Synthesis, Structural, Magnetic and Computational Studies of a One-Dimensional Ferromagnetic Cu(II) Chain Assembled from a New Schiff Base Ligand
Abstract
1. Introduction
2. Materials & Methods
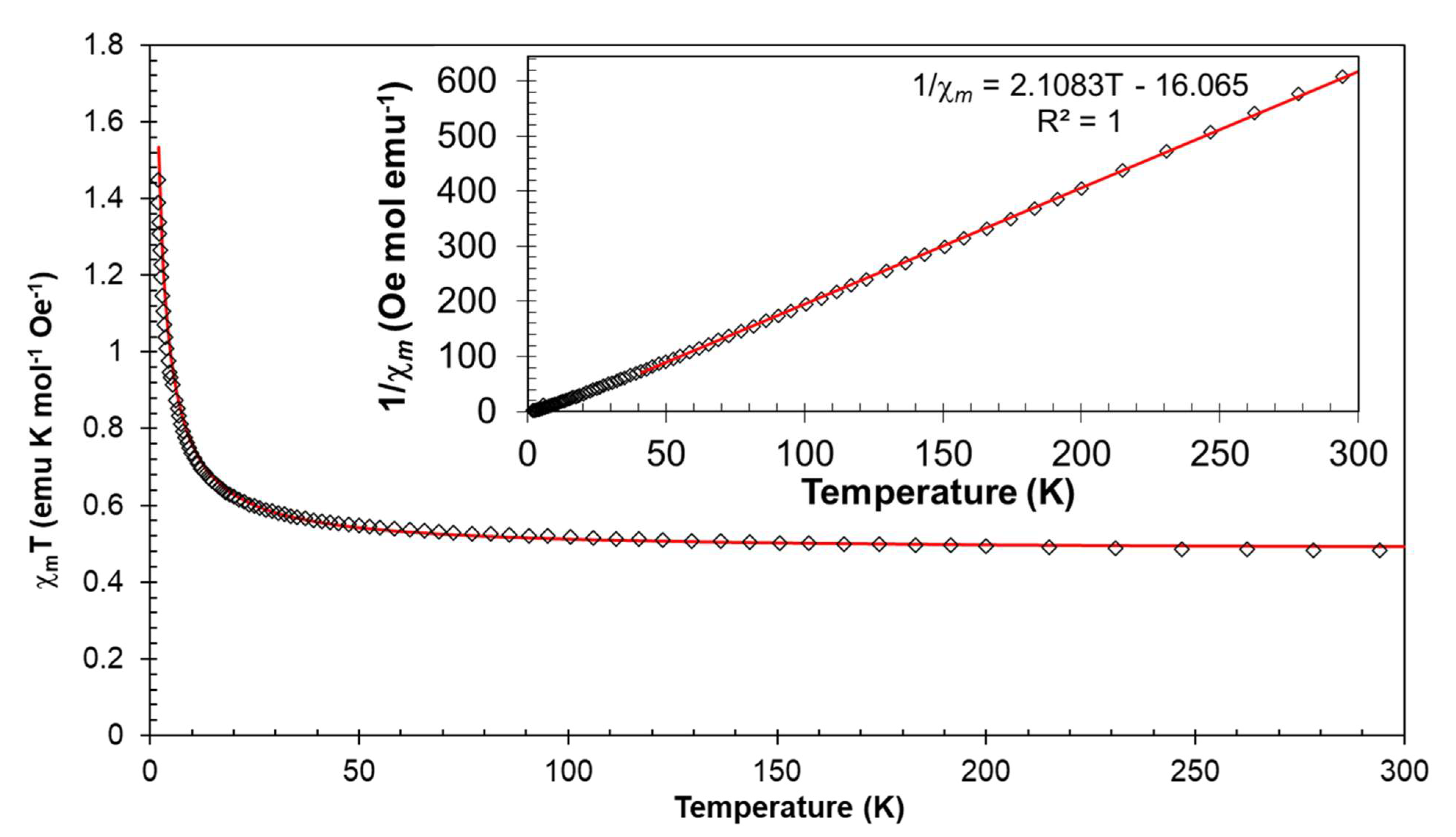
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaliyappan, T.; Kannan, P. Co-Ordination Polymers. Prog. Polym. Sci. 2000, 25, 343–370. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Gao, S.; Su, G.; Xu, G.-X. Cyano-Bridged 4f–3d Coordination Polymers with a Unique Two-Dimensional Topological Architecture and Unusual Magnetic Behavior. Angew. Chem. Int. Ed. 2001, 40, 434–437. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Tong, M.-L.; Zhang, W.-X.; Chen, X.-M. Assembling Magnetic Nanowires into Networks: A Layered CoII Carboxylate Coordination Polymer Exhibiting Single-Chain-Magnet Behavior. Angew. Chem. Int. Ed. 2006, 45, 6310–6314. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhang, D.-Q.; Zhu, D.-B. 1D Coordination Polymers Constructed from Anti–anti Carboxylato-Bridged MnIII3O(Brppz)3 Units: From Long-Range Magnetic Ordering to Single-Chain Magnet Behaviors. Inorg. Chem. 2009, 48, 4980–4987. [Google Scholar] [CrossRef] [PubMed]
- Herringer, S.N.; Deumal, M.; Ribas-Arino, J.; Novoa, J.J.; Landee, C.P.; Wikaira, J.L.; Turnbull, M.M. S=1/2 One-Dimensional Random-Exchange Ferromagnetic Zigzag Ladder, Which Exhibits Competing Interactions in a Critical Regime. Eur. J. Chem. 2014, 20, 8355–8362. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, L.; Zhou, H.; Cen, P.; Jin, X.; Xie, G.; Chen, S.; Hu, Q. Single-Ion-Magnet Behavior in a Two-Dimensional Coordination Polymer Constructed from CoII Nodes and a Pyridylhydrazone Derivative. Inorg. Chem. 2015, 54, 8884–8886. [Google Scholar] [CrossRef]
- Jochim, A.; Lohmiller, T.; Rams, M.; Böhme, M.; Ceglarska, M.; Schnegg, A.; Plass, W.; Näther, C. Influence of the Coligand onto the Magnetic Anisotropy and the Magnetic Behavior of One-Dimensional Coordination Polymers. Inorg. Chem. 2020, 59, 8971–8982. [Google Scholar] [CrossRef]
- Hu, J.-J.; Peng, Y.; Liu, S.-J.; Wen, H.-R. Recent Advances in Lanthanide Coordination Polymers and Clusters with Magnetocaloric Effect or Single-Molecule Magnet Behavior. Dalton Trans. 2021, 50, 15473–15487. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal–Organic and Covalent Organic Frameworks as Single-Site Catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef]
- Wu, M.-X.; Yang, Y.-W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.; Uflyand, I.E. Design and Synthesis of Coordination Polymers with Chelated Units and Their Application in Nanomaterials Science. RSC Adv. 2017, 7, 42242–42288. [Google Scholar] [CrossRef]
- Acar, Y.; Coban, M.B.; Gungor, E.; Kara, H. Two New NIR Luminescencent Er(III) Coordination Polymers with Potential Application Optical Amplification Devices. J. Clust. Sci. 2020, 31, 117–124. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent Developments in Luminescent Coordination Polymers: Designing Strategies, Sensing Application and Theoretical Evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Li, Y.-M.; Xiao, C.-Y.; Zhang, X.-D.; Xu, Y.-Q.; Lun, H.-J.; Niu, J.-Y. MnII, CuII and CoII Coordination Polymers Showing Antiferromagnetism, and the Coexistence of Spin Frustration and Long Range Magnetic Ordering. CrystEngComm 2013, 15, 7756. [Google Scholar] [CrossRef]
- Nath, A.; Islam, S.S.; Mukharjee, P.K.; Nath, R.; Mandal, S. Reentrant Spin-Glass Behavior in Cobalt(II) Based Coordination Polymers. Cryst. Growth Des. 2019, 19, 6463–6471. [Google Scholar] [CrossRef]
- Journaux, Y.; Ferrando-Soria, J.; Pardo, E.; Ruiz-Garcia, R.; Julve, M.; Lloret, F.; Cano, J.; Li, Y.; Lisnard, L.; Yu, P.; et al. Design of Magnetic Coordination Polymers Built from Polyoxalamide Ligands: A Thirty Year Story. Eur. J. Inorg. Chem. 2018, 2018, 228–247. [Google Scholar] [CrossRef]
- Veríssimo, L.M.; Pereira, M.S.S.; Strečka, J.; Lyra, M.L. Kosterlitz-Thouless and Gaussian Criticalities in a Mixed Spin-(1/2, 5/2, 1/2) Heisenberg Branched Chain with Exchange Anisotropy. Phys. Rev. B 2019, 99, 134408. [Google Scholar] [CrossRef]
- Keene, T.D.; Hursthouse, M.B.; Price, D.J. Two-Dimensional Metal−Organic Frameworks: A System with Competing Chelating Ligands. Cryst. Growth Des. 2009, 9, 2604–2609. [Google Scholar] [CrossRef]
- Saines, P.J.; Bristowe, N.C. Probing Magnetic Interactions in Metal–Organic Frameworks and Coordination Polymers Microscopically. Dalton Trans. 2018, 47, 13257–13280. [Google Scholar] [CrossRef] [PubMed]
- Chiari, B.; Hatfield, W.E.; Piovesana, O.; Tarantelli, T.; Ter Haar, L.W.; Zanazzi, P.F. Exchange Interaction in Multinuclear Transition-Metal Complexes. 3. Synthesis, x-Ray Structure, and Magnetic Properties of Cu2L(CH3COO)2.2CH3OH (L2− = Anion of N,N′-Bis((2-(o-Hydroxybenzhydrylidene)Amino)Ethyl)-1,2-Ethanediamine), a One-Dimensional Heisenberg Antiferromagnet Having through-Bond Coupled Copper(II) Ions. Inorg. Chem. 1983, 22, 1468–1473. [Google Scholar] [CrossRef]
- Gao, E.-Q.; Bai, S.-Q.; Yue, Y.-F.; Wang, Z.-M.; Yan, C.-H. New One-Dimensional Azido-Bridged Manganese(II) Coordination Polymers Exhibiting Alternating Ferromagnetic−Antiferromagnetic Interactions: Structural and Magnetic Studies. Inorg. Chem. 2003, 42, 3642–3649. [Google Scholar] [CrossRef]
- Papatriantafyllopoulou, C.; Zartilas, S.; Manos, M.J.; Pichon, C.; Clérac, R.; Tasiopoulos, A.J. A Single-Chain Magnet Based on Linear [MnIII2MnII] Units. Chem. Commun. 2014, 50, 14873–14876. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Dolinar, B.S.; Liu, S.; Brown, A.J.; Zhang, X.; Wang, Z.-X.; Dunbar, K.R. Enforcing Ising-like Magnetic Anisotropy via Trigonal Distortion in the Design of a W(V)–Co(II) Cyanide Single-Chain Magnet. Chem. Sci. 2018, 9, 119–124. [Google Scholar] [CrossRef]
- Monroe, J.C.; Landee, C.P.; Turnbull, M.M.; Wikaira, J.L. Well-Isolated Pyrazine-Bridged Copper(II) Chains: Synthesis and Magneto-Structural Analysis. J. Coord. Chem. 2020, 73, 2645–2663. [Google Scholar] [CrossRef]
- Amaral, S.; Jensen, W.E.; Landee, C.P.; Turnbull, M.M.; Matthew Woodward, F. Quantum Linear Magnetic Chains: Structure and Magnetic Behavior of (2-Methylpyrazine)Copper(II) Nitrate. Polyhedron 2001, 20, 1317–1322. [Google Scholar] [CrossRef]
- Breunig, O.; Garst, M.; Klümper, A.; Rohrkamp, J.; Turnbull, M.M.; Lorenz, T. Quantum Criticality in the spin-1/2 Heisenberg chain system copper pyrazine dinitrate. Sci. Adv. 2017, 3, eaao3773. [Google Scholar] [CrossRef] [PubMed]
- Bethe, H. Zur Theorie der Metalle: I. Eigenwerte und Eigenfunktionen der linearen Atomkette. Z. Physik 1931, 71, 205–226. [Google Scholar] [CrossRef]
- Eggert, S.; Affleck, I.; Takahashi, M. Susceptibility of the Spin 1/2 Heisenberg Antiferromagnetic Chain. Phys. Rev. Lett. 1994, 73, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.C.; Kremer, R.K.; Troyer, M.; Wang, X.; Klümper, A.; Bud’ko, S.L.; Panchula, A.F.; Canfield, P.C. Thermodynamics of Spin S=1/2 Antiferromagnetic Uniform and Alternating-Exchange Heisenberg Chains. Phys. Rev. B 2000, 61, 9558–9606. [Google Scholar] [CrossRef]
- Landee, C.P.; Turnbull, M.M. Review: A Gentle Introduction to Magnetism: Units, Fields, Theory, and Experiment. J. Coord. Chem. 2014, 67, 375–439. [Google Scholar] [CrossRef]
- Landee, C.P.; Turnbull, M.M. Recent Developments in Low-Dimensional Copper(II) Molecular Magnets. Eur. J. Inorg. Chem. 2013, 2013, 2266–2285. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chui, S.S.-Y.; Williams, I.D. Cooperative Magnetic Behavior in the Coordination Polymers [Cu3(TMA)2L3] (L=H2O, Pyridine). J. Appl. Phys. 2000, 87, 6007–6009. [Google Scholar] [CrossRef]
- Kirkman-Davis, E.; Witkos, F.E.; Selmani, V.; Monroe, J.C.; Landee, C.P.; Turnbull, M.M.; Dawe, L.N.; Polson, M.I.J.; Wikaira, J.L. Pyrazine-Bridged Cu(II) Chains: Diaquabis(n-Methyl-2-Pyridone)Copper(II) Perchlorate Complexes. Dalton Trans. 2020, 49, 13693–13703. [Google Scholar] [CrossRef]
- Santoro, A.; Mighell, A.D.; Reimann, C.W. The Crystal Structure of a 1:1 Cupric Nitrate–Pyrazine Complex Cu(NO3)2·(C4N2H4). Acta Cryst. B 1970, 26, 979–984. [Google Scholar] [CrossRef]
- Kono, Y.; Sakakibara, T.; Aoyama, C.P.; Hotta, C.; Turnbull, M.M.; Landee, C.P.; Takano, Y. Field-Induced Quantum Criticality and Universal Temperature Dependence of the Magnetization of a Spin-1/2 Heisenberg Chain. Phys. Rev. Lett. 2015, 114, 037202. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.R.; Varughese, P.A.; Olejniczak, I.; Pigos, J.M.; Musfeldt, J.L.; Landee, C.P.; Turnbull, M.M.; Carr, G.L. Vibrational Properties of the One-Dimensional, S = 1/2, Heisenberg Antiferromagnet Copper Pyrazine Dinitrate. Chem. Mater. 2001, 13, 2127–2134. [Google Scholar] [CrossRef]
- Jornet-Somoza, J.; Deumal, M.; Robb, M.A.; Landee, C.P.; Turnbull, M.M.; Feyerherm, R.; Novoa, J.J. First-Principles Bottom-up Study of 1D to 3D Magnetic Transformation in the Copper Pyrazine Dinitrate S=1/2 Antiferromagnetic Crystal. Inorg. Chem. 2010, 49, 1750–1760. [Google Scholar] [CrossRef]
- Lu, J.Y.; Babb, A.M. A Simultaneous Reduction, Substitution, and Self-Assembly Reaction under Hydrothermal Conditions Afforded the First Diiodopyridine Copper(I) Coordination Polymer. Inorg. Chem. 2002, 41, 1339–1341. [Google Scholar] [CrossRef]
- Erxleben, A. Structures and Properties of Zn(II) Coordination Polymers. Coord. Chem. Rev. 2003, 246, 203–228. [Google Scholar] [CrossRef]
- Horikoshi, R.; Mikuriya, M. One-Dimensional Coordination Polymers from the Self-Assembly of Copper(II) Carboxylates and 4,4′-Dithiobis(Pyridine). Bull. Chem. Soc. Jpn. 2005, 78, 827–834. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Wu, H.; Ma, J.-F.; Liu, Y.-Y.; Liu, B.; Yang, J. Syntheses, Structures, and Photoluminescence of Zinc(II) Coordination Polymers Based on Carboxylates and Flexible Bis-[(Pyridyl)-Benzimidazole] Ligands. Cryst. Growth Des. 2010, 10, 4795–4805. [Google Scholar] [CrossRef]
- Wang, J.-P.; Su, B.; Li, J.-H.; Wang, G.-M. Diverse Architectures and Luminescence Properties of Three Low-Dimensional Zn(II)/Cd(II) Coordination Polymers Based on a Pyridine-Imidazole Ligand. Inorg. Chem. Commun. 2018, 90, 29–33. [Google Scholar] [CrossRef]
- Kumar, S.; Dhar, D.N.; Saxena, P.N. Applications of Metal Complexes of Schiff Bases—A Review. J. Sci. Ind. Res. 2009, 68, 181–187. [Google Scholar]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Dhahagani, K.; Mathan Kumar, S.; Chakkaravarthi, G.; Anitha, K.; Rajesh, J.; Ramu, A.; Rajagopal, G. Synthesis and Spectral Characterization of Schiff Base Complexes of Cu(II), Co(II), Zn(II) and VO(IV) Containing 4-(4-Aminophenyl)Morpholine Derivatives: Antimicrobial Evaluation and Anticancer Studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, X.; Huang, Y.; Xu, S.; Su, X.; Pan, X.; Xu, J.; Wang, A.; Liang, C.; Wang, X.; et al. A Schiff Base Modified Gold Catalyst for Green and Efficient H2 Production from Formic Acid. Energy Environ. Sci. 2015, 8, 3204–3207. [Google Scholar] [CrossRef]
- Nair, M.S.; Arish, D.; Joseyphus, R.S. Synthesis, Characterization, Antifungal, Antibacterial and DNA Cleavage Studies of Some Heterocyclic Schiff Base Metal Complexes. J. Saudi Chem. Soc. 2012, 16, 83–88. [Google Scholar] [CrossRef]
- Hossain, M.S.; Roy, P.K.; Zakaria, C.M.; Kudrat-E-Zahan, M. Selected Schiff Base Coordination Complexes and their Microbial Application: A Review. Int. J. Chem. Stud. 2018, 6, 19–31. [Google Scholar]
- Kolcu, F.; Erdener, D.; Kaya, İ. A Schiff Base Based on Triphenylamine and Thiophene Moieties as a Fluorescent Sensor for Cr(III) Ions: Synthesis, Characterization and Fluorescent Applications. Inorg. Chim. Acta. 2020, 509, 119676. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal Complexes Driven from Schiff Bases and Semicarbazones for Biomedical and Allied Applications: A Review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Hadadzadeh, H.; Fejfarova, K.; Dusek, M. Metal-Dependent Assembly of a Tetranuclear Copper(II) Complex versus a 1D Chain Coordination Polymer of Cobalt(III) Complex with N2O2-Chelating Schiff-Base Ligand: Synthesis, Characterization and Crystal Structures. Polyhedron 2010, 29, 807–812. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, Characterization and Biological Studies of Metal(II) Complexes of (3E)-3-[(2-{(E)-[1-(2,4-Dihydroxyphenyl)Ethylidene]Amino}ethyl)Imino]-1-Phenylbutan-1-One Schiff Base. Molecules 2015, 20, 9788–9802. [Google Scholar] [CrossRef] [PubMed]
- Novoa, N.; Manzur, C.; Roisnel, T.; Kahlal, S.; Saillard, J.-Y.; Carrillo, D.; Hamon, J.-R. Nickel(II)-Based Building Blocks with Schiff Base Derivatives: Experimental Insights and DFT Calculations. Molecules 2021, 26, 5316. [Google Scholar] [CrossRef]
- Protasenko, N.A.; Baryshnikova, S.V.; Astaf’eva, T.V.; Cherkasov, A.V.; Poddel’sky, A.I. Mono- and Binuclear Zinc Complexes with a Bidentate Phenol-Containing 2-Benzylideneamino-5-Methylphenol Schiff Base. Russ. J. Coord. Chem. 2021, 47, 417–423. [Google Scholar] [CrossRef]
- Celedon, S.; Roisnel, T.; Carrillo, D.; Ledoux-Rak, I.; Hamon, J.-R.; Manzur, C. Transition Metal(II) Complexes Featuring Push-Pull Dianionic Schiff Base Ligands: Synthesis, Crystal Structure, Electrochemical, and NLO Studies. J. Coord. Chem. 2020, 73, 3079–3094. [Google Scholar] [CrossRef]
- Ghosh, P.; Dey, S.K.; Ara, M.H.; Karim, K.; Islam, A.B.M.N. A Review on Synthesis and Versatile Applications of Some Selected Schiff Bases with Their Transition Metal Complexes. Egypt. J. Chem. 2019, 62, 523–547. [Google Scholar] [CrossRef]
- Zare, N.; Zabardasti, A. A New Nano-Sized Mononuclear Cu(II) Complex with N,N-Donor Schiff Base Ligands: Sonochemical Synthesis, Characterization, Molecular Modeling and Biological Activity. Appl. Organomet. Chem. 2019, 33, e4687. [Google Scholar] [CrossRef]
- El-Bindary, A.A.; El-Sonbati, A.Z.; Diab, M.A.; Ghoneim, M.M.; Serag, L.S. Polymeric Complexes—LXII. Coordination Chemistry of Supramolecular Schiff Base Polymer Complexes—A Review. J. Mol. Liq. 2016, 216, 318–329. [Google Scholar] [CrossRef]
- Sadhukhan, D.; Ray, A.; Butcher, R.J.; Gómez García, C.J.; Dede, B.; Mitra, S. Magnetic and Catalytic Properties of a New Copper(II)–Schiff Base 2D Coordination Polymer Formed by Connected Helical Chains. Inorg. Chim. Acta 2011, 376, 245–254. [Google Scholar] [CrossRef]
- Shi, S.-M.; Gu, Y.-Q.; Chen, Z.-F.; Liu, Y.-C.; Liang, H. One-Dimensional Chain Copper(II) and Nickel(II) Coordination Polymers With N-Salicylideneglycine Schiff Base Ligand. Synth. React. Inorg. M 2012, 42, 1262–1266. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R.; Zorlu, Y. NOO-Type Tridentate Schiff Base Ligand and Its One-Dimensional Cu(II) Coordination Polymer: Synthesis, Crystal Structure, Biomacromolecular Interactions and Radical Scavenging Activities. Inorg. Chim. Acta 2021, 514, 119994. [Google Scholar] [CrossRef]
- Nabei, A.; Kuroda-Sowa, T.; Okubo, T.; Maekawa, M.; Munakata, M. The Effect of Molecular Packing on the Occurrence of Spin Crossover Phenomena in One-Dimensional Fe(II)-Bis-Schiff Base Complexes. Inorg. Chim. Acta 2008, 361, 3489–3493. [Google Scholar] [CrossRef]
- Choi, S.W.; Kwak, H.Y.; Yoon, J.H.; Kim, H.C.; Koh, E.K.; Hong, C.S. Intermolecular Contact-Tuned Magnetic Nature in One-Dimensional 3d−5d Bimetallic Systems: From a Metamagnet to a Single-Chain Magnet. Inorg. Chem. 2008, 47, 10214–10216. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, K.; Yoon, J.; Ryu, D.; Koo, B.; Koh, E.; Hong, C. Cyanide-Bridged WVMnIII Single-Chain Magnet Based on an Octacoordinate [W(CN)6(Phen)]− Anion. Sci. China Chem. 2012, 55, 1012–1017. [Google Scholar] [CrossRef]
- Lochenie, C.; Gebauer, A.; Klimm, O.; Puchtler, F.; Weber, B. Iron(II) Spin Crossover Complexes with Diaminonaphthalene-Based Schiff Base-like Ligands: 1D Coordination Polymers. New J. Chem. 2016, 40, 4687–4695. [Google Scholar] [CrossRef]
- APEX Suite of Crystallographic Software, APEX 2 Version 4; Bruker AXS Inc.: Madison, WI, USA, 2008.
- Bruker SAINT, version V8.34A; Bruker AXS Inc.: Madison, WI, USA, 2013.
- Sheldrick, G.M. SADABS, version 2.03; University of Göttingen: Göttingen, Germany, 2002.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, 1986; ISBN 9783642707353 9783642707339. [Google Scholar]
- Jaguar, version 11.4; Schrödinger, Inc.: New York, NY, USA, 2021.
- Becke, A.D. A New Mixing of Hartree–Fock and Local Density-functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, L.; Zhang, L.; Li, X.-L.; Guo, M.; Powell, A.K.; Tang, J. Macroscopic Hexagonal Tubes of 3d-4f Metallocycles. Angew. Chem. Int. Ed. 2016, 55, 15574–15578. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.K.; Chilton, N.F.; Moubaraki, B.; Hooper, T.; Brechin, E.K.; Evangelisti, M.; Murray, K.S. Molecular Coolers: The Case for [CuII5GdIII4]. Chem. Sci. 2011, 2, 1166. [Google Scholar] [CrossRef]
- Leng, J.-D.; Liu, J.-L.; Tong, M.-L. Unique Nanoscale {CuII36LnIII24} (Ln = Dy and Gd) Metallo-Rings. Chem. Commun. 2012, 48, 5286. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Casanova, J.; Alzuet, G.; Ferrer, S.; Latorre, J.; Antonio Ramírez, J.; Borrás, J. Superoxide Dismutase Activity of Ternary Copper Complexes of Sulfathiazole and Imidazole Derivatives. Synthesis and Properties of [CuL2(R-Him)2] [HL=4-Amino-N-(Thiazol-2-Yl)Benzenesulfonamide, R-Him=4-Methylimidazole, 4,4-Dimethylimidazoline or 1,2-Dimethylimidazole]. Crystal Structure of [CuL2(4,4-Dimethylimidazoline)2]. Inorg. Chim. Acta 2000, 304, 170–177. [Google Scholar] [CrossRef]
- Blundell, S. Magnetism in Condensed Matter; Oxford master series in condensed matter physics; Oxford University Press: New York, NY, USA, 2001; pp. 171–172. ISBN 9780198505914. [Google Scholar]
- Yamaguchi, K.; Takahara, Y.; Fueno, T. Ab-Initio Molecular Orbital Studies of Structure and Reactivity of Transition Metal-OXO Compounds. In Applied Quantum Chemistry; Smith, V.H., Schaefer, H.F., Morokuma, K., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 155–184. ISBN 9789401086097 9789400947467. [Google Scholar]
- Soda, T.; Kitagawa, Y.; Onishi, T.; Takano, Y.; Shigeta, Y.; Nagao, H.; Yoshioka, Y.; Yamaguchi, K. Ab Initio Computations of Effective Exchange Integrals for H–H, H–He–H and Mn2O2 Complex: Comparison of Broken-Symmetry Approaches. Chem. Phys. Lett. 2000, 319, 223–230. [Google Scholar] [CrossRef]
- Ruiz, E.; Cano, J.; Alvarez, S.; Alemany, P. Broken Symmetry Approach to Calculation of Exchange Coupling Constants for Homobinuclear and Heterobinuclear Transition Metal Complexes. J. Comput. Chem. 1999, 20, 1391–1400. [Google Scholar] [CrossRef]
- Onofrio, N.; Mouesca, J.-M. Analysis of the Singlet–Triplet Splitting Computed by the Density Functional Theory–Broken-Symmetry Method: Is It an Exchange Coupling Constant? Inorg. Chem. 2011, 50, 5577–5586. [Google Scholar] [CrossRef]
- Ruiz-Pérez, C.; Sanchiz, J.; Molina, M.H.; Lloret, F.; Julve, M. Ferromagnetism in Malonato-Bridged Copper(II) Complexes. Synthesis, Crystal Structures, and Magnetic Properties of {[Cu(H2O)3][Cu(Mal)2(H2O)]}n and {[Cu(H2O)4]2[Cu(Mal)2(H2O)]}[Cu(Mal)2(H2O)2]{[Cu(H2O)4][Cu(Mal)2(H2O)2]} (H2mal=Malonic Acid). Inorg. Chem. 2000, 39, 1363–1370. [Google Scholar] [CrossRef]
- Towle, D.K.; Hoffmann, S.K.; Hatfield, W.E.; Singh, P.; Chaudhuri, P. Magnetic and Structural Properties of Acetato(Dien)Copper(1+) Perchlorate: A µ-Acetato-Bridged Quasi-One-Dimensional Complex. Inorg. Chem. 1988, 27, 394–399. [Google Scholar] [CrossRef]
- Colacio, E.; Costes, J.P.; Kivekas, R.; Laurent, J.P.; Ruiz, J. A Quasi-Tetrahedral Tetracopper Cluster with Syn-Anti Bridging Carboxylate Groups: Crystal and Molecular Structure and Magnetic Properties. Inorg. Chem. 1990, 29, 4240–4246. [Google Scholar] [CrossRef]
- Colacio, E.; Dominguez-Vera, J.M.; Costes, J.P.; Kivekas, R.; Laurent, J.P.; Ruiz, J.; Sundberg, M. Structural and Magnetic Studies of a Syn-Anti Carboxylate-Bridged Helix-like Chain Copper(II) Complex. Inorg. Chem. 1992, 31, 774–778. [Google Scholar] [CrossRef]
- Colacio, E.; Dominguez-Vera, J.M.; Moreno, J.M.; Ruiz, J.; Kivekäs, R.; Romerosa, A. Structure and Magnetic Properties of a Syn-Anti Carboxylate Bridged Linear Trinuclear Copper(II) Complex with Ferromagnetic Exchange Interaction. Inorg. Chim. Acta 1993, 212, 115–121. [Google Scholar] [CrossRef]
- Konar, S.; Mukherjee, P.S.; Drew, M.G.B.; Ribas, J.; Ray Chaudhuri, N. Syntheses of Two New 1D and 3D Networks of Cu(II) and Co(II) Using Malonate and Urotropine as Bridging Ligands: Crystal Structures and Magnetic Studies. Inorg. Chem. 2003, 42, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
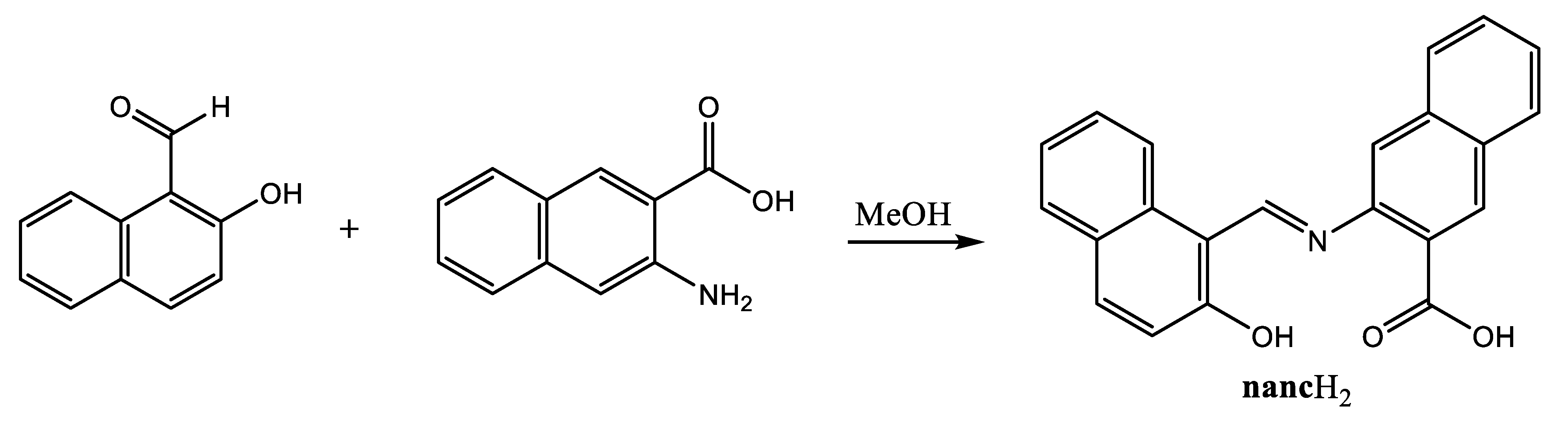
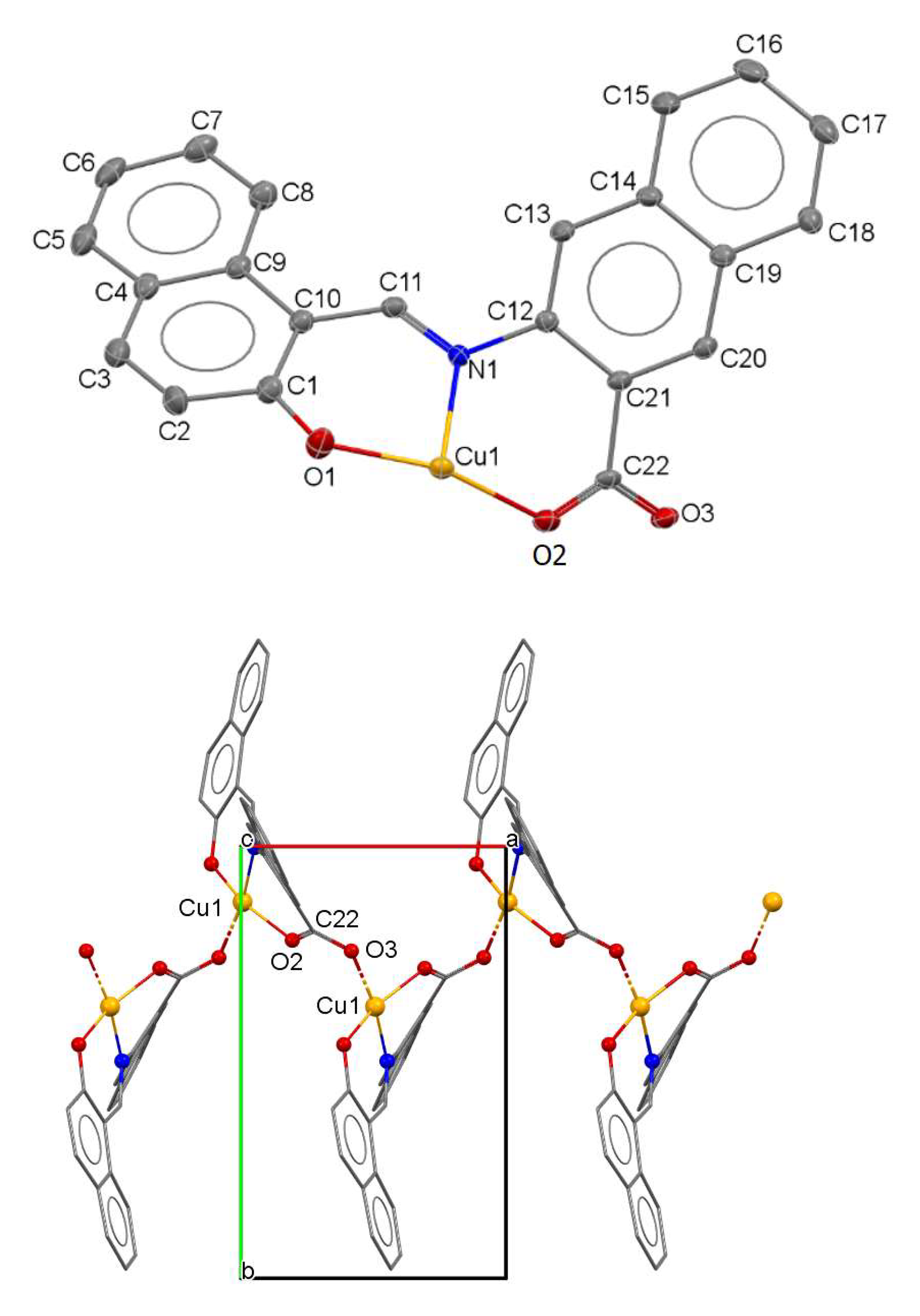
| Chemical Formula | C22H13CuNO3 |
|---|---|
| Mr | 402.87 |
| Crystal system, space group | Orthorhombic, P212121 |
| Temperature (K) | 152(1) |
| a, b, c (Å) | 7.3981 (5), 12.0652 (10), 18.5949 (13) |
| V (Å3) | 1659.8 (2) |
| Z | 4 |
| Radiation type | Mo Kα |
| µ (mm−1) | 1.34 |
| Crystal size (mm) | 0.5 × 0.5 × 0.5 |
| Tmin, Tmax | 0.561, 0.746 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 12654, 4985, 4680 |
| Rint | 0.038 |
| (sin θ/λ)max (Å−1) | 0.715 |
| R [F2 > 2σ(F2)], wR(F2), S | 0.034, 0.076, 1.03 |
| No. of reflections | 4985 |
| No. of parameters | 245 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.49, −0.35 |
| Absolute structure | Refined as an inversion twin. |
| Absolute structure parameter | 0.081 (15) |
| Cu1—O1 | 1.890 (2) | Cu1—O3i | 1.947 (2) |
| Cu1—N1 | 1.933 (2) | O3—Cu1ii | 1.9469 (19) |
| Cu1—O2 | 1.943 (2) | ||
| O1—Cu1—N1 | 92.29 (9) | O1—Cu1—O3i | 87.21 (9) |
| O1—Cu1—O2 | 159.44 (10) | N1—Cu1—O3i | 169.02 (9) |
| N1—Cu1—O2 | 93.17 (9) | O2—Cu1—O3i | 91.09 (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worrell, A.; Delle Monache, G.; Turnbull, M.M.; Rawson, J.M.; Stamatatos, T.C.; Pilkington, M. Synthesis, Structural, Magnetic and Computational Studies of a One-Dimensional Ferromagnetic Cu(II) Chain Assembled from a New Schiff Base Ligand. Chemistry 2023, 5, 85-96. https://doi.org/10.3390/chemistry5010007
Worrell A, Delle Monache G, Turnbull MM, Rawson JM, Stamatatos TC, Pilkington M. Synthesis, Structural, Magnetic and Computational Studies of a One-Dimensional Ferromagnetic Cu(II) Chain Assembled from a New Schiff Base Ligand. Chemistry. 2023; 5(1):85-96. https://doi.org/10.3390/chemistry5010007
Chicago/Turabian StyleWorrell, Anne, Gabriele Delle Monache, Mark M. Turnbull, Jeremy M. Rawson, Theocharis C. Stamatatos, and Melanie Pilkington. 2023. "Synthesis, Structural, Magnetic and Computational Studies of a One-Dimensional Ferromagnetic Cu(II) Chain Assembled from a New Schiff Base Ligand" Chemistry 5, no. 1: 85-96. https://doi.org/10.3390/chemistry5010007
APA StyleWorrell, A., Delle Monache, G., Turnbull, M. M., Rawson, J. M., Stamatatos, T. C., & Pilkington, M. (2023). Synthesis, Structural, Magnetic and Computational Studies of a One-Dimensional Ferromagnetic Cu(II) Chain Assembled from a New Schiff Base Ligand. Chemistry, 5(1), 85-96. https://doi.org/10.3390/chemistry5010007







