Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines
Abstract
1. Introduction
2. Chemical Characters of Quinoxalines
3. Methods of Preparation of Quinoxalines
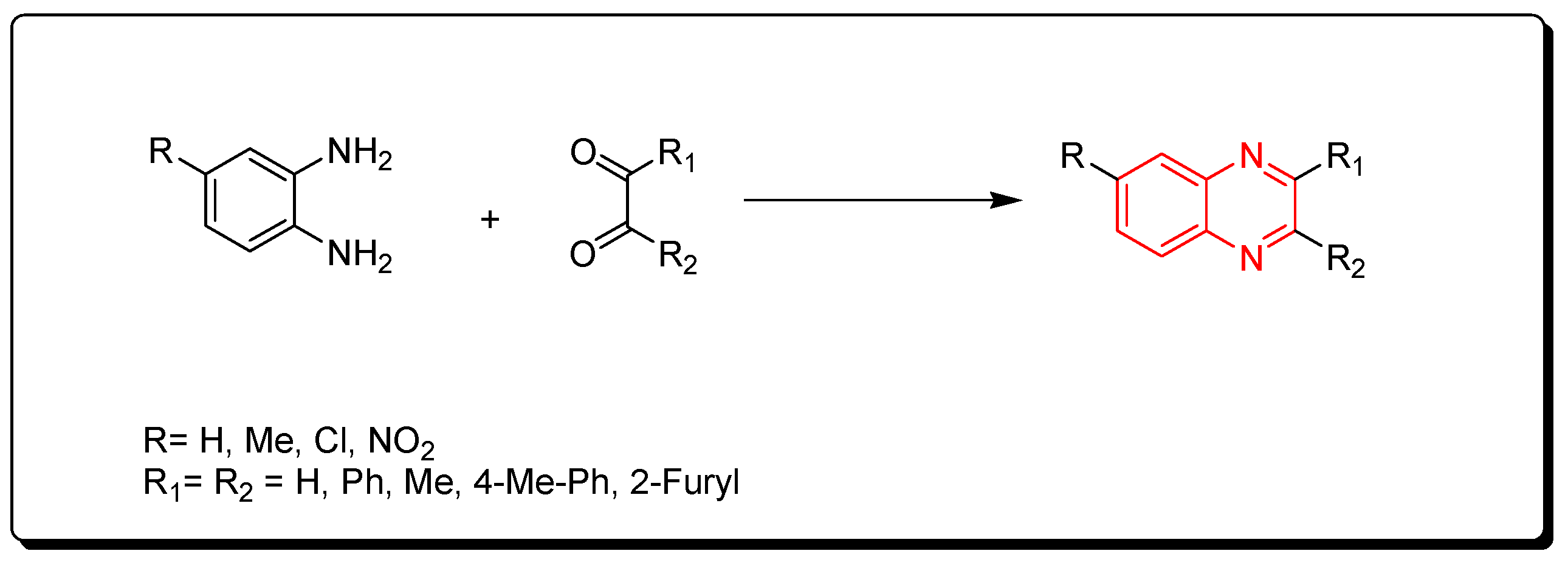
- The traditional chemistry pathway, which is based on the condensation between o-phenylenediamines and dicarbonyl compounds in the presence of special conditions such as organic solvents, high temperatures, long times, or strong catalysts. Additionally, the reaction yield may be low and side products may be produced. These types of reactions have negative effects on the environment.
- The green chemistry pathway, which is a cost-effective pathway through using green chemistry methodologies to produce quinoxalines. This pathway is characterized by using an environmentally friendly recyclable catalyst, a low cost, lower consumption of energy, one-pot synthesis, no side products, short time, and high yield. It can be performed in an aqueous medium at room temperature or by the microwave reactor.
3.1. Traditional Chemistry Pathway
3.1.1. Condensation of o-Phenylenediamine and 1,2-Dicarbonyl Derivatives
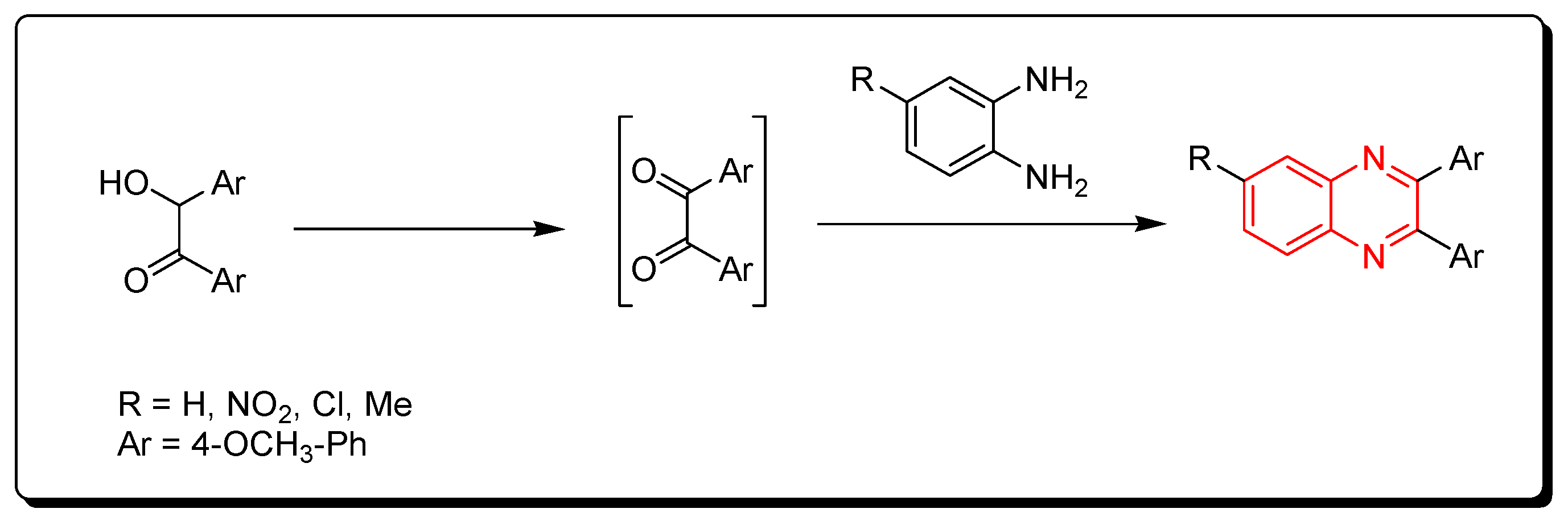
3.1.2. O-Phenylenediamine and In Situ Produced 1,2-Dicarbonyls
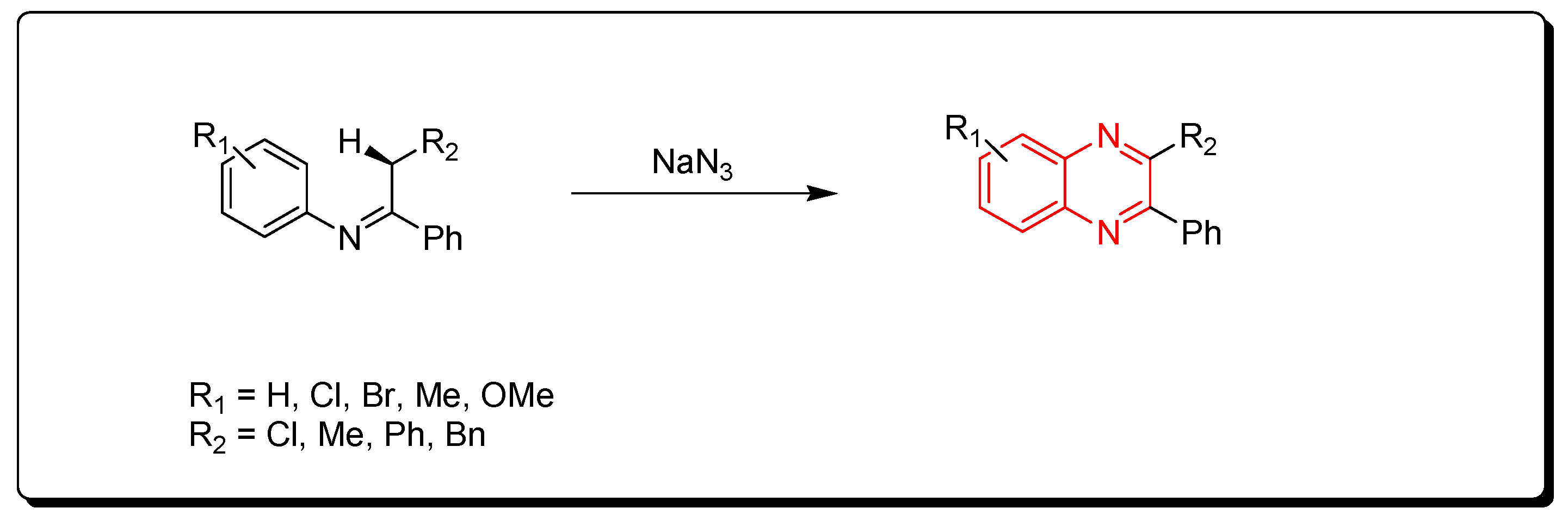
3.1.3. Metal-Catalyzed Cyclization of Imines and Azides
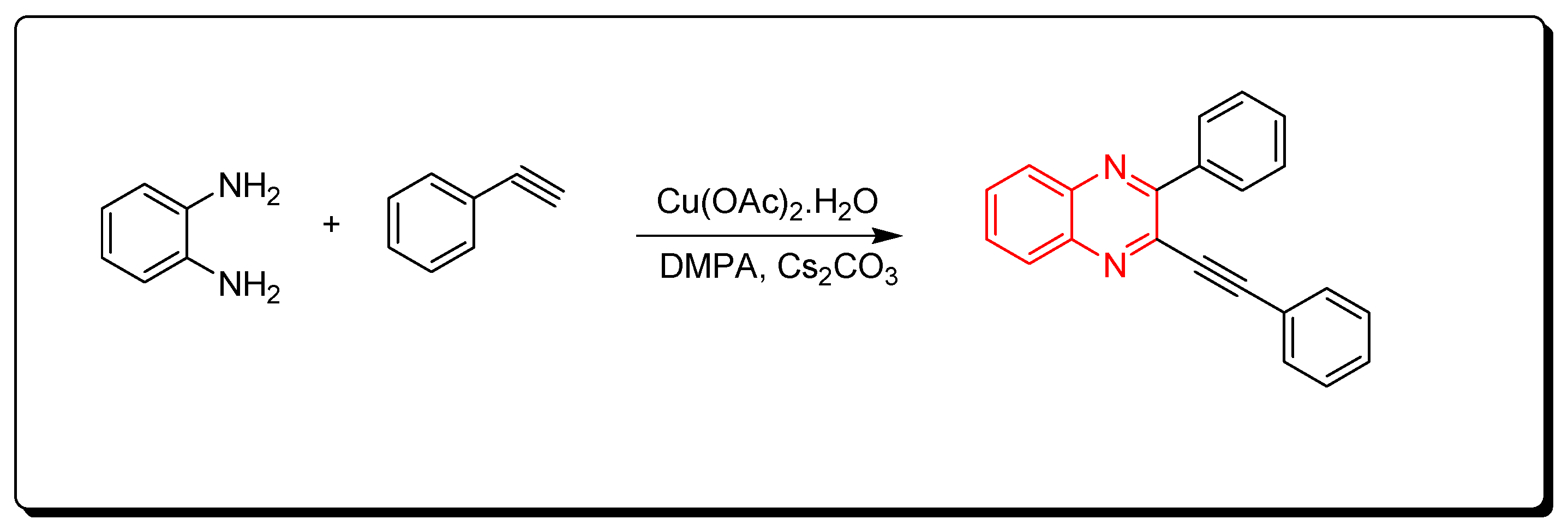
3.1.4. Cyclocondensation of o-Phenylenediamine and Aromatic Alkynes
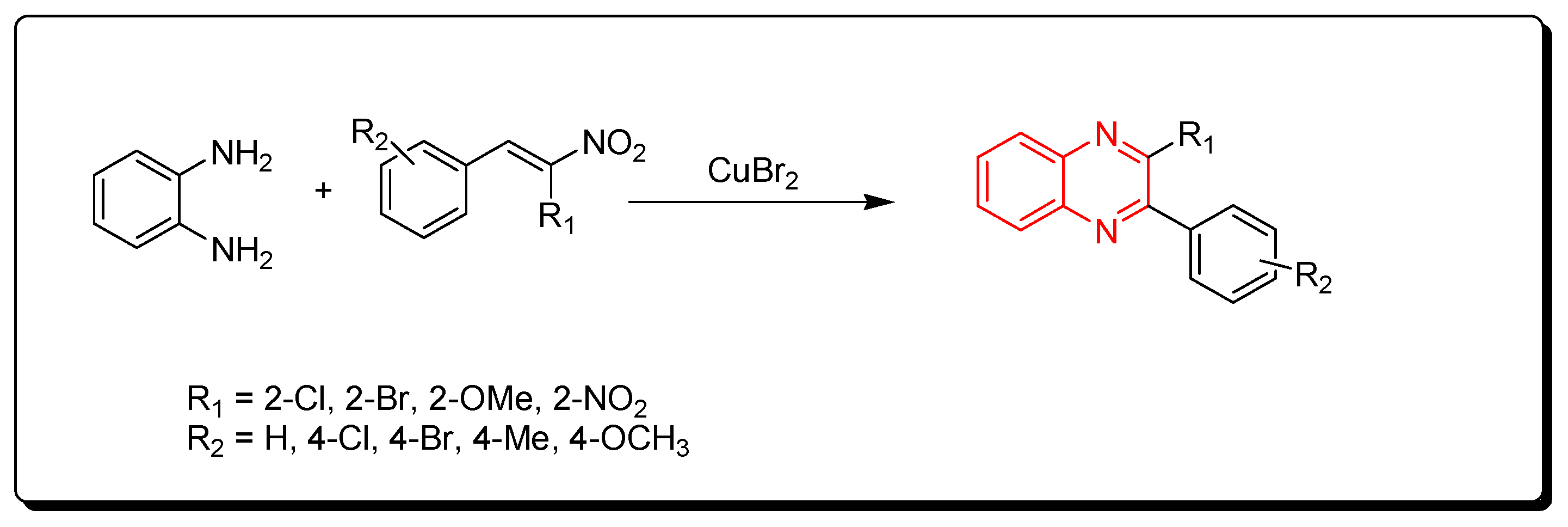
3.1.5. Cyclocondensation of o-Phenylenediamine and Nitro-Olefins
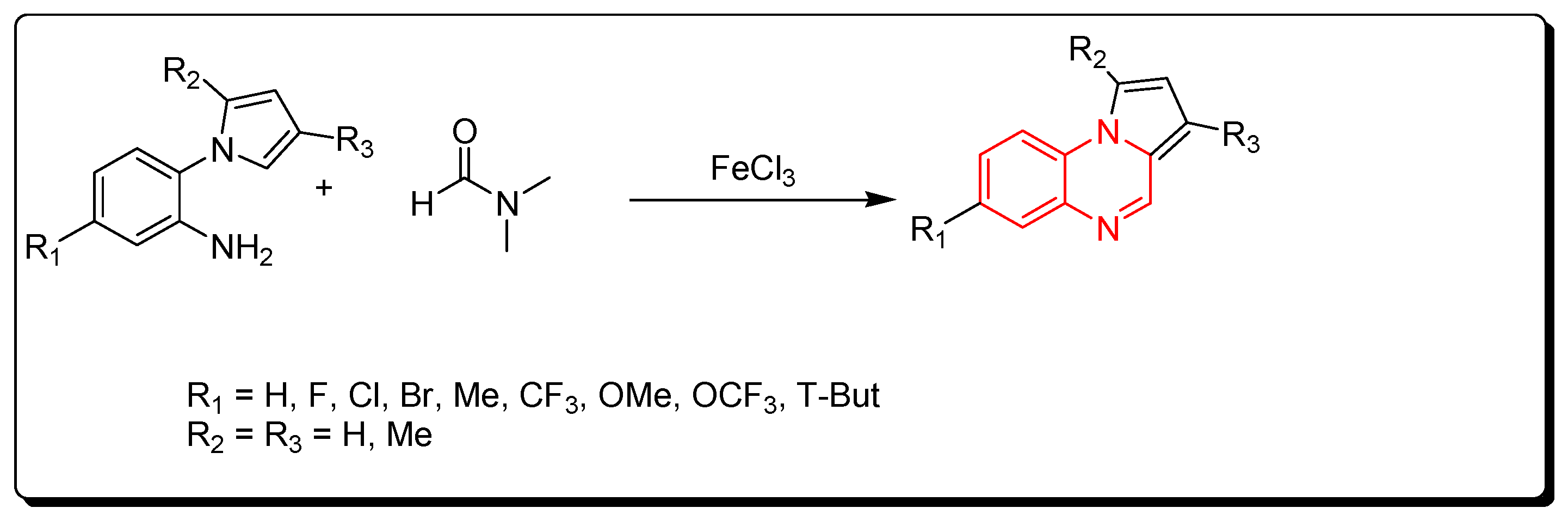
3.1.6. Cyclocondensation of Aromatic Amines and DMF
3.2. Green Chemistry Pathway
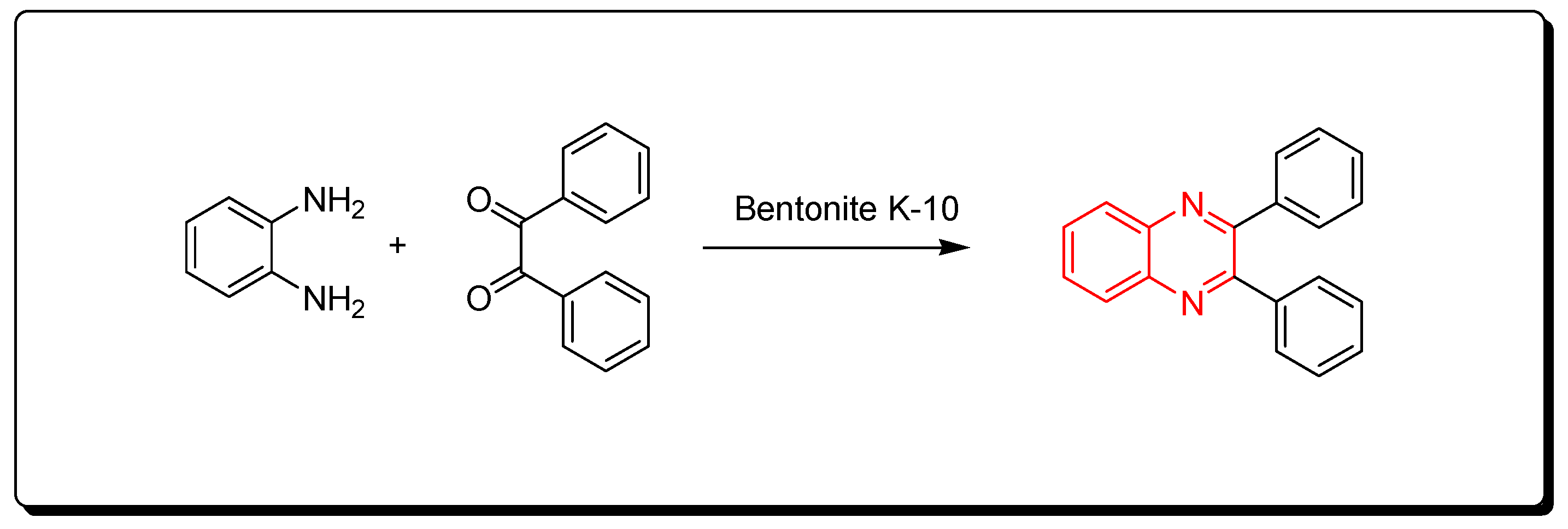
3.2.1. Clay-10 Based Method
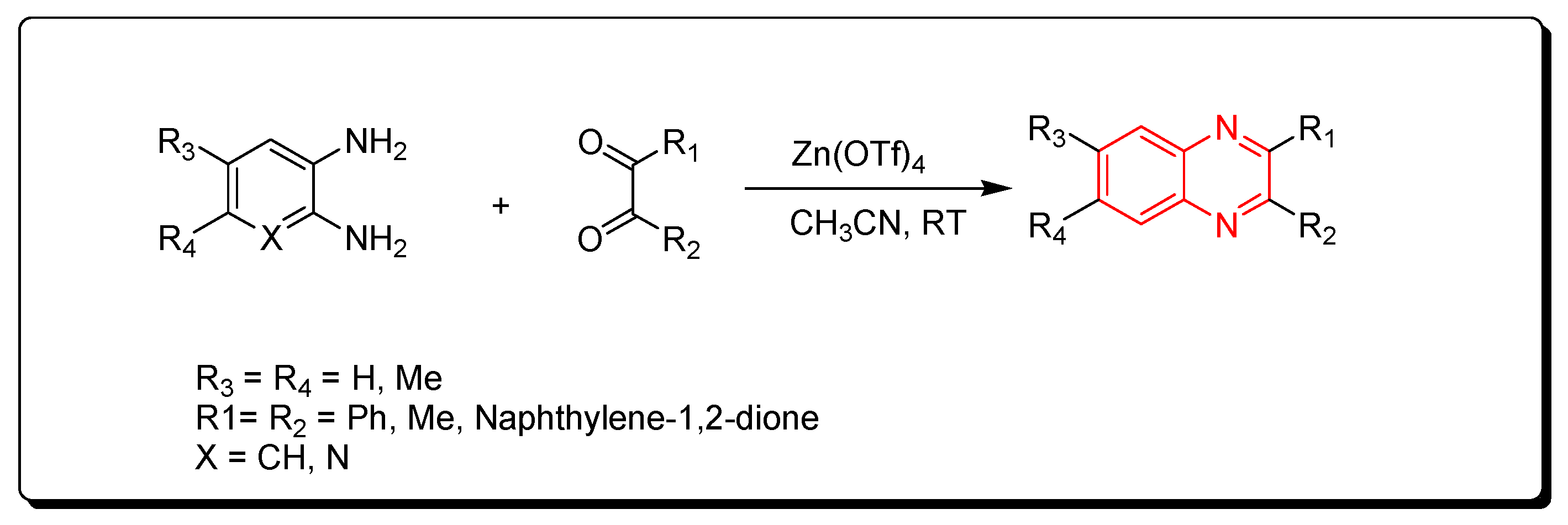
3.2.2. Zinc Triflate Catalyst
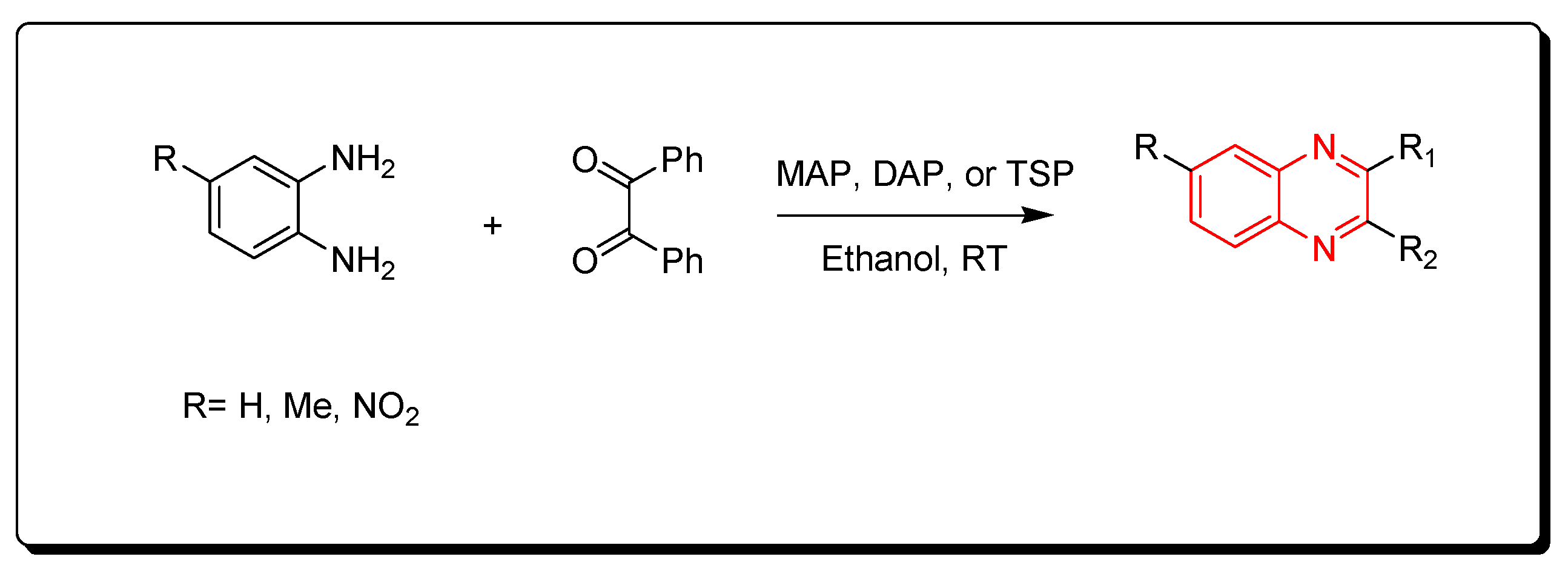
3.2.3. Phosphate-Based Catalyst
3.2.4. Lanthanide-Based Catalyst
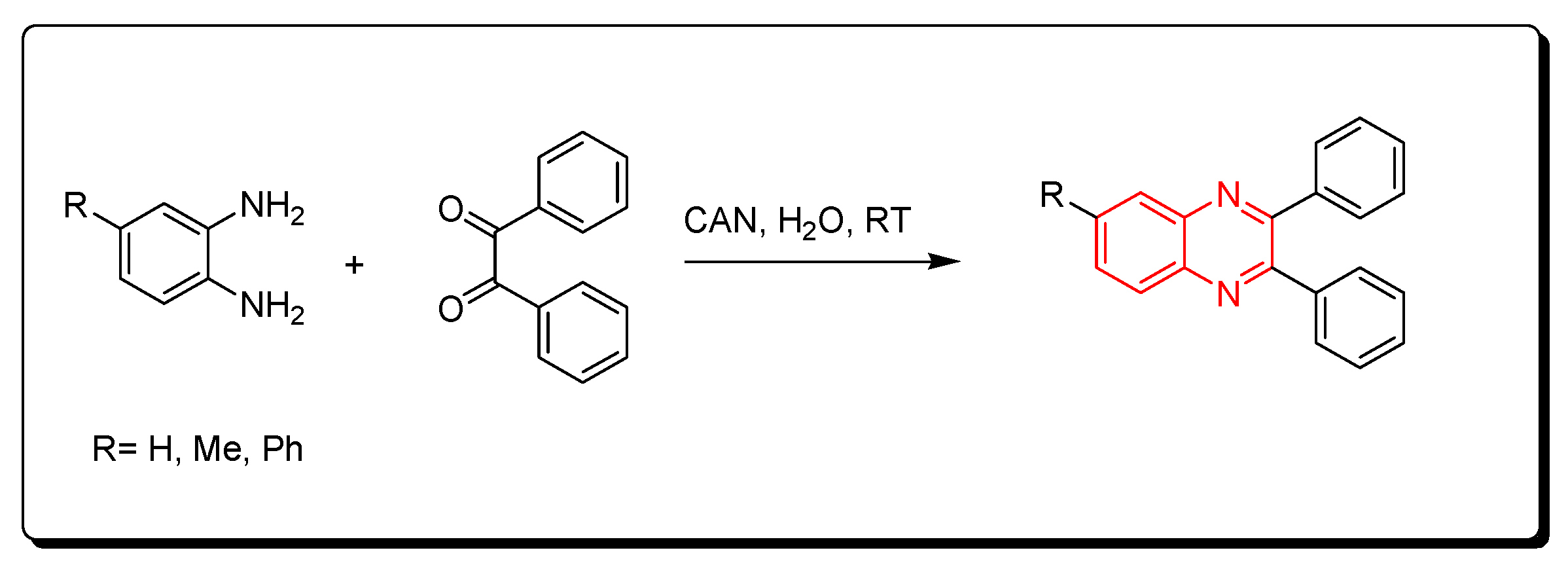
3.2.5. Fluorinated Alcohols Catalyst (HFIP)
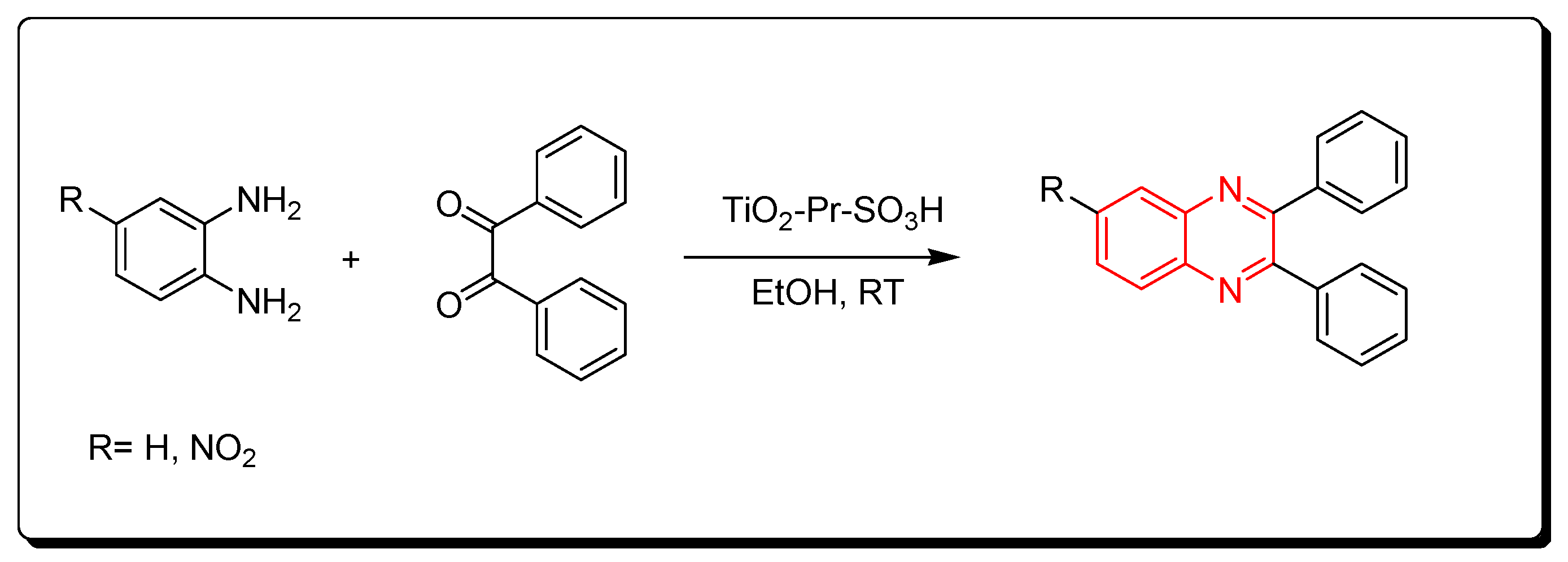
3.2.6. Solid Acid Catalyst (TiO2-Pr-SO3H)
3.3. Reaction of Quinoxalines
3.3.1. Intramolecular Arylation Using Lewis Acid Catalyst
3.3.2. Intramolecular Cyclization of Quinoxalines
4. Pharmaceutical Products of Anticancer Quinoxalines
- Erdafitinib is an inhibitor of the subgroup tyrosine kinase fibroblast (FGFR). These receptors become unregulated and are exposed to angiogenesis, differentiation, and proliferation in certain types of tumors. Erdafitinib is used for the treatment of malignancy and some types of solid tumors. It has the brand name Balversa. It was discovered to overcome the toxicity profiles of other anticancer agents used for the treatment of gastric cancer, bile duct cancer, and lung cancer. It was invented for the first time by the Astex Pharmaceutical Company. The FDA approved it in 2018 for the management of urothelial tumors. In 2019 it was approved for the treatment of other types of tumors. It inhibits FGFR-1, FGFR-2, FGFR-3, and FGFR-4 with a strong IC50 = 1.2, 2.5, 3, and 5.7 nM, respectively [14].
- Chloroquinoxaline sulfonamide was listed as CQS, and it was used in the treatment of different types of tumors. It completed clinical trials (phase II) on colorectal and lung cancer cell lines. It works via the inhibition of topoisomerase IIα and topoisomerase IIβ. Therefore, it inhibits DNA replication. It showed a high toxicity profile, so it was discontinued after this phase II. It showed IC50 = 1.8 μM against B16 murine melanoma cells [14].
- Tyrophostin is a tyrosine kinase inhibitor. It was used for the treatment of resistant melanoma cell platelet-derived growth factor receptor kinase (PDGFR), activates apoptosis, and reduces capability and movement of resistant melanoma cells of skin cancer. It has no effect on the epidermal growth factor receptor (EGFR), but it strongly inhibits PDGFR with an IC50 = 0.3 to 0.5 μM. It also works via the activation of apoptosis in tumor cell lines. It is used in the treatment of melanoma [14].
- Pilaralisib is an effective and favorably selective inhibitor of class I phosphatidylinositol 3-kinase (PI3K). It inhibits the formation of PIP3 in the cell membrane, which leads to the inhibition of cell differentiation and proliferation. It was invented for the treatment of solid tumors by Sanofi and Exelixis. It significantly inhibited tumor growth but showed a high toxicity profile, so it was discontinued after phase II. It displayed an IC50 of 39, 383, 23, and 36 nM against PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ [14].
- 2-(4-Chlorophenyl)-5-Quinoxalinecarboxamide is an example of an antineoplastic agent that inhibits the poly(ADP-ribose) polymerase enzyme. This enzyme participates in the base excision repair (BER) pathway by facilitating the poly(ADP-ribosyl)action of a select few acceptor proteins that are important for DNA metabolism and chromatin architecture. The quinoxaline derivative is still in the experimental stage [90].
- PQ-10 is a quinoxaline and quinazoline derivative that works via the inhibition of cAMP and cAMP-inhibited cGMP 3′,5′-cyclic phosphodiesterase 10A. The latter enzyme controls the amount of cyclic nucleotides inside cells, which aids in signal transduction. It is capable of hydrolyzing both cAMP and cGMP but prefers cAMP more highly. This derivative is in the experimental stage as a new anticancer treatment with a quinoxaline system [90].
| Molecular Structure | Generic Name | Chemical Name | Molecular Formula/ Molecular Weight |
|---|---|---|---|
 | Erdafitinib | N’-(3,5-dimethoxyphenyl)-N’-[3-(1-methylpyrazol-4-yl)quinoxalin-6-yl]-N-propan-2-ylethane-1,2-diamine | C25H30N6O2 446.2 |
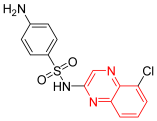 | Chloroquinoxaline sulfonamide | 4-amino-N-(5-chloro-2-quinoxalinyl)-benzenesulfonamide | C14H11ClN4O2S 334.03 |
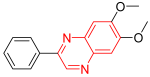 | Tyrophostin | 6,7-Dimethoxy-2-phenylquinoxaline | C16H14N2O2 266.1 |
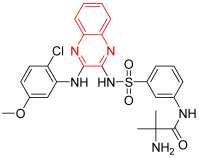 | Pilaralisib | 2-amino-N-[3-[[3-(2-chloro-5-methoxyanilino)quinoxalin-2-yl]sulfamoyl]phenyl]-2-methylpropanamide | C25H25ClN6O4S 540.13 |
 | NA | 2-(4-Chlorophenyl)-5-Quinoxalinecarboxamide | C15H10ClN3O 283.05 |
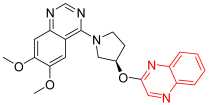 | PQ-10 | 6,7-Dimethoxy-4-[(3R)-3-(2-quinoxalinyloxy)-1-pyrrolidinyl]-quinazoline | C22H21N5O3 403.43 |
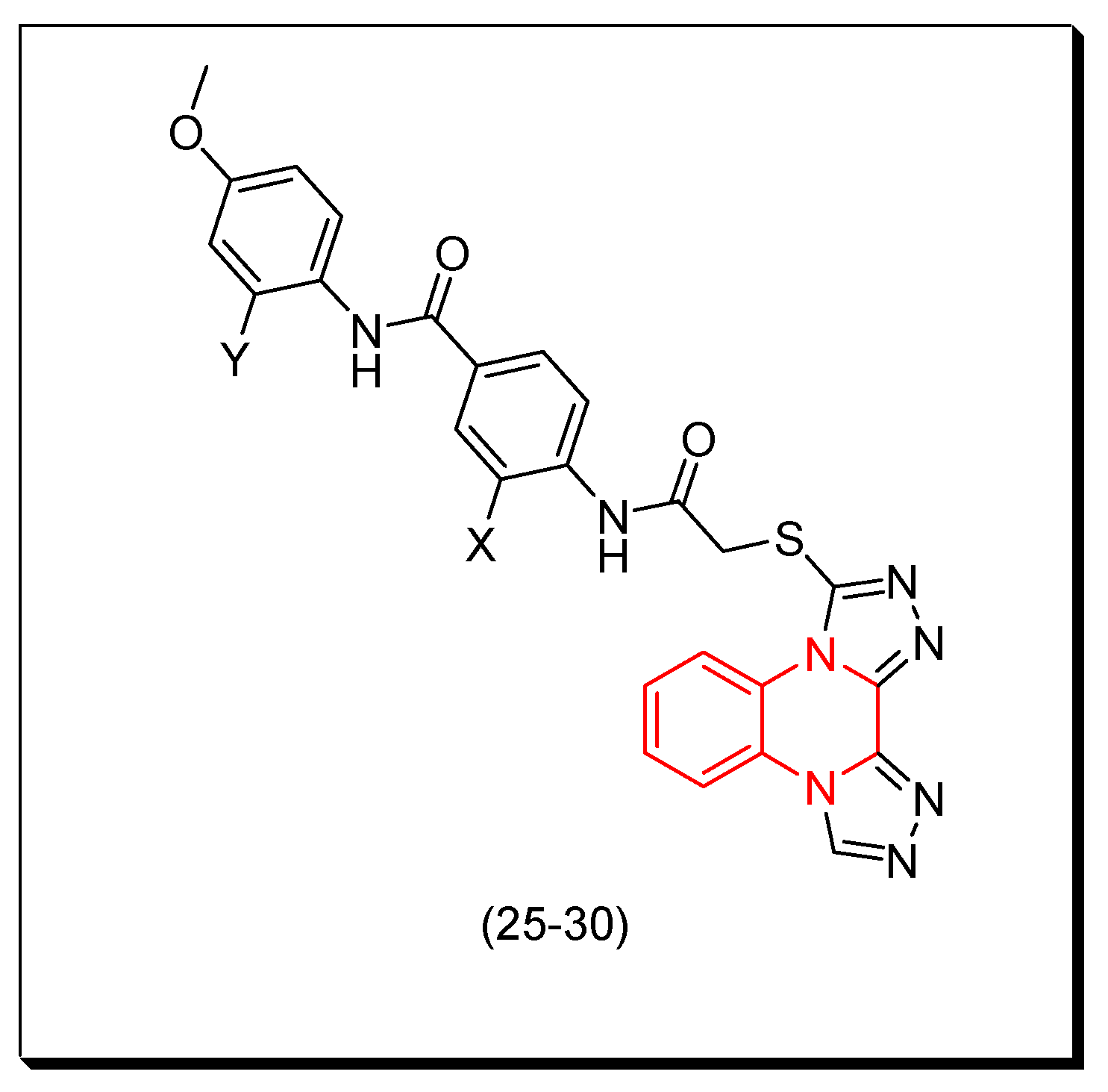
5. Anticancer Quinoxalines
6. Quantitative Structure–Activity Relationship (QSAR) Modeling of Anticancer Quinoxalines
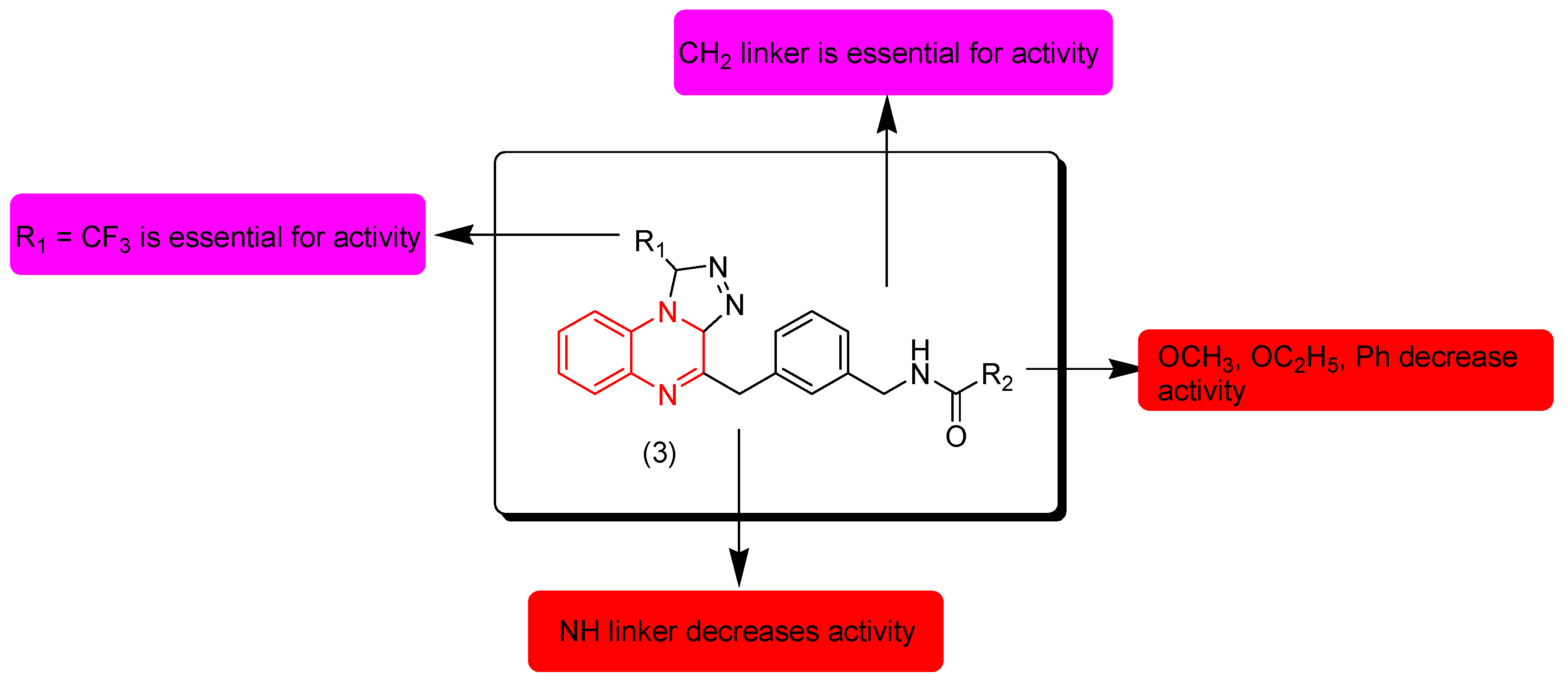
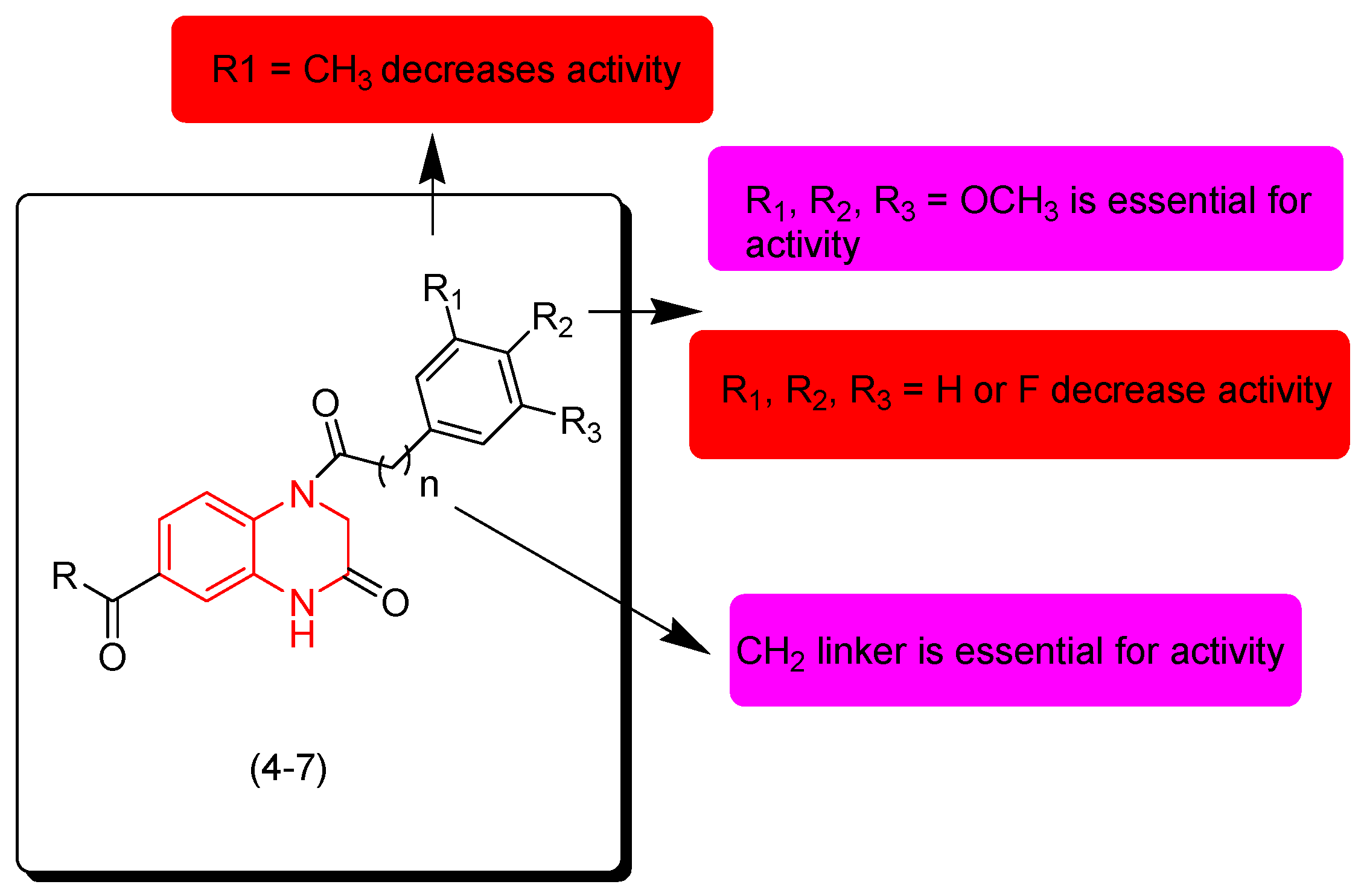
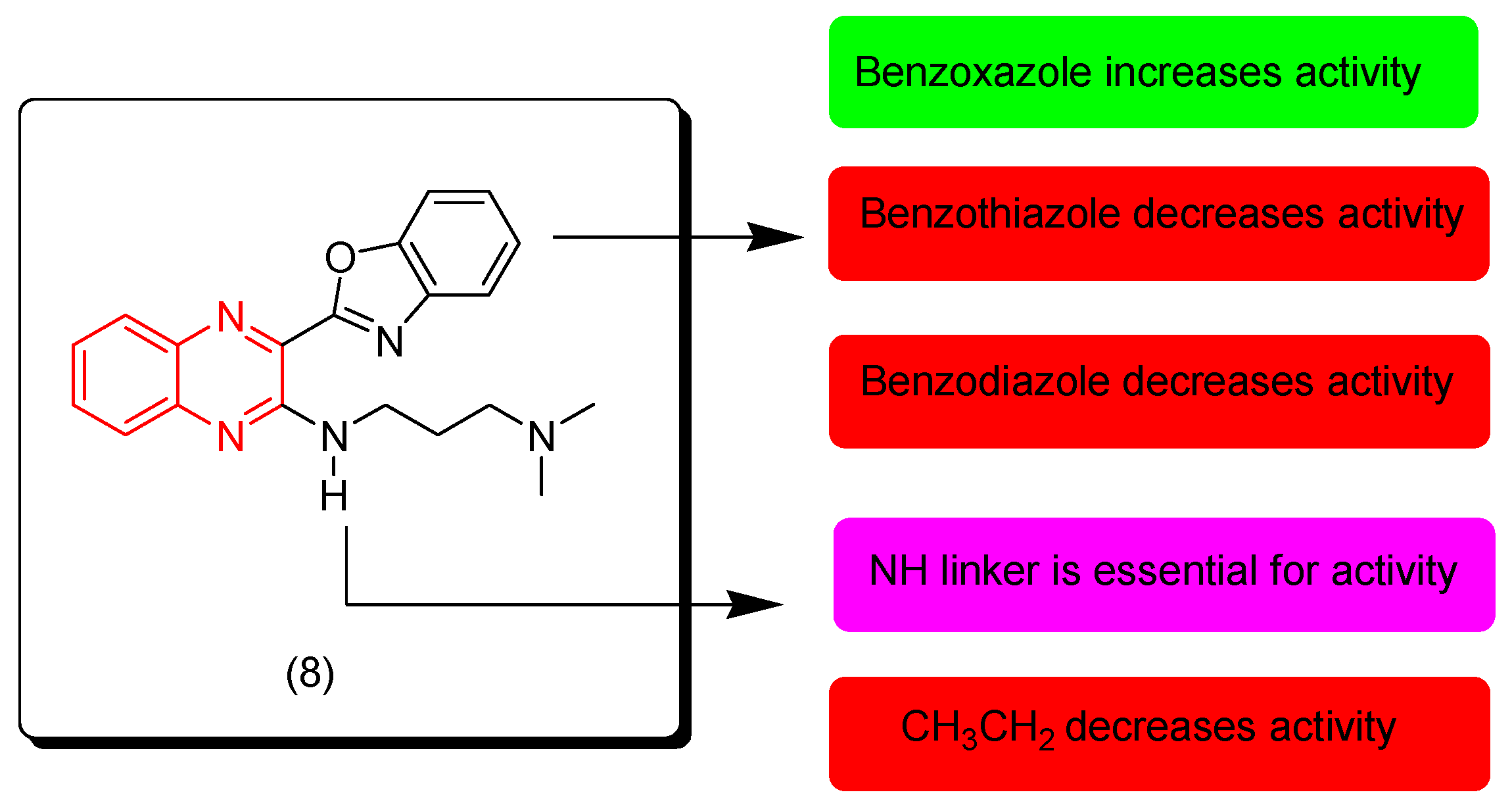
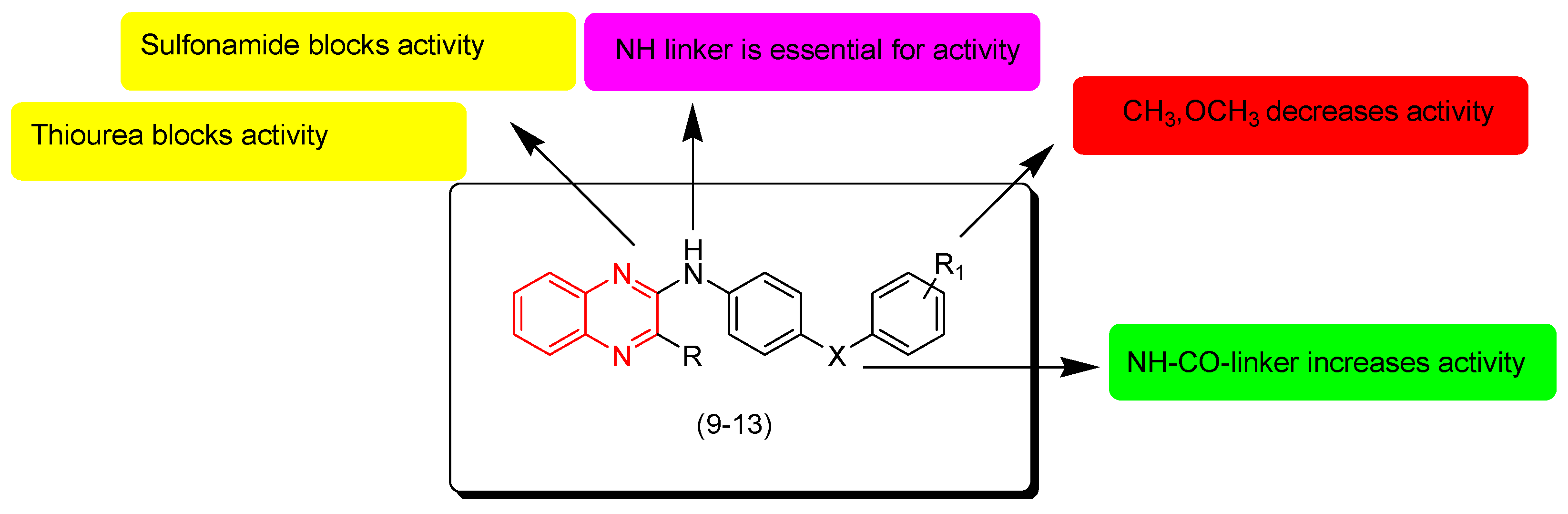
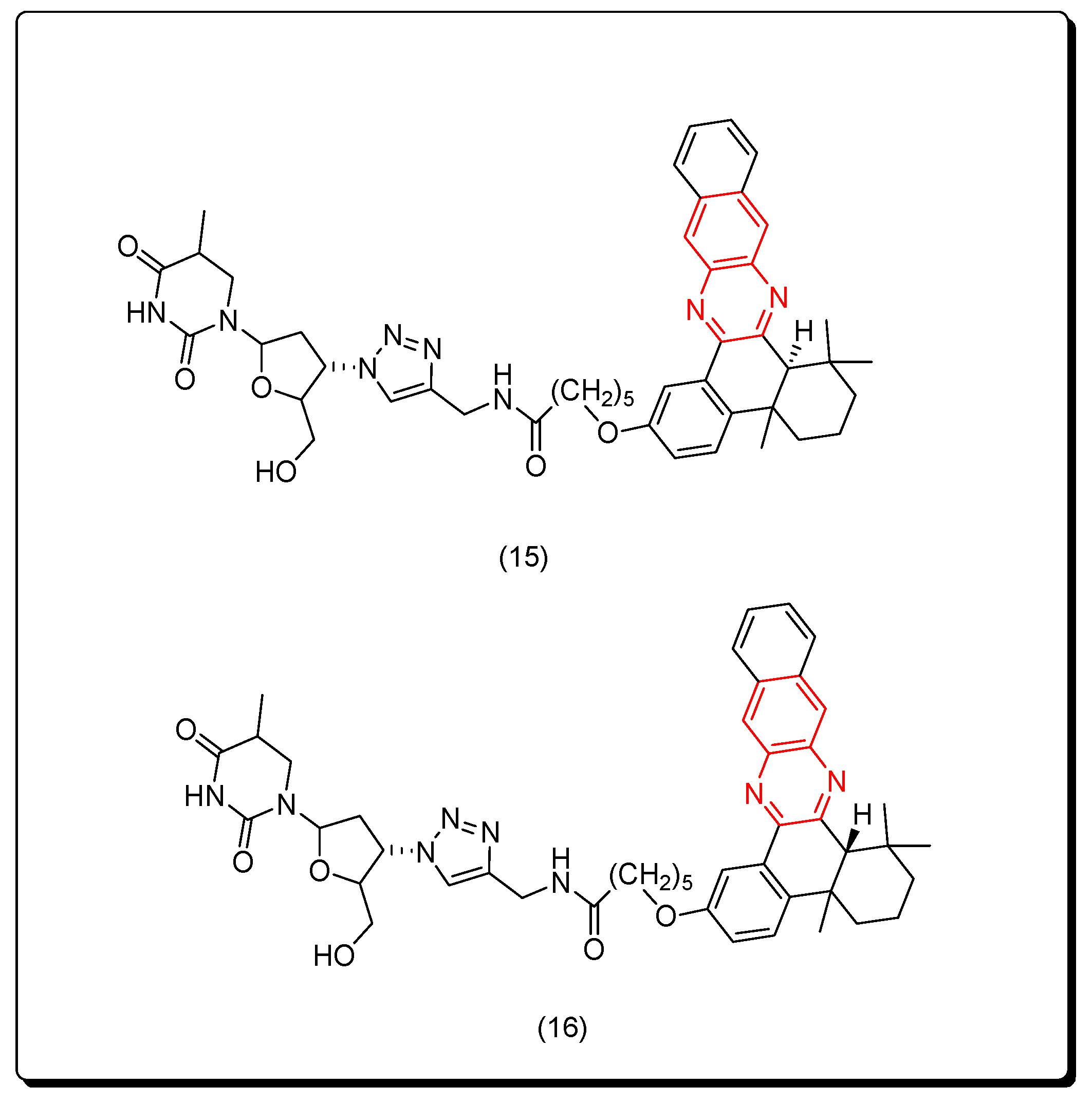
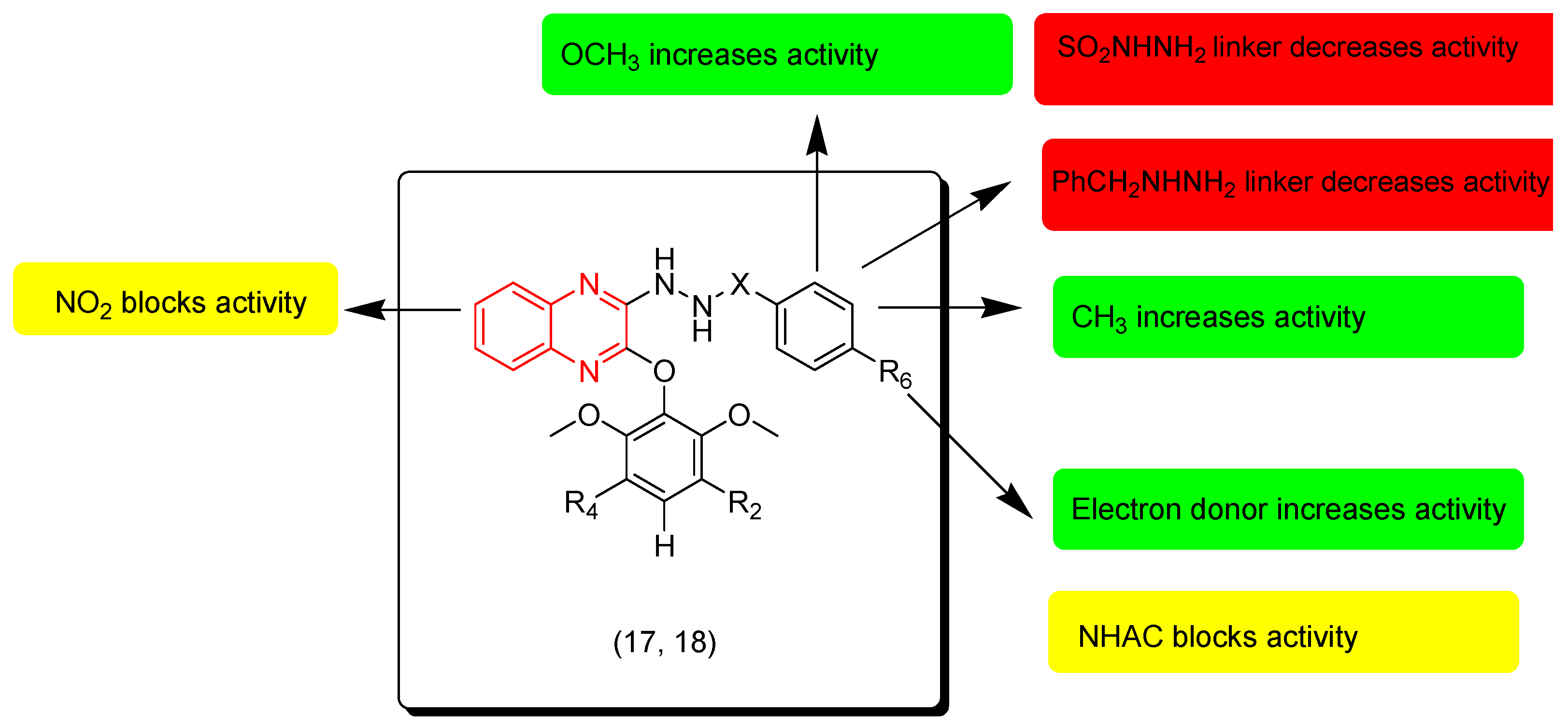
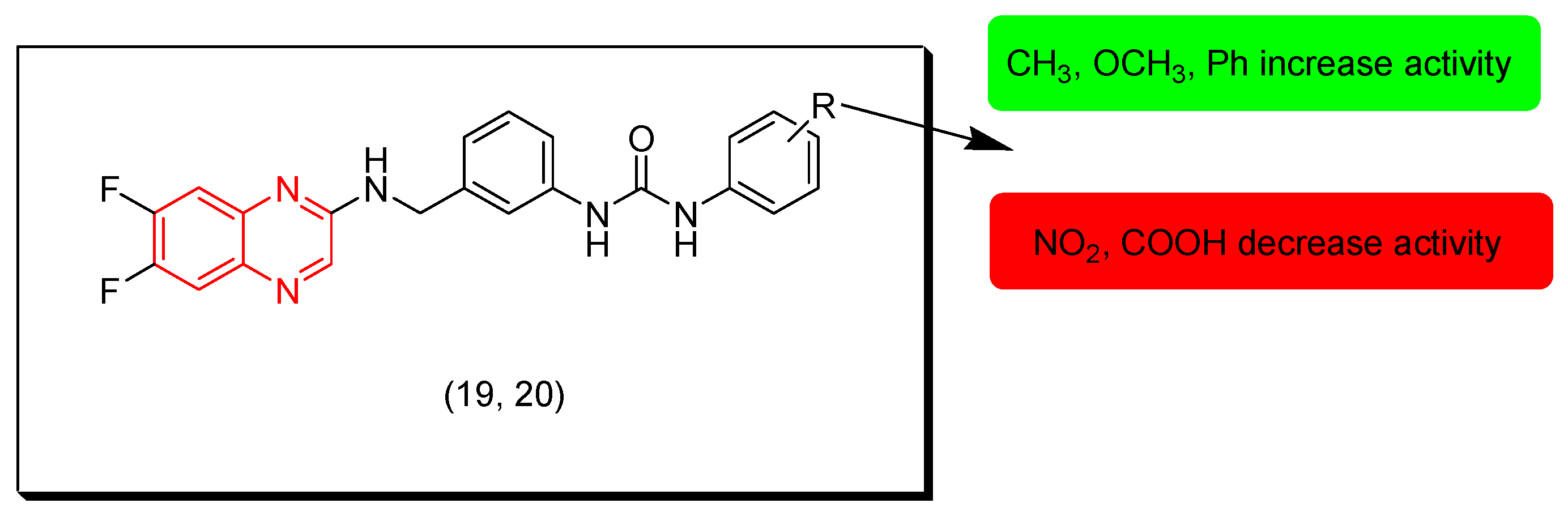
7. SAR of Anticancer Quinoxalines
- Quinoxaline moiety is an essential pharmacophore for the anticancer activity.
- The main sites of substitutions are first, second, and third, sixth, and/or seventh positions.
- The quinoxaline nucleus can be part of a hybrid molecule through a molecular hybridization process to potentiate the anticancer activity.
- The quinoxaline system can be joined with a polycyclic aromatic system at the (B) junction.
- There are two types of linkers that can be fused to the quinoxaline nucleus; in most cases the aliphatic linker is more reactive than the hetero-atomic linker.
- The third position from the quinoxaline nucleus can be fused to the heterocyclic system or aromatic system via an aliphatic linker.
- The aromatic ring of the quinoxaline nucleus can be substituted with halogens such as Cl or F at the sixth and/or seventh position/s to increase the activity.
8. Future Potentials
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mabrouk, R.R.; Abdallaha, A.E.; Mahdy, H.A.; El-Kalyoubi, S.A.; Kamal, O.J.; Abdelghany, T.M.; Zayed, M.F.; Alshaeri, H.K.; Alasmari, M.M.; El-Zahabi, M.A. Design, Synthesis, and Biological Evaluation of New Potential Unusual Modified Anticancer Immunomodulators for Possible Non-Teratogenic Quinazoline-Based Thalidomide Analogs. Int. J. Mol. Sci. 2023, 24, 12416. [Google Scholar] [CrossRef] [PubMed]
- Ihmaid, S.; Ahmed, H.E.A.; Zayed, M.F. The Design and Development of Potent Small Molecules as Anticancer Agents Targeting EGFR TK and Tubulin Polymerization. Int. J. Mol. Sci. 2018, 19, 408. [Google Scholar] [CrossRef] [PubMed]
- El-Helby, A.A.; Ayyad, A.R.; Zayed, M.F.; Abulkhir, S.H.; Elkady, H.; El-Adl, K. Design, synthesis, in silico ADMET profile and GABA-A docking of novel phthalazines as potent anticonvulsants. Arch. Pharm. 2019, 352, 1800373. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases. Sci. Pharm. 2023, 91, 18. [Google Scholar] [CrossRef]
- El-Zahabia, M.A.; Bamanie, H.F.; Ghareeb, S.; Alshaeri, K.H.; Alasmari, M.M.; Muostafa, M.; Al-Marzoki, Z.; Zayed, M.F. Design, Synthesis, Molecular Modeling andAnti-Hyperglycemic Evaluation of Quinazoline-Sulfonylurea Hybrids as Peroxisome Proliferator-Activated Receptor Gamma (PPAR) and Sulfonylurea Receptor (SUR) Agonists. Int. J. Mol. Sci. 2022, 23, 9605. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Ibrahim, S.; Habib, E.E.; Hassan, M.H.; Ahmed, S.; Rateb, H.S. Design, synthesis, antimicrobial and anti-biofilm evaluation, and molecular docking of new substituted fluoroquinazolinones. J. Med. Chem. 2019, 15, 657–673. [Google Scholar]
- Ghorab, M.M.; Abdel-Kader, M.S.; Alqahtani, A.S.; Soliman, A.M. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J. Enzym. Inhib. Med. Chem. 2021, 36, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Ayyad, R.R. Some Novel Anticonvulsant Agents Derived from Phthalazinedione. Arzneimittelforschung 2012, 62, 532–536. [Google Scholar] [CrossRef]
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Analgesic and Anti-Inflammatory Agents. ChemEngineering 2022, 6, 94. [Google Scholar] [CrossRef]
- Abulkhair, S.H.; Elmeligie, S.; Ghiaty, A.; El-Morsy, A.; Bayoumi, H.A.; Ahmed, A.E.H.; El-Adl, K.; Zayed, M.F.; Hassan, H.M.; Akl, E.N.; et al. In vivo- and in silico-driven identification of novel synthetic quinoxalines as anticonvulsants and AMPA inhibitors. Arch. Pharm. 2021, 354, 2000449. [Google Scholar] [CrossRef]
- Elhelby, A.A.; Ayyad, R.R.; Zayed, M.F. Synthesis and biological evaluation of some quinoxaline derivatives as anticonvulsant agents. Arzneimittelforschung 2011, 61, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Iazzetti, A.; Fabrizi, G.; Goggiamani, A.; Marrone, F.; Sferrazza, A.; Ullah, K. Synthesis of Functionalized 3H-pyrrolo-[1,2,3-de] Quinoxalines via Gold-Catalyzed Intramolecular Hydroamination of Alkynes. Molecules 2023, 28, 5831. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Deep, A.; Marwaha, M.; Marwaha, R.K. Quinoxaline: A chemical moiety with spectrum of interesting biological activities. Mini Rev. Med. Chem. 2022, 22, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Suthar, K.S.; NChundawat, S.N.; Singh, P.G.; Padrón, M.J.; Jhala, K.Y. Quinoxaline: A comprehension of current pharmacological advancement in medicinal chemistry. Eur. J. Med. Chem. Rep. 2022, 5, 100040. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline Derivatives as Antiviral Agents: A Systematic Review. Molecules 2020, 25, 2784. [Google Scholar] [CrossRef] [PubMed]
- Patinote, C.; Raevens, S.; Baumann, A.; Pellegrin, E.; Bonnet, P.-A.; Deleuze-Masquéfa, C. [1,2,4]triazolo[4,3-a]quinoxaline as Novel Scaffold in the Imiqualines Family: Candidates with Cytotoxic Activities on Melanoma Cell Lines. Molecules 2023, 28, 5478. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Fillová, N.; Moreau, S.; Baylot, V.; Desplat, V. Synthesis, Crystal Structure and Anti-Leukemic Activity of (E)-Pyrrolo[1,2-a]quinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one. Molbank 2023, 2023, M1691. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Wang, X.; Lei, Y. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions. Molecules 2023, 28, 5030. [Google Scholar] [CrossRef]
- Matveevskaya, V.V.; Pavlov, D.I.; Kovrizhina, A.R.; Sukhikh, T.S.; Sadykov, E.H.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khlebnikov, A.I.; Potapov, A.S. Experimental and Computational Investigation of the Oxime Bond Stereochemistry in c-Jun N-terminal Kinase 3 Inhibitors 11H-Indeno[1,2-b]quinoxalin-11-one Oxime and Tryptanthrin-6-oxime. Pharmaceutics 2023, 15, 1802. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Quinoxaline-Based Photoinitiators of Polymerization. Catalysts 2023, 13, 718. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Wu, M.; Lei, Y.-Z. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules 2023, 28, 2513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.P.; Pathak, P.; Grishina, M.; et al. Nitrogen Containing Heterocycles as Anticancer Agents: A Medicinal Chemistry Perspective. Pharmaceuticals 2023, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Zhdankina, A.A.; Tikhonov, D.I.; Logvinov, S.V.; Plotnikov, M.B.; Khlebnikov, A.I.; Kolosova, N.G. Suppression of Age-Related Macular Degeneration-like Pathology by c-Jun N-Terminal Kinase Inhibitor IQ-1S. Biomedicines 2023, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Pooladian, F.; Crawford, P.W.; Kessler, J.M.; Casey, G.R.; Ragain, C.M. Reduction Potential Predictions for Thirty-Seven 1,4-di-N-Oxide Quinoxaline-2-Carboxamide Derivatives with Anti-Tuberculosis Activity. Compounds 2023, 3, 83–95. [Google Scholar] [CrossRef]
- Goel, K.K.; Hussain, A.; Altamimi, M.A.; Rajput, S.K.; Sharma, P.P.; Kharb, R.; Mahdi, W.A.; Imam, S.S.; Alshehri, S.; Alnemer, O.A.; et al. Identification of Potential Antitubulin Agents with Anticancer Assets from a Series of Imidazo[1,2-a]quinoxaline Derivatives: In Silico and In Vitro Approaches. Molecules 2023, 28, 802. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.V.; Skripka, M.O.; Spasov, A.A.; Vassiliev, P.M.; Perfiliev, M.A.; Divaeva, L.N.; Zubenko, A.A.; Morkovnik, A.S.; Klimenko, A.I.; Miroshnikov, M.V.; et al. Design, Synthesis and Pharmacological Evaluation of Novel C2,C3-Quinoxaline Derivatives as Promising Anxiolytic Agents. Int. J. Mol. Sci. 2022, 23, 14401. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Liu, P.; Jiang, Y.; He, X.; Zhang, L.; Wang, L.; Yang, T. Discovery and SAR Study of Quinoxaline–Arylfuran Derivatives as a New Class of Antitumor Agents. Pharmaceutics 2022, 14, 2420. [Google Scholar] [CrossRef]
- González-González, A.; Sánchez-Sánchez, O.; Krauth-Siegel, R.L.; Bolognesi, M.L.; Gớmez-Escobedo, R.; Nogueda-Torres, B.; Vázquez-Jiménez, L.K.; Saavedra, E.; Encalada, R.; Espinoza-Hicks, J.C.; et al. In Vitro and In Silico Analysis of New n-Butyl and Isobutyl Quinoxaline-7-carboxylate 1,4-di-N-oxide Derivatives against Trypanosoma cruzi as Trypanothione Reductase Inhibitors. Int. J. Mol. Sci. 2022, 23, 13315. [Google Scholar] [CrossRef]
- Bouali, N.; Hammouda, M.B.; Ahmad, I.; Ghannay, S.; Thouri, A.; Dbeibia, A.; Patel, H.; Hamadou, W.S.; Hosni, K.; Snoussi, M.; et al. Multifunctional Derivatives of Spiropyrrolidine Tethered Indeno-Quinoxaline Heterocyclic Hybrids as Potent Antimicrobial, Antioxidant and Antidiabetic Agents: Design, Synthesis, In Vitro and In Silico Approaches. Molecules 2022, 27, 7248. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Arutiunov, N.A.; Aksenov, D.A.; Samovolov, A.V.; Kurenkov, I.A.; Aksenov, N.A.; Aleksandrova, E.A.; Momotova, D.S.; Rubin, M. A Convenient Way to Quinoxaline Derivatives through the Reaction of 2-(3-Oxoindolin-2-yl)-2-phenylacetonitriles with Benzene-1,2-diamines. Int. J. Mol. Sci. 2022, 23, 11120. [Google Scholar] [CrossRef]
- Bhat, Z.R.; Kumar, M.; Sharma, N.; Yadav, U.P.; Singh, T.; Joshi, G.; Pujala, B.; Raja, M.; Chatterjee, J.; Tikoo, K.; et al. In Vivo Anticancer Evaluation of 6b, a Non-Covalent Imidazo[1,2-a]quinoxaline-Based Epidermal Growth Factor Receptor Inhibitor against Human Xenograft Tumor in Nude Mice. Molecules 2022, 27, 5540. [Google Scholar] [CrossRef] [PubMed]
- Syam, Y.M.; Anwar, M.M.; Abd El-Karim, S.S.; Elokely, K.M.; Abdelwahed, S.H. New Quinoxaline-Based Derivatives as PARP-1 Inhibitors: Design, Synthesis, Antiproliferative, and Computational Studies. Molecules 2022, 27, 4924. [Google Scholar] [CrossRef] [PubMed]
- Montero, V.; Montana, M.; Khoumeri, O.; Correard, F.; Estève, M.-A.; Vanelle, P. Synthesis, In Vitro Antiproliferative Activity, and In Silico Evaluation of Novel Oxiranyl-Quinoxaline Derivatives. Pharmaceuticals 2022, 15, 781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Inwati, G.K.; Ahmed, E.M.; Lal, C.; Makwana, B.; Yadav, V.K.; Islam, S.; Ahn, H.-J.; Yadav, K.K.; Jeon, B.-H. Modified 7-Chloro-11H-indeno[1,2-b]quinoxaline Heterocyclic System for Biological Activities. Catalysts 2022, 12, 213. [Google Scholar] [CrossRef]
- Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Moreau, S.; Desplat, V. Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one. Molbank 2022, 2022, M1333. [Google Scholar] [CrossRef]
- Sharma, B.K.; Shaikh, A.M.; Chacko, S.; Kamble, M.R. Synthesis, spectral, electrochemical and theoretical investigation of indolo[2,3-b]quinoxaline dyes derived from anthraquinone for n–type materials. J. Chem. Sci. 2017, 129, 483–494. [Google Scholar] [CrossRef]
- Peppas, A.; Sokalis, D.; Perganti, D.; Schnakenburg, G.; Falaras, P.; Philippopoulos, I.A. Sterically demanding pyridine-quinoline anchoring ligands as building blocks for copper(i)-based dye-sensitized solar cell (DSSC) complexes. Dalton Trans. 2022, 51, 15049. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Al-Hussain, S.A. Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents. Molecules 2022, 27, 835. [Google Scholar] [CrossRef]
- Liakhov, S.A.; Schepetkin, I.A.; Karpenko, O.S.; Duma, H.I.; Haidarzhy, N.M.; Kirpotina, L.N.; Kovrizhina, A.R.; Khlebnikov, A.I.; Bagryanskaya, I.Y.; Quinn, M.T. Novel c-Jun N-Terminal Kinase (JNK) Inhibitors with an 11H-Indeno[1,2-b]quinoxalin-11-one Scaffold. Molecules 2021, 26, 5688. [Google Scholar] [CrossRef]
- Suwanhom, P.; Saetang, J.; Khongkow, P.; Nualnoi, T.; Tipmanee, V.; Lomlim, L. Synthesis, Biological Evaluation, and In Silico Studies of New Acetylcholinesterase Inhibitors Based on Quinoxaline Scaffold. Molecules 2021, 26, 4895. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline Moiety: A Potential Scaffold against Mycobacterium tuberculosis. Molecules 2021, 26, 4742. [Google Scholar] [CrossRef] [PubMed]
- Bouz, G.; Bouz, S.; Janďourek, O.; Konečná, K.; Bárta, P.; Vinšová, J.; Doležal, M.; Zitko, J. Synthesis, Biological Evaluation, and In Silico Modeling of N-Substituted Quinoxaline-2-Carboxamides. Pharmaceuticals 2021, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Amrane, D.; Arnold, C.-S.; Hutter, S.; Sanz-Serrano, J.; Collia, M.; Azqueta, A.; Paloque, L.; Cohen, A.; Amanzougaghene, N.; Tajeri, S.; et al. 2-Phenoxy-3-Trichloromethylquinoxalines Are Antiplasmodial Derivatives with Activity against the Apicoplast of Plasmodium falciparum. Pharmaceuticals 2021, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.; Ahmad, S.; Hussain, S.; Batool, F.; Riaz, H.; Zafar, R.; Kotwica-Mojzych, K.; Mojzych, M. Recent Updates on the Synthesis of Bioactive Quinoxaline-Containing Sulfonamides. Appl. Sci. 2021, 11, 5702. [Google Scholar] [CrossRef]
- El-Sayed, N.N.E.; Al-Otaibi, T.M.; Alonazi, M.; Masand, V.H.; Barakat, A.; Almarhoon, Z.M.; Ben Bacha, A. Synthesis and Characterization of Some New Quinoxalin-2(1H)one and 2-Methyl-3H-quinazolin-4-one Derivatives Targeting the Onset and Progression of CRC with SAR, Molecular Docking, and ADMET Analyses. Molecules 2021, 26, 3121. [Google Scholar] [CrossRef]
- Mehmood, R.; Rashid, N.; Ullah, S.; Amaldoss, M.J.N.; Sorrell, C.C. Synthesis and Structure-Chirality Relationship Analysis of Steroidal Quinoxalines to Design and Develop New Chiral Drugs. Chemistry 2021, 3, 402–410. [Google Scholar] [CrossRef]
- Kumar, M.; Joshi, G.; Arora, S.; Singh, T.; Biswas, S.; Sharma, N.; Bhat, Z.R.; Tikoo, K.; Singh, S.; Kumar, R. Design and Synthesis of Non-Covalent Imidazo[1,2-a]quinoxaline-Based Inhibitors of EGFR and Their Anti-Cancer Assessment. Molecules 2021, 26, 1490. [Google Scholar] [CrossRef]
- Khatoon, H.; Abdulmalek, E. Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades. Molecules 2021, 26, 1055. [Google Scholar] [CrossRef]
- Oyallon, B.; Brachet-Botineau, M.; Logé, C.; Robert, T.; Bach, S.; Ibrahim, S.; Raoul, W.; Croix, C.; Berthelot, P.; Guillon, J.; et al. New Quinoxaline Derivatives as Dual Pim-1/2 Kinase Inhibitors: Design, Synthesis and Biological Evaluation. Molecules 2021, 26, 867. [Google Scholar] [CrossRef]
- Nam, K.Y.; Damodar, K.; Lee, Y.; Park, L.S.; Gim, J.G.; Park, J.P.; Jeon, S.H.; Lee, J.T. Design and Synthesis of π-Extended Resveratrol Analogues and In Vitro Antioxidant and Anti-Inflammatory Activity Evaluation. Molecules 2021, 26, 646. [Google Scholar] [CrossRef]
- Giuglio-Tonolo, A.G.; Curti, C.; Terme, T.; Vanelle, P. A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]quinoxaline-5,10-diones. Molecules 2020, 25, 5922. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Moreau, S.; Pinaud, N.; Marchivie, M.; Desplat, V. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-Leukemic Activity. Molbank 2020, 2020, M1113. [Google Scholar] [CrossRef]
- Palos, I.; Luna-Herrera, J.; Lara-Ramírez, E.E.; Loera-Piedra, A.; Fernández-Ramírez, E.; Aguilera-Arreola, M.G.; Paz-González, A.D.; Monge, A.; Wan, B.; Franzblau, S.; et al. Anti-Mycobacterium tuberculosis Activity of Esters of Quinoxaline 1,4-Di-N-Oxide. Molecules 2018, 23, 1453. [Google Scholar] [CrossRef] [PubMed]
- Alswah, M.; Bayoumi, A.H.; Elgamal, K.; Elmorsy, A.; Ihmaid, S.; Ahmed, H.E.A. Design, Synthesis and Cytotoxic Evaluation of Novel Chalcone Derivatives Bearing Triazolo[4,3-a]-quinoxaline Moieties as Potent Anticancer Agents with Dual EGFR Kinase and Tubulin Polymerization Inhibitory Effects. Molecules 2018, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, S.; Jin, X.; Zhang, Y.; Hua, D.; Miao, T.; Tao, X.; Wang, S. Synthesis and Evaluation of New Quinoxaline Derivatives of Dehydroabietic Acid as Potential Antitumor Agents. Molecules 2017, 22, 1154. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Vargas, K.F.; Nogueda-Torres, B.; Sánchez-Torres, L.E.; Suarez-Contreras, E.; Villalobos-Rocha, J.C.; Torres-Martinez, Y.; Lara-Ramirez, E.E.; Fiorani, G.; Krauth-Siegel, R.L.; Bolognesi, M.L.; et al. Trypanocidal Activity of Quinoxaline 1,4 Di-N-oxide Derivatives as Trypanothione Reductase Inhibitors. Molecules 2017, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Al-Marhabi, A.R.; Abbas, H.-A.S.; Ammar, Y.A. Synthesis, Characterization and Biological Evaluation of Some Quinoxaline Derivatives: A Promising and Potent New Class of Antitumor and Antimicrobial Agents. Molecules 2015, 20, 19805–19822. [Google Scholar] [CrossRef]
- Mickevičienė, K.; Baranauskaitė, R.; Kantminienė, K.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Novikov, V. Synthesis and Antimicrobial Activity of N-Substituted-β-amino Acid Derivatives Containing 2-Hydroxyphenyl, Benzo[b]phenoxazine and Quinoxaline Moieties. Molecules 2015, 20, 3170–3189. [Google Scholar] [CrossRef]
- Radwan, A.A.; Abdel-Mageed, W.M. In Silico Studies of Quinoxaline-2-Carboxamide 1,4-di-N-Oxide Derivatives as Antimycobacterial Agents. Molecules 2014, 19, 2247–2260. [Google Scholar] [CrossRef]
- Gil, A.; Pabón, A.; Galiano, S.; Burguete, A.; Pérez-Silanes, S.; Deharo, E.; Monge, A.; Aldana, I. Synthesis, Biological Evaluation and Structure-Activity Relationships of New Quinoxaline Derivatives as Anti-Plasmodium falciparum Agents. Molecules 2014, 19, 2166–2180. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Pérez-Silanes, S.; Galiano, S.; Gonzalez, G.; Monge, A.; Deharo, E.; Aldana, I. New Amide Derivatives of Quinoxaline 1,4-di-N-Oxide with Leishmanicidal and Antiplasmodial Activities. Molecules 2013, 18, 4718–4727. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Q.; Shangguan, S.; Liu, X.; Hu, Y.; Sheng, R. Synthesis and Biological Evaluation of 3-Aryl-quinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives as Hypoxic Selective Anti-tumor Agents. Molecules 2012, 17, 9683–9696. [Google Scholar] [CrossRef] [PubMed]
- Barea, C.; Pabón, A.; Galiano, S.; Pérez-Silanes, S.; Gonzalez, G.; Deyssard, C.; Monge, A.; Deharo, E.; Aldana, I. Antiplasmodial and Leishmanicidal Activities of 2-Cyano-3-(4-phenylpiperazine-1-carboxamido) Quinoxaline 1,4-Dioxide Derivatives. Molecules 2012, 17, 9451–9461. [Google Scholar] [CrossRef]
- Ancizu, S.; Castrillo, N.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Delagrange, P.; Caignard, D.-H.; Galiano, S. New Quinoxaline Derivatives as Potential MT1 and MT2 Receptor Ligands. Molecules 2012, 17, 7737–7757. [Google Scholar] [CrossRef] [PubMed]
- Morales-Castellanos, J.J.; Ramírez-Hernández, K.; Gómez-Flores, N.S.; Rodas-Suárez, O.R.; Peralta-Cruz, J. Microwave-assisted Solvent-free Synthesis and in Vitro Antibacterial Screening of Quinoxalines and Pyrido[2,3b]pyrazines. Molecules 2012, 17, 5164–5176. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE) Chemical Computing Group. Available online: http://www.chemcomp.com (accessed on 9 July 2023).
- Swiss Institute of Bioinformatics (SwissADME). Available online: http://www.swissADME.ch (accessed on 10 July 2023).
- Singh, D.P.; Deivedi, S.K.; Hashim, S.R.; Singhal, R.G. Synthesis and Antimicrobial Activity of Some New Quinoxaline Derivatives. Pharmaceuticals 2010, 3, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Mukherjee, S.; Rodriguez, R.R.; Banik, B.K. An Effective Microwave-Induced Iodine-Catalyzed Method for the Synthesis of Quinoxalines via Condensation of 1,2-Diamines with 1,2-Dicarbonyl Compounds. Molecules 2010, 15, 4207–4212. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Charnaud, S.; Bongard, E.; Villar, R.; Burguete, A.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Vivas, L.; et al. Synthesis and Antiplasmodial Activity of 3-Furyl and 3-Thienylquinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives. Molecules 2008, 13, 69–77. [Google Scholar] [CrossRef] [PubMed]
- EI-Bendary, E.R.; Goda, F.E.; Maarouf, A.R.; Badria, F.A. Synthesis and antimicrobial evaluation of 3-hydrazino-quinoxaline derivatives and their cyclic analoaues. Sci. Pharm. 2004, 72, 175–185. [Google Scholar] [CrossRef][Green Version]
- Nasr, M.N.; Gineinaha, M.M.; Maarouf, A.R. Synthesis and Anticonvulsant Activity of Novel 2- and 3-14-(Trisubstituted Pyridy1)-phenylaminol- and 2-[3- and 4-(Trisubstituted Pyridy1)-phenoxylquinoxaline Derivatives. Sci. Pharm. 2003, 71, 9–18. [Google Scholar] [CrossRef]
- Waring, M.J.; Ben-Hadda, T.; Kotchevar, A.T.; Ramdani, A.; Touzani, R.; Elkadiri, S.; Hakkou, A.; Bouakka, M.; Ellis, T. 2,3-Bifunctionalized Quinoxalines: Synthesis, DNA Interactions and Evaluation of Anticancer, Anti-tuberculosis and Antifungal Activity. Molecules 2002, 7, 641–656. [Google Scholar] [CrossRef]
- Rodrigo, G.A.; Bekerman, D.G.; Robinsohn, A.E.; Fernández, B.M. Synthesis and Physicochemical Study of a Quinoxaline Derivative with Potencial Antineoplasic or Anti-HIV Activity. Molecules 2000, 5, 358–359. [Google Scholar] [CrossRef]
- Veisi, H. Molecular iodine: Recent application in heterocyclic synthesis. Curr. Org. Chem. 2011, 15, 2438–2468. [Google Scholar] [CrossRef]
- Aichhorn, S.; Himmelsbach, M.; Schofberger, W. Synthesis of quinoxalines or quinolin-8-amines from N-propargyl aniline derivatives employing tin and indium chlorides. Org. Biomol. Chem. 2015, 13, 9373–9380. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bakhtiari, K.; Tehrani, M.H.; Javadi, N.M.; Oskooie, A.H. Facile synthesis of quinoxaline derivatives using o-iodoxybenzoic acid (IBX) at room temperature. ARKIVOC 2006, XVI, 16–22. [Google Scholar] [CrossRef]
- Jeganathan, M.; Dhakshinamoorthy, A.; Pitchumani, K. One-pot synthesis of 2- substituted quinoxalines using K10-montmorillonite as heterogeneous catalyst. Tetrahedron Lett. 2014, 55, 1616–1620. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Wei, J.; Lin, D.; Xie, Y.; Zeng, W. Copper-catalyzed cascade Cycloamination of α-csp3–H Bond of N-aryl Ketimines with azides: Access to Quinoxalines. Org. Lett. 2016, 18, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, Y.; Meng, X.; Zhao, M.; Chen, Y.; Chen, B. Copper-catalyzed synthesis of quinoxalines with o-phenylenediamine and terminal alkyne in the presence of bases. Org. Lett. 2011, 13, 4514–4517. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, K.; Zhao, M.; Li, Y.; Chen, B. Cu(II)-catalyzed synthesis of quinoxalines from o-phenylene diamines and nitroolefins. Tetrahedron Lett. 2013, 54, 1627–1630. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Xie, C.; Zhou, F.; Chen, M. Terminal methyl as a one-carbon synthon: Synthesis of quinoxaline derivatives via radical-type transformation. New J. Chem. 2020, 44, 2465–2470. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Zare, A.; Shekouhy, M.; Moosavi-Zare, A.R. Bentonite clay K-10 as an efficient reagent for the synthesis of quinoxaline derivatives at room temperature. J. Chem. 2009, 6, S247–S253. [Google Scholar] [CrossRef]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; Volume 30. [Google Scholar]
- Subrahmanyam, S.C.; Narayanan, S. Synthesis of Quinoxalines in Presence of Zinc Triflate. Asian J. Chem. 2011, 23, 1331–1333. [Google Scholar]
- An, Z.; Wu, M.; Ni, J.; Qi, Z.; Yu, G.; Yan, R.; Zhao, L.-B. FeCl3-Catalyzed synthesis of pyrrolo[1,2-a]quinoxaline derivatives from 1-(2-aminophenyl)pyrroles through annulation and cleavage of cyclic ethers. Chem. Commun. 2017, 53, 11572–11575. [Google Scholar] [CrossRef] [PubMed]
- Atghia, S.V.; Beigbaghlou, S.S. Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) as a new, highly efficient, and recyclable solid acid catalyst for the preparation of quinoxaline derivatives. J. Nanostruct. Chem. 2013, 3, 38. [Google Scholar] [CrossRef]
- Kumar, K.S.; Rambabu, D.; Sandra, S.; Kapavarapu, R.; Krishna, R.G.G.; Rao, B.V.M.; Chatti, K.; Reddy, M.C.; Misra, P.; Pal, M. AlCl3 induced (hetero)arylation of 2,3-dichloroquinoxaline: A one-pot synthesis of mono/disubstituted quinoxalines as potential antitubercular agents. Bioorg. Med. Chem. 2012, 20, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.P.; Mukherjee, S.; Deora, S.G.; Chennubhotla, S.K.; Medisetti, R.; Yellanki, S.; Kulkarni, P.P.; Sripelly, S.; Parsa, L.V.K.; Chatti, K.; et al. Ligand/PTCfree intramolecular Heck reaction: Synthesis of pyrroloquinoxalines and their evaluation against PDE4/luciferase/oral cancer cell growth in vitro and zebrafish in vivo. Org. Biomol. Chem. 2013, 11, 6680. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Available online: https://go.drugbank.com/ (accessed on 28 September 2023).
- Kamble, A.A.; Kamble, R.R.; Kumbar, M.N.; Tegginamath, G. Pyridine-catalyzed synthesis of quinoxalines as anticancer and anti-tubercular agents. Med. Chem. Res. 2016, 25, 1163–1174. [Google Scholar] [CrossRef]
- Ali, I.; Lee, J.; Choi, G.G.A.; Lee, K. Discovery of novel [1,2,4]triazolo[4,3-a] quinoxaline aminophenyl derivatives as BET inhibitors for cancer treatment. Bioorg. J. Med. Chem. Lett. 2017, 27, 4606–4613. [Google Scholar] [CrossRef]
- Dong, H.Q.J.; Huang, J.; Zhang, S.; Niu, L.; Zhang, Y.; Wang, J. Synthesis and biological evaluation of N-substituted3-oxo-1,2,3,4-tetrahydro-quinoxaline-6- carboxylic acid derivatives as tubulin polymerization inhibitors. Eur. J. Med. Chem. 2018, 143, 8–20. [Google Scholar]
- Liu, J.; Wang, P.; Zhang, Z.; Xue, G.; Ahmed, A.E.; Mamdouh, F.A.; Omran, M.A.; Salah, H. Synthesis, EGFR-TK inhibition and anticancer activity of new quinoxaline derivatives. Synth. Commun. 2020, 50, 2924–2940. [Google Scholar]
- Newahie, E.S.M.A.; Nissan, M.; Ismail, M.S.N.; Ella, E.A.A.D.; Khojah, M.S.; Abouzid, M.A.K. Design and synthesis of new quinoxaline derivatives as anticancer agents and apoptotic inducers. Molecules 2019, 24, 1175. [Google Scholar] [CrossRef] [PubMed]
- Gobouri, A.A. Synthesis and biological evaluation of some N-substituted quinoxaline derivatives as antitumor agents. Russ. J. Bioorg. Chem. 2020, 46, 409–416. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Yang, R.; Qian, T.; Zhou, Q. Naphthyl quinoxaline thymidine conjugate is a potent anticancer agent post UVA activation and elicits marked inhibition of tumor growth through vaccination. Eur. J. Med. Chem. 2019, 1, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, P.; Zhang, Z.; Xue, G.; Zha, D.; Wang, J.; Xu, X. Facile synthesis and anti-proliferative activity evaluation of quinoxaline derivatives. Synth. Commun. 2020, 50, 823–830. [Google Scholar]
- Li, G.Z.; Ouyang, X.L.; Mo, Z.Y.; Ju, Y.; Cao, X.L.; Yang, L.; Tang, H.T.; Pan, Y.M. Synthesis and biological evaluation of novel 1,3-diphenylurea quinoxaline derivatives as potent anticancer agents. Med. Chem. Res. 2021, 30, 1496–1511. [Google Scholar] [CrossRef]
- Patinote, C.; Masquefa, D.C.; Kaddour, H.K.; Vincent, A.L.; Larive, R.; Zghaib, Z.; Guichou, F.J.; Assaf, D.M.; Cuq, P.; Bonnet, A.P. Imidazo[1,2-a]quinoxalines for melanoma treatment with original mechanism of action. Eur. J. Med. Chem. 2021, 212, 113031. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, H.S.; Uzairu, A.; Danazumi, U.A.; Finbarrs-Bello, E.; Umar, B.A.; Shallangwa, A.G.; Uba, S. Computational design of quinoxaline molecules as VEGFR-2 inhibitors: QSAR modelling, pharmacokinetics, molecular docking, and dynamics simulation studies. Biocatal. Agric. Biotechnol. 2023, 51, 102787. [Google Scholar] [CrossRef]


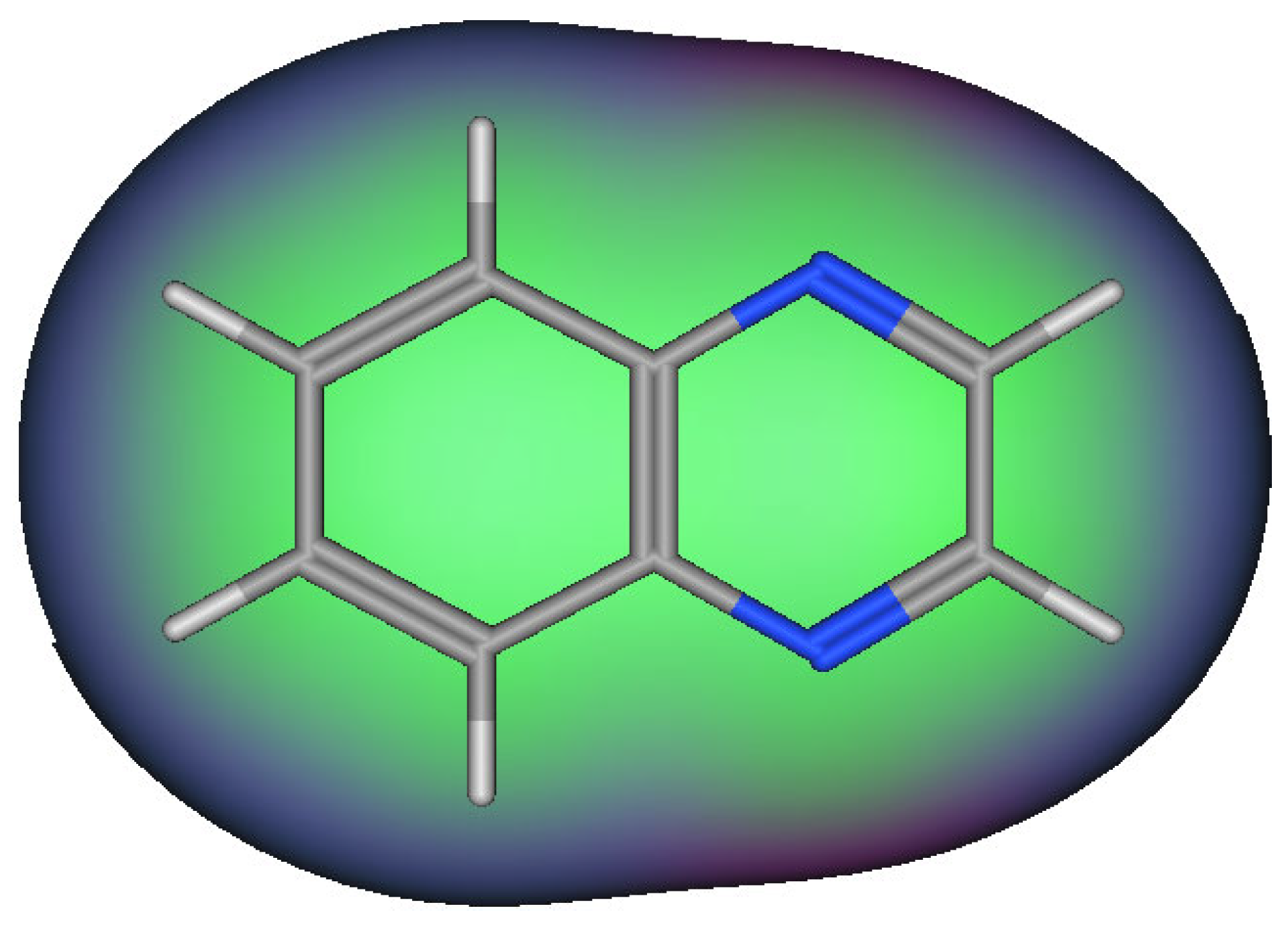

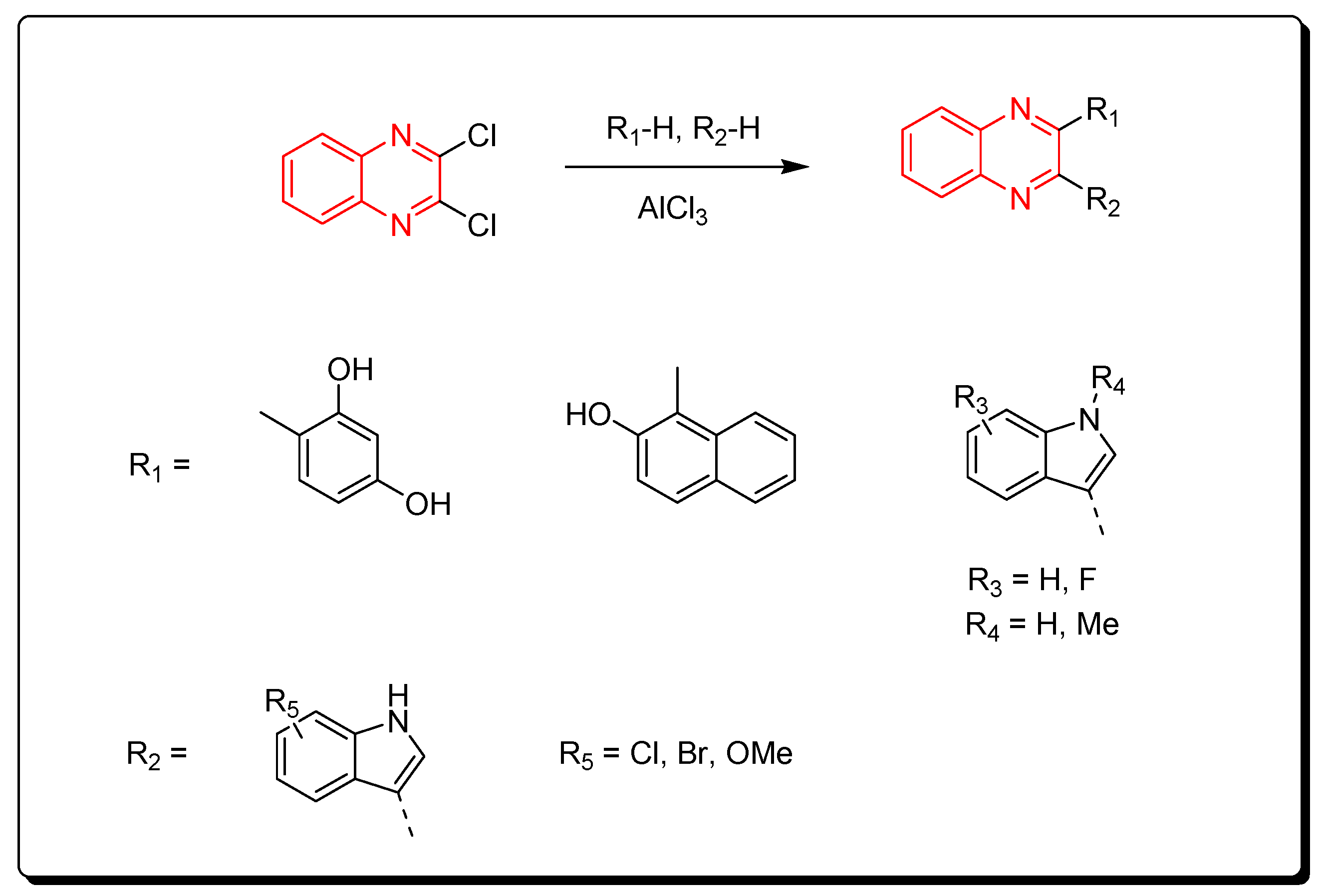




| Characteristic | Quinoxaline |
|---|---|
| Molecular formula | C8H6N2 |
| Molecular weight | 130.15 g/mol |
| Number of heavy atoms | 10 |
| Number of aromatic heavy atoms | 10 |
| Fraction Csp3 | 0 |
| Number of rotatable bonds | 0 |
| Number of H-bond acceptors | 2 |
| Number of H-bond donors | 0 |
| Molar refractivity | 39.54 |
| Tropological polar surface area | 25.78 A2 |
| Lipophilicity | 1.47 |
| Water solubility | Soluble |
| GI absorption | High |
| BBB permeation | Yes |
| Bioavailability score | 0.55 |
| Lipinski | Yes |
| Synthetic accessibility | Easy |
| ID | GATS5e | GATS3i | GATS8i | SpMax8_Bhs | VR2_Dt | Pred. pIC50 |
|---|---|---|---|---|---|---|
| 25 | - | - | - | - | - | 5.270 |
| 26 | 0.84815 | 1.14294 | 1.10205 | 3.268138 | 13.5029 | 6.16 |
| 27 | 0.77563 | 1.11386 | 1.01902 | 3.511698 | 13.5029 | 5.59 |
| 28 | 0.78409 | 1.11669 | 0.93430 | 3.26897 | 12.6848 | 5.56 |
| 29 | 0.79970 | 1.12572 | 1.04414 | 3.274876 | 13.5029 | 6.13 |
| 30 | 0.82951 | 1.15402 | 1.03294 | 3.407978 | 12.3978 | 5.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.F. Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines. Chemistry 2023, 5, 2566-2587. https://doi.org/10.3390/chemistry5040166
Zayed MF. Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines. Chemistry. 2023; 5(4):2566-2587. https://doi.org/10.3390/chemistry5040166
Chicago/Turabian StyleZayed, Mohamed F. 2023. "Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines" Chemistry 5, no. 4: 2566-2587. https://doi.org/10.3390/chemistry5040166
APA StyleZayed, M. F. (2023). Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines. Chemistry, 5(4), 2566-2587. https://doi.org/10.3390/chemistry5040166






