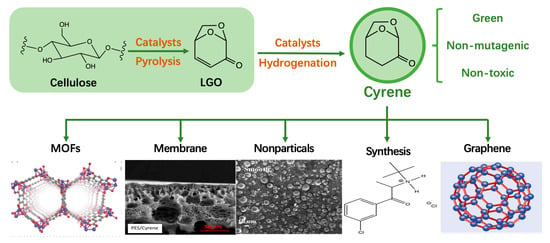
Preparation and Application of Green Sustainable Solvent Cyrene
Abstract
:1. Introduction
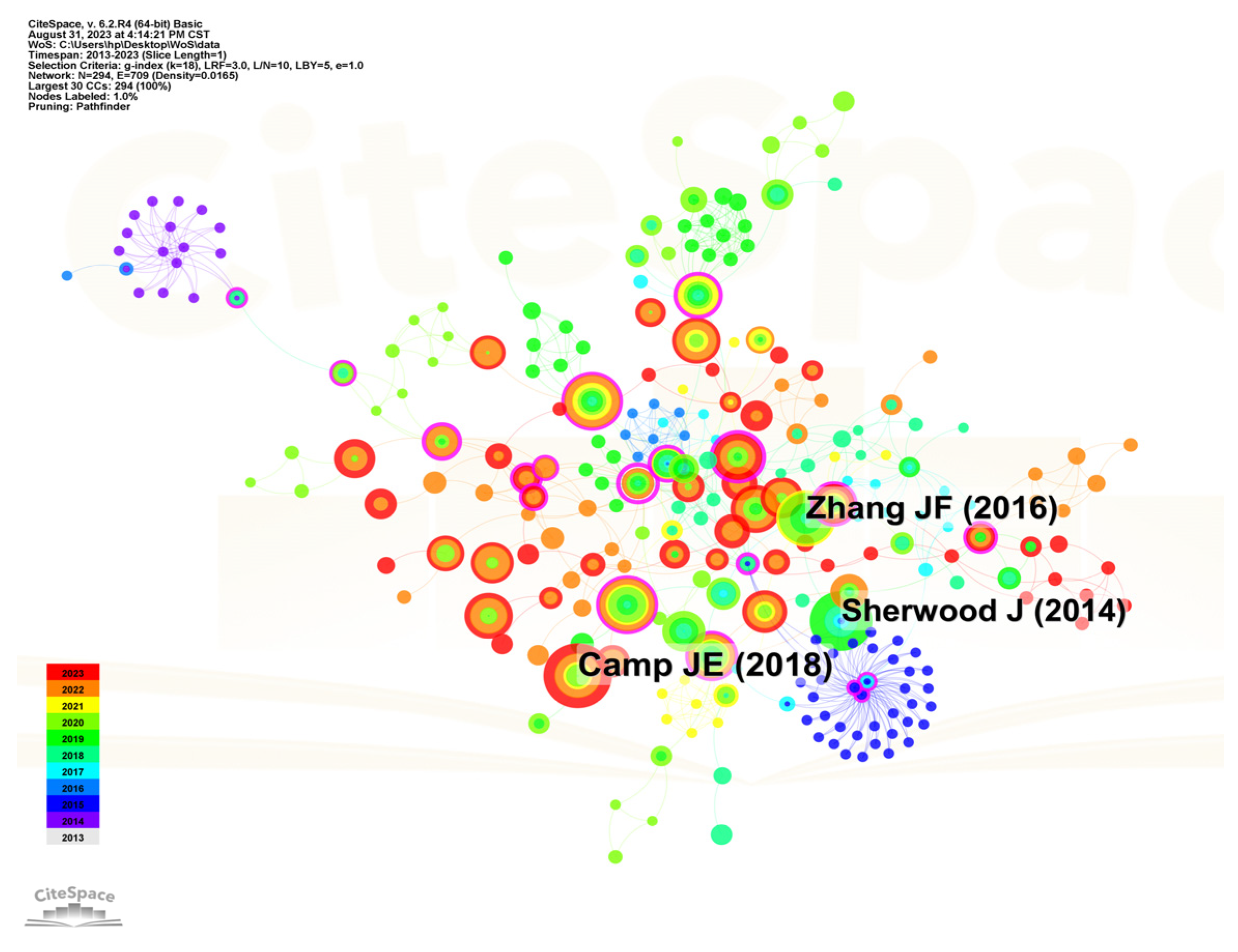
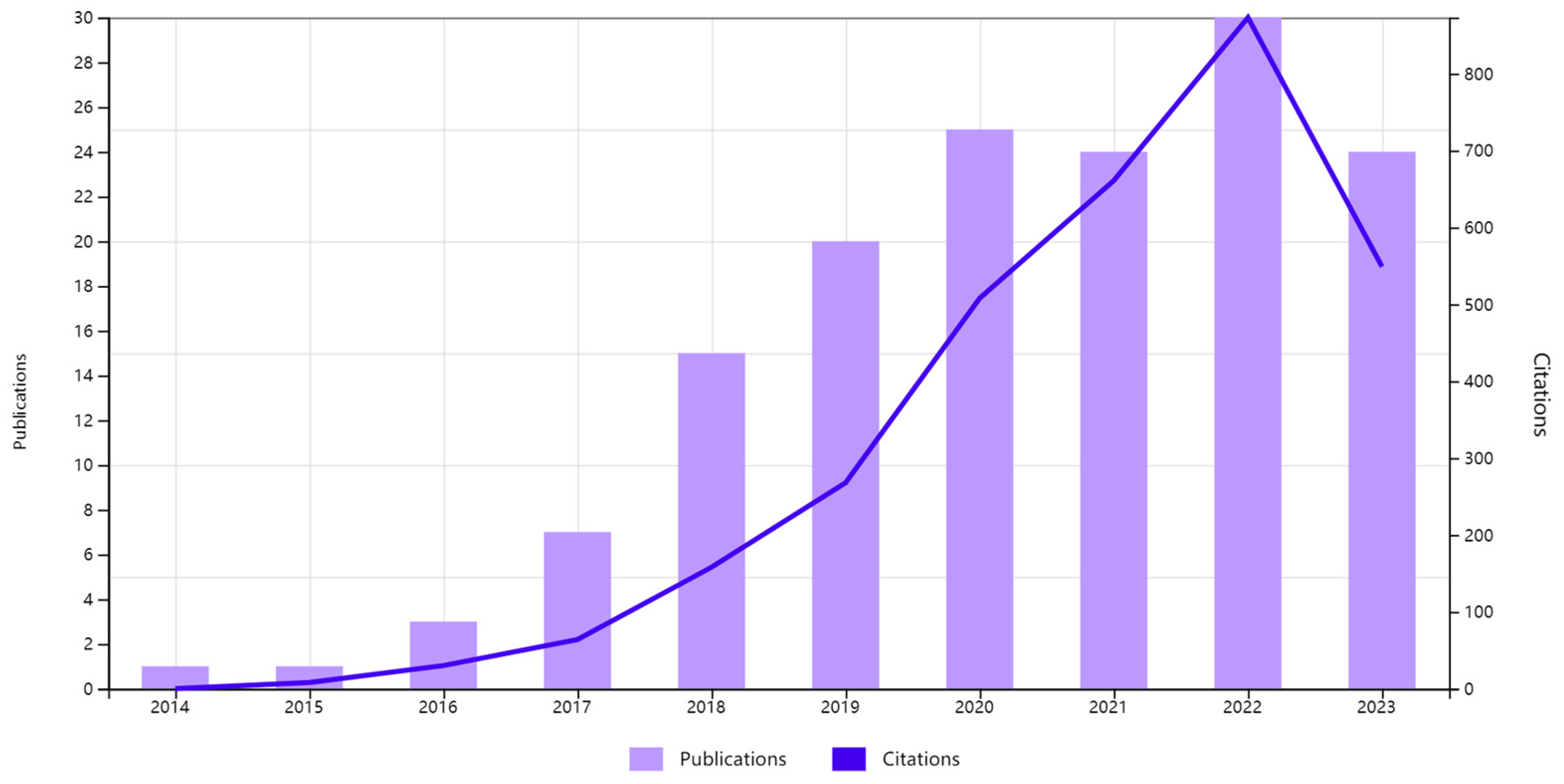
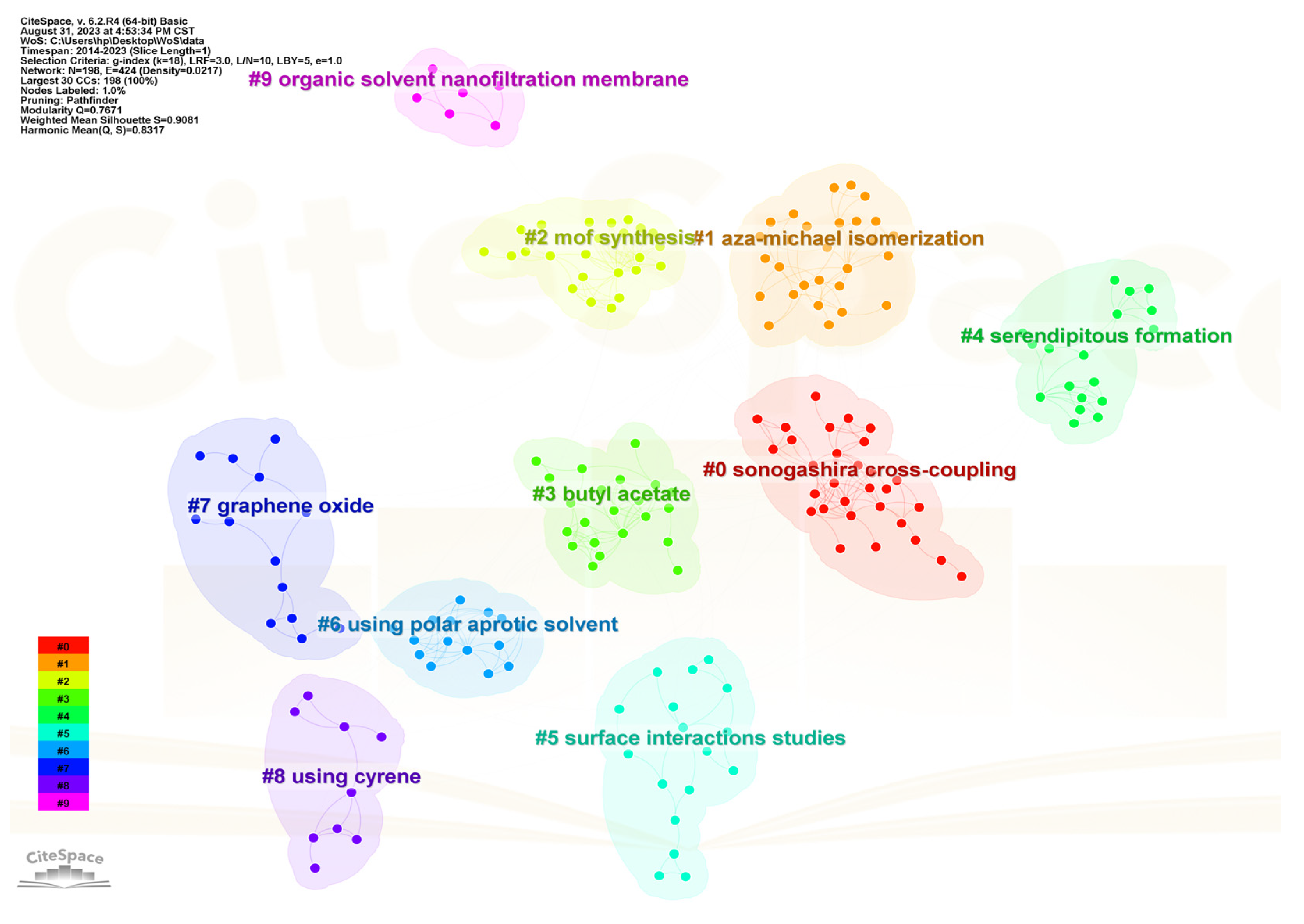
2. Literature Analysis Report
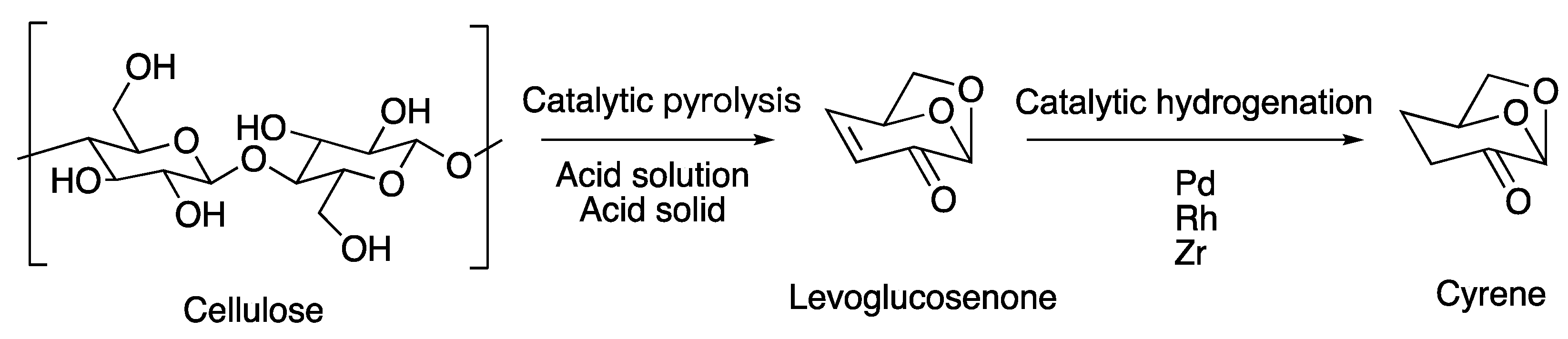
3. Preparation of Cyrene
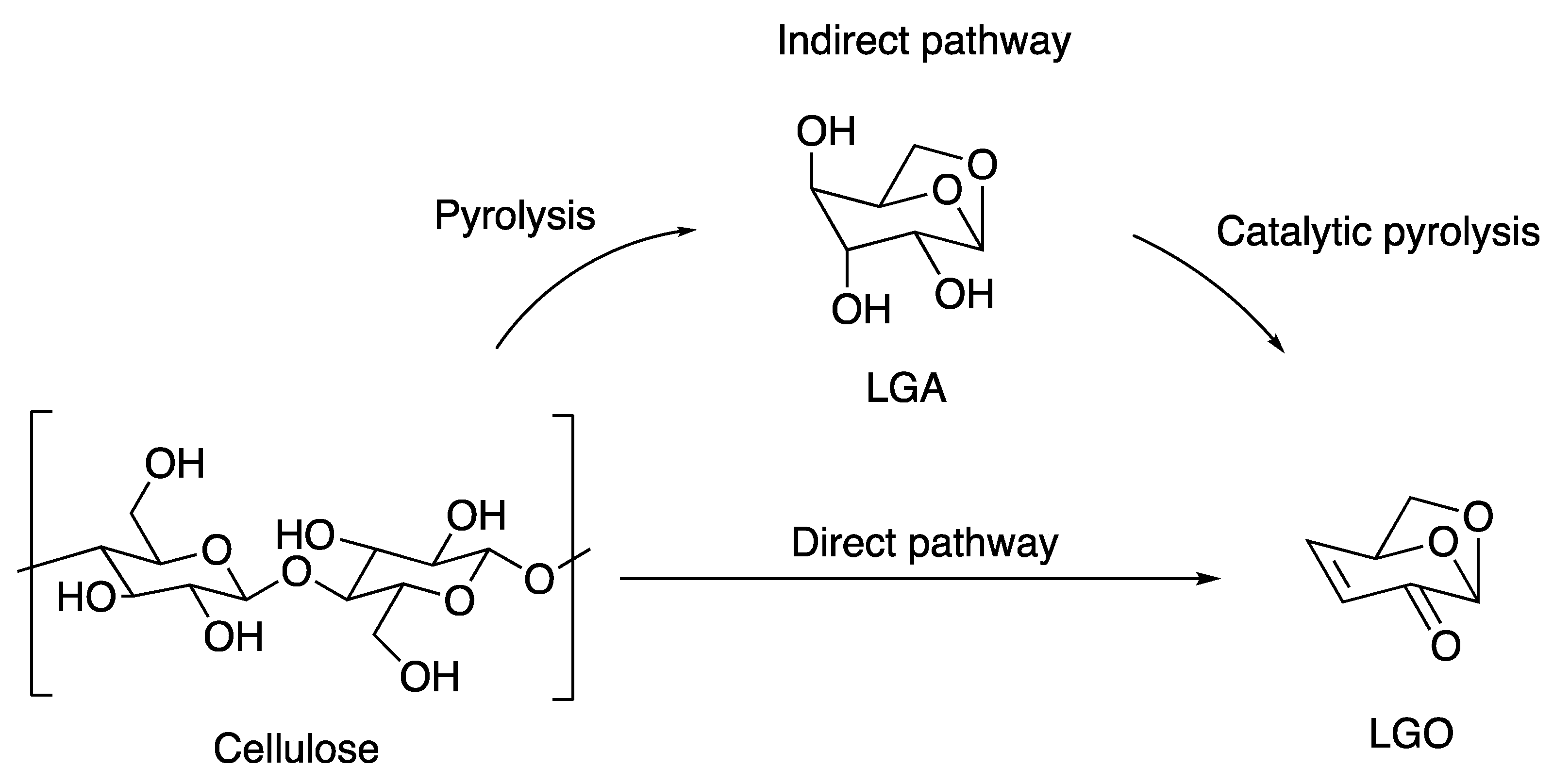
3.1. Preparation of LGO
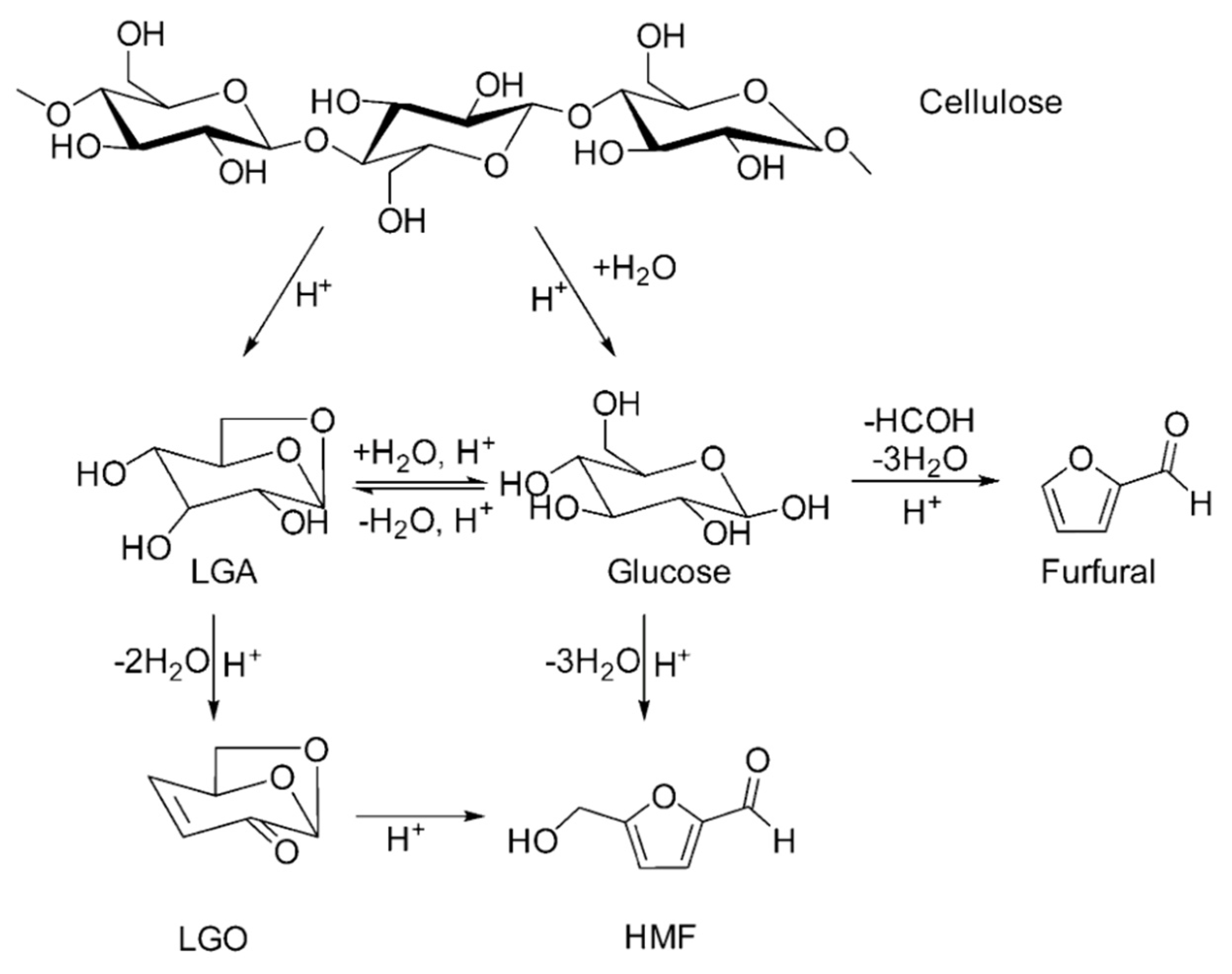
3.1.1. Acid Impregnation Followed by Pyrolysis to Produce LGO Directly
3.1.2. Indirect Generation of LGO Using Solid Catalysts
3.1.3. Indirect Generation of LGO by Pyrolysis in Liquid Phase
3.2. Catalytic Hydrogenation of LGO to Produce Cyrene
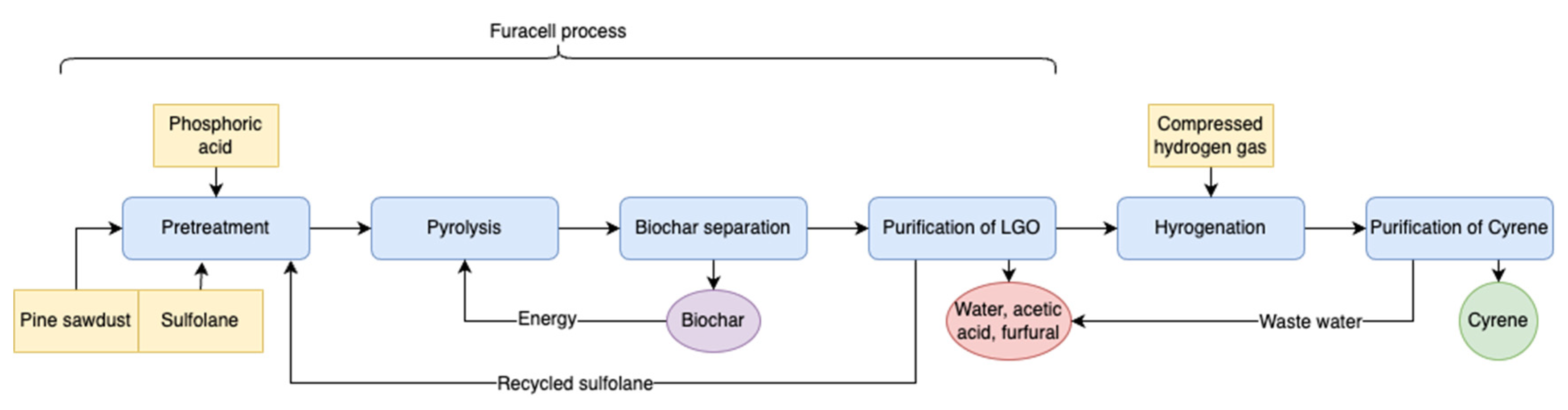
3.3. Furacell Process
4. Applications of Cyrene
4.1. Cyrene for the Synthesis of Materials
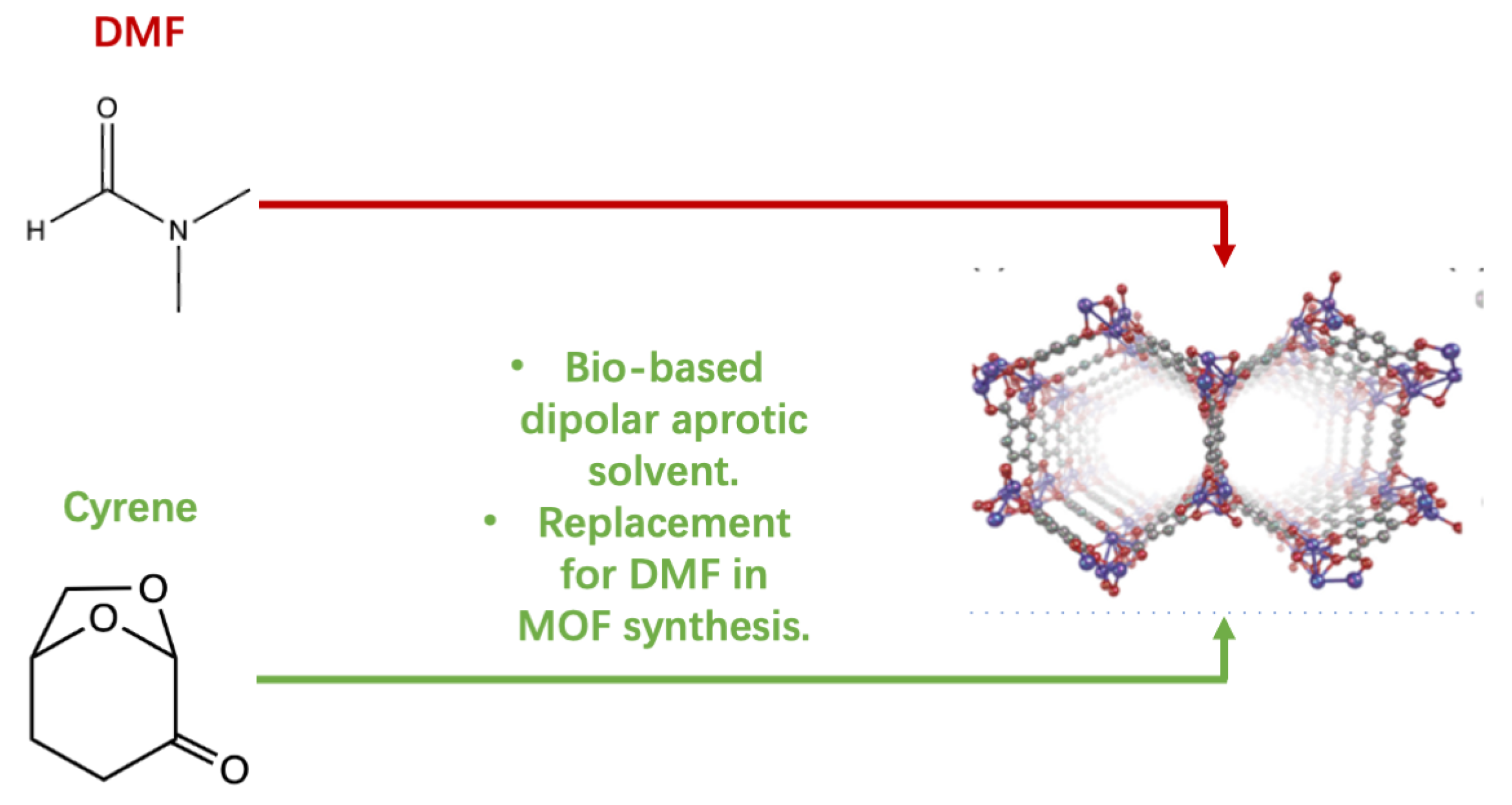
4.1.1. Cyrene in MOFs Synthesis
4.1.2. Cyrene for the Fabrication of Membranes
4.1.3. Cyrene for the Dispersion of Graphene and Carbon Nanotube
4.2. Cyrene for Extraction and Separation
4.3. Cyrene in Chemical Synthesis
4.3.1. Cyrene as a Reactant in Reactions
4.3.2. Cyrene as Replacement of Dipolar Aprotic Solvents in Reactions
4.3.3. Cyrene Application in the Biomedical Field
4.3.4. Cyrene Application in Biocatalysis
4.4. Cyrene for Non-Materials and Lithium-Ion Batteries Materials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sherwood, J.; De Bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunta, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A bio-based novel and sustainable solvent for organic synthesis. Green Chem. 2022, 24, 6435–6449. [Google Scholar]
- Camp, J.E. Bio-available Solvent Cyrene: Synthesis, Derivatization, and Applications. ChemSusChem 2018, 11, 3048–3055. [Google Scholar]
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101, 5303–5310. [Google Scholar] [PubMed]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Chen, C.; Leydesdorff, L. Patterns of connections and movements in dual-map overlays: A new method of publication portfolio analysis. J. Assoc. Inf. Sci. Technol. 2014, 65, 334–351. [Google Scholar]
- Zhang, J.F.; White, G.B.; Ryan, M.D.; Hunt, A.J.; Katz, M.J. Dihydrolevoglucosenone (Cyrene) As a Green Alternative to N,N-Dimethylformamide (DMF) in MOF Synthesis. ACS Sustain. Chem. Eng. 2016, 4, 7186–7192. [Google Scholar] [CrossRef]
- Chen, C. Science Mapping: A Systematic Review of the Literature. J. Data Inf. Sci. 2017, 2, 1–40. [Google Scholar]
- Kudo, S.; Huang, X.; Asano, S.; Hayashi, J. Catalytic Strategies for Levoglucosenone Production by Pyrolysis of Cellulose and Lignocellulosic Biomass. Energy Fuels 2021, 35, 9809–9824. [Google Scholar]
- Lu, Q.; Zhang, Y.; Dong, C.; Yang, Y.; Yu, H. The mechanism for the formation of levoglucosenone during pyrolysis of β-d-glucopyranose and cellobiose: A density functional theory study. J. Anal. Appl. Pyrolysis 2014, 110, 34–43. [Google Scholar]
- Assary, R.S.; Curtiss, L.A. Thermochemistry and Reaction Barriers for the Formation of Levoglucosenone from Cellobiose. ChemCatChem 2012, 4, 200–205. [Google Scholar]
- Halpern, Y.; Riffer, R.; Broido, A. Levoglucosenone (1,6-anhydro-3,4-dideoxy-.DELTA.3-.beta.-D-pyranosen-2-one). Major product of the acid-catalyzed pyrolysis of cellulose and related carbohydrates. J. Org. Chem. 1973, 38, 204–209. [Google Scholar]
- Dobele, G.; Rossinskaja, G.; Telysheva, G.; Meier, D.; Faix, O. Cellulose dehydration and depolymerization reactions during pyrolysis in the presence of phosphoric acid. J. Anal. Appl. Pyrolysis 1999, 49, 307–317. [Google Scholar]
- Dobele, G.; Meier, D.; Faix, O.; Radtke, S.; Rossinskaja, G.; Telysheva, G. Volatile products of catalytic flash pyrolysis of celluloses. J. Anal. Appl. Pyrolysis 2001, 58–59, 453–463. [Google Scholar] [CrossRef]
- Dobele, G.; Dizhbite, T.; Rossinskaja, G.; Telysheva, G.; Meier, D.; Radtke, S.; Faix, O. Pre-treatment of biomass with phosphoric acid prior to fast pyrolysis: A promising method for obtaining 1,6-anhydrosaccharides in high yields. J. Anal. Appl. Pyrolysis 2003, 68–69, 197–211. [Google Scholar] [CrossRef]
- Dobele, G.; Rossinskaja, G.; Dizhbite, T.; Telysheva, G.; Meier, D.; Faix, O. Application of catalysts for obtaining 1,6-anhydrosaccharides from cellulose and wood by fast pyrolysis. J. Anal. Appl. Pyrolysis 2005, 74, 401–405. [Google Scholar] [CrossRef]
- Sarotti, A.M.; Spanevello, R.A.; Suárez, A.G. An efficient microwave-assisted green transformation of cellulose into levoglucosenone. Advantages of the use of an experimental design approach. Green Chem. 2007, 9, 1137–1140. [Google Scholar]
- Kudo, S.; Zhou, Z.; Norinaga, K.; Hayashi, J. Efficient levoglucosenone production by catalytic pyrolysis of cellulose mixed with ionic liquid. Green Chem. 2011, 13, 3306–3311. [Google Scholar]
- Zandersons, J.; Zhurinsh, A.; Dobele, G.; Jurkjane, V.; Rizhikovs, J.; Spince, B.; Pazhe, A. Feasibility of broadening the feedstock choice for levoglucosenone production by acid pre-treatment of wood and catalytic pyrolysis of the obtained lignocellulose. J. Anal. Appl. Pyrolysis 2013, 103, 222–226. [Google Scholar]
- Dobele, G.; Zhurinsh, A.; Volperts, A.; Jurkjane, V.; Pomilovskis, R.; Meile, K. Study of levoglucosenone obtained in analytical pyrolysis and screw-type reactor, separation and distillation. Wood Sci. Technol. 2020, 54, 383–400. [Google Scholar]
- Fan, J.; De Bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.; Macquarrie, D.J.; Clark, J.H. Direct Microwave-Assisted Hydrothermal Depolymerization of Cellulose. J. Am. Chem. Soc. 2013, 135, 11728–11731. [Google Scholar] [PubMed]
- Bouxin, F.P.; Clark, J.H.; Fan, J.; Budarin, V. Combining steam distillation with microwave-assisted pyrolysis to maximise direct production of levoglucosenone from agricultural wastes. Green Chem. 2019, 21, 1282–1291. [Google Scholar]
- Casoni, A.I.; Nievas, M.L.; Moyano, E.L.; Álvarez, M.; Diez, A.; Dennehy, M.; Volpe, M.A. Catalytic pyrolysis of cellulose using MCM-41 type catalysts. Appl. Catal. A Gen. 2016, 514, 235–240. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Q.; Zhu, X.-F.; Zhang, Y. Catalytic Fast Pyrolysis of Cellulose to Prepare Levoglucosenone Using Sulfated Zirconia. ChemSusChem 2011, 4, 79–84. [Google Scholar]
- Wei, X.; Wang, Z.; Wu, Y.; Yu, Z.; Jin, J.; Wu, K. Fast pyrolysis of cellulose with solid acid catalysts for levoglucosanone. J. Anal. Appl. Pyrolysis 2014, 107, 150–154. [Google Scholar] [CrossRef]
- Lu, Q.; Ye, X.; Zhang, Z.; Dong, C.; Zhang, Y. Catalytic fast pyrolysis of cellulose and biomass to produce levoglucosenone using magnetic SO42−/TiO2–Fe3O4. Bioresour. Technol. 2014, 171, 10–15. [Google Scholar]
- Zhang, Z.; Lu, Q.; Ye, X.; Wang, T.; Wang, X.; Dong, C. Selective Production of Levoglucosenone from Catalytic Fast Pyrolysis of Biomass Mechanically Mixed with Solid Phosphoric Acid Catalysts. Bioenergy Res. 2015, 8, 1263–1274. [Google Scholar]
- Li, K.; Wang, B.; Bolatibieke, D.; Nan, D.-H.; Zhang, Z.-X.; Cui, M.-S.; Lu, Q. Catalytic fast pyrolysis of biomass with Ni-P-MCM-41 to selectively produce levoglucosanone. J. Anal. Appl. Pyrolysis 2020, 148, 104824. [Google Scholar]
- Li, Y.; Hu, B.; Fu, H.; Wu, Y.; Zhang, Z.; Liu, J.; Zhang, B.; Lu, Q. Catalytic Fast Pyrolysis of Cellulose for the Selective Production of Levoglucosenone Using Phosphorus Molybdenum Tin Mixed Metal Oxides. Energy Fuels 2022, 36, 10251–10260. [Google Scholar] [CrossRef]
- Cao, F.; Schwartz, T.J.; McClelland, D.J.; Krishna, S.H.; Dumesic, J.A.; Huber, G.W. Dehydration of cellulose to levoglucosenone using polar aprotic solvents. Energy Environ. Sci. 2015, 8, 1808–1815. [Google Scholar]
- Kawamoto, H.; Saito, S.; Hatanaka, W.; Saka, S. Catalytic pyrolysis of cellulose in sulfolane with some acidic catalysts. J. Wood Sci. 2007, 53, 127–133. [Google Scholar] [CrossRef]
- Lusi, A.; Radhakrishnan, H.; Hu, H.; Hu, H.; Bai, X. Plasma electrolysis of cellulose in polar aprotic solvents for production of levoglucosanone. Green Chem. 2020, 22, 7871–7883. [Google Scholar]
- Huang, X.; Liu, T.; Wang, J.; Wei, F.; Ran, J.; Kudo, S. Selective hydrogenation of levoglucosenone over Pd/C using formic acid as a hydrogen source. J. Energy Inst. 2020, 93, 2505–2510. [Google Scholar] [CrossRef]
- Mazarío, J.; Romero, M.P.; Concepción, P.; Chávez-Sifontes, M.; Spanevello, R.A.; Comba, M.B.; Suárez, A.G.; Domine, M.E. Tuning zirconia-supported metal catalysts for selective one-step hydrogenation of levoglucosanone. Green Chem. 2019, 21, 4769–4785. [Google Scholar] [CrossRef]
- Mouterde, L.M.M.; Allais, F.; Stewart, J.D. Enzymatic reduction of levoglucosenone by an alkene reductase (OYE 2.6): A sustainable metal- and dihydrogen-free access to the bio-based solvent Cyrene (R). Green Chem. 2018, 20, 5528–5532. [Google Scholar] [CrossRef]
- Byrne, F.; Forier, B.; Bossaert, G.; Hoebers, C.; Farmer, T.J.; Clark, J.H.; Hunt, A.J. 2,2,5,5-Tetramethyltetrahydrofuran (TMTHF): A non-polar, non-peroxide forming ether replacement for hazardous hydrocarbon solvents. Green Chem. 2017, 19, 3671–3678. [Google Scholar] [CrossRef]
- Milescu, R.A.; Segatto, M.L.; Stahl, A.; McElroy, C.R.; Farmer, T.J.; Clark, J.H.; Zuin, V.G. Sustainable Single-Stage Solid–Liquid Extraction of Hesperidin and Rutin from Agro-Products Using Cyrene. ACS Sustain. Chem. Eng. 2020, 8, 18245–18257. [Google Scholar] [CrossRef]
- Richardson, D.E.; Raverty, W.D. Predicted environmental effects from liquid emissions in the manufacture of levoglucosenone and Cyrene (TM). Appita 2016, 69, 344–351. [Google Scholar]
- Court, G.R.; Lawrence, C.H.; Raverty, W.D.; Duncan, A.J. Method for Converting Lignocellulosic Materials into Useful Chemicals. U.S. Patent 2012/0111714 A1, 6 January 2012. [Google Scholar]
- El-Sayed, E.S.; Yuan, D. Waste to MOFs: Sustainable linker, metal, and solvent sources for value-added MOF synthesis and applications. Green Chem. 2020, 22, 4082. [Google Scholar] [CrossRef]
- Bhindi, M.; Massengo, L.; Hammerton, J.; Derry, M.J.; Worrall, S.D. Structure Control Using Bioderived Solvents inElectrochemical Metal-OrganicFramework Synthesis. Appl. Sci 2023, 13, 720. [Google Scholar] [CrossRef]
- Škrjanc, A.; Byrne, C.; Logar, N.Z. Green Solvents as an Alternative to DMF in ZIF-90 Synthesis. Molecules 2021, 26, 1573. [Google Scholar]
- Marinoa, T.; Galianoa, F.; Molinob, A.; Figol, A. New frontiers in sustainable membrane preparation: CyreneTM as green bioderived solvent. J. Membr. Sci. 2019, 580, 224–234. [Google Scholar] [CrossRef]
- Milescu, R.A.; McElroy, C.R.; Farmer, T.J.; Williams, P.M.; Walters, M.J.; Clark, J.H. Fabrication of PES/PVP water filtration membranes using Cyrene®, a safer bio-based polar aprotic solvent. Adv. Polym. Technol. 2019, 15, 9692859. [Google Scholar] [CrossRef]
- Milescu, R.A.; Zhenova, A.; Vastano, M.; Gammons, R.; Lin, S.; Lau, C.H.; Clark, J.H.; McElroy, C.R.; Pellis, A. Polymer Chemistry Applications of Cyrene and its Derivative Cygnet 0.0 as Safer Replacements for Polar Aprotic Solvents. ChemSusChem 2021, 14, 3367–3381. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Izzati Ayob, N.A.; Blanford, C.F.; Mohammad Rawi, N.F.; Szekely, G. Nonwoven Membrane supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Fei, F.; Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Tailoring the Performance of Organic Solvent Nanofiltration Membranes with Biophenol Coatings. ACS Appl. Polym. Mater. 2019, 1, 452–460. [Google Scholar] [CrossRef]
- Carner, C.A.; Croft, C.F.; Kolev, S.D.; Almeida, M.I.G.S. Green solvents for the fabrication of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2020, 239, 116486. [Google Scholar] [CrossRef]
- Foong, Y.X.; Yew, L.H.; Chai, P.V. Green approaches to polysulfone based membrane reparation via dimethyl sulfoxide and eco-friendly natural additive gum Arabic. Mater. Today Proc. 2021, 46, 2092–2097. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Leaper, S.; Shokri, J.; Alberto, M.; Gorgojo, P. Effect of graphene oxide in the formation of polymeric asymmetric membranes via phase inversion. J. Membr. Sci. 2022, 641, 119924. [Google Scholar] [CrossRef]
- Tomietto, P.; Russo, F.; Galiano, F.; Loulergue, P.; Salerno, S.; Paugam, L.; Audic, J.L.; De Bartolo, L.; Figoli, A. Sustainable fabrication and pervaporation application of bio-based membranes: Combining a polyhydroxyalkanoate (PHA) as biopolymer and CyreneTM as green solvent. J. Membr. Sci. 2022, 643, 120061. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Sherwood, J.; Budarin, V.L.; Ellis, G.J.; Clark, J.H.; Shuttleworth, P.S. Identification of high performance solvents for the sustainable processing of graphene. Green Chem. 2017, 19, 2550. [Google Scholar] [CrossRef]
- Pan, K.W.; Fan, Y.Y.; Hu, Z.R.; Leng, T.; Li, J.; Xin, Z.Y.; Zhang, J.W.; Hao, L.; Gallop, J.; Novoselov, K.S. Sustainable production of highly conductive multilayer graphene ink for wireless connectivity and IoT applications. Nat. Commun. 2018, 9, 5197–5206. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Motealleh, A.; Costa, C.M.; Hilliou, L.; Perinka, N.; Ribeiro, C.; Viana, J.C.; Costa, P.; Mendez, S.L. Environmentally Friendly Conductive Screen-Printable Inks Based on N-Doped Graphene and Polyvinylpyrrolidone. Adv. Eng. Mater. 2022, 24, 2101258. [Google Scholar] [CrossRef]
- Yang, J.L.; Ling, K.; Liu, L.H.; Zeng, X.H.; Xu, X.W.; Li, Z.L.; He, P. Printable and Wearable Graphene-Based Strain Sensor With High Sensitivity for Human Motion Monitoring. IEEE Sens. J. 2022, 22, 14. [Google Scholar] [CrossRef]
- Tkachev, S.; Monteiro, M.; Santos, J.; Placidi, E.; Hassine, M.B.; Marques, P.; Ferreira, P.; Alpuim, P.; Capasso, A. Environmentally Friendly Graphene Inks for Touch Screen Sensors. Adv. Funct. Mater. 2021, 31, 3287. [Google Scholar] [CrossRef]
- Fernandes, J.; Nemala, S.S.; Bellis, G.D.; Capasso, A. Green Solvents for the Liquid Phase Exfoliation Production of Graphene: The Promising Case of Cyrene. Front. Chem. 2022, 10, 878799. [Google Scholar] [CrossRef]
- Poon, R.; Zhitomirsky, I. Application of Cyrene as a solvent and dispersing agent for fabrication of Mn3O4-carbon nanotube supercapacitor electrodes. Colloid Interface Sci. 2020, 34, 100226. [Google Scholar] [CrossRef]
- Das, N.K.; Mishra, D.K.; Banerjee, T.; Naik, P.K.; Dehury, P.; Bose, S.; Banerjee, T. Dihydrolevoglycosenone as a novel bio-based nanofluid for thermal energy storage: Physiochemical and quantum chemical insights. J. Energy Storage 2023, 59, 106365. [Google Scholar] [CrossRef]
- Meng, X.Z.; Pu, Y.Q.; Ragauskas, A.J.; Lic, M.; Ragauskas, A.J. A biomass pretreatment using cellulose-derived solvent Cyrene. Green Chem. 2020, 22, 2862. [Google Scholar] [CrossRef]
- Abranches, D.O.; Benfica, J.; Coutinho, J.A.P.; Abranches, D.O.; Shimizu, J.B.S.; Coutinho, J.A.P. Solubility Enhancement of Hydrophobic Substances in Water/Cyrene Mixtures: A Computational Study. Ind. Eng. Chem. Res. 2020, 59, 18247–18253. [Google Scholar] [CrossRef]
- Mohan, M.; Sale, K.L.; Kalb, R.S.; Simmons, B.A.; Gladden, J.M.; Singh, S. Multiscale Molecular Simulation Strategies for Understanding the Delignification Mechanism of Biomass in Cyrene. ACS Sustain. Chem. Eng. 2022, 10, 11016–11029. [Google Scholar] [CrossRef]
- Duval, A.; Avérous, L. Dihydrolevoglucosenone (Cyrene™) as a versatile biobased solvent for lignin fractionation, processing, and chemistry. Green Chem. 2022, 24, 338–349. [Google Scholar] [CrossRef]
- Elhami, V.; Beek, N.; Wang, L.S.; Picken, S.J.; Tamis, J.; Sousa, J.A.B.; Hempenius, M.A.; Schuur, B. Extraction of low molecular weight polyhydroxyalkanoates from mixed microbial cultures using bio-based solvents. Sep. Purif. Technol. 2022, 299, 121773. [Google Scholar] [CrossRef]
- Yin, X.Y.; Cai, T.T.; Liu, C.; Jiang, J.C.; Wang, K. Directional Decomposition of Bamboo Powder in Bio-based Polar Aprotic Solvent / Aqueous p-Toluenesulfonic Acid Coupling Systems. Chem. Ind. For. Prod. 2022, 1, 0253–2417. [Google Scholar]
- Yin, X.Y.; Cai, T.T.; Wang, K. A novel solvothermal biorefinery for production of lignocellulosic xylooligosaccharides, fermentable sugars and lignin nano-particles in biphasic system. Carbohydr. Polym. 2022, 295, 119901. [Google Scholar] [CrossRef]
- Kisanthiaa, R.; Huntb, A.J.; Sherwoodc, J.; Somsakeesitd, L.; Phaosiri, C. Impact of conventional and sustainable solvents on the yield, selectivity and recovery of curcuminoids from turmeric. ACS Sustain. Chem. Eng. 2021, 10, 104–114. [Google Scholar] [CrossRef]
- Arefmanesh, M.; Nikafshar, S.; Master, E.R.; Nejad, M. From acetone fractionation to lignin-based phenolic and polyurethane resins. Ind. Crops Prod. 2022, 178, 114604. [Google Scholar] [CrossRef]
- Brouwer, T.; Schuur, B. Dihydrolevoglucosenone (Cyrene), a Biobased Solvent for Liquid-Liquid Extraction Applications. ACS Sustain. Chem. Eng. 2020, 8, 14807–14817. [Google Scholar] [CrossRef]
- Brouwer, T. Schuur, Comparison of solvent-based affinity separation processes using Cyrene and Sulfolane for aromatic/aliphatic separations. J. Chem. Tech. Biotech. 2021, 96, 2630–2646. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Vovers, J.; Lu, H.T.; Stevens, G.W.; Mumford, K.A. Investigation of green solvents for the extraction of phenol and natural alkaloids: Solvent and extractant selection. Chem. Eng. J. 2022, 442, 136054. [Google Scholar] [CrossRef]
- Wilson, K.L.; Kennedy, A.R.; Murray, J.; Greatrex, B.; Jamieson, C.; Watson, A.J.B. Scope and limitations of a DMF bio-alternative within Sonogashira cross-coupling and Cacchi-type annulation. Org. Chem. 2016, 12, 2005–2011. [Google Scholar]
- Alhifthi, A.; Harris, B.L.; Goerigk, L.; White, J.M.; Williams, S.J. Structure–reactivity correlations of the abnormal Beckmann reaction of dihydrolevoglucosenone oxime. Org. Biomol. Chem. 2017, 15, 10105–10115. [Google Scholar] [CrossRef]
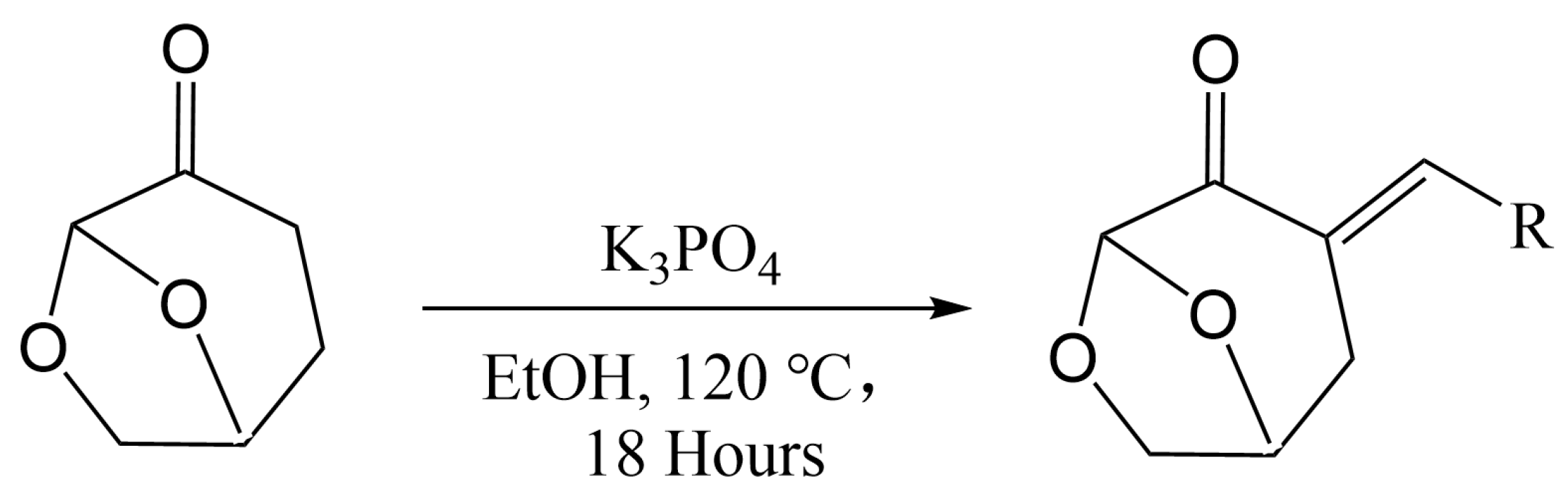
- Hughes, L.; McElroy, C.R.; Whitwood, A.C.; Hun, A.J. Development of pharmaceutically relevant bio-based intermediates though aldol condensation and Claisen-Schmidt reactions of dihydrolevoglucosenone (Cyrene®). Green Chem. 2018, 20, 4423–4427. [Google Scholar] [CrossRef]
- Bonneau, G.; Peru, A.M.; Flourat, A.L.; Allais, F. Organic solvent- and catalyst-free Baeyer–Villiger oxidation of levoglucosenone and dihydrolevoglucosenone (Cyrene®): A sustainable route to (S)-γ-hydroxymethyl-α,β-butenolide and (S)-γ-hydroxymethyl-γ-butyrolactone. Green Chem. 2018, 20, 2455–2458. [Google Scholar] [CrossRef]
- Bruyn, M.D.; Sener, C.; Petrolini, D.D.; McClelland, D.J.; He, J.Y.; Ball, M.R.; Liu, Y.F.; Martins, L.; Dumesic, J.A.; Huber, G.W.; et al. Catalytic hydrogenation of dihydrolevoglucosenone to levoglucosanol with a hydrotalcite/mixed oxide copper catalyst. Green Chem. 2019, 21, 5000–5007. [Google Scholar] [CrossRef]
- Klepp, J.; Sumby, C.J.; Greatrex, B.W. Synthesis of a Chiral Auxiliary Family from Levoglucosenone and Evaluation in the Diels-Alder Reaction. Synlett 2018, 29, 1441–1446. [Google Scholar]
- Klepp, J.; Podversnik, H.; Puschnig, J.; Greatex, B.W. Diastereoselective sulfa-Michael reactions controlled by a biomassderived chiral auxiliary. Tetrahedron Lett. 2019, 75, 3894–3903. [Google Scholar] [CrossRef]
- Sharipov, B.T.; Davydova, A.N.; Faizullina, L.K.; Valeev, F.A. Preparation of the diastereomerically pure 2S-hydroxy derivative of dihydrolevoglucosenone (cyrene). Mendeleev Commun. 2019, 29, 200–202. [Google Scholar] [CrossRef]
- Martinho, L.A.; Rosalba, T.P.F.; Sousa, G.G.; Gatto, C.C.; Politi, J.R.S.; Andrade, C.K.Z. Cyrene: A very reactive bio-based chiral ketone in diastereoselective Passerini reactions. Mol. Divers. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hohol, R.E.; Arcure, H.; Witczak, Z.J.; Bielski, R.; Kirschbaum, K.; Andreana, P.; Mencer, D.E. One-pot synthesis of carbohydrate exo-cyclic enones and hemiketals with 6,8-dioxabicyclo- [3.2.1] octane moieties. Serendipitous formation of a spironolactone when 2-pyridinecarboxaldehyde is used as the reactant. Part II*. Tetrahedron Lett. 2018, 74, 7303–7309. [Google Scholar] [CrossRef]
- Ray, P.; Hughes, T.; Smith, C.; Hibbert, M.; Saito, K.; Simon, G.P. Development of bio-acrylic polymers from Cyrene™: Transforming a green solvent to a green polymer. Polym. Chem. 2019, 10, 3334–3341. [Google Scholar] [CrossRef]
- Néant, F.D.; Mouterde, L.; Fadlallah, S.; Miller, S.A.; Allais, F. Sustainable Synthesis and Polycondensation of Levoglucosenone-Cyrene-Based Bicyclic Diol Monomer: Access to Renewable Polyesters. ChemSusChem 2020, 10, 1002. [Google Scholar]
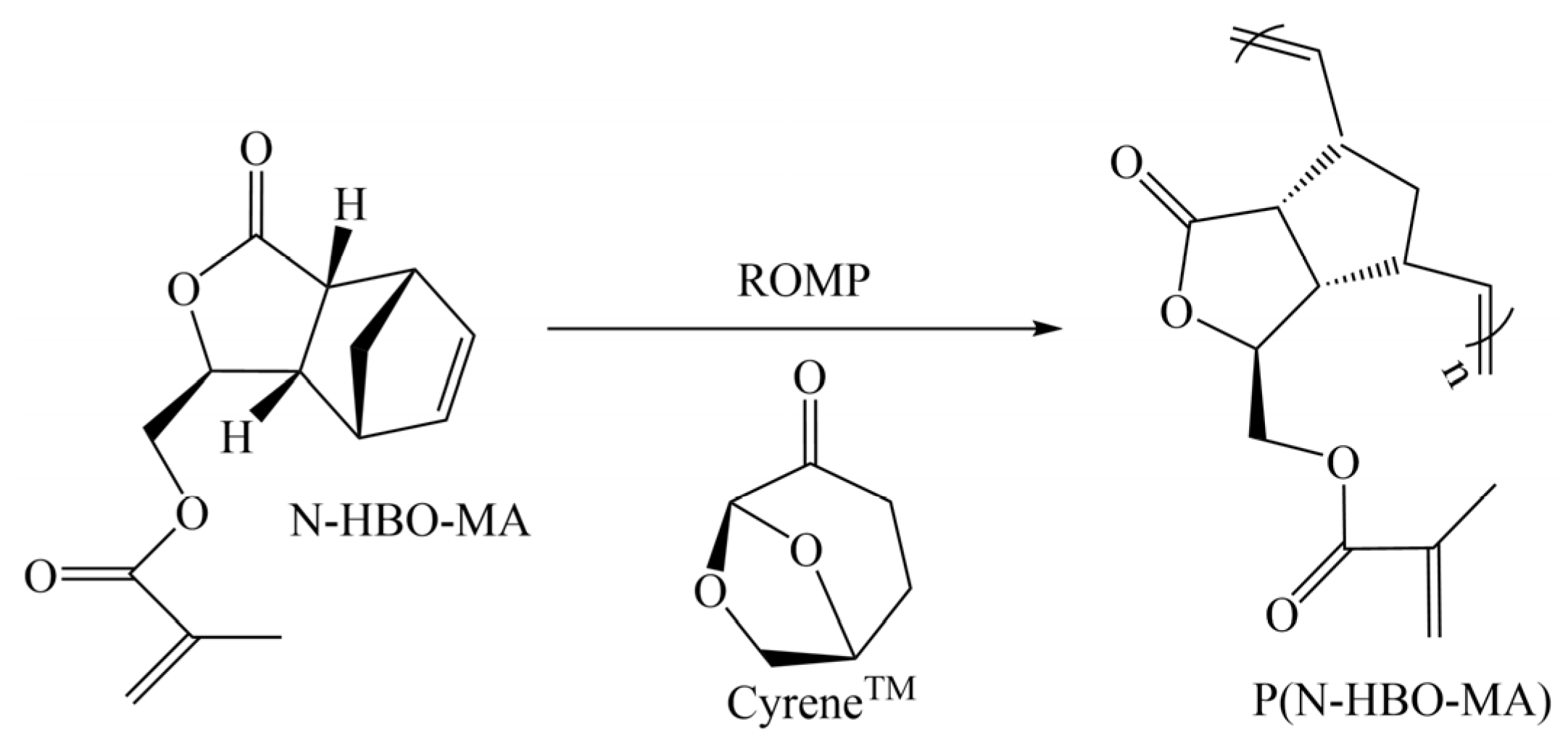
- Fadlallah, S.; Peru, A.A.M.; Allais, L.L.F. Chemo-enzymatic synthesis of a levoglucosenone-derived bi-functional monomer and its ring-opening metathesis polymerization in the green solvent CyreneTM. Polym. Chem. 2020, 11, 7471. [Google Scholar] [CrossRef]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A bio-based solvent for the Mizoroki–Heck reaction of aryl iodides. Org. Biomol. Chem. 2023, 21, 351. [Google Scholar] [CrossRef] [PubMed]
- Galaverna, R.S.; Fernandes, L.P.; Silva, V.H.M.; Siervo, A.; Pastre, J.C. Humins-Like Solid Support for Palladium Immobilization: Highly Efficient and Recyclable Catalyst for Cross-Coupling eactions. Org. Chem. 2022, 24, 47–57. [Google Scholar] [CrossRef]
- Ferrazzano, L.; Martelli, G.; Fantoni, T.; Daka, A.; Corbisiero, D.; Viola, A.; Ricci, A.; Cabri, W.; Tolomelli, A. Fast Heck-Cassar-Sonogashira (HCS) Reactions in Green Solvents. Org. Lett. 2020, 22, 3969–3973. [Google Scholar] [CrossRef]
- Mistry, L.; Mapesa, K.; Bousfield, T.W.; Camp, J.E. Synthesis of Ureas in the Bio-alternative Solvent Cyrene. Green Chem. 2017, 19, 2123–2128. [Google Scholar] [CrossRef]
- Wilson, K.L.; Murray, J.; Jamieson, C.; Watson, A.J.B. Cyrene as a Bio-Based Solvent for the Suzuki-Miyaura Cross Coupling. Synlett 2018, 29, 650–654. [Google Scholar]
- Sherwood, J. Suzuki—Miyaura cross coupling is not an informative reaction to demonstrate the performance of new solvents. Org. Chem. 2020, 16, 1001–1005. [Google Scholar] [CrossRef]
- Mention, M.M.; Flourat, A.L.; Peyrot, C.; Allais, F. Biomimetic regioselective and high-yielding Cu(I)-catalyzed dimerization of sinapate esters in green solvent Cyrene™: Towards sustainable antioxidant and anti-UV ingredients. Green Chem. 2020, 22, 2077–2085. [Google Scholar] [CrossRef]
- Marathianos, A.; Liarou, E.; Hancox, E.; Grace, J.L.; Haddleton, D.M. Dihydrolevoglucosenone (Cyrene™) as a biorenewable solvent for Cu(0)wire-mediated reversible deactivation radical polymerization (RDRP) without external deoxygenation. Green Chem. 2020, 22, 5833. [Google Scholar] [CrossRef]
- Veerabagu, U.; Jaikumar, G.; Lu, F.S.; Quero, F. High yield and greener C-H difluoromethylation reactions using copper iodide nanoparticles/boron nitride nanosheets as versatile and recyclable heterogeneous catalyst. React. Chem. Eng. 2021, 6, 1900–1910. [Google Scholar] [CrossRef]
- Nickisch, R.; Conen, P.; Gabrielsen, S.M.; Meier, M.A.R. A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur. RSC Adv. 2021, 11, 3134–3142. [Google Scholar] [CrossRef]
- Sangon, S.; Supanchaiyamat, N.; Sherwood, J.; McElroy, C.R.; Hunt, A.J. Direct comparison of safer or sustainable alternative dipolar aprotic solvents for use in carbon-carbon bond formation. Chem. Eng. 2020, 5, 1798. [Google Scholar] [CrossRef]
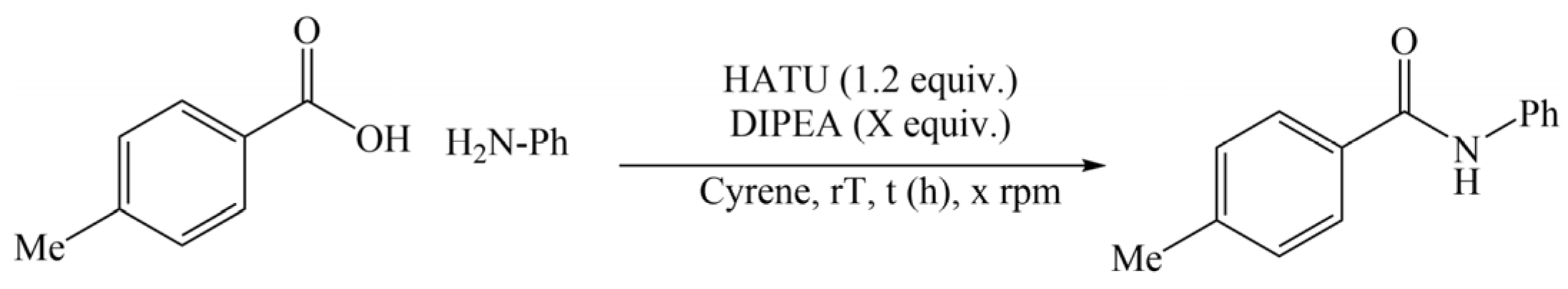
- Wilson, K.L.; Murray, J.; Jamieson, C.; Watson, A.J.B. Cyrene as a bio-based solvent for HATU mediated amide coupling. Org. Biomol. Chem. 2018, 16, 2851–2854. [Google Scholar] [CrossRef]
- Bousfield, T.W.; Pearcea, K.P.R.; Nyaminia, S.B.; Dimakisa, A.A.; Camp, J.E. Synthesis of Amides from Acid Chlorides and Amines in the Bio-based Solvent CyreneTM. Green Chem. 2019, 21, 3675–3681. [Google Scholar] [CrossRef]
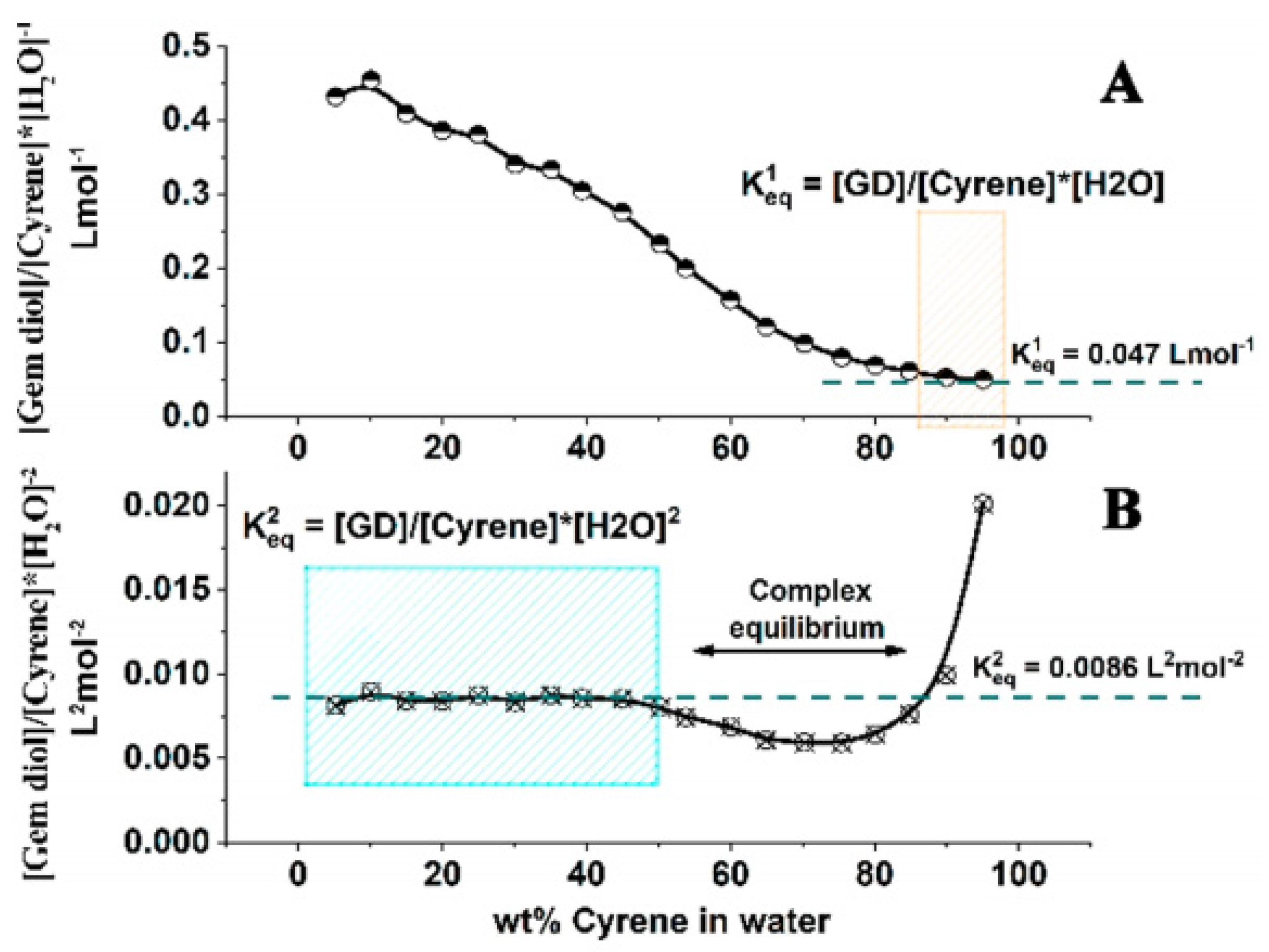
- De Bruyn, M.; Budarin, V.L.; Misefari, A.; Shimizu, S.; Fish, H.; Cockett, M.; Hunt, A.J.; Hofstetter, H.; Weckhuysen, B.M.; Clark, J.H. Geminal Diol of Dihydrolevoglucosenone as a Switchable Hydrotrope: A Continuum of Green Nanostructured Solvents. ACS Sustain. Chem. Eng. 2019, 7, 7878–7883. [Google Scholar] [CrossRef]
- Abranches, D.O.; Benfica, J.; Shimizu, S.; Coutinho, J.A. The Perspective of Cooperative Hydrotropy on the Solubility in Aqueous Solutions of Cyrene. Ind. Eng. Chem. Res. 2020, 10, 0888–5885. [Google Scholar] [CrossRef]
- Chen, Y.; Terazono, Y.C.; Fefer, M.; Liu, J.; Gale, C.B.; Brook, M.A. A simple route to photodynamic chlorin e6 amide derivatives. J. Porphyr. Phthalocyanines 2022, 26, 56–64. [Google Scholar] [CrossRef]
- Lawrenson, S.; North, M.; Peigneguy, F.; Routledge, A. Greener solvents for solid-phase synthesis. Green Chem. 2017, 19, 952. [Google Scholar] [CrossRef]
- Ferrazzano, L.; Corbisiero, D.; Martelli, G.; Tolomelli, A.; Viola, A.; Ricci, A.; Cabri, W. Green Solvent Mixtures for Solid-Phase Peptide Synthesis:A Dimethylformamide-Free Highly Efficient Synthesis of Pharmaceutical-Grade Peptides. ACS Sustain. Chem. Eng. 2019, 7, 11036–11043. [Google Scholar]
- Camp, J.E.; Nyaminia, S.B.; Scott, F.J. Cyrene™ is a green alternative to DMSO as a solvent for antibacterial drug discovery against ESKAPE pathogens. RSC Med. Chem. 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Mouterde, L.M.M.; Couvreur, J.; Langlait, M.M.J.; Brunois, F.; Allais, F. Identification and expression of a CHMO from the Pseudomonas aeruginosa strain Pa1242: Application to the bioconversion of Cyrene™ into a key precursor (S)-γ-hydroxymethylbutyrolactone. Green Chem. 2021, 23, 2694. [Google Scholar] [CrossRef]
- Andrew, O.B.; Sherwood, J.; Hurst, G.A. A Greener Synthesis of the Antidepressant Bupropion Hydrochloride. J. Chem. Educ. 2022, 99, 3277–3282. [Google Scholar] [CrossRef]
- Citarella, A.; Amenta, A.; Passarella, D.; Micale, N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. Int. J. Mol. Sci. 2022, 23, 15960. [Google Scholar] [CrossRef] [PubMed]
- Witczak, Z.J.; Bielski, R.; Mencer, D.E. Concise and efficient synthesis of E-stereoisomers of exo-cyclic carbohydrate enones. Aldol condensation of dihydrolevoglucosenone with five-membered aromatic aldehydes1 Part 1. Tetrahedron Lett. 2017, 58, 4069–4072. [Google Scholar] [CrossRef]
- Yu, H.T.; Yu, D.K.; Xue, Z.M.; Zhang, B.L. Dihydrolevoglucosenone as a bio-based catalytic solvent for efficient reductive-transformation of CO2 with amines into formamides and benzothiazoles. J. Chem. Eng. 2022, 431, 133397. [Google Scholar] [CrossRef]
- Gonzalo, G. Biocatalysed reductions of a-ketoesters employing CyreneTM as cosolvent. Biocatal. Biotransformation 2022, 40, 252–257. [Google Scholar] [CrossRef]
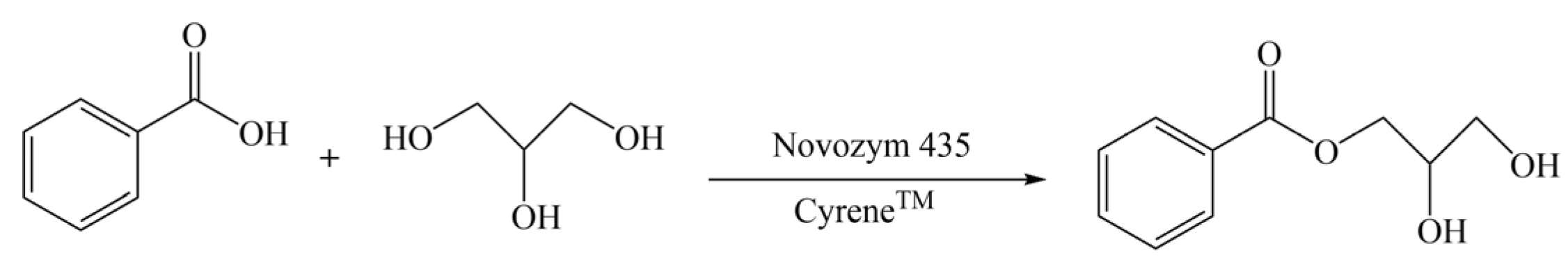
- Guajardoa, N.; María, P.D. Assessing biocatalysis using dihydrolevoglucosenone (Cyrene™) as versatile bio-based (co)solvent. Mol. Catal. 2020, 485, 110813. [Google Scholar] [CrossRef]
- Kuhl, N.; Turnbull, B.W.H.; Thaisrivongs, D.A.; Ji, Y.N.; Larson, R.T.; Shevlin, M.; Prier, C.K.; Chung, C.K.; Desmond, R.; Guetschow, E.; et al. Utilizing biocatalysis and a sulfolane-mediated reductive acetal opening to access nemtabrutinib from Cyrene. Green Chem. 2023, 25, 606. [Google Scholar] [CrossRef]
- Tamargo, R.J.I.; Rubio, P.Y.M.; Mohandoss, S.; Shim, J.J.; Lee, Y.R. Cyrene™ as a Neoteric Bio-Based Solvent for Catalyst-Free Microwave-Assisted Construction of Diverse Bipyridine Analogues for Heavy-Metal Sensing. ChemSusChem 2021, 14, 2133–2140. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nano-composite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Grune, C.; Thamm, J.; Werz, O.; Fischer, D. Cyrene™ as an Alternative Sustainable Solvent for the Preparation of Poly(lactic-co-glycolic acid) Nanoparticles. J. Pharm. Sci. 2021, 110, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Czapka, A.; Grune, C.; Schädel, D.P.; Bachmann, V.; Scheuer, K.; Dirauf, M.; Weber, C.; Skaltsounis, A.L.; Jandt, K.D.; Schubert, U.S.; et al. Drug delivery of 6-bromoindirubin-3′-glycerol-oxime ether employing poly(D,L-lactide-co-glycolide)-based nanoencapsulation techniques with sustainable solvents. J. Nanobiotechnol. 2022, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- German, L.; Cuevas, J.M.; Cobos, R.; Alvare, L.P.; Vilela, J.L.V. Green alternative cosolvents to N-methyl-2-pyrrolidone in water polyurethane dispersions. RSC Adv. 2021, 11, 19070. [Google Scholar] [CrossRef]
- Adama, J.; Sorbob, M.R.D.; Kaur, J.; Romano, R.; Singh, M.; Altucci, C. Surface Interactions Studies of Novel Two-Dimensional Molybdenum Disulfide with Gram-Negative and GramPositive Bacteria. Anal. Lett. 2023, 56, 357–371. [Google Scholar] [CrossRef]
- Itawi, H.E.; Fadlallah, S.; Perré, P.; Leephakphumphanich, W.; Ruscassier, N.; Zoghlami, A.; Allais, F.; Perré, P. Online Microfluidic Production of Sustainable Cyrene™-Derived Porous Microparticles. Sustainability 2023, 15, 2023. [Google Scholar] [CrossRef]
- Bai, Y.C.; Hawley, W.B.; Jafta, C.J.; Muralidharan, N.; Polzin, B.J.; Belharouak, I. Sustainable recycling of cathode scraps via Cyrene-based separation. Sustain. Mater. Technol. 2020, 25, e00202. [Google Scholar] [CrossRef]
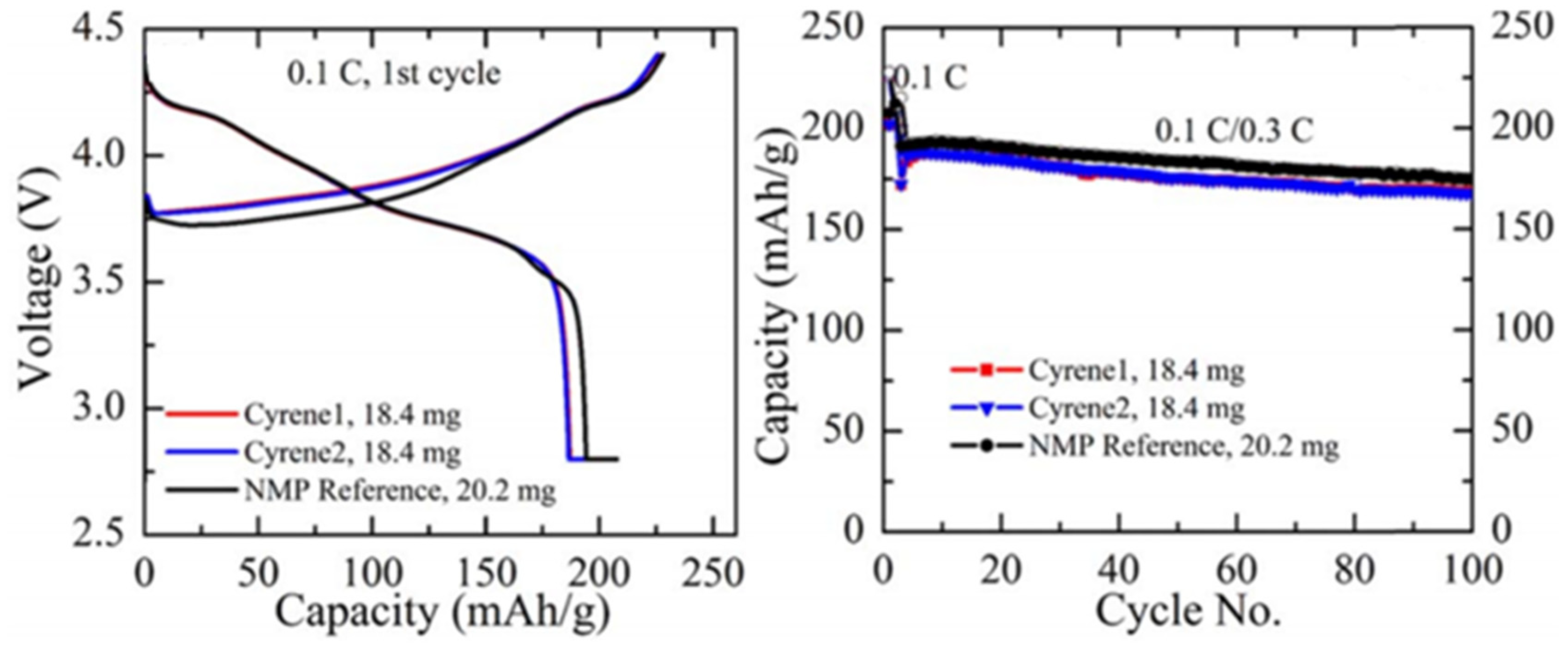
- Zhou, H.; Pei, B.; Fan, Q.L.; Xin, F.X. Whittingham, Can Greener Cyrene Replace NMP for Electrode Preparation of NMC 811 Cathodes? J. Electrochem. Soc. 2021, 168, 040536. [Google Scholar] [CrossRef]
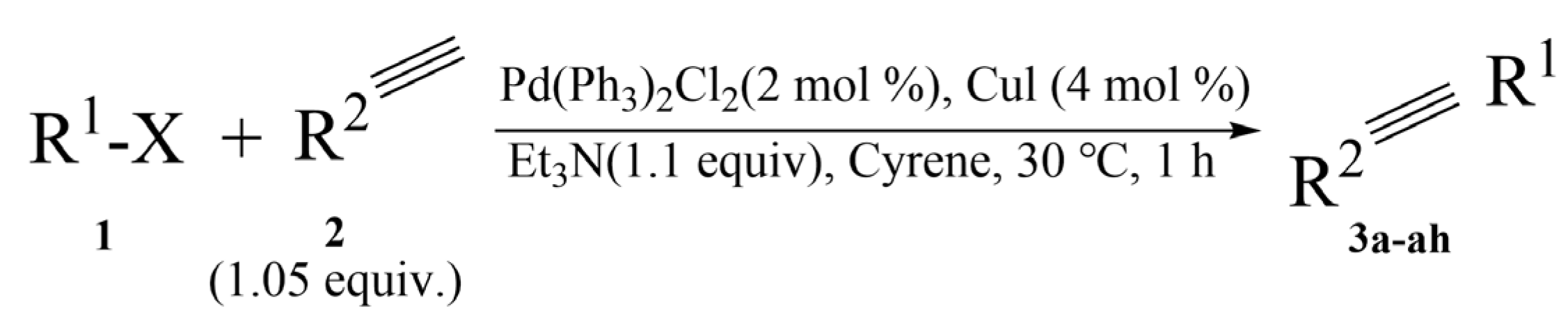

| References | Year | Strength | Begin | End | 2014–2023 |
|---|---|---|---|---|---|
| Sherwood J, 2014, CHEM COMMUN, V50, P9650, DOI 10.1039/c4cc04133j, DOI | 2014 | 5.94 | 2016 | 2017 | ▂▂▃▃▂▂▂▂▂▂ |
| Alder CM, 2016, GREEN CHEM, V18, P3879, DOI 10.1039/c6gc00611f, DOI | 2016 | 2.6 | 2016 | 2019 | ▂▂▃▃▃▃▂▂▂▂ |
| Zhang JF, 2016, ACS SUSTAIN CHEM ENG, V4, P7186, DOI 10.1021/acssuschemeng.6b02115, DOI | 2016 | 6.64 | 2017 | 2021 | ▂▂▂▃▃▃▃▃▂▂ |
| Cao F, 2015, ENERG ENVIRON SCI, V8, P1808, DOI 10.1039/c5ee00353a, DOI | 2015 | 5.85 | 2017 | 2018 | ▂▂▂▃▃▂▂▂▂▂ |
| Wilson KL, 2016, BEILSTEIN J ORG CHEM, V12, P2005, DOI 10.3762/bjoc.12.187, DOI | 2016 | 4.3 | 2017 | 2020 | ▂▂▂▃▃▃▃▂▂▂ |
| Prat D, 2016, GREEN CHEM, V18, P288, DOI 10.1039/c5gc01008j, DOI | 2016 | 3.08 | 2017 | 2021 | ▂▂▂▃▃▃▃▃▂▂ |
| De Bruyn M, 2016, ENERG ENVIRON SCI, V9, P2571, DOI 10.1039/c6ee01352j, DOI | 2016 | 2.31 | 2017 | 2019 | ▂▂▂▃▃▃▂▂▂▂ |
| Byrne F, 2017, GREEN CHEM, V19, P3671, DOI 10.1039/c7gc01392b, DOI | 2017 | 2.32 | 2018 | 2020 | ▂▂▂▂▃▃▃▂▂▂ |
| Sherwood J, 2016, GREEN CHEM, V18, P3990, DOI 10.1039/c6gc00932h, DOI | 2016 | 2.71 | 2019 | 2020 | ▂▂▂▂▂▃▃▂▂▂ |
| Marino T, 2019, J MEMBRANE SCI, V580, P224, DOI 10.1016/j.memsci.2019.03.034, DOI | 2019 | 2.34 | 2020 | 2021 | ▂▂▂▂▂▂▃▃▂▂ |
| Entry | Materials | Catalyst | Conditions | Reactor | Yield | Ref |
|---|---|---|---|---|---|---|
| 1 | Cellulose | NaHSO4 | Slow pyrolysis at 300 °C | Horizontal tube reactor | 6.9% | [12] |
| 2 | Cellulose | 5.4 wt% H3PO4 | Slow pyrolysis at 350 °C | vertical flow reactor | 22.3% | [13] |
| 3 | Cellulose | 2 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 34% | [15] |
| 4 | Newsprint | 1 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 21% | [15] |
| 5 | Kraft pulp | 2 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 19% | [15] |
| 6 | Birchwood | 2.5 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 17% | [15] |
| 7 | Cellulose | Ionic liquids | Slow pyrolysis at 300 °C | Horizontal reactor | 38.2% | [18] |
| 8 | Cellulose | SO42−/ZrO2 | Fast pyrolysis at 335 °C | Vertical reactor | 8.14% | [24] |
| 9 | Cellulose | HZSM-5 | Fast pyrolysis at 335 °C | Vertical reactor | 1.7% | [24] |
| 10 | Cellulose | TiO2 | Fast pyrolysis at 335 °C | Vertical reactor | 5% | [24] |
| 11 | Cellulose | SO42−/TiO2-Fe3O4 | Fast pyrolysis at 300 °C | CDS Pyroprobe 5200HP pyrolyser | 15.43% | [26] |
| 12 | Poplar wood | SO42−/TiO2-Fe3O4 | Fast pyrolysis at 300 °C | CDS Pyroprobe 5200HP pyrolyser | 7.06% | [26] |
| 13 | Cellulose | SPA | Fast pyrolysis at 280 °C | CDS Pyroprobe 5200HP pyrolyser | 16.1% | [27] |
| 14 | Cellulose | Al-MCM-41 | Fast pyrolysis at 400 °C | Vertical reactor | 53% | [23] |
| 15 | Cellulose | Ni-P-MCM-41 | Fast pyrolysis at 350 °C | Vertical reactor | 21.4% | [28] |
| 16 | Pine wood | Ni-P-MCM-41 | Fast pyrolysis at 350 °C | Vertical reactor | 10.7% | [28] |
| 17 | Cellulose | P-Mo/SnO2 | Fast pyrolysis at 300 °C | Micropyrolyser | 18% | [29] |
| 18 | Cellulose | P-Mo/SnO2 | Fast pyrolysis at 300 °C | Vertical reactor | 12.7% | [29] |
| 19 | Cellulose | 1 wt% H3PO4 | In sulfolane at 200–280 °C | Round flask | 38% | [31] |
| 20 | Cellulose | 20 mM H2SO4 | In THF at 210 °C | Hastelloy autoclave | 51% | [30] |
| 21 | Cellulose | 7 mM H2SO4 | Plasma electrolysis in GVL at 160 °C | Round-bottom flask | 43% | [32] |
| 22 | Brichwood | 5 wt% H3PO4 | Analytical pyrolysis at 350 °C | Micro Double-shot Pyrolyser | 21.08% | [20] |
| 23 | Crude waste softwood hydrolysis lignin | H2SO4 | Microwave-assisted pyrolysis at 180 °C | CEM ‘Discover’ MW generator | 8% | [21] |
| Entry | Catalyst | Solvent | Conditions | Reactor | Yield | Ref |
|---|---|---|---|---|---|---|
| 1 | 10% Pd/C | / | 1.1 atm H2, 8 days | / | >90% | [1] |
| 2 | 10% Pd/C | EtOAc | 1.1 atm H2, 96 h | / | >90% | [1] |
| 3 | 10% Pd/C | EtOAc | 3 to 80 bar, 2 to 48 h | High-pressure reactor | >90% | [1] |
| 4 | 10% Pd/C | / | 80 bar, less than 2 h | High-pressure reactor | >90% | [1] |
| 5 | 5% Pd/C | THF | Stirring at 66 °C, 2 h | Autoclave | >99% | [33] |
| 6 | Pd/t-ZrO2 | Water | 10 bar H2, 80 °C | Batch glass micro-reactor | >99% | [34] |
| 7 | OYE 2.6 Tyr78Trp | EtOH | Room temperature | / | >99% | [37] |
| Entry | MOFs | Expected () | Observed in DMF () | Observed in Cyrene () |
|---|---|---|---|---|
| 1 | HKUST-1 | 1740 | 1400 | 1500 |
| 2 | UiO-66 | 1700 | 1300 | 500 |
| 3 | Co-MOF-74 | 1572 | 800 | 200 |
| 4 | ZIF-8 | 1950 | 1700 | 600 |
| 5 | Zn(BDC)(DABCO) | 1750 | 1950 | 1300 |
| Entry | Membranes | Conditions | Conclusions | Ref |
|---|---|---|---|---|
| 1 | PES, PVDF | 13 wt% of PES/PVDF, 87 wt% Cyrene, exposure time to RH 0–5 min, NIPS and VIPS-NIPS. | Without pore former agent, Cyrene can be used for the preparation of PES and PVDF membranes. | [43] |
| 2 | PES, PVP | 20 wt% PES, 0–7.6 wt% PVP 80 wt% Cyrene or NMP, 70 °C, 4 h, NIPS | With PVP and PEG, membranes produced with Cyrene are more sustainable, with less of both polymers’ loss and tunable pore size and contact angles. | [44] |
| 3 | PVC/PIMs, PVDF-HFP/PIM | 2 g CTA in 30 mL Cyrene, 50 °C, 3–4 days, | PVC dissolved at 60 °C with 30 mL solvent, PVDF-HFP not dissolved. | [48] |
| 4 | PES/GO | PES concentration 9%, GO concentration in the dope solution: 0.1, 0.3, 0.5 | The highest porosity and pure water flux (PWF) occurs at a loading of 0.3 wt% GO for systems PES/GO/Cyrene. | [50] |
| Entry | Conditions | Conclusions | Ref |
|---|---|---|---|
| 1 | ~4.5 mg of graphite, 3 mL Cyrene, ultrasonic mixed 15 min, exfoliation at 7000 rpm for 10 min | 92.5% of the flakes ≤ 10, 75% of the flakes < 5%, 7.5% are monolayer, ≤10 layer flakes: 0.725 ± 0.406 μm in width, 1.323 ± 0.647 μm in length. | [52] |
| 2 | 1 g TPU powder, 10 mL Cyrene, 0.2 wt% fluorosurfactant 3000 rpm for 10 min, 1 g graphene was added, 3000 rpm for 10 min. For comparison, weight ratio of graphene to TPU at 1:2 and 2:1. | The graphene/TPU/PDMS wide tensile range (∼80%), excellent sensitivity (GF > 3905), extremely low sensing limit (∼0.1 ‰) and good durability over 5000 cycles. | [55] |
| 3 | 30 g of natural graphite powder, 600 mL Cyrene, 9000 rpm for 40 min. | Cyrene had the highest graphene flake concentration, smallest flake sizes and a generally narrower flake size distribution. Touch sensor prototype: optical transmittance of 78%, sheet resistance of 290 Ω−1 and no significant change in sheet resistance when bent to a curvature radius of 28 mm. | [56] |
| Entry | Conditions | Solvent | Yield of Hesperidin | Solvent | Yield of Hesperidin | Ref | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Orange peel: 250 mg black tea: 500 mg solvent: 5 mL stirred 2 min 14,450 rpm RT or 65 °C for 2 h | Cyrene | RT | Hot | Cyrene + EtOH (43/57) | RT | Hot | [37] |
| 3.23 | 6.18 | 0.09 | 0.92 | |||||
| Cyrene + EtOH (30/70) | 11.61 | / | Cyrene + MeOH (51/49) | 0.34 | 1.54 | |||
| Cyrene + H2O (70/30) | 20.71 | 23.43 | Cyrene + H2O (70/30) | 1.53 | 1.68 | |||
| Cyrene + Acetic add + H2O (49/22/29) | 21.43 | 30.46 | Cyrene + H2O (30/70) | 1.52 | 1.17 | |||
| (μM) | ||||||
|---|---|---|---|---|---|---|
| aureus | E. faecalis | E. coli | ||||
| Compound | DMSO | Cyrene | DMSO | Cyrene | DMSO | Cyrene |
| Tetracycline | 0.78 | 0.78 | NA | NA | 3.13 | 3.13 |
| Vancomycin hydrochloride | 0.39 | 0.39 | 13 | 13 | NA | NA |
| Ciprofloxacin hydrochloride | 1.6 | 1.6 | 0.39 | 0.39 | 0.019 | 0.019 |
| Colistin sulfate | NA | NA | NA | NA | 0.78 | 0.78 |
| Penicillin G sodium | 34 | 34 | NA | NA | NA | NA |
| Polymixin B sulfate | NA | NA | NA | NA | 1.6 | 1.6 |
| Tobramycin | NA | NA | NA | NA | 0.78 | 0.78 |
| Levofloxacin | 0.78 | 0.78 | 0.19 | 0.39 | 0.019 | 0.019 |
| MIC80 (μM) | ||||||
| P. aeruginosa | A. baumanii | K. pneumoniae | ||||
| Compound | DMSO | Cyrene | DMSO | DMSO | Cyrene | DMSO |
| Tetracycline | 25 | 25 | 25 | 25 | 25 | 25 |
| Vancomycin hydrochloride | NA | NA | NA | NA | NA | NA |
| Ciprofloxacin hydrochloride | 0.39 | 0.39 | 1.6 | 0.39 | 0.39 | 1.6 |
| Colistin sulfate | 3.1 | 3.1 | 1.6 | 3.1 | 3.1 | 1.6 |
| Penicillin G sodium | NA | NA | NA | NA | NA | NA |
| Polymixin B sulfate | 3.1 | 3.1 | 1.6 | 3.1 | 3.1 | 1.6 |
| Tobramycin | 1.6 | 1.6 | 3.1 | 1.6 | 1.6 | 3.1 |
| Levofloxacin | 1.6 | 1.6 | 0.39 | 1.6 | 1.6 | 0.39 |
| Entry | Solvent | T [°C] | t [h] | Yield [a] [%] |
|---|---|---|---|---|
| 1 | - | 90 | 2 | 40 |
| 2 | H2O | 90 | 1 | - |
| 3 | EtOH | 90 | 1 | 48 |
| 4 | DMF | 90 | 1 | 66 |
| 5 | CyreneTM | 90 | 1 | 76 |
| 6 | CyreneTM | 110 | 1 | 83 |
| 7 | CyreneTM | 130 | 1 | 94 |
| 8 | CyreneTM | 150 | 1 | 91 |
| 9 [b] | CyreneTM | 130 | 24 | 35 |
| 10 | DMF | 130 | 1 | 85 |
| 11 | - | 130 | 1 | 32 |
| Entry | Conditions | Solvent | Yield | Ref |
|---|---|---|---|---|
| 1 | 10 mg Resomer® RG 502, 1 mg atorvastatin Stirred 3 h, RT or 37 °C Homogenisation vs. ultrasonication (30 s, 1 and 2 min, 50% and 100% cycle) | Cyrene | 60% | [114] |
| 2 | 50 mg PLGA, Stirred 3 h, RT Homogenised at 24,000 rpm for 15 min | 4.5 mL ethyl acetate + atorvastatin (5 mg) dissolved in poly(ethylene glycol) 400 g/mol as cosolvent | 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dai, M.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. Preparation and Application of Green Sustainable Solvent Cyrene. Chemistry 2023, 5, 2322-2346. https://doi.org/10.3390/chemistry5040154
Wang Y, Dai M, Luo G, Fan J, Clark JH, Zhang S. Preparation and Application of Green Sustainable Solvent Cyrene. Chemistry. 2023; 5(4):2322-2346. https://doi.org/10.3390/chemistry5040154
Chicago/Turabian StyleWang, Yadong, Mingfei Dai, Gang Luo, Jiajun Fan, James H. Clark, and Shicheng Zhang. 2023. "Preparation and Application of Green Sustainable Solvent Cyrene" Chemistry 5, no. 4: 2322-2346. https://doi.org/10.3390/chemistry5040154
APA StyleWang, Y., Dai, M., Luo, G., Fan, J., Clark, J. H., & Zhang, S. (2023). Preparation and Application of Green Sustainable Solvent Cyrene. Chemistry, 5(4), 2322-2346. https://doi.org/10.3390/chemistry5040154