Modification of the Bridging Unit in Luminescent Pt(II) Complexes Bearing C^N*N and C^N*N^C Ligands
Abstract
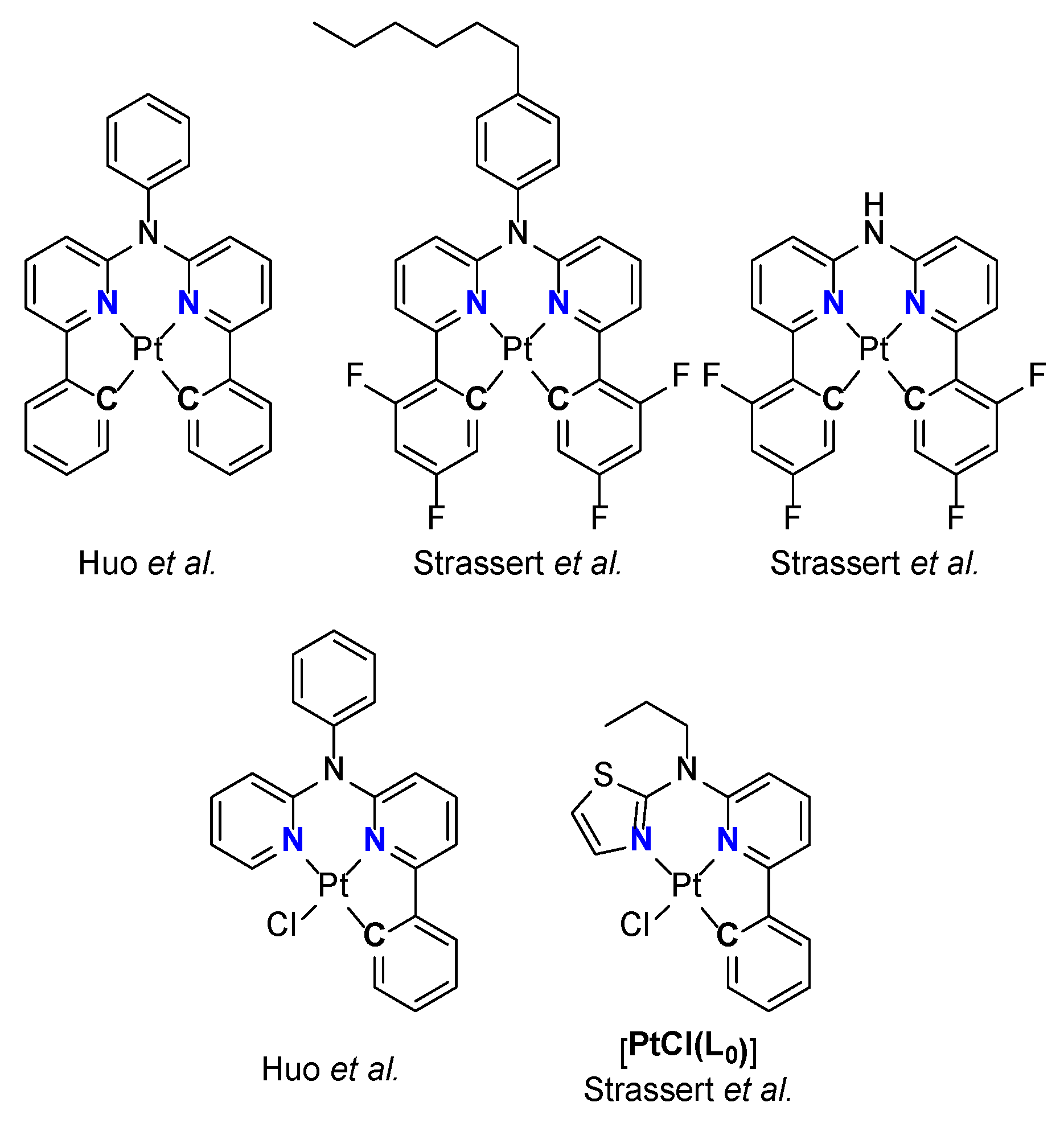
1. Introduction
2. Experimental Section
3. Results and Discussion
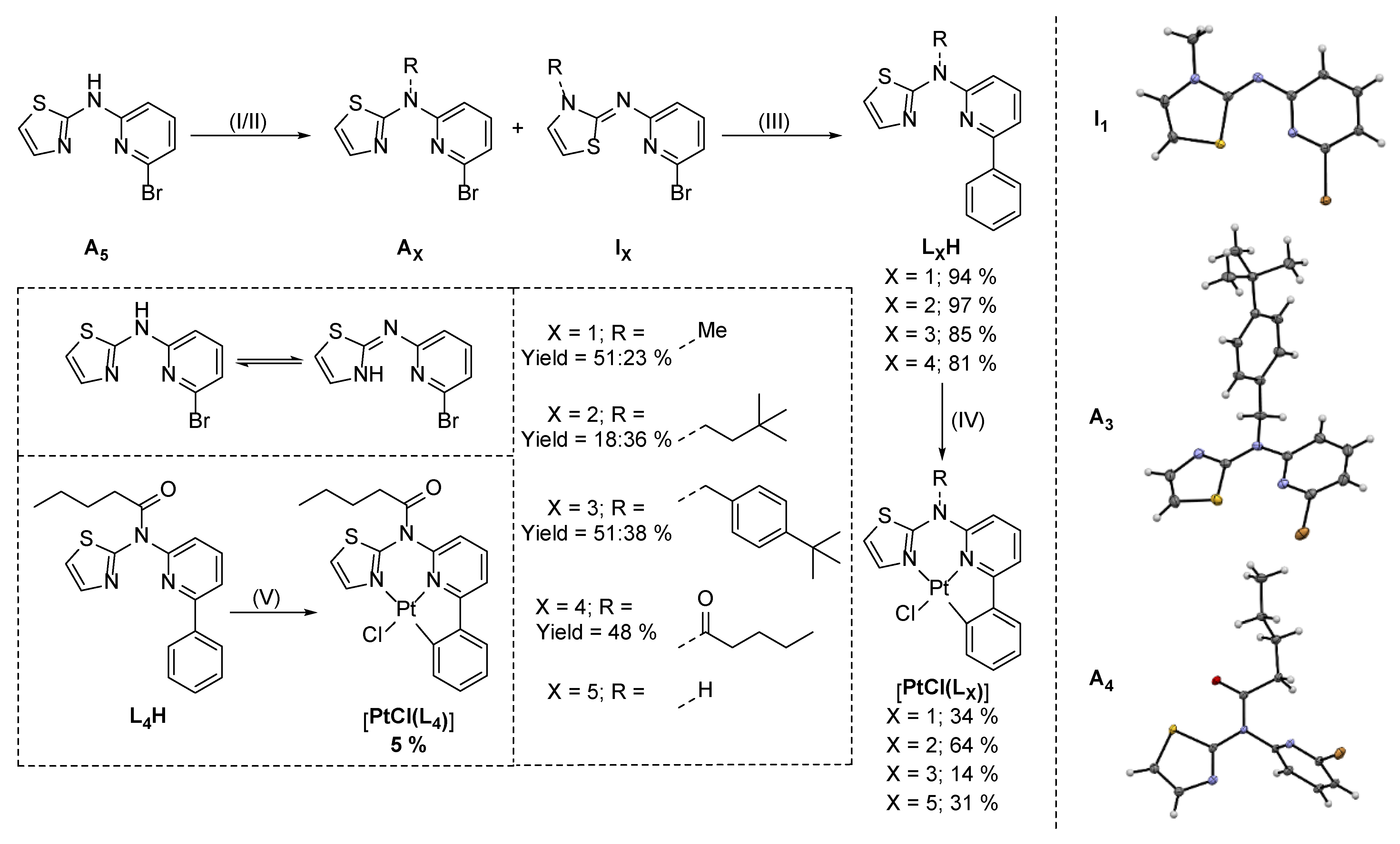
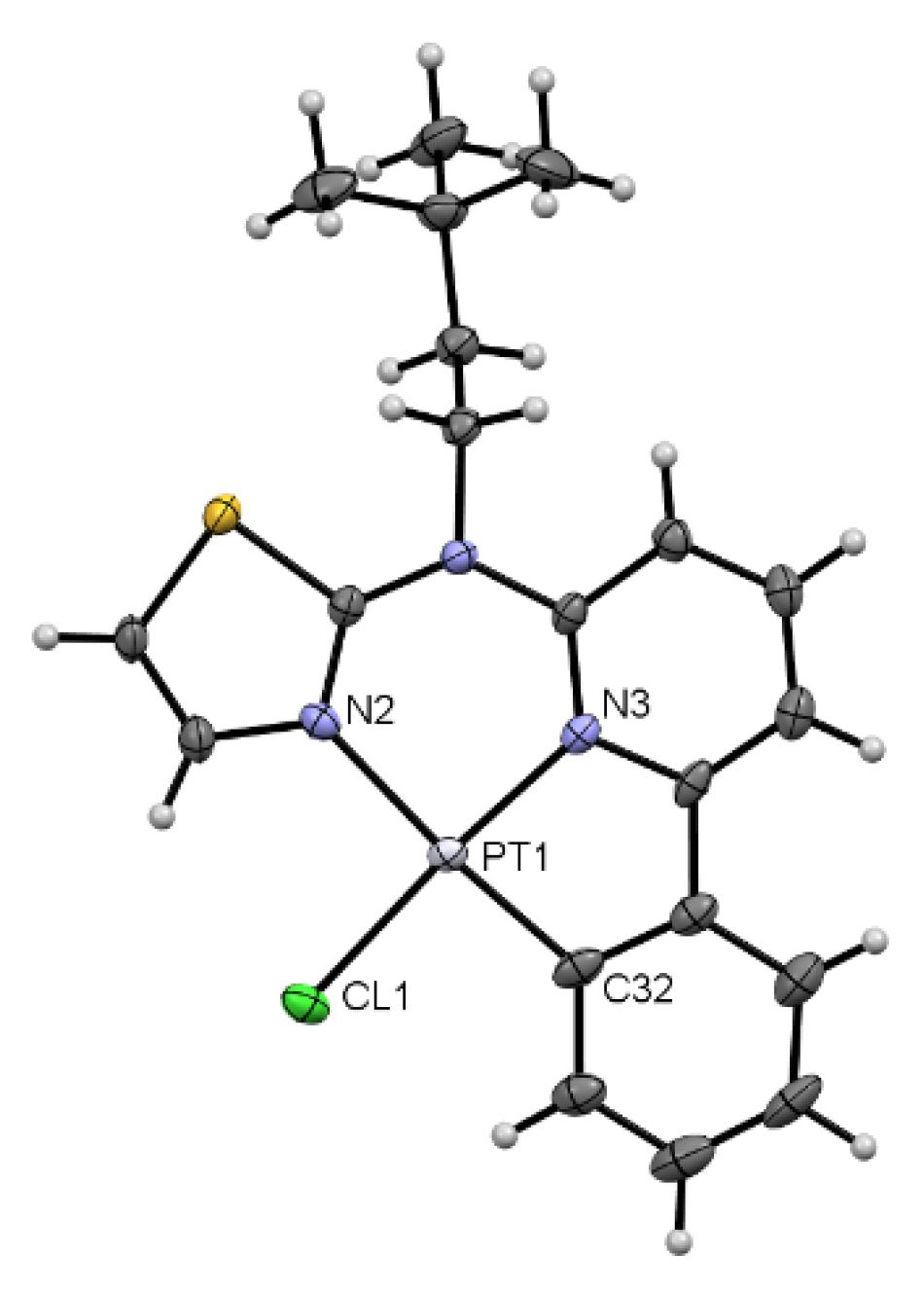
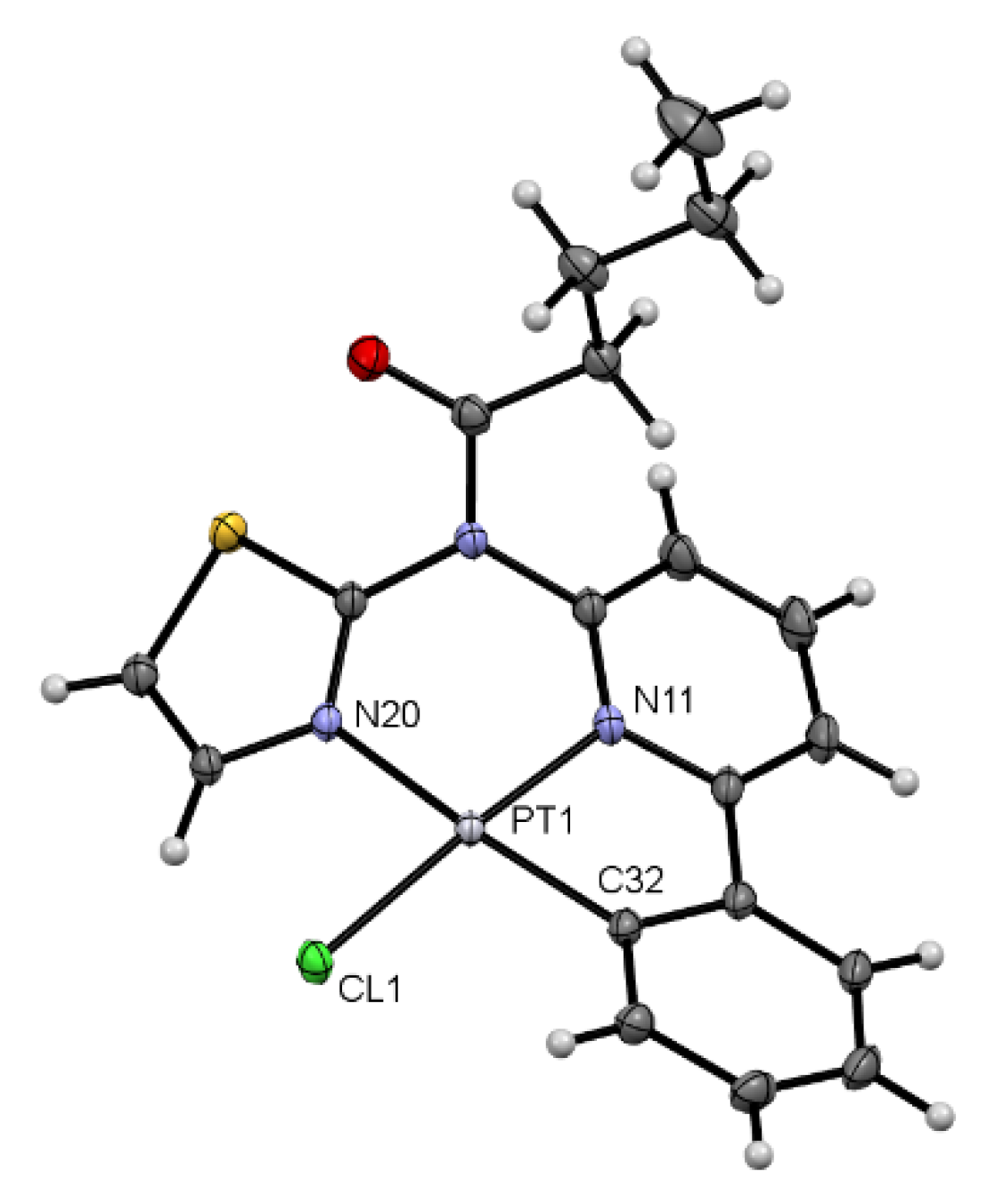
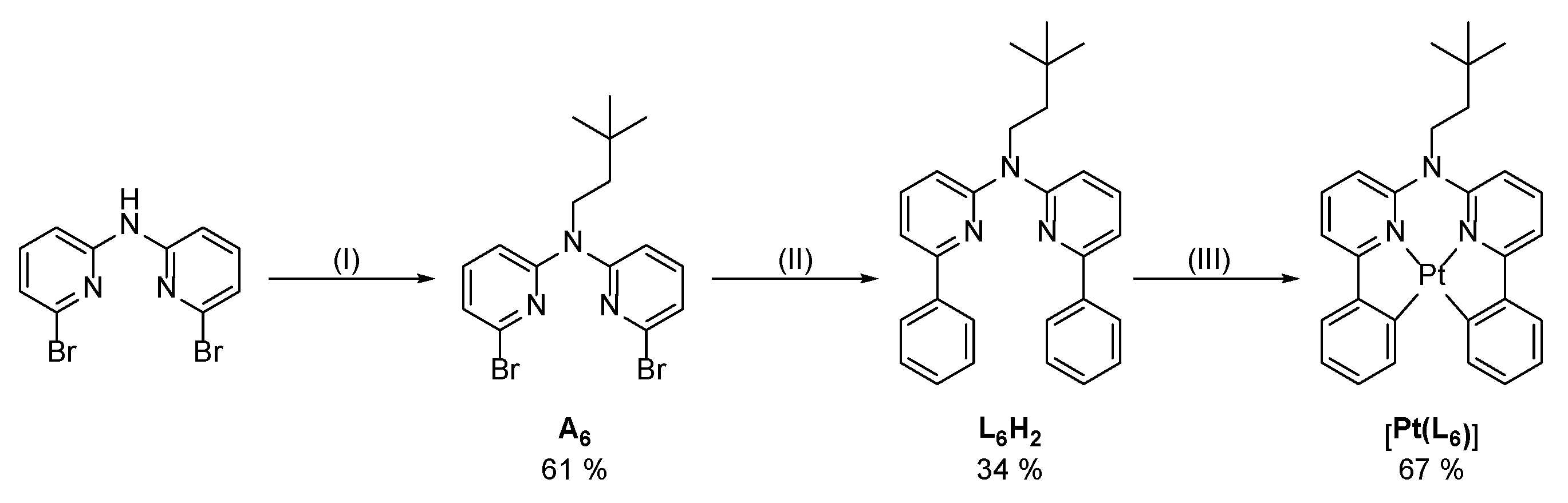
3.1. Tridentate Coordination
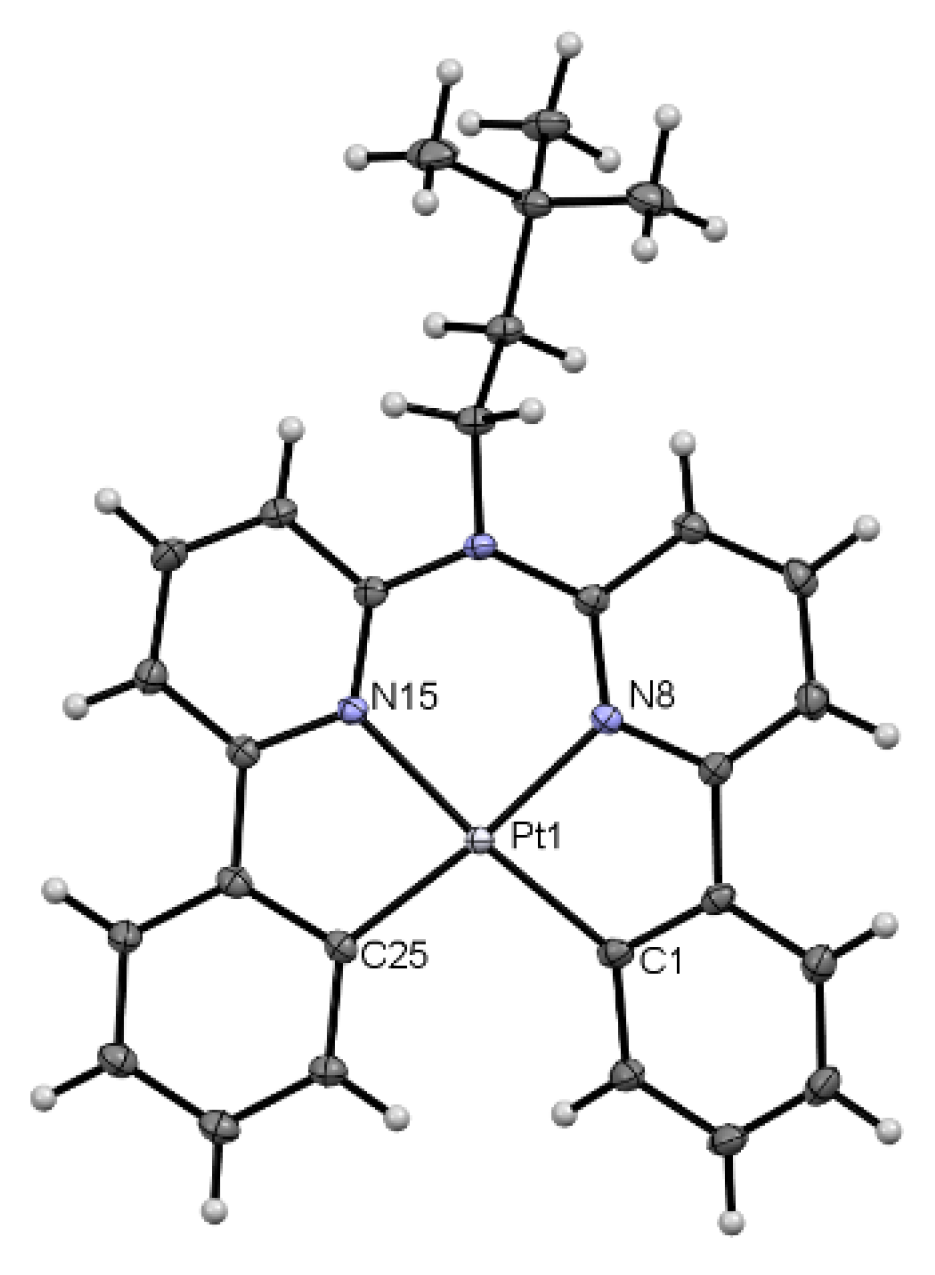
3.2. Tetradentate Coordination
3.3. Photophysics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wunschel, K.R.; Ohnesorge, W.E. Luminescence of Iridium(II) Chelates with 2,2’-bipyridine and with 1,10-phenanthroline. J. Am. Chem. Soc. 1967, 89, 2777–2778. [Google Scholar] [CrossRef]
- Baldo, M.A.; Lamansky, S.; Thompson, M.E.; Forrest, S.R. Very High-Efficiency Green Organic Light-Emitting Devices Based on Electrophosphorescence. Appl. Phys. Lett. 1999, 75, 4–6. [Google Scholar] [CrossRef]
- Cheung, T.-C.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Photoluminescent Cyclometallated Diplatinum(II,II) Complexes: Photophysical Properties and Crystal Structures of [PtL(PPh3)]ClO4 and [Pt2L2(µ-dppm)][ClO4]2(HL = 6-phenyl-2,2′-bipyridine, dppm = Ph2PCH2PPh2). J. Chem. Soc., Dalton Trans. 1996, 1645–1651. [Google Scholar] [CrossRef]
- Wong, Y.S.; Tang, M.C.; Ng, M.; Yam, V.W.W. Toward the Design of Phosphorescent Emitters of Cyclometalated Earth Abundant Nickel(II) and Their Supramolecular Study. J. Am. Chem. Soc. 2020, 142, 7638–7646. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.G.; Beeby, A.; Davies, E.S.; Weinstein, J.A.; Wilson, C. An Alternantive Rout to Highly Luminescent Platinum(II) Complexes: Cyclometalation with N^C^N-Coordinating Dipyridylbenzene Ligands. Inorg. Chem. 2003, 42, 8609–8611. [Google Scholar] [CrossRef]
- Otto, S.; Grabolle, M.; Förster, C.; Kreitner, C.; Resch-Genger, U.; Heinze, U. [Cr(ddpd)2]3+: A Molecular, Water-Soluble, Highly NIR-Emissive Ruby Analogue. Angew. Chem. Int. Ed. 2015, 54, 11572–11576. [Google Scholar] [CrossRef]
- Chow, P.-K.; Cheng, G.; Tong, G.S.M.; To, W.-P.; Kwong, W.-L.; Low, K.-H.; Kwok, C.-C.; Ma, C.; Che, C.-M. Luminescent Pincer Platinum(II) Complexes with Emission Quantum Yields up to Almost Unity: Photophysics, Photoreductive C-C Bond Formation, and Materials Applications. Angew. Chem. Int. Ed. 2015, 54, 2084–2089. [Google Scholar] [CrossRef]
- Sanning, J.; Ewen, P.; Stegemann, L.; Schmidt, J.; Daniliuc, C.G.; Koch, T.; Doltsinis, N.L.; Wegner, D.; Strassert, C.A. Scanning-Tunneling-Spectroscopy-Directed Design of Tailored Deep-Blue Emitters. Angew. Chem. Int. Ed. 2015, 54, 786–791. [Google Scholar] [CrossRef]
- Rossi, E.; Colombo, A.; Dragonetti, C.; Roberto, D.; Ugo, R.; Valore, A.; Falciola, L.; Brulatti, P.; Cocchi, M.; Williams, J.A.G. Novel N^C^N-Cyclometallated Platinum Complexes with Acetylide Co-Ligands as Efficient Phosphors for OLEDs. J. Mater. Chem. 2012, 22, 10650–10655. [Google Scholar] [CrossRef]
- Kayano, T.; Takayasu, S.; Sato, K.; Shinozaki, K. Luminescence Color Tuning of PtII Complexes and a Kinetic Study of Trimer Formation in the Photoexcited State. Chem. Eur. J. 2014, 20, 16583–16589. [Google Scholar] [CrossRef]
- Cebrián, C.; Mauro, M. Recent Advances in Phosphorescent Platinum Complexes for Organic Light-Emitting Diodes, Beilstein. J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar]
- Cheng, G.; Kwak, Y.; To, W.P.; Lam, T.L.; Tong, G.S.M.; Sit, M.K.; Gong, S.; Choi, B.; Choi, W.I.; Yang, C.; et al. High-Efficiency Solution-Processed Organic Light-Emitting Diodes with Tetradentate Platinum(II) Emitters. ACS Appl. Mater. Interfaces 2019, 11, 45161–45170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Song, J.; Qu, J.; Li, B.; Zhu, W.; Wong, W.-Y. Near-Infrared Emitting Materials via Harvesting Triplet Excitons: Molecular Design, Properties, and Application in Organic Light Emitting Diodes. Adv. Opt. Mater. 2018, 6, 1800466. [Google Scholar] [CrossRef]
- Sanning, J.; Stegemann, L.; Ewen, P.R.; Schwermann, C.; Daniliuc, C.G.; Zhang, D.; Lin, N.; Duan, L.; Wegner, D.; Doltsinis, N.L.; et al. Colour-Tunable Asymmetric Cyclometalated Pt(II) Complexes and STM-Assisted Stability Assessment of Ancillary Ligands for OLEDs. J. Mater. Chem. C 2016, 4, 2560–2565. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-Covalent Intramolecular Interactions through Ligand-Design Promoting Efficient Luminescence from Transition Metal Complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Ravotto, L.; Ceroni, P. Aggregation induced phosphorescence of metal complexes: From principles to applications. Coord. Chem. Rev. 2017, 346, 62–76. [Google Scholar] [CrossRef]
- Nisic, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Valore, A.; Malicka, J.M.; Cocchi, M.; Freeman, G.R.; Williams, J.A.G. Platinum(II) Complexes with Cyclometallated 5-π-Delocalized-Donor-1,3-di(2-pyridyl)benzene Ligands as Efficient Phosphors for NIROLEDs. J. Mater. Chem. C 2014, 2, 1791–1800. [Google Scholar] [CrossRef]
- Tam, A.Y.-Y.; Tsang, D.P.-K.; Chan, M.-Y.; Zhu, N.; Yam, V.W.-W. A Luminescent Cyclometalated Platinum(II) Complex and its Green Organic Light Emitting Device with High Device Performance. Chem. Commun. 2011, 47, 3383–3385. [Google Scholar] [CrossRef]
- Lu, W.; Mi, B.-X.; Chan, M.C.W.; Hui, Z.; Zhu, N.; Lee, S.-T.; Che, C.-M. [(C^N^N)Pt(C≡C)nR] (HC^N^N = 6-aryl-2,2′-bipyridine, n = 1-4, R = aryl, SiMe3) as a New Class of Light Emitting Materials and their Applications in Electrophosphorescent Devices. Chem. Commun. 2002, 206–207. [Google Scholar] [CrossRef]
- Mao, M.; Peng, J.; Lam, T.-L.; Ang, W.-H.; Li, H.; Cheng, G.; Che, C.-M. High-performance organic light-emitting diodes with low-efficiency roll-off using bulky tetradentate [Pt(O^N^C^N)] emitters. J. Mater. Chem. C 2019, 7, 7230–7236. [Google Scholar] [CrossRef]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar] [CrossRef]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. [Google Scholar] [CrossRef]
- Zhong, J.-J.; Meng, Q.-Y.; Wang, G.-X.; Liu, Q.; Chen, B.; Feng, K.; Tung, C.-H.; Wu, L.-Z. A Highly Efficient and Selective Aerobic Cross-Dehydrogenative-Coupling Reaction Photocatalyzed by a Platinum(II) Terpyridyl Complex. Chem. Eur. J. 2013, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Yamashita, H. Metal Complexes Supported on Solid Matrices for Visible-Light-Driven Molecular Transformations. Chem. Eur. J. 2016, 22, 11122–11137. [Google Scholar] [CrossRef] [PubMed]
- Parasram, M.; Gevorgyan, V. Visible light-induced transition metal-catalyzed transformations: Beyond conventional photosensitizers. Chem. Soc. Rev. 2017, 46, 6227–6240. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Choi, S.; Ohkubo, K.; Fukuzumi, S.; Cho, E.J.; You, Y. Mechanisms and applications of cyclometalated Pt(II) complexes in photoredox catalytic trifluoromethylation. Chem. Sci. 2015, 6, 1454–1464. [Google Scholar] [CrossRef]
- Wu, P.; Wong, E.L.-M.; Ma, D.-L.; Tong, G.S.-M.; Ng, K.-M.; Che, C.-M. Cyclometalated Platinum(II) Complexes as Highly Sensitive Luminescent Switch-On Probes for Practical Application in Protein Staining and Cell Imaging. Chem. Eur. J. 2009, 15, 3652–3656. [Google Scholar] [CrossRef]
- Chung, C.Y.-S.; Li, S.P.-Y.; Louie, M.-W.; Lo, K.K.-W.; Yam, V.W.-W. Induced Self-Assembly and Disassembly of Water-Soluble Alkynylplatinum(II) Terpyridyl Complexes with “Switchable“ NearInfrared (NIR) Emission Modulated by Metal-Metal Interaction over Physiological pH: Demonstration of pH-Responsive NIR Luminescent Probes in Cell-Imaging Studies. Chem. Sci. 2013, 4, 2453–2462. [Google Scholar] [CrossRef]
- Baggaley, E.; Botchway, S.W.; Haycock, J.W.; Morris, H.; Sazanovich, I.V.; Williams, J.A.G.; Weinstein, J.A. Long-Lived Metal Complexes open up Microsecond Lifetime Imaging Microscopy under Multiphoton Excitation: From FLIM to PLIM and beyond. Chem. Sci. 2014, 5, 879–886. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef]
- Guerchais, V.; Fillaut, J.-L. Sensory Luminescent Iridium(III) and Platinum(II) Complexes for Cation Recognition. Coord. Chem. Rev. 2011, 255, 2448–2457. [Google Scholar] [CrossRef]
- Ma, D.-L.; Ma, V.P.-Y.; Chan, D.S.-H.; Leung, K.-H.; He, H.-Z.; Leung, C.-H. Recent Advances in Luminescent Heavy Metal Complexes for Sensing. Coord. Chem. Rev. 2012, 256, 3087–3113. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Hussain, F.; Zeng, C.; Wang, B.; Li, Z.; Kozin, I.; Wang, S. Multiresponsive Tetradentate Phosphorescent Metal Complexes as Highly Sensitive and Robust Luminescent Oxygen Sensors: Pd(II) Versus Pt(II) and 1,2,3-Triazolyl Versus 1,2,4-Triazolyl. ACS Appl. Mater. Interfaces 2019, 11, 12666–12674. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tong, G.S.M.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Highly Phosphorescent Platinum(II) Emitters: Photophysics, Materials and Biological Application. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef]
- Williams, J.A.G. Photochemistry and Photophysics of Coordination Compounds II. Top. Curr. Chem. 2007, 281, 205–268. [Google Scholar]
- Wu, W.; Huang, D.; Zhao, J. Tridentate Cyclometalated Platinum(II) Complexes with Strong Absorption of Visible Light and Long-Lived Triplet Excited States as Photosensitizers for Triplet–Triplet Annihilation Upconversion. Dyes Pigm. 2013, 96, 220–231. [Google Scholar] [CrossRef]
- Ravindranathan, D.; Vezzu, D.A.K.; Bartolotti, L.; Boyle, P.D.; Hou, S. Improvement in Phosphorescence Efficiency through Tuning of Coordination Geometry of Tridentate Cyclometalated Platinum(II) Complexes. Inorg. Chem. 2010, 49, 8922–8928. [Google Scholar] [CrossRef]
- Vezzu, D.A.K.; Deaton, J.C.; Jones, J.S.; Bartolotti, L.; Harris, C.F.; Marchetti, A.P.; Kondakova, M.; Pike, R.D.; Huo, S. Highly Luminescent Tetradentate Bis-Cyclometalated Platinum Complexes: Design, Synthesis, Structure, Photophysics, and Electroluminescence Application. Inorg. Chem. 2010, 49, 5107–5119. [Google Scholar] [CrossRef]
- Wilde, S.; Ma, D.; Koch, T.; Bakker, A.; Gonzalez-Abradelo, D.; Stegemann, L.; Daniliuc, C.G.; Fuchs, H.; Gao, H.; Doltsinis, N.L.; et al. Toward Tunable Electroluminescent Devices by Correlating Function and Submolecular Structure in 3D Crystals, 2D-Confined Monolayers, and Dimers. ACS Appl. Mater. Interfaces 2018, 10, 22460–22473. [Google Scholar] [CrossRef]
- Ren, J.; Cnudde, M.; Brünink, D.; Buss, S.; Daniliuc, C.G.; Liu, L.; Fuchs, H.; Strassert, C.A.; Gao, H.-Y.; Doltsinis, N.L. On-Surface Reactive Planarization of Pt(II) Complexes. Angew. Chem. Int. Ed. 2019, 58, 15396–15400. [Google Scholar] [CrossRef]
- Knedel, T.-O.; Buss, S.; Maisuls, I.; Daniliuc, C.G.; Schlüsener, C.; Brandt, P.; Weingart, O.; Vollrath, A.; Janiak, C.; Strassert, C.A. Encapsulation of Phosphorescent Pt(II) Complexes in Zn-Based Metal–Organic Frameworks toward Oxygen-Sensing Porous Materials. Inorg. Chem. 2020, 59, 7252–7264. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Schnürch, M.; Waldner, B.; Hilber, K.; Mihovilovic, M.D. Synthesis of 5-arylated N-arylthiazole-2-amines as Potential Skeletal Muscle Cell Differentiation Promoters. Bioorg. Med. Chem. Lett. 2011, 21, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Dao-Huy, T.; Waldner, B.; Wimmer, L.; Schnürch, M.; Mihovilovic, M.D. Synthesis of endo- and exo-N-Protected 5-Arylated 2-Aminothiazols through Direct Arylation: An Efficient Route to Cell Differentiation Accelerators. Eur. J. Org. Chem. 2015, 4765–4771. [Google Scholar] [CrossRef]
- Sintenis, F. Beiträge zur Kenntniss der Benzyläther. Liebigs Ann. Chem. 1872, 161, 329–346. [Google Scholar] [CrossRef]
- Lai, S.-W.; Cheung, T.-C.; Chan, M.C.W.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Luminescent Mononuclear and Binuclear Cyclometalated Palladium(II) Complexes of 6-Phenyl-2,2‘-Bipyridines: Spectroscopic and Structural Comparisons with Platinum(II) Analogues 1,2. Inorg. Chem. 2000, 39, 255–262. [Google Scholar] [CrossRef]
- Zhang, E.-X.; Wang, D.-X.; Huang, Z.-T.; Wang, M.-X. Synthesis of (NH)m(NMe)4−m-Bridged Calix [4]pyridines and the Effect of NH Bridge on Structure and Properties. J. Org. Chem. 2009, 74, 8595–8603. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Galenko, E.E.; Kozina, D.O.; Kalinichev, A.A.; Baigildin, V.A.; Prudovskaya, N.A.; Shakirova, J.R.; Khlebnikov, A.F.; Prosev, V.V.; Evarestov, R.A.; et al. Nonsymmetric [Pt(C^N*N′^C′)] Complexes: Aggregation-Induced Emission in the Solid State and in Nanoparticles Tuned by Ligand Structure. Chem. Eur. J. 2022, 28, e202202207. [Google Scholar] [CrossRef]
- Sillen, A.; Engelborghs, Y. The Correct Use of “Average” Fluorescence Parameters. Photochem. Photobiol. 1998, 67, 475–486. [Google Scholar] [CrossRef]
- Wilde, S.; Mittelberg, L.; Daniliuc, C.G.; Koch, T.; Doltsinis, N.L.; Strassert, C.A. Studie über den Einfluss des Fluorierungsgrades an einem tetradentaten C^N*N^C-Luminophor auf die photophysikalischen Eigenschaften seiner Platin(II)-Komplexe und deren Aggregation. Z. Naturforsch. 2018, 73, 849–863. [Google Scholar] [CrossRef]
- Strassert, C.A.; Dicelio, L.E.; Awruch, J. Reduction of an Amido Zinc(II) Phthalocyanine by Diborane. Synthesis 2006, 5, 799–802. [Google Scholar] [CrossRef]
- Strassert, C.A.; Awruch, J. Conversion of Phthalimides to Isoinddolines by Diborane. Monatsh. Chem. 2006, 137, 1499–1503. [Google Scholar] [CrossRef]

| Complex | λabs / nm (ε / 103 M−1 cm−1) | Medium (T / K) | λem / nm | τav a | ΦL ± 0.02 / ± 0.05 b |
|---|---|---|---|---|---|
| [PtCl(L0)] [40] | 246 (12.5), 266 (20.1), 278 (18.1), 316 (8.1), 348 (8.3), 370 (5.2) | DCM, Ar (298) | 496 | 19.1 ns | <0.02 |
| Glassy matrix (77) | 487 | 31.8 µs | 0.98 | ||
| [PtCl(L1)] | 266 (17.8), 279 (17.4), 314 (6.7), 348 (6.6), 370 (3.7) | DCM, Ar (298) | 495 | 14.58 ns | <0.02 |
| Glassy matrix (77) | 483 | 23.52 µs | 0.98 | ||
| [PtCl(L2)] | 266 (19.9), 280 (17.4), 291 (13.5), 315 (8.2), 349 (8.3), 371 (5.0) | DCM, Ar (298) | 495 | 15.94 ns | <0.02 |
| Glassy matrix (77) | 487 | 32.86 µs | 0.98 | ||
| [PtCl(L3)] | 267 (30.1), 267 (27.7), 316 (10.9), 348 (11.7), 368 (6.7) | DCM, Ar (298) | 496 | 25.7 ns | <0.02 |
| Glassy matrix (77) | 484 | 24.879 µs | 0.98 | ||
| [PtCl(L4)] | 273 (17.7), 293 (15.0), 345 (5.4), 378 (3.3) | DCM, Ar (298) | 504 | n.d. | n.d. |
| Glassy matrix (77) | 487 | 12.42 µs | 0.98 | ||
| [PtCl(L5)] | 262 (22.6), 276 (20.9), 288 (16.1), 313 (9.0), 346 (8.6), 370 (5.1) | DCM, Ar (298) | 493 | 0.2695 µs | <0.02 |
| Glassy matrix (77) | 488 | 45.7 µs | 0.98 | ||
| [Pt(L6)] | 274 (43.9), 290 (33.0), 318 (23.5), 334 (22.1), 366 (16.8), 404 (7.2) | DCM, Ar (298) | 510 | 4.2035 µs | 0.54 |
| Glassy matrix (77) | 500 | 11.436 µs | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buss, S.; Cappellari, M.V.; Hepp, A.; Kösters, J.; Strassert, C.A. Modification of the Bridging Unit in Luminescent Pt(II) Complexes Bearing C^N*N and C^N*N^C Ligands. Chemistry 2023, 5, 1243-1255. https://doi.org/10.3390/chemistry5020084
Buss S, Cappellari MV, Hepp A, Kösters J, Strassert CA. Modification of the Bridging Unit in Luminescent Pt(II) Complexes Bearing C^N*N and C^N*N^C Ligands. Chemistry. 2023; 5(2):1243-1255. https://doi.org/10.3390/chemistry5020084
Chicago/Turabian StyleBuss, Stefan, María Victoria Cappellari, Alexander Hepp, Jutta Kösters, and Cristian A. Strassert. 2023. "Modification of the Bridging Unit in Luminescent Pt(II) Complexes Bearing C^N*N and C^N*N^C Ligands" Chemistry 5, no. 2: 1243-1255. https://doi.org/10.3390/chemistry5020084
APA StyleBuss, S., Cappellari, M. V., Hepp, A., Kösters, J., & Strassert, C. A. (2023). Modification of the Bridging Unit in Luminescent Pt(II) Complexes Bearing C^N*N and C^N*N^C Ligands. Chemistry, 5(2), 1243-1255. https://doi.org/10.3390/chemistry5020084






