On the Redox Equilibrium of TPP/TPPO Containing Cu(I) and Cu(II) Complexes
Abstract
1. Introduction
2. Materials and Methods
3. Results
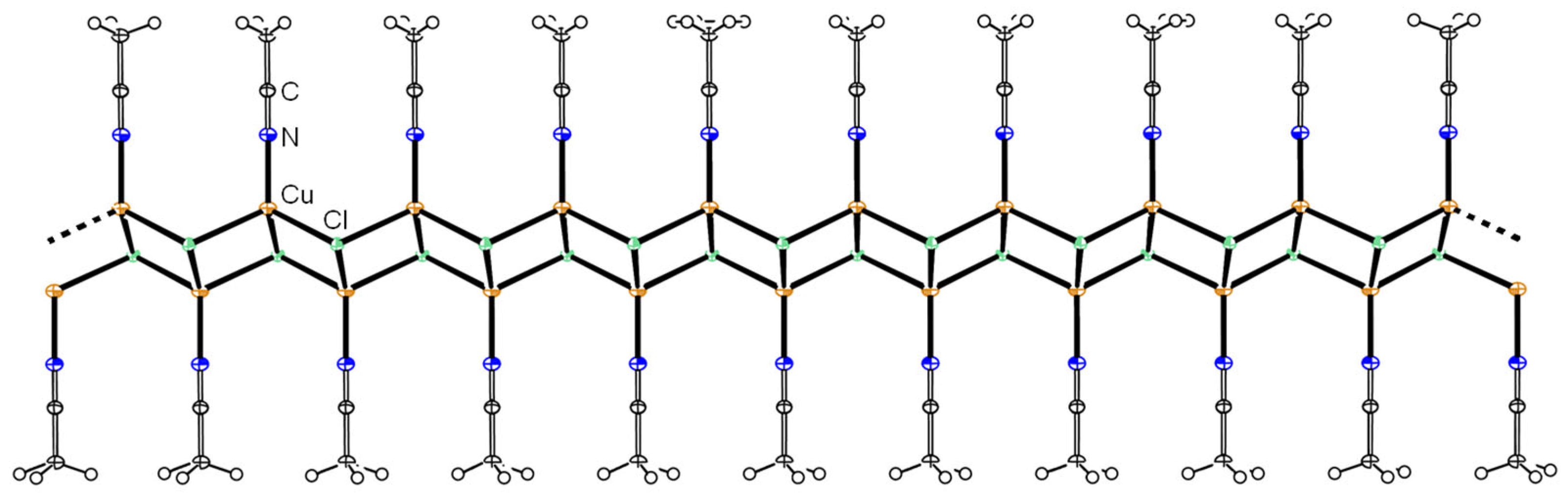
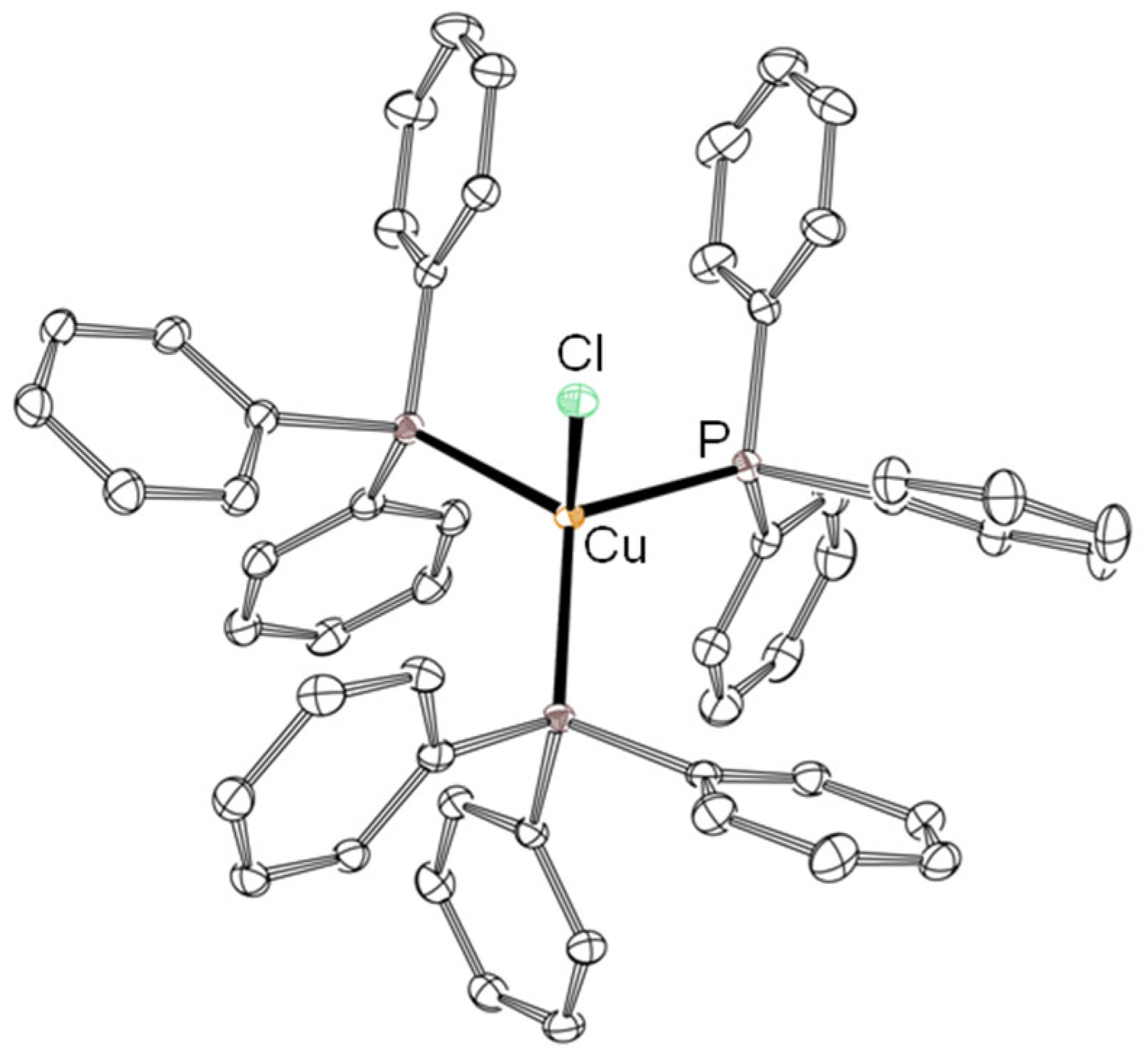
3.1. Template Reactions of [CuII4OCl6(MeOH)4] with TPP/TPPO
3.2. Reaction of CuO, CuCl2, and TPP/TPPO
3.3. Reaction of CuCl2·2H2O and TPP/TPPO under Atmospheric Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haiduc, I. ReviewInverse coordination̶̶ – An emerging new chemical concept. Oxygen and other chalcogens as coodination centers. Coord. Chem. Rev. 2017, 338, 1. [Google Scholar] [CrossRef]
- Melník, M.; Kabešová, M.; Koman, M.; Macáškova, L.; Holloway, C.E. Copper(II) coordination compounds: Classification and analysis of crystallographic and structural data IV. Trimeric and oligomeric compounds. J. Coord. Chem. 1999, 48, 271. [Google Scholar] [CrossRef]
- Melník, M.; Koman, M.; Ondrejovič, G. Tetramers Cu4 (μ4-O)(η-X)6 (L4): Analysis of structural data. Coord. Chem. Rev. 2011, 255, 1581. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Kelley, J.A. Five-coordinate complexes. II. 1 Trigonal Bipyramidal Copper(II) in a Metal Atom Cluster. J. Am. Chem. Soc. 1966, 88, 4746. [Google Scholar] [CrossRef]
- Bertrand, J.A. Five-coordinate complexes. III. Structure and properties of. µ4-oxohexa-µ-chlorotetrakis {(triphenylphosphine oxide)copper (II)}1. Inorg. Chem. 1967, 6, 495. [Google Scholar] [CrossRef]
- Lines, M.E.; Ginsberg, A.P.; Martin, R.L.; Sherwood, R.C. Magnetic Exchange in Transition Metal Complexes. VII. Spin Coupling in μ4-Oxohexa-μ-halotetrakis [(Triphenylphosphine Oxide or Pyridine) Copper (II)]: Evidence for Antisymmetric Exchange. J. Chem. Phys. 1972, 57, 1. [Google Scholar] [CrossRef]
- Wong, H.; Dieck, H.T.; O’Connor, C.J.; Sinn, E. Magnetic exchange interactions in tetranuclear copper(II) complexes. Effect of ligand electronegativity. J. Chem. Soc. Dalton Trans. 1980, 5, 786. [Google Scholar] [CrossRef]
- Reim, J.; Griesar, K.; Haase, W.; Krebs, B. Structure and magnetism of novel tetranuclear µ4-oxo-bridged copper(II) complexes. J. Chem. Soc. Dalton Trans. 1995, 16, 2649. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kozlov, Y.N.; Shul’pina, L.S.; Lyakin, O.Y.; Kirillov, A.M.; Talsi, E.P.; Pombeiro, A.J.L.; Shul’pin, G.B. Remarkably fast oxidation of alkanes by hydrogen peroxide catalyzed by a tetracopper(II) triethanolaminate complex: Promoting effects of acid co-catalysts and water, kinetic and mechanistic features. J. Catal. 2009, 268, 26. [Google Scholar] [CrossRef]
- Sun, H.; Harms, K.; Sundermeyer, J. Aerobic oxidation of 2,3,6-Trimethylphenol to Trimethyl-1,4-benzoquinone with Copper(II) Chloride as Catalyst in Ionic Liquid and Structure of the Active Species. J. Am. Chem. Soc. 2004, 126, 9550. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kopylovich, M.N.; Kirillova, M.V.; Haukka, M.; Da Silva, M.F.C.G.; Pombeiro, A.J.L. Mild Peroxidative Oxidation of Cyclohexane Catalyzed by Mono-, Di-, Tri-, Tetra-and Polynuclear Copper Triethanolamine Complexes. Angew. Chem. 2005, 117, 4419. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kopylovich, M.N.; Kirillova, M.V.; Haukka, M.; Da Silva, M.F.C.G.; Pombeiro, A.J.L. Mild Peroxidative Oxidation of Cyclohexane Catalyzed by Mono-, Di-, Tri-, Tetra-and Polynuclear Copper Triethanolamine Complexes. Angew. Chem. Int. Ed. 2005, 44, 4345. [Google Scholar] [CrossRef] [PubMed]
- Gawlig, C.; Schindler, S.; Becker, S. One-Pot Conversion of Cyclohexane to Adipic Acid Using a µ4-Oxido-Copper Cluster as Catalyst Together with Hydrogen Peroxide. Eur. J. Inorg. Chem. 2020, 2020, 248. [Google Scholar] [CrossRef]
- Löw, S.; Becker, J.; Würtele, C.; Miska, A.; Kleeberg, C.; Behrens, U.; Walter, O.; Schindler, S. Reactions of Copper(II) Chloride in Solution: Facile Formation of Tetranuclear Copper Clusters and Other Complexes That Are Relevant in Catalytic Redox Processes. Chem. Eur. J. 2013, 19, 5342. [Google Scholar] [CrossRef]
- Becker, S.; Behrens, U.; Schindler, S. Investigations Concerning [Cu4OX6L4] Cluster Formation of Copper(II) Chloride with Amine Ligands Related to Benzylamine. Eur. J. Inorg. Chem. 2015, 14, 2437. [Google Scholar] [CrossRef]
- Becker, S.; Dürr, M.; Miska, A.; Becker, J.; Gawlig, C.; Behrens, U.; Ivanović-Burmazović, I.; Schindler, S. Copper Chloride Catalysis: Do μ4-Oxido Copper Clusters Play a Significant Role? Inorg. Chem. 2016, 55, 3759. [Google Scholar] [CrossRef]
- Makáňová, D.; Ondrejovič, G.; Gažo, J. Reaktionen des Kupfer(II)-chlorids mit Triphenylphosphin Isolierung und Identifizierung einiger Oxidoreduktionsprodukte. Chem. Zvesti 1973, 27, 4. [Google Scholar]
- Berners-Price, S.J.; Sadler, P.J. Bioinorganic Chemistry; Springer: Berlin/Heidelberg, Germany, 1988; Volume 70, p. 27. [Google Scholar] [CrossRef]
- Cahours, A.; Gal, H. Untersuchungen über neue Derivate des Triäthylphosphins. Ann. Chem. Pharm. 1870, 155, 355. [Google Scholar] [CrossRef]
- Boden, P.; Di Martino-Fumo, P.; Busch, J.M.; Rehak, F.R.; Steiger, S.; Fuhr, O.; Nieger, M.; Volz, D.; Klopper, W.; Bräse, S.; et al. Cover Feature: Investigation of Luminescent Triplet States in Tetranuclear CuI Complexes: Thermochromism and Structural Characterization. Chem. Eur. J. 2021, 27, 5309. [Google Scholar] [CrossRef]
- Lapprand, A.; Dutartre, M.; Khiri, N.; Levert, E.; Fortin, D.; Rousselin, Y.; Soldera, A.; Jugé, S.; Harvey, P.D. Luminescent P-chirogenic copper clusters. Inorg. Chem. 2013, 52, 7958. [Google Scholar] [CrossRef]
- Perruchas, S.; Le Goff, X.F.; Maron, S.; Maurin, I.; Guillen, F.; Garcia, A.; Gacoin, T.; Boilot, J.-P. Mechanochromic and Thermochromic Luminescence of a Copper Iodide Cluster. J. Am. Chem. Soc. 2010, 132, 10967. [Google Scholar] [CrossRef] [PubMed]
- Perruchas, S.; Tard, C.; Le Goff, X.F.; Fargues, A.; Garcia, A.; Kahlal, S.; Saillard, J.-Y.; Gacoin, T.; Boilot, J.-P. Thermochromic luminescence of Copper Iodide Clusters: The Case of Phosphine Ligands. Inorg. Chem. 2011, 50, 10682. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625. [Google Scholar] [CrossRef] [PubMed]
- Dieck, H.T.; Brehm, H. Vierkernige Komplexe mit trigonal-bipyramidal koordiniertem Kupfer (II), II. Synthese und Substitution an der axialen Position. Chem. Ber. 1969, 102, 3577. [Google Scholar] [CrossRef]
- Zelonka, R.A.; Baird, M.C. Five-coordinate Tertiary Phosphine and Arsine Adducts of Copper(hexafluoroacetylacetonate)2. Can. J. Chem. 1972, 50, 1269. [Google Scholar] [CrossRef]
- Jardine, F.H.; Rule, L.; Vohra, A.G. The chemistry of copper (I) complexes. Part I. Halogeno-complexes. J. Chem. Soc. A 1970, 238–240. [Google Scholar] [CrossRef]
- Bock, H.; Dieck, H.T.; Pyttlik, H.; Schnöller, M. Über Kupfer-Komplexe Cu4O(Amin)4(Hal)6 mit tetraedrisch koodiniertem Sauerstoff. Z. Anorg. Allg. Chem. 1968, 357, 54. [Google Scholar] [CrossRef]
- Dieck, H.T. Tetranuclear complexes of Trigonal-bipyramidal Copper(II). III. Electronic and Infrared Spectra. Inorg. Chim. Acta 1973, 7, 397. [Google Scholar] [CrossRef]
- Ondrejovič, G.; Melník, M.; Makáňová, D.; Gažo, J. Darstellung und Eigenschaften zweier isomerer Formen des Dichloro-bis[triphenylphosphinoxid—Kupfer(II)]-Komplexes. Monatsh. Chem. 1977, 108, 1047. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Kalyanaraman, A.R. The Crystal and Molecular Structure of Dichlorobis(Triphenylphosphine Oxide)Copper(II). Inorg. Chim. Acta 1971, 5, 341. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Cotton, F.A. 445. Phosphine Oxide Complexes. Part IV. Tetrahedral, Planar, and Binuclear Complexes of Copper(II) with Phosphine Oxides, and some Arsine Oxide Analogues. J. Chem. Soc. 1961, 2298–2305. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper (I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ 4. Dalton Trans. 2007, 9, 955. [Google Scholar] [CrossRef]
- Churchill, M.R.; DeBoer, B.G.; Donovan, D.J. Molecules with an M4X4 core. IV. Crystallographic Detection of a Step Configuration for the Cu4I4 Core in Tetrameric Triphenylphosphinecopper (I) Iodide, [PPh3CuI] 4. Inorg. Chem. 1975, 14, 617. [Google Scholar] [CrossRef]
- Churchill, M.R.; Kalra, K.L. Molecules with an M4X4 core. II. X-ray Crystallographic Determination of the Molecular Structure of Tetrameric Triphenylphosphinecopper(I) Bromide in Crystalline [PPH3CuBr]4·2 CHCl3. Inorg. Chem. 1974, 13, 1427. [Google Scholar] [CrossRef]
- Churchill, M.R.; Kalra, K.L. Crystallographic Studies on Sulfur Dioxide Insertion compounds. V.1Elucidation of the Molecular Geometry of cis-µ-carbonyl-µ-(sulfur dioxide)-bis (π-cyclopentadienylcarbonyliron), cis-(π.-C5H5)2Fe2 (CO)3 (SO2). Inorg. Chem. 1973, 12, 1650. [Google Scholar] [CrossRef]
- Takemura, Y.; Nakajima, T.; Tanase, T. Interconversion between ladder-type octanuclear and linear tetranuclear copper(I) complexes supported by tetraphosphine ligands. Dalton Trans. 2009, 46, 10231. [Google Scholar] [CrossRef]
- Costa, G.; Pellizer, G.; Rubessa, F. Complex of copper-methyl with triphenylphosphine and other copper(I) complexes with triphenylphosphine. J. Inorg. Nucl. Chem. 1964, 26, 961. [Google Scholar] [CrossRef]
- Albano, V.G.; Bellon, P.L.; Ciani, G.; Manassero, M. Crystal and Molecular Structure of Monoclinic Di-µ-chloro-tris- (triphenylphosphine) dicopper(I) Cu2Cl2(PPh3)3. J. Chem. Soc., Dalton Trans. 1972, 171–175. [Google Scholar] [CrossRef]
- Hartley, S.B.; Holmes, W.S.; Jacques, J.K.; Mole, M.F.; McCoubrey, J.C. Thermochemical properties of phosphorus compounds. Q. Rev. Chem. Soc. 1963, 17, 204. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S.G. Organische Chemie; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Ogata, Y.; Yamashita, M. Kinetics of the Autoxidation of Trimethyl Phosphite, Methyl Diphenyl-phosphinite, and Triphenylphosphine. J. Chem. Soc. Perkin Trans. 1972, 2, 730. [Google Scholar] [CrossRef]
- Costa, G.; Reisenhofer, E.; Stefani, L. Complexes of copper(I) with triphenylphosphine. J. Inorg. Nucl. Chem. 1965, 27, 2581. [Google Scholar] [CrossRef]
- Favarin, L.R.V.; Rosa, P.P.; Pizzuti, L.; Machulek, A.; Caires, A.R.L.; Bezerra, L.S.; Pinto, L.M.C.; Maia, G.; Gatto, C.C.; Back, D.F.; et al. Synthesis and Structural Characterization of New Heteroleptic Copper(I) Complexes Based on Mixed Phosphine/Thiocarbamoyl-pyrazoline Ligands. Polyhedron 2017, 121, 185. [Google Scholar] [CrossRef]
- Fife, D.J.; Moore, W.M.; Morse, K.W. Solution Equilibria of Tertiary Phosphine Complexes of Copper(I) Halides. Inorg. Chem. 1984, 23, 1684. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- APEX2, version 2; Bruker AXS, Inc.: Madison, WI, USA, 2007.
- XPREP, Bruker AXS, Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- PLATON, Multipurpose Crystallographic Tool, Spek, A.L.; University of Utrecht: Utrecht, The Netherlands, 2008.
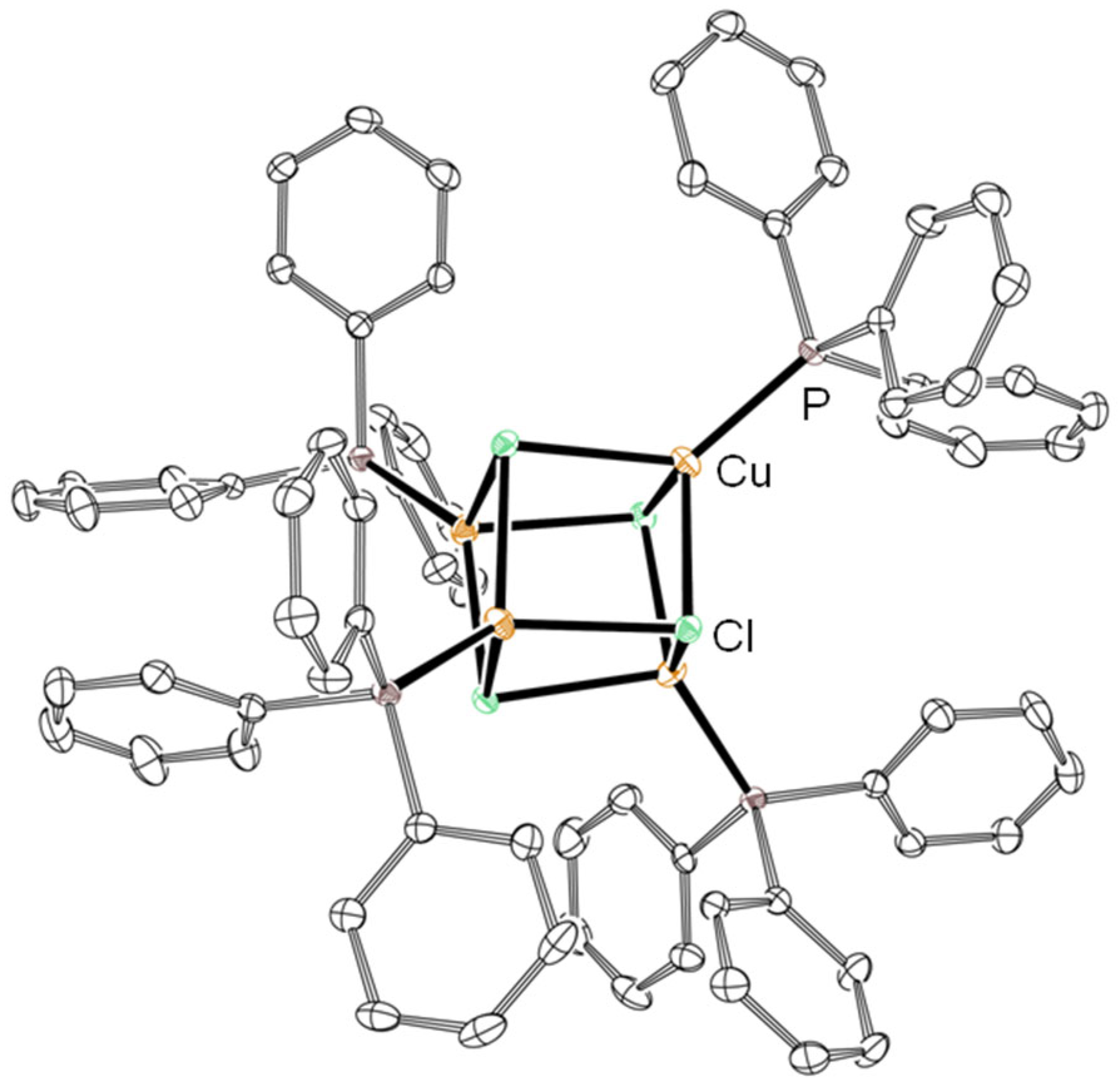
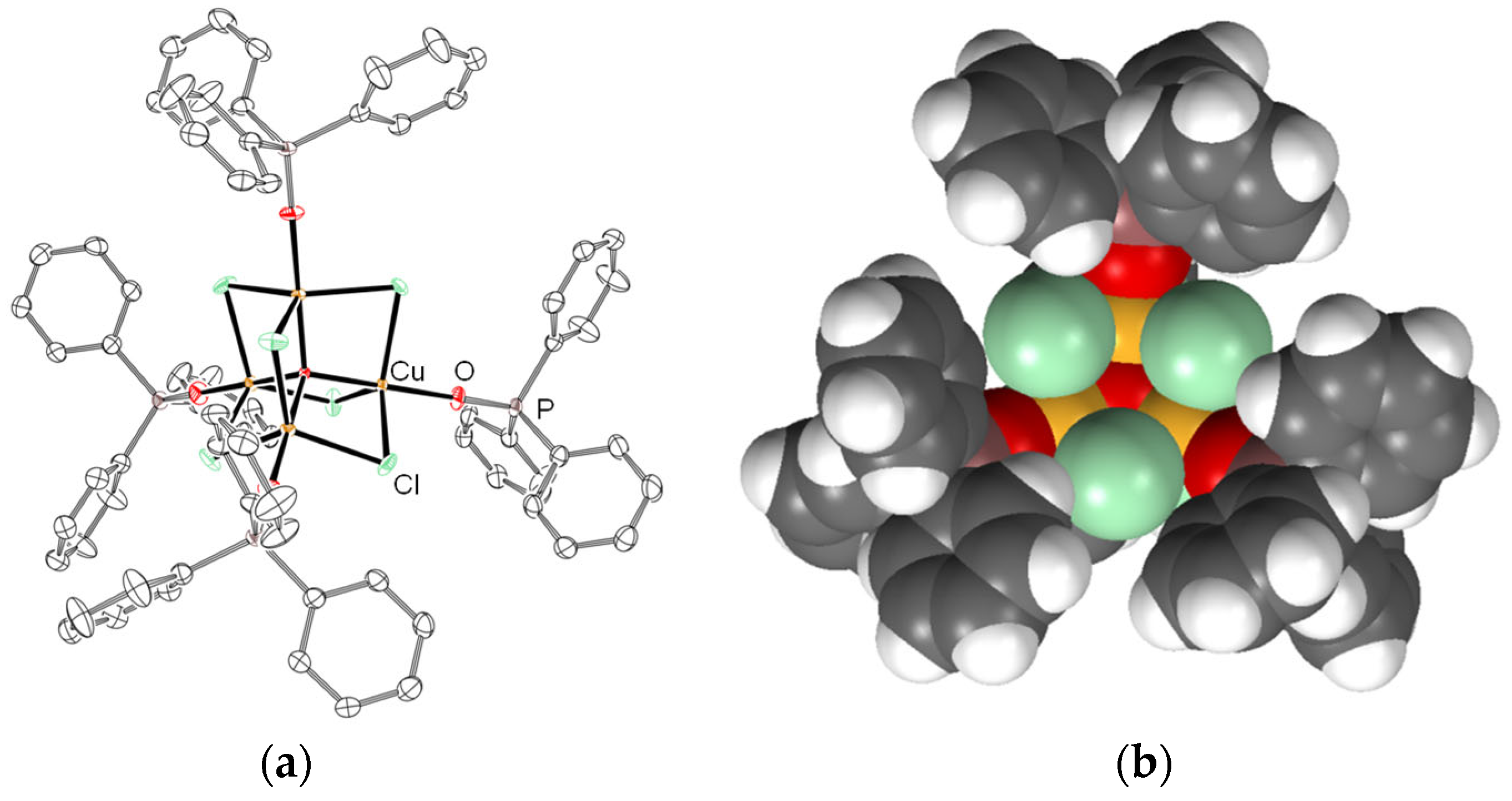
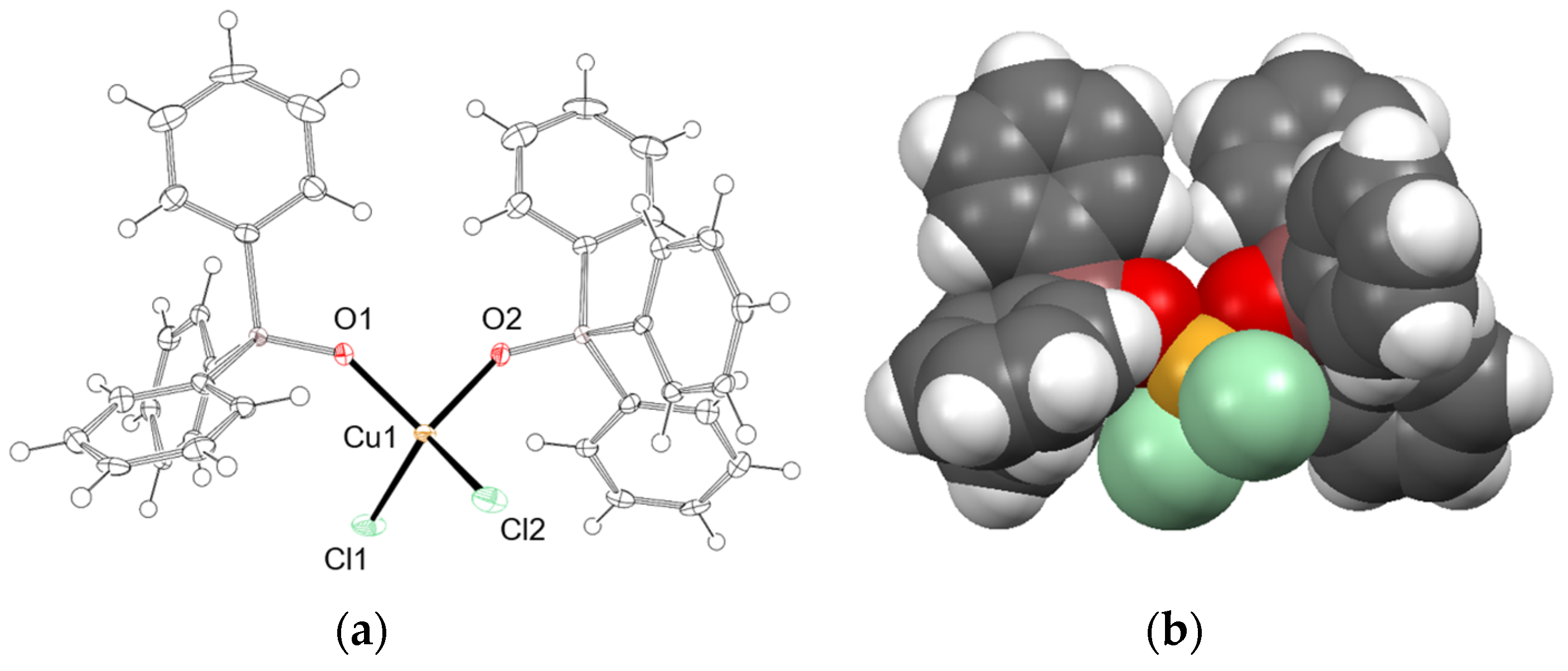

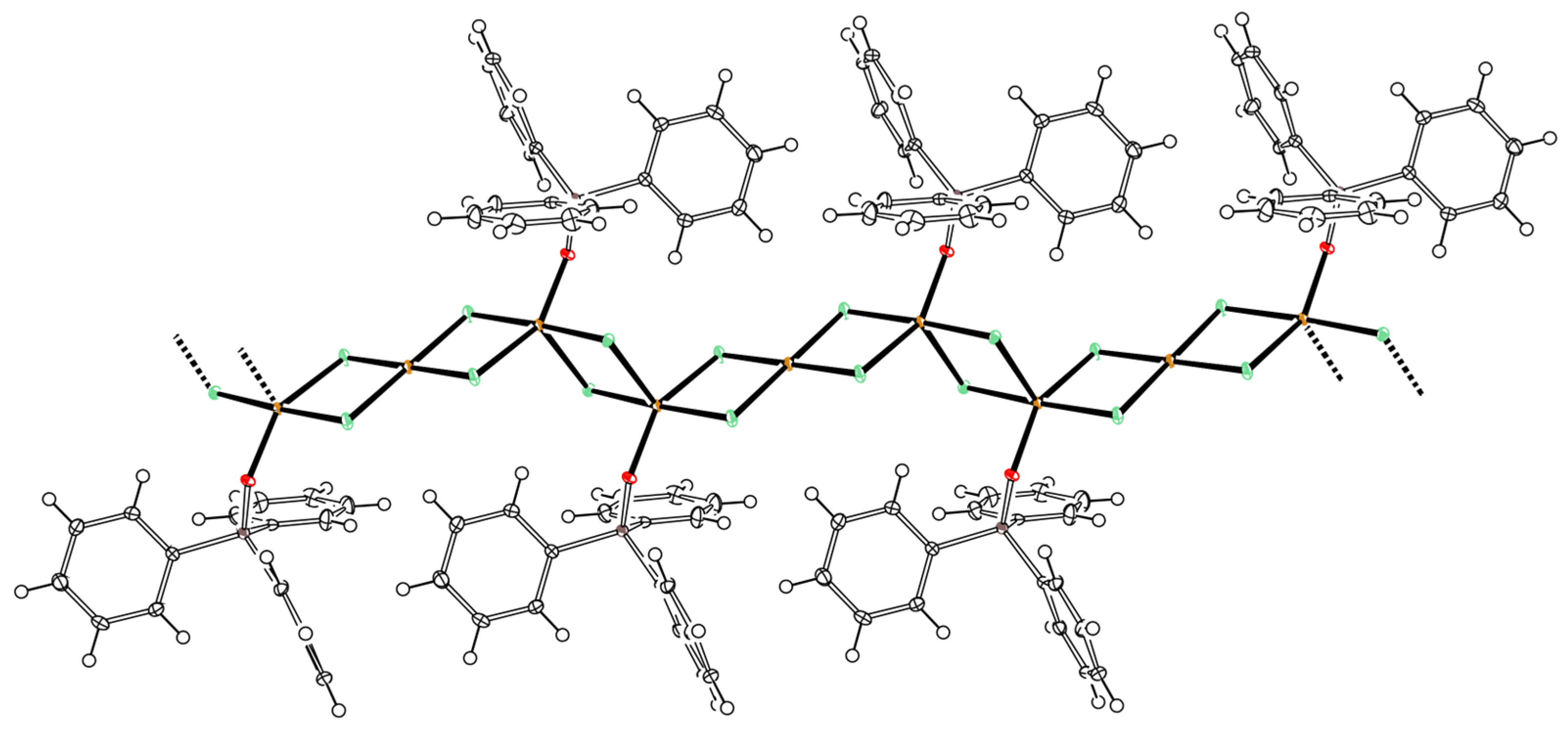
| Entry | Cu(II):TPP | Isolated Product | Yield |
|---|---|---|---|
| 1 | 1:0.5 | [CuII4OCl6(TPPO)4] (2) [CuICl(TPP)] (1a) | Few crystals 12% |
| 2 | 1:1 | [CuICl(TPP)] (1a) | 37% |
| 3 | 1:2 | [CuICl(TPP)2] (1b) | 63% |
| 4 | 1:4 | [CuICl(TPP)3] (1c) | 78% |
| Entry | Starting Compound | Ligand Added | Products |
|---|---|---|---|
| 1 | [CuII4OCl6(MeOH)4] | TPP | [CuICl(TPP)n] (n = 1–3, 1a–1c) [CuII4OCl6(TPP)4] (1) [CuII4OCl6(TPPO)4] (2) [{CuI4Cl4}(TPP)4] (3) |
| 2 | [CuII4OCl6(CH3CN)4] | TPPO | [CuII4OCl6(CH3CN)n-4(TPPO)n] |
| 3 | CuO, CuCl2 | TPP | [CuIICl2(TPPO)2] (2a) [{CuI4Cl4}(TPP)4] (3) CuICl·CH3CN |
| 4 | CuO, CuCl2 | TPPO | [CuII4OCl6(TPPO)4] (2) [CuIICl2(TPPO)2] (2a) [{CuIICl2}2(TPPO)2] (2b) [{CuIICl2}3(TPPO)2] (2c) |
| 5 | CuCl2·2H2O | TPP | [CuII4OCl6(TPPO)4] (2) [CuICl(TPP)] (1a) [CuICl(TPP)2] (1b) [CuICl(TPP)3] (1c) |
| 6 | CuCl2·2H2O | TPPO | [CuII4OCl6(TPPO)4] (2) [CuIICl2(TPPO)2] (2a) CuCl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faber, S.L.; Dilmen, N.I.; Becker, S. On the Redox Equilibrium of TPP/TPPO Containing Cu(I) and Cu(II) Complexes. Chemistry 2023, 5, 1288-1301. https://doi.org/10.3390/chemistry5020087
Faber SL, Dilmen NI, Becker S. On the Redox Equilibrium of TPP/TPPO Containing Cu(I) and Cu(II) Complexes. Chemistry. 2023; 5(2):1288-1301. https://doi.org/10.3390/chemistry5020087
Chicago/Turabian StyleFaber, Stephanie L., Nesrin I. Dilmen, and Sabine Becker. 2023. "On the Redox Equilibrium of TPP/TPPO Containing Cu(I) and Cu(II) Complexes" Chemistry 5, no. 2: 1288-1301. https://doi.org/10.3390/chemistry5020087
APA StyleFaber, S. L., Dilmen, N. I., & Becker, S. (2023). On the Redox Equilibrium of TPP/TPPO Containing Cu(I) and Cu(II) Complexes. Chemistry, 5(2), 1288-1301. https://doi.org/10.3390/chemistry5020087









