Diverse Biological Activities of 1,3,4-Thiadiazole Scaffold
Abstract
:1. Introduction
2. Pharmacological Activities
2.1. Anti-Convulsant Agents
2.2. Anti-Alzheimer Agents
2.3. Anti-Cancer and Anti-Tumor Agents
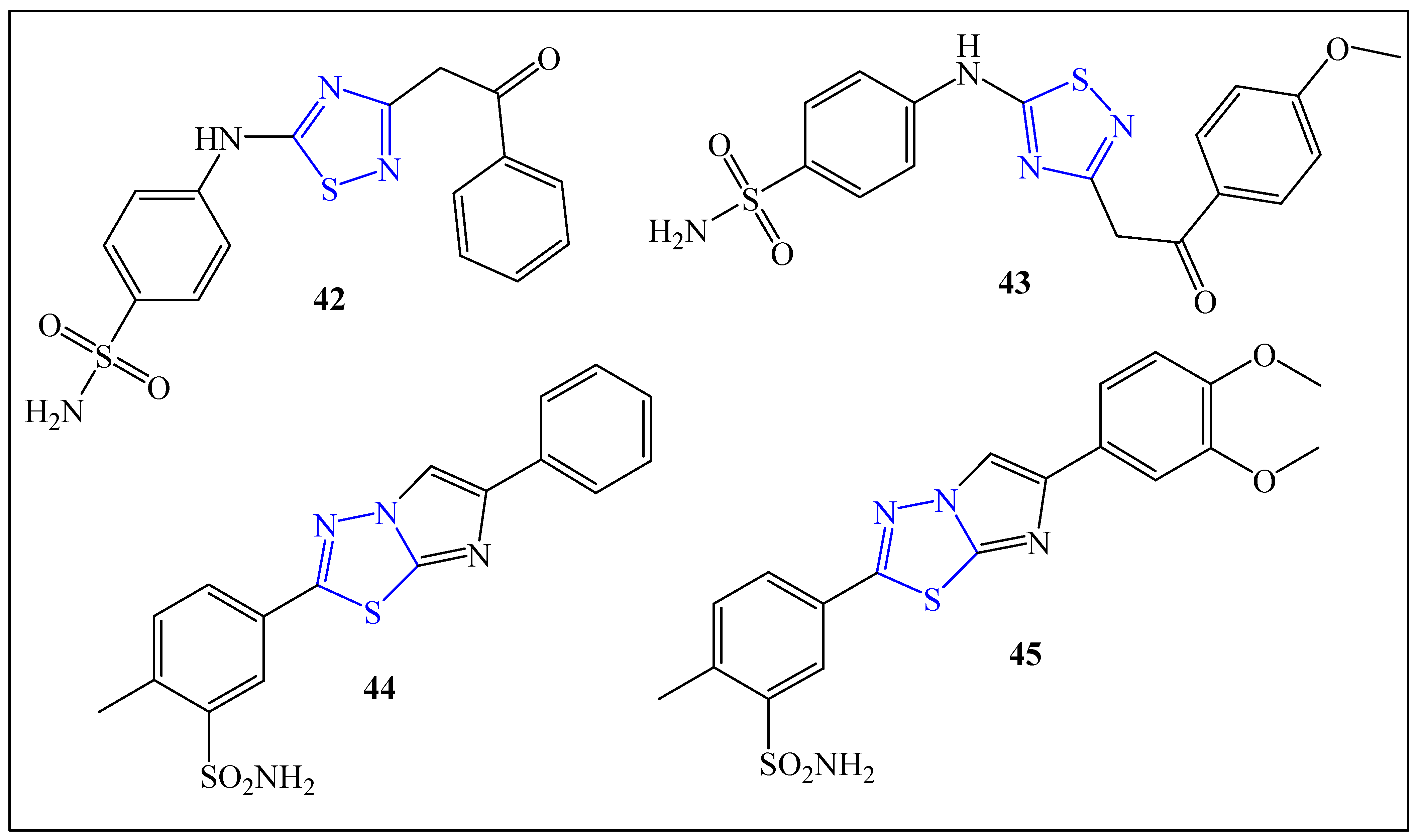
2.4. Anti-Diabetic Agents
2.5. Anti-Viral Agents
2.5.1. Treatment of SARS-CoV-2 Virus
2.5.2. Treatment of Hepatitis B Virus (HBV) and Human Immunodeficiency Virus (HIV)
2.6. Anti-Platelet Agents
2.7. Anti-Tuberculosis Agents
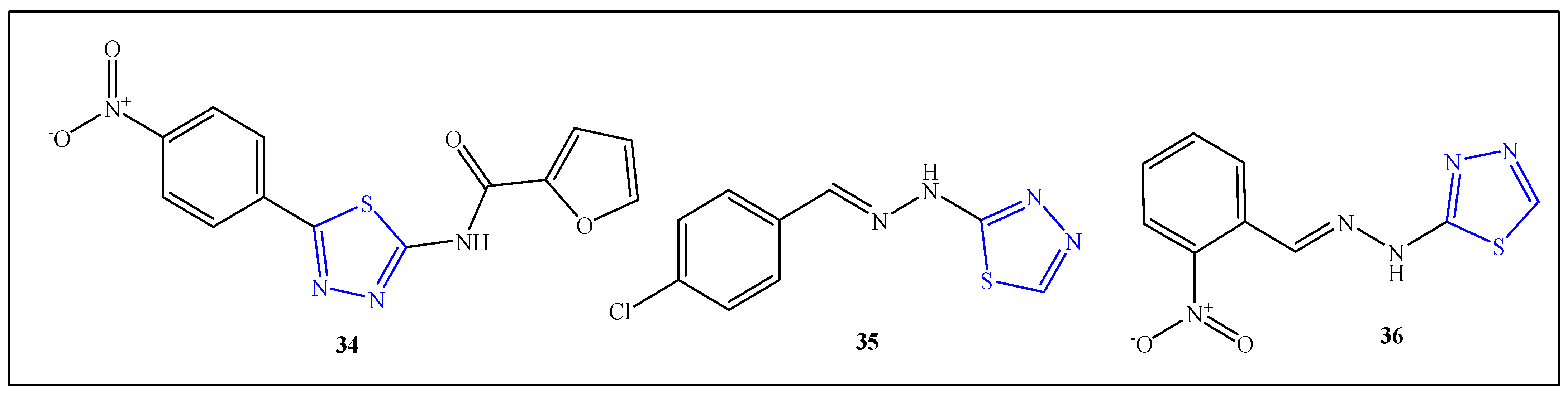
2.8. Anti-Microbial Agents
3. Others Uses
3.1. Carbonic Anhydrase Inhibitor Agents
3.2. Diuretic Agents
3.3. In the Treatment of Obesity
3.4. Insectiside and Acaraside Agents
3.5. Rodenticide Agents
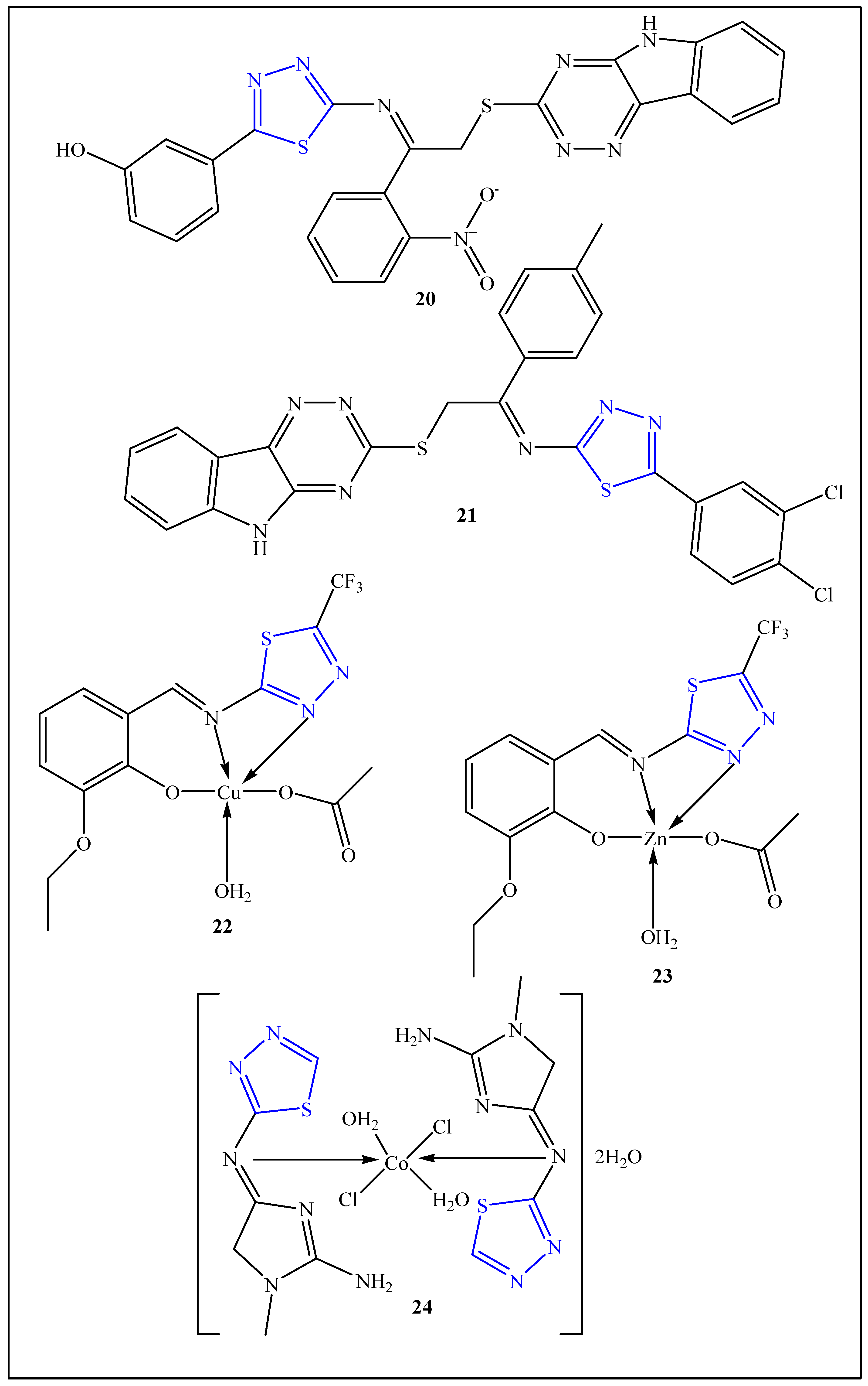
3.6. Plant Growth Stimulator Agents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serban, G.; Stanasel, O.; Serban, E.; Bota, S. 2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents. Drug Des. Dev. Ther. 2018, 31, 1545–1566. [Google Scholar] [CrossRef]
- Omar, E.M.M.A.; Wafa, A.M.O. Synthesis and in vitro antimicrobial and antifungal properties of some novel 1,3,4-thiadiazole and s-triazolo[3,4-b][1,3,4]thiadiazole derivatives. J. Heterocycl. Chem. 1986, 23, 1339–1341. [Google Scholar] [CrossRef]
- Anthwal, T.; Singh, H.; Nain, S. 1,3,4-thiadiazole scaffold: Anti-microbial agents. Pharm. Chem. J. 2022, 55, 1345–1358. [Google Scholar] [CrossRef]
- Zhang, R.; Lil, B.; Chil, C.; Liu, Y.; Liu, X.; Li, X.; Li, J. Design, synthesis, antiproliferative and antimicrobial evaluation of a new class of disulfides containing 1,3,4-thiadiazole units. Med. Chem. Res. 2022, 31, 1571–1583. [Google Scholar] [CrossRef]
- Sassiver, L.M.; Shepherd, R.G. 2-Sulfanilamido-5-methoxy-1,3,4-thiadiazole and related compounds. J. Med. Chem. 1966, 9, 541–545. [Google Scholar] [CrossRef]
- Sharma, B.; Verma, A.; Prajapati, S.; Sharma, K.U. Synthetic methods, chemistry, and the anticonvulsant activity of thiadiazoles. Int. J. Med. Chem. 2013, 2013, 348948. [Google Scholar] [CrossRef]
- Raj, V.; Rai, A.; Singh, M.; Kumar, R.; Kumar, A.; Kumar, V.; Sharma, S.K. Recent update on 1, 3, 4-thiadiazole derivatives: As anticonvulsant agents. Am. Res. J. Pharm. 2015, 1, 34–61. [Google Scholar]
- Anthwal, T.; Nain, S. 1,3,4-thiadiazole scaffold: As anti-epileptic agents. Front. Chem. 2022, 9, 671212. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, M. Thiadiazole derivatives as anticancer agents. Pharmacol. Rep. 2020, 72, 1079–1100. [Google Scholar] [CrossRef] [PubMed]
- Alireza, A. 1,3,4-thiadiazole based anticancer agents. Anti-Cancer Agents. Med. Chem. 2016, 16, 1301–1314. [Google Scholar]
- Serban, G. Synthetic compounds with 2-amino-1,3,4-thiadiazole moiety against viral infections. Molecules 2020, 25, 942. [Google Scholar] [CrossRef] [PubMed]
- Tatar, E.; Kucukguzel, S.; Karakus, S.; De Clercq, E.; Andrei, G.; Snoeck, R.; Pannecouque, C.; Oktem-okullu, S.; Unubol, N.; Kocagoz, T.; et al. Synthesis and biological evaluation of some new 1,3,4-thiadiazole and 1,2,4-triazole derivatives from L-methionine as antituberculosis and antiviral agents. Marmara. Pharm. J. 2015, 19, 88–102. [Google Scholar] [CrossRef]
- Oruc, E.E.; Rollas, S.; Kandemirli, F.; Shvets, N.; Dimoglo, S.A. 1,3,4-thiadiazole derivatives. synthesis, structure elucidation, and structure−antituberculosis activity relationship investigation. J. Med. Chem. 2004, 47, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Shkair, H.M.A.; Shakya, K.A.; Raghavendra, M.N.; Naik, R.R. Molecular modeling, synthesis and pharmacological evaluation of 1,3,4-thiadiazoles as anti-inflammatory and analgesic agents. Med. Chem. 2016, 12, 90–100. [Google Scholar] [CrossRef]
- Hafez, N.H.; Hegab, I.M.; Ahmed-Farag, S.I.; El-Gazzar, A.B.A. A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′,2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorganic Med. Chem. Lett. 2008, 18, 4538–4543. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Ghada, M.S.Z.; Mohamed, A.M.; Magda, A.A.; Thoraya, F.A. Anti-inflammatory, analgesic and anti-ulcerogenic activities of novel bis-thiadiazoles, bis-thiazoles and bis-formazanes. Med. Chem. 2017, 13, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Malik, S.; Jain, B.; Ganesh, N. Synthesis, Characterization and biological studies of Zn(II) complex of schiff base derived from 5-acetazolamido-1,3,4- thiadiazole-2- sulphonamide, a diuretic drug. Asian J. Exp. Sci. 2009, 23, 189–192. [Google Scholar]
- Datar, P.A.; Deokule, T.A. Design and synthesis of thiadiazole derivatives as antidiabetic agents. Med. Chem. 2014, 4, 390–399. [Google Scholar] [CrossRef]
- Datar, P.A.; Deokule, T.A. Development of thiadiazole as an antidiabetic agent- a review. Mini-Rev. Med. Chem. 2014, 14, 136–153. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, A.R.; Ahmed, B. Syntheses and anti-depressant activity of 5-amino-1,3,4-thiadiazole-2-thiol imines and thiobenzyl derivatives. Bioorganic Med. Chem. 2008, 16, 8029–8034. [Google Scholar] [CrossRef]
- Clerici, F.; Pocar, D.; Guido, M.; Loche, A.; Perlini, V.; Brufani, M. Synthesis of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001, 44, 931–936. [Google Scholar] [CrossRef]
- Gomha, M.S.; Edress, M.M.; Muhammad, A.Z.; Gaber, M.H.; Amin, M.M.; Matar, K.I. Synthesis under microwave irradiation and molecular docking of some novel bioactive thiadiazoles. Mini-Rev. Med. Chem. 2019, 19, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, A.; Babalouei, F. Thiadiazoles: The appropriate pharmacological scaffolds with leishmanicidal and antimalarial activities: A review. Iran. J. Basic. Med. Sci. 2017, 20, 613–622. [Google Scholar]
- Bekhit, A.A.; Hassan, M.M.A.; Abd-El-Razik, A.H.; El-Miligy, M.M.M.; El-Agroudy, J.E.; Bekhit, A.E.A. New heterocyclic hybrids of pyrazole and its bioisosteres: Design, synthesis and biological evaluation as dual acting antimalarial-antileishmanial agents. Eur. J. Med. Chem. 2015, 94, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Pei, Q.; Wang, P.; Ma, Q.; Hu, W. Optimized POCl 3 -assisted synthesis of 2-amino-1,3,4-thiadiazole/1,3,4 oxadiazole derivatives as anti-influenza agents. Arab. J. Chem. 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Tawfik, S.S.; Farahat, A.A.; El-Sayeda, A.M.; Tantawya, S.A.; Bagatob, O.; Ali, A.M. Synthesis and anti-influenza activity of novel thiadiazole, oxadiazole and triazole based scaffolds. Lett. Drug Des. Discov. 2018, 15, 363–374. [Google Scholar] [CrossRef]
- Hamadneh, A.L.; Sabbah, A.D.; Hikmat, J.S.; Al-Samad, A.L.; Hasan, M.; Al-Qirim, M.T.; Hamadneh, M.I.; Ammar, H.; Al-Dujaili, H.A. Hypolipidemic effect of novel 2,5-bis(4-hydroxybenzylidenamino)-1,3,4-thiadiazole as potentialperoxisome proliferation-activated receptor-α agonist in acute hyperlipidemic rat model. Mol. Cell. Biochem. 2019, 458, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Thomas, B.; Santhosh, N. Design, synthesis and biological evaluation of novel thiadiazole derivatives as antihyperlipidemic agents. Int. J. Pharm. Chem. Anal. 2018, 5, 16–23. [Google Scholar]
- Samel, B.A.; Pai, R.N. Synthesis of novel aryloxy propanoyl thiadiazoles as potential antihypertensive agents. J. Chin. Chem. Soc. 2010, 57, 1327–1330. [Google Scholar] [CrossRef]
- Manimaran, T.; Anand, R.M.; Jishala, M.I.; Gopalasatheeskumar, K. Review on substituted 1,3,4-thiadiazole compounds. IJPAR 2017, 6, 222–231. [Google Scholar]
- Joseph, L.; George, M.; Mathews, P. A review on various biological activities of 1,3,4-thiadiazole derivatives. J. Pharm. Chem. Biol. Sci. 2015, 3, 329–345. [Google Scholar]
- Loscher, W.; Schmidt, D. New horizons in the development of antiepileptic drugs. Epilepsy Res. 2002, 50, 3–16. [Google Scholar] [CrossRef]
- Rishipathak, D.; Patil, D.; Chikhale, H. Synthesis and biological evaluation of some newer 1h-Benzo[b][1,5]diazepin-2(3h)-One Derivatives as Potential Anticonvulsant Agents. Pharm. Sci. 2022, 28, 630–637. [Google Scholar] [CrossRef]
- Aliyu, A.; Idris, A.Y.; Musa, M.A.; Hamza, N.A.; Ahmadu, O.J.; Shehu, A.; Salisu, A. Design, synthesis and in-vivo anticonvulsant evaluation of 5-[(E)- (3,4,5-trimethoxybenzylidene) amino]-1,3,4-thiadiazole-2-thiol. IJSGS 2021, 7, 120–127. [Google Scholar]
- Nordberg, A.; Svensson, A. Cholinesterase inhibitors in the treatment of alzheimer’s disease a comparison of tolerability and pharmacology. Drug Saf. 1998, 19, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Jain, S.; Chopra, N. Hybrids of thiazolidin-4-ones and 1,3,4-thiadiazole: Synthesis and biological screening of a potential new class of acetylcholinesterase inhibitors. Biointereface Res. Appl. Chem. 2022, 12, 2800–2812. [Google Scholar]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kaminski, D.; Creaven, B.; Milos, A.; Jenkins, H.; Slusarczyk, L.; Matwijczuk, A. Design, spectroscopy, and assessment of cholinesterase inhibition and antimicrobial activities of novel coumarin–thiadiazole hybrids. Int. J. Mol. Sci. 2022, 23, 6314. [Google Scholar] [CrossRef] [PubMed]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kaminski, D.; Creaven, B.; Jenkins, H.; Radomski, P.; Milos, A.; Slusarczyk, L.; et al. Novel coumarin-thiadiazole hybrids and their Cu(II) and Zn(II) complexes as potential antimicrobial agents and acetylcholinesterase inhibitors. Int. J. Mol. Sci. 2021, 22, 9709. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Anastassova, N.; Georgieva, I.; Milanova, V.; Tzoneva, R.; Radev, K.; Denitsa, Y.; Mavrova, A. Synthesis of new triazole and thiadiazole derivatives of the n,n′-disubstituted benzimidazole-2-thione and evaluation of their antitumor potential. J. Chem. Technol. Metall. 2022, 57, 709–717. [Google Scholar]
- Karakus, S.L.K.; Ozbas, S.; Rollas, S.; Akbuga, J. Synthesis, structure elucidation and cytotoxic activities of 2,5-disubstituted-1,3,4-thiadiazole and 1,2,4-triazole-3-thione derivatives. J. Res. Pharm. 2022, 26, 941–953. [Google Scholar]
- Liu, Y.; Li, J.; Liu, X.; Li, Z.; Men, Y.; Sun, Y.; Chen, B. Design, synthesis, and screening for the antiproliferative activity of new 1,3,4-thiadiazole scaffold linked to substituted phenacyl derivatives and disulfides. J. Sulfur. Chem. 2022, 43, 426–442. [Google Scholar] [CrossRef]
- Chen, C.; Chu, H.; Wang, A.; Yin, H.; Gao, Y.; Liu, S.; Li, W.; Han, L. Discovery of 2,5-diphenyl-1,3,4-thiadiazole derivatives as HDAC inhibitors with DNA binding affinity. Eur. J. Med. Chem. 2022, 241, 114634. [Google Scholar] [CrossRef]
- Khan, A.A.; Rahim, F.; Taha, M.; Rehman, W.; Iqbal, N.; Wadood, A.; Ahmad, N.; Shah, A.A.S.; Ghoneim, M.M.; Alshehri, S.; et al. New biologically dynamic hybrid pharmacophore triazinoindole-based-thiadiazole as potent α-glucosidase inhibitors: In vitro and in silico study. Int. J. Biol. Macromol. 2022, 199, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Deswal, Y.; Asijaa, S.; Dubey, A.; Deswal, L.; Kumar, D.; Jindal, K.D.; Devi, J.J. Cobalt(II), nickel(II), copper(II) and zinc(II) complexes of thiadiazole based Schiff base ligands: Synthesis, structural characterization, DFT, antidiabetic and molecular docking studies. Mol. Struct. 2022, 1253, 132266. [Google Scholar] [CrossRef]
- Radha, P.V.; Prabakaran, M. Novel thiadiazole-derived schiff base ligand and its transition metal complexes: Thermal behaviour, theoretical study, chemo-sensor, antimicrobial, antidiabetic and anticancer activity. Appl. Organomet. Chem. 2022, 36, e6872. [Google Scholar] [CrossRef]
- Baloch, S.; Baloch, A.M.; Zheng, T.; Pei, X. The coronavirus disease 2019 (COVID-19) pandemic. TJEM 2020, 250, 271–278. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, P.S.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS. Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef]
- Rashdan, M.R.H.; Abdelmonsef, A.H. In silico study to identify novel potential thiadiazole-based molecules as anti-Covid-19 candidates by hierarchical virtual screening and molecular dynamics simulations. Struct. Chem. 2022, 33, 1727–1739. [Google Scholar] [CrossRef]
- Rashdan, M.R.H.; Abdelmonsef, A.H. Towards covid-19 TMPRSS2 enzyme inhibitors and antimicrobial agents: Synthesis, antimicrobial potency, molecular docking, and drug-likeness prediction of thiadiazole-triazole hybrids. J. Mol. Struct. 2022, 1268, 133659. [Google Scholar] [CrossRef] [PubMed]
- Lavanchy, D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral. Hepat. 2004, 11, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Klimas, N.; Koneru, O.A.; Fletcher, A.M. Overview of HIV. Psychosom. Med. 2008, 70, 523–530. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, G.; Ma, Z.; Xu, C.; Guo, Z.; Gong, P.; Xu, L. Synthesis and anti-hepatitis B virus evaluation of 7-methoxy-3-heterocyclic quinolin-6-ols. Arch. Pharm. Chem. Life Sci. 2015, 348, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Shafique, M.; Hameed, S.; Naseer, M.M.; Al-Masoudi, A.N. Synthesis of new chiral 1,3,4-thiadiazole-based di and tri-arylsulfonamide residues and evaluation of in vitro anti-HIV activity and cytotoxicity. Mol. Divers. 2018, 22, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, E.M.H.K.; Sardari, S.; Mashayekhi, V.; Zadeh, E.M.; Azerang, P.; Kobarfard, F. One pot synthesis and biological activity evaluation of novel schiff bases derived from 2-hydrazinyl-1,3,4-thiadiazole. Chem. Pharm. Bull. 2013, 61, 160–166. [Google Scholar] [CrossRef]
- Ruel, R.; L’Heureux, A.; Thibeault, C.; Daris, P.J.; Martel, A.; Price, A.L.; Wu, Q.; Hua, J.; Wexler, R.R.; Rehfuss, R.; et al. New azole antagonists with high affinity for the P2Y1 receptor. Bioorganic Med. Chem. Lett. 2013, 23, 3519–3522. [Google Scholar] [CrossRef]
- Patel, H.; Jadhav, H.; Ansari, I.; Pawara, R.; Sanjay Surana, S. Pyridine and nitro-phenyl linked 1,3,4-thiadiazoles as MDR-TB inhibitors. Eur. J. Med. Chem. 2019, 167, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.Z.; Alshaye, N.A.; Mosa, T.M.; El-Sadany, S.K.; Hamed, E.A.; El-Atawy, M.A. Synthesis and antimicrobial activity screening of piperazines bearing N,N0-bis(1,3,4-thiadiazole) moiety as probable enoyl-ACP reductase inhibitors. Molecules 2022, 27, 3698. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Abdelrahman, M.T.; Shehadi, I.A.; El-Tanany, S.S.; Hemdan, B.A. Novel thiadiazole-based molecules as promising inhibitors of black fungi and pathogenic bacteria: In vitro antimicrobial evaluation and molecular docking studies. Molecules 2022, 27, 3613. [Google Scholar] [CrossRef]
- Kamel, G.M.; Sroor, M.F.; Othman, M.A.; Hassaneen, M.H.; Abdallah, A.T.; Saleh, M.F.; Teleb, M.A.M. Synthesis and biological evaluation of new 1,3,4-thiadiazole derivatives as potent antimicrobial agents. Monatsh. Chem. 2022, 153, 929–937. [Google Scholar] [CrossRef]
- Pan, N.; Liu, C.; Wu, R.; Fei, Q.; Wu, W. Novel pyrimidine derivatives bearing a 1,3,4-thiadiazole skeleton: Design, synthesis, and antifungal activity. Front. Chem. 2022, 10, 922813. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Ram, S.; Angeli, A.; Bonardi, A.; Nocentini, A.; Gratteri, P.; Supuran, T.C.; Sharma, K.P. Novel benzenesulfonamide-bearing pyrazoles and 1,2,4-thiadiazoles as selective carbonic anhydrase inhibitors. Arch. Pharm. 2022, 355, 2100241. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Aashritha, K.; Singh, P.; Angeli, A.; Kothari, A.; Sigalapalli, K.D.; Yaddanapudi, M.V.; Supuran, T.C.; Arifuddin, M. Design and synthesis of benzenesulfonamide-linked imidazo [2,1-b][1,3,4]thiadiazole derivatives as carbonic anhydrase I and II inhibitors. Arch. Pharm. 2021, 354, 2100028. [Google Scholar] [CrossRef] [PubMed]
- Ergena, A.; Rajeshwar, Y.; Solomon, G. Synthesis and diuretic activity of substituted 1,3,4-thiadiazoles. Scientifica 2022, 2022, 3011531. [Google Scholar] [CrossRef]
- Drapak, V.; Zimenkovsky, S.B.; Slabyy, V.M.; Holota, M.S.; Perekhoda, O.L.; Yaremkevych, V.R.; Nektegayev, O.I. Synthesis and diuretic activity of novel 5-amino-1,3,4-thiadiazole2-thiol derivatives. Biopolym. Cell. 2021, 37, 33–45. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, J.M.; Song, K.; Lee, S.; Jung, E.M.; Kim, M.; Park, H.; Yoo, J.; Chang, C.; Kim, J.; et al. Methylsulfonylpyrazolyl oxadiazoles and thiadiazoles as potent, orally bioavailable cannabinoid-1 receptor antagonists for the treatment of obesity. Future Med. Chem. 2009, 1, 947–967. [Google Scholar] [CrossRef]
- Ismail, F.M.H.; Salem, S.M.; Mohamed, M.M.A.; Aly, F.A. Design, synthesis and insecticidal activity of new 1,3,4-thiadiazole and 1,3,4-thiadiazolo [3,2-a]pyrimidine derivatives under solvent-free conditions. Synth. Commun. 2021, 51, 2644–2660. [Google Scholar] [CrossRef]
- Mohamed, M.M.A.; Ismail, F.M.; Madkour, F.M.H.; Aly, F.A.; Salem, S.M. Straightforward synthesis of 2-chloro-N-(5-(cyanomethyl)-1,3,4-thiadiazol-2-yl)benzamide as a precursor for synthesis of novel heterocyclic compounds with insecticidal activity. Synth. Commun. 2020, 50, 3424–3442. [Google Scholar] [CrossRef]
- Lv, M.; Liu, G.; Jia, M.; Xu, H. Synthesis of matrinic amide derivatives containing 1,3,4-thiadiazole scaffold as insecticidal/acaricidal agents. Bioorg. Chem. 2018, 81, 88–92. [Google Scholar] [CrossRef]
- Fadda, A.A.; Salam, A.M.; Tawfik, H.E.; Anwarb, M.E.; Etmana, H.A. Synthesis and insecticidal assessment of some innovative heterocycles incorporating a thiadiazole moiety against the cotton leafworm, spodoptera littoralis. RSC. Adv. 2017, 7, 39773–39785. [Google Scholar] [CrossRef]
- Witmer, W.G. The changing role of rodenticides and their alternatives in the management of commensal rodents. Hum–Wildl. Interact. 2019, 13, 186–199. [Google Scholar]
- Witmer, G.; Horak, K.; Moulton, R.; Baldwin, A.R. New rodenticides: An update on recent research trials. In Proceedings of the 15th Wildlife Damage Management Conference, Clemson, SC, USA, 27 March 2013; pp. 79–85. [Google Scholar]
- Conole, D.; Beck, M.T.; Jay-Smith, M.; Tingle, D.M.; Eason, T.C.; Brimble, M.A.; Rennison, D. Synthesis and methemoglobinemia-inducing properties of benzocaine isosteres designed as humane rodenticides. Bioorg. Med. Chem. 2014, 22, 2220–2235. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, N.E.; Vorskanyan, A.S.; Grigoryan, A.A.; Yengoyan, A.P. Synthesis and biological evaluation of novel nonfused heterocyclic systems derivatives based on 5-(alkylthio)-1, 3, 4-thiadiazole-2(3H)-thions. J. Chem. Biol. Phys. Sci. 2016, 6, 434–444. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anthwal, T.; Paliwal, S.; Nain, S. Diverse Biological Activities of 1,3,4-Thiadiazole Scaffold. Chemistry 2022, 4, 1654-1671. https://doi.org/10.3390/chemistry4040107
Anthwal T, Paliwal S, Nain S. Diverse Biological Activities of 1,3,4-Thiadiazole Scaffold. Chemistry. 2022; 4(4):1654-1671. https://doi.org/10.3390/chemistry4040107
Chicago/Turabian StyleAnthwal, Tulika, Sarvesh Paliwal, and Sumitra Nain. 2022. "Diverse Biological Activities of 1,3,4-Thiadiazole Scaffold" Chemistry 4, no. 4: 1654-1671. https://doi.org/10.3390/chemistry4040107
APA StyleAnthwal, T., Paliwal, S., & Nain, S. (2022). Diverse Biological Activities of 1,3,4-Thiadiazole Scaffold. Chemistry, 4(4), 1654-1671. https://doi.org/10.3390/chemistry4040107




