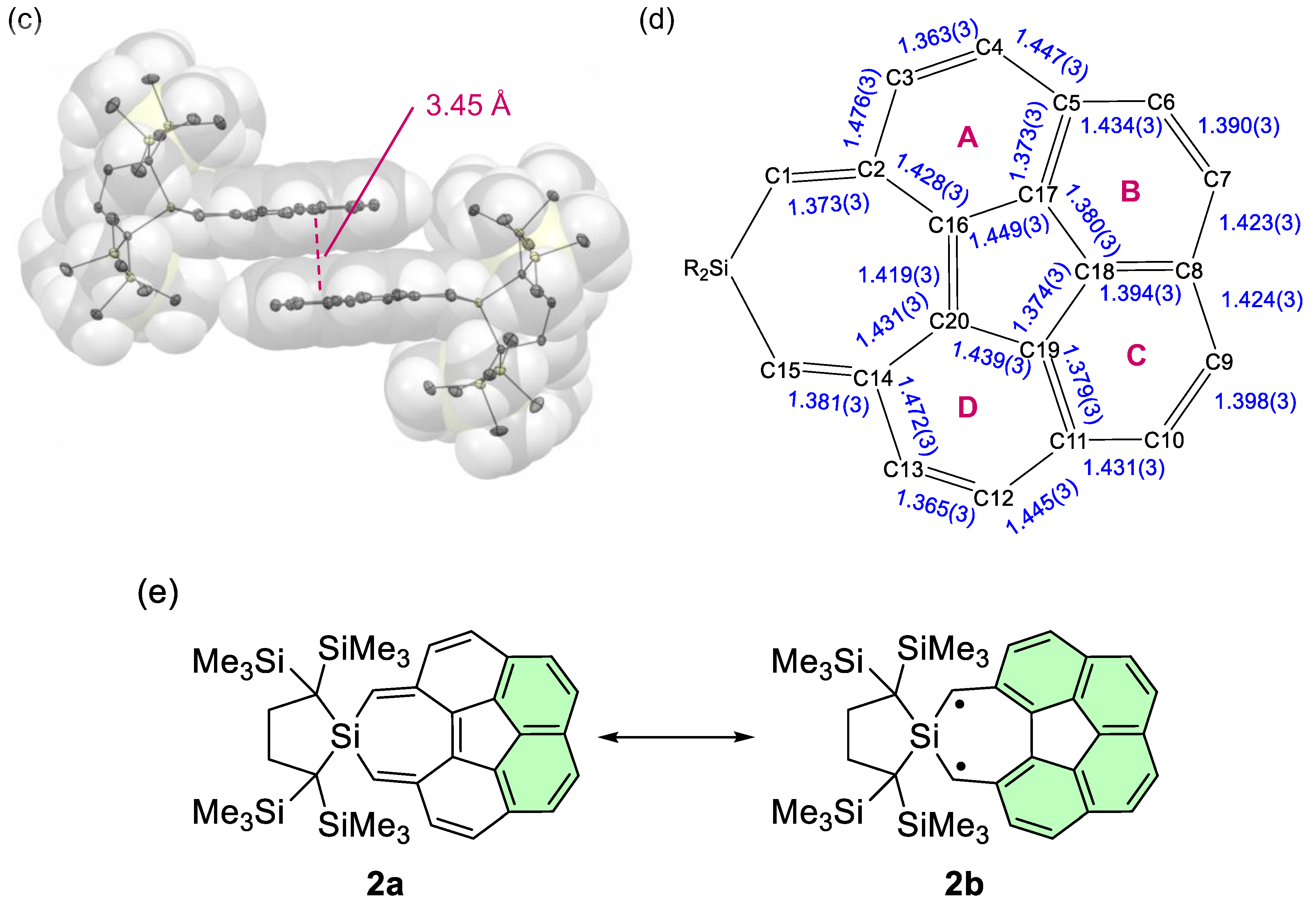
2.1. Synthesis and Structure
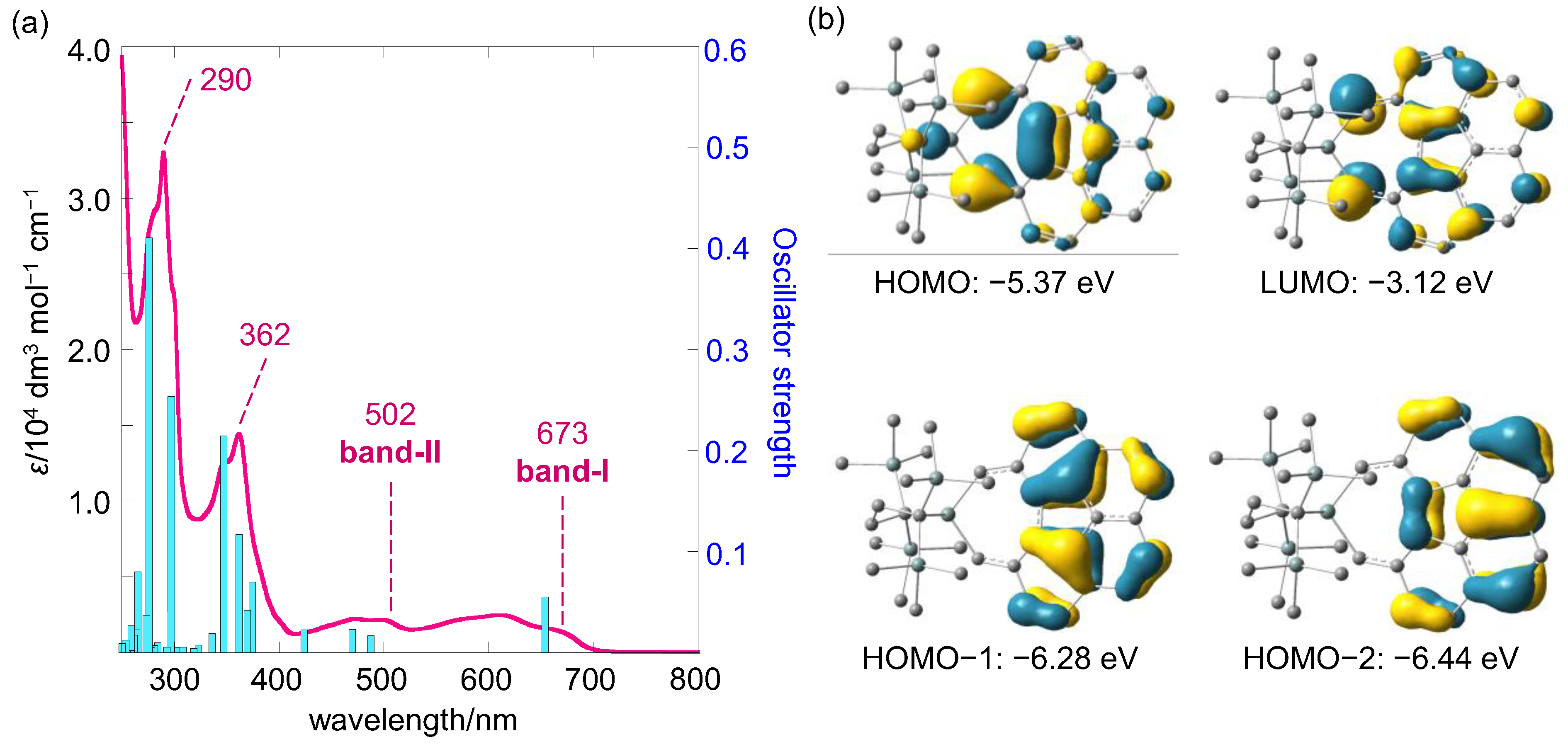
2.2. UV-vis Absorption Spectrum and Electronic Structure
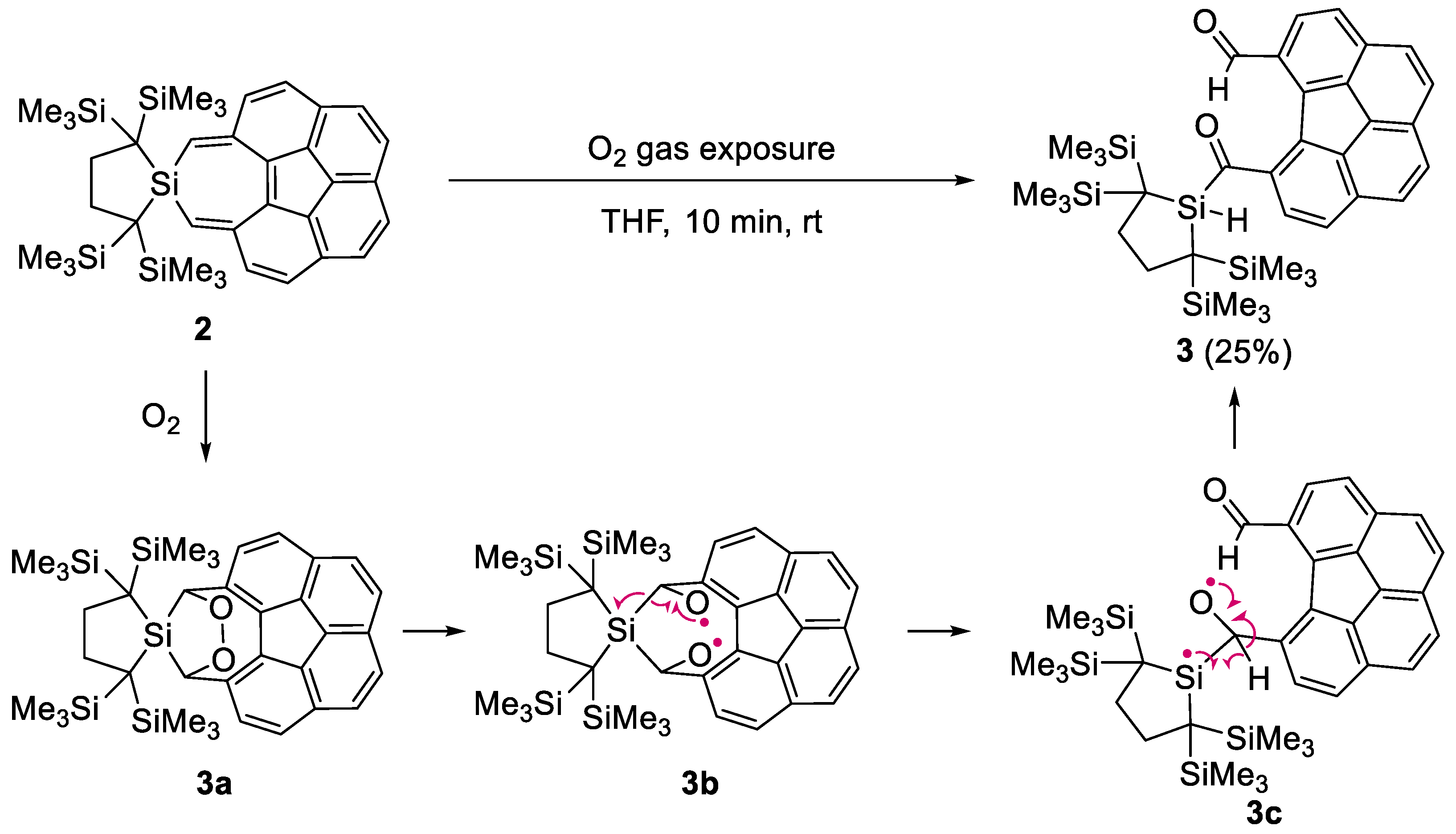
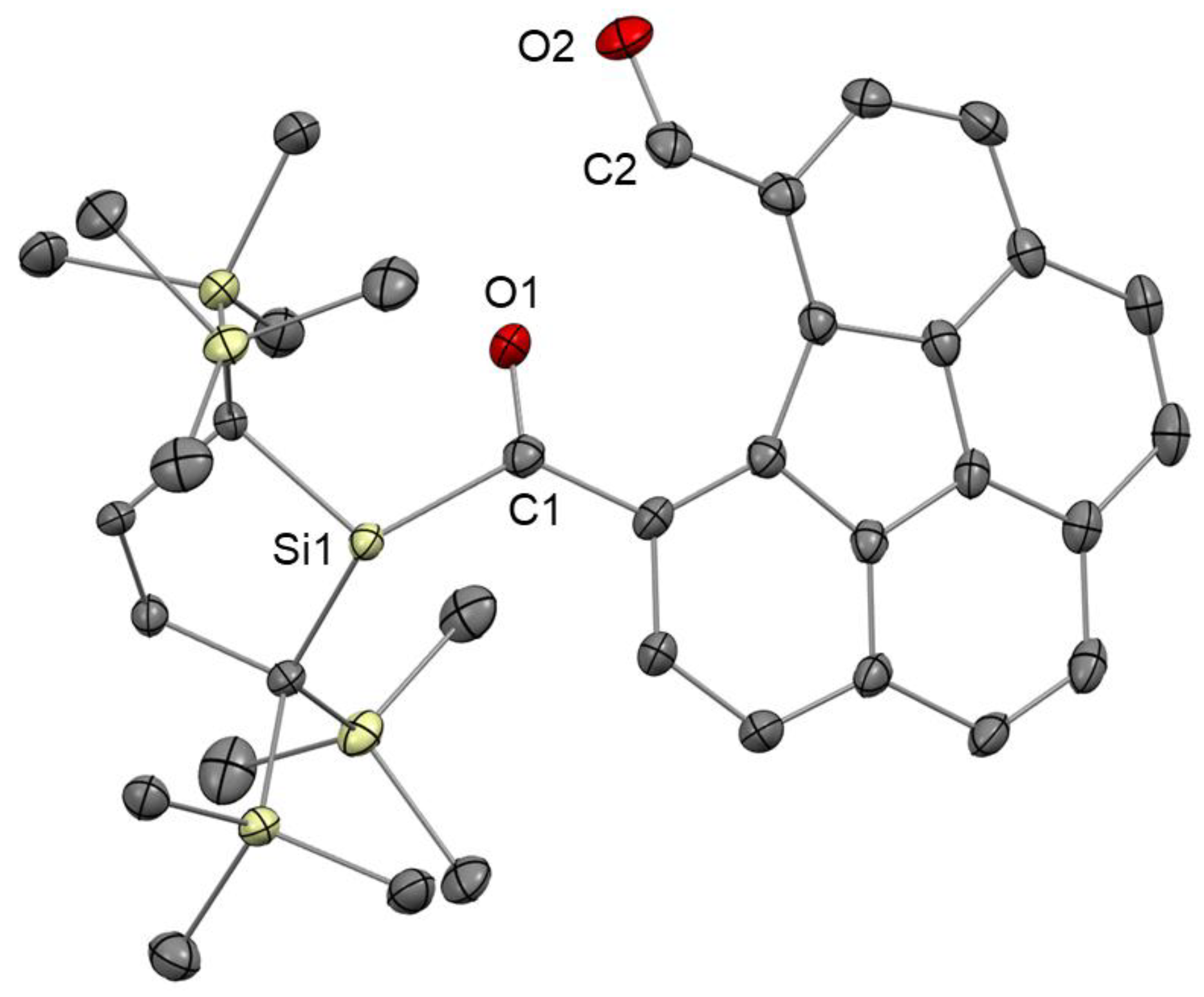
2.3. Decomposition of 2 in Air
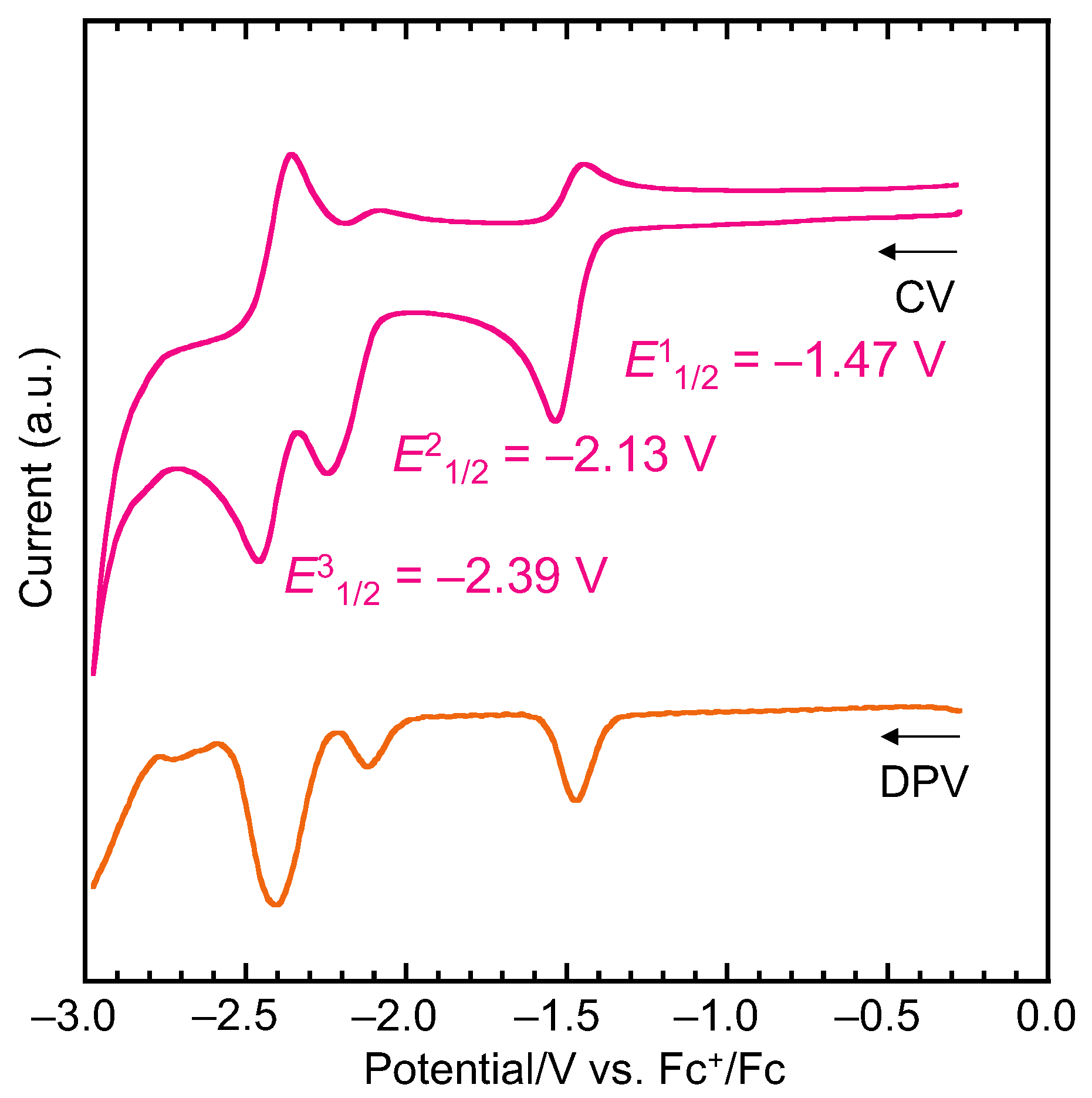
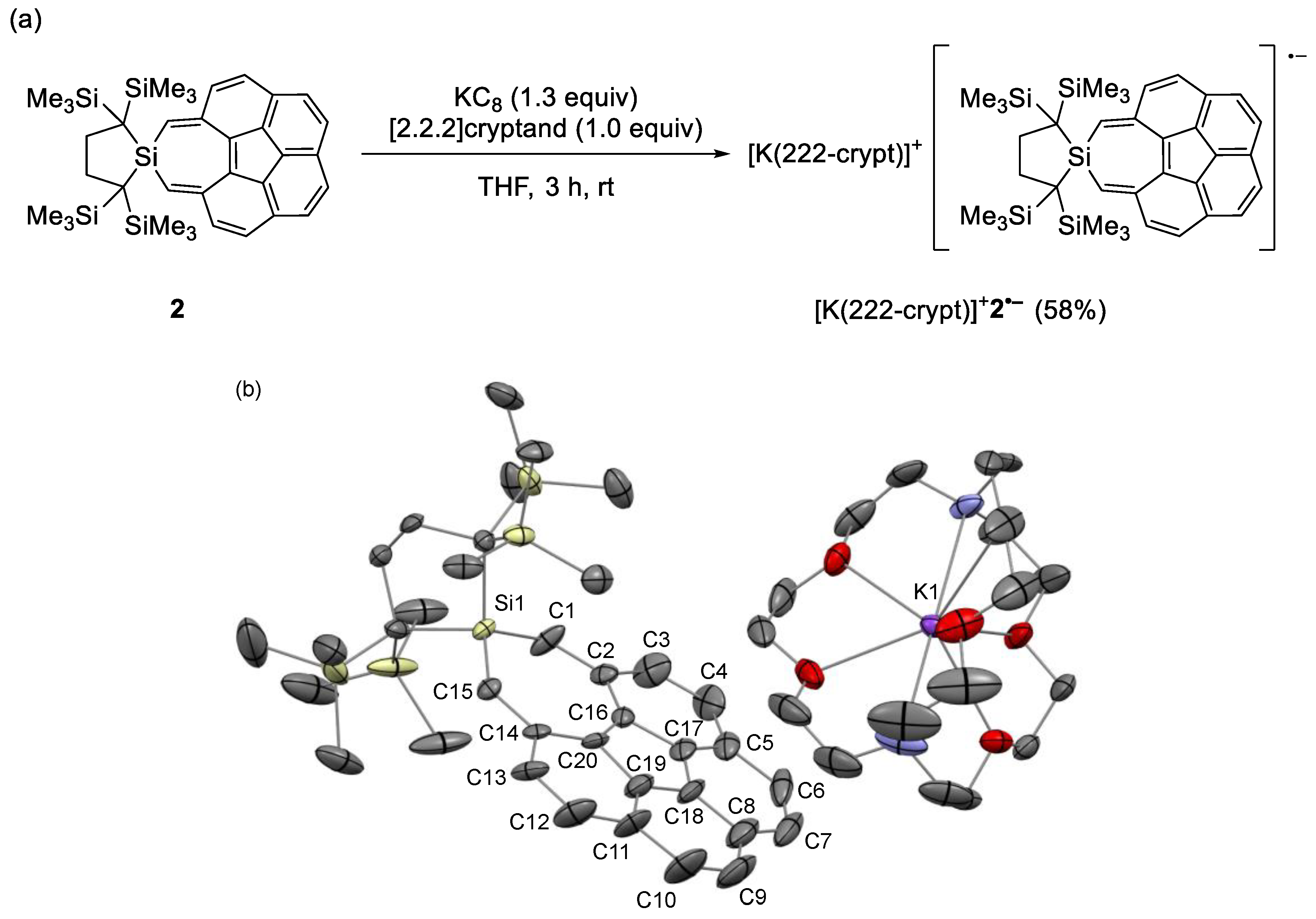
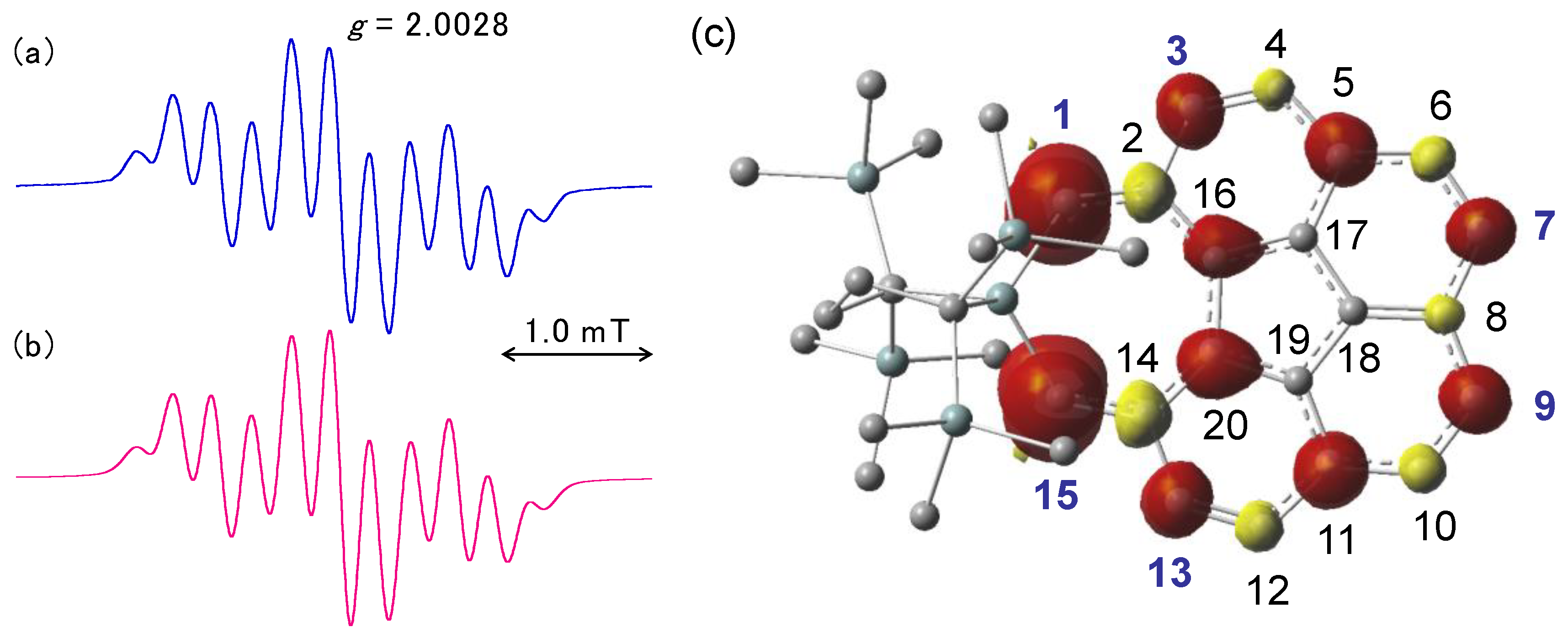
2.4. Cyclic Voltammetry and Chemical One-Electron Reduction
3. Materials and Methods
3.1. General
3.2. Materials
3.3. Synthesis of 2′,2′,5′,5′-Tetrakis(trimethylsilyl)spiro[benzo[6,7]fluorantheno[1,10-cde]silepine-2,1′-silolane] (2)
3.4. Synthesis of (2,2,5,5-Tetrakis(trimethylsilyl)silolane-1-carbonyl)benzo[ghi]fluoranthene-5-carbaldehyde (3)
3.5. One-Electron Reduction of 2
3.6. X-Ray Crystallographic Analysis
3.7. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Mes | Mesityl |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| HONO | Highest occupied natural orbital |
| LUNO | Lowest unoccupied natural orbital |
References
- Martin, N.; Seoane, C.; Hanack, M. Recent Advances in o-Quinodimethane Chemistry. Org. Prep. Proced. Int. 1991, 23, 237–272. [Google Scholar] [CrossRef]
- Segura, J.L.; Martin, N. o-Quinodimethanes: Efficient Intermediates in Organic Synthesis. Chem. Rev. 1999, 99, 3199–3246. [Google Scholar] [CrossRef]
- Konishi, A.; Kubo, T. Benzenoid Quinodimethanes. Top. Curr. Chem. 2017, 375, 83. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Gao, S. Recent advances in the application of Diels–Alder reactions involving o-quinodimethanes, aza-o-quinone methides and o-quinone methides in natural product total synthesis. Chem. Soc. Rev. 2018, 47, 7926–7953. [Google Scholar] [CrossRef]
- Sun, Z.; Ye, Q.; Chi, C.; Wu, J. Low band gap polycyclic hydrocarbons: From closed-shell near infrared dyes and semiconductors to open-shell radicals. Chem. Soc. Rev. 2012, 41, 7857–7889. [Google Scholar] [CrossRef]
- Iwashita, S.; Ohta, E.; Higuchi, H.; Kawai, H.; Fujiwara, K.; Ono, K.; Takenaka, M.; Suzuki, T. First stable 7,7,8,8-tetraaryl-o-quinodimethane: Isolation, X-ray structure, electrochromic response of 9,10-bis(dianisylmethylene)-9,10-dihydrophenanthrene. Chem. Commun. 2004, 2076–2077. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakano, Y.; Iwai, T.; Iwashita, S.; Miura, Y.; Katoono, R.; Kawai, H.; Fujiwara, K.; Tsuji, Y.; Fukushima, T. 7,7,8,8-Tetraaryl-o-quinodimethane Stabilized by Dibenzo Annulation: A Helical π-Electron System That Exhibits Electrochromic and Unique Chiroptical Properties. Chem. Eur. J. 2013, 19, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ghereg, D.; Ech-Cherif El Kettani, S.; Lazraq, M.; Ranaivonjatovo, H.; Schoeller, W.W.; Escudié, J.; Gornitzka, H. An isolable o-quinodimethane and its fixation of molecular oxygen to give an endoperoxide. Chem. Commun. 2009, 4821–4823. [Google Scholar] [CrossRef]
- Ghereg, D.; Gornitzka, H.; Ranaivonjatovo, H.; Escudié, J. Reactions of germenes with various naphthoquinones controlled by substituent effects. Dalton Trans. 2010, 39, 2016–2022. [Google Scholar] [CrossRef]
- Shimizu, A.; Tobe, Y. Indeno [2,1-a]fluorene: An Air-Stable ortho-Quinodimethane Derivative. Angew. Chem. Int. Ed. 2011, 50, 6906–6910. [Google Scholar] [CrossRef]
- Miyoshi, H.; Nobusue, S.; Shimizu, A.; Hisaki, I.; Miyata, M.; Tobe, Y. Benz[c]indeno [2,1-a]fluorene: A 2,3-naphthoquinodimethane incorporated into an indenofluorene frame. Chem. Sci. 2014, 5, 163–168. [Google Scholar] [CrossRef]
- Shimizu, A.; Nobusue, S.; Miyoshi, M.; Tobe, Y. Indenofluorene congeners: Biradicaloids and beyond. Pure Appl. Chem. 2014, 86, 517–528. [Google Scholar] [CrossRef]
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Suzuki, S.; Kozaki, M.; Okada, K. 2,11-Dibromo-13,14-dimesityl-5,8-dioxapentaphene: A Stable and Twisted Polycyclic System Containing the o-Quinodimethane Skeleton. Org. Lett. 2016, 18, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Hirose, S.; Ueda, Y.; Uekusa, H.; Hamura, T. Thermodynamically Stable o-Quinodimethane: Synthesis, Structure, and Reactivity. Chem. Eur. J. 2021, 27, 3665–3669. [Google Scholar] [CrossRef]
- Ariai, J.; Gellrich, U. An Acceptor-Substituted N-Heterocyclic ortho-Quinodimethane: Pushing the Boundaries of Polarization in Donor−Acceptor Substituted Polyenes. J. Am. Chem. Soc. 2024, 146, 32859–32869. [Google Scholar] [CrossRef]
- Sahara, K.; Abe, M.; Zipse, H.; Kubo, T. Duality of Reactivity of a Biradicaloid Compound with an o-Quinodimethane Scaffold. J. Am. Chem. Soc. 2020, 142, 5408–5418. [Google Scholar] [CrossRef]
- Zhu, H.; Fujimori, S.; Kostenko, A.; Inoue, S. Dearomatization of C 6 Aromatic Hydrocarbons by Main Group Complexes. Chem. Eur. J. 2023, 29, e202301973. [Google Scholar] [CrossRef]
- Suzuki, H.; Tokitoh, N.; Okazaki, R. A Novel Reactivity of a Silylene: The First Examples of [1 + 2] Cycloaddition with Aromatic Compounds. J. Am. Chem. Soc. 1994, 116, 11572–11573. [Google Scholar] [CrossRef]
- Lim, Y.M.; Lee, M.E.; Lee, J.; Do, Y. Reactivity of Bromodilithiosilane to Naphthalene and Anthracene. Organometallics 2008, 27, 6375–6378. [Google Scholar] [CrossRef]
- Mizuhata, Y.; Sato, T.; Tokitoh, N. Reactions of an overcrowded silylene with pyridines: Formation of a novel 2H-1,2-azasilepine and its further cycloaddition. Heterocycles 2012, 84, 413–418. [Google Scholar] [CrossRef]
- Kosai, T.; Ishida, S.; Iwamoto, T. A Two-Coordinate Cyclic (Alkyl)(Amino)Silylene: Balancing Thermal Stability and Reactivity. Angew. Chem. Int. Ed. 2016, 55, 15554–15558. [Google Scholar] [CrossRef]
- Wendel, D.; Porzelt, A.; Herz, F.A.D.; Sarkar, D.; Jandl, C.; Inoue, S.; Rieger, B. From Si(II) to Si(IV) and Back: Reversible Intramolecular Carbon–Carbon Bond Activation by an Acyclic Iminosilylene. J. Am. Chem. Soc. 2017, 139, 8134–8137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, J.; Yang, H.; Cui, C. Synthesis of Silaketenimine Anion and Its Coupling with Isocyanide. J. Am. Chem. Soc. 2019, 141, 19600–19604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, J.; Cui, C. Intramolecular Cyclopropanation of Alkali-Metal-Substituted Silylene with the Aryl Substituent of an N-Heterocyclic Framework. Inorg. Chem. 2019, 58, 12007–12010. [Google Scholar] [CrossRef]
- Holzner, R.; Reiter, D.; Frisch, P.; Inoue, S. DMAP-stabilized bis(silyl)silylenes as versatile synthons for organosilicon compounds. RSC Adv. 2020, 10, 3402–3406. [Google Scholar] [CrossRef]
- Reiter, D.; Frisch, P.; Wendel, D.; Hörmann, F.M.; Inoue, S. Oxidation reactions of a versatile, two-coordinate, acyclic iminosiloxysilylene. Dalton Trans. 2020, 49, 7060–7068. [Google Scholar] [CrossRef]
- Xu, C.; Ye, Z.; Xiang, L.; Yang, S.; Peng, Q.; Leng, X.; Chen, Y. Insertion of Metal-Substituted Silylene into Naphthalene’s Aromatic Ring and Subsequent Rearrangement for Silaspiro-Benzocycloheptenyl and Cyclobutenosilaindan Derivatives. Angew. Chem. Int. Ed. 2021, 60, 3189–3195. [Google Scholar] [CrossRef]
- Zhu, H.; Kostenko, A.; Franz, D.; Hanusch, F.; Inoue, S. Room Temperature Intermolecular Dearomatization of Arenes by an Acyclic Iminosilylene. J. Am. Chem. Soc. 2023, 145, 1011–1021. [Google Scholar] [CrossRef]
- Ding, Y.; Jin, W.; Zhang, J.; Cui, C. A Masked Boryl-Substituted Oxo-Bridged Bis-Silylene: Synthesis and Reductive-Elimination and Synergistic Oxidative-Addition Reactivity. J. Am. Chem. Soc. 2024, 146, 27925–27934. [Google Scholar] [CrossRef]
- Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C. The First Isolable Dialkylsilylene. J. Am. Chem. Soc. 1999, 121, 9722–9723. [Google Scholar] [CrossRef]
- Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C. Excited-State Reactions of an Isolable Silylene with Aromatic Compounds. J. Am. Chem. Soc. 2002, 124, 3830–3831. [Google Scholar] [CrossRef]
- Kira, M.; Ishida, S.; Iwamoto, T.; de Meijere, A.; Fujitsuka, M.; Ito, O. The Singlet Excited State of a Stable Dialkylsilylene is Responsible for Its Photoreactions. Angew. Chem. Int. Ed. Engl. 2004, 43, 4510–4512. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Uchida, K.; Sung, Y.M.; Shin, J.-Y.; Ishida, S.; Lim, J.M.; Hiroto, S.; Furukawa, K.; Kim, D.; Iwamoto, T.; et al. Near-IR Absorbing Nickel(II) Porphyrinoids Prepared by Regioselective Insertion of Silylenes into Antiaromatic Nickel(II) Norcorrole. Angew. Chem. Int. Ed. 2014, 53, 1506–1509. [Google Scholar] [CrossRef]
- Kosai, T.; Ishida, S.; Iwamoto, T. Transformation of azulenes to bicyclic [4]dendralene and heptafulvene derivatives by photochemical cycloaddition of dialkylsilylene. Chem. Commun. 2015, 51, 10707–10709. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Tamura, T.; Iwamoto, T. Dearomative cycloadditions of a silylene with pyrazine and quinoxaline. Dalton Trans. 2018, 47, 11317–11321. [Google Scholar] [CrossRef]
- Su, M.-D. A Theoretical Investigation of Photochemical Reactions of an Isolable Silylene with Benzene. Chem. Eur. J. 2014, 20, 9419–9423. [Google Scholar] [CrossRef]
- Li, X.; Kang, F.; Inagaki, M. Buckybowls: Corannulene and Its Derivatives. Small 2016, 12, 3206–3223. [Google Scholar] [CrossRef]
- Nestoros, E.; Stuparu, M.C. Corannulene: A molecular bowl of carbon with multifaceted properties and diverse applications. Chem. Commun. 2018, 54, 6503–6519. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyrański, M.K. Structural aspects of aromaticity. Chem. Rev. 2001, 101, 1385–1419. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Szatylowicz, H.; Stasyuk, O.A.; Dominikowska, J.; Palusiak, M. Aromaticity from the viewpoint of molecular geometry: Application to planar systems. Chem. Rev. 2014, 114, 6383–6422. [Google Scholar] [CrossRef]
- Clar, E. The Aromatic Sextet; Wiley: London, UK, 1972. [Google Scholar]
- Balaban, A.T.; Klein, D.J. Claromatic Carbon Nanostructures. J. Phys. Chem. C 2009, 113, 19123–19133. [Google Scholar] [CrossRef]
- Wassmann, T.; Seitsonen, A.P.; Saitta, A.M.; Lazzeri, M.; Mauri, F. Clar’s Theory, Pi-Electron Distribution, and Geometry of Graphene Nanoribbons. J. Am. Chem. Soc. 2010, 132, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kishi, R.; Nitta, T.; Kubo, T.; Nakasuji, K.; Kamada, K.; Ohta, K.; Yamaguchi, K. Second Hyperpolarizability (γ) of Singlet Diradical System: Dependence of γ on the Diradical Character. J. Phys. Chem. A 2005, 109, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kamada, K.; Ohta, K.; Shimizu, A.; Kubo, T.; Kishi, R.; Takahashi, H.; Botek, E.; Champagne, B.; Nakano, M. Singlet Diradical Character from Experiment. J. Phys. Chem. Lett. 2010, 1, 937–940. [Google Scholar] [CrossRef]
- Nakano, M. Electronic Structure of Open-Shell Singlet Molecules: Diradical Character Viewpoint. Top. Curr. Chem. 2017, 375, 47. [Google Scholar] [CrossRef]
- Tashiro, S.; Yamada, M.; Shionoya, M. Iridium-Catalyzed Reductive Carbon–Carbon Bond Cleavage Reaction on a Curved Pyridylcorannulene Skeleton. Angew. Chem. Int. Ed. 2015, 54, 5351–5354. [Google Scholar] [CrossRef]
- Guo, W.; Ding, W.; Yao, Y.; Rajca, S.; Li, Q.; Jiang, H.; Rajca, A.; Wang, Y. Aromatic C−C Bond Cleavage in a Curved π-System of Aminocorannulene. Org. Lett. 2023, 25, 3972–3977. [Google Scholar] [CrossRef]
- Balci, M. Bicyclic Endoperoxides and Synthetic Applications. Chem. Rev. 1981, 81, 91–108. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Empirical Absorption Correction Program; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXL-2019, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2019. [Google Scholar]
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of Software (Yadokari-XG 2009) for Crystal Structure Analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Maeda, S.; Ohno, K.; Morokuma, K. Systematic exploration of the mechanism of chemical reactions: The global reaction route mapping (GRRM) strategy using the ADDF and AFIR methods. Phys. Chem. Chem. Phys. 2013, 15, 3683–3701. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Maeda, S. A scaled hypersphere search method for the topography of reaction pathways on the potential energy surface. Chem. Phys. Lett. 2004, 384, 277–282. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K. Global Mapping of Equilibrium and Transition Structures on Potential Energy Surfaces bythe Scaled Hypersphere Search Method: Applications to ab Initio Surfaces of Formaldehyde and Propyne Molecules. J. Phys. Chem. A 2005, 109, 5742–5753. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Maeda, S. Global Reaction Route Mapping on Potential Energy Surfaces of Formaldehyde, Formic Acid, and Their Metal-Substituted Analogues. J. Phys. Chem. A 2006, 110, 8933–8941. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K. Structures of Water Octamers (H2O)8: Exploration on Ab Initio Potential Energy Surfaces by the Scaled Hypersphere Search MethodClick to copy article link. J. Phys. Chem. A 2007, 111, 4527–4534. [Google Scholar] [CrossRef]
- Maeda, S.; Harabuchi, Y.; Osada, Y.; Taketsugu, T.; Morokuma, K.; Ohno, K. GRRM14. Available online: https://iqce.jp/GRRM/index_e.shtml (accessed on 24 September 2020).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, S.; Mori, M.; Honda, S.; Iwamoto, T. A Stable π-Expanded o-Quinodimethane via the Photochemical Dearomative Cycloaddition of Corannulene with an Isolable Dialkylsilylene. Chemistry 2025, 7, 37. https://doi.org/10.3390/chemistry7020037
Ishida S, Mori M, Honda S, Iwamoto T. A Stable π-Expanded o-Quinodimethane via the Photochemical Dearomative Cycloaddition of Corannulene with an Isolable Dialkylsilylene. Chemistry. 2025; 7(2):37. https://doi.org/10.3390/chemistry7020037
Chicago/Turabian StyleIshida, Shintaro, Maiko Mori, Shunya Honda, and Takeaki Iwamoto. 2025. "A Stable π-Expanded o-Quinodimethane via the Photochemical Dearomative Cycloaddition of Corannulene with an Isolable Dialkylsilylene" Chemistry 7, no. 2: 37. https://doi.org/10.3390/chemistry7020037
APA StyleIshida, S., Mori, M., Honda, S., & Iwamoto, T. (2025). A Stable π-Expanded o-Quinodimethane via the Photochemical Dearomative Cycloaddition of Corannulene with an Isolable Dialkylsilylene. Chemistry, 7(2), 37. https://doi.org/10.3390/chemistry7020037





