Fe Doping Enhances the Peroxidase-Like Activity of CuO for Ascorbic Acid Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus and Characterization
2.3. Preparation of CuO and Fe-CuO
2.4. POD-like Activity Assay for Fe-CuO Nanozymes
2.5. Detection of AA Using Fe-CuO Nanozymes
2.6. TAC Assay
3. Results and Discussion
3.1. Characterization of CuO and Fe-CuO Nanozymes
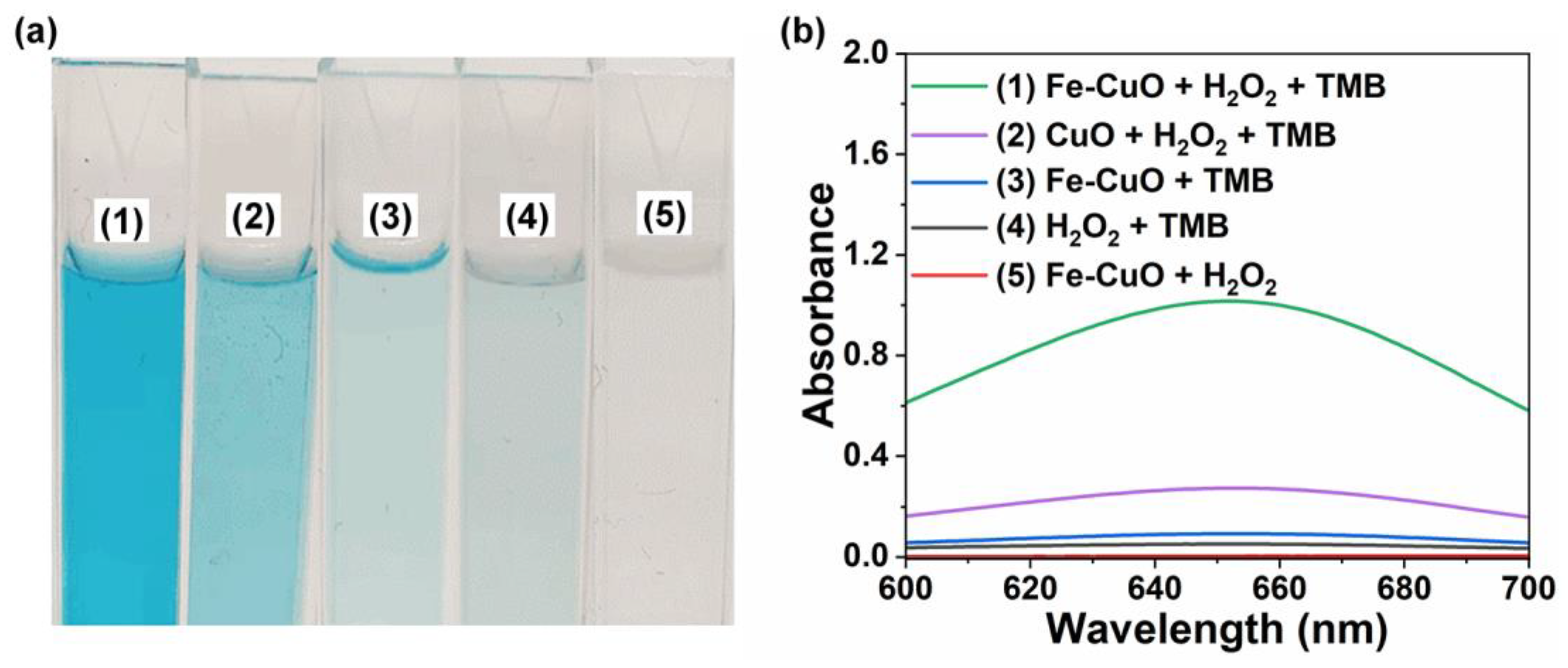
3.2. Fe-Doping-Enhanced Peroxidase-like Activity of CuO
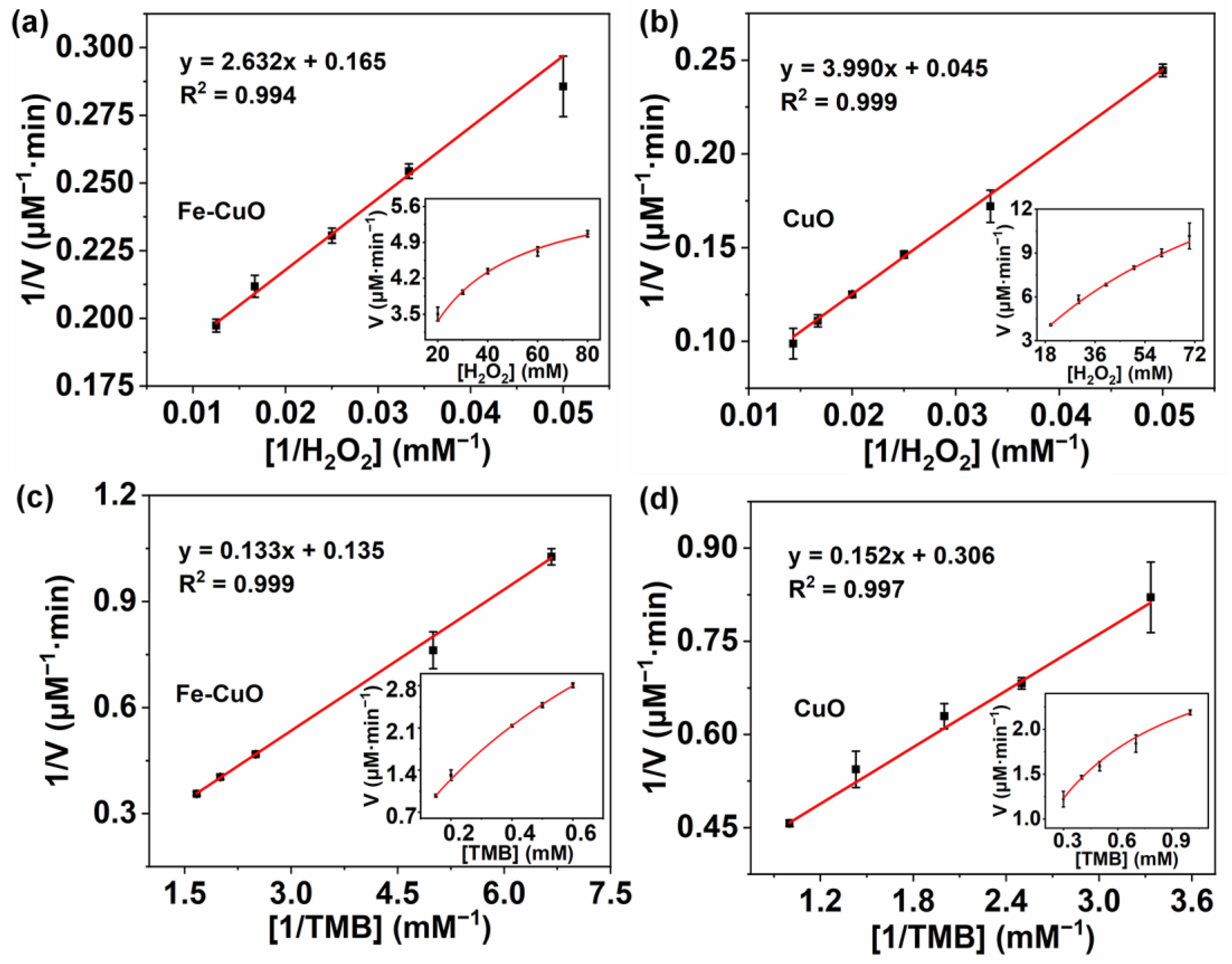
3.3. Steady-State Kinetic Assay
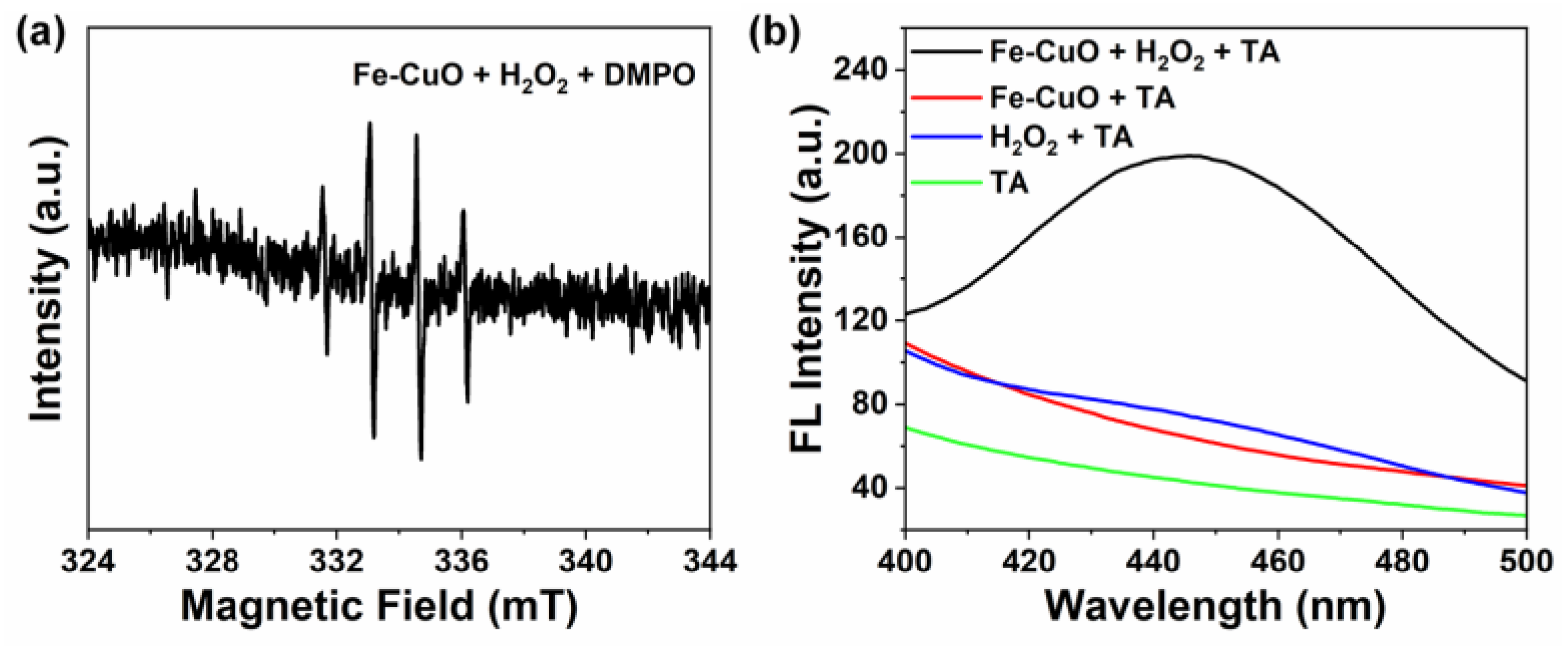
3.4. Reactive Oxygen Species in the Nanozymatic Catalysis
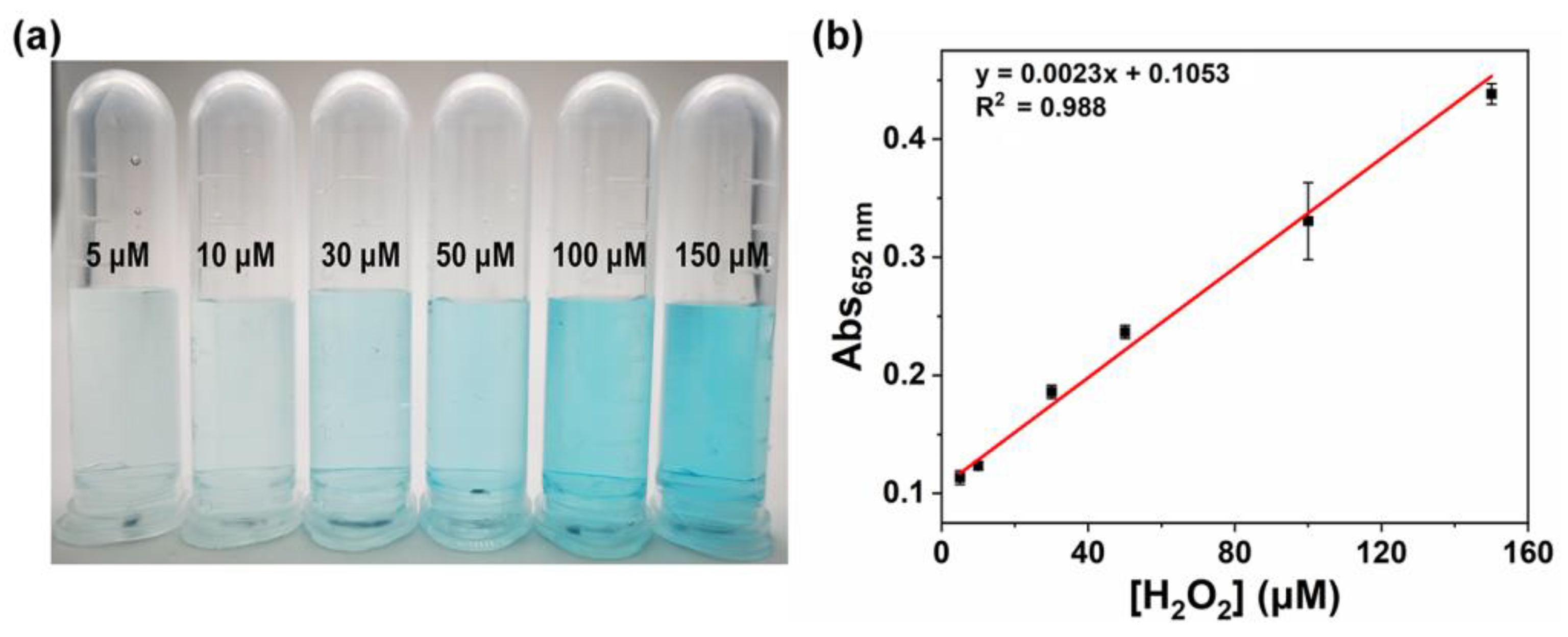
3.5. H2O2 Concentration-Dependent Colorimetric Signals Using Fe-CuO Nanozyme
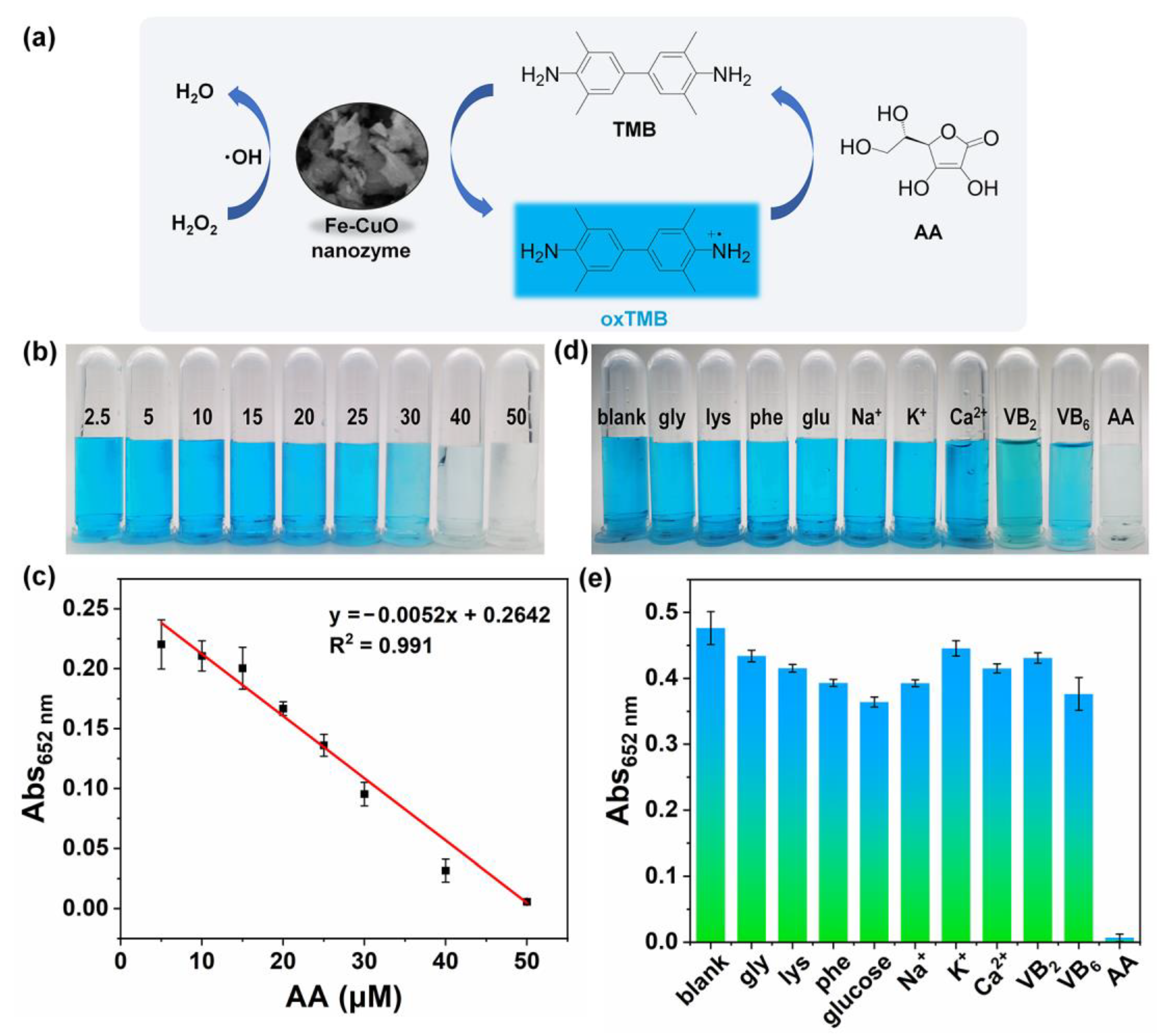
3.6. Analytical Performance of Fe-CuO Nanozyme-Based AA Detection
3.7. Practicability for TAC Assay in Real-World Scenarios
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geng, C.; Huang, Y.; Li, B.; Wang, Y.; Zhu, L.; Xu, Y.; Gao, K.; Mu, Y.; Su, Y.; Deng, S.; et al. Point-of-Care Testing of Chloramphenicol in Food Production Using Smartphone-Based Electrochemical Detector. J. Anal. Test. 2022, 7, 33–39. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, K.; Yan, A.; Wang, J.G.; Zhang, Z.; Li, D. Intelligent Probabilistic System for Digital Tracing Cellular Origin of Individual Clinical Extracellular Vesicles. Anal. Chem. 2021, 93, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-X.; Qin, X.; Qin, Y.; Xiang, Y.; Zhang, G.-J.; Yang, F. Bioprobes-regulated precision biosensing of exosomes: From the nanovesicle surface to the inside. Coord. Chem. Rev. 2022, 463, 214538. [Google Scholar] [CrossRef]
- Liang, T.-T.; Qin, X.; Xiang, Y.; Tang, Y.; Yang, F. Advances in nucleic acids-scaffolded electrical sensing of extracellular vesicle biomarkers. TrAC Trends Anal. Chem. 2022, 148, 116532. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Zhu, F.; Zhu, Y.; Lu, X.; Wan, Y.; Su, S.; Chao, J.; Wang, L.; Zhu, D. Programmably engineered FRET-nanoflare for ratiometric live-cell ATP imaging with anti-interference capability. Chem. Commun. 2023, 59, 4047–4050. [Google Scholar] [CrossRef]
- Wang, C.; Yan, A.; Wang, H.; Su, Y.; Li, D. DNA-Mediated Membrane Fusion and Its Biological Applications: Sensing, Reaction Control and Drug Delivery. Anal. Sens. 2022, 2, e202200024. [Google Scholar] [CrossRef]
- Pan, H.; Dong, Y.; Gong, L.; Zhai, J.; Song, C.; Ge, Z.; Su, Y.; Zhu, D.; Chao, J.; Su, S.; et al. Sensing gastric cancer exosomes with MoS2-based SERS aptasensor. Biosens. Bioelectron. 2022, 215, 114553. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Dong, N.; Kang, W.; Li, K.; Nie, Z. Titanium Carbide MXenes Mediated In Situ Reduction Allows Label-Free and Visualized Nanoplasmonic Sensing of Silver Ions. Anal. Chem. 2020, 92, 4623–4629. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wang, H.W.a.E. Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; Yu, Y.; Yin, M.; Sun, J.; Huang, J.; Chen, N.; Wang, H.; Fan, C.; Song, H. An Organelle-Specific Nanozyme for Diabetes Care in Genetically or Diet-Induced Models. Adv. Mater. 2020, 32, e2003708. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ke, Z.; Zhang, J.; Feng, G. Dual-signal output paper sensor based on coordinative self-assembly biomimetic nanozyme for point-of-care detection of biomarker. Biosens. Bioelectron. 2022, 216, 114656. [Google Scholar] [CrossRef] [PubMed]
- Zandieh, M.; Liu, J. Removal and Degradation of Microplastics Using the Magnetic and Nanozyme Activities of Bare Iron Oxide Nanoaggregates. Angew. Chem. Int. Ed. 2022, 61, e202212013. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Tan, W.; Han, P.; Li, X.; Li, J.; Nie, Z.; Li, K. A photonanozyme with light-empowered specific peroxidase-mimicking activity. Nano Res. 2022, 15, 9073–9081. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Tan, W.; Jin, P.; Zhang, P.; Li, K. Bioinspired Coassembly of Copper Ions and Nicotinamide Adenine Dinucleotides for Single-Site Nanozyme with Dual Catalytic Functions. Anal. Chem. 2023, 95, 2865–2873. [Google Scholar] [CrossRef]
- Wu, J.; Wei, H. Efficient Design Strategies for Nanozymes. Prog. Chem. 2020, 33, 42–51. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Surface Science of Nanozymes and Defining a Nanozyme Unit. Langmuir 2022, 38, 3617–3622. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Nanozymes: Definition, Activity, and Mechanisms. Adv. Mater. 2023, e2211041. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Gao, M.; Lu, X.; Chen, S.; Tian, D.; Zhu, Y.; Wang, C. Enhanced Peroxidase-like Activity of Mo6+-Doped Co3O4 Nanotubes for Ultrasensitive and Colorimetric l-Cysteine Detection. ACS Appl. Nano Mater. 2018, 1, 4703–4715. [Google Scholar] [CrossRef]
- Chaudhary, M.; Singh, M.; Kumar, A.; Prachi; Gautam, Y.K.; Malik, A.K.; Kumar, Y.; Singh, B.P. Experimental investigation of Co and Fe-Doped CuO nanostructured electrode material for remarkable electrochemical performance. Ceram. Int. 2021, 47, 2094–2106. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q.; Lang, Y.; Jiang, X.; Wu, P. Rationale of 3,3′,5,5′-Tetramethylbenzidine as the Chromogenic Substrate in Colorimetric Analysis. Anal. Chem. 2020, 92, 12400–12406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Y.; Zhou, Q.; Hu, L.; Fu, W.; Wang, Y. Peroxidase-like Activity of Metal-Organic Framework [Cu(PDA)(DMF)] and Its Application for Colorimetric Detection of Dopamine. ACS Appl. Mater. Interfaces 2019, 11, 44466–44473. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, N.; Zhang, J.; Yan, R.; Li, J.; Wang, L.; Wang, N.; Lv, M.; Zhang, M. Ultrasensitive aptamer-based protein assays based on one-dimensional core-shell nanozymes. Biosens Bioelectron. 2020, 150, 111881. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Zeng, C.; Zhou, C.; Li, Q.; Lu, N.; Wang, L. One-Dimensional Synergistic Core-Shell Nanozymes with Superior Peroxidase-like Activity for Ultrasensitive Colorimetric Detection of Blood Cholesterol. ACS Appl. Bio Mater. 2020, 3, 5111–5119. [Google Scholar] [CrossRef]
- He, Y.; Li, N.; Liu, X.; Chen, W.; Zhu, X.; Liu, Q. 5,10,15,20-tetrakis (4-carboxyl phenyl) porphyrin-functionalized urchin-like CuCo2O4 as an excellent artificial nanozyme for determination of dopamine. Microchim. Acta 2021, 188, 171. [Google Scholar] [CrossRef]
- Jiao, A.; Xu, L.; Tian, Y.; Cui, Q.; Liu, X.; Chen, M. Cu2O nanocubes-grafted highly dense Au nanoparticles with modulated electronic structures for improving peroxidase catalytic performances. Talanta 2021, 225, 121990. [Google Scholar] [CrossRef]
- Zhu, Z.; Gong, L.; Miao, X.; Chen, C.; Su, S. Prussian Blue Nanoparticle Supported MoS2 Nanocomposites as a Peroxidase-Like Nanozyme for Colorimetric Sensing of Dopamine. Biosensors 2022, 12, 260. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Gong, W.; Zhang, M.; Lu, N. Modulating the Biomimetic and Fluorescence Quenching Activities of Metal-Organic Framework/Platinum Nanoparticle Composites and Their Applications in Molecular Biosensing. ACS Appl. Mater. Interfaces 2022, 14, 21677–21686. [Google Scholar] [CrossRef]
- Hao, P.; Liu, Y.; Wang, L.; Zhu, X.; Liu, Q. Nanozyme Sensor Array Based on Porphyrin-Modified CoMoO4 to Detect and Distinguish Biologically Important Thiols. ACS Appl. Nano Mater. 2023, 6, 1937–1947. [Google Scholar] [CrossRef]
- Hao, P.; Liu, Y.; Dong, S.; Fan, G.; Li, G.; Xie, M.; Liu, Q. Enhanced peroxidase-like activity of 2(3), 9(10), 16(17), 23(24)-octamethoxyphthalocyanine modified CoFe LDH for a sensor array for reducing substances with catechol structure. Anal. Bioanal. Chem. 2023, 415, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, Y.-N.; Yang, B.; Liu, Z.; Zhang, X.; Liu, Q. Iron Doped CuSn(OH)6 Microspheres as a Peroxidase-Mimicking Artificial Enzyme for H2O2 Colorimetric Detection. ACS Sustain. Chem. Eng. 2018, 6, 14383–14393. [Google Scholar] [CrossRef]
- Qileng, A.; Zhu, H.; Liu, S.; He, L.; Qin, W.; Liu, W.; Xu, Z.; Liu, Y. Machine learning: Assisted multivariate detection and visual image matching to build broad-specificity immunosensor. Sens. Actuators B Chem. 2021, 339, 129872. [Google Scholar] [CrossRef]
- Chen, H.; Cai, Z.; Gui, J.; Tang, Y.; Yin, P.; Zhu, X.; Zhang, Y.; Li, H.; Liu, M.; Yao, S. A redox reaction-induced ratiometric fluorescence platform for the specific detection of ascorbic acid based on Ag2S quantum dots and multifunctional CoOOH nanoflakes. J. Mater. Chem. B 2023, 11, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhao, S.; Wang, Q.; Wei, H. N-Doped Carbon As Peroxidase-Like Nanozymes for Total Antioxidant Capacity Assay. Anal. Chem. 2019, 91, 15267–15274. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, L.; Yan, H.; Xu, W.; Wu, Y.; Wang, H.; Gu, W.; Zhu, C. Hierarchically Porous S/N Codoped Carbon Nanozymes with Enhanced Peroxidase-like Activity for Total Antioxidant Capacity Biosensing. Anal. Chem. 2020, 92, 13518–13524. [Google Scholar] [CrossRef]
- Han, S.; Chen, X.; Fan, Y.; Zhang, Y.; Yang, Z.; Kong, X.; Liu, Z.; Liu, Q.; Zhang, X. The excellent peroxidase-like activity of uniform CuCo2O4 microspheres with oxygen vacancy for fast sensing of hydrogen peroxide and ascorbic acid. New J. Chem. 2021, 45, 2030–2037. [Google Scholar] [CrossRef]
- He, Y.; Li, N.; Lian, J.; Yang, Z.; Liu, Z.; Liu, Q.; Zhang, X.; Zhang, X. Colorimetric ascorbic acid sensing from a synergetic catalytic strategy based on 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphyrin functionalized CuS nanohexahedrons with the enhanced peroxidase-like activity. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 598, 124855. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Hu, X.; Chen, H.; Tang, Y.; Zhu, Y.; Zhu, X.; Zhang, Y.; Liu, M.; Yao, S. Polyoxometalate Nanostructures Decorated with CuO Nanoparticles for Sensing Ascorbic Acid and Fe2+ Ions. ACS Appl. Nano Mater. 2021, 4, 8302–8313. [Google Scholar] [CrossRef]
- Ma, C.B.; Xu, Y.; Wu, L.; Wang, Q.; Zheng, J.J.; Ren, G.; Wang, X.; Gao, X.; Zhou, M.; Wang, M.; et al. Guided Synthesis of a Mo/Zn Dual Single-Atom Nanozyme with Synergistic Effect and Peroxidase-like Activity. Angew. Chem. Int. Ed. 2022, 61, e202116170. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, Y.; Cao, Z.; Lin, W.; Lu, N. DNA-Programmed Tuning of the Growth and Enzyme-Like Activity of a Bimetallic Nanozyme and Its Biosensing Applications. ACS Appl. Mater. Interfaces 2023, 15, 18620–18629. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J. What Is a “Real Sample”? ACS Sens. 2018, 3, 1609. [Google Scholar] [CrossRef] [PubMed]




| Catalysts | Km (mM) | Vmax (μM/min) | References | ||
|---|---|---|---|---|---|
| H2O2 | TMB | H2O2 | TMB | ||
| Fe-CuO | 15.9 | 0.986 | 6.05 | 7.40 | This work |
| CuO | 87.9 | 0.497 | 22.03 | 3.27 | |
| HRP | 3.7 | 0.434 | 5.23 | 6.0 | [9] |
| Cu(PDA)(DMF) | 28.6 | 0.169 | 1.9 | 1.32 | [24] |
| Fe3O4@C NWs | 0.23 | 0.20 | 1.45 | 0.80 | [25] |
| Fe3O4@C/Ni NW | 0.059 | 0.10 | 2.17 | 2.34 | [26] |
| Por-CuCo2O4 | 9.96 | 0.044 | 4.97 | 5.96 | [27] |
| Au/Cu2O | 10.56 | 0.21 | 4.01 | 3.65 | [28] |
| MoS2-CPBNPs | 3.17 | 0.22 | 0.89 | 3.82 | [29] |
| MIL-88B-NH2/Pt | 0.026 | 0.00213 | 1.24 | 0.62 | [30] |
| Por-CoMoO4 | 0.63 | 0.26 | 1.23 | 56.81 | [31] |
| Pc(OH)8-CoFe LDH | 1.55 | 0.361 | 2.86 | 7.47 | [32] |
| Materials | Linear Range | LOD | Reference |
|---|---|---|---|
| Fe-CuO | 5–50 μM | 4.66 µM | This work |
| CP (600C−6) | 0.8–80 μM | 35 μM | [36] |
| SNC-900 | 100–5000 μM | 80 μM | [37] |
| CuCo2O4 | 1–10 μM | 0.573 μM | [38] |
| TPyP-CuS | 1–30 μM | 0.419 μM | [39] |
| CuO NPs-POM | 0.02–500 μM | 0.015 μM | [40] |
| Zn/Mo DSAC-SMA | 0.1–5000 μM | 0.76 μM | [41] |
| Au/T15/Pt | 1.25−22.5 μM | 0.853 μM | [42] |
| Sample | Detected (μM) * | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Mizone | 14.78 ± 0.58 | 10.00 | 9.872 ± 0.916 | 98.70 | 9.3 |
| 15.00 | 15.26 ± 0.35 | 101.7 | 2.3 | ||
| 20.00 | 20.77 ± 1.24 | 103.8 | 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Yang, Y.; Xie, Y.; Li, J.; Li, K. Fe Doping Enhances the Peroxidase-Like Activity of CuO for Ascorbic Acid Sensing. Chemistry 2023, 5, 1302-1316. https://doi.org/10.3390/chemistry5020088
Yan B, Yang Y, Xie Y, Li J, Li K. Fe Doping Enhances the Peroxidase-Like Activity of CuO for Ascorbic Acid Sensing. Chemistry. 2023; 5(2):1302-1316. https://doi.org/10.3390/chemistry5020088
Chicago/Turabian StyleYan, Boyu, Ying Yang, Yinyun Xie, Jinzhao Li, and Kun Li. 2023. "Fe Doping Enhances the Peroxidase-Like Activity of CuO for Ascorbic Acid Sensing" Chemistry 5, no. 2: 1302-1316. https://doi.org/10.3390/chemistry5020088
APA StyleYan, B., Yang, Y., Xie, Y., Li, J., & Li, K. (2023). Fe Doping Enhances the Peroxidase-Like Activity of CuO for Ascorbic Acid Sensing. Chemistry, 5(2), 1302-1316. https://doi.org/10.3390/chemistry5020088







