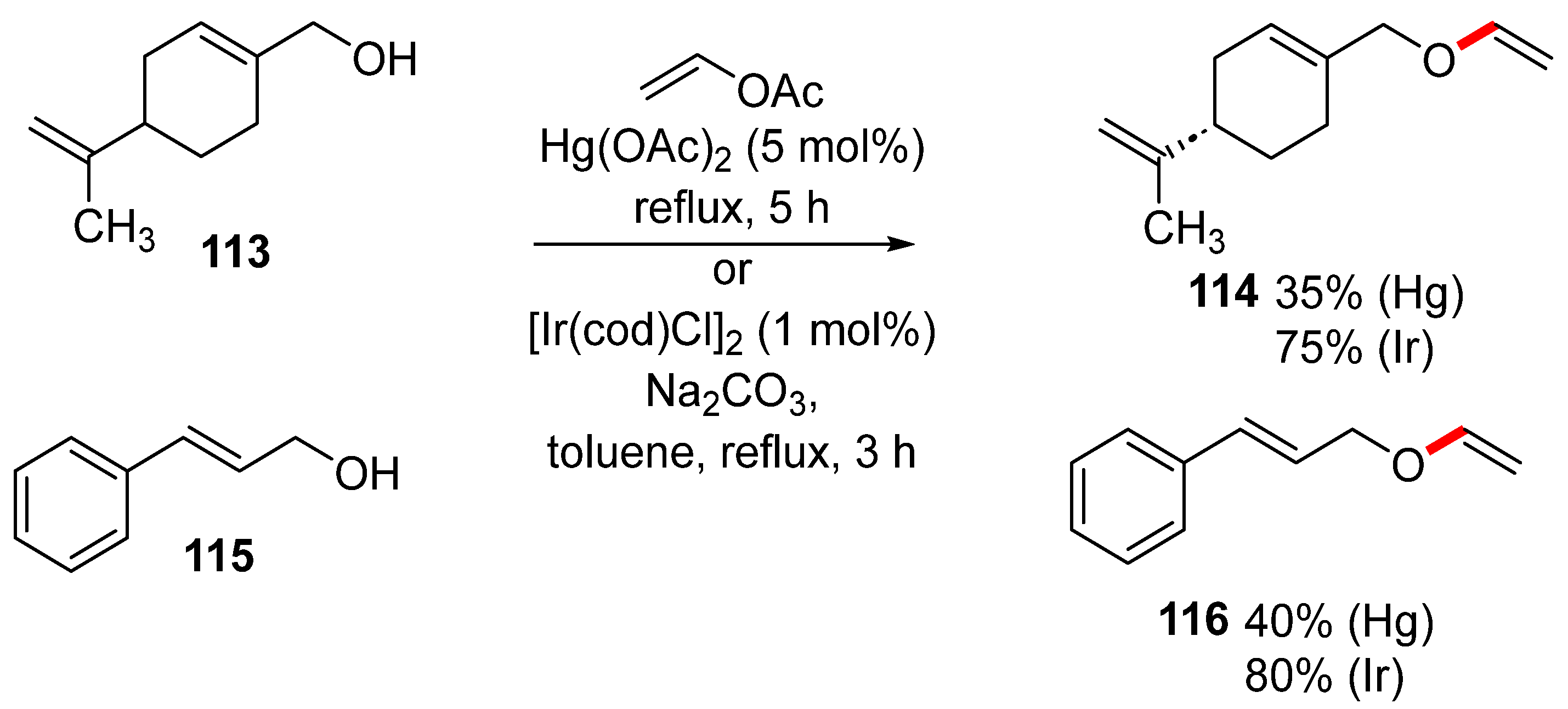Abstract
This review summarizes the applications of vinyl sulfonate and vinyl acetate as green alternatives for vinyl bromide in cross-coupling reactions. In the first part, the preparation of vinyl sulfonates and their cross-coupling reactions are briefly discussed. Then, a brief review of vinyl acetate cross-coupling reactions, including cyclization reactions, the Fujiware–Moritani reaction, and transvinylation reactions are described.
1. Introduction
Cross-coupling reactions have become an indispensable aspect of modern organic synthesis [1,2,3,4,5]. The applications of cross-coupling reactions involve the formation of C–C and C–heteroatom bonds, including C–B [6,7,8,9,10], C–N [11,12,13,14,15], C–O [16,17,18,19], C–P [20,21,22,23,24], and C–S bonds [25,26,27,28]. A wide range of compounds with applications in medical or material science has been synthesized by means of cross-coupling reactions. Here, the synthesis of tetrasubstituted alkenes [29,30,31,32,33,34,35,36,37] and the preparation of heterocyclic compounds [38,39,40,41,42,43] are examples of such syntheses. A typical reaction scheme for the formation of a C–atom bond via a cross-coupling reaction involves a reaction between an electrophile and a nucleophile. Alternatively, it is also possible to form bonds between two electrophiles or two nucleophiles, with the related reactions being known as oxidative [44,45,46] or reductive [1,47,48,49] cross-coupling reactions, respectively.
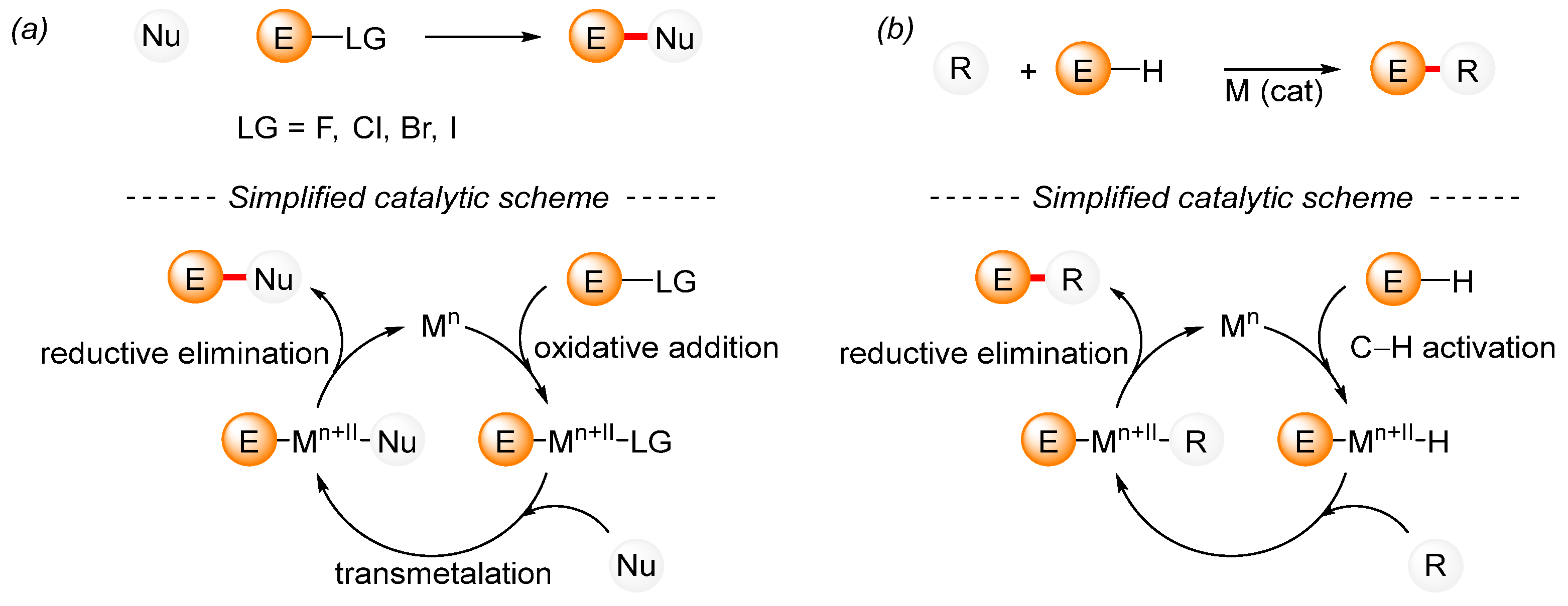
A traditional cross-coupling reaction requires a mutual interaction between the nucleophile and the electrophilic reagent, which is catalyzed by a transition metal complex (Scheme 1a). Initially, the transition metal complex reacts with the electrophile to produce the product of the oxidative addition. This is followed by the transmetalation step, while in the final step, which is known as reductive elimination, the reaction product is formed alongside the active catalytic species, which represents the beginning of the next catalytic cycle. Among the wide range of available nucleophilic reagents, boronic acids, organostannanes, organozinc compounds, Grignard reagents, alkenes, and alkynes are commonly used for the formation of C–C bonds. In contrast, organohalides are typical examples of electrophiles that have been widely used in cross-coupling reactions during the past century. In addition to substrates with activated C–N [50,51,52,53], C–S [54,55,56], or C–P [22] bonds, substrates with activated C–O [51,57,58,59,60,61] bonds are often used. Together with traditional cross-coupling reactions, C–H bond activation reactions are being intensively developed (Scheme 1b) [62]. A simplified mechanism for cross-coupling reactions with C–H bond activation illustrates the unpretentiousness of C–H bond activation on the structure of the starting materials. However, the mechanisms of C–H bond activation are considerably more complex [63].
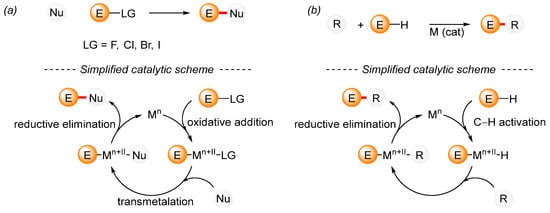
Scheme 1.
A simplified mechanism for a general transition-metal-catalyzed cross-coupling reaction. (a) General cross-coupling reaction; (b) General cross-coupling reaction including C–H activation.
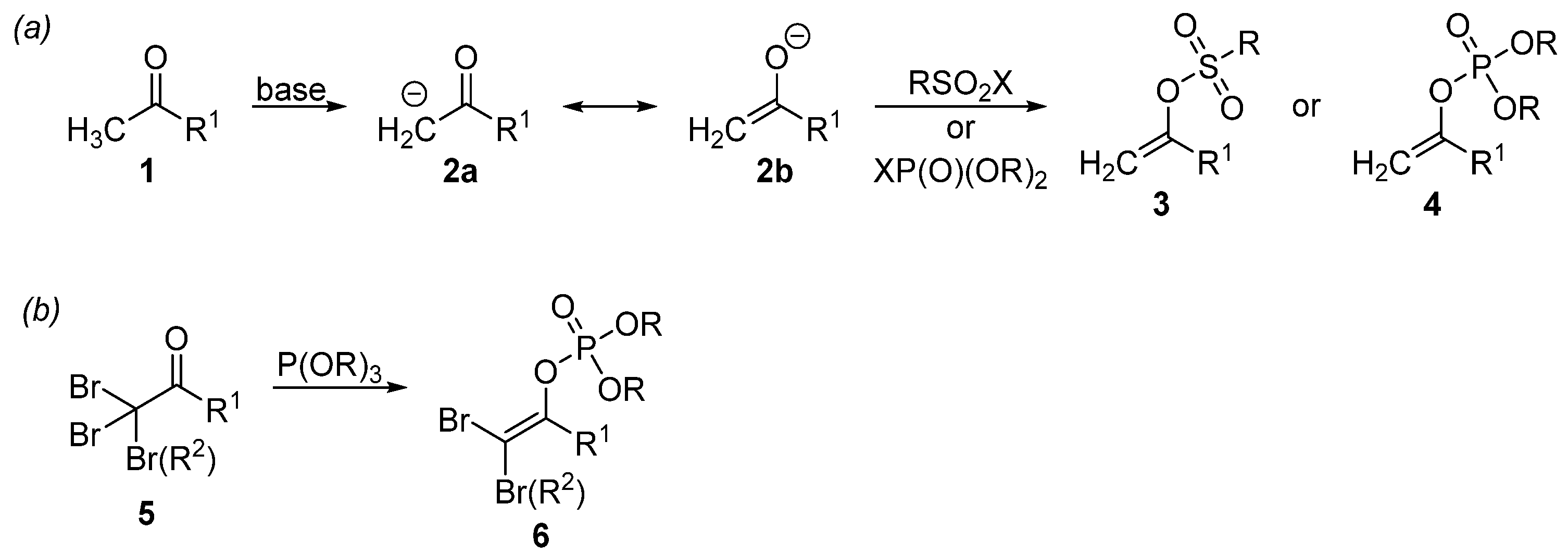
From a practical perspective, substrates with activated C–O bonds are of particular interest. These substances are represented by various esters, sulfonates, and phosphates. Organophosphates and organosulfonates are often used as electrophiles in cross-coupling reactions due to their easy availability. Indeed, the easy availability of organosulfonates and organophosphates is demonstrated by the preparation of vinyl derivatives 3 and 4 (Scheme 2a). The starting ketone 1 is enolized by means of a base and then the formed enol 2b is phosphorylated [64] or sulfonylated [65] at the O-terminus. A variation of this approach is used to synthesize aryl sulfonates [66] and aryl phosphates [67]. In addition, the Perkow reaction [68], which is based on the reaction of halogenated ketones with phosphites, can also be used to prepare vinyl phosphates (Scheme 2b). This is particularly advantageous for the preparation of triple [69,70] and double [71,72,73,74,75,76] electrophilic templates 6 using brominated ketone 5.

Scheme 2.
Synthesis of vinyl sulfonates and vinyl phosphates. (a) An acid-base reaction for the preparation of vinyl sulfonates and vinyl phosphates; (b) The Perkow reaction for the preparation of vinyl phosphates.
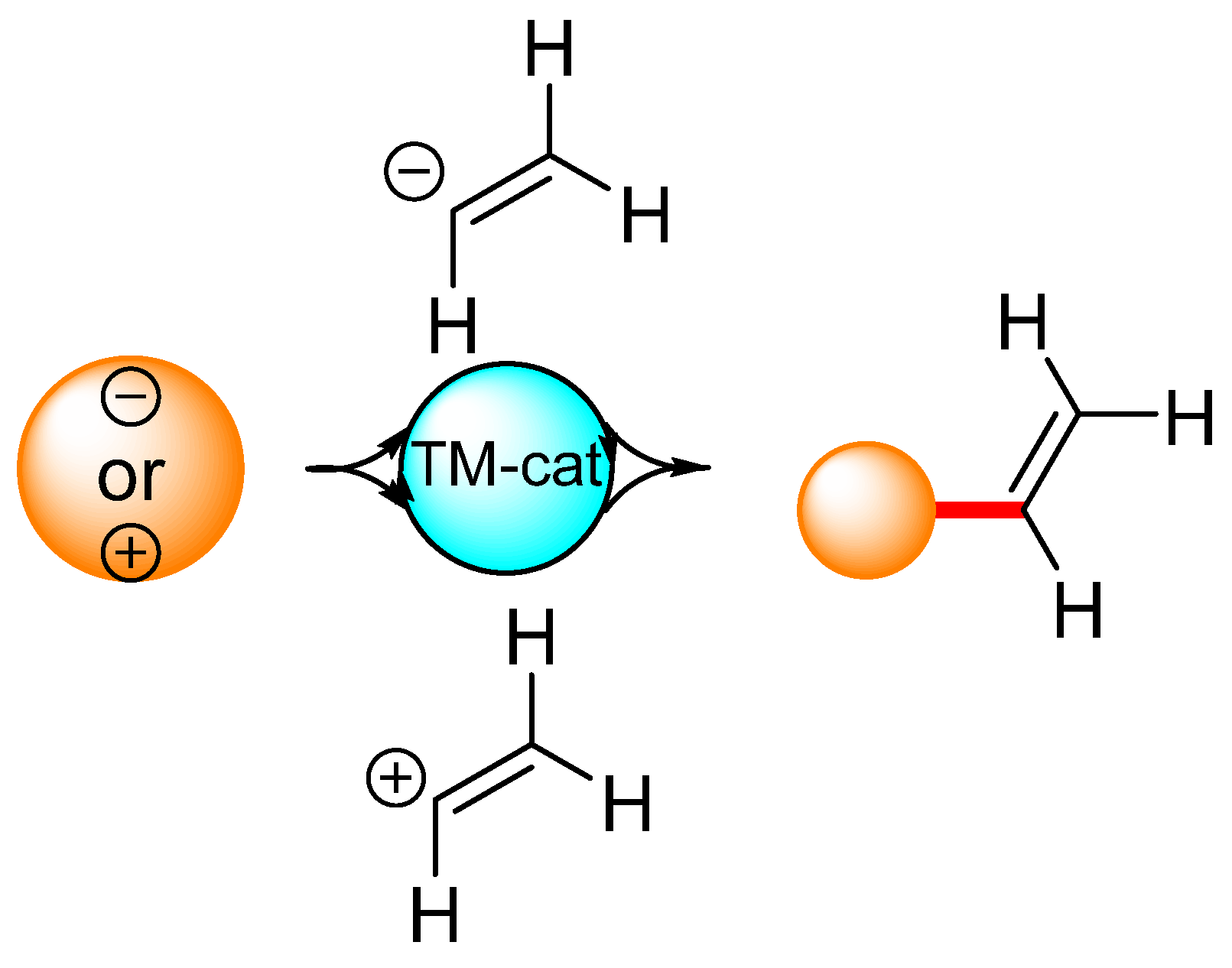
Cross-coupling reactions are also useful for the introduction of simple fragments—namely, the vinyl group. Traditionally, dehydrohalogenation [77], the Wittig reaction [78,79,80,81,82], or the Peterson olefination [83,84] were commonly used to introduce the vinyl group, although the discovery of cross-coupling reactions has expanded the available vinylation methods. In principle, vinylation can be performed via the reaction of metalated ethylene with an electrophilic reagent in the presence of a catalytic amount of a transition metal complex (Scheme 3). Vinylboronic acid [85,86,87], vinylmagnesium bromide [88], and vinylstannanes [89,90] have all been used en route to monosubstituted ethylene. Another procedure—that is, a reaction of organometallic reagents with vinyl-type electrophiles, mainly vinyl bromide—is also widely used. The opposite approach, which is based on the cross-coupling reactions of vinylic electrophiles, is also feasible. The Sonogashira reaction [91,92,93,94], radical hydroarylation [95], and the Suzuki cross-coupling reaction [96] are typical examples of the use of vinyl bromide in organic synthesis. However, vinyl bromide has a low boiling point (15.8 °C) [97], while the International Agency for Research on Cancer has listed vinyl bromide as a suspected human carcinogen [98].

Scheme 3.
General scheme representing vinylation under transition metal catalysis.
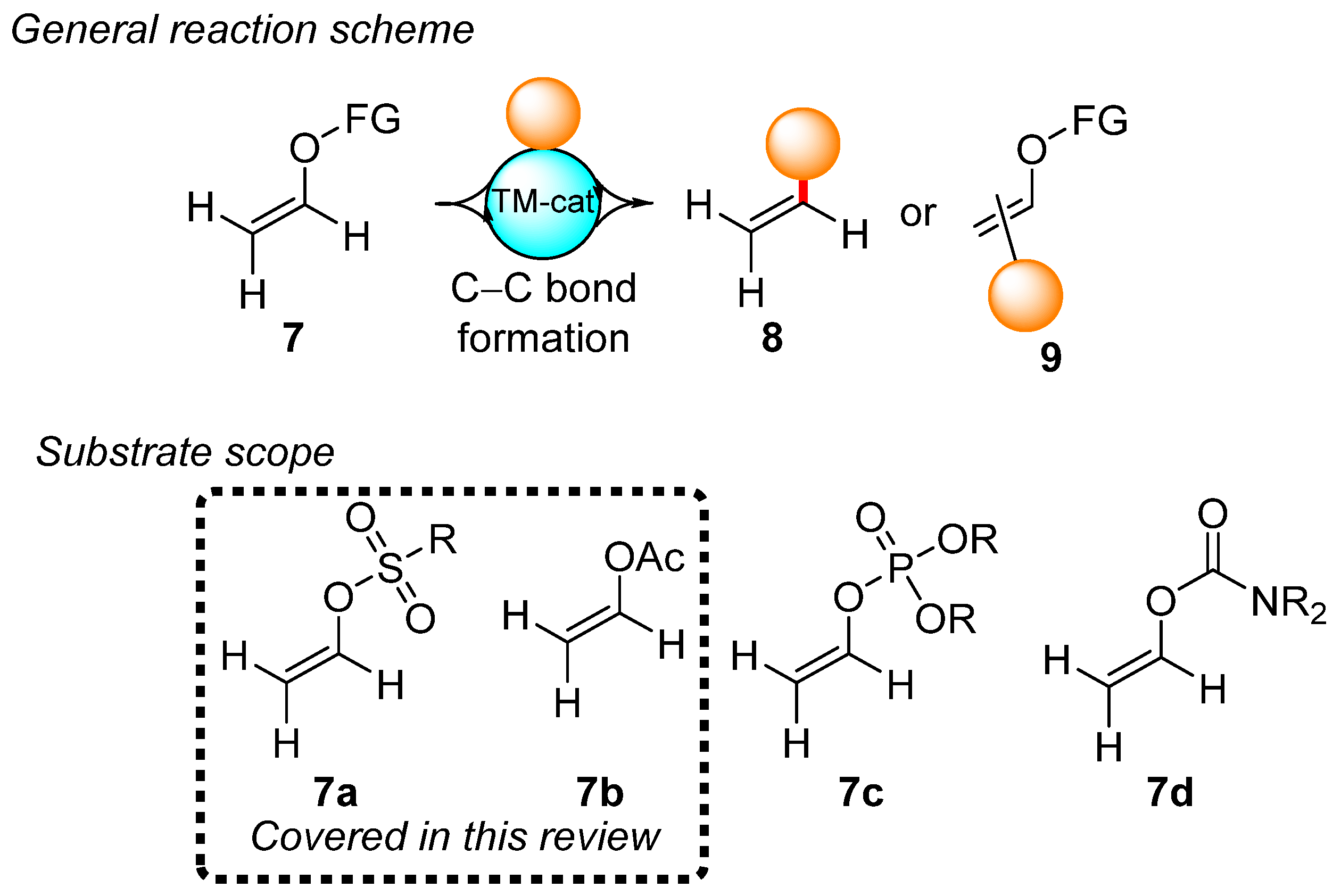
The aforementioned properties of vinyl bromide make it a difficult and dangerous substrate to manipulate. Fortunately, vinyl electrophiles with activated C–O bonds can be considered a suitable alternative to vinyl bromide for use in cross-coupling reactions, especially in the formation of C–C bonds. As no prior review has summarized the use of vinyl esters with activated C–O bonds, the aim of this review is to summarize the preparation and use of the compounds listed in Scheme 4 in terms of the preparation of monosubstituted ethylenes 8 and disubstituted alkenes 9. Vinyl sulfonates 7a and vinyl acetates 7b are easily available and frequently used in organic synthesis, while vinyl phosphates 7c and vinyl carbamates 7d are readily available but are characterized by undeveloped chemistry. Vinyl carbamates are frequently used as acetylene surrogates due to their α-lithiation-elimination sequence [99,100], whereas vinyl phosphates 7c are rarely used in organic synthesis [101]. Therefore, this review focuses on the chemistry of vinyl sulfonates 7a and vinyl acetates 7b and covers the 2015–2023 period. Although the C–H bond activation reactions of substrates 7a and 7b do not directly provide the vinylation products, selected C–H bond activation reactions for these substrates are covered by this review.

Scheme 4.
Scope of this review.
2. Cross-Coupling Reactions of Vinyl Sulfonates
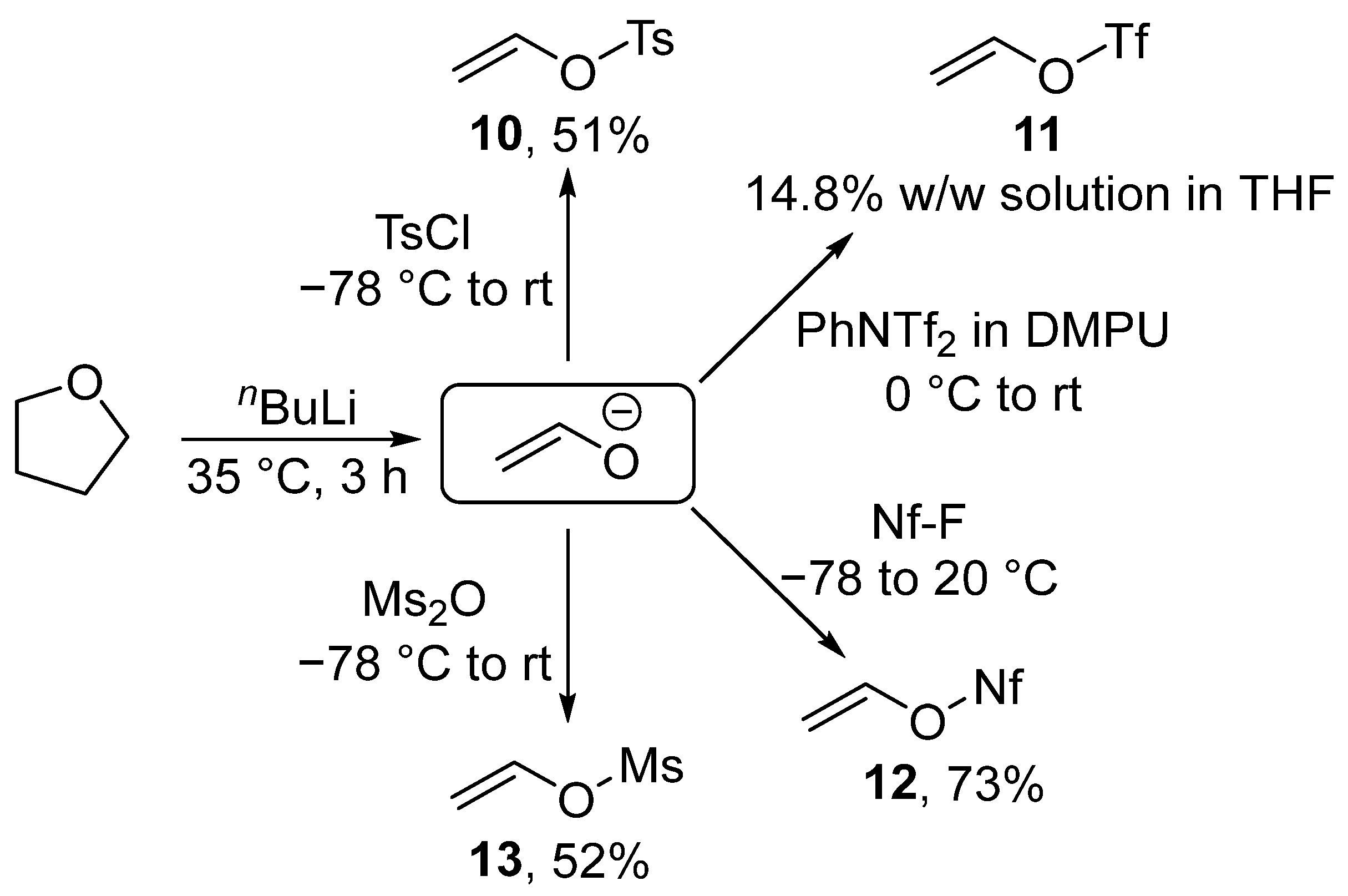
Despite the existence of alternative processes, most vinyl sulfonates are currently prepared via the decomposition of tetrahydrofuran with butyllithium at 35 °C. The resulting enolate can be sulfonylated with tosyl chloride [102,103], nonafluorobutanesulfonyl fluoride [104,105], mesyl anhydride [106], or N-phenyl-bis(trifluoromethanesulfonimide) [107]. Sulfonates 10, 12, and 13 were isolated as a liquid with a significantly higher boiling point than that of vinyl bromide. Only triflate 11 was isolated as a solution in tetrahydrofuran (THF) (Scheme 5).
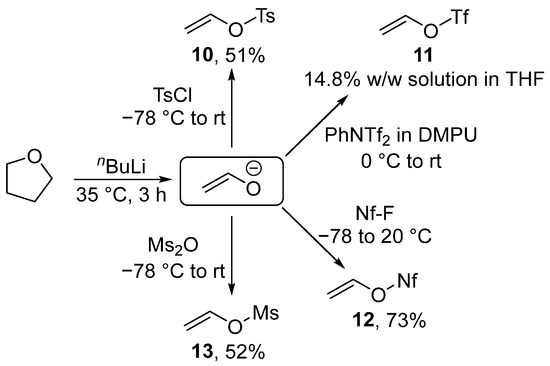
Scheme 5.
Synthesis of vinyl sulfonates.
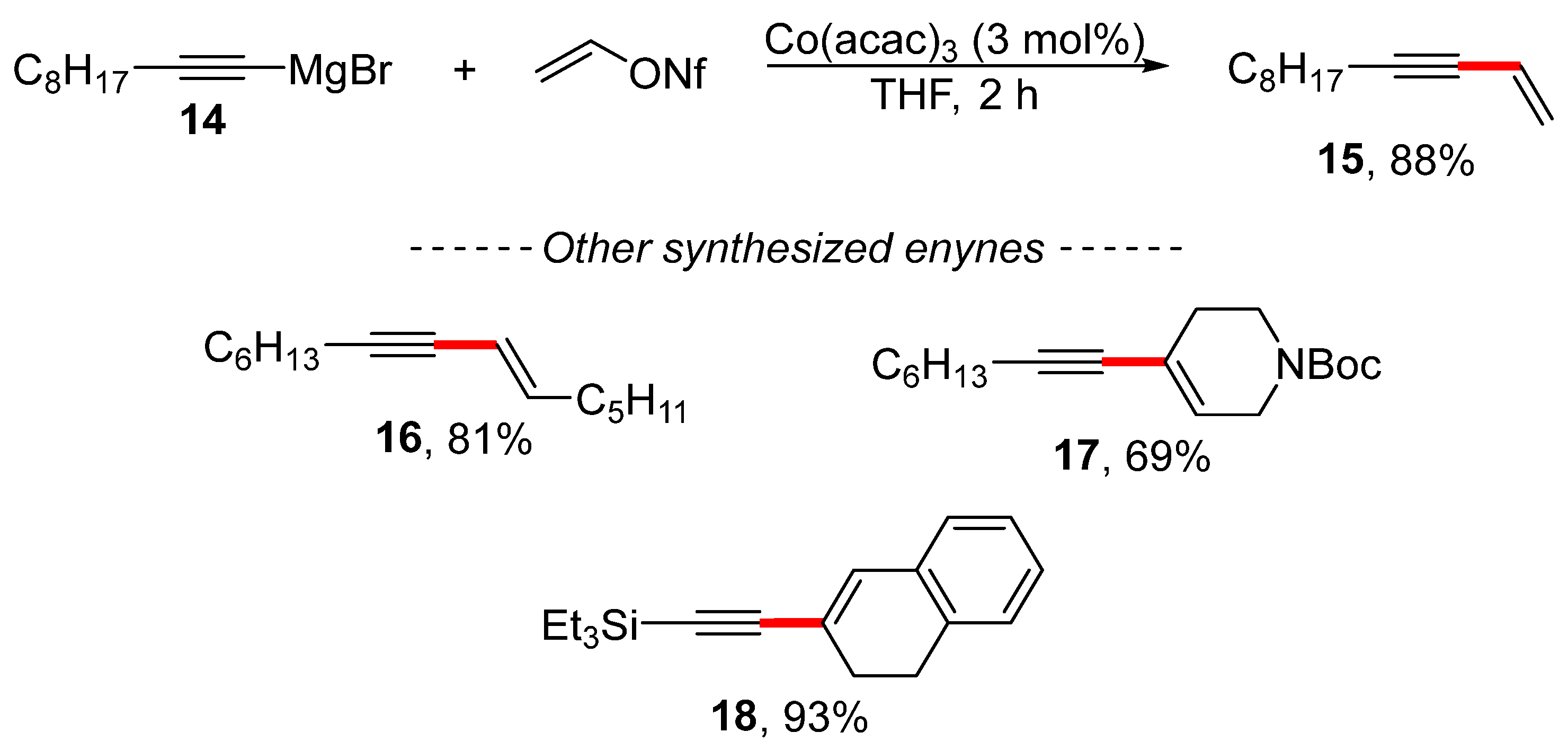
The vinylation of alkynylmagnesium bromide 14 can be performed using vinyl nonaflate in the presence of a cobalt catalyst to produce enyne 15 (Scheme 6) [108]. The optimized reaction conditions have a significantly broader scope, although enyne 15 was prepared in a near-quantitative isolated yield. In addition, examples of other selected substrates illustrate how the use of Grignard reagents results in the limited tolerance of the functional groups during this reaction.

Scheme 6.
Cobalt-catalyzed vinylation of a Grignard reagent.
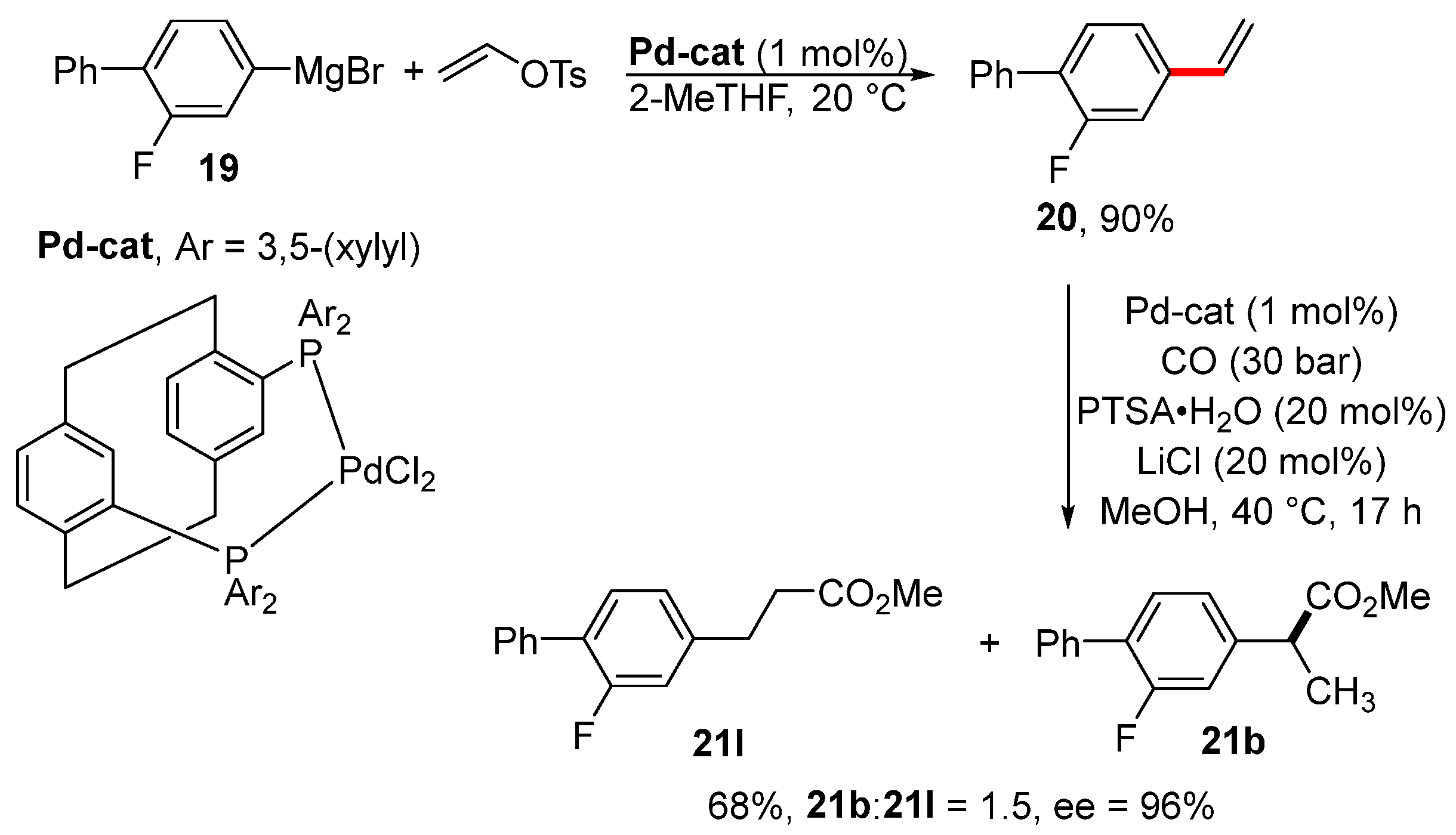
Styrene derivative 20 was obtained via the vinylation of arylmagnesium bromide 19 in the presence of a catalytic amount of palladium complex (Scheme 7) [109]. The same catalyst was also used for the asymmetric methoxycarbonylation of styrene 20 to provide the prodrug of flurbiprofen 21b. The disadvantage of this procedure for the preparation of the prodrug of flurbiprofen is the formation of an equimolar mixture of linear 21l and branched product 21b.
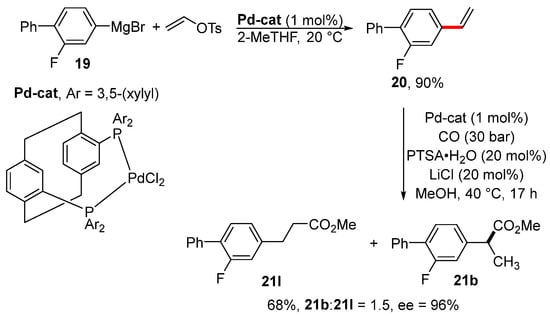
Scheme 7.
Preparation of the prodrug of flurbiprofen 21b via the vinylation of Grignard reagent 19.
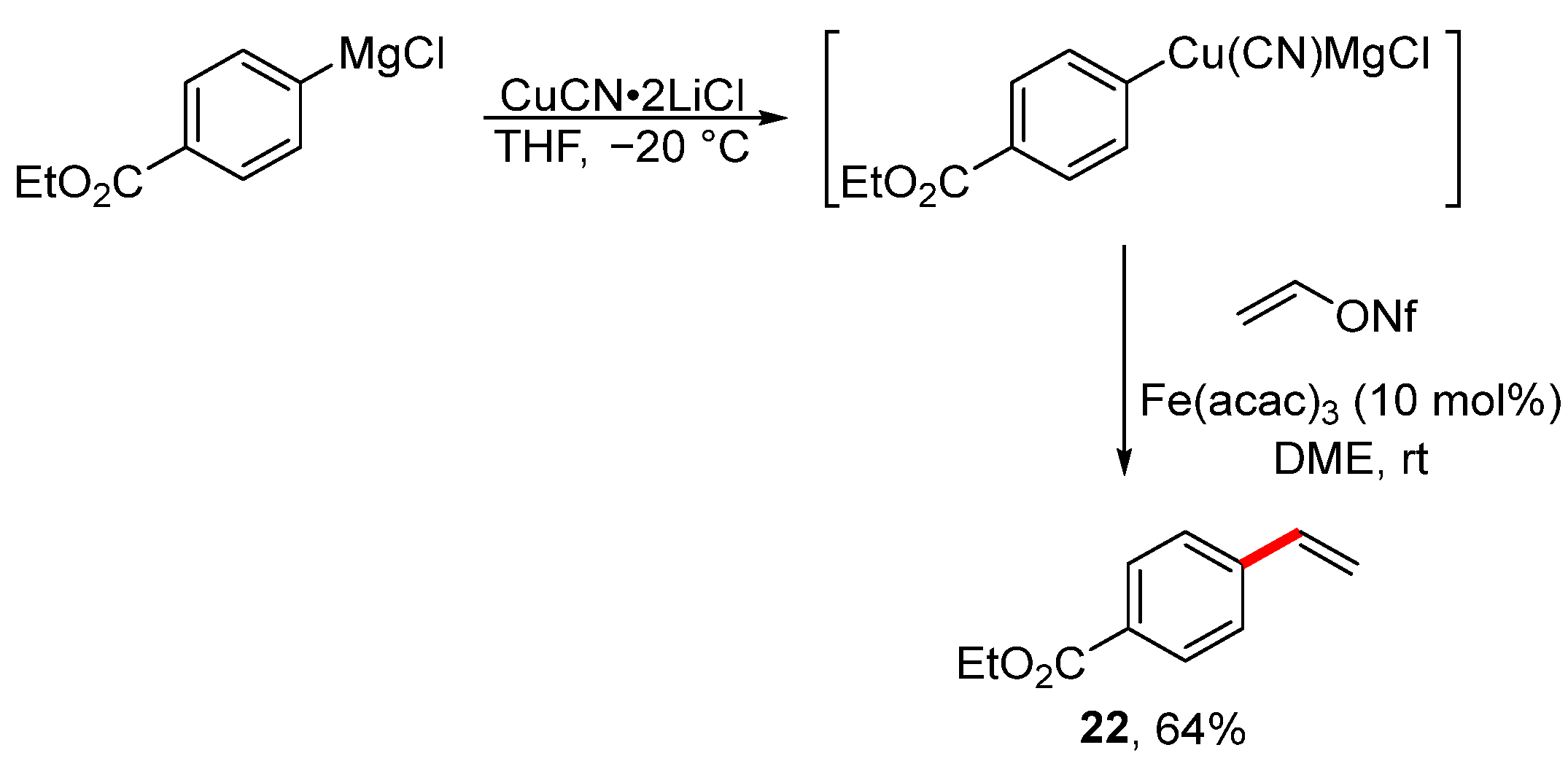
Significantly better tolerance of the functional groups is achieved during the cross-coupling reaction of vinyl nonaflate with the organocopper reagent when catalyzed by an iron complex (Scheme 8) [110]. For the preparation of functionalized Grignard reagents, it is necessary to use the approach developed by Professor Knochel [111,112,113,114]. The scope of the published cross-coupling reaction is remarkably broad, although only nitrile and ester groups have been used to date.

Scheme 8.
Iron-catalyzed reaction of vinyl nonaflate with an organocopper reagent.
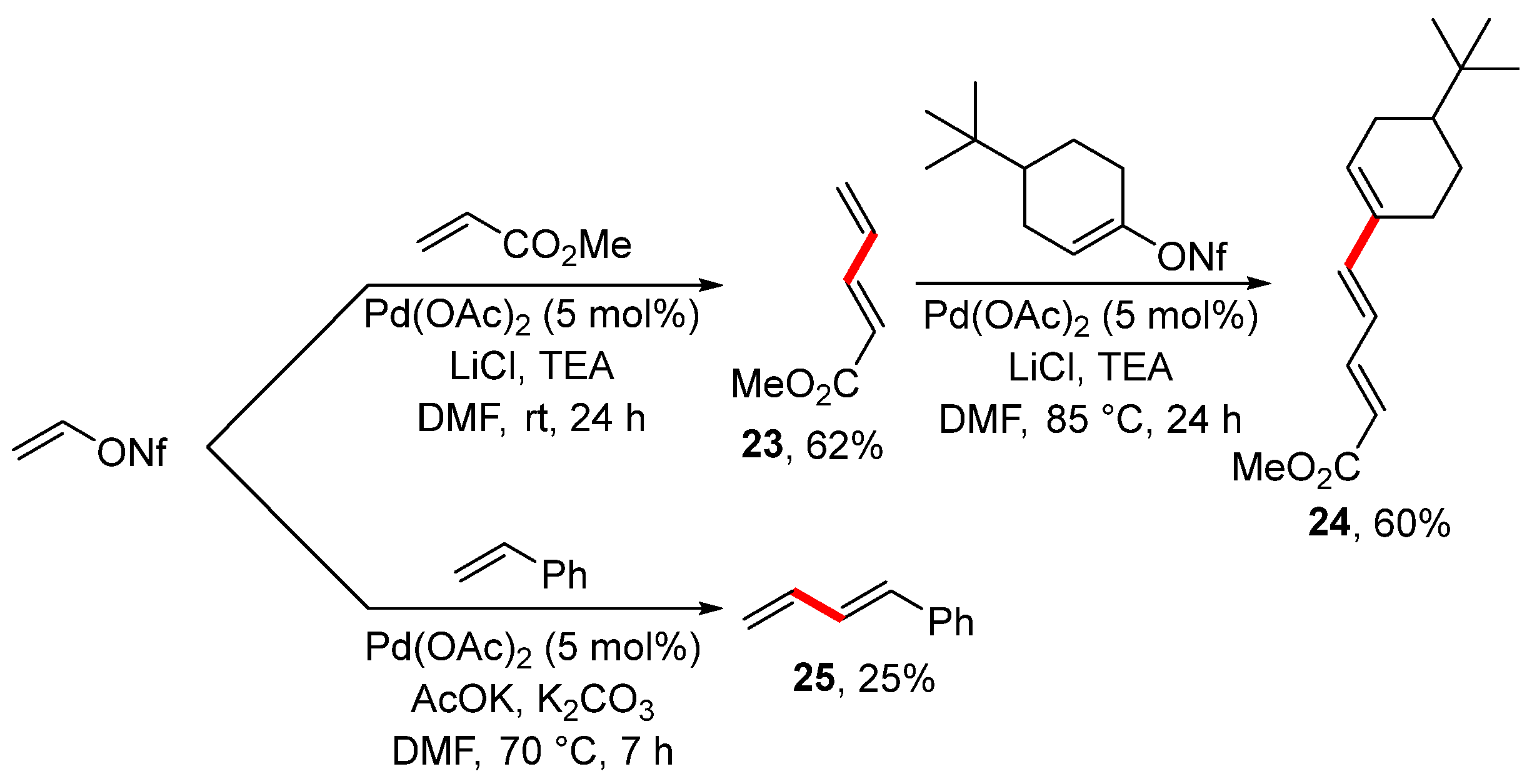
The Heck reaction is another popular alternative for the preparation of alkenes. For instance, the Heck reaction of vinyl nonaflate with methyl acrylate and styrene led to alkenes 23 and 25 (Scheme 9), as reported in 2001 [105]. In the case of alkene 23, the vinylation was further extended to prepare conjugated triene 24 under similar reaction conditions and with a good overall yield. Surprisingly, styrene provided substituted buta-1,3-diene in a low isolated yield. This was an unexpected finding because styrene reacts very well under Heck reaction conditions with heteroaryl [115], aryl [116], and vinyl halides [117]. The observed reactivity of styrene was explained by the competitive elimination of NfOH under the utilized reaction conditions.
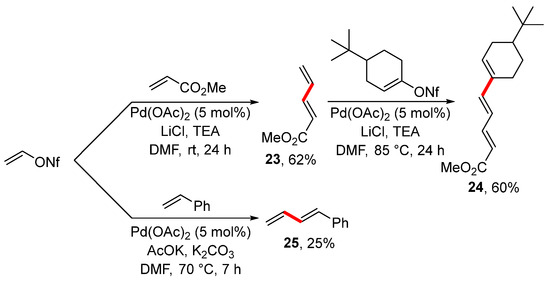
Scheme 9.
Heck reaction of vinyl nonaflate.
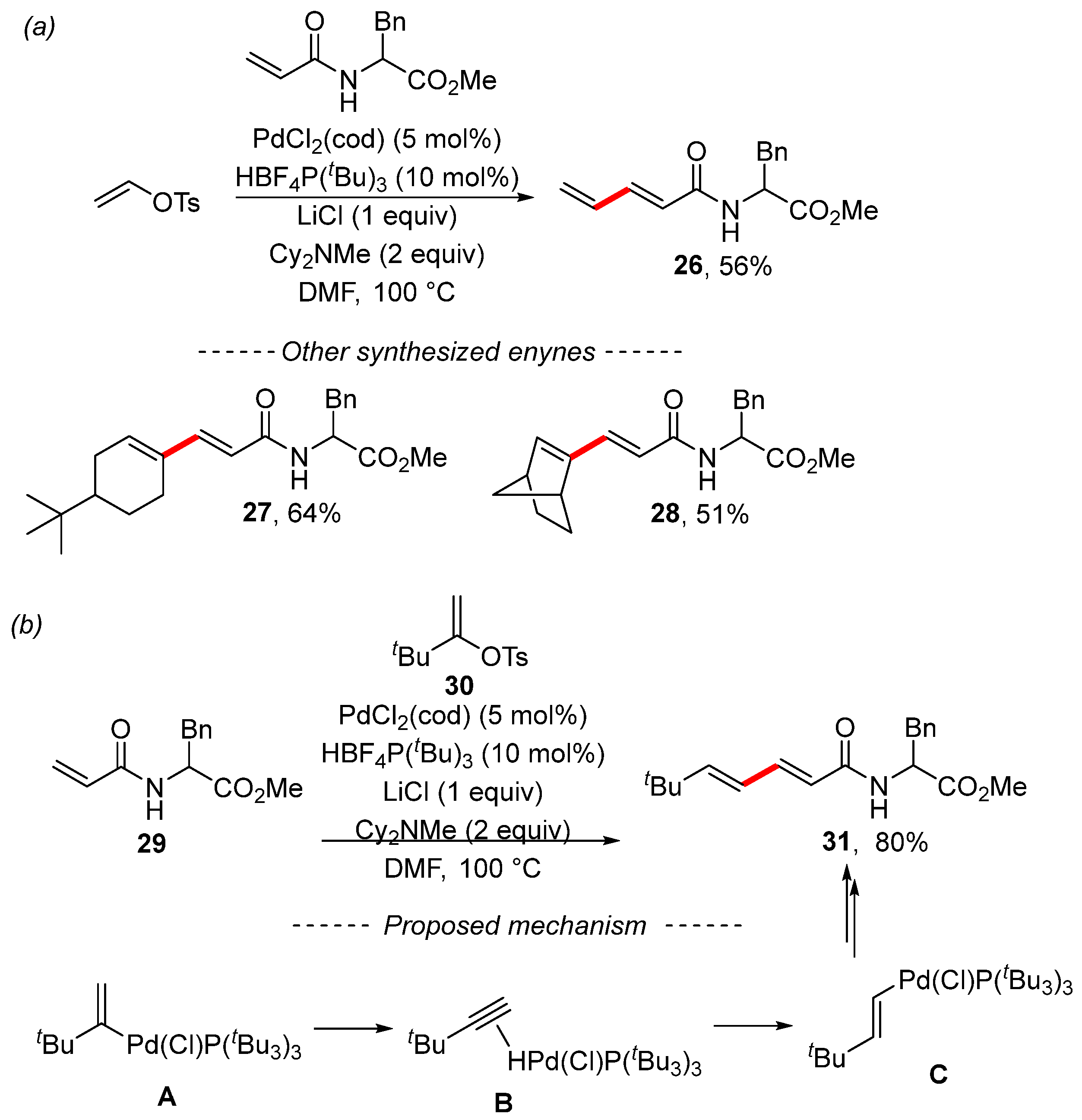
An extensive study concerning the Heck reaction was published by the Skrydstrup group [118]. The Heck reaction of vinyl tosylate gave the expected diene 26. Cyclic tosylates react similarly, as illustrated by examples 27 and 28 (Scheme 10a). However, the Heck reaction of tosylate with the bulky tert-butyl group 30 provided the isomerized product 31 (Scheme 10b). Study of the reaction mechanism by means of quantum chemical calculations showed that the isomerization begins with the oxidative addition of the palladium complex to form the T-shaped complex A. Subsequently, the β-elimination of the H–Pd moiety forms complex B. Then, the isomerization to complex C occurs through reinsertion of tert-butylacetylene with the opposite regioselectivity. Complex C further undergoes the Heck reaction.
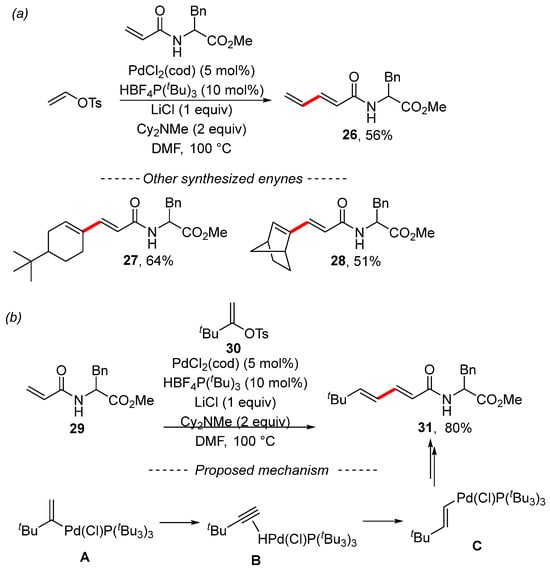
Scheme 10.
Study of the Heck reactions of various vinyl tosylates. (a) The Heck reaction of vinyl tosylate; (b) 1,2-Migration during the Heck reaction.
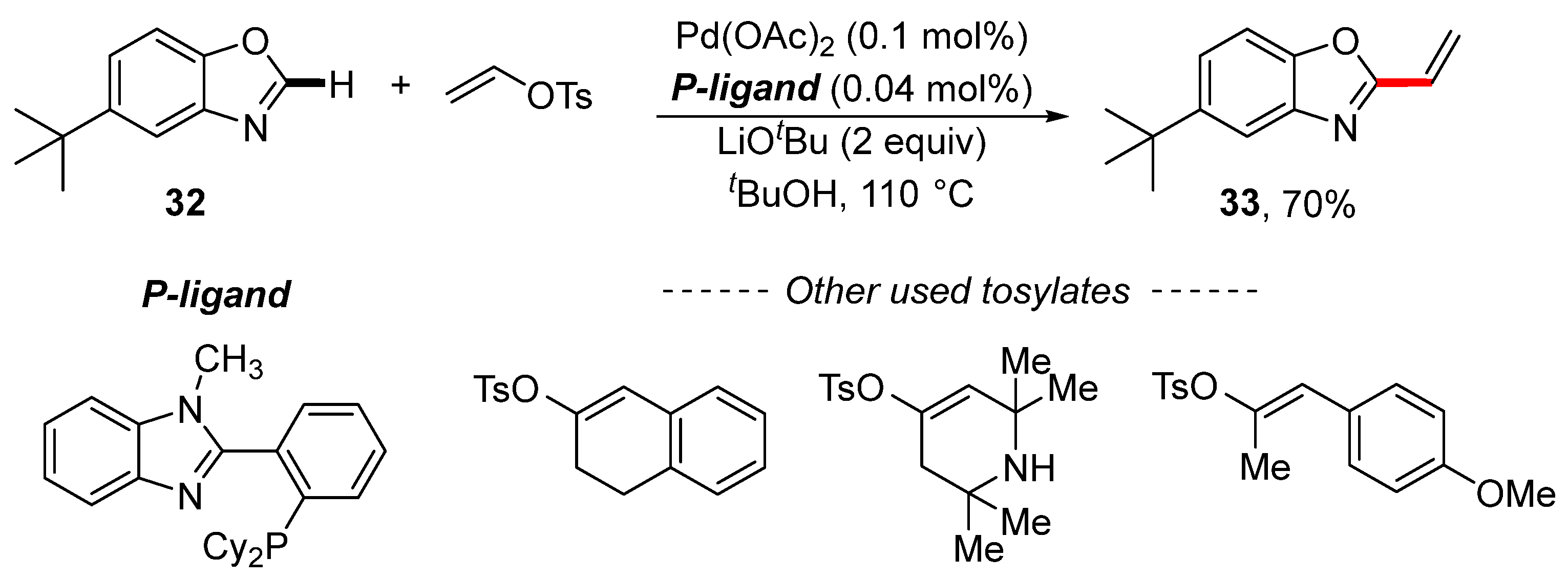
Vinyl tosylate has been successfully used in C–H activation reactions. The palladium-catalyzed vinylation of benzoxazole 32 was described by Lei and Kwong (Scheme 11) [119]. The product of vinylation reaction 33 was isolated in a 70% yield. The scope of the described reaction is limited to simple vinyl tosylates, as illustrated by the other used tosylates. In addition, selected synthesized 2-substituted benzoxazoles were used for the preparation of cyclometalated Ir(III) complexes.

Scheme 11.
Palladium-catalyzed C–H activation for the vinylation of benzoxazole.
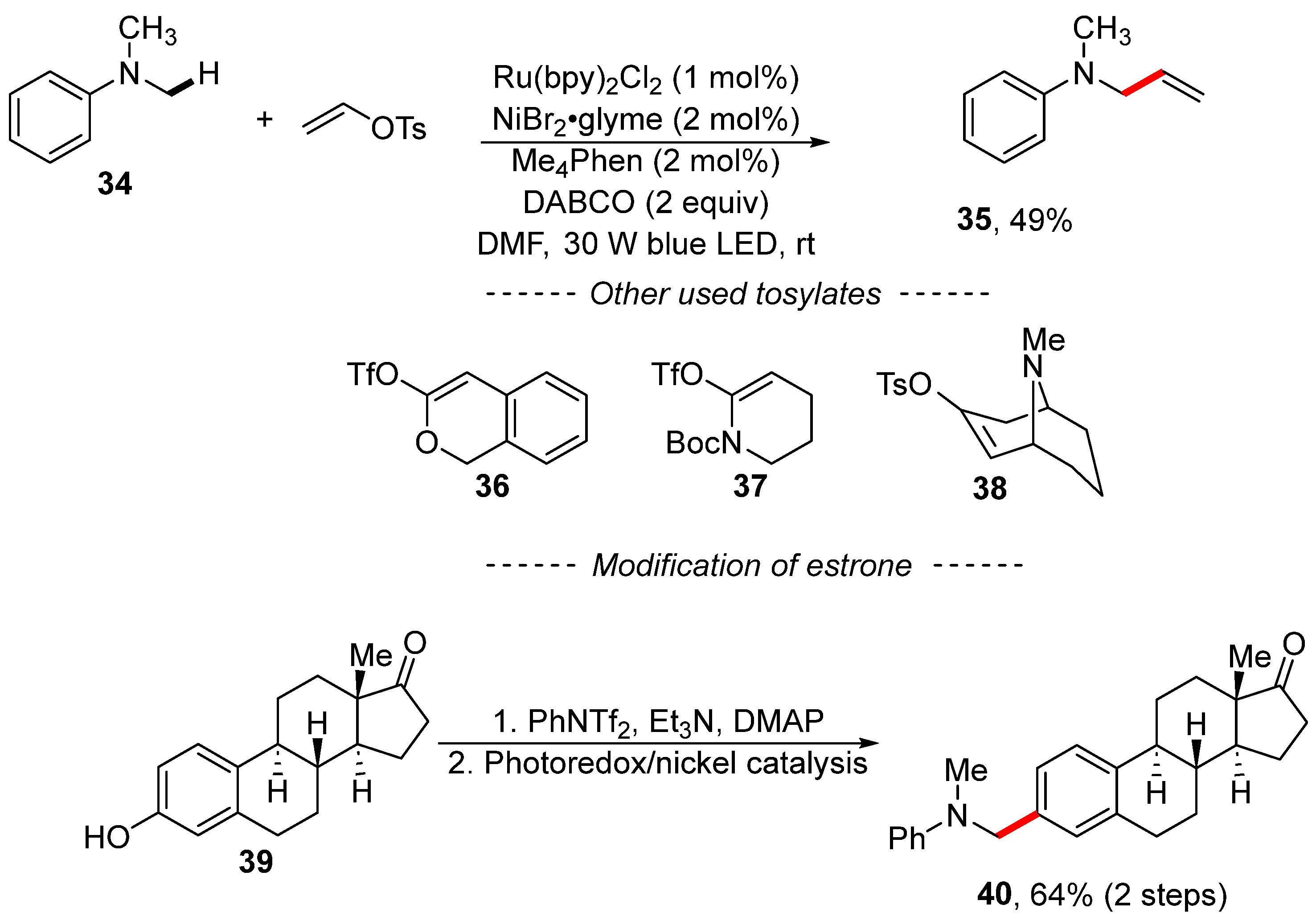
Yu developed reaction conditions for the photoredox nickel-catalyzed vinylation and arylation of anilines (Scheme 12) [120]. Here, N,N-dimethylaniline (34) reacted with vinyl tosylate in the presence of ruthenium and nickel complexes to yield N-allylaniline 35. This reaction is characterized by a wide range with respect to vinyl sulfonates, including triflates derived from lactone 36 and lactame 37. The detailed mechanism of the transformation was not discussed; however, the authors showed that the procedure can be used, for example, for the modification of estrone 39 via its conversion into the corresponding triflate followed by photoredox nickel-catalyzed C–H arylation.
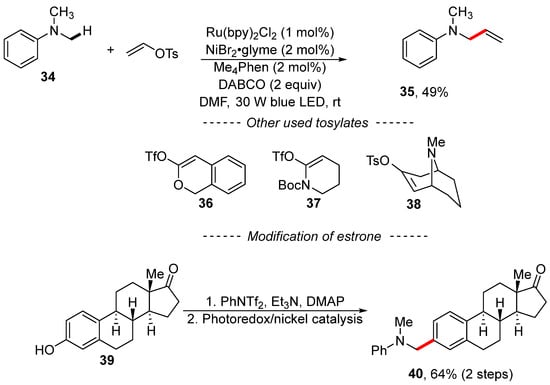
Scheme 12.
Photoredox/nickel dual catalysis en route to C–H vinylation.
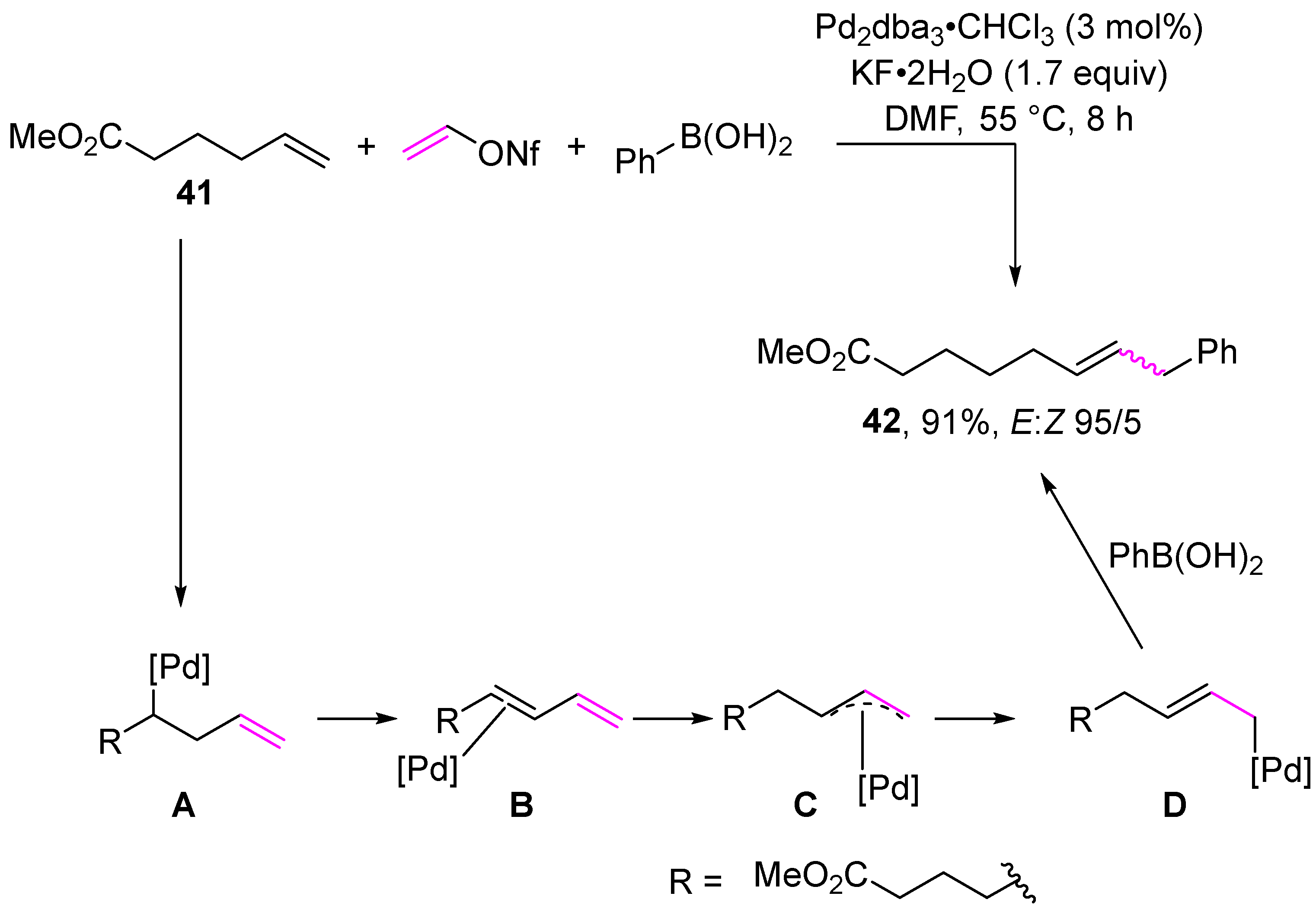
Multicomponent reactions represent another class of transformation of vinyl sulfonates. Examples of multicomponent reactions are presented in Scheme 13 and Scheme 14. The first multicomponent reaction of vinyl sulfonates involves the reaction of vinyl nonaflate, phenylboronic acid, and alkene in the presence of a catalytic amount of palladium complex to give alkene 41 in a quantitative yield (Scheme 13) [104]. The proposed mechanism begins with the formation of complex A via the oxidative addition of the palladium(0) complex followed by migratory insertion. Then, β-hydride elimination forms complex B, which is hydropalladated to provide the η3-allyl complex C. Subsequently, transmetalation followed by reductive elimination gives the product of reaction 41.
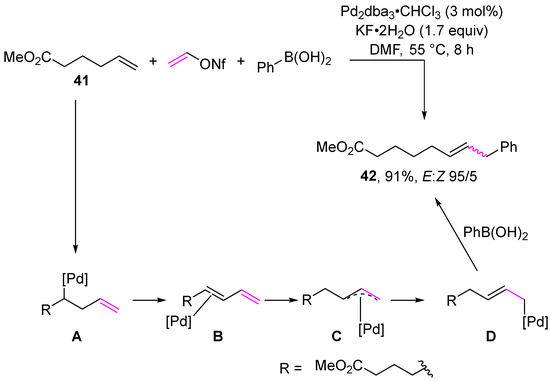
Scheme 13.
The use of vinyl nonaflate in a multicomponent reaction.
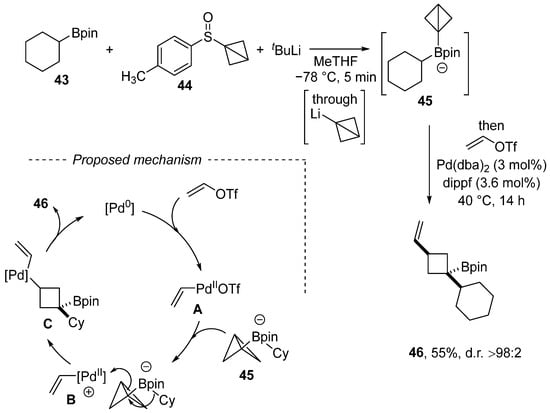
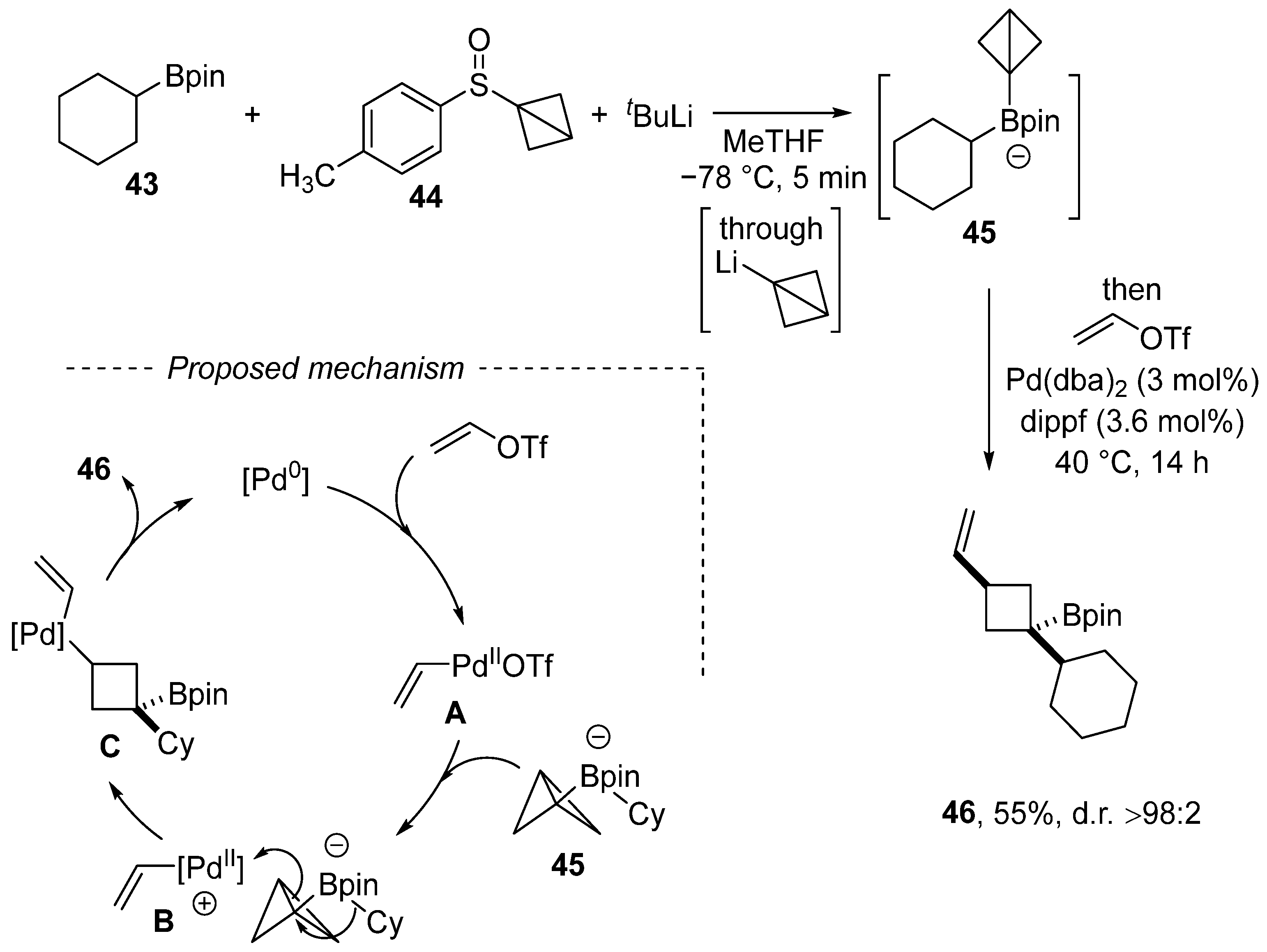
Scheme 14.
Carbopalladation of a C–C bond en route to vinylated cyclobutene.
Aggarwal et al. discovered a new multicomponent reaction of vinyl triflate, which involves the C–C bond carbopalladation of strained bicyclo[1.1.0]butyl boronate (Scheme 14) [107]. A selected example of vinylation involves the reaction of vinyl triflate with the ate complex 44 generated from sulfon 44, tert-butyllithium, and cyclohexan-1-ylboronic acid pinacol ester 43. The reaction mechanism entails the oxidative addition of the Pd(0) complex to vinyl triflate, which is followed by carbopalladation by means of the cationic Pd complex B. The final product 46 is formed via reductive elimination. Although the presented example is limited to vinyl triflate, the paper focused on aryl triflates and high diastereoselectivity (d.r. > 98:2) was achieved in all cases.
3. Cross-Coupling Reactions of Vinyl Acetates
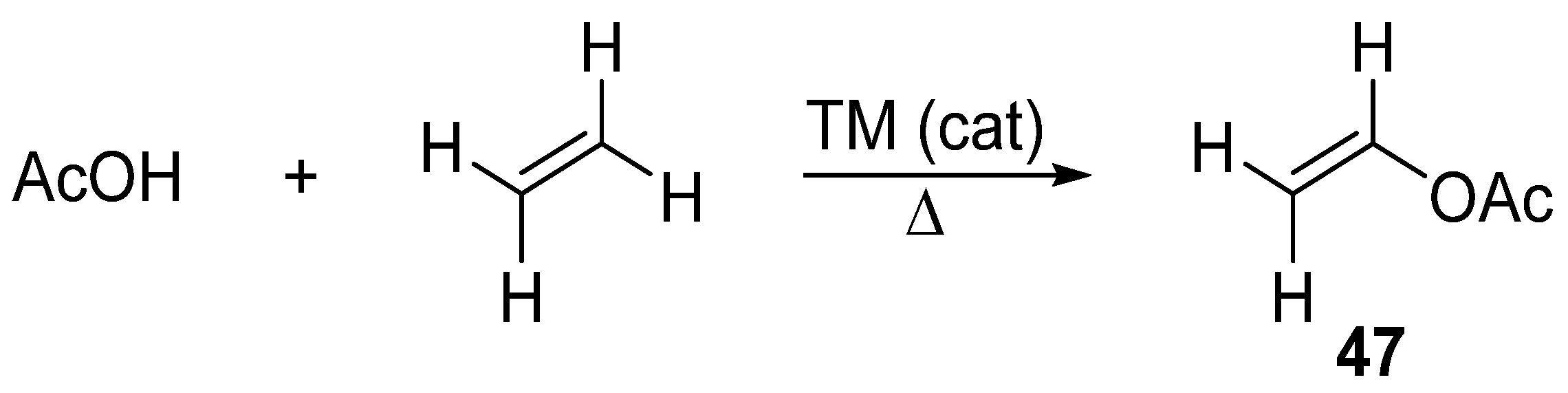
Vinyl acetate is a popular reagent for the vinylation of various substrates. The reason for its popularity is its commercial availability and ease of preparation from acetic acid and ethylene under transition metal catalysis, including the use of Pd–Au catalysts (Scheme 15) [121].

Scheme 15.
Synthesis of vinyl acetate.
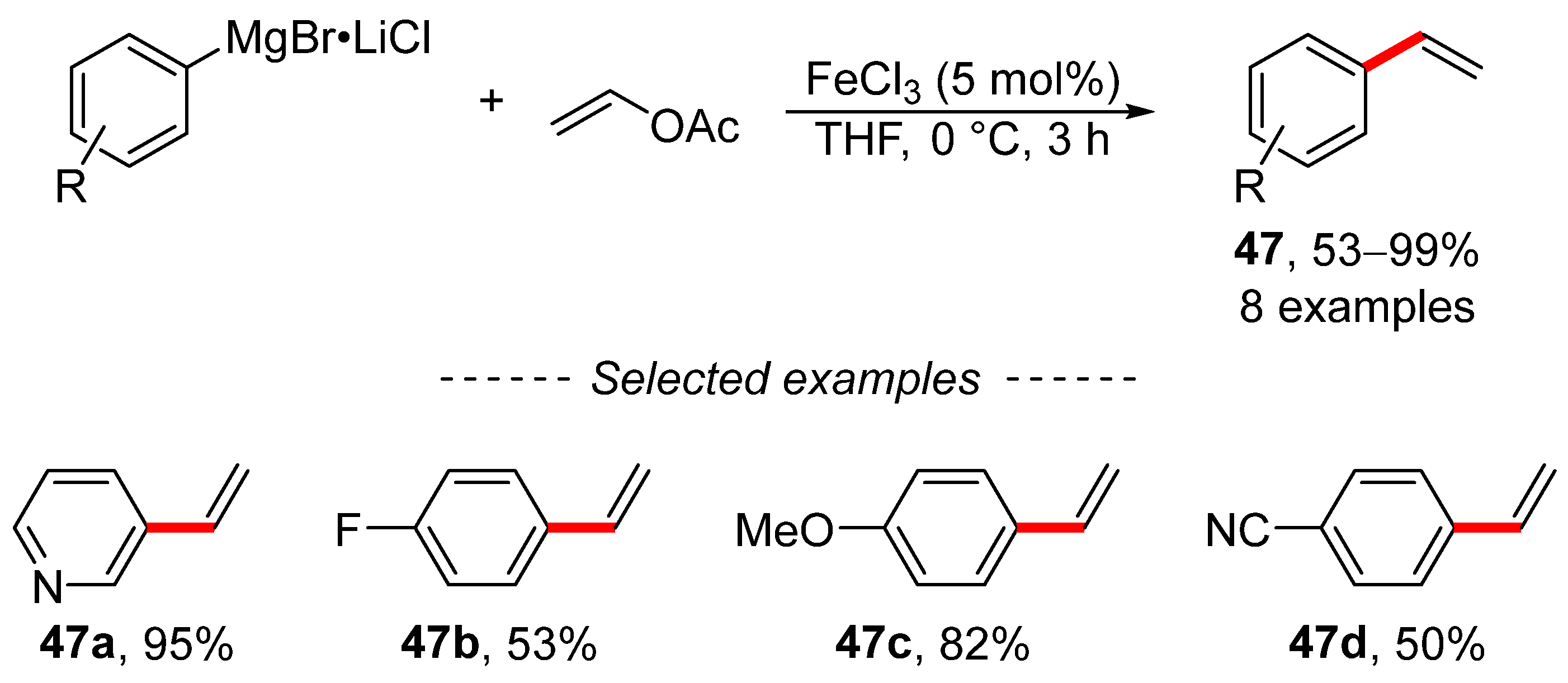
The Kumada reaction of vinyl acetate, as an example of traditional cross-coupling chemistry, was extensively studied by Wangelin (Scheme 16) [122]. The reaction was catalyzed by ferric chloride and the optimized reaction conditions were used to prepare a series of di- and trisubstituted alkenes, including monosubstituted ethylene derivatives 47. The authors obtained valuable data through mechanistic studies. The study of the reaction mechanism revealed the following patterns: (i) kinetic poisoning studies showed that the studied reaction is a homogeneously catalyzed reaction; (ii) the coordination of the alkene is the rate determining step; and (iii) vinyl acetates are more reactive than vinyl pivalates and vinyl carbamates.
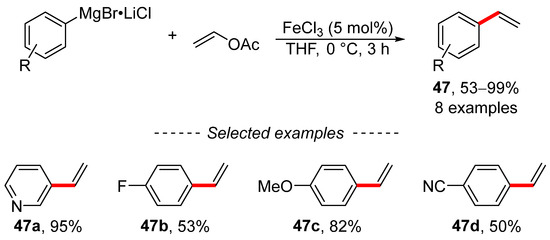
Scheme 16.
Cross-coupling reaction of vinyl acetate with arylmagnesium bromides.
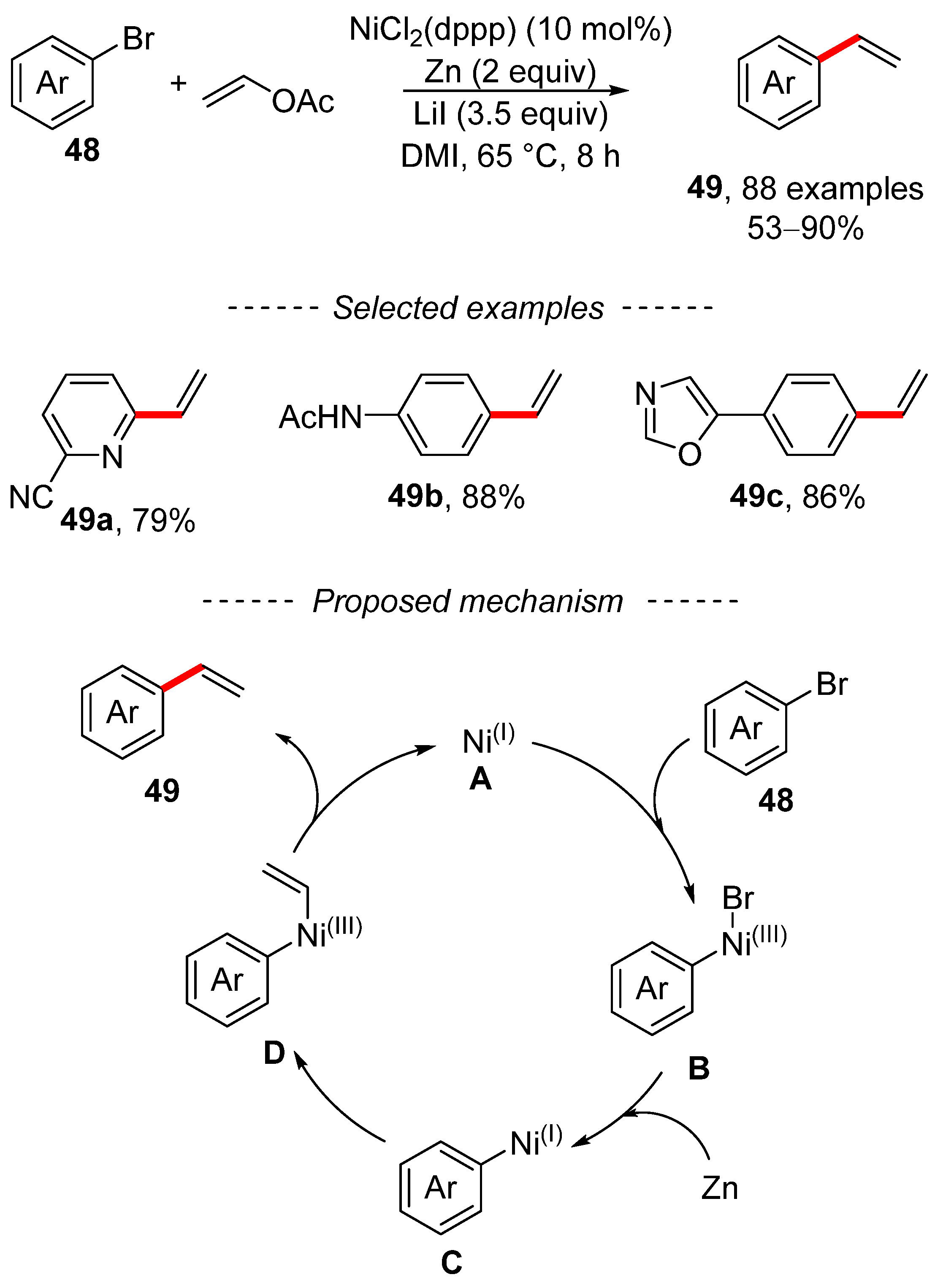
In addition to the Kumada reaction, vinyl acetate can also be used in reductive cross-coupling reactions. A recent example describes the vinylation of organobromide 48 when catalyzed by a nickel complex (Scheme 17) [123]. The described vinylation is characterized by simple experimental conditions with a wide scope. Moreover, 88 compounds were prepared, including vinylated aromatic and heteroaromatic hydrocarbons. The proposed mechanism is based on standard knowledge in the field of reductive cross-coupling reactions.

Scheme 17.
Nickel-catalyzed reductive cross-coupling of vinyl acetate with aryl bromides.
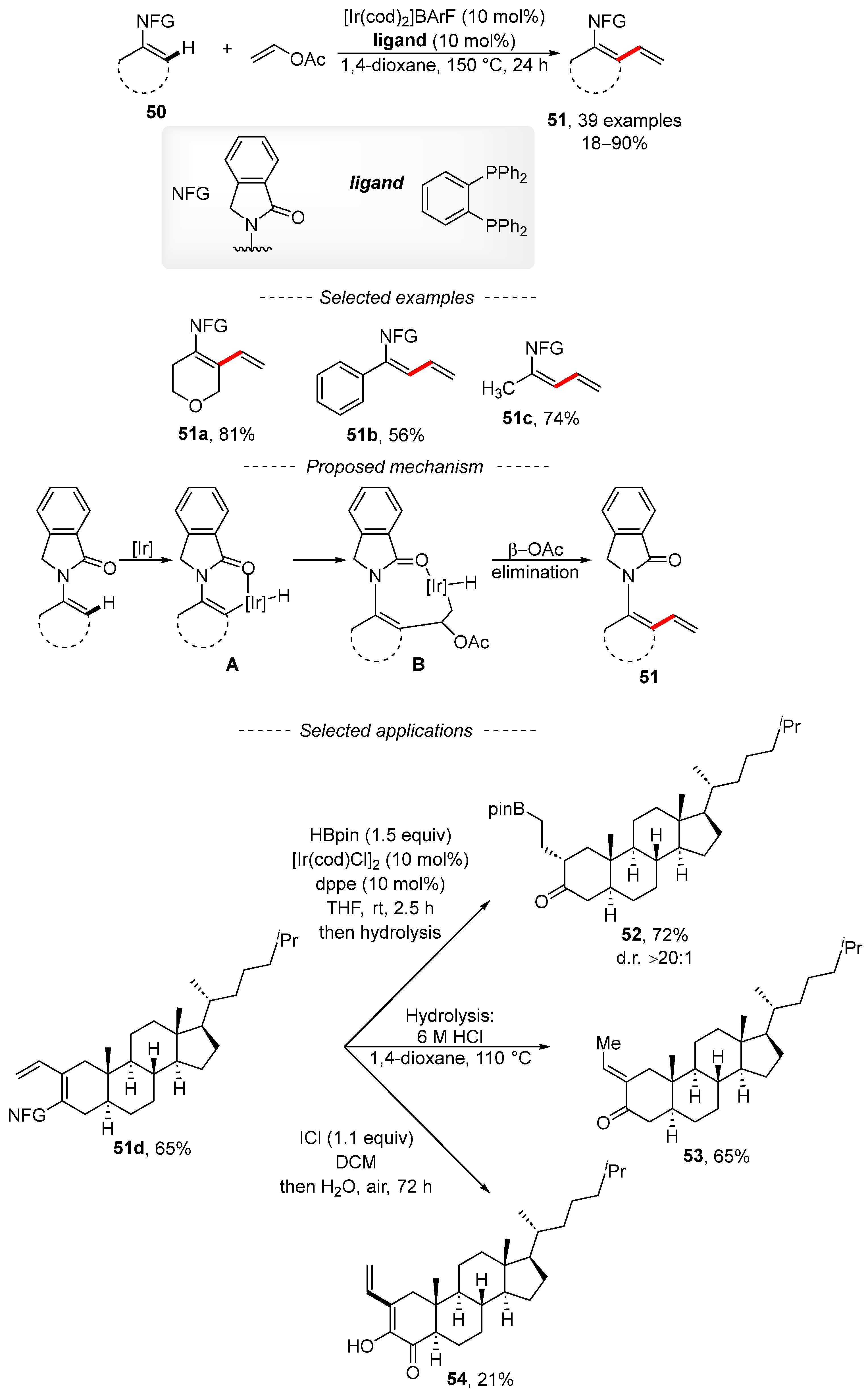
The activation of the C–H bond is a popular method for the introduction of a vinyl group by means of vinyl acetate together with rhodium, iridium cobalt, or palladium complexes. The published iridium-catalyzed reaction describes the vinylation of enamides 50 (Scheme 18) [124]. The reaction conditions tolerate a variety of functional groups, including nitro, ester, halogen, nitrile, and Bpin groups. The scope of the reaction is not limited to the preparation of cyclic dienes 51a, as the acyclic compounds 51b and 51c are also available via this method. Based on quantum chemical calculations and experimental results, a reasonable mechanism was proposed. Initially, the activation of the C–H bond gives complex A. Subsequently, the migratory insertion of vinyl acetate into the Ir–C bond is followed by β-elimination to form the reaction product 51. The authors also showed that the reaction can be used for the preparation of cholestan-3-one derivatives. First, cholestan-3- was converted into the conjugated diene 51d under the developed reaction conditions. Then, the exocyclic vinyl group was modified by means of hydroboration to give borane 52. Diene 51d can also be hydrolyzed to unsaturated ketone 53, with its oxidation affording the cholestane derivative 54 in only a 21% isolated yield.
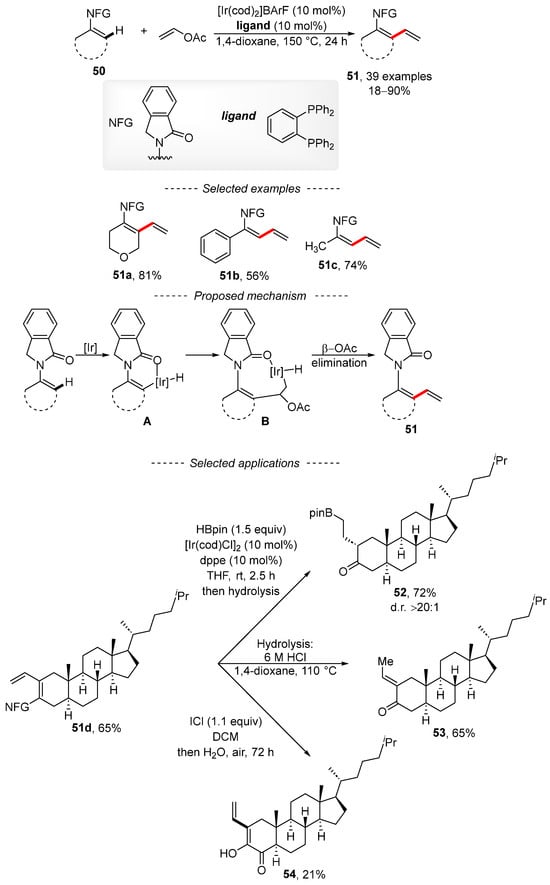
Scheme 18.
Iridium-catalyzed C–H vinylation of enamides.
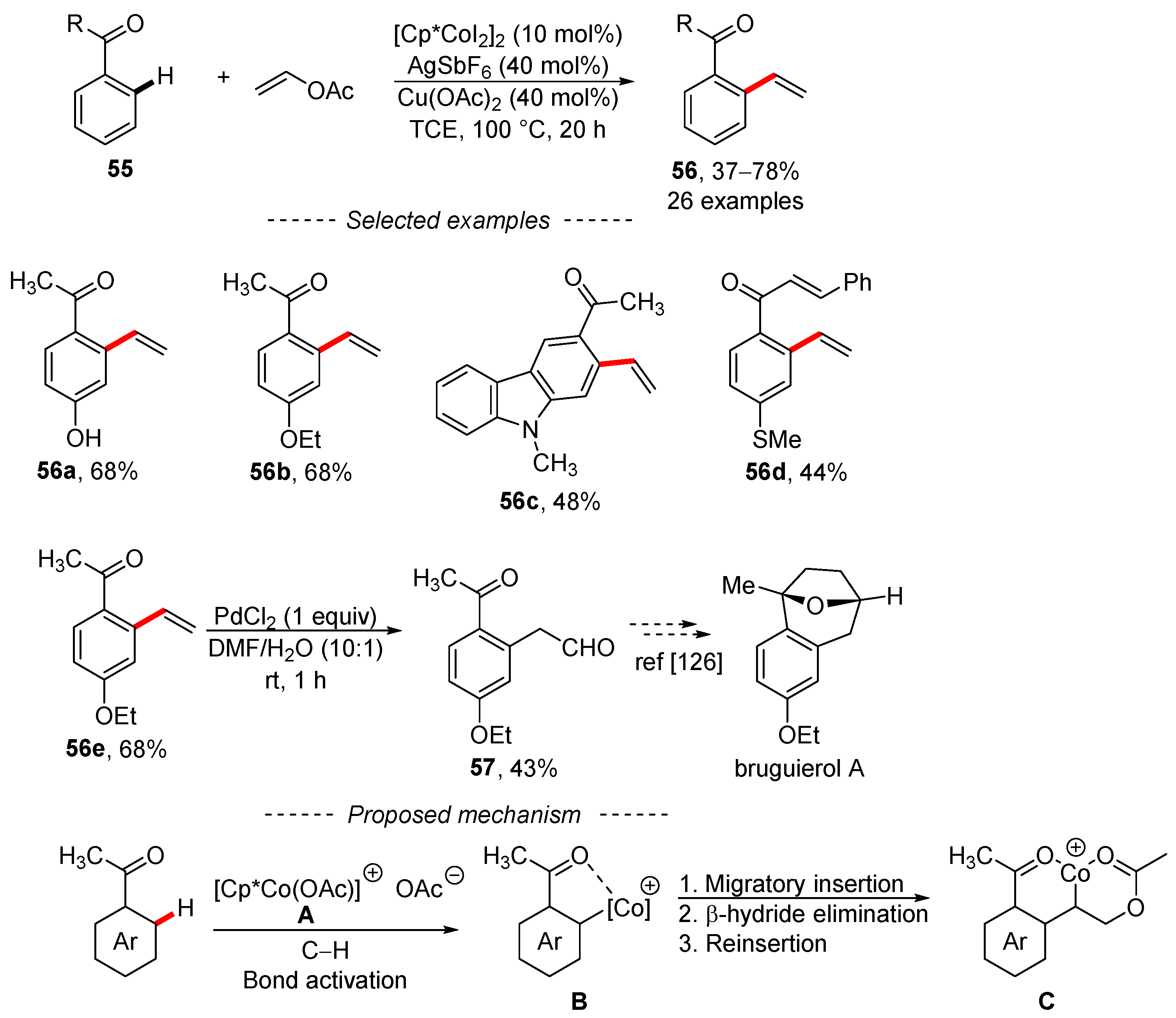
The C–H vinylation of aromatic ketones can also be catalyzed by a cobalt complex under ligandless conditions (Scheme 19) [125]. The optimized reaction conditions were mostly used for the modification of substituted acetophenones 55, although the successful vinylation of carbazole 56c showed that heteroaromatic ketones can also be used. Modification of the vinyl group afforded aldehyde 57, which completed the formal synthesis of bruguierol A [126]. The results of mechanistic studies are in agreement with the findings reported in the literature. Thus, the presence of silver salt and copper acetate is essential for the formation of catalyst A from the starting complex [Cp*CoI2]2. Activation of the C–H bond followed by migratory insertion, β-hydride elimination, and reinsertion gives complex C. Subsequent β-acetate elimination provides the main reaction product 56.
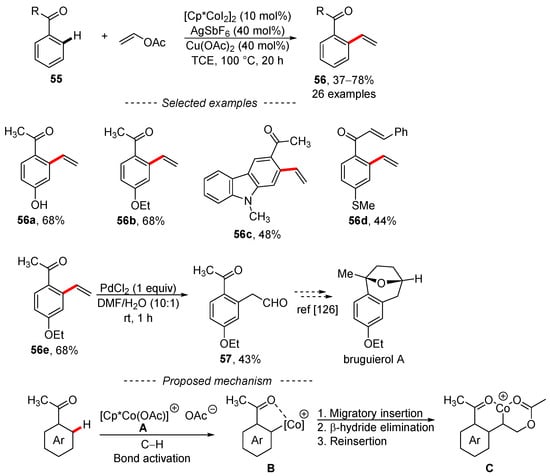
Scheme 19.
Cobalt-catalyzed C–H vinylation of acetophenones.
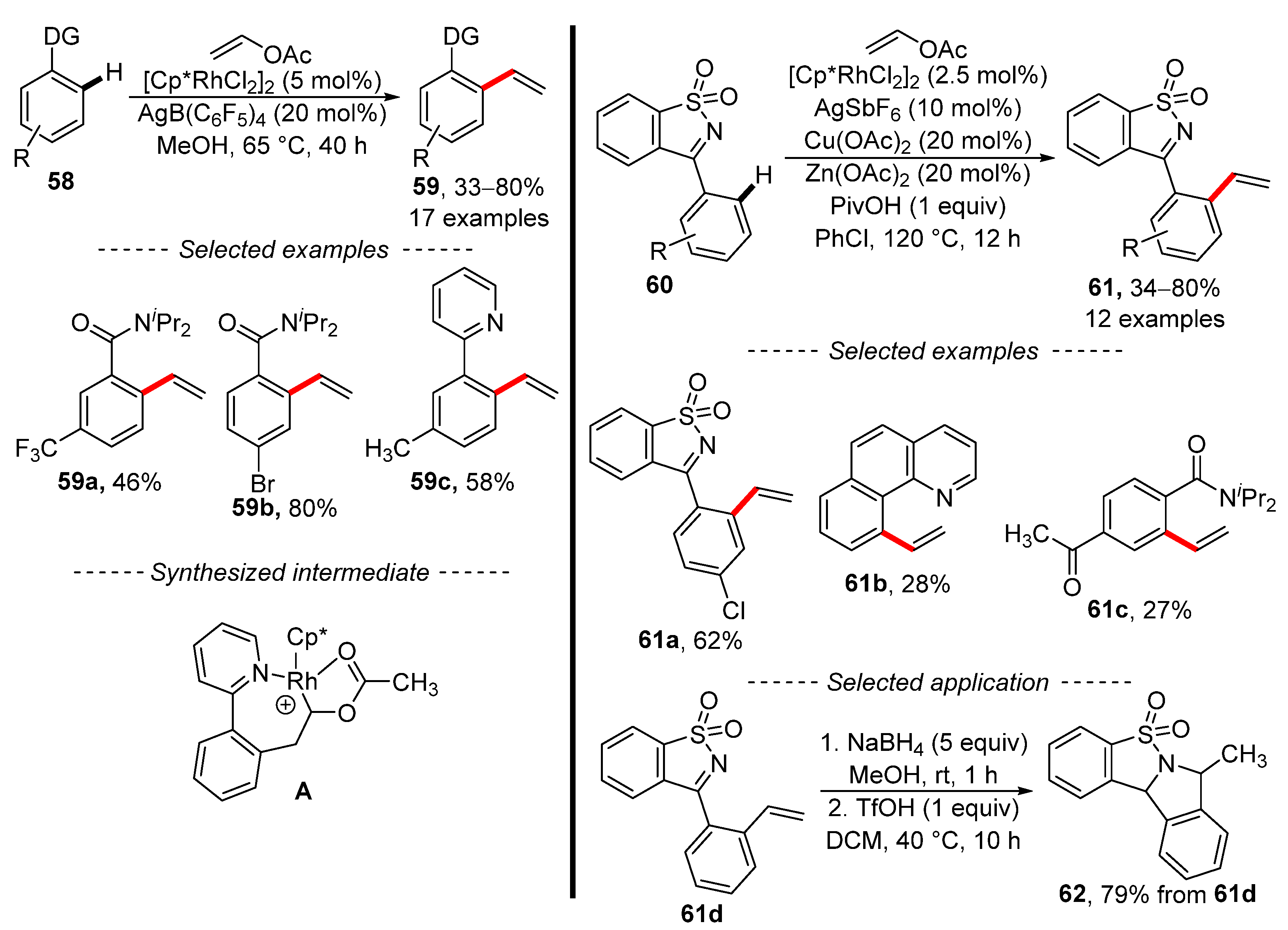
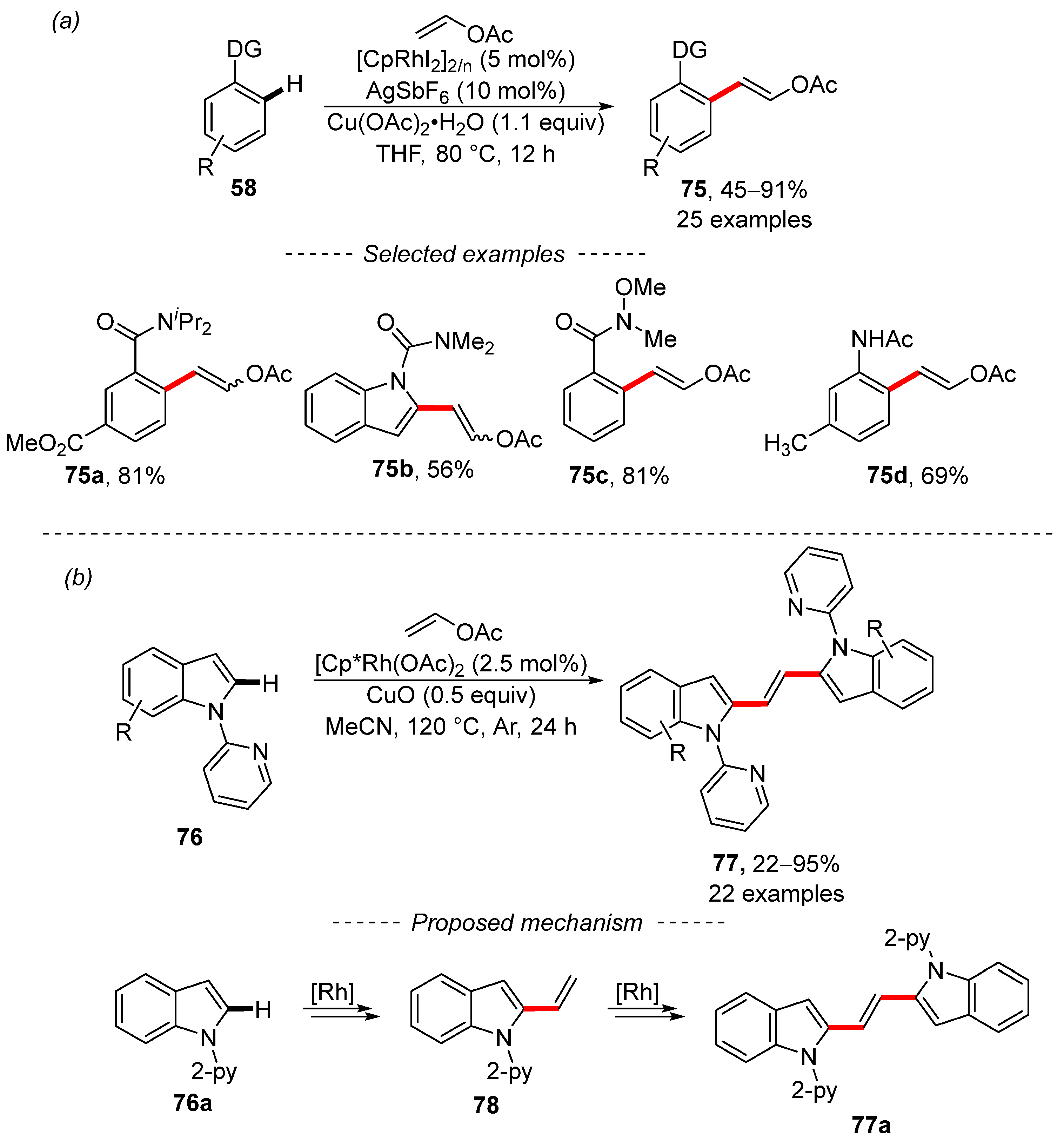
Ellman [127] and Wei [128] used vinyl acetate for the chemoselective vinylation of aromatic substrates under the catalysis of the same rhodium complex (Scheme 20). In the first case, substituted benzenes 58 with an amide or 2-pyridyl directing group were vinylated. Unfortunately, the scope of this reaction is limited to a few functional groups, which are shown as selected examples 59a, 59b, and 59c. However, the authors were able to demonstrate that rhodacycle A is a catalytic resting-state species and that it catalyzes the described reaction. In a similar work, Wei et al. showed that the same rhodium complex catalyzed the vinylation of aromatic hydrocarbons with a sulfonyl ketimine-directing group 60. The optimized reaction conditions are significantly more complex than in previous work [127]. In addition, the developed reaction conditions can be used for the vinylation of heteroaromatic compounds 61b and benzamides 61c. The applications of synthesized styrenes include the reduction of the imino group of styrene 61d followed by acid-mediated cyclization into the fused heterocyclic compound 62.

Scheme 20.
Selective rhodium-catalyzed vinylation of aromates by means of vinyl acetates.
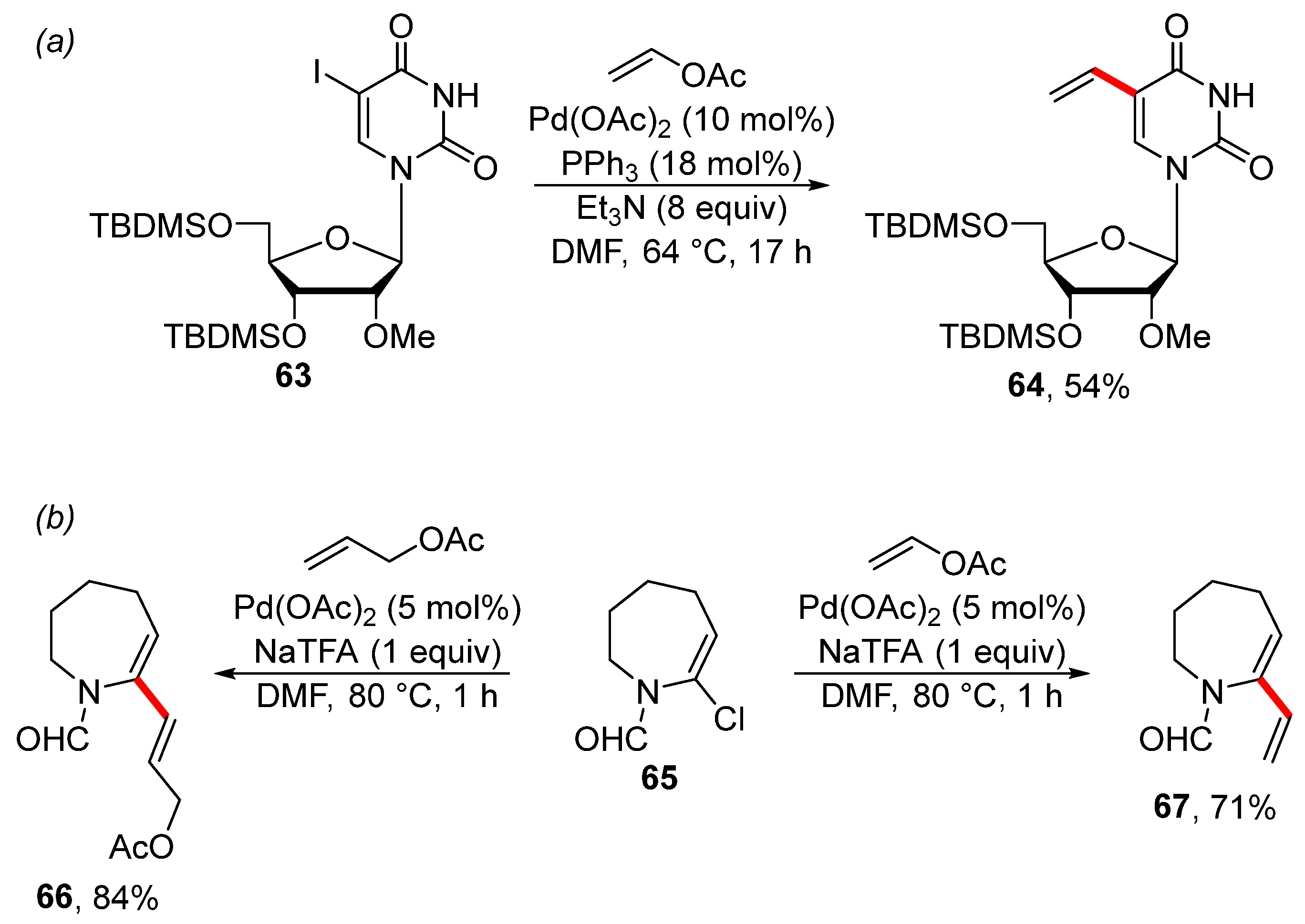
Vinyl acetate was used for the efficient vinylation of iodouridine 63, giving vinylated analogue 64 in a 54% isolated yield (Scheme 21a). The isolated alkene 64 was further modified into a new isoindoline-derived benzimidazole nitroxide spin label [129]. An interesting dichotomy was observed during the Heck coupling of chlorinated enamine 65. The use of allyl acetate gave the Fujiwara–Moritani reaction product 66, while the use of vinyl acetate gave the product of β-OAc elimination 67 (Scheme 21b) [130].

Scheme 21.
Palladium-catalyzed vinylation of organohalides. (a) Palladium-catalyzed vinylation of iodouridine; (b) The Heck reaction of chlorinated enamine.
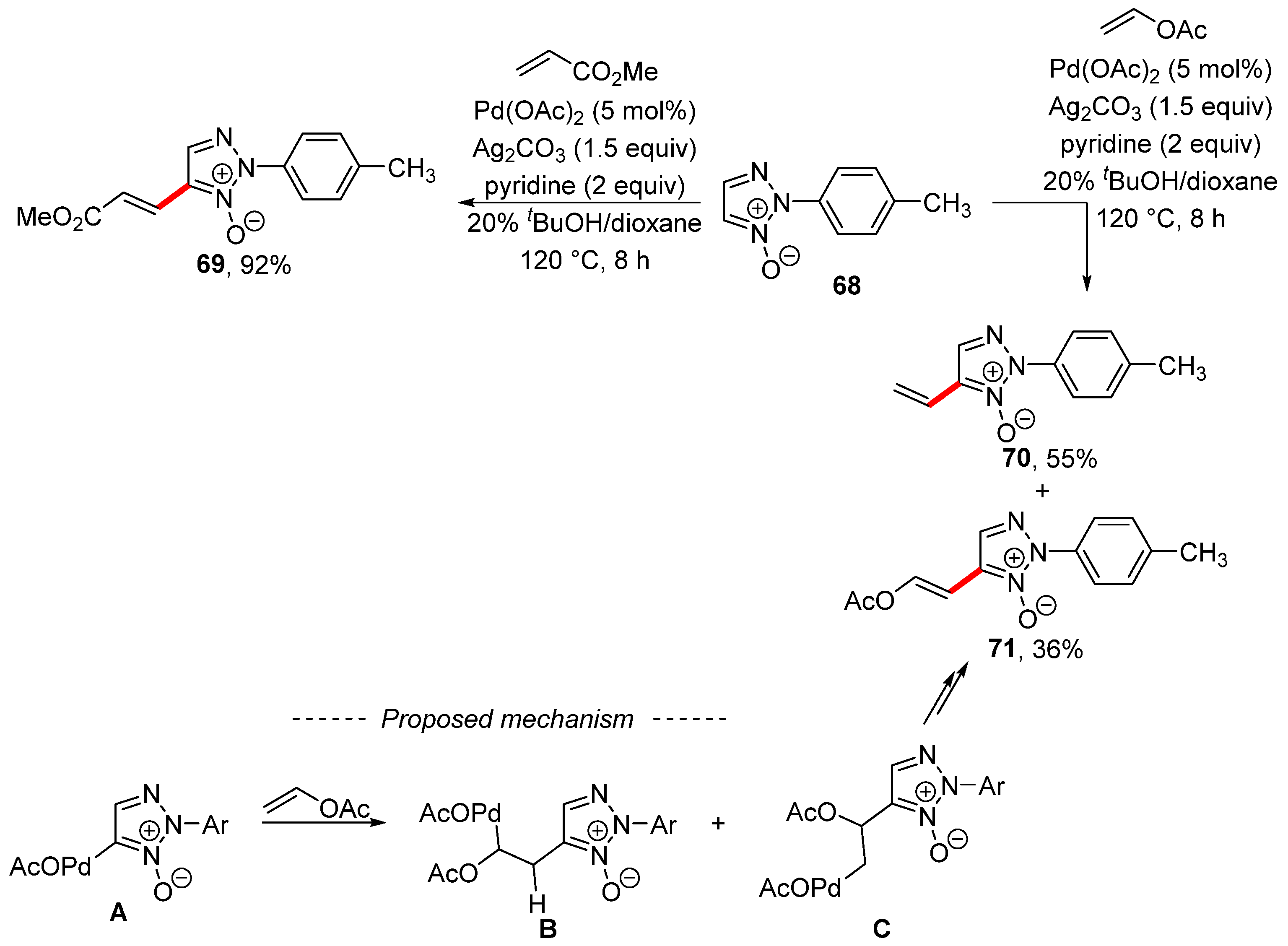
Interesting reactivity was observed during the palladium-catalyzed modification of 1,2,3-triazole N-oxide 68 in the presence of silver carbonate as an oxidant (Scheme 22) [131]. The starting triazole 68 reacted with styrene or methyl acrylate, as well as with other acrylic acid esters, to produce the Fujiwara–Moritani reaction product 69. However, the use of an electron-rich alkene—namely, vinyl acetate—gave a mixture of the Fujiwara–Moritani reaction product 71 and the vinylation product 70. The formation of both regioisomers was explained by the activation of the C–H bond by means of palladium acetate. Subsequent migratory insertion of the formed complex A and vinyl acetate gave regioisomers B and C. Then, products 70 and 71 were obtained via β-elimination. It is important to note that the same group described the formation of 4-vinyl-3-(p-tolyl)sydnone during an analogous palladium-catalyzed reaction of vinyl acetate with 3-(p-tolyl)sydnone [132].
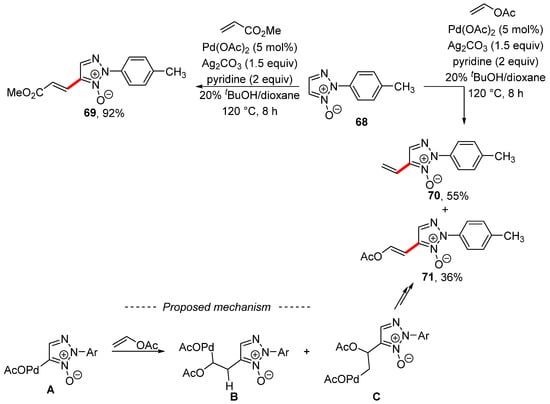
Scheme 22.
Vinylation versus Heck-type coupling during the C–H activation of 1,2,3-triazole N-oxide.
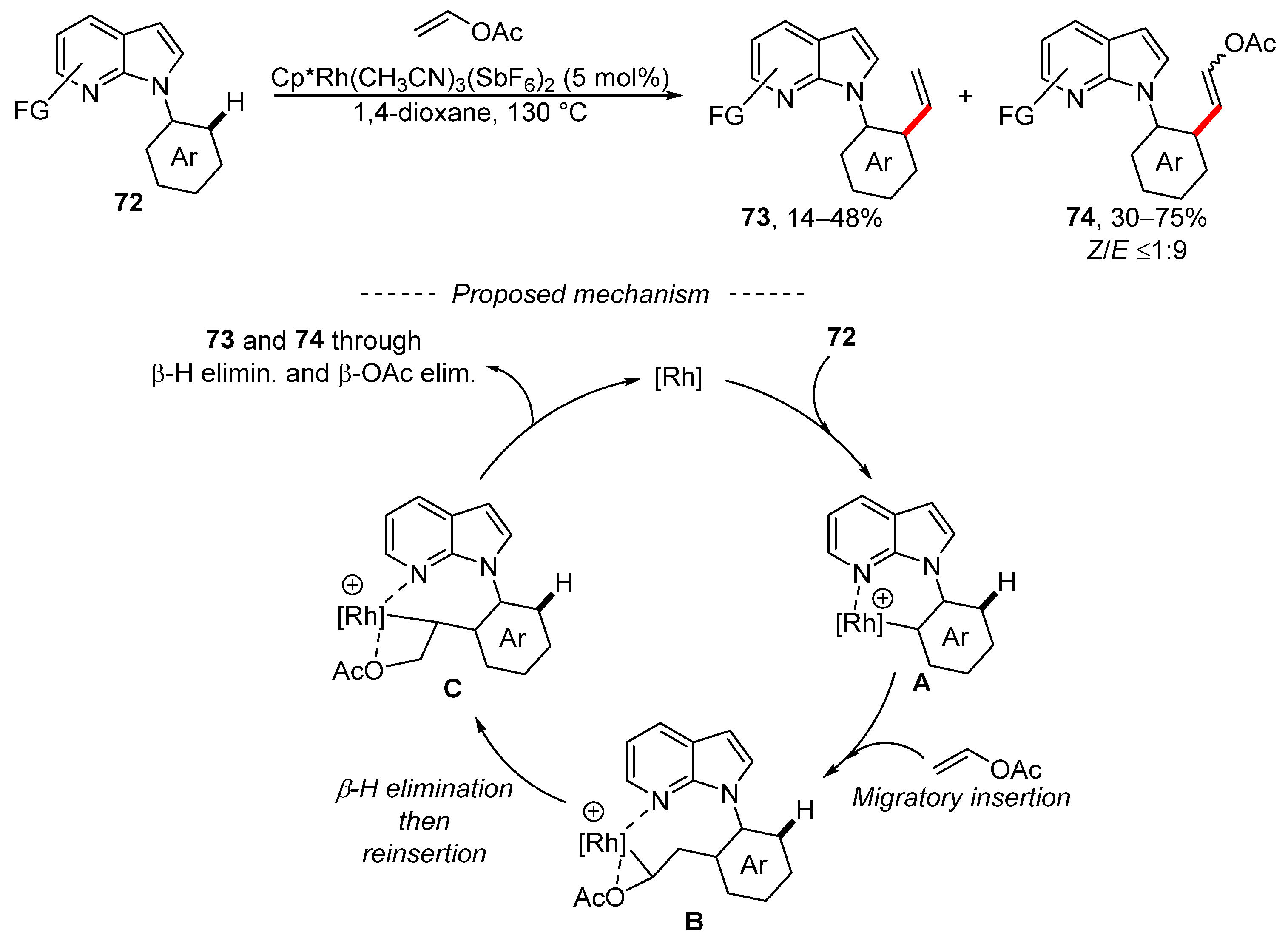
An analogous competition between vinylation and Fujiwara–Moritani alkenylation was also observed during the rhodium-catalyzed reaction of vinyl acetate with 7-azaindoles 72 (Scheme 23) [133]. Optimization of the reaction conditions gave regioisomers 73 and 74 in all cases. The optimized reaction conditions were also successfully used for substituted 7-azaindoles, vinyl benzoate, vinyl pivalate, vinyl butanoate, and substituted vinyl acetate. In total, 13 substituted 7-azaindoles were prepared as a mixture of isomeric products 73 and 74, where the latter was the major product. The disubstituted alkenes 74 were always formed as a mixture of the two stereoisomers, where the E isomer dominates. The proposed mechanism for the formation of both products involves several important steps. Initially, complex A is formed via the activation of the C–H bond. Then, the coordination of vinyl acetate to complex A and the subsequent migratory insertion gives complex B, which isomerizes into complex C. Both stereoisomers are formed by means of β-H elimination or β-OAc elimination.
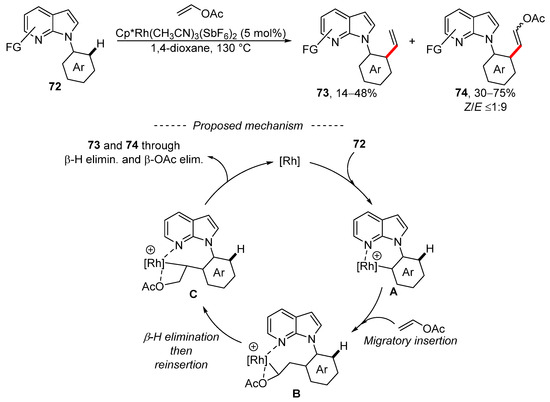
Scheme 23.
Rhodium-catalyzed vinylation of carboxamides.
Wen and Zhang succeeded in the selective C–H olefination of electron-rich alkenes, including vinyl acetate, when catalyzed by rhodium complexes (Scheme 24a) [134]. An amide group serves as the main directing group, as illustrated by selected examples 75a and 75b and the sophisticated Weinreb amide group 75c. By contrast, acetamido group 75d can also be used to facilitate C–H activation. A series of mechanistic studies was also performed in this work, the results of which essentially support previous results [133]. In further work, the same authors again used the rhodium complex for the bisindolylation of vinyl acetate (Scheme 24b) [135]. This later work is characterized by the extensive scope and good tolerance of the sensitive functional groups, including the aldehyde, ester, and nitrile groups. Moreover, 2-pyridyl is used as the directing group. The proposed catalytic cycle assumes the formation of vinylated indole 78 as the key intermediate, which is further converted into the product. In a subsequent publication, the authors showed that the rhodium complex [Cp*RhCl2]2 in combination with AgSbF6, Cu(OAc)2, and vinyl acetate is a suitable system for Heck-type coupling, leading to substituted vinyl acetates [136].
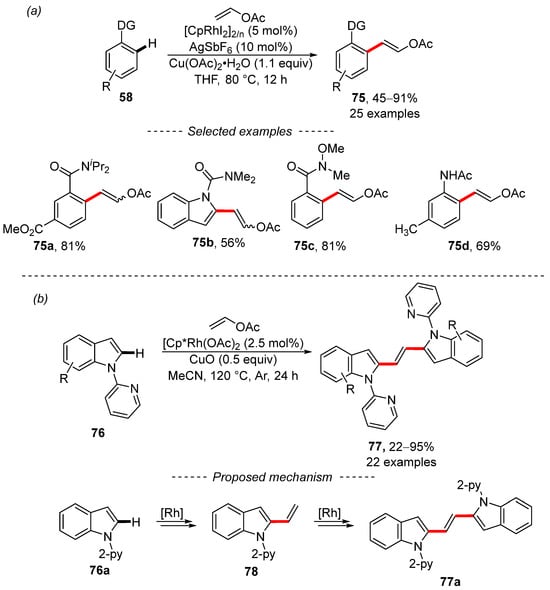
Scheme 24.
Rhodium-catalyzed Fujiwara–Moritani coupling. (a) Rhodium-catalyzed C–H olefination of aromates; (b) Rhodium-catalyzed C–H olefination of indole.
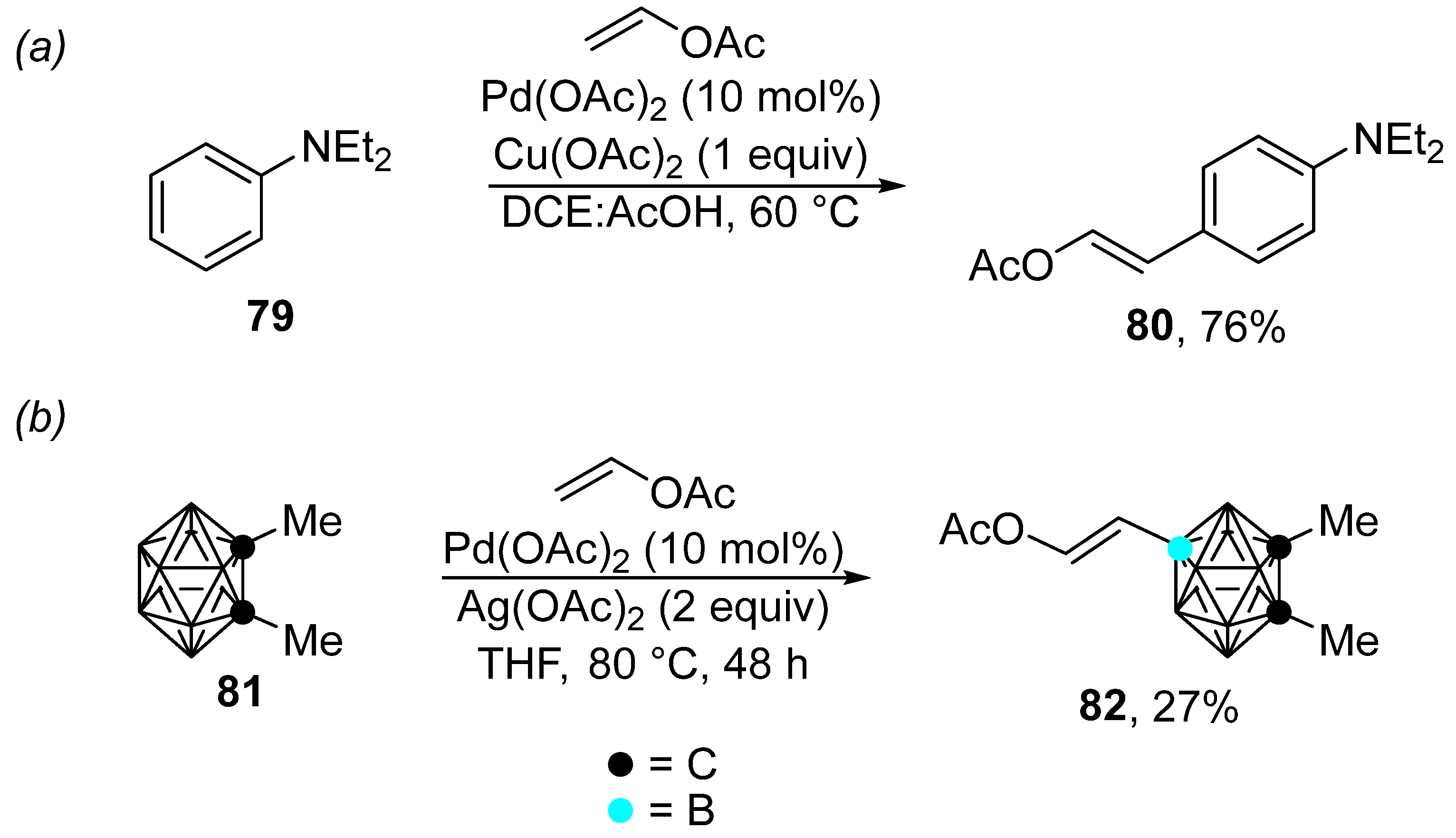
Recently, unusual cases of the Fujiwara–Moritani reaction catalyzed by palladium acetate have been described (Scheme 25). For instance, the reaction shown in Scheme 25a is characterized by the unusual regioselectivity of the C–H bond activation, which takes place in the para position [137]. The reaction conditions tolerate a wide range of olefins, including vinyl acetate. However, the anilines used are limited to N,N-dimethyl- and N,N-diethylaniline. The proposed mechanism was supported by density functional theory (DFT) calculations, and the authors suggested that the para selectivity of the C–H bond activation stems from the presence of acetic acid, which protonates the nitrogen atom. Additionally, 1,2-diMe-o-carborane 81 can be modified by means of Fujiwara–Moritani coupling through the palladium-catalyzed activation of the B–H bond (Scheme 25b) [138].
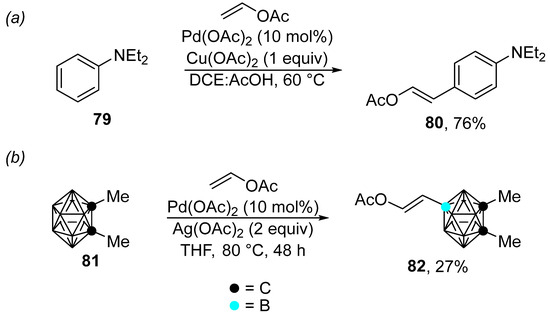
Scheme 25.
Examples of the palladium-catalyzed Fujiwara–Moritani reaction. (a) Palladium-catalyzed C–H olefination of N,N-diethylaniline; (b) Palladium-catalyzed B–H modification of o-carborane.
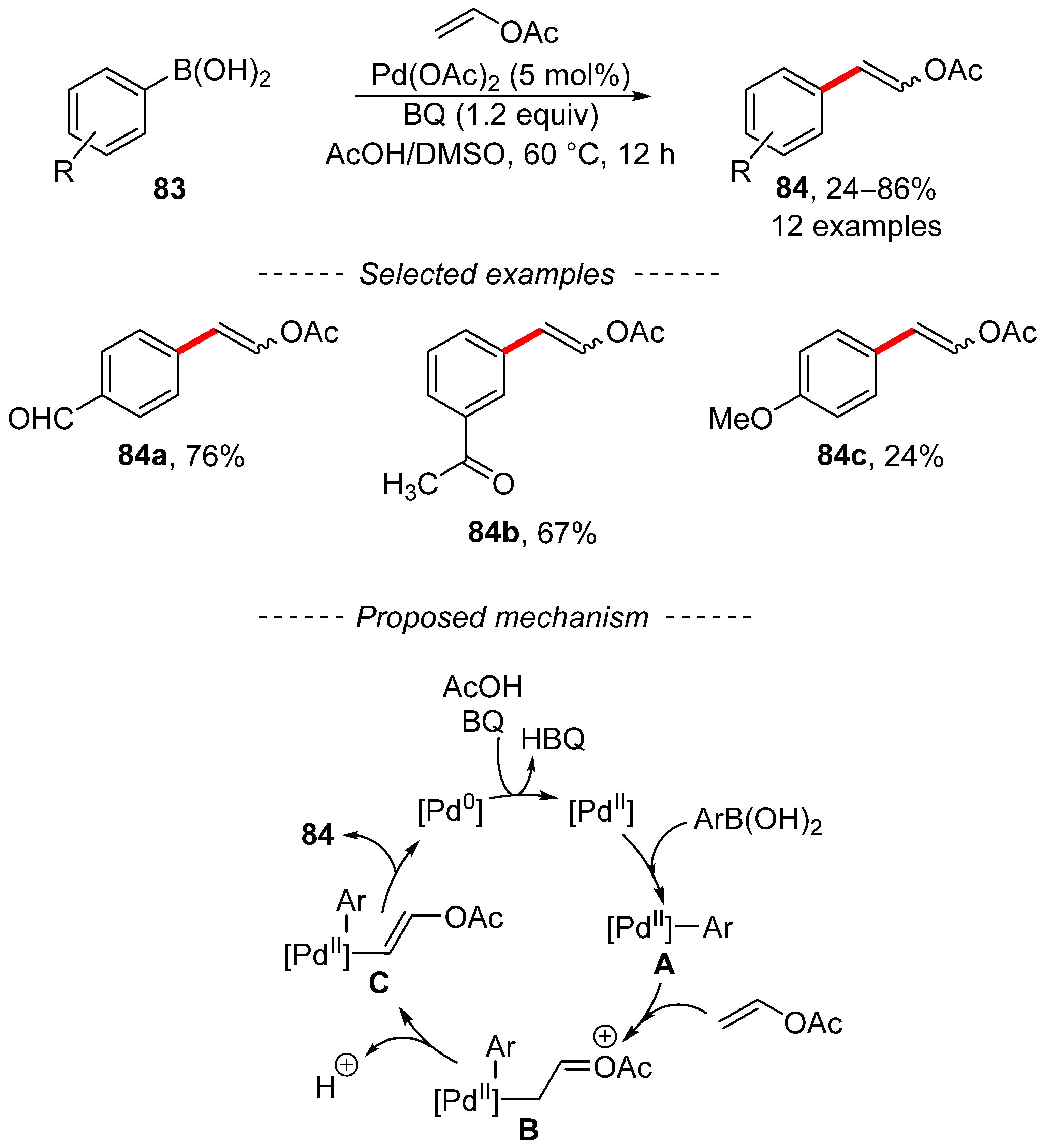
A different procedure for the modification of vinyl acetate is based on an oxidative Heck reaction using boronic acids. This process has been extensively studied in the past [139]. A recent report that used an oxidative Heck reaction between boronic acids and vinyl acetate was published by Lei (Scheme 26) [140]. This reaction is catalyzed by palladium acetate in the presence of benzoquinone, which is used as a reoxidant. The proposed catalytic cycle involves the formation of the key intermediates A, B, and C. In addition, the beginning of the catalyzed reaction is accelerated by the presence of an acidic environment, which renders the palladium catalyst more electron-deficient. An interesting extension of the oxidative Heck reaction between vinyl acetate and boronic acids entails its implementation in a continuous flow reactor [141,142].
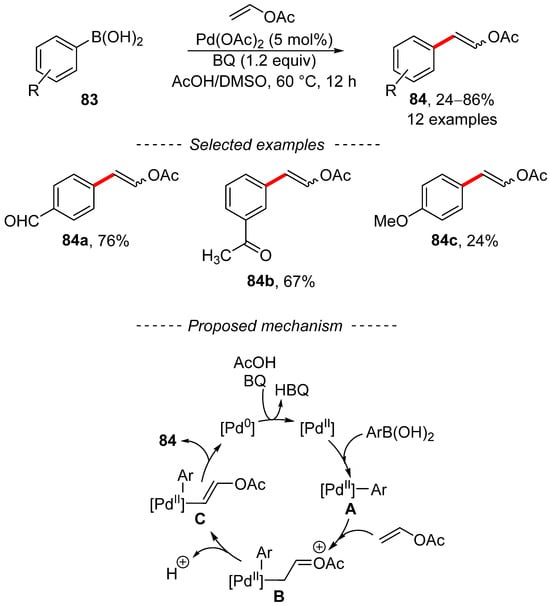
Scheme 26.
Oxidative Heck reaction for the synthesis of vinyl acetates.
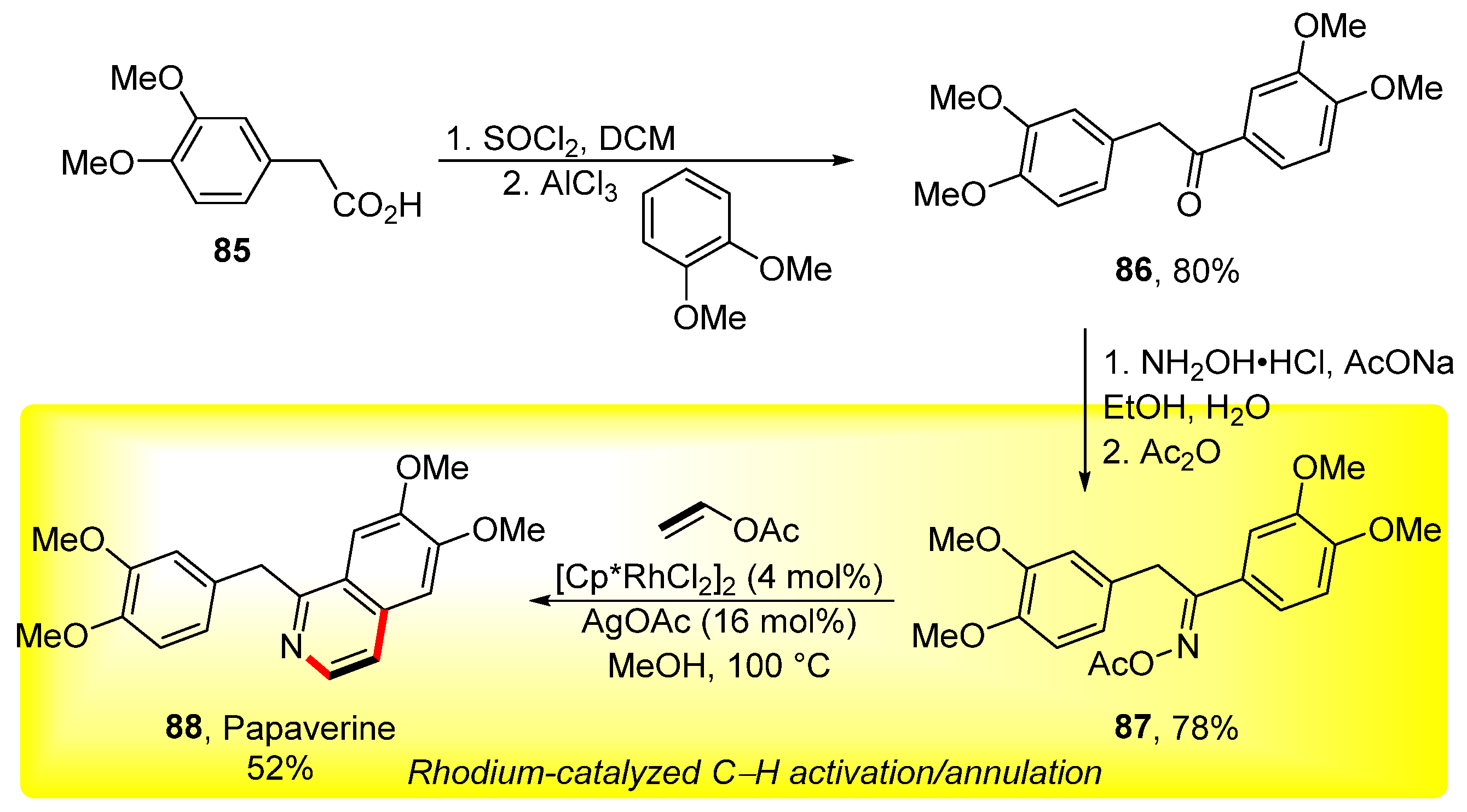
Vinyl acetates represent the substrate of choice for use in cyclization reactions. In this case, the activation of the C–H bond and the suitable structure of the starting material comprise the method of choice. A report published in 2015 described the synthesis of isoquinolines via rhodium-catalyzed cyclization between acetophenone acetyl oximes and vinyl acetates or 1-substituted vinyl acetates. At the end of an extensive study, the cyclization reaction was used to prepare papaverine 88 (Scheme 27) [143]. The synthesis of the target compound 88 starts with carboxylic acid 85, which is converted into ketone 86 by Friedel–Craft acylation of 1,2-dimethoxybenzene. Acetyl oxime 87 is then obtained via a standard procedure in a 78% yield. The final cyclization makes use of the optimized conditions and vinyl acetate to give papaverine in a 52% yield. Subsequently, Marsden used similar reaction conditions to prepare isoquinolines by means of vinyl acetate, rhodium catalyst, and acetophenone acetyl oxime [144].

Scheme 27.
Total synthesis of papaverine via the rhodium-catalyzed cyclization of acetyl oxime 87.
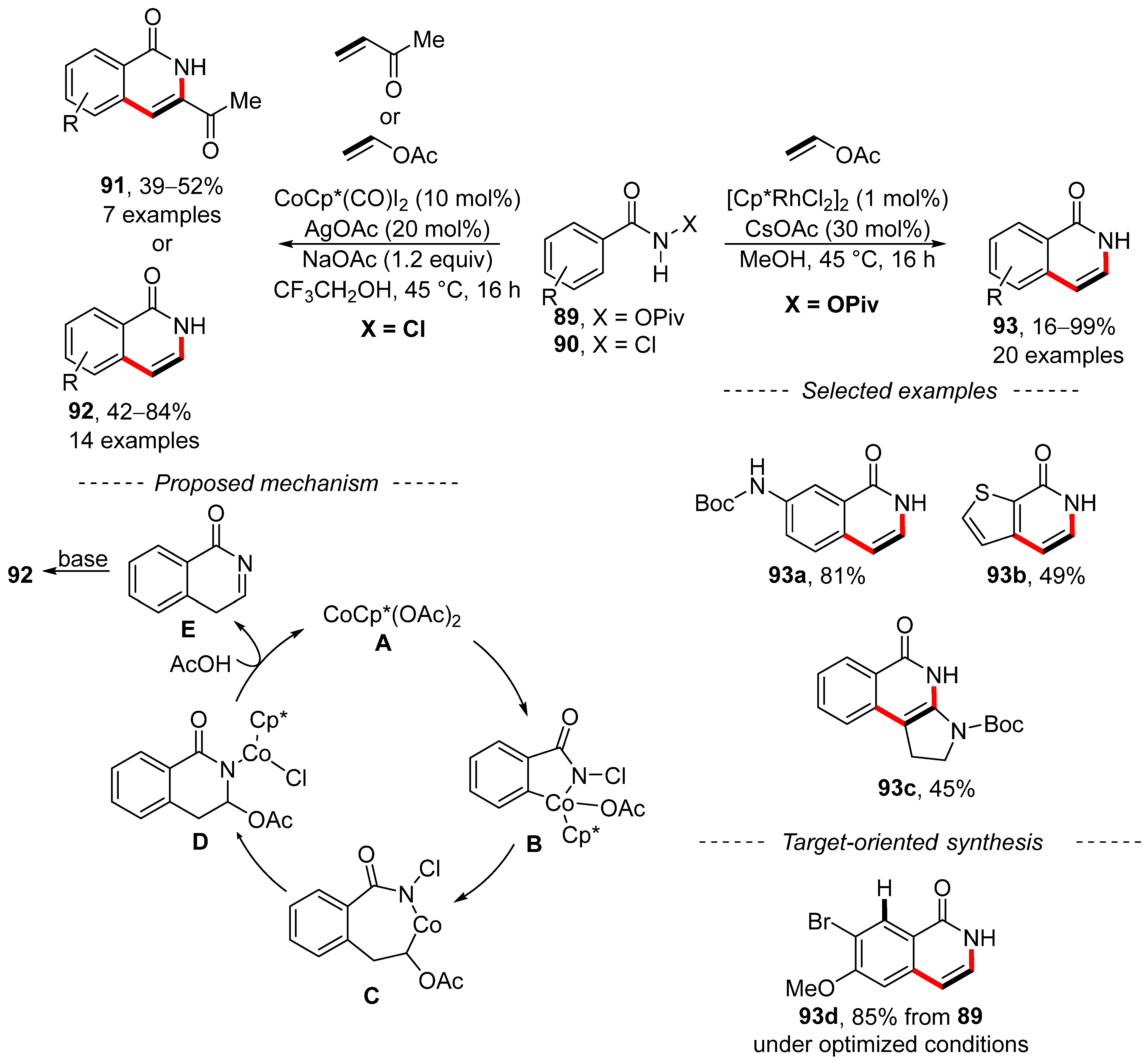
Two complementary procedures for the annulation of benzamides have been reported independently by Jeganmohan [145] and Marsden [146] (Scheme 28). Jeganmohan used N-chlorobenzamide 90 together with a cobalt complex and vinyl acetate to prepare isoquinolones 92. Alternatively, methyl vinyl ketone can be used rather than vinyl acetate to give substituted isoquinolones 91. After a series of experiments, a reasonable mechanism was proposed. Here, the reaction starts with the formation of catalytic species A by means of silver acetate. The subsequent ortho-cobaltation and insertion of vinyl acetate yield complex C. Then, reductive elimination and re-oxidative addition yield complex D. Protonation with acetic acid releases catalyst A together with intermediate E, which is transferred into reaction product 92 via basic isomerization. Similar steps can be used to explain the formation of substituted isoquinolones 91. By contrast, Marsden took advantage of rhodium-catalyzed annulation to accomplish the synthesis of similar isoquinolones 93 from the corresponding pivalates 89. A total of 20 examples of isoquinolones 93 was prepared under the optimized reaction conditions, including protected isoquinolone 93a. In addition, fused heterocycles were obtained from thiofen-2-carboxylic acid amide 93b or enamide 93c. A mechanism for the described conversion has not yet been proposed. However, the intermediate 93d used for the synthesis of the hepatitis C protease inhibitor MK-1220 was prepared as a single regioisomer in an 85% yield from amide 89a.
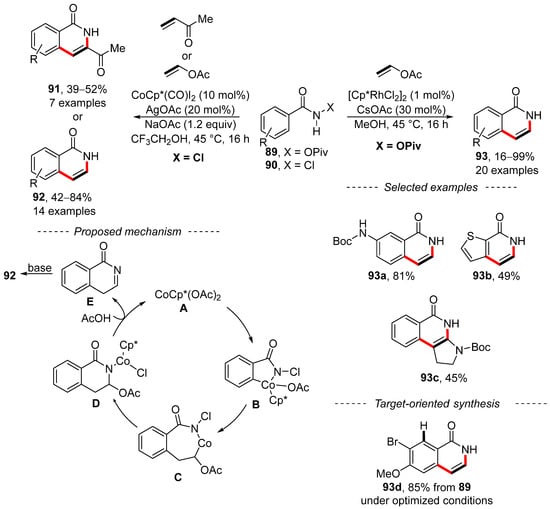
Scheme 28.
Complementary C–H activation/annulation of benzamides.
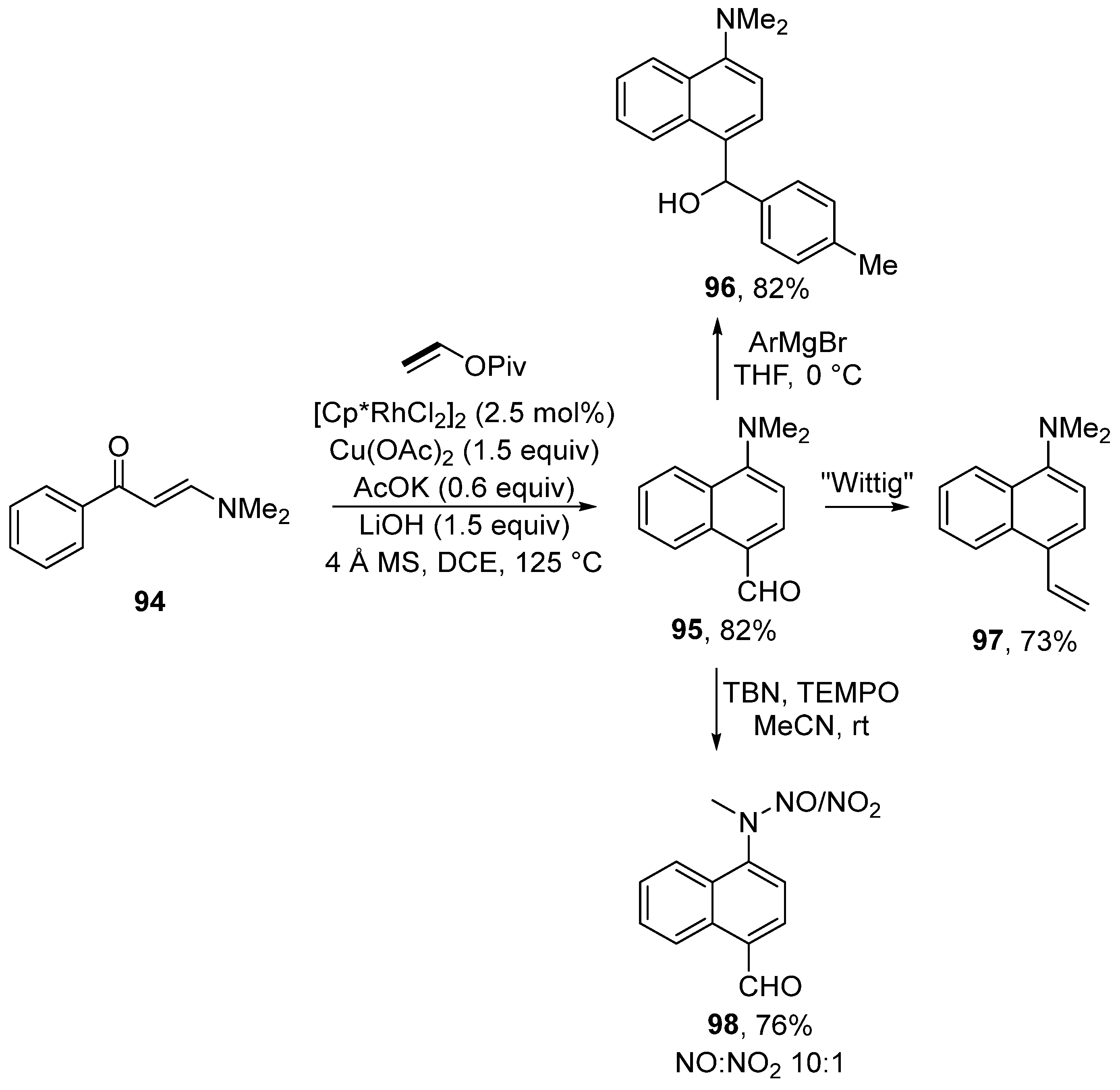
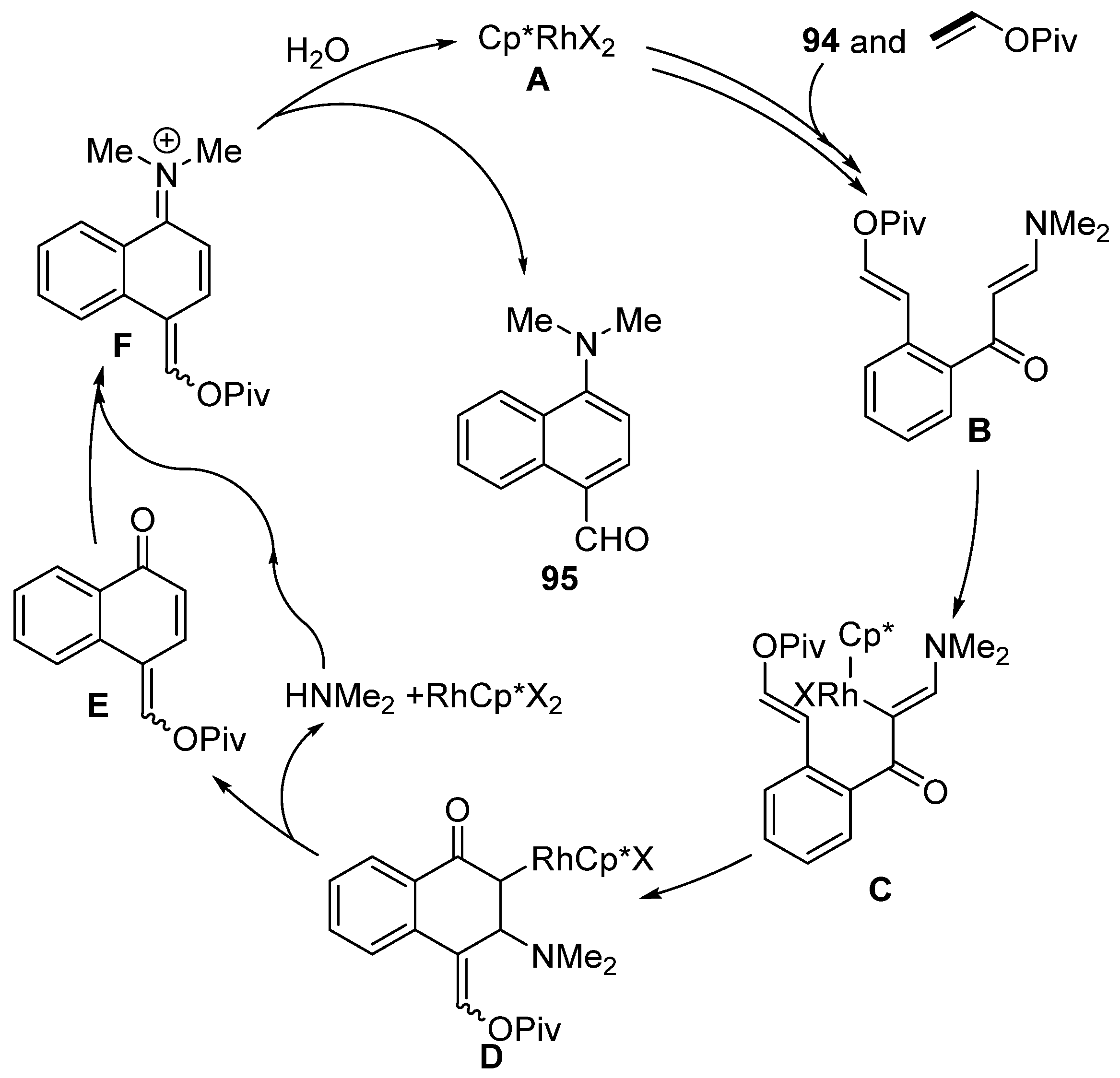
Moreover, enaminones can be used for the synthesis of mainly push–pull substituted naphthalenes via rhodium-catalyzed [5 + 1] annulation of enaminones 94 (Scheme 29) [147]. During the reaction, naphthalene derivatives with formyl and N,N-dimethylamino groups are formed. These groups can be further modified by, for example, the addition of a Grignard reagent, Wittig olefination, or oxidation into a nitroso compound. The proposed mechanism for this transformation is shown in Scheme 30. The authors assumed that the catalytic particle A is formed by means of potassium acetate. Subsequently, a series of typical steps (C–H activation, migratory insertion, and β–H elimination) produces the intermediate B. Further activation of the C–H bond yields the intermediate C. Migratory insertion then produces compound D, which β-eliminates dimethyl amine and ketone E. Overall, the catalytic cycle is completed by the condensation of dimethylamine and ketone E and the subsequent hydrolysis of iminium salt F.
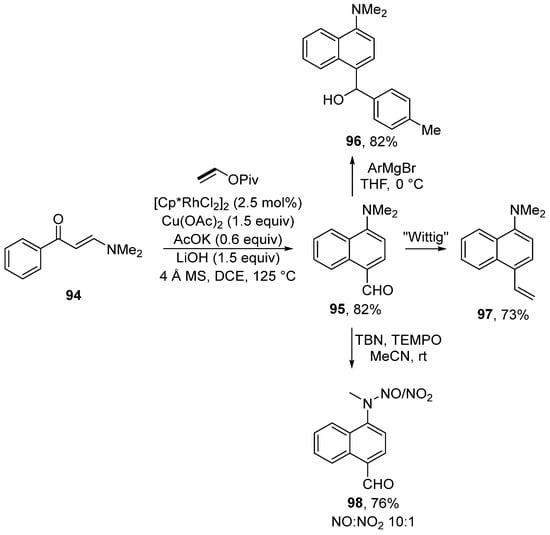
Scheme 29.
Rhodium-catalyzed functionalization of enaminones en route to 1,4-disubstituted naphthalenes.
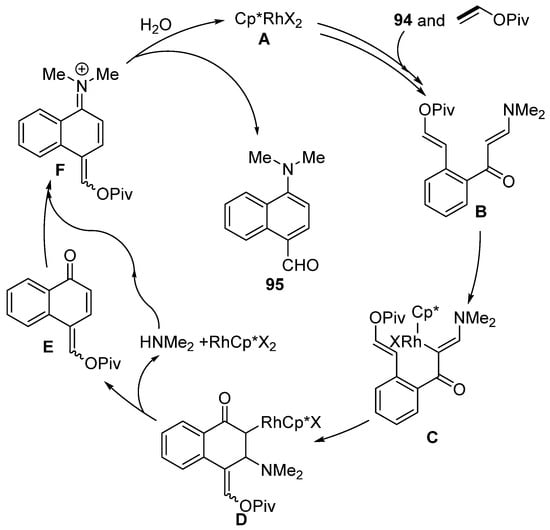
Scheme 30.
Proposed mechanism for the formation of 1,4-disubstituted naphthalenes.
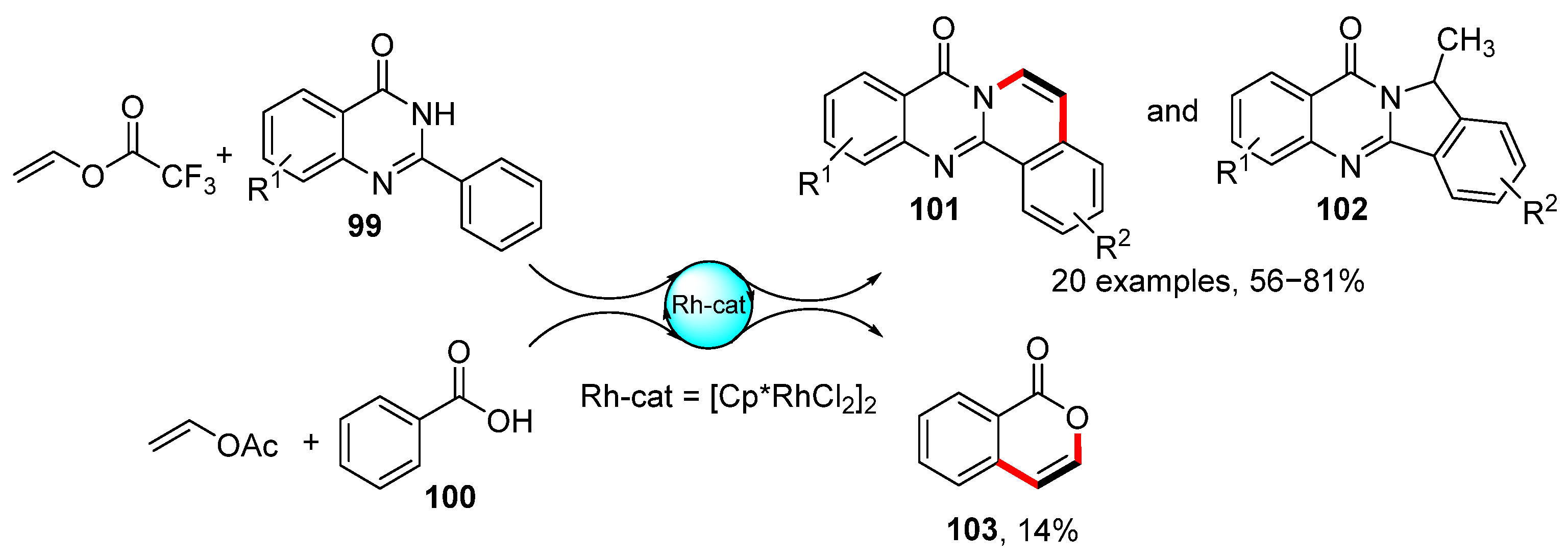
In addition to enaminones and acyl oxime (Scheme 27 and Scheme 29), the C–H activation/annulation sequence was applied to the synthesis of both isocoumarin 103 [148] and 2-arylquinazolin-4-ones 101 [149] from the corresponding benzoic acid (100) or carboxamides 99 (Scheme 31). Moreover, 2-arylquinazolin-4-ones 101 were obtained as a mixture with isomeric product 102 in high overall isolated yields and with limited functional group tolerance (CF3, F, OMe, CH3). By contrast, isocoumarin 103 was obtained in a low isolated yield from vinyl acetate. Substantially better isolated yields were obtained for 1-substituted vinyl acetates. Both reactions were catalyzed by the rhodium complex [RhCp*Cl2]2, meaning that the proposed mechanisms for the preparation of the target compounds are similar to previous proposals. Additionally, 1-substituted vinyl esters were used for the rhodium-catalyzed transformation of 7-azaindoles into π-conjugated 7-azaindole [150].

Scheme 31.
Ruthenium-catalyzed C–H activation/annulation transformation of carboxylic acids and carboxamides.
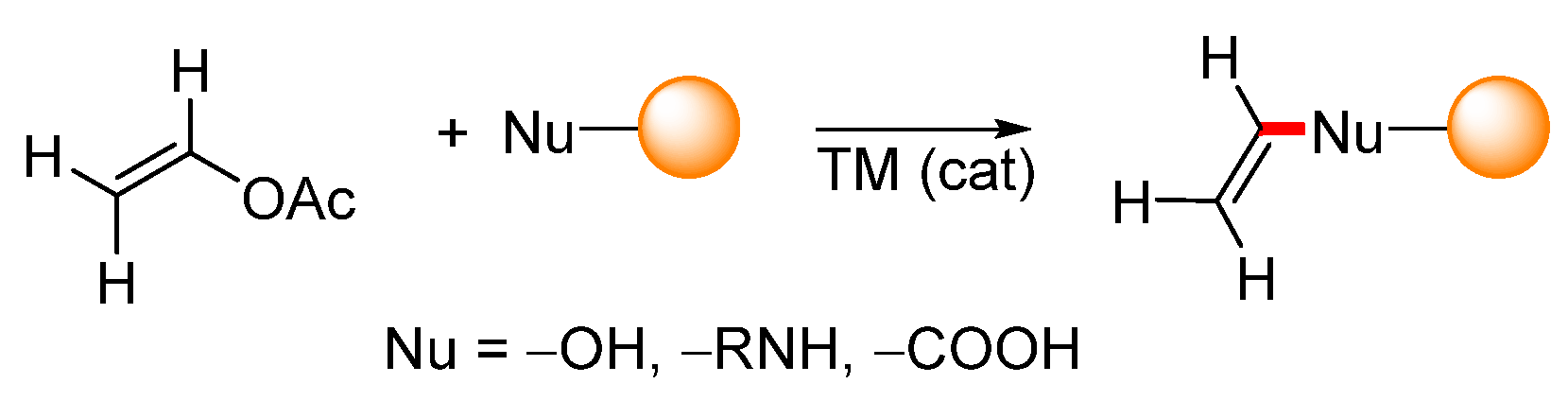
Aside from the above-mentioned C–C bond-forming cross-coupling reactions, vinyl acetate can be used in transvinylation reactions. Transvinylation is typically a transition-metal-catalyzed reaction during which a vinyl group is transferred from vinyl acetate to heteronucleophiles such as carboxylic acids, alcohols, phenols, or amines (Scheme 32).
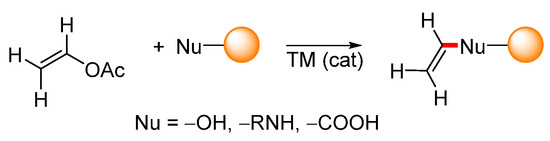
Scheme 32.
General scheme representing transition-metal-catalyzed transvinylation.
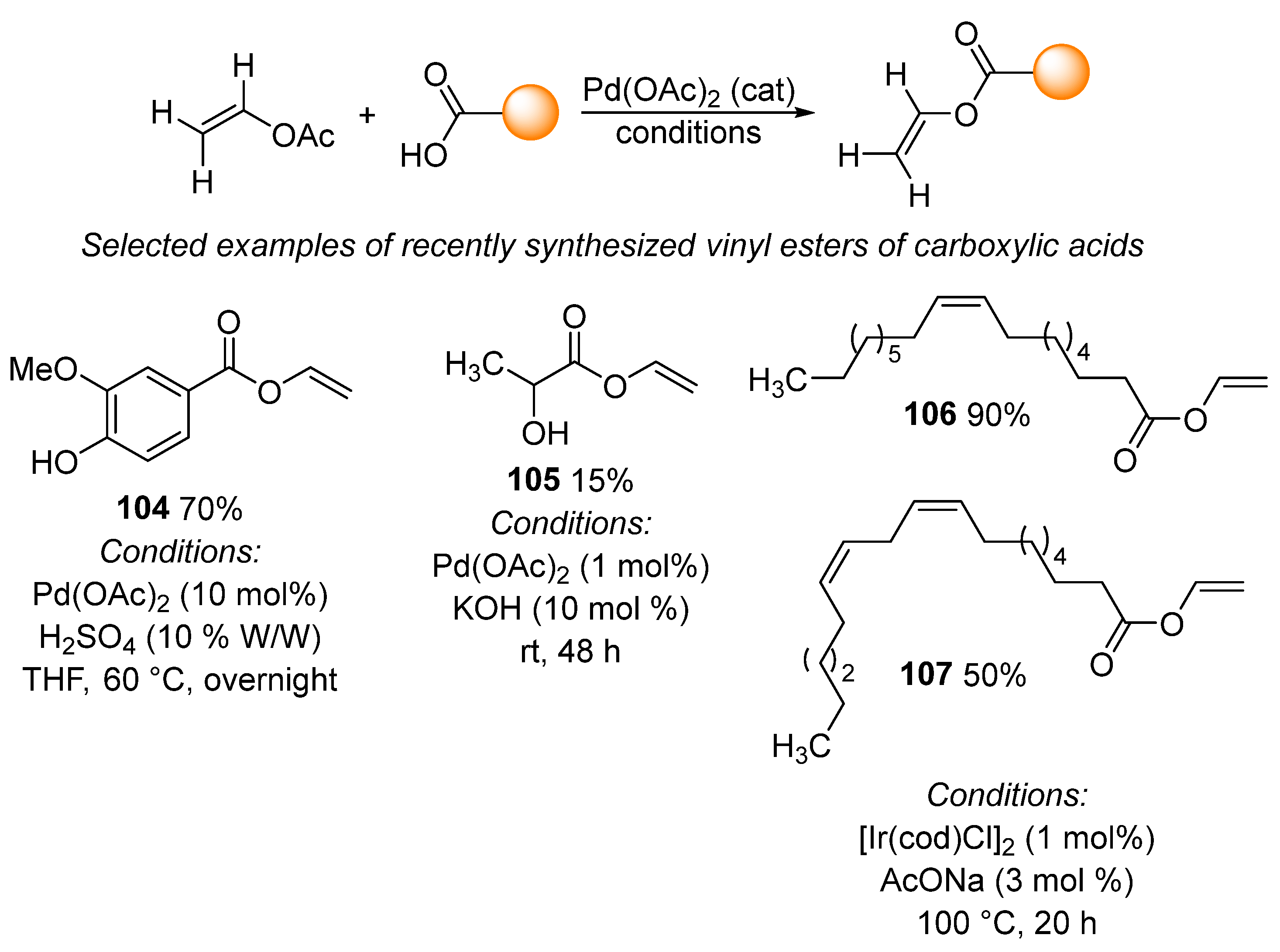
A frequently used transvinylation reaction entails the preparation of vinyl esters of carboxylic acids. During this reaction, vinyl acetate reacts with carboxylic acid in the presence of a transition metal complex. Palladium acetate is the catalyst of choice for this transvinylation reaction, together with catalytic amounts of protonic acid or potassium hydroxide, as seen in selected examples 104 [151] and 105 [152] (Scheme 33). The reaction is most commonly performed in tetrahydrofuran or with vinyl acetate used as a solvent. In addition to examples 104 and 105, analogous reaction conditions were used to prepare a wide variety of vinyl esters of carboxylic acids [152,153,154,155,156,157,158,159,160,161,162], including the synthesis of vinyl cinnamates via the flow process [163] and the preparation of vinyl L-leucinate and vinyl L-valinate in the presence of benzoquinone [153]. Transvinylation can also be catalyzed by an iridium complex. This process has recently been used for the preparation of vinyl oleate 106 and vinyl linoleate 107 under harsher reaction conditions when compared with palladium-catalyzed reactions [164,165]. Furthermore, transvinylation between vinyl acetate and adipic [166] or azalaic [167] acid when catalyzed by mercuric acetate and copper acetate has recently been described. This process has only limited use due to the high toxicity of mercuric acetate. In addition, mercury-catalyzed transvinylation proceeds by a different mechanism involving the formation of an acetylene–mercury complex [168].
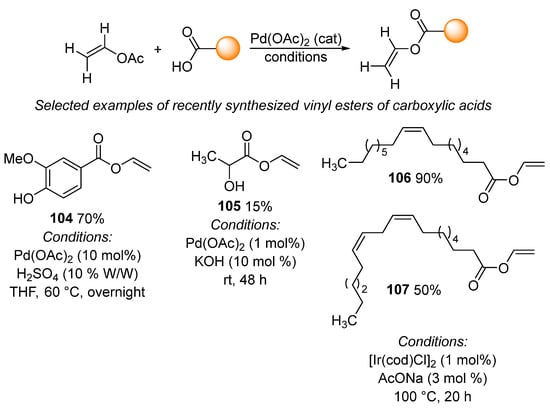
Scheme 33.
Selected applications of transvinylation for the synthesis vinyl esters.
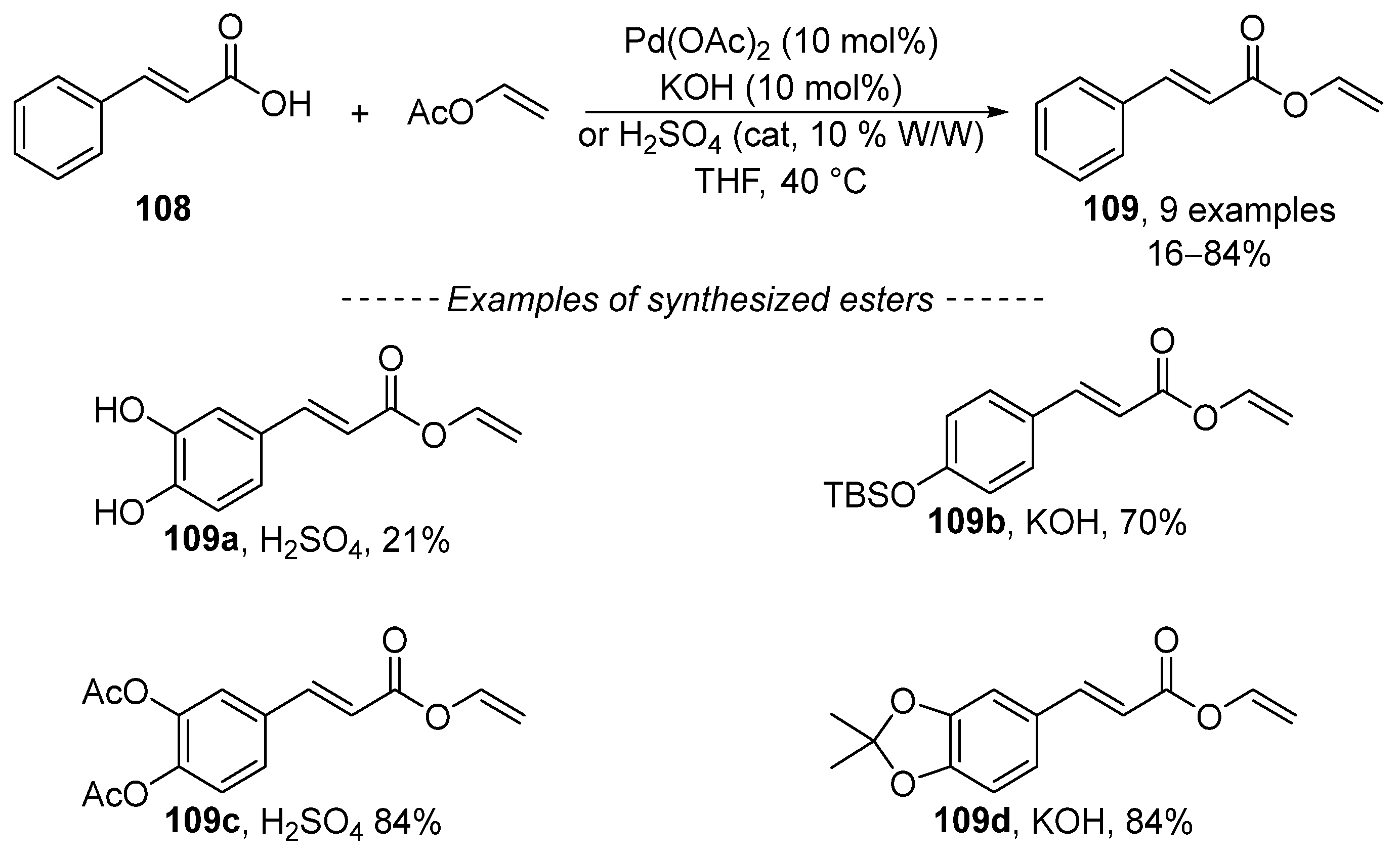
Although the transvinylation reaction has been known for a long time, papers continue to be published that study its course. In 2018, Kadidae et al. described transvinylation as a method suitable for the preparation of vinyl cinnamates 109 (Scheme 34) [157]. The reaction is catalyzed by palladium acetate in the presence of catalytic amounts of potassium hydroxide or sulfuric acid. The choice between basic and acidic catalysis is influenced by the structure of the substrate. Carboxylic acids 109b and 109d, with an acid-sensitive functional group, can be vinylated in the presence of potassium hydroxide (and vice versa).
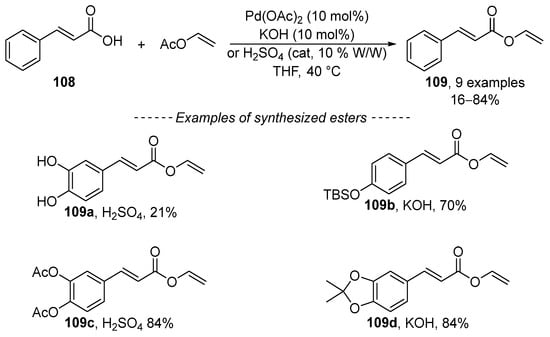
Scheme 34.
Recent study concerning the transvinylation of hydroxycinnamic acids.
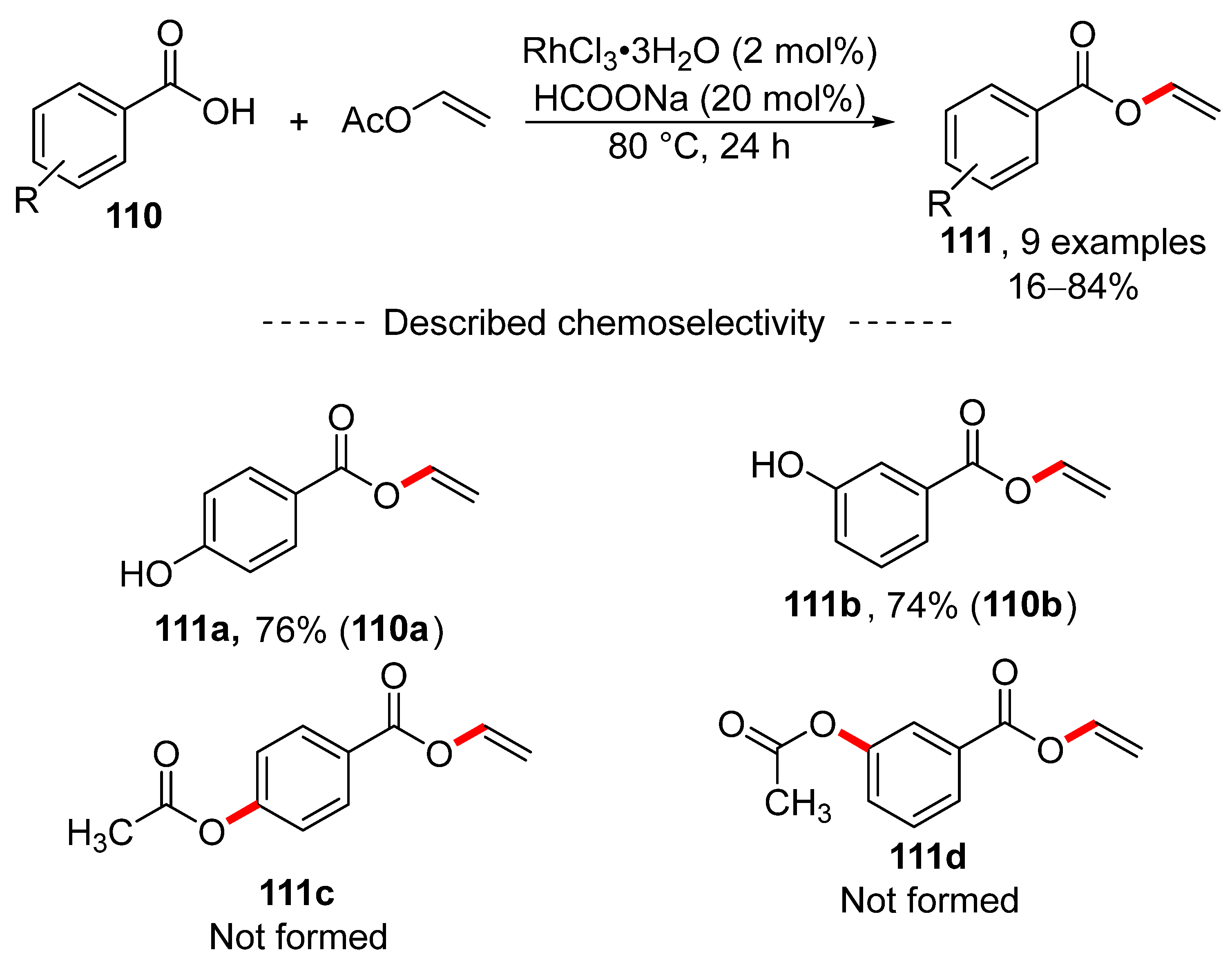
A study published in 2018 showed that transvinylation can be catalyzed by rhodium chloride trihydrate (Scheme 35) [169]. Here, excellent transvinylation results were obtained for simple benzoic acid derivatives. Moreover, excellent chemoselectivity was observed for hydroxybenzoic acids 110a and 110b, where exclusive formation of vinyl esters 111a and 111b was detected.
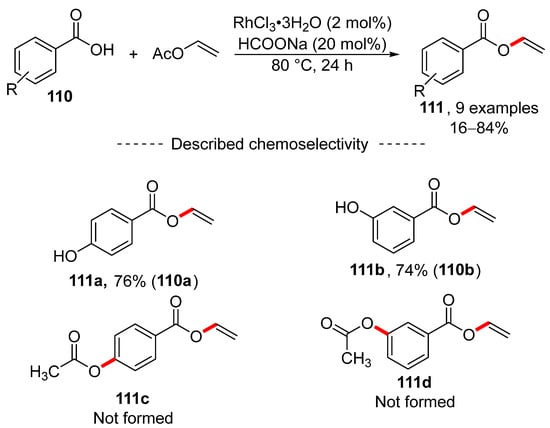
Scheme 35.
Rhodium-catalyzed transvinylation.
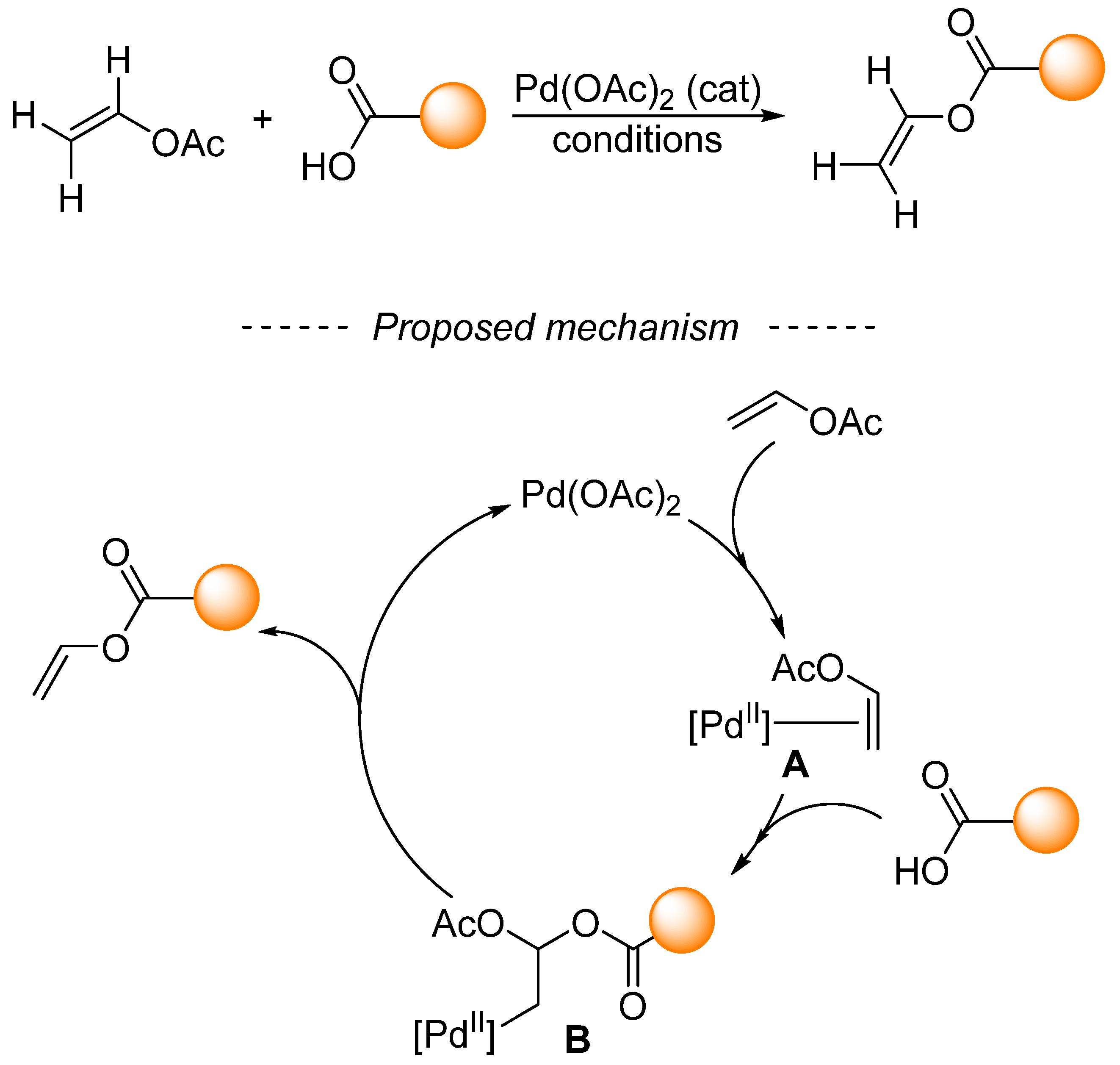
The mechanism behind palladium-catalyzed transvinylation was studied in the second half of the last century [170,171]. Based on the findings of these studies, it was proposed that the mechanism consists of an oxypalladation–deoxypalladation sequence (Scheme 36). In the first step, vinyl acetate coordinates with palladium acetate to form complex A. Then, the activated double bond is attacked by carboxylic acid or carboxylate anion, which leads to complex B. Oxypalladation is considered to be the rate-determining step of transvinylation [170]. The resulting complex B undergoes deoxypalladation to form both palladium acetate and the reaction product. Transvinylation is also significantly retarded by the substitution of the vinyl group of vinyl acetate [171]. With some simplification, ruthenium- [172] and rhodium-catalyzed [169] transvinylation proceed via analogous mechanisms.
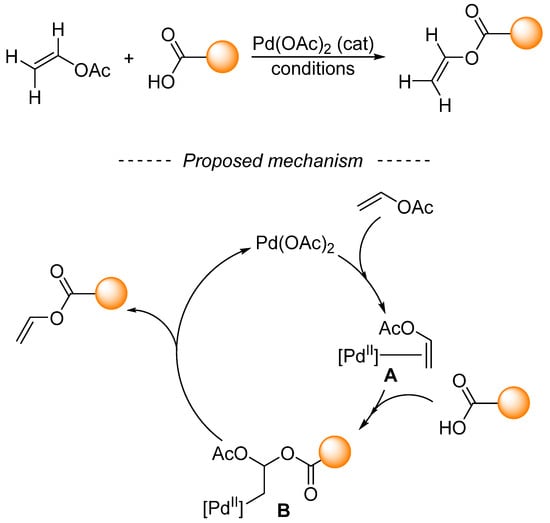
Scheme 36.
Oxypalladation–deoxypalladation sequence en route to transvinylation products.
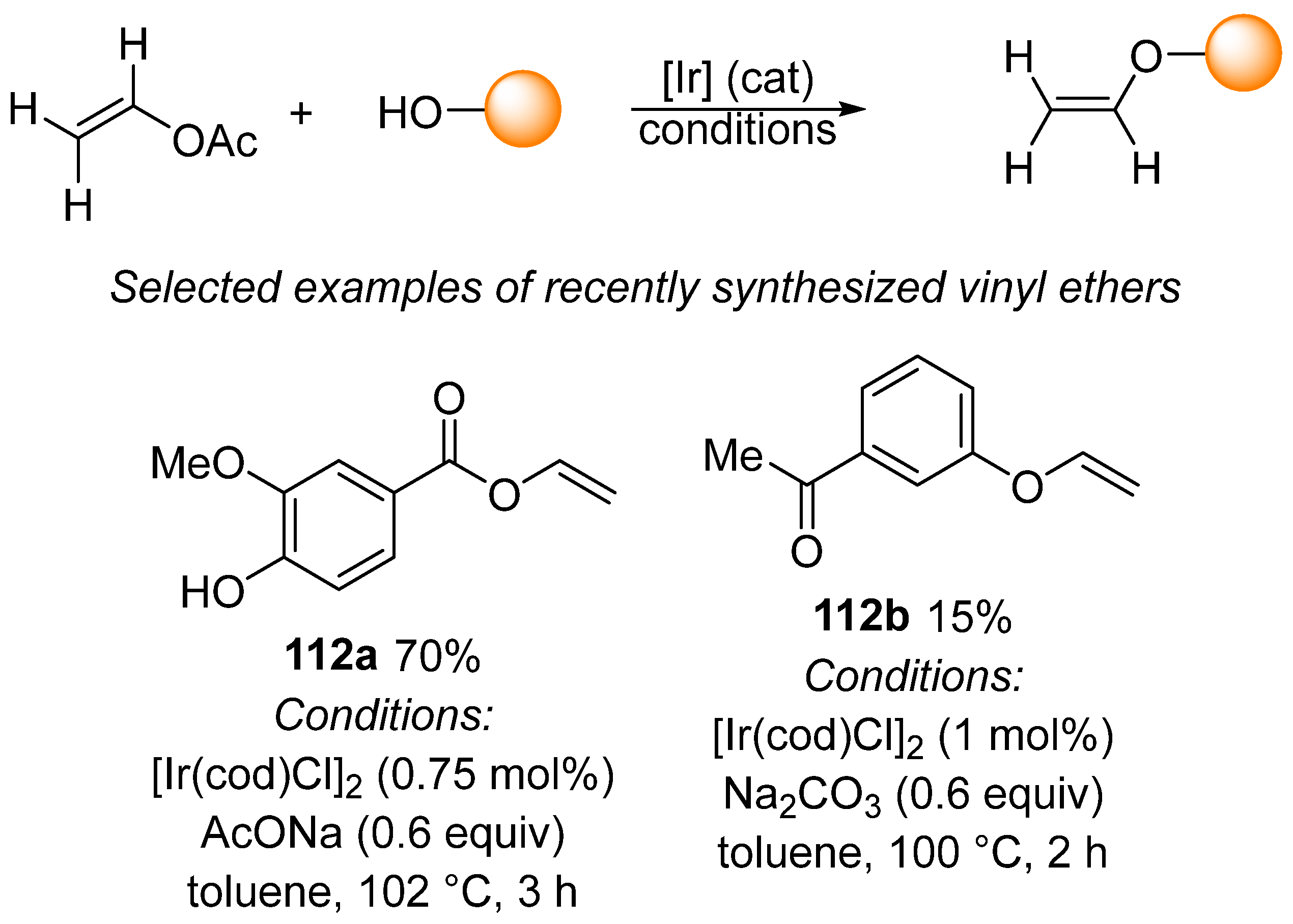
Another popular use of vinyl esters, especially vinyl acetates, entails the preparation of vinyl ethers by means of transvinylation. An earlier review demonstrated that transvinylation can be catalyzed by, for example, palladium, gold, and iridium complexes [173]. However, recent examples 112a [174] and 112b [175] illustrate how iridium has become a popular catalyst for use in the preparation of vinyl ethers via transvinylation reactions (Scheme 37).

Scheme 37.
Selected examples of transvinylation for the synthesis of vinyl ethers.
The main advantage of iridium-catalyzed transvinylation for the preparation of vinyl ethers is the use of a low catalytic amount of the iridium complex and simple reaction conditions. Moreover, Flores-Pérez demonstrated that the iridium complex provided significantly higher yields of vinyl ethers 114 and 116 when compared with mercuric acetate-catalyzed transvinylation (Scheme 38) [176]. In addition, cationic Ir(cod)2BF4 was used for the synthesis of biobased vinyl ethers [177].

Scheme 38.
Iridium- versus mercury-catalyzed transvinylation for ether synthesis.
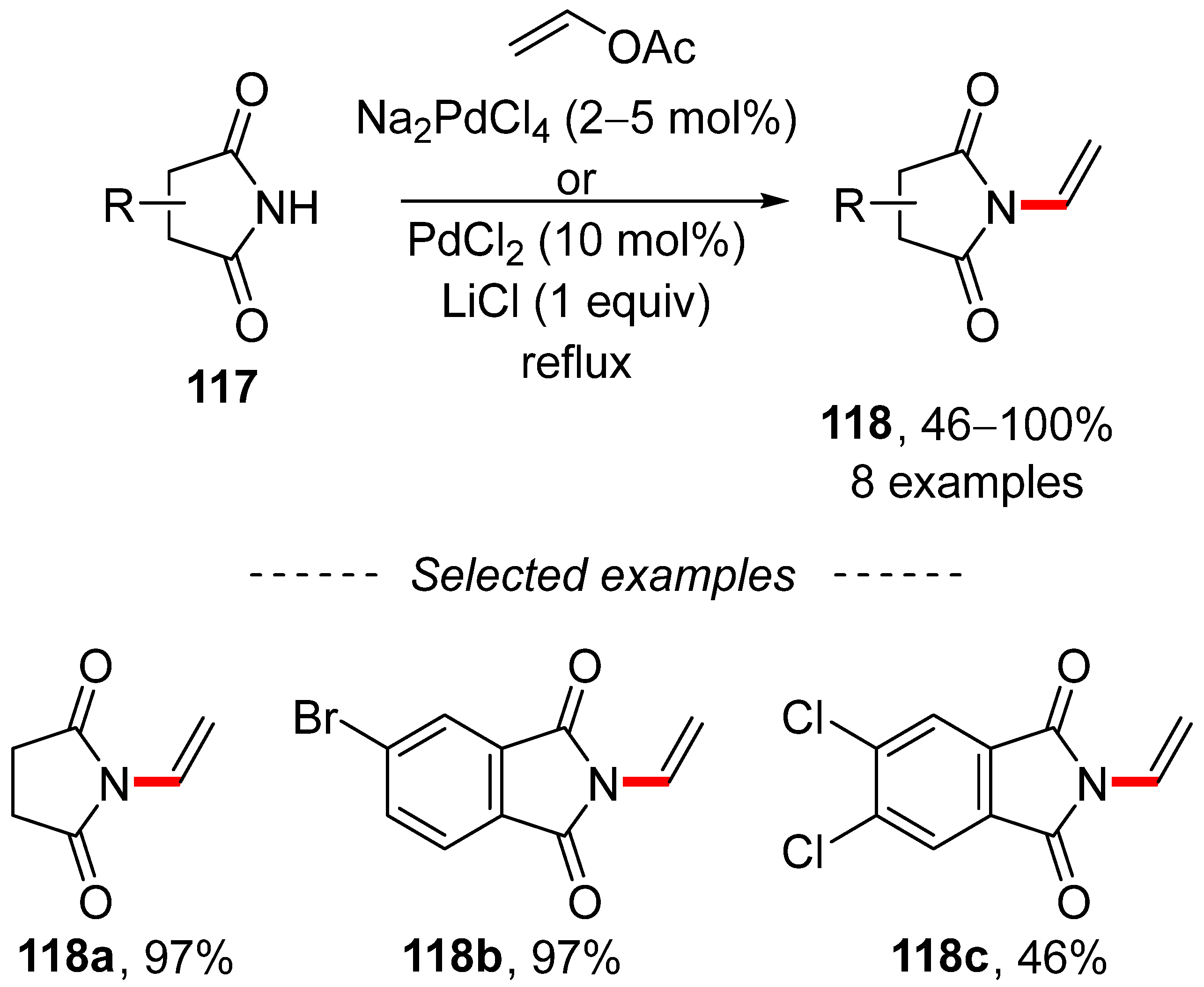
The synthesis of enamines and vinyl amides is another area in which vinyl acetate can be employed. A well-established procedure based on the copper-catalyzed reaction of organohalogens with carboxylic acid amides is termed the Goldberg reaction [178,179]. Aside from organohalogenides, vinyl acetates can also be used. Furthermore, a palladium-catalyzed transvinylation reaction for the preparation of enamides was recently described by Waser et al. (Scheme 39) [180]. This reaction is performed in refluxing vinyl acetate and the isolated yields of vinyl amides 118 are high.

Scheme 39.
Palladium-catalyzed transvinylation for the synthesis of enamides.
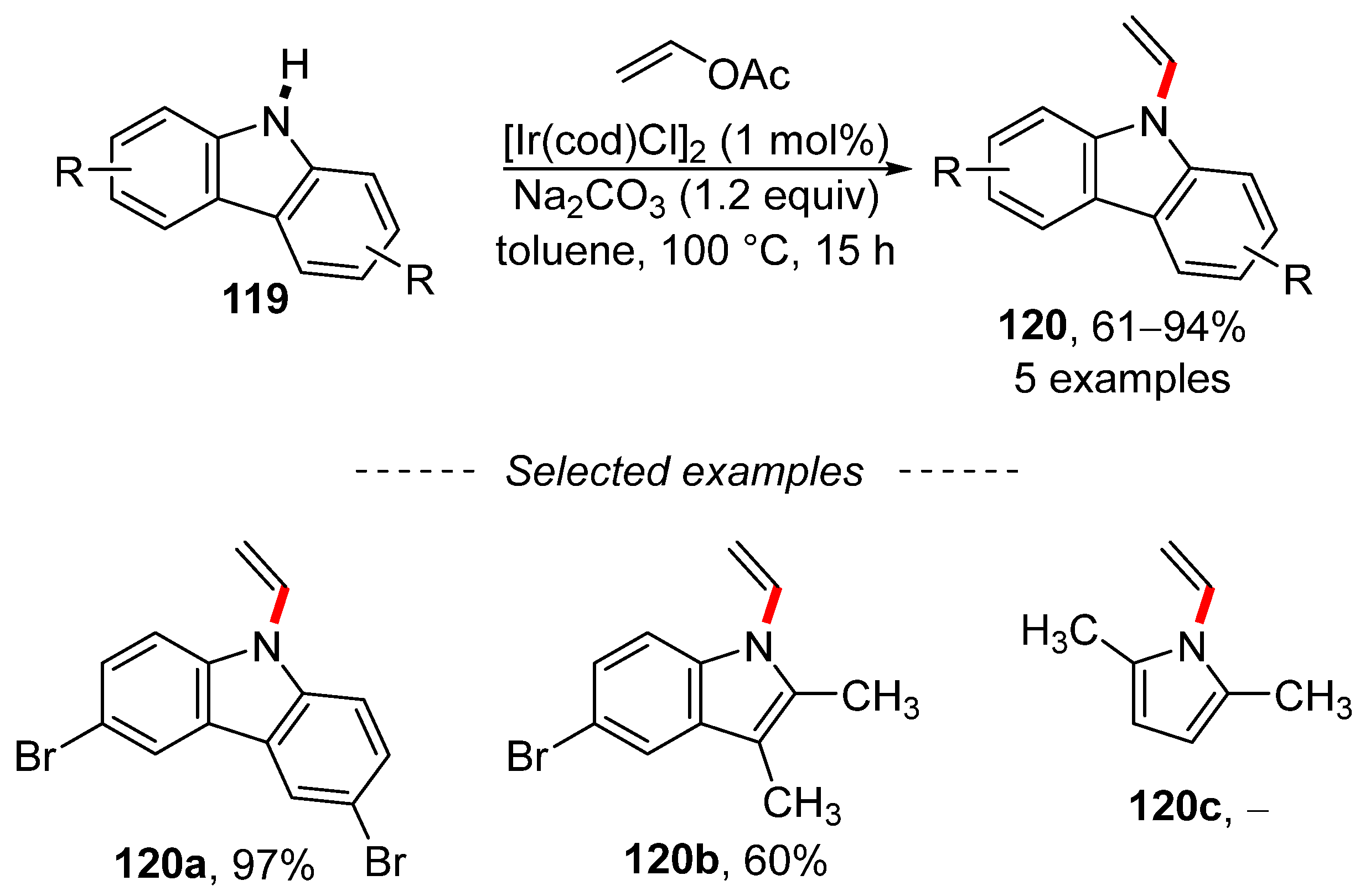
The analogous vinylation reaction can also be used to vinylate N-heterocyclic compounds, including carbazole 119 (Scheme 40) [181]. The extension of the optimized conditions to other pyrrole derivatives is limited by the substitution of all positions of the pyrrole ring. Thus, 2,5-dimethylpyrrole did not react under the optimized conditions and the indole derivative was isolated in a 60% yield.

Scheme 40.
Iridium-catalyzed vinylation of N-heterocycles with vinyl acetate.
4. Conclusions
This review summarized the available processes for the preparation of mono- and disubstituted ethene by means of electrophilic vinyl templates with activated C–O bonds. Currently, except for low-boiling-point vinyl bromide, vinyl sulfonates and vinyl acetate are most commonly used for simple vinylation. Thus, vinyl sulfonates and vinyl acetates are used to form a single C–C bond via transition-metal-catalyzed reactions. For vinyl acetates, the Fujiwara–Moritani reaction is widely used to prepare monosubstituted vinyl acetates. Due to their easy availability, vinyl acetates are also frequently used for the preparation of vinyl esters and ethers via transvinylation reactions. The reactivity of other electrophiles, particularly vinyl phosphates, is undeveloped, which severely limits the development of multicomponent and cyclization reactions using vinyl templates as a C2 synthon source.
Funding
This research received no external funding.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The author declare no conflict of interest.
References
- Knappke, C.E.I.; Grupe, S.; Gärtner, D.; Corpet, M.; Gosmini, C.; Jacobi von Wangelin, A. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J. 2014, 20, 6828–6842. [Google Scholar] [CrossRef]
- Lucas, E.L.; Jarvo, E.R. Stereospecific and Stereoconvergent Cross-Couplings Between Alkyl Electrophiles. Nat. Rev. Chem. 2017, 1, 0065. [Google Scholar] [CrossRef]
- Noël, T.; Buchwald, S.L. Cross-Coupling in Flow. Chem. Soc. Rev. 2011, 40, 5010–5029. [Google Scholar] [CrossRef]
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.-M.; Garg, N.K.; Percec, V. Nickel-Catalyzed Cross-Couplings Involving Carbon–Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. [Google Scholar] [CrossRef]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef]
- Friese, F.W.; Studer, A. New Avenues for C–B Bond Formation via Radical Intermediates. Chem. Sci. 2019, 10, 8503–8518. [Google Scholar] [CrossRef]
- Chen, K.; Wang, L.; Meng, G.; Li, P. Recent Advances in Transition-Metal-Free Aryl C–B Bond Formation. Synthesis 2017, 49, 4719–4730. [Google Scholar] [CrossRef]
- Verma, P.K.; Shegavi, M.L.; Bose, S.K.; Geetharani, K. A Nano-Catalytic Approach for C–B Bond Formation Reactions. Org. Biomol. Chem. 2018, 16, 857–873. [Google Scholar] [CrossRef]
- Yan, G.; Huang, D.; Wu, X. Recent Advances in C–B Bond Formation through a Free Radical Pathway. Adv. Synth. Catal. 2018, 360, 1040–1053. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, G.; Hashmi, A.S.K. Carbene B–H Insertion Reactions for C–B Bond Formation. ChemCatChem 2021, 13, 4299–4312. [Google Scholar] [CrossRef]
- Kärkäs, M.D. Electrochemical Strategies for C–H Functionalization and C–N Bond Formation. Chem. Soc. Rev. 2018, 47, 5786–5865. [Google Scholar] [CrossRef]
- Kim, H.; Chang, S. Transition-Metal-Mediated Direct C–H Amination of Hydrocarbons with Amine Reactants: The Most Desirable but Challenging C–N Bond-Formation Approach. ACS Catal. 2016, 6, 2341–2351. [Google Scholar] [CrossRef]
- Oeser, P.; Koudelka, J.; Petrenko, A.; Tobrman, T. Recent Progress Concerning the N-Arylation of Indoles. Molecules 2021, 26, 5079. [Google Scholar] [CrossRef]
- Shin, K.; Kim, H.; Chang, S. Transition-Metal-Catalyzed C–N Bond Forming Reactions Using Organic Azides as the Nitrogen Source: A Journey for the Mild and Versatile C–H Amination. Acc. Chem. Res. 2015, 48, 1040–1052. [Google Scholar] [CrossRef]
- Song, G.; Wang, F.; Li, X. C–C, C–O and C–N Bond Formation via Rhodium(iii)-Catalyzed Oxidative C–H Activation. Chem. Soc. Rev. 2012, 41, 3651–3678. [Google Scholar] [CrossRef]
- Krylov, I.B.; Vil, V.A.; Terent’ev, A.O. Cross-Dehydrogenative Coupling for the Intermolecular C–O Bond Formation. Beilstein J. Org. Chem. 2015, 11, 92–146. [Google Scholar] [CrossRef]
- Lefèvre, G.; Franc, G.; Tlili, A.; Adamo, C.; Taillefer, M.; Ciofini, I.; Jutand, A. Contribution to the Mechanism of Copper-Catalyzed C–N and C–O Bond Formation. Organometallics 2012, 31, 7694–7707. [Google Scholar] [CrossRef]
- Stürmer, R. Take the Right Catalyst: Palladium-Catalyzed C–C, C–N, and C–O Bond Formation on Chloroarenes. Angew. Chem. Int. Ed. 1999, 38, 3307–3308. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H. Zinc-Catalyzed Organic Synthesis: C–C, C–N, C–O Bond Formation Reactions. Adv. Synth. Catal. 2012, 354, 3141–3160. [Google Scholar] [CrossRef]
- Pan, X.-Q.; Zou, J.-P.; Yi, W.-B.; Zhang, W. Recent Advances in Sulfur- and Phosphorous-Centered Radical Reactions for the Formation of S–C and P–C Bonds. Tetrahedron 2015, 71, 7481–7529. [Google Scholar] [CrossRef]
- Tappe, F.M.J.; Trepohl, V.T.; Oestreich, M. Transition-Metal-Catalyzed C–P Cross-Coupling Reactions. Synthesis 2010, 3037–3062. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Duan, Z. Synthetic Applications of Transition-Metal-Catalyzed C–P Bond Cleavage. Chem. Asian J. 2018, 13, 2164–2173. [Google Scholar] [CrossRef]
- Wauters, I.; Debrouwer, W.; Stevens, C.V. Preparation of Phosphines Through C–P Bond Formation. Beilstein J. Org. Chem. 2014, 10, 1064–1096. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.-Y.; Dong, D.-Q.; Wang, Z.-L. Copper-Catalyzed Cross-Coupling Reactions for C–P Bond Formation. RSC Adv. 2015, 5, 52824–52831. [Google Scholar] [CrossRef]
- Bhunia, S.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C–N, C–O, C–S, and C–C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179. [Google Scholar] [CrossRef]
- Eichman, C.C.; Stambuli, J.P. Transition-Metal-Catalyzed Synthesis of Aryl Sulfides. Molecules 2011, 16, 590–608. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Wu, W.; Jiang, H. Recent Developments in Palladium-Catalyzed C–S Bond Formation. Org. Chem. Front. 2020, 7, 1395–1417. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T.S.A.; Liu, X. Recent Advances in C–S Bond Formation via C–H Bond Functionalization and Decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. [Google Scholar] [CrossRef]
- Buttard, F.; Sharma, J.; Champagne, P.A. Recent Advances in the Stereoselective Synthesis of Acyclic All-Carbon Tetrasubstituted alkenes. Chem. Commun. 2021, 57, 4071–4088. [Google Scholar] [CrossRef]
- Edlová, T.; Čubiňák, M.; Tobrman, T. Cross-Coupling Reactions of Double or Triple Electrophilic Templates for Alkene Synthesis. Synthesis 2020, 53, 255–266. [Google Scholar]
- Flynn, A.B.; Ogilvie, W.W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745. [Google Scholar] [CrossRef]
- Mukherjee, N.; Planer, S.; Grela, K. Formation of Tetrasubstituted C–C Double Bonds via Olefin Metathesis: Challenges, Catalysts, and Applications in Natural Product Synthesis. Org. Chem. Front. 2018, 5, 494–516. [Google Scholar] [CrossRef]
- Negishi, E.-i.; Huang, Z.; Wang, G.; Mohan, S.; Wang, C.; Hattori, H. Recent Advances in Efficient and Selective Synthesis of Di-, Tri-, and Tetrasubstituted Alkenes via Pd-Catalyzed Alkenylation–Carbonyl Olefination Synergy. Acc. Chem. Res. 2008, 41, 1474–1485. [Google Scholar] [CrossRef]
- Paek, S.M. Synthesis of Tetrasubstituted Alkenes via Metathesis. Molecules 2012, 17, 3348–3358. [Google Scholar] [CrossRef]
- Polák, P.; Váňová, H.; Dvořák, D.; Tobrman, T. Recent Progress in Transition Metal-Catalyzed Stereoselective Synthesis of Acyclic All-Carbon Tetrasubstituted Alkenes. Tetrahedron Lett. 2016, 57, 3684–3693. [Google Scholar] [CrossRef]
- Reiser, O. Palladium-Catalyzed Coupling Reactions for the Stereoselective Synthesis of Tri- and Tetrasubstituted Alkenes. Angew. Chem. Int. Ed. 2006, 45, 2838–2840. [Google Scholar] [CrossRef]
- Tobrman, T.; Mrkobrada, S. Palladium-Catalyzed Cross-Coupling Reactions of Borylated Alkenes for the Stereoselective Synthesis of Tetrasubstituted Double Bond. Organics 2022, 3, 210–239. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Padusha, M.S.A.; Sajith, A.M. Application of Palladium Based Precatalytic Systems in the Suzuki-Miyaura Cross-Coupling Reactions of Chloro- Heterocycles. ChemistrySelect 2020, 5, 9005–9016. [Google Scholar] [CrossRef]
- Fairlamb, I.J.S. Regioselective (Site-Selective) Functionalisation of Unsaturated Halogenated Nitrogen, Oxygen and Sulfur Heterocycles by Pd-Catalysed Cross-Couplings and Direct Arylation Processes. Chem. Soc. Rev. 2007, 36, 1036–1045. [Google Scholar] [CrossRef]
- Gandhi, S. Catalytic Enantioselective Cross Dehydrogenative Coupling of sp3 C–H of Heterocycles. Org. Biomol. Chem. 2019, 17, 9683–9692. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E. Recent Advances in Application of Intramolecular Suzuki Cross-Coupling in Cyclization and Heterocyclization. Monatsh. Chem. 2012, 143, 861–880. [Google Scholar] [CrossRef]
- Langer, P. Cross-Coupling Reactions of Polyhalogenated Heterocycles. Synlett 2022, 33, 1029–1051. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, J.-P. Tandem Reactions Initiated by Copper-Catalyzed Cross-Coupling: A New Strategy Towards Heterocycle Synthesis. Org. Biomol. Chem. 2011, 9, 6873–6894. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Gee, A.D.; Gouverneur, V. AuI/AuIII Catalysis: An Alternative Approach for C–C Oxidative Coupling. Chem. Eur. J. 2011, 17, 8248–8262. [Google Scholar] [CrossRef]
- Jones, K.M.; Klussmann, M. Oxidative Coupling of Tertiary Amines: Scope, Mechanism and Challenges. Synlett 2012, 159–162. [Google Scholar] [CrossRef]
- Kozlowski, M.C. Oxidative Coupling in Complexity Building Transforms. Acc. Chem. Res. 2017, 50, 638–643. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Y.; Sessler, J.L.; Gong, H. Cross-Electrophile Couplings of Activated and Sterically Hindered Halides and Alcohol Derivatives. Acc. Chem. Res. 2020, 53, 1833–1845. [Google Scholar] [CrossRef]
- Pang, X.; Peng, X.; Shu, X.-Z. Reductive Cross-Coupling of Vinyl Electrophiles. Synthesis 2020, 52, 3751–3763. [Google Scholar] [CrossRef]
- Pang, X.; Su, P.-F.; Shu, X.-Z. Reductive Cross-Coupling of Unreactive Electrophiles. Acc. Chem. Res. 2022, 55, 2491–2509. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Szostak, M. Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar] [CrossRef]
- Pound, S.M.; Watson, M.P. Asymmetric Synthesis via Stereospecific C–N and C–O Bond Activation of Alkyl Amine and Alcohol Derivatives. Chem. Commun. 2018, 54, 12286–12301. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.-Q.; Hong, X. Computational Studies on Ni-Catalyzed Amide C–N Bond Activation. Chem. Commun. 2019, 55, 11330–11341. [Google Scholar] [CrossRef]
- Wang, Q.; Su, Y.; Li, L.; Huang, H. Transition-Metal Catalysed C–N Bond Activation. Chem. Soc. Rev. 2016, 45, 1257–1272. [Google Scholar] [CrossRef]
- Cheng, H.-G.; Chen, H.; Liu, Y.; Zhou, Q. The Liebeskind–Srogl Cross-Coupling Reaction and its Synthetic Applications. Asian J. Org. Chem. 2018, 7, 490–508. [Google Scholar] [CrossRef]
- Otsuka, S.; Nogi, K.; Yorimitsu, H. C–S Bond Activation. Top. Curr. Chem. 2018, 376, 13. [Google Scholar] [CrossRef]
- Pan, F.; Shi, Z.-J. Recent Advances in Transition-Metal-Catalyzed C–S Activation: From Thioester to (Hetero)aryl Thioether. ACS Catal. 2014, 4, 280–288. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. Iron-Catalyzed C–O Bond Activation: Opportunity for Sustainable Catalysis. ChemSusChem 2017, 10, 3964–3981. [Google Scholar] [CrossRef]
- Sellars, J.D.; Steel, P.G. Transition Metal-Catalysed Cross-Coupling Reactions of P-activated Enols. Chem. Soc. Rev. 2011, 40, 5170–5180. [Google Scholar] [CrossRef]
- Tobisu, M.; Chatani, N. Cross-Couplings Using Aryl Ethers via C–O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726. [Google Scholar] [CrossRef]
- Tobisu, M.; Chatani, N. Metal-Catalyzed Aromatic C–O Bond Activation/Transformation. In Organometallics for Green Catalysis; Dixneuf, P.H., Soulé, J.-F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 103–140. [Google Scholar]
- Cornella, J.; Zarate, C.; Martin, R. Metal-Catalyzed Activation of Ethers via C–O Bond Cleavage: A New Strategy for Molecular Diversity. Chem. Soc. Rev. 2014, 43, 8081–8097. [Google Scholar] [CrossRef]
- Gandeepans, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef]
- Boutadla, Y.; Davies, D.L.; Macgregor, S.A.; Poblador-Bahamonde, A.I. Mechanisms of C–H bond activation: Rich synergy between computation and experiment. Dalton Trans. 2009, 30, 5820–5831. [Google Scholar] [CrossRef]
- Kotek, V.; Polák, P.; Tobrman, T. Efficient and Simple Preparation of Functionalized 1,1-Dibromoenol Phosphates. Monat. Chem. 2016, 147, 405–412. [Google Scholar] [CrossRef]
- Ashida, Y.; Tanabe, Y. Cover Picture: Stereocomplementary and Parallel Syntheses of Multi-Substituted (E)-, (Z)-Stereodefined α,β-Unsaturated Esters: Application to Drug Syntheses. Chem. Rec. 2020, 20, 1409. [Google Scholar] [CrossRef]
- Chassaing, S.; Specklin, S.; Weibel, J.-M.; Pale, P. Vinyl and Aryl Sulfonates: Preparations and Applications in Total Synthesis. Curr. Org. Synth. 2012, 9, 806–827. [Google Scholar] [CrossRef]
- Li, Z.; Ning, Z.; Yu, H.; Jiao, L. Research Progress in the Preparation of Aryl and Alkyl Mixed Phosphates. Chin. J. Org. Chem. 2021, 41, 4180–4191. [Google Scholar] [CrossRef]
- Perkow, W.; Ullerich, K.; Meyer, F. Neue Phosphorsäureester mit pupillenverengender Wirkung. Naturwissenschaften 1952, 39, 353. [Google Scholar] [CrossRef]
- Kotek, V.; Polák, P.; Dvořáková, H.; Tobrman, T. Aluminum Chloride Promoted Cross-Coupling of Trisubstituted Enol Phosphates with Organozinc Reagents En Route to the Stereoselective Synthesis of Tamoxifen and Its Analogues. Eur. J. Org. Chem. 2016, 5037–5044. [Google Scholar] [CrossRef]
- Kotek, V.; Dvořáková, H.; Tobrman, T. Modular and Highly Stereoselective Approach to All-Carbon Tetrasubstituted Alkenes. Org. Lett. 2015, 17, 608–611. [Google Scholar] [CrossRef]
- Koudelka, J.; Tobrman, T. Synthesis of 2-Substituted Cyclobutanones by a Suzuki Reaction and Dephosphorylation Sequence. Eur. J. Org. Chem. 2021, 3260–3269. [Google Scholar] [CrossRef]
- Edlová, T.; Dvořáková, H.; Eigner, V.; Tobrman, T. Substrate-Controlled Regioselective Bromination of 1,2-Disubstituted Cyclobutenes: An Application in the Synthesis of 2,3-Disubstituted Cyclobutenones. J. Org. Chem. 2021, 86, 5820–5831. [Google Scholar] [CrossRef]
- Čubiňák, M.; Bigeon, J.; Galář, P.; Ondič, L.; Tobrman, T. The Synthesis of Tetrasubstituted Cycloalkenes Bearing π-Conjugated Substituents and Their Optical Properties. ChemistrySelect 2021, 6, 9904–9910. [Google Scholar] [CrossRef]
- Čubiňák, M.; Tobrman, T. Room-Temperature Negishi Reaction of Trisubstituted Vinyl Phosphates for the Synthesis of Tetrasubstituted Alkenes. J. Org. Chem. 2020, 85, 10728–10739. [Google Scholar] [CrossRef]
- Polák, P.; Tobrman, T. Novel Selective Approach to Terminally Substituted [n]Dendralenes. Eur. J. Org. Chem. 2019, 957–968. [Google Scholar] [CrossRef]
- Polák, P.; Tobrman, T. The synthesis of polysubstituted indoles from 3-bromo-2-indolyl phosphates. Org. Biomol. Chem. 2017, 15, 6233–6241. [Google Scholar] [CrossRef]
- Barkley, L.B.; Knowles, W.S.; Raffelson, H.; Thompson, Q.E. Studies in Steroid Total Synthesis. IV. A Stereoselective Ring A Synthesis1. J. Am. Chem. Soc. 1956, 78, 4111–4116. [Google Scholar] [CrossRef]
- Kumar, S.; Khatri, V.; Mangla, P.; Chhatwal, R.J.; Parmar, V.S.; Prasad, A.K. C-Glycopyranosyl Aldehydes: Emerging Chiral Synthons in Organic Synthesis. RSC Adv. 2023, 13, 19898–19954. [Google Scholar] [CrossRef]
- Ilia, G.; Simulescu, V.; Plesu, N.; Chiriac, V.; Merghes, P. Wittig and Wittig–Horner Reactions under Sonication Conditions. Molecules 2023, 28, 1958. [Google Scholar] [CrossRef]
- Morodo, R.; Bianchi, P.; Monbaliu, J.-C.M. Continuous Flow Organophosphorus Chemistry. Eur. J. Org. Chem. 2020, 5236–5277. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Daraie, M.; Ghanbarian, M. Applications of Wittig Reaction in the Total Synthesis of Natural Macrolides. ChemistrySelect 2020, 5, 9654–9690. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ghanbarian, M.; Zadsirjan, V.; Alimadadi Jani, B. Recent Advances in the Applications of Wittig Reaction in the Total Synthesis of Natural Products Containing Lactone, Pyrone, and Lactam as a Scaffold. Monatsh. Chem. 2019, 150, 1365–1407. [Google Scholar] [CrossRef]
- Ager, D.J. Peterson Alkenation. Sci. Synth. 2010, 47, 85. [Google Scholar] [CrossRef]
- Frances van Staden, L.; Gravestock, D.; Ager, D.J. New developments in the Peterson olefination reaction. Chem. Soc. Rev. 2002, 31, 195–200. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Lia, Y.; Tang, Y. Denitrogenative Suzuki and carbonylative Suzuki coupling reactions of benzotriazoles with boronic acids. Chem. Sci. 2017, 8, 3852–3857. [Google Scholar] [CrossRef]
- Kálai, T.; Jekő, J.; Hideg, K. Synthesis of Isoindoline Nitroxides by Electrocyclic Reactions. Synthesis 2009, 2591–2595. [Google Scholar] [CrossRef]
- Peyroux, E.; Berthiol, F.; Doucet, H.; Santelli, M. Suzuki Cross-Coupling Reactions between Alkenylboronic Acids and Aryl Bromides Catalysed by a Tetraphosphane-Palladium Catalyst. Eur. J. Org. Chem. 2004, 1075–1082. [Google Scholar] [CrossRef]
- Okazaki, R.; Ooka, M.; Tokitoh, N.; Inamoto, N. Synthesis and Reactions of 1,6-Dithiocyanato- and 1,6-Diiodo-1,3,5-cycloheptatrienes. J. Org. Chem. 1985, 50, 180–185. [Google Scholar] [CrossRef]
- McLaughlin, M.L.; McKinney, J.A.; Paquette, L.A. Efficient Preparation of Homochiral Bicyclo-Annulated Cyclopentadienes via the Skattebøl Rearrangement. Avoidance of limitations due to angle strain. Tetrahedron Lett. 1986, 27, 5595–5598. [Google Scholar] [CrossRef]
- Labadie, J.W.; Stille, J.K. Mechanisms of the Palladium-Catalyzed Couplings of Acid Chlorides with Organotin Reagents. J. Am. Chem. Soc. 1983, 105, 6129–6137. [Google Scholar] [CrossRef]
- Adamson, N.J.; Jeddi, H.; Malcolmson, S.J. Preparation of Chiral Allenes through Pd-Catalyzed Intermolecular Hydroamination of Conjugated Enynes: Enantioselective Synthesis Enabled by Catalyst Design. J. Am. Chem. Soc. 2019, 141, 8574–8583. [Google Scholar] [CrossRef]
- Du, F.; Yin, L.; Ning, Y.; Peng, Y. Catalytic Asymmetric Synthesis of Phosphoryl-1,4-dihydropyridazines via an Enantioselective Allylic Alkylation/1,3-Dipolar Cycloaddition/Rearrangement Reaction Sequence. Adv. Synth. Catal. 2016, 358, 2280–2285. [Google Scholar] [CrossRef]
- Sirotkina, J.; Grigorjeva, L.; Jirgensons, A. Synthesis of Alkynyl-Glycinols by Lewis Acid Catalyzed Propargylic Substitution of Bis-Imidates. Eur. J. Org. Chem. 2015, 6900–6908. [Google Scholar] [CrossRef]
- Cheng, J.-K.; Loh, T.-P. Copper- and Cobalt-Catalyzed Direct Coupling of sp3 α-Carbon of Alcohols with Alkenes and Hydroperoxides. J. Am. Chem. Soc. 2015, 137, 42–45. [Google Scholar] [CrossRef]
- Seath, C.P.; Vogt, D.B.; Xu, Z.; Boyington, A.J.; Jui, N.T. Radical Hydroarylation of Functionalized Olefins and Mechanistic Investigation of Photocatalytic Pyridyl Radical Reactions. J. Am. Chem. Soc. 2018, 140, 15525–15534. [Google Scholar] [CrossRef]
- Groves, A.; Martínez, J.I.; Smith, J.J.; Lam, H.W. Remote Nucleophilic Allylation by Allylrhodium Chain Walking. Chem. Eur. J. 2018, 24, 13432–13436. [Google Scholar] [CrossRef]
- Le Fèvre, R.J.W.; Sundaram, A. 787. Molecular polarisability: The conformations of ten cyclic dibasic acid anhydrides indicated by their dipole moments, molar Kerr constants, etc. J. Chem. Soc. (Resumed) 1962, 4009–4019. [Google Scholar] [CrossRef]
- Agents Classified by the IARC Monographs, Volumes 1–134. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 20 August 2023).
- Mykura, R.C.; Songara, P.; Luc, E.; Rogers, J.; Stammers, E.; Aggarwal, V.K. Studies on the Lithiation, Borylation, and 1,2-Metalate Rearrangement of O-Cycloalkyl 2,4,6-Triisopropylbenzoates. Angew. Chem. Int. Ed. 2021, 60, 11436–11441. [Google Scholar] [CrossRef]
- Wang, Y.; Noble, A.; Myers, E.L.; Aggarwal, V.K. Enantiospecific Alkynylation of Alkylboronic Esters. Angew. Chem. Int. Ed. 2016, 55, 4270–4274. [Google Scholar] [CrossRef]
- Kumpulainen, H.; Järvinen, T.; Saari, R.; Lehtonen, M.; Vepsäläinen, J. An Efficient Strategy for the Synthesis of 1-Chloroethyl Phosphates and Phosphoramidates. J. Org. Chem. 2005, 70, 9056–9058. [Google Scholar] [CrossRef]
- Zhao, S.; Cai, X.; Lu, Y.; Hu, J.; Xiong, Z.; Jin, J.; Li, Y.; Wang, H.; Wu, J.-Q. Cp*Ir(III) and Cp*Rh(III)-Catalyzed Annulation of Salicylaldehydes with Fluorinated Vinyl Tosylates. Chem. Commun. 2022, 58, 8966–8969. [Google Scholar] [CrossRef]
- Gøgsig, T.M.; Søbjerg, L.S.; Lindhardt, A.T.; Jensen, K.L.; Skrydstrup, T. Direct Vinylation and Difluorovinylation of Arylboronic Acids Using Vinyl- and 2,2-Difluorovinyl Tosylates via the Suzuki–Miyaura Cross Coupling. J. Org. Chem. 2008, 73, 3404–3410. [Google Scholar] [CrossRef]
- McCammant, M.S.; Shigeta, T.; Sigman, M.S. Palladium-Catalyzed 1,3-Difunctionalization Using Terminal Alkenes with Alkenyl Nonaflates and Aryl Boronic Acids. Org. Lett. 2016, 18, 1792–1795. [Google Scholar] [CrossRef]
- Lyapkalo, I.M.; Webel, M.; Reißig, H.-U. Synthesis and Heck Reactions of Ethenyl- and (Z)-Butadien-1-yl Nonaflate Obtained by the Fragmentation of Furan Derivatives. Eur. J. Org. Chem. 2001, 4189–4194. [Google Scholar] [CrossRef]
- Song, B.; Knauber, T.; Gooßen, L.J. Decarboxylative Cross-Coupling of Mesylates Catalyzed by Copper/Palladium Systems with Customized Imidazolyl Phosphine Ligands. Angew. Chem. Int. Ed. 2013, 52, 2954–2958. [Google Scholar] [CrossRef]
- Fawcett, A.; Biberger, T.; Aggarwal, V.K. Carbopalladation of C–C σ-Bonds Enabled by Strained Boronate Complexes. Nat. Chem. 2019, 11, 117–122. [Google Scholar] [CrossRef]
- Shirakawa, E.; Sato, T.; Imazaki, Y.; Kimura, T.; Hayashi, T. Cobalt-Catalyzed Cross-Coupling of Alkynyl Grignard Reagents with Alkenyl Triflates. Chem. Commun. 2007, 43, 4513–4515. [Google Scholar] [CrossRef]
- Harkness, G.J.; Clarke, M.L. A Highly Enantioselective Alkene Methoxycarbonylation Enables a Concise Synthesis of (S)-Flurbiprofen. Eur. J. Org. Chem. 2017, 4859–4863. [Google Scholar] [CrossRef]
- Dunet, G.; Knochel, P. Iron-Catalyzed Cross-Coupling between Alkenyl and Dienyl Sulfonates and Functionalized Arylcopper Reagents. Synlett 2006, 3, 0407–0410. [Google Scholar] [CrossRef]
- Li-Yuan Bao, R.; Zhao, R.; Shi, L. Progress and Developments in the Turbo Grignard Reagent i-PrMgCl·LiCl: A Ten-Year Journey. Chem. Commun. 2015, 51, 6884–6900. [Google Scholar] [CrossRef]
- Adrio, J.; Carretero, J.C. Functionalized Grignard Reagents in Kumada Cross-Coupling Reactions. ChemCatChem 2010, 2, 1384–1386. [Google Scholar] [CrossRef]
- Ila, H.; Baron, O.; Wagner, A.J.; Knochel, P. Functionalized Magnesium Organometallics as Versatile Intermediates for the Synthesis of Polyfunctional Heterocycles. Chem. Commun. 2006, 6, 583–593. [Google Scholar] [CrossRef]
- Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F.F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V.A. Highly Functionalized Organomagnesium Reagents Prepared through Halogen–Metal Exchange. Angew. Chem. Int. Ed. 2003, 42, 4302–4320. [Google Scholar] [CrossRef]
- Tobrman, T.; Dvořák, D. Heck reactions of 6- and 2-halopurines. Eur. J. Org. Chem. 2008, 2923–2928. [Google Scholar] [CrossRef]
- Khan, R.I.; Pitchumani, K. A Pyridinium Modified β-Cyclodextrin: An Ionic Supramolecular Ligand for Palladium Acetate in C–C Coupling Reactions in Water. Green Chem. 2016, 18, 5518–5528. [Google Scholar] [CrossRef]
- Berthiol, F.; Doucet, H.; Santelli, M. Heck Reaction of Vinyl Bromides with Alkenes in the Presence of a Tetra phosphine/Palladium Catalyst. Synlett 2003, 0841–0844. [Google Scholar] [CrossRef]
- Hansen, A.L.; Ebran, J.-P.; Ahlquist, M.; Norrby, P.-O.; Skrydstrup, T. Heck Coupling with Nonactivated Alkenyl Tosylates and Phosphates: Examples of Effective 1,2-Migrations of the Alkenyl Palladium(II) Intermediates. Angew. Chem. Int. Ed. 2006, 45, 3349–3353. [Google Scholar] [CrossRef]
- Fu, W.C.; Wu, Y.; So, C.M.; Wong, S.M.; Lei, A.; Kwong, F.Y. Catalytic Direct C2-Alkenylation of Oxazoles at Parts per Million Levels of Palladium/PhMezole-Phos Complex. Org. Lett. 2016, 18, 5300–5303. [Google Scholar] [CrossRef]
- Gui, Y.-Y.; Liao, L.-L.; Sun, L.; Zhang, Z.; Ye, J.-H.; Shen, G.; Lu, Z.-P.; Zhou, W.-J.; Yu, D.-G. Coupling of C(sp3)–H bonds with C(sp2)–O Electrophiles: Mild, General and Selective. Chem. Commun. 2017, 53, 1192–1195. [Google Scholar] [CrossRef]
- Kumar, D.; Chen, M.S.; Goodman, D.W. Synthesis of Vinyl Acetate on Pd-Based Catalysts. Catal. Today 2007, 123, 77–85. [Google Scholar] [CrossRef]
- Gärtner, D.; Stein, A.L.; Grupe, S.; Arp, J.; Jacobi von Wangelin, A. Iron-Catalyzed Cross-Coupling of Alkenyl Acetates. Angew. Chem. Int. Ed. 2015, 54, 10545–10549. [Google Scholar] [CrossRef]
- Su, M.; Huang, X.; Lei, C.; Jin, J. Nickel-Catalyzed Reductive Cross-Coupling of Aryl Bromides with Vinyl Acetate in Dimethyl Isosorbide as a Sustainable Solvent. Org. Lett. 2022, 24, 354–358. [Google Scholar] [CrossRef]
- Zhou, B.; Qi, X.; Liu, P.; Dong, G. Development and Mechanistic Studies of the Iridium-Catalyzed C–H Alkenylation of Enamides with Vinyl Acetates: A Versatile Approach for Ketone Functionalization. Angew. Chem. Int. Ed. 2021, 60, 20926–20934. [Google Scholar] [CrossRef]
- Sk, M.R.; Maji, M.S. Cobalt(III)-Catalyzed Ketone-Directed C–H Vinylation Using Vinyl Acetate. Org. Chem. Front. 2020, 7, 19–24. [Google Scholar] [CrossRef]
- Sarkar, D.; Venkateswaran, R.V. Synthesis of Bruguierol A Employing Ring-Closing Metathesis. Tetrahedron Lett. 2011, 52, 3232–3233. [Google Scholar] [CrossRef]
- Otley, K.D.; Ellman, J.A. An Efficient Method for the Preparation of Styrene Derivatives via Rh(III)-Catalyzed Direct C–H Vinylation. Org. Lett. 2015, 17, 1332–1335. [Google Scholar] [CrossRef]
- Mei, S.-T.; Jiang, K.; Wang, N.-J.; Shuai, L.; Yuan, Y.; Wei, Y. Rhodium-Catalyzed Direct C–H Vinylation of Arenes To Access Styrenes with Vinyl Acetate as a Vinyl Source. Eur. J. Org. Chem. 2015, 6135–6140. [Google Scholar] [CrossRef]
- Gophane, D.B.; Endeward, B.; Prisner, T.F.; Sigurdsson, S.T. A Semi-Rigid Isoindoline-Derived Nitroxide Spin Label for RNA. Org. Biomol. Chem. 2018, 16, 816–824. [Google Scholar] [CrossRef]
- Beng, T.K.; Wilkerson-Hill, S.M.; Sarpong, R. Direct Access to Functionalized Azepanes by Cross-Coupling with α-Halo Eneformamides. Org. Lett. 2014, 16, 916–919. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Xu, B.; Kuang, C. Palladium-Catalyzed Olefination and Arylation of 2-Substituted 1,2,3-Triazole N-Oxides. Org. Lett. 2013, 15, 2342–2345. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, C. Room-Temperature Direct Alkenylation of 3-Arylsydnones. Eur. J. Org. Chem. 2014, 7810–7813. [Google Scholar] [CrossRef]
- Li, S.-S.; Wang, C.-Q.; Lin, H.; Zhang, X.-M.; Dong, L. Rhodium(III)-Catalyzed C–C Coupling of 7-Azaindoles with Vinyl Acetates and Allyl Acetates. Org. Biomol. Chem. 2016, 14, 229–237. [Google Scholar] [CrossRef]
- Lin, W.; Li, W.; Lu, D.; Su, F.; Wen, T.-B.; Zhang, H.-J. Dual Effects of Cyclopentadienyl Ligands on Rh(III)-Catalyzed Dehydrogenative Arylation of Electron-Rich Alkenes. ACS Catal. 2018, 8, 8070–8076. [Google Scholar] [CrossRef]
- Qin, W.-B.; Li, W.-W.; Zhu, P.-F.; Mo, X.-G.; Zhang, H.-J.; Wen, T.-B. Rh(III)-Catalyzed Regio- and Stereoselective Bisindolylation of Vinyl Acetate: An Efficient Approach toward (E)-1,2-Bis(2-indolyl)ethenes. Org. Chem. Front. 2018, 5, 1096–1100. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Lin, W.; Su, F.; Wen, T.-B. Rhodium-Catalyzed β-Selective Oxidative Heck-Type Coupling of Vinyl Acetate via C–H Activation. Org. Lett. 2016, 18, 6356–6359. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Pourkaveh, R.; Karimi, A. Oxidative Heck Reaction as a Tool for Para-selective Olefination of Aniline: A DFT Supported Mechanism. J. Org. Chem. 2017, 82, 10635–10640. [Google Scholar] [CrossRef]
- Wu, J.; Cao, K.; Xu, T.-T.; Zhang, X.-J.; Jiang, L.; Yang, J.; Huang, Y. Palladium-Catalyzed Regioselective Mono-Alkenylation of o-Carboranes via Heck Type Coupling Reaction of a Cage B–H Bond. RSC Adv. 2015, 5, 91683–91685. [Google Scholar] [CrossRef]
- Karimi, B.; Behzadnia, H.; Elhamifar, D.; Akhavan, P.F.; Esfahani, F.K.; Zamani, A. Transition-Metal-Catalyzed Oxidative Heck Reactions. Synthesis 2010, 1399–1427. [Google Scholar] [CrossRef]
- Meng, L.; Liu, C.; Zhang, W.; Zhou, C.; Lei, A. Palladium-Catalysed β-Selective Oxidative Heck Reaction of an Electron-Rich Olefin. Chem. Commun. 2014, 50, 1110–1112. [Google Scholar] [CrossRef]
- Odell, L.R.; Lindh, J.; Gustafsson, T.; Larhed, M. Continuous Flow Palladium(II)-Catalyzed Oxidative Heck Reactions with Arylboronic Acids. Eur. J. Org. Chem. 2010, 2270–2274. [Google Scholar] [CrossRef]
- Öhrngren, P.; Fardost, A.; Russo, F.; Schanche, J.-S.; Fagrell, M.; Larhed, M. Evaluation of a Nonresonant Microwave Applicator for Continuous-Flow Chemistry Applications. Org. Process Res. Dev. 2012, 16, 1053–1063. [Google Scholar] [CrossRef]
- Chu, H.; Sun, S.; Yu, J.-T.; Cheng, J. Rh-Catalyzed Sequential Oxidative C–H Activation/Annulation with Geminal-Substituted Vinyl Acetates to Access Isoquinolines. Chem. Commun. 2015, 51, 13327–13329. [Google Scholar] [CrossRef]
- Webb, N.J.; Raw, S.A.; Marsden, S.P. Isoquinoline Synthesis by C–H Activation/Annulation Using Vinyl Acetate as an Acetylene Equivalent. Tetrahedron 2018, 74, 5200–5205. [Google Scholar] [CrossRef]
- Jothi Murugan, S.; Jeganmohan, M. Cp*Co(III)-Catalyzed Regioselective [4 + 2]-Annulation of N-Chlorobenzamides with Vinyl Acetate/Vinyl Ketones. J. Org. Chem. 2023, 88, 1578–1589. [Google Scholar] [CrossRef]
- Webb, N.J.; Marsden, S.P.; Raw, S.A. Rhodium(III)-Catalyzed C–H Activation/Annulation with Vinyl Esters as an Acetylene Equivalent. Org. Lett. 2014, 16, 4718–4721. [Google Scholar] [CrossRef]
- Liang, G.; Rong, J.; Sun, W.; Chen, G.; Jiang, Y.; Loh, T.-P. Synthesis of Polyaromatic Rings: Rh(III)-Catalyzed [5 + 1] Annulation of Enaminones with Vinyl Esters through C–H Bond Functionalization. Org. Lett. 2018, 20, 7326–7331. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.-J.; Han, T.; Ruan, W.; Wen, T.-B. Rh(III)-Catalyzed Oxidative Coupling of Benzoic Acids with Geminal-Substituted Vinyl Acetates: Synthesis of 3-Substituted Isocoumarins. J. Org. Chem. 2015, 80, 620–627. [Google Scholar] [CrossRef]
- Lou, M.; Deng, Z.; Mao, X.; Fu, Y.; Yang, Q.; Peng, Y. Rhodium-catalyzed C–H bond activation alkylation and cyclization of 2-arylquinazolin-4-ones. Org. Biomol. Chem. 2018, 16, 1851–1859. [Google Scholar] [CrossRef]
- Li, S.-S.; Liu, C.-F.; Xia, Y.-Q.; Li, W.-H.; Zhang, G.-T.; Zhang, X.-M.; Dong, L. A Unique Annulation of 7-Azaindoles with Alkenyl Esters to Produce π-Conjugated 7-Azaindole Derivatives. Org. Biomol. Chem. 2016, 14, 5214–5218. [Google Scholar] [CrossRef]
- Pinna, C.; Martino, P.A.; Meroni, G.; Sora, V.M.; Tamborini, L.; Dallavalle, S.; Contente, M.L.; Pinto, A. Biocatalyzed Synthesis of Vanillamides and Evaluation of Their Antimicrobial Activity. J. Agric. Food Chem. 2022, 70, 223–228. [Google Scholar] [CrossRef]
- Cavallari, E.; Carrera, C.; Aime, S.; Reineri, F. 13C MR Hyperpolarization of Lactate by Using ParaHydrogen and Metabolic Transformation in Vitro. Chem. Eur. J. 2017, 23, 1200–1204. [Google Scholar] [CrossRef]
- Gregorić, T.; Makarević, J.; Štefanić, Z.; Žinić, M.; Frkanec, L. Gamma Radiation- and Ultraviolet-Induced Polymerization of Bis(amino acid)fumaramide Gel Assemblies. Polymers 2022, 14, 214. [Google Scholar] [CrossRef]
- Deng, W.; Hu, Y.; Hu, J.; Li, X.; Li, Y.; Huang, Y. Electrochemically induced Markovnikov-type selective hydro/deuterophosphonylation of electron-rich alkenes. Chem. Commun. 2022, 58, 12094–12097. [Google Scholar] [CrossRef]
- Zeng, T.; You, W.; Chen, G.; Nie, X.; Zhang, Z.; Xia, L.; Hong, C.; Chen, C.; You, Y. Degradable PE-Based Copolymer with Controlled Ester Structure Incorporation by Cobalt-Mediated Radical Copolymerization under Mild Condition. iScience 2020, 23, 100904. [Google Scholar] [CrossRef]
- Young, C.M.; Taylor, J.E.; Smith, A.D. Evaluating Aryl Esters as Bench-Stable C(1)-Ammonium Enolate Precursors in Catalytic, Enantioselective Michael Addition–Lactonisations. Org. Biomol. Chem. 2019, 17, 4747–4752. [Google Scholar] [CrossRef]
- Kadidae, L.O.; Usami, A.; Honda, M. Palladium(II) Acetate as Catalyst in Transvinylation Reactions of Hydroxycinnamic Acid and Its Derivatives. Asian J. Chem. 2018, 30, 589–593. [Google Scholar] [CrossRef]
- Sato, K.; Kobayashi, S.; Sekishita, A.; Wakui, M.; Tanaka, M. Synthesis and Thrombogenicity Evaluation of Poly(3-methoxypropionic acid vinyl ester): A Candidate for Blood-Compatible Polymers. Biomacromolecules 2017, 18, 1609–1616. [Google Scholar] [CrossRef]
- Hibert, G.; Grau, E.; Pintori, D.; Lecommandoux, S.; Cramail, H. ADMET Polymerization of α,ω-Unsaturated Glycolipids: Synthesis and Physico-Chemical Properties of the Resulting Polymers. Polym. Chem. 2017, 8, 3731–3739. [Google Scholar] [CrossRef]
- Hedir, G.G.; Arno, M.C.; Langlais, M.; Husband, J.T.; O’Reilly, R.K.; Dove, A.P. Poly(oligo(ethylene glycol) vinyl acetate)s: A Versatile Class of Thermoresponsive and Biocompatible Polymers. Angew. Chem. Int. Ed. 2017, 56, 9178–9182. [Google Scholar] [CrossRef]
- Krejzová, J.; Šimon, P.; Vavříková, E.; Slámová, K.; Pelantová, H.; Riva, S.; Spiwok, V.; Křen, V. Enzymatic Synthesis of New C-6-acylated Derivatives of NAG-thiazoline and Evaluation of their Inhibitor Activities Towards Fungal β-N-acetylhexosaminidase. J. Mol. Catal. B Enzym. 2013, 87, 128–134. [Google Scholar] [CrossRef]
- Martínez-Montero, S.; Fernández, S.; Sanghvi, Y.S.; Gotor, V.; Ferrero, M. An Expedient Biocatalytic Procedure for Abasic Site Precursors Useful in Oligonucleotide Synthesis. Org. Biomol. Chem. 2011, 9, 5960–5966. [Google Scholar] [CrossRef]
- Padrosa, D.R.; Contente, M.L. Multi-Gram Preparation of Cinnamoyl Tryptamines as Skin Whitening Agents Through a Chemo-Enzymatic Flow Process. Tetrahedron Lett. 2021, 86, 153453. [Google Scholar] [CrossRef]
- Jebrane, M.; Terziev, N.; Heinmaa, I. Biobased and Sustainable Alternative Route to Long-Chain Cellulose Esters. Biomacromolecules 2017, 18, 498–504. [Google Scholar] [CrossRef]
- Vilela, C.; Rua, R.; Silvestre, A.J.D.; Gandini, A. Polymers and Copolymers from Fatty Acid-Based Monomers. Ind. Crops Prod. 2010, 32, 97–104. [Google Scholar] [CrossRef]
- Lou, S.; Gao, S.; Wang, W.; Zhang, M.; Zhang, J.; Wang, C.; Li, C.; Kong, D.; Zhao, Q. Galactose-Functionalized Multi-Responsive Nanogels for Hepatoma-Targeted Drug Delivery. Nanoscale 2015, 7, 3137–3146. [Google Scholar] [CrossRef]
- Shen, F.-W.; Zhou, K.-C.; Cai, H.; Zhang, Y.-N.; Zheng, Y.-L.; Quan, J. One-Pot Synthesis of Thermosensitive Glycopolymers Grafted Gold Nanoparticles and Their Lectin Recognition. Colloids Surf. B 2019, 173, 504–511. [Google Scholar] [CrossRef]
- Adelman, R.L. The Interchange Reaction of Vinyl Acetate with Organic Acids. J. Org. Chem. 1949, 14, 1057–1077. [Google Scholar] [CrossRef]
- Jiang, R.; Chen, Z.; Zhan, K.; Liu, L.; Zhou, J.; Ai, Y.; Li, S.; Bao, H.; Hu, Z.n.; Qi, L.; et al. Reusable Rhodium Catalyst for the Selective Transvinylation of sp2-C Linked Carboxylic Acid. Tetrahedron Lett. 2018, 59, 3279–3282. [Google Scholar] [CrossRef]
- Ketterling, A.A.; Lisitsyn, A.S.; Nosov, A.V.; Likholobov, V.A. Carboxylic Acid Transvinylation as Catalysed by Complexes of Palladium Acetate with Phenanthroline-Like Ligands. Appl. Catal. 1990, 66, 123–131. [Google Scholar] [CrossRef]
- Henry, P.M. Palladium(II)-Catalyzed Exchange and Isomerization Reactions. I. Exchange of Enol Acetates with Acetic Acid Catalyzed by Palladium(II) Chloride. J. Am. Chem. Soc. 1971, 93, 3853–3859. [Google Scholar] [CrossRef]
- Ziriakus, J.; Zimmermann, T.K.; Pöthig, A.; Drees, M.; Haslinger, S.; Jantke, D.; Kühn, F.E. Ruthenium-Catalyzed Transvinylation—New Insights. Adv. Synth. Catal. 2013, 355, 2845–2859. [Google Scholar] [CrossRef]
- Winternheimer, D.J.; Shade, R.E.; Merlic, C.A. Methods for Vinyl Ether Synthesis. Synthesis 2010, 2497–2511. [Google Scholar] [CrossRef]
- Ober, M.S.; Romer, D.R.; Etienne, J.; Thomas, P.J.; Jain, V.; Cameron, J.F.; Thackeray, J.W. Backbone Degradable Poly(aryl acetal) Photoresist Polymers: Synthesis, Acid Sensitivity, and Extreme Ultraviolet Lithography Performance. Macromolecules 2019, 52, 886–895. [Google Scholar] [CrossRef]
- Cluzeau, J.; Nettekoven, U.; Kovačevič, M.P.; Časar, Z. Concise Six-Step Asymmetric Approach to Ramelteon from an Acetophenone Derivative Using Ir, Rh, Cu, and Ni Catalysis. J. Org. Chem. 2022, 87, 2129–2135. [Google Scholar] [CrossRef]
- Alavez-Rosas, D.; Maldonado-Domínguez, M.; González-Antonio, O.; Romero-Ávila, M.; Méndez-Stivalet, J.; Flores-Pérez, B. Synthesis of 1,3- and 1,2,3-Functionalized Pyrroles via Ir(I)-Catalyzed Vinylation of Allyl Alcohols. Chem. Heterocycl. Compd. 2017, 53, 526–531. [Google Scholar] [CrossRef]
- Spiegelberg, B.; Jiao, H.; Grauke, R.; Kubis, C.; Spannenberg, A.; Brandt, A.; Taden, A.; Beck, H.; Tin, S.; de Vries, J.G. Use of Iridium-Catalyzed Transfer Vinylation for the Synthesis of Bio-Based (bis)-Vinyl Ethers. Adv. Synth. Catal. 2022, 364, 1251–1263. [Google Scholar] [CrossRef]
- Jena, S.; Chanda, K. Copper Catalyzed Synthesis of Heterocyclic Molecules via C–N and C–O Bond Formation under Microwaves: A Mini-Review. ACS Omega 2023, 8, 23240–23256. [Google Scholar] [CrossRef]
- Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Ber. Dtsch. Chem. Ges. 1906, 39, 1691–1692. [Google Scholar] [CrossRef]
- Serrano, E.; de Nanteuil, F.; Waser, J. Diester-Substituted Aminocyclopropanes: Synthesis and Use in [3+2]-Annulation Reactions. Synlett 2014, 25, 2285–2288. [Google Scholar] [CrossRef]
- Kimura, J.; Nakamichi, S.; Ogawa, S.; Obora, Y. Iridium-Catalyzed Vinylation of Carbazole Derivatives with Vinyl Acetate. Synlett 2017, 28, 719–723. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).