New Materials and Phenomena in Membrane Distillation
Abstract
1. Introduction
2. Membrane Distillation
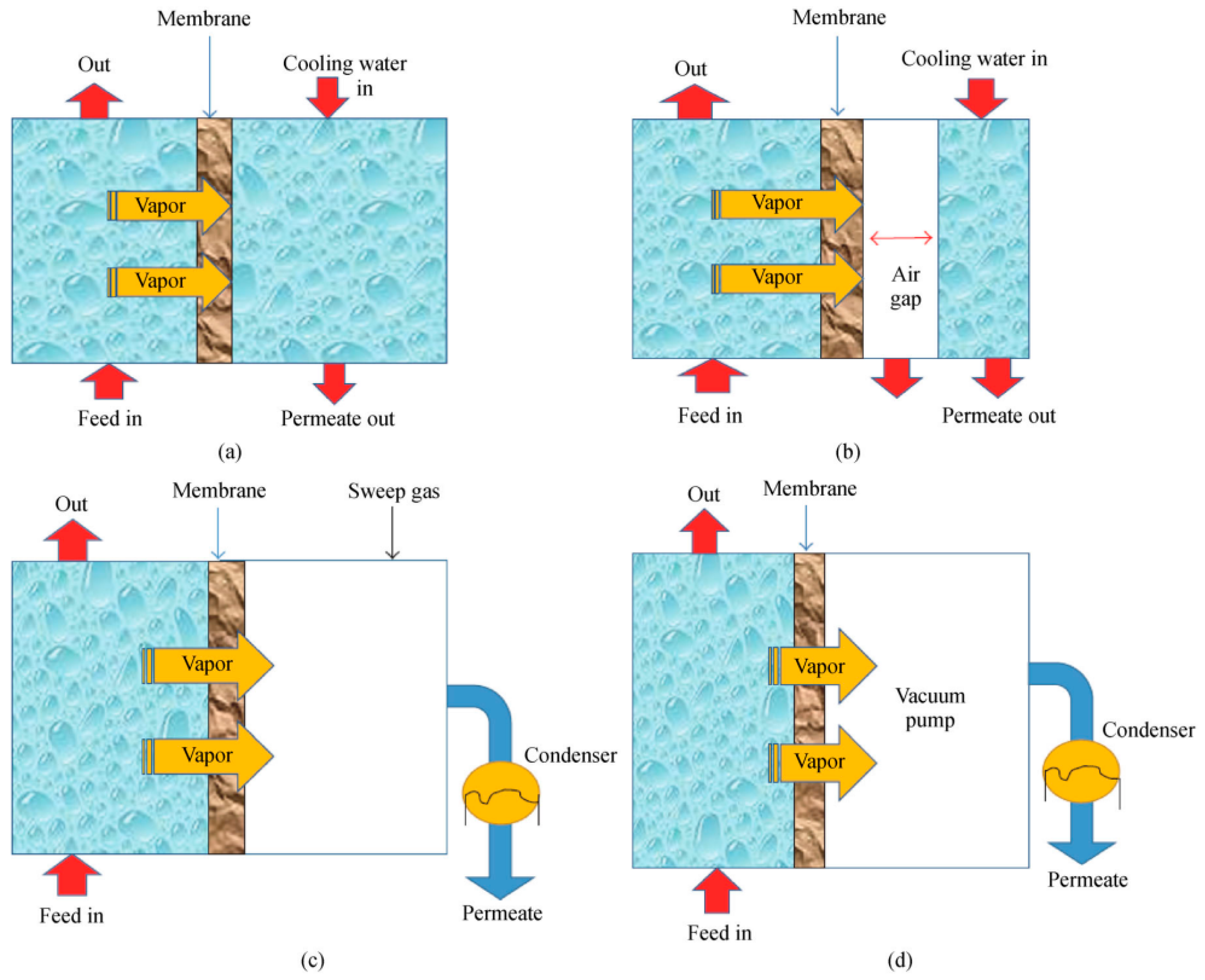
2.1. Membrane Distillation Configurations
2.2. Membrane Modules
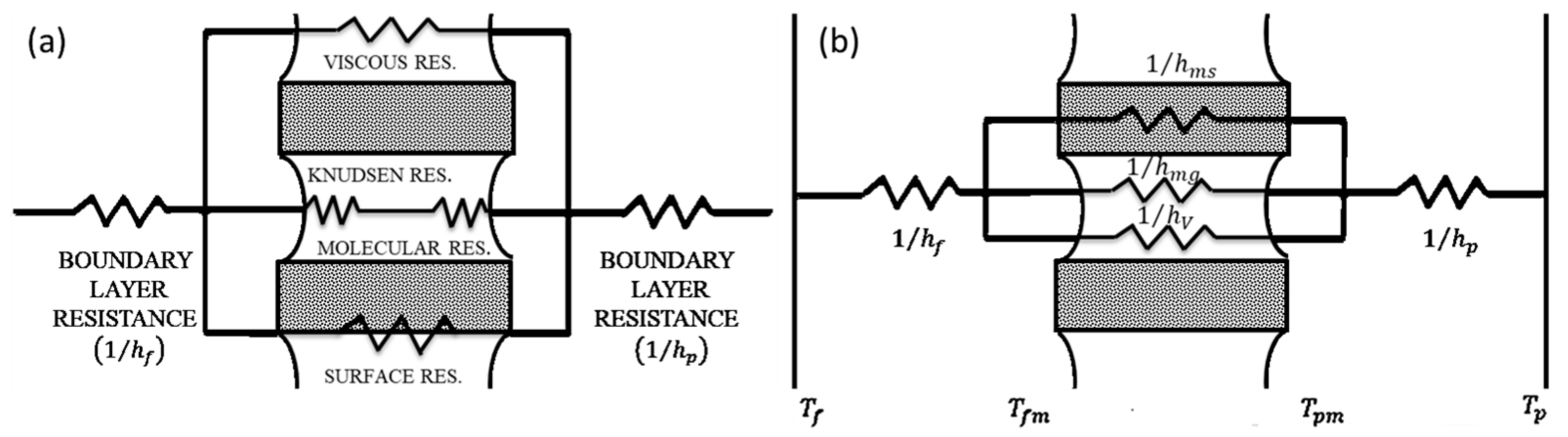
2.3. Mass and Heat Transfer
2.3.1. Mass Transfer
- Sh is the Sherwood number, (d: hydraulic number, D: diffusion coefficient)
- Re is the Reynolds number, (ρ: fluid density; ν: fluid velocity, μ: fluid viscosity)
- Sc is the Schmidt number,
2.3.2. Heat Transfer
- (1)
- convection from the feed bulk to the vapour–liquid interface at the membrane surface [61]
- (2)
- evaporation and conduction through the microporous membrane
- (3)
- convection from the vapour–liquid interface at the membrane surface to the permeate side [61]
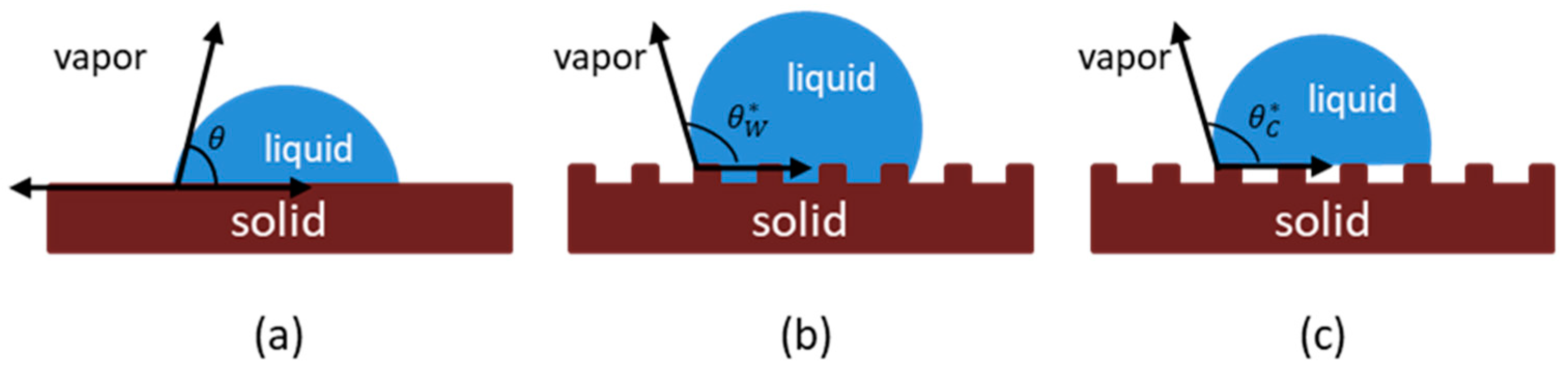
3. Membrane Properties
- (iii)
- Low surface free energy. Surface-free energy can provide useful information about wetting potential of the feed solution with surface tension.
- (iv)
- Low thermal conductivity. High thermal conductivities reduce vapour flux increasing heat transfer.
- (v)
- High porosity. Membrane porosity is the volume fraction of the pores of the membrane. Membranes with greater porosity have a larger surface area for evaporation. So, a membrane with high porosity has higher permeate flux and lower conductive heat loss. Nevertheless, high-porosity membranes tend to break because of their low mechanical resistance. This results in a loss of membrane performance [52].
4. Advances on MD Membrane Materials
4.1. Polymeric Membranes
4.2. Ceramic Membranes
4.3. Omniphobic Ceramic Membranes
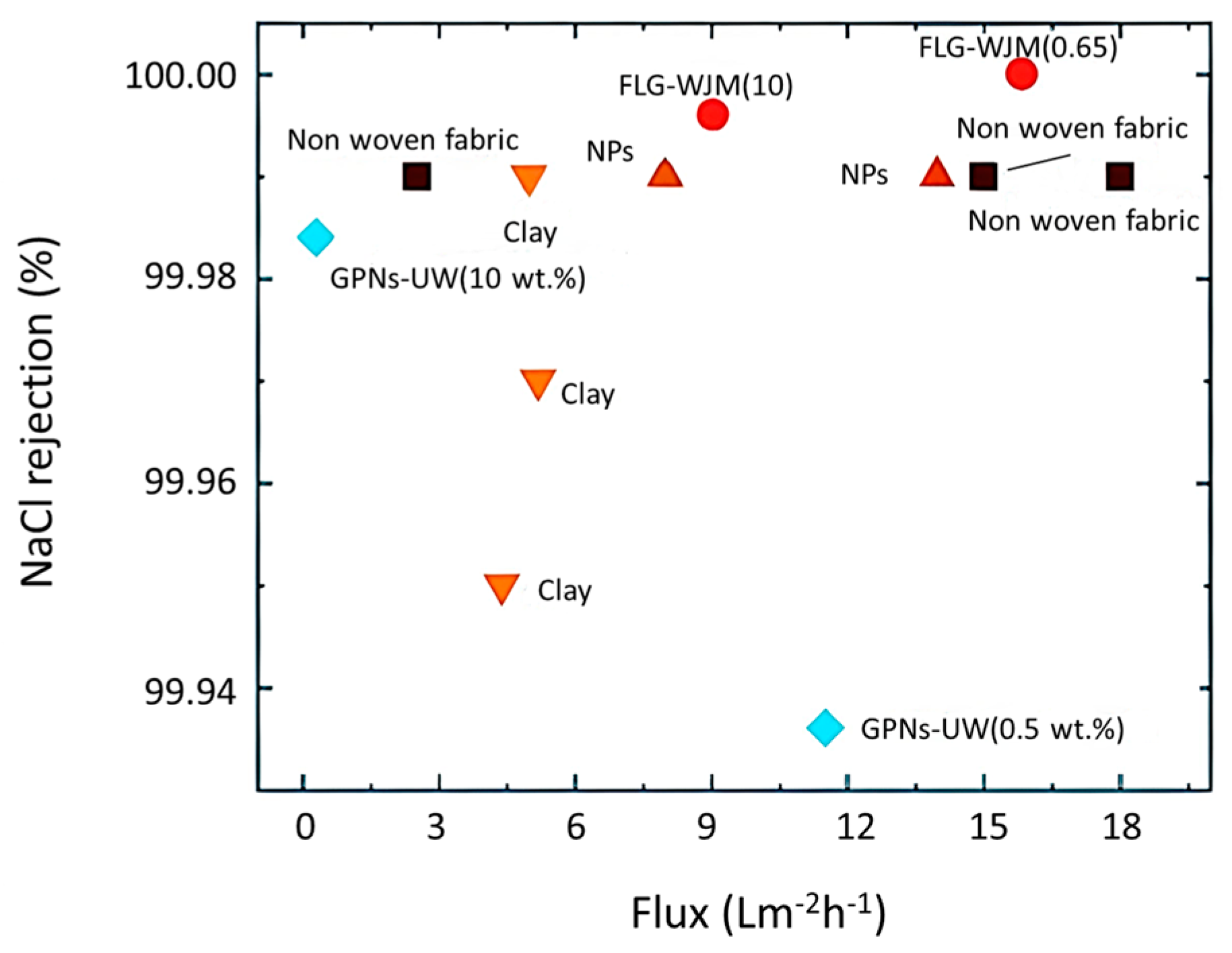
4.4. New Materials
4.4.1. Graphene
4.4.2. Two-Dimensional Materials beyond Graphene
4.5. Carbon Nanotubes
4.6. Hydrophilic/Hydrophobic Membranes
5. Photothermal Membrane for Membrane Distillation
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drioli, E.; Curcio, E. Membrane engineering for process intensification: A perspective. J. Chem. Technol. Biotechnol. 2007, 82, 223–227. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and new perspectives on integrated membrane operations for sustainable industrial growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Lado, J.J.; Cartolano, V.; García-Quismondo, E.; García, G.; Almonacid, I.; Senatore, V.; Naddeo, V.; Palma, J.; Anderson, M.A. Performance analysis of a capacitive deionization stack for brackish water desalination. Desalination 2021, 501, 114912. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ooi, B.S.; Ahmad, A.L.; Leo, C.P.; Low, S.C. Vacuum membrane distillation for desalination: Scaling phenomena of brackish water at elevated temperature. Sep. Purif. Technol. 2021, 254, 117572. [Google Scholar] [CrossRef]
- Tufa, R.A.; Noviello, Y.; Di Profio, G.; Macedonio, F.; Ali, A.; Drioli, E.; Fontananova, E.; Bouzek, K.; Curcio, E. Integrated membrane distillation-reverse electrodialysis system for energy-efficient seawater desalination. Appl. Energy 2019, 253, 113551. [Google Scholar] [CrossRef]
- Arola, K.; Van der Bruggen, B.; Mänttäri, M.; Kallioinen, M. Treatment options for nanofiltration and reverse osmosis concentrates from municipal wastewater treatment: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2049–2116. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Maltos, R.A.; Regnery, J.; Almaraz, N.; Fox, S.; Schutter, M.; Cath, T.J.; Veres, M.; Coday, B.D.; Cath, T.Y. Produced water impact on membrane integrity during extended pilot testing of forward osmosis–reverse osmosis treatment. Desalination 2018, 440, 99–110. [Google Scholar] [CrossRef]
- Zou, L.; Gusnawan, P.; Zhang, G.; Yu, J. Novel Janus composite hollow fiber membrane-based direct contact membrane distillation (DCMD) process for produced water desalination. J. Membr. Sci. 2020, 597, 117756. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Drioli, E.; Macedonio, F. Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment. Desalination 2018, 434, 161–168. [Google Scholar] [CrossRef]
- Chen, G.Q.; Gras, S.L.; Kentish, S.E. The application of forward osmosis to dairy processing. Sep. Purif. Technol. 2020, 246, 116900. [Google Scholar] [CrossRef]
- Hausmann, A.; Sanciolo, P.; Vasiljevic, T.; Ponnampalam, E.; Quispe-Chavez, N.; Weeks, M.; Duke, M. Direct contact membrane distillation of dairy process streams. Membranes 2011, 1, 48–58. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. A comprehensive review of membrane distillation and osmotic distillation in agro-food applications. J. Membr. Sci. Res. 2020, 6, 304–318. [Google Scholar]
- Destani, F.; Naccarato, A.; Tagarelli, A.; Cassano, A. Recovery of aromatics from orange juice evaporator condensate streams by reverse osmosis. Membranes 2020, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, J.; Guillen-Burrieza, E.; Arafat, H.A.; Kurzawa, M.; Wolan, A.; Kujawski, W. Raw juice concentration by osmotic membrane distillation process with hydrophobic polymeric membranes. Food Bioprocess Technol. 2015, 8, 2146–2158. [Google Scholar] [CrossRef]
- Julian, H.; Yaohanny, F.; Devina, A.; Purwadi, R.; Wenten, I.G. Apple juice concentration using submerged direct contact membrane distillation (SDCMD). J. Food Eng. 2020, 272, 109807. [Google Scholar] [CrossRef]
- Fortunato, L.; Elcik, H.; Blankert, B.; Ghaffour, N.; Vrouwenvelder, J. Textile dye wastewater treatment by direct contact membrane distillation: Membrane performance and detailed fouling analysis. J. Membr. Sci. 2021, 636, 119552. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Gad-Allah, T.A.; Gorgojo, P. Air-gap membrane distillation as a one-step process for textile wastewater treatment. Chem. Eng. J. 2019, 360, 1330–1340. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Porter, C.J.; Cheng, W.; Zhang, X.; Wang, L.; Elimelech, M. Concentration and recovery of dyes from textile wastewater using a self-standing, support-free forward osmosis membrane. Environ. Sci. Technol. 2019, 53, 3078–3086. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Urea recovery from fresh human urine by forward osmosis and membrane distillation (FO–MD). Environ. Sci. Water Res. Technol. 2020, 5, 1993–2003. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, H.; Qu, F.; Zhang, H.; Rong, H.; Yu, H.; Liang, H.; Ding, A.; Li, G.; Van der Bruggen, B. Application of membrane distillation to anaerobic digestion effluent treatment: Identifying culprits of membrane fouling and scaling. Sci. Total Environ. 2019, 688, 880–889. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, P.; Labhasetwar, P.K.; Shahi, V.K. CNT functionalized ZIF-8 impregnated poly (vinylidene fluoride-co-hexafluoropropylene) mixed matrix membranes for antibiotics removal from pharmaceutical industry wastewater by vacuum membrane distillation. J. Environ. Chem. Eng. 2021, 9, 106560. [Google Scholar] [CrossRef]
- Woldemariam, D.; Kullab, A.; Fortkamp, U.; Magner, J.; Royen, H.; Martin, A. Membrane distillation pilot plant trials with pharmaceutical residues and energy demand analysis. Chem. Eng. J. 2016, 306, 471–483. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Novel hydrophobic PVDF membranes prepared by nonsolvent induced phase separation for membrane distillation. J. Membr. Sci. 2020, 596, 117575. [Google Scholar] [CrossRef]
- Liu, C.; Dong, G.; Tsuru, T.; Matsuyama, H. Organic solvent reverse osmosis membranes for organic liquid mixture separation: A review. J. Membr. Sci. 2021, 620, 118882. [Google Scholar] [CrossRef]
- Boo, C.; Lee, J.; Elimelech, M. Omniphobic polyvinylidene fluoride (PVDF) membrane for desalination of shale gas produced water by membrane distillation. Environ. Sci. Technol. 2016, 50, 12275–12282. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. (Eds.) Comprehensive Membrane Science and Engineering; Newnes: Boston, MA, USA, 2010; Volume 1. [Google Scholar]
- Sun, A.C.; Kosar, W.; Zhang, Y.; Feng, X. Vacuum membrane distillation for desalination of water using hollow fiber membranes. J. Membr. Sci. 2014, 455, 131–142. [Google Scholar] [CrossRef]
- Donato, L.; Garofalo, A.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Improved performance of vacuum membrane distillation in desalination with zeolite membranes. Sep. Purif. Technol. 2020, 237, 116376. [Google Scholar] [CrossRef]
- Gopi, G.; Arthanareeswaran, G.; Ismail, A.F. Perspective of renewable desalination by using membrane distillation. Chem. Eng. Res. Des. 2019, 144, 520–537. [Google Scholar]
- Ali, A. Evaluation of Membrane Characteristics and Thermal Polarization in Membrane Distillation. Ph.D. Thesis, Université Paul Sabatier-Toulouse III, Toulouse, France, 2015. [Google Scholar]
- Chafidz, A.; Al-Zahrani, S.; Al-Otaibi, M.N.; Hoong, C.F.; Lai, T.F.; Prabu, M. Portable and integrated solar-driven desalination system using membrane distillation for arid remote areas in Saudi Arabia. Desalination 2014, 345, 36–49. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Zhang, C.; Hong, S.; Shi, L.; Chang, J.; Li, R.; Jin, Y.; Ong, C.; Zhuo, S.; et al. Simultaneous production of fresh water and electricity via multistage solar photovoltaic membrane distillation. Nat. Commun. 2019, 10, 3012. [Google Scholar] [CrossRef] [PubMed]
- Khayet, M. Treatment of radioactive wastewater solutions by direct contact membrane distillation using surface modified membranes. Desalination 2013, 321, 60–66. [Google Scholar] [CrossRef]
- Liu, C.; Martin, A.R. The use of membrane distillation in hugh-purity water production for the semiconductor industry. Ultrapure Water 2006, 23, 32–38. [Google Scholar]
- Parani, S.; Oluwafemi, O.S. Membrane distillation: Recent configurations, membrane surface engineering, and applications. Membranes 2021, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzza, A.; Basile, A. Membrane contactors: Fundamentals, membrane materials and key operations. In Handbook of Membrane Reactors; Woodhead Publishing: Sawston, UK, 2013; pp. 54–106. [Google Scholar]
- Noamani, S.; Niroomand, S.; Rastgar, M.; Azhdarzadeh, M.; Sadrzadeh, M. Modeling of Air-Gap Membrane Distillation and Comparative Study with Direct Contact Membrane Distillation. Ind. Eng. Chem. Res. 2020, 59, 21930–21947. [Google Scholar] [CrossRef]
- Ansari, A.; Galogahi, F.M.; Thiel, D.V.; Helfer, F.; Millar, G.; Soukane, S.; Ghaffour, N. Downstream variations of air-gap membrane distillation and comparative study with direct contact membrane distillation: A modelling approach. Desalination 2022, 526, 115539. [Google Scholar] [CrossRef]
- Yang, C.; Peng, X.; Zhao, Y.; Wang, X.; Cheng, L.; Wang, F.; Li, Y.; Li, P. Experimental study on VMD and its performance comparison with AGMD for treating copper-containing solution. Chem. Eng. Sci. 2019, 207, 876–891. [Google Scholar] [CrossRef]
- Kalla, S.; Upadhyaya, S.; Singh, K. Principles and advancements of air gap membrane distillation. Rev. Chem. Eng. 2019, 35, 817–859. [Google Scholar] [CrossRef]
- Xie, Z.; Duong, T.; Hoang, M.; Nguyen, C.; Bolto, B. Ammonia removal by sweep gas membrane distillation. Water Res. 2009, 43, 1693–1699. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Membrane distillation—Principles, applications, configurations, design, and implementation. In Emerging Technologies for Sustainable Desalination Handbook; Butterworth-Heinemann: Oxford, UK, 2018; pp. 55–106. [Google Scholar]
- Alhathal Alanezi, A.; Abdallah, H.; El-Zanati, E.; Ahmad, A.; Sharif, A.O. Performance investigation of O-ring vacuum membrane distillation module for water desalination. J. Chem. 2016, 2016, 9378460. [Google Scholar] [CrossRef]
- Criscuoli, A.; Bafaro, P.; Drioli, E. Vacuum membrane distillation for purifying waters containing arsenic. Desalination 2013, 323, 17–21. [Google Scholar] [CrossRef]
- Kaleekkal, N.J.; Mural, P.K.S.; Vigneswaran, S.; Ghosh, U. (Eds.) . Sustainable Technologies for Water and Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Jansen, A.E.; Assink, J.W.; Hanemaaijer, J.H.; Van Medevoort, J.; Van Sonsbeek, E. Development and pilot testing of full-scale membrane distillation modules for deployment of waste heat. Desalination 2013, 323, 55–65. [Google Scholar] [CrossRef]
- Zhani, K.; Zarzoum, K.; Ben Bacha, H.; Koschikowski, J.; Pfeifle, D. Autonomous solar powered membrane distillation systems: State of the art. Desalination Water Treat. 2016, 57, 23038–23051. [Google Scholar] [CrossRef]
- Guillén-Burrieza, E.; Blanco, J.; Zaragoza, G.; Alarcón, D.C.; Palenzuela, P.; Ibarra, M.; Gernjak, W. Experimental analysis of an air gap membrane distillation solar desalination pilot system. J. Membr. Sci. 2011, 379, 386–396. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.D.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Köhler, W.; Wiegand, S. (Eds.) . Thermal Nonequilibrium Phenomena in Fluid Mixtures; Springer: Cham, Switzerland, 2008; Volume 584. [Google Scholar]
- Würger, A. Is Soret equilibrium a non-equilibrium effect? C. R. Mécanique 2013, 341, 438–448. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination Using Membrane Distillation: Experimental Studies on Full Scale Spiral Wound Modules. J. Membr. Sci. 2011, 370, 104–112. [Google Scholar] [CrossRef]
- Ali, A.; Macedonio, F.; Drioli, E.; Aljlil, S.; Alharbi, O.A. Experimental and Theoretical Evaluation of Temperature Polarization Phenomenon in Direct Contact Membrane Distillation. Chem. Eng. Res. Des. 2013, 91, 1966–1977. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane Distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; Wiley: New York, NY, USA, 1960. [Google Scholar]
- Mason, E.A.; Malinauskas, A.P. Gas Transport in Porous Media: The Dusty-Gas Model; Elsevier: New York, NY, USA, 1983. [Google Scholar]
- Al-Anezi, A.A.H.; Sharif, A.O.; Sanduk, M.I.; Khan, A.R. Experimental investigation of heat and mass transfer in tubular membrane distillation module for desalination. Int. Sch. Res. Notices 2012, 2012, 738731. [Google Scholar] [CrossRef][Green Version]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.; Loh, C.H.; Permogorov, N.; Wang, R. Surfactant effects on water recovery from produced water via direct-contact membrane distillation. J. Membr. Sci. 2017, 528, 126–134. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, R.; Shi, B.; Li, G.; Wu, Y. Factors affecting pore structure and performance of poly (vinylidene fluoride-co-hexafluoro propylene) asymmetric porous membrane. J. Membr. Sci. 2006, 277, 55–64. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Li, N.N.; Fane, A.G.; Ho, W.W.; Matsuura, T. (Eds.) . Advanced Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Drioli, E.; Giorno, L.; Fontananova, E. (Eds.) Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; Di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Kim, J.F.; Jung, J.T.; Wang, H.H.; Lee, S.Y.; Moore, T.; Sanguineti, A.; Drioli, E.; Lee, Y.M. Microporous PVDF membranes via thermally induced phase separation (TIPS) and stretching methods. J. Membr. Sci. 2016, 509, 94–104. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the Production and Modification of PVDF Membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, J.; Lee, S.; Kim, S.; Kyong, H. Recent Progress of Membrane Distillation Using Electrospun Nanofibrous Membrane. J. Membr. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Drioli, E. New performance of hydrophobic fluorinated porous membranes exhibiting particulate-like morphology. Desalination 2009, 240, 14. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F.; Veerasamy, D. Membrane distillation technology for treatment of wastewater from rubber industry in Malaysia. Procedia CIRP 2015, 26, 792–796. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F. Dye wastewater treatment by direct contact membrane distillation using polyvinylidene fluoride hollow fiber membranes. J. Polym. Eng. 2015, 35, 471–479. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Ragunath, S.; Roy, S.; Mitra, S. Carbon nanotube immobilized membrane with controlled nanotube incorporation via phase inversion polymerization for membrane distillation based desalination. Sep. Purif. Technol. 2018, 194, 249–255. [Google Scholar] [CrossRef]
- Liu, G.; Pan, J.; Xu, X.; Wang, Z.; Cui, Z. Preparation of ECTFE porous membrane with a green diluent TOTM and performance in VMD process. J. Membr. Sci. 2020, 612, 118375. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Drioli, E. PVDF and HYFLON AD Membranes: Ideal Interfaces for Contactor Applications. J. Membr. Sci. 2007, 300, 51–62. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Y.; Li, X.; Wang, X.; Drioli, E.; Wang, Z.; Zhao, S. Optimization of novel composite membranes for water and mineral recovery by vacuum membrane distillation. Desalination 2018, 440, 39–47. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Tai, Z.S.; Othman, M.H.D.; Harun, Z.; Jamalludin, M.R.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Hydrophobic ceramic membrane for membrane distillation: A mini review on preparation, characterization, and applications. Sep. Purif. Technol. 2019, 217, 71–84. [Google Scholar] [CrossRef]
- Cerneaux, S.; Strużyńska, I.; Kujawski, W.M.; Persin, M.; Larbot, A. Comparison of various membrane distillation methods for desalination using hydrophobic ceramic membranes. J. Membr. Sci. 2009, 337, 55–60. [Google Scholar] [CrossRef]
- Ramlow, H.; Ferreira, R.K.M.; Marangoni, C.; Machado, R.A.F. Ceramic membranes applied to membrane distillation: A comprehensive review. Int. J. Appl. Ceram. Technol. 2019, 16, 2161–2172. [Google Scholar] [CrossRef]
- Larbot, A.; Gazagnes, L.; Krajewski, S.; Bukowska, M.; Kujawski, W. Water desalination using ceramic membrane distillation. Desalination 2004, 168, 367–372. [Google Scholar] [CrossRef]
- Fang, H.; Gao, J.F.; Wang, H.T.; Chen, C.S. Hydrophobic porous alumina hollow fiber for water desalination via membrane distillation process. J. Membr. Sci. 2012, 403, 41–46. [Google Scholar] [CrossRef]
- Ko, C.C.; Ali, A.; Drioli, E.; Tung, K.L.; Chen, C.H.; Chen, Y.R.; Macedonio, F. Performance of ceramic membrane in vacuum membrane distillation and in vacuum membrane crystallization. Desalination 2018, 440, 48–58. [Google Scholar] [CrossRef]
- Maaskant, E.; de Wit, P.; Benes, N.E. Direct interfacial polymerization onto thin ceramic hollow fibers. J. Membr. Sci. 2018, 550, 296–301. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Effect of raw multi-wall carbon nanotubes on morphology and separation properties of polyimide membranes. Sep. Sci. Technol. 2010, 45, 2287–2297. [Google Scholar] [CrossRef]
- Chen, L.H.; Chen, Y.R.; Huang, A.; Chen, C.H.; Su, D.Y.; Hsu, C.C.; Tsai, F.Y.; Tung, K.L. Nanostructure depositions on alumina hollow fiber membranes for enhanced wetting resistance during membrane distillation. J. Membr. Sci. 2018, 564, 227–236. [Google Scholar] [CrossRef]
- Sabir, A.; Islam, A.; Shafiq, M.; Shafeeq, A.; Butt, M.T.Z.; Ahmad, N.M.; Sanaullah, K.; Jamil, T. Novel polymer matrix composite membrane doped with fumed silica particles for reverse osmosis desalination. Desalination 2015, 368, 159–170. [Google Scholar] [CrossRef]
- Camacho, L.M.; Pinion, T.A.; Olatunji, S.O. Behavior of mixed-matrix graphene oxide–polysulfone membranes in the process of direct contact membrane distillation. Sep. Purif. Technol. 2020, 240, 116645. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Bonyadi, S.; Chung, T.S. Flux enhancement in membrane distillation by fabrication of dual layer hydrophilic–hydrophobic hollow fiber membranes. J. Membr. Sci. 2007, 306, 134–146. [Google Scholar] [CrossRef]
- Dastbaz, A.; Karimi-Sabet, J.; Ahadi, H.; Amini, Y. Preparation and characterization of novel modified PVDF-HFP/GO/ODS composite hollow fiber membrane for Caspian Sea water desalination. Desalination 2017, 424, 62–73. [Google Scholar] [CrossRef]
- Gontarek, E.; Macedonio, F.; Militano, F.; Giorno, L.; Lieder, M.; Politano, A.; Drioli, E.; Gugliuzza, A. Adsorption-assisted transport of water vapour in super-hydrophobic membranes filled with multilayer graphene platelets. Nanoscale 2019, 11, 11521–11529. [Google Scholar] [CrossRef]
- Frappa, M.; Castillo, A.D.R.; Macedonio, F.; Politano, A.; Drioli, E.; Bonaccorso, F.; Pellegrini, V.; Gugliuzza, A. A few-layer graphene for advanced composite PVDF membranes dedicated to water desalination: A comparative study. Nanoscale Adv. 2020, 2, 4728–4739. [Google Scholar] [CrossRef]
- Politano, A.; Bonaccorso, F.; Del Rio Castillo, A.E.; Drioli, E.; Gugliuzza, A.; Macedonio, F.; Pellegrini, V. A Procedure to Fabricate a Nanocomposite Membrane with Bidimensional Crystals Obtained through Exfoliation of Layered Materials by Wet-Jet Milling Technique. Italian Patent IT102018000020641, 22 November 2020. [Google Scholar]
- Li, Y.X.; Cao, Y.; Wang, M.; Xu, Z.L.; Zhang, H.Z.; Liu, X.W.; Li, Z. Novel high-flux polyamide/TiO2 composite nanofiltration membranes on ceramic hollow fibre substrates. J. Membr. Sci. 2018, 565, 322–330. [Google Scholar] [CrossRef]
- Prince, J.A.; Singh, G.; Rana, D.; Matsuura, T.; Anbharasi, V.; Shanmugasundaram, T.S. Preparation and characterization of highly hydrophobic poly (vinylidene fluoride)–Clay nanocomposite nanofiber membranes (PVDF–clay NNMs) for desalination using direct contact membrane distillation. J. Membr. Sci. 2012, 397, 80–86. [Google Scholar] [CrossRef]
- Hou, D.; Dai, G.; Wang, J.; Fan, H.; Zhang, L.; Luan, Z. Preparation and characterization of PVDF/nonwoven fabric flat-sheet composite membranes for desalination through direct contact membrane distillation. Sep. Purif. Technol. 2012, 101, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, B.; Wang, Q.; Wang, S. Fabrication and characterization of superhydrophobic poly (vinylidene fluoride) membrane for direct contact membrane distillation. Desalination 2013, 324, 1–9. [Google Scholar] [CrossRef]
- Meng, B.; Liu, G.; Mao, Y.; Liang, F.; Liu, G.; Jin, W. Fabrication of surface-charged MXene membrane and its application for water desalination. J. Membr. Sci. 2021, 623, 119076. [Google Scholar] [CrossRef]
- Garofalo, A.; Carnevale, M.C.; Donato, L.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Scale-up of MFI zeolite membranes for desalination by vacuum membrane distillation. Desalination 2016, 397, 205–212. [Google Scholar] [CrossRef]
- Wu, X.Q.; Mirza, N.R.; Huang, Z.; Zhang, J.; Zheng, Y.M.; Xiang, J.; Xie, Z. Enhanced desalination performance of aluminium fumarate MOF-incorporated electrospun nanofiber membrane with bead-on-string structure for membrane distillation. Desalination 2021, 520, 115338. [Google Scholar] [CrossRef]
- Frappa, M.; Castillo, A.D.R.; Macedonio, F.; Di Luca, G.; Drioli, E.; Gugliuzza, A. Exfoliated Bi2Te3-enabled membranes for new concept water desalination: Freshwater production meets new routes. Water Res. 2021, 203, 117503. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, M.; Roy, S.; Mitra, S. Flux enhancement in direct contact membrane distillation by implementing carbon nanotube immobilized PTFE membrane. Sep. Purif. Technol. 2016, 161, 136–143. [Google Scholar] [CrossRef]
- Roy, S.; Bhadra, M.; Mitra, S. Enhanced desalination via functionalized carbon nanotube immobilized membrane in direct contact membrane distillation. Sep. Purif. Technol. 2014, 136, 58–65. [Google Scholar] [CrossRef]
- Silva, T.L.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M. Multi-walled carbon nanotube/PVDF blended membranes with sponge-and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- Dumée, L.F.; Sears, K.; Schütz, J.; Finn, N.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Characterization and evaluation of carbon nanotube Bucky-Paper membranes for direct contact membrane distillation. J. Membr. Sci. 2010, 351, 36–43. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon nanotube membranes in water treatment applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Cheng, D.Y.; Wiersma, S.J. Composite membrane for a membrane distillation system. U.S. Patent 4,419,242, 6 December 1983. [Google Scholar]
- Zuo, J.; Chung, T.S.; O’Brien, G.S.; Kosar, W. Hydrophobic/hydrophilic PVDF/Ultem® dual-layer hollow fiber membranes with enhanced mechanical properties for vacuum membrane distillation. J. Membr. Sci. 2017, 523, 103–110. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, X.; Gusnawan, P.; Zhang, G.; Yu, J. Crosslinked PVDF based hydrophilic-hydrophobic dual-layer hollow fiber membranes for direct contact membrane distillation desalination: From the seawater to oilfield produced water. J. Membr. Sci. 2021, 619, 118802. [Google Scholar] [CrossRef]
- Santoro, S.; Avci, A.H.; Politano, A.; Curcio, E. The advent of thermoplasmonic membrane distillation. Chem. Soc. Rev. 2022, 51, 6087–6125. [Google Scholar] [CrossRef] [PubMed]
- Koschikowski, J.; Wieghaus, M.; Rommel, M. Solar thermal-driven desalination plants based on membrane distillation. Desalination 2003, 156, 295–304. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Yang, H.; Chen, H. Feasibility research of potable water production via solar-heated hollow fiber membrane distillation system. Desalination 2009, 247, 403–411. [Google Scholar] [CrossRef]
- Saffarini, R.B.; Summers, E.K.; Arafat, H.A. Technical evaluation of stand-alone solar powered membrane distillation systems. Desalination 2012, 286, 332–341. [Google Scholar] [CrossRef]
- Hejazi, M.A.A.; Bamaga, O.A.; Al-Beirutty, M.H.; Gzara, L.; Abulkhair, H. Effect of intermittent operation on performance of a solar-powered membrane distillation system. Sep. Purif. Technol. 2019, 220, 300–308. [Google Scholar] [CrossRef]
- Zhang, P.; Liao, Q.; Yao, H.; Huang, Y.; Cheng, H.; Qu, L. Direct solar steam generation system for clean water production. Energy Storage Mat. 2019, 18, 429–446. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Hu, Y.; He, Y.; Yan, Y. Solar-thermal conversion and steam generation: A review. Appl. Therm. Eng. 2020, 179, 115691. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, X.; Yan, C.; Liu, Y. Review of interface solar-driven steam generation systems: High-efficiency strategies, applications and challenges. Appl. Energy 2021, 283, 116361. [Google Scholar] [CrossRef]
- Cazalilla, M.A.; Dolado, J.S.; Rubio, A.; Echenique, P.M. Plasmonic excitations in noble metals: The case of Ag. Phys. Rev. B 2000, 61, 8033. [Google Scholar] [CrossRef]
- Politano, A. Interplay of structural and temperature effects on plasmonic excitations at noble-metal interfaces. Philos. Mag. 2012, 92, 768–778. [Google Scholar] [CrossRef]
- Politano, A.; Chiarello, G. The influence of electron confinement, quantum size effects, and film morphology on the dispersion and the damping of plasmonic modes in Ag and Au thin films. Prog. Surf. Sci. 2015, 90, 144–193. [Google Scholar]
- Rodríguez-Oliveros, R.; Sánchez-Gil, J.A. Gold nanostars as thermoplasmonic nanoparticles for optical heating. Opt. Express 2012, 20, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Lyvers, D.P.; Moon, J.M.; Kildishev, A.V.; Shalaev, V.M.; Wei, A. Gold nanorod arrays as plasmonic cavity resonators. ACS Nano 2008, 2, 2569–2576. [Google Scholar] [CrossRef]
- Politano, A.; Formoso, V.; Chiarello, G. Dispersion and damping of gold surface plasmon. Plasmonics 2008, 3, 165–170. [Google Scholar] [CrossRef]
- Politano, A.; Argurio, P.; Di Profio, G.; Sanna, V.; Cupolillo, A.; Chakraborty, S.; Arafat, H.A.; Curcio, E. Photothermal membrane distillation for seawater desalination. Adv. Mater. 2017, 29, 1603504. [Google Scholar] [CrossRef]
- Susman, M.D.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Chemical deposition and stabilization of plasmonic copper nanoparticle films on transparent substrates. Chem. Mater. 2012, 24, 2501–2508. [Google Scholar] [CrossRef]
- Chong, X.; Abboud, J.; Zhang, Z. Plasmonics resonance enhanced active photothermal effects of aluminum and iron nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 2234–2240. [Google Scholar] [CrossRef]
- Amendola, V.; Saija, R.; Maragò, O.M.; Iatì, M.A. Superior plasmon absorption in iron-doped gold nanoparticles. Nanoscale 2015, 7, 8782–8792. [Google Scholar] [CrossRef]
- Kravets, V.G.; Jalil, R.; Kim, Y.J.; Ansell, D.; Aznakayeva, D.E.; Thackray, B.; Britnell, L.; Belle, B.D.; Withers, F.; Radko, I.P.; et al. Graphene-protected copper and silver plasmonics. Scientific reports 2014, 4, 1–8. [Google Scholar]
- Sarina, S.; Zhu, H.; Jaatinen, E.; Xiao, Q.; Liu, H.; Jia, J.; Chen, C.; Zhao, J. Enhancing catalytic performance of palladium in gold and palladium alloy nanoparticles for organic synthesis reactions through visible light irradiation at ambient temperatures. J. Am. Chem. Soc. 2013, 135, 5793–5801. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yang, C.; Wang, Z.; Yin, H.; Lü, X.; Huang, F.; Lin, J.; Xie, X.; Jiang, M. Effective nonmetal incorporation in black titania with enhanced solar energy utilization. Energy Environ. Sci. 2014, 7, 967–972. [Google Scholar] [CrossRef]
- Farid, M.U.; Kharraz, J.A.; An, A.K. Plasmonic titanium nitride nano-enabled membranes with high structural stability for efficient photothermal desalination. ACS Appl. Mater. Interfaces 2021, 13, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Robson, M.E.; Phelps, J.L.; Tan, J.S.; Shao, B.; Owens, G.; Xu, H. A flexible photothermal cotton-CuS nanocage-agarose aerogel towards portable solar steam generation. Nano Energy 2019, 56, 708–715. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Fu, L.; Zou, M.; Li, Z.; Cao, A.; Yuan, Q. An ultrathin flexible 2D membrane based on single-walled nanotube–MoS2 hybrid film for high-performance solar steam generation. Adv. Funct. Mater. 2018, 28, 1704505. [Google Scholar] [CrossRef]
- Ghim, D.; Wu, X.; Suazo, M.; Jun, Y.S. Achieving maximum recovery of latent heat in photothermally driven multi-layer stacked membrane distillation. Nano Energy 2021, 80, 105444. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Song, X.; Megarajan, S.K.; Jiang, H. A facile nanocomposite strategy to fabricate a rGO–MWCNT photothermal layer for efficient water evaporation. J. Mater. Chem. A 2018, 6, 963–971. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, X.; Zhu, J. Enhanced direct steam generation via a bio-inspired solar heating method using carbon nanotube films. Powder Technol. 2017, 321, 276–285. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Pedersen, S.; Zodrow, K.R.; Hogan, N.J.; Neumann, O.; Wu, J.; Wang, T.; Deshmukh, A.; Elimelech, M.; et al. Nanophotonics-enabled solar membrane distillation for off-grid water purification. Proc. Natl. Acad. Sci. USA 2017, 114, 6936–6941. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Zuo, K.; Chen, L.; Yuan, L.; Liang, J.; Li, Q.; Ajayan, P.M.; Zhao, Y.; Lou, J. Bio-derived ultrathin membrane for solar driven water purification. Nano Energy 2019, 60, 567–575. [Google Scholar] [CrossRef]
- Jiang, B.P.; Zhang, L.; Zhu, Y.; Shen, X.C.; Ji, S.C.; Tan, X.Y.; Cheng, L.; Liang, H. Water-soluble hyaluronic acid–hybridized polyaniline nanoparticles for effectively targeted photothermal therapy. J. Mater. Chem. B 2015, 3, 3767–3776. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Huang, J.; Zhang, X. One-step synthesis of iodinated polypyrrole nanoparticles for CT imaging guided photothermal therapy of tumors. Small 2018, 14, 1803101. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Li, W.; Jin, J. Bio-inspired vertically aligned polyaniline nanofiber layers enabling extremely high-efficiency solar membrane distillation for water purification. J. Mater. Chem. A 2021, 9, 10678–10684. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandro, F.; Macedonio, F.; Drioli, E. New Materials and Phenomena in Membrane Distillation. Chemistry 2023, 5, 65-84. https://doi.org/10.3390/chemistry5010006
Alessandro F, Macedonio F, Drioli E. New Materials and Phenomena in Membrane Distillation. Chemistry. 2023; 5(1):65-84. https://doi.org/10.3390/chemistry5010006
Chicago/Turabian StyleAlessandro, Francesca, Francesca Macedonio, and Enrico Drioli. 2023. "New Materials and Phenomena in Membrane Distillation" Chemistry 5, no. 1: 65-84. https://doi.org/10.3390/chemistry5010006
APA StyleAlessandro, F., Macedonio, F., & Drioli, E. (2023). New Materials and Phenomena in Membrane Distillation. Chemistry, 5(1), 65-84. https://doi.org/10.3390/chemistry5010006







