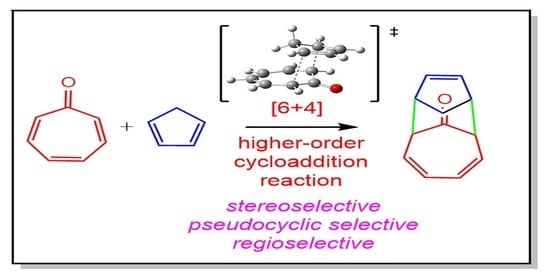
Unveiling the Chemistry of Higher-Order Cycloaddition Reactions within the Molecular Electron Density Theory
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
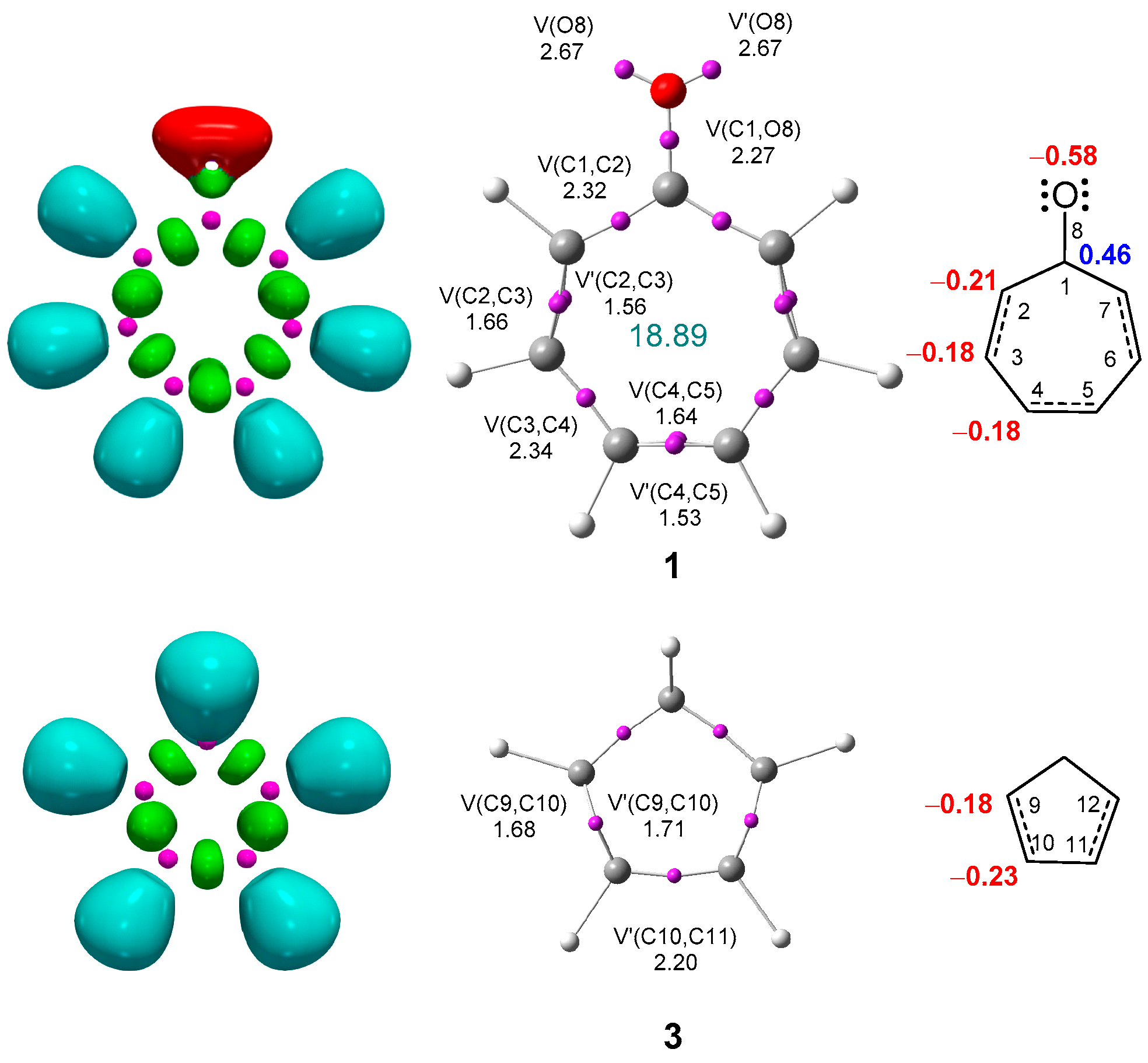
3.1. Analysis of the Ground State Electronic Structures of the Reagents
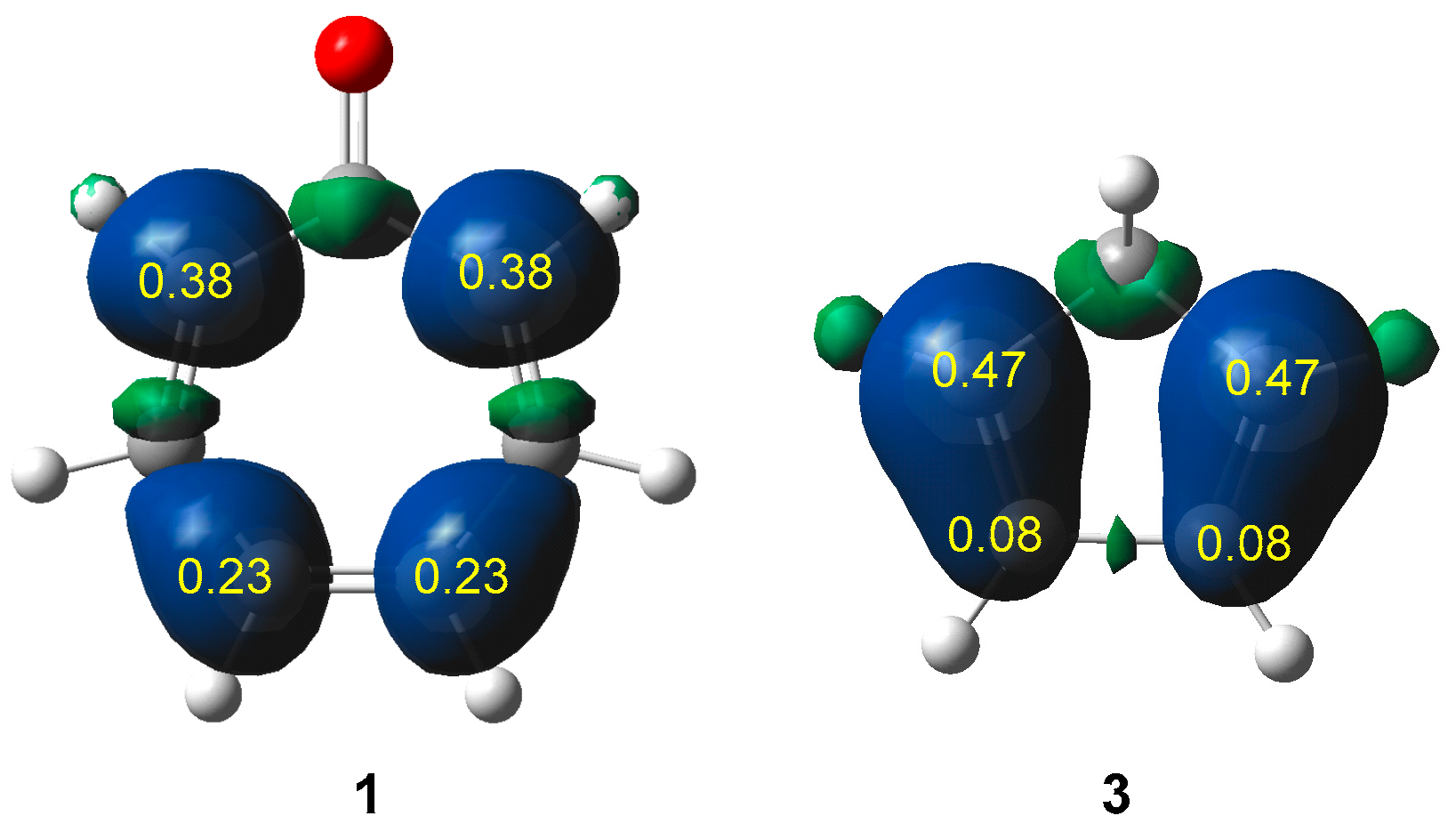
3.2. Analysis of the CDFT Reactivity Indices of the Reagents
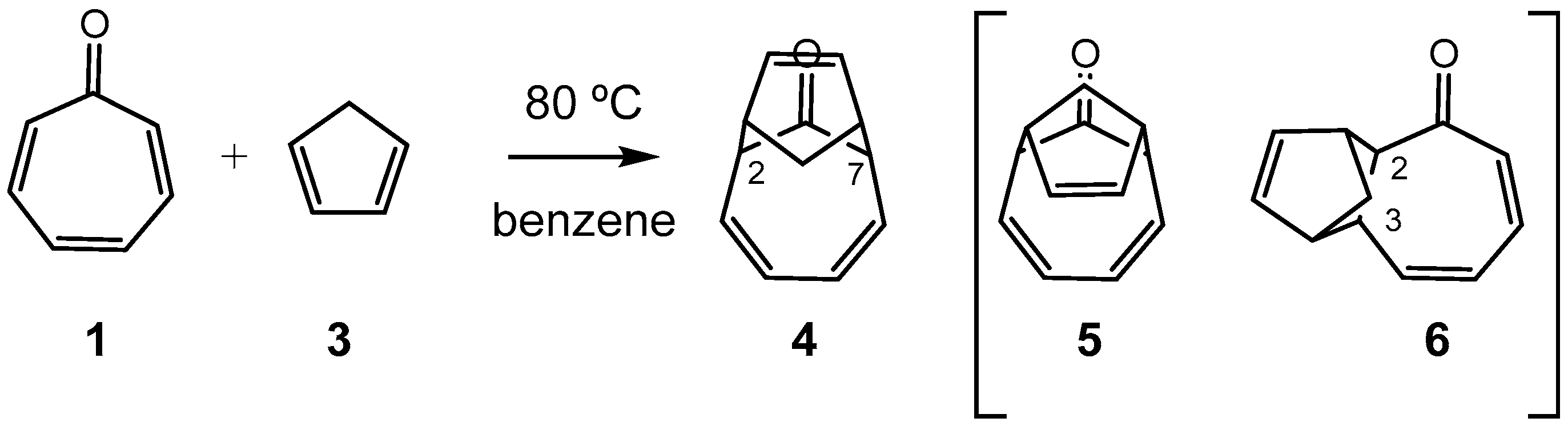
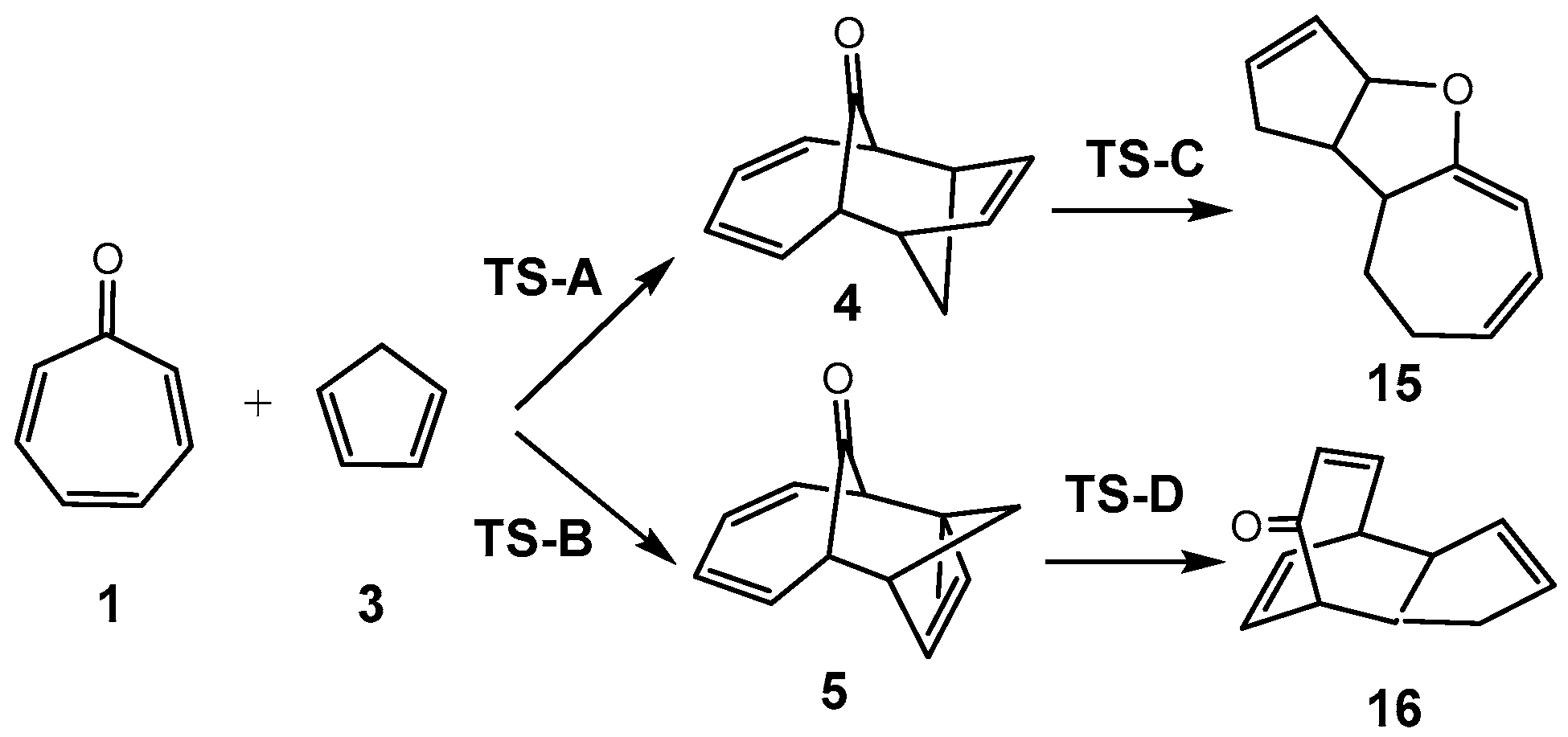
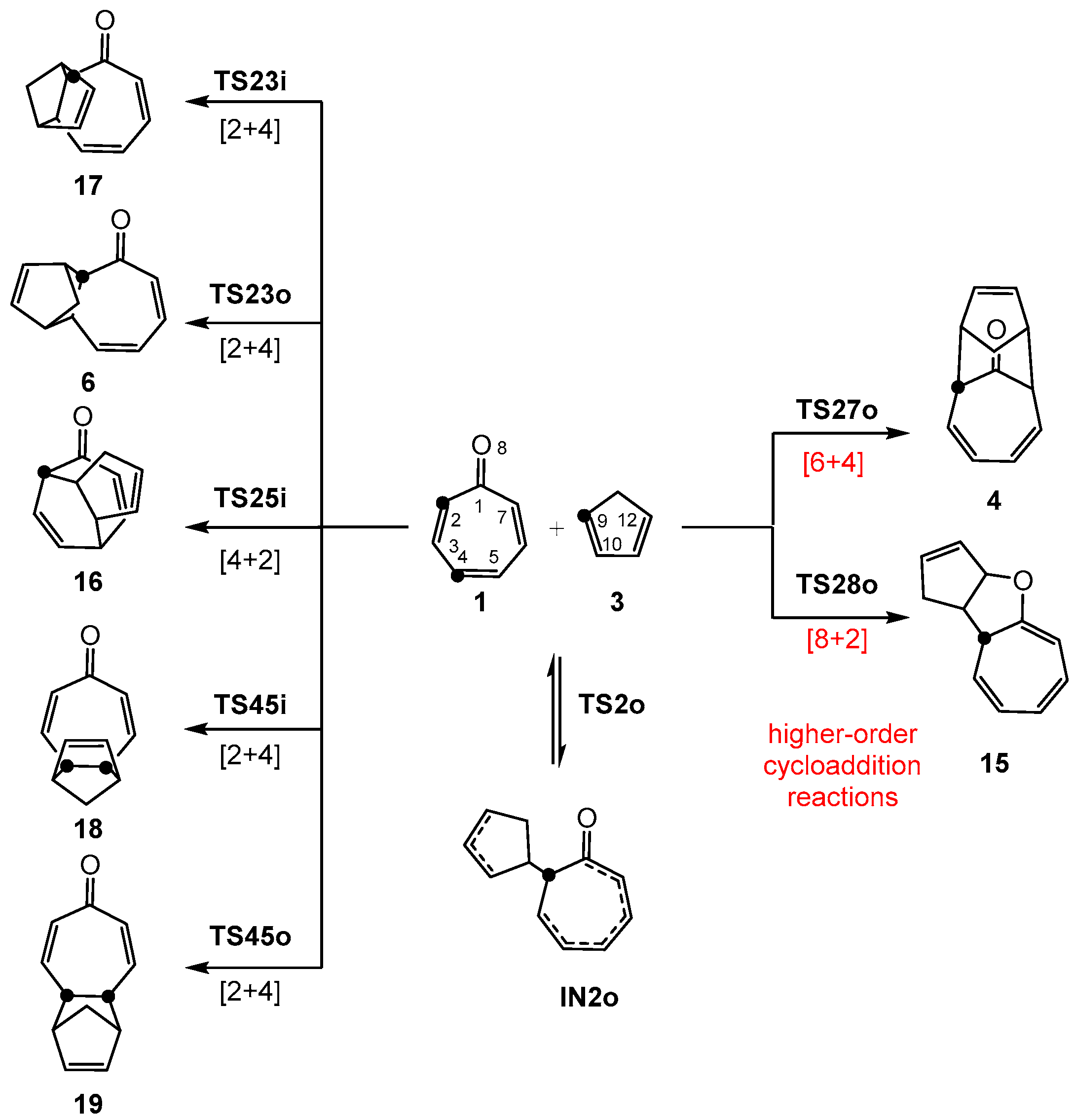
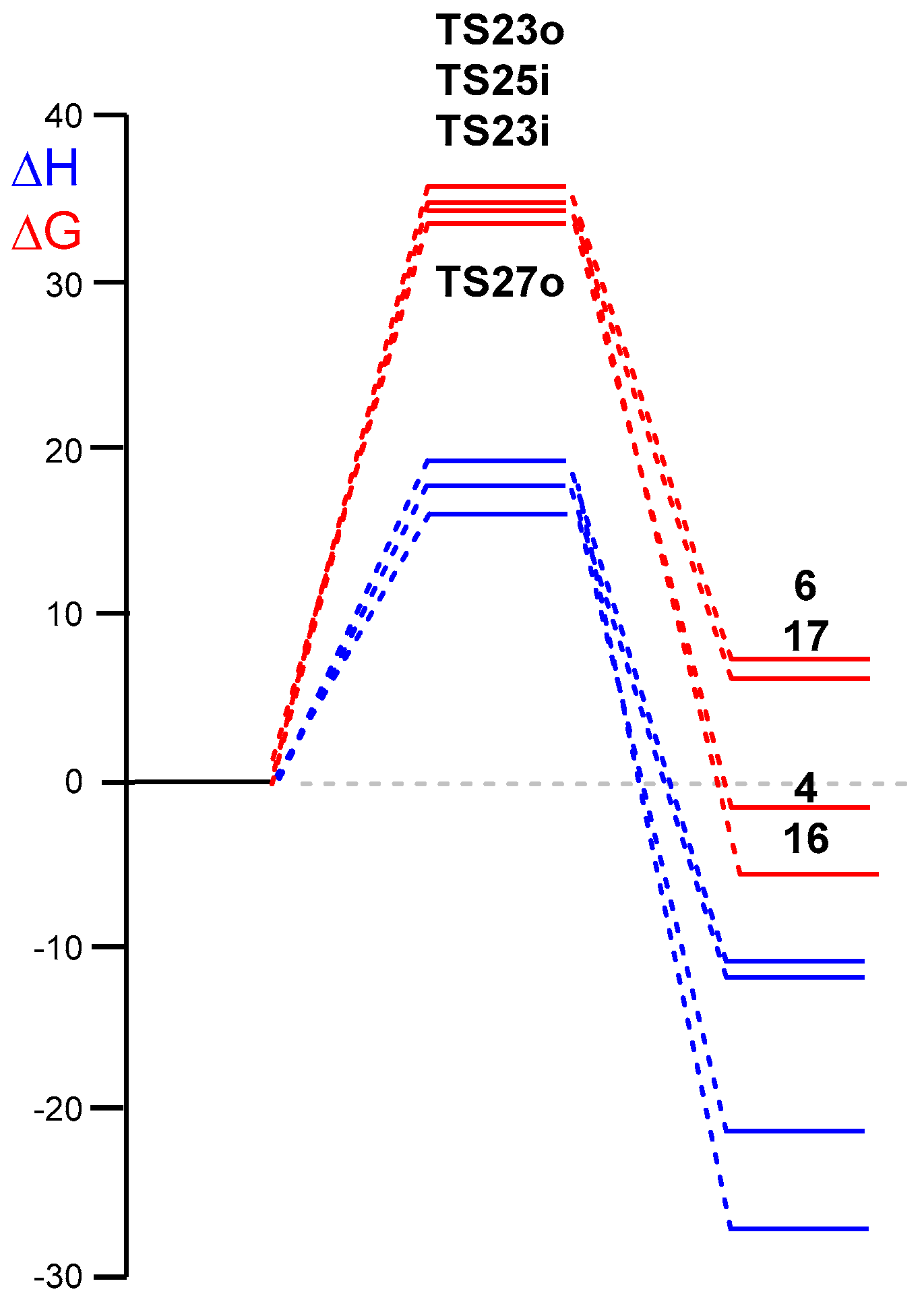
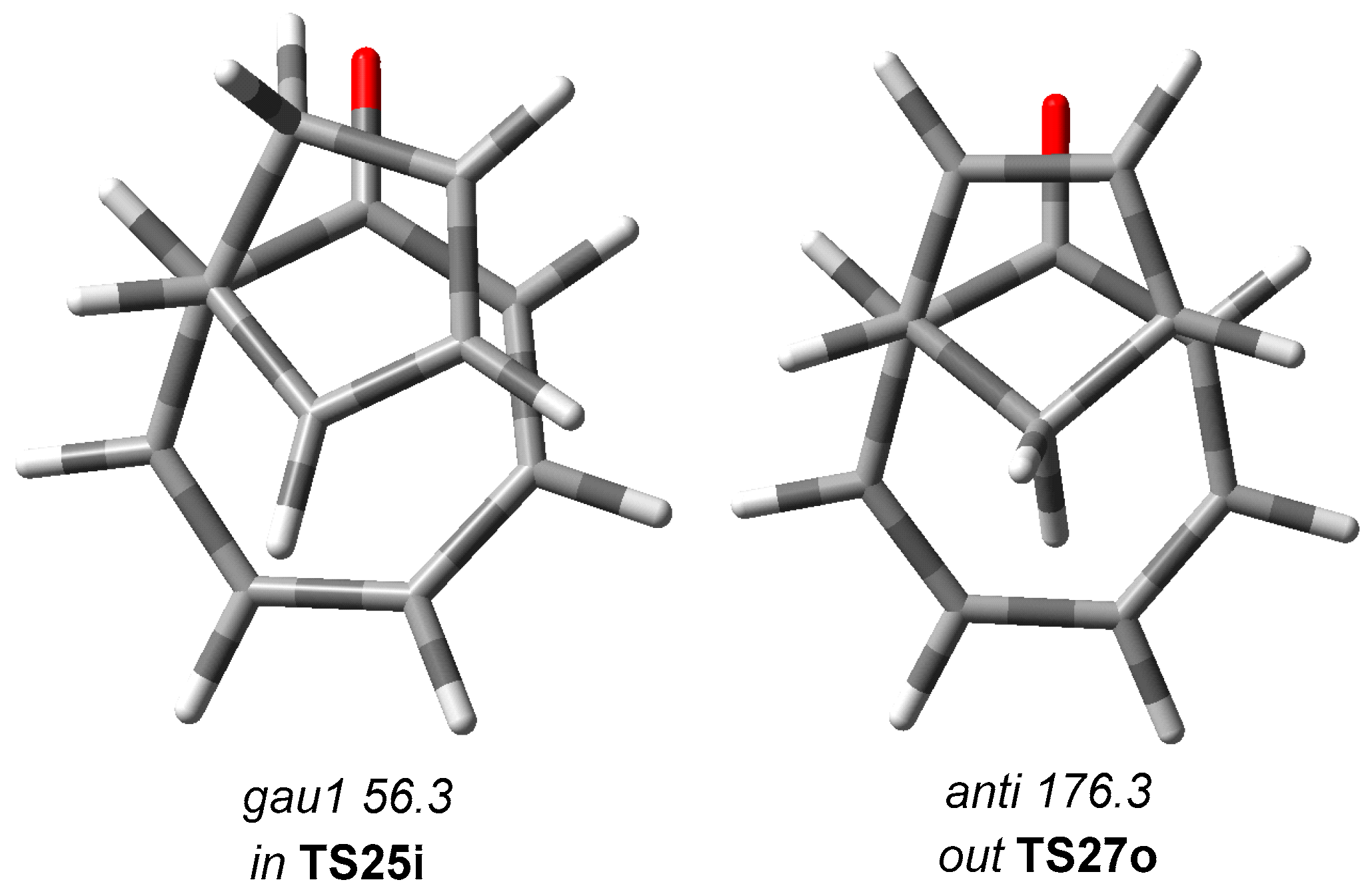
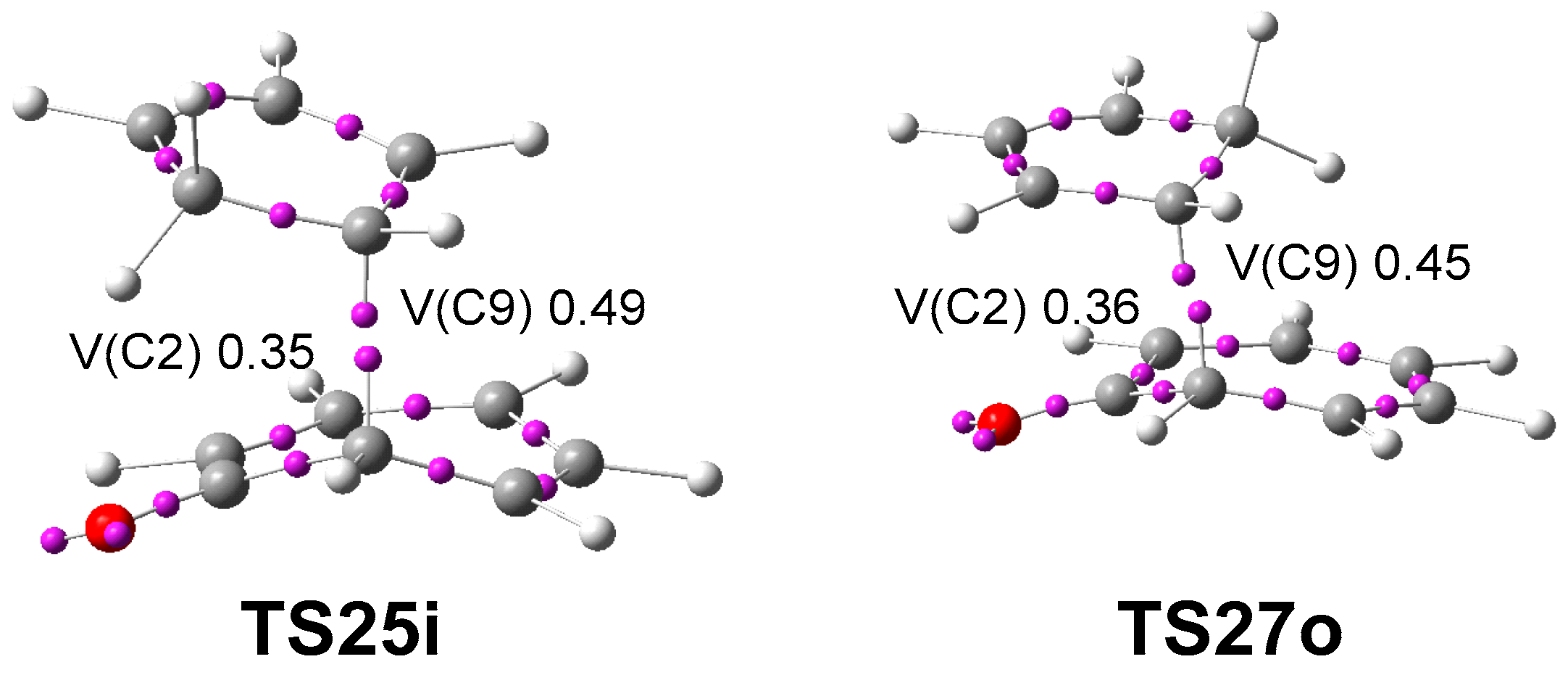
3.3. Study of the Competitive Reaction Paths Associated with the Cycloaddition Reaction of Tropone 1 with Cp 3
3.4. BET Analysis of the Most Favorable Reaction Path Associated with the HOCA Reaction of Tropone 1 with Cp 3
3.5. What Is the Origin of the Pseudocyclic Selectivity in this [6 + 4] HOCA Reaction?
3.6. Why Is It Not Possible to Form the Formal in [6 + 4] CA 5 in the HOCA Reaction of Tropone 1 with Cp 3?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carruthers, W. Some Modern Methods of Organic Synthesis, 2nd ed.; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Rigby, J.H. Higher-Order Cycloaddition Reactions in Organic Synthesis: Stereoselective Preparation of 7- to 10-Membered Ring Systems; John Wiley & Sons Inc.: Weinheim, Germany, 2008. [Google Scholar]
- Itô, S.; Fujise, Y.; Okuda, T.; Inoue, Y. Reaction of Tropone with Cyclopentadiene. Bull. Chem. Soc. Jpn. 1966, 39, 1351. [Google Scholar] [CrossRef] [Green Version]
- Cookson, R.C.; Drake, B.V.; Hudec, J.; Morrison, A. The adduct of tropone and cyclopentadiene: A new type of cyclic reaction. Chem. Commun. 1966, 1, 15. [Google Scholar] [CrossRef]
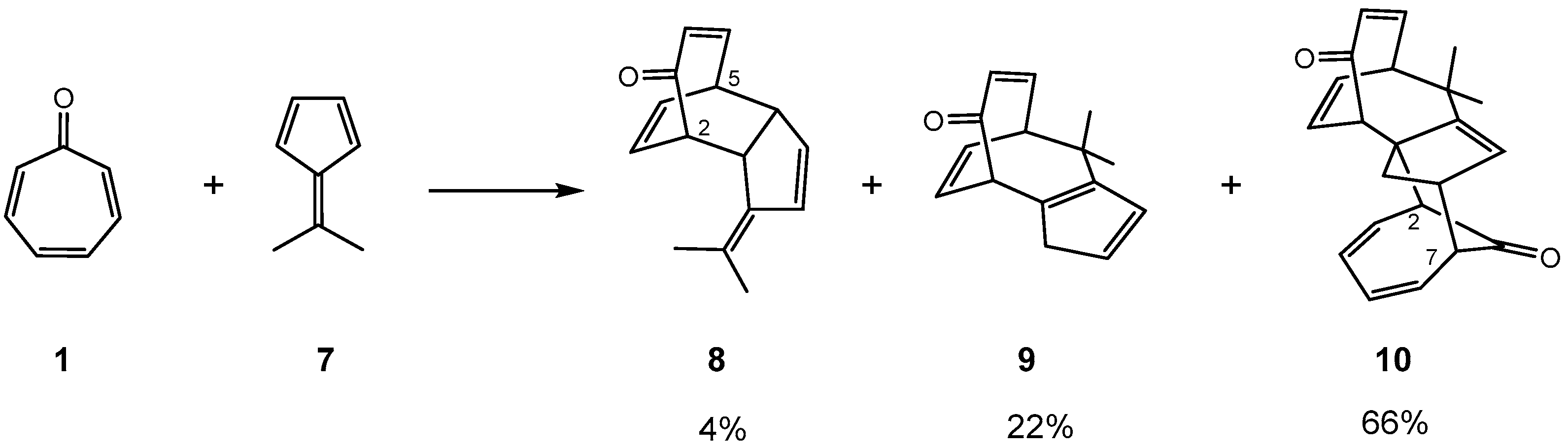
- Houk, K.N.; Luskus, L.J.; Bhacca, N.S. Novel Double [6 + 4] Cycloaddition of Tropone to Dimethylfulvene. J. Am. Chem. Soc. 1970, 92, 6392–6394. [Google Scholar] [CrossRef]
- Bhacca, N.S.; Luskus, L.J.; Houk, K.N. Elucidation of the Structure of the Double [6 + 4] Adduct of Tropone and Dimethylfulvene by Nuclear Magnetic Resonance and The Nuclear Overhauser Effect. J. Chem. Soc. D 1971, 109–111. [Google Scholar] [CrossRef]
- Yamabe, S.; Nishihara, Y.; Minato, T. Asymmetry in symmetric cycloadditions. J. Phys. Chem. A 2002, 106, 4980–4987. [Google Scholar] [CrossRef]
- Rivero, A.R.; Fernández, I.; Sierra, M.A. Regio- and Diastereoselective Stepwise [8 + 3]-Cycloaddition Reaction between Tropone Derivatives and Donor–Acceptor Cyclopropanes. Org. Lett. 2013, 15, 4928–4931. [Google Scholar] [CrossRef]
- Yu, P.; Chen, T.Q.; Yang, Z.; He, C.Q.; Patel, A.; Lam, Y.; Liu, C.-Y.; Houk, K.N. Mechanisms and Origins of Periselectivity of the Ambimodal [6 + 4] Cycloadditions of Tropone to Dimethylfulvene. J. Am. Chem. Soc. 2017, 139, 8251–8258. [Google Scholar] [CrossRef]
- Yang, L.C.; Wang, Y.N.; Liu, R. Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions. Nat. Chem. 2020, 12, 860–868. [Google Scholar] [CrossRef]
- McLeod, D.; Thøgersen, M.K.; Jessen, N.I.; Jørgensen, K.A.; Jamieson, C.S.; Xue, X.-S.; Houk, K.N.; Liu, F.; Hoffmann, R. Expanding the frontiers of higher-order cycloadditions. Acc. Chem. Res. 2019, 52, 3488–3501. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Thøgersen, M.K.; Yang, L.; Lauridsen, R.F.; Xue, X.-S.; Jørgensen, K.A.; Houk, K.N. [8 + 2] vs. [4 + 2] Cycloadditions of Cyclohexadienamines to Tropone and Heptafulvenes—Mechanisms and Selectivities. J. Am. Chem. Soc. 2021, 143, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.B.; Hoffmann, R. The Conservation of Orbital Symmetry. Angew. Chem. Int. Ed. Engl. 1969, 8, 781–853. [Google Scholar] [CrossRef]
- Fukui, K. Molecular Orbitals in Chemistry, Physics, and Biology; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Domingo, L.R. Molecular electron density theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. Atoms in Molecules. A Quantum Theory; Claredon Press: Oxford, UK, 1990. [Google Scholar]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular-systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
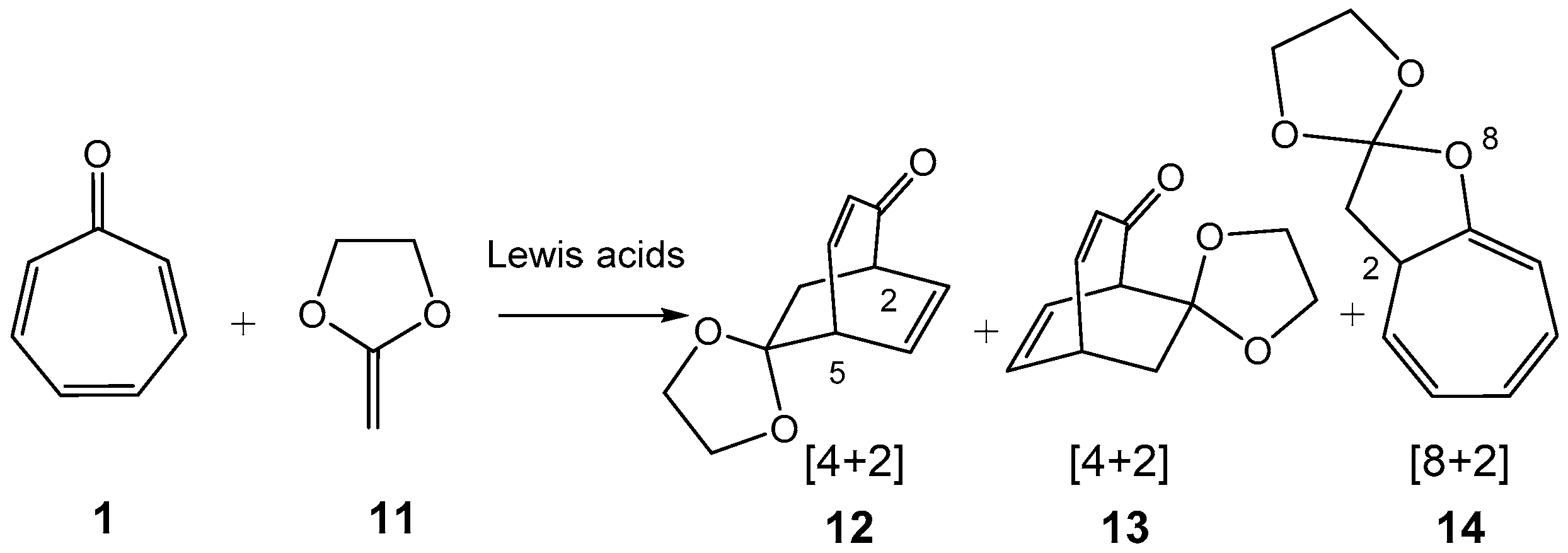
- Domingo, L.R.; Pérez, P. A Molecular Electron Density Theory Study of the Higher–Order Cycloaddition Reactions of Tropone with Electron-rich Ethylene. The Role of the Lewis Acid Catalyst in the Mechanism and Pseudocyclic Selectivity. New J. Chem. 2022, 46, 294–308. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, A. Molecular Electron Density Theory Study of the Reactivity of Tetrazines in Aza-Diels-Alder Reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef] [Green Version]
- Domingo, L.R.; Sáez, J.A.; Zaragoza, R.J.; Arnó, M. Understanding the Participation of Quadricyclane as Nucleophile in Polar [2 sigma+2 sigma+2 pi] Cycloadditions toward Electrophilic pi Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef]
- Li, P.; Yamamoto, H. Lewis acid catalyzed inverse-electron-demand Diels–Alder reaction of tropones. J. Am. Chem. Soc. 2009, 131, 16628–16629. [Google Scholar] [CrossRef] [Green Version]
- Cooper, S.; Jamieson, C.S.; Sengupta, A.; Houk, K.N. Cycloadditions of cyclopentadiene and cycloheptatriene with tropones: All Endo-[6 + 4] Cycloadditions are ambimodal. J. Am. Chem. Soc. 2021, 143, 3918–3926. [Google Scholar]
- Gleiter, R.; Bohm, M.C. Regio- and stereoselectivity in Diels-Alder reactions. Theor. Consid. Pure Appl. Chem. 1983, 55, 237–244. [Google Scholar] [CrossRef]
- Polo, V.; Andrés, J. A joint study based on the electron localization function and catastrophe theory of the chameleonic and centauric models for the Cope rearrangement of 1,5-hexadiene and its cyano derivatives. J. Comput. Chem. 2005, 26, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, E.; Santos, J.C.; Gómez, B.; Contreras, R.; Fuentealba, P. The Bonding Nature of Some Simple Sigmatropic Transition States from the Topological Analysis of the Electron Localization Function. J. Phys. Chem. A 2002, 106, 11533–11539. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Silvi, B.; Pérez, P. The mysticism of pericyclic reactions. A contemporary rationalisation of organic reactivity based on the electron density analysis. Eur. J. Org. Chem. 2018, 2018, 1107–1120. [Google Scholar] [CrossRef]
- Houk, K.N.; Sims, J.; Watts, C.R.; Luskus, L.J. Origin of reactivity, regioselectivity, and periselectivity in 1,3-dipolar cycloadditions. J. Am. Chem. Soc. 1973, 95, 7301–7315. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory study of the competitiveness of polar Diels-Alder and polar Alder ene reactions. Molecules 2018, 23, 1913. [Google Scholar] [CrossRef] [Green Version]
- Domingo, L.R.; Pérez, P. Understanding the Higher–Order Cycloaddition Reactions of Heptafulvene, Tropone and its Nitrogen Derivatives with Electrophilic and Nucleophilic Ethylenes inside the Molecular Electron Density Theory. New J. Chem. 2022, 46, 11520–11530. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Hehre, M.J.; Radom, L.; Schleyer, P.V.R.; Pople, J. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B. Modern Electronic Structure Theory; Yarkony, D.R., Ed.; World Scientific Publishing: Singapore, 1994. [Google Scholar]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, J.; Persico, M. Molecular interactions in solution: And overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Simkin, B.Y.; Sheikhet, I.I. Quantum Chemical and Statistical Theory of Solutions—Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef] [Green Version]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. (Eds.) GaussView, Version 6.0; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Silvi, B.; Savín, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory study of the enhanced reactivity of aza aromatic compounds participating in Diels-Alder reactions. Org. Biomol. Chem. 2020, 18, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Sáez, J.A. Understanding the Origin of the Asynchronicity in Bond-Formation in Polar Cycloaddition Reactions. A DFT Study of the 1,3-Dipolar Cycloaddition Reaction of Carbonyl Ylides with 1,2-Benzoquinones. RSC Adv. 2012, 2, 1334–1342. [Google Scholar] [CrossRef]
- Ess, D.H.; Wheeler, S.E.; Iafe, R.G.; Xu, L.; Çelebi-Ölçüm, N.; Houk, K.N. Bifurcations on Potential Energy Surfaces of Organic Reactions. Angew. Chem. Int. Ed. Engl. 2008, 47, 7592–7601. [Google Scholar] [CrossRef]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Perez, P.; Contreras, R. Origin of the synchronicity on the transition structures of polar Diels-Alder reactions. Are these reactions [4 + 2] processes? J. Org. Chem. 2003, 68, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Sáez, J.A. Understanding the Electronic Reorganization along the Non-polar [3 + 2] cycloaddition reactions of carbonyl ylides. J. Org. Chem. 2011, 76, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.; Brett Beno, B.; Houk, K.N. Density Functional Theory prediction of the relative energies and isotope effects for the concerted and stepwise mechanisms of the Diels-Alder reaction of butadiene and ethylene. J. Am. Chem. Soc. 1996, 118, 6036–6043. [Google Scholar] [CrossRef]
- Domingo, L.R.; Arnó, M.; Andrés, J. Influence of reactant polarity on the course of the inverse-electron-demand Diels-Alder reaction. A DFT study of regio- and stereoselectivity, presence of Lewis acid catalyst, and inclusion of solvent effects in the reaction between nitroethene and substituted ethenes. J. Org. Chem. 1999, 64, 5867–5875. [Google Scholar]
- García, J.I.; Mayoral, J.A.; Salvatella, L. Do Secondary Orbital Interactions Really Exist? Acc. Chem. Res. 2000, 33, 658–664. [Google Scholar] [CrossRef]
- Lewis, G.N. Valence and the Structure of Atoms and Molecules; Chemical Catalog Co.: New York, NY, USA, 1923. [Google Scholar]
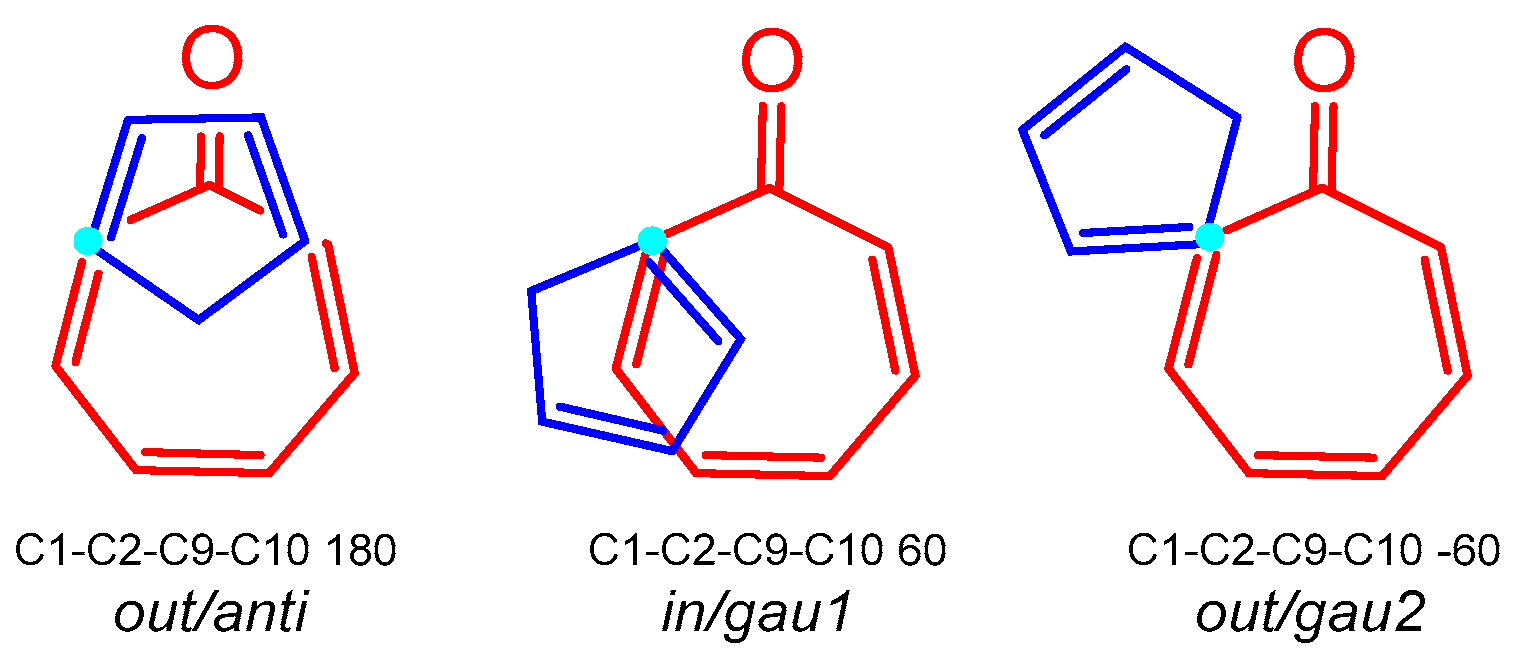


| μ | η | ω | N | |
|---|---|---|---|---|
| tropone 1 | −4.28 | 4.20 | 2.18 | 2.75 |
| Cp 3 | −3.01 | 5.48 | 0.83 | 3.37 |
| Gas Phase | Benzene | Gas Phase | Benzene | ||
|---|---|---|---|---|---|
| TS25i | 17.0 | 17.4 | 16 | −29.4 | −28.3 |
| TS27o | 14.8 | 15.3 | 4 | −25.8 | −24.7 |
| TS23i | 16.7 | 16.9 | 17 | −16.4 | −15.3 |
| TS23o | 17.5 | 18.1 | 6 | −15.5 | −14.4 |
| TS28o | 25.1 | 25.5 | 15 | −21.5 | −19.9 |
| TS2o | 28.7 | 28.4 | IN2o | 28.6 | 27.9 |
| TS45i | 19.1 | 19.2 | 18 | −16.0 | −15.2 |
| TS45o | 19.3 | 19.5 | 19 | −14.9 | −14.0 |
| ∆H | ∆S | ∆G | |
|---|---|---|---|
| TS25i | 18.0 | −48.4 | 35.1 |
| TS27o | 16.2 | −50.4 | 34.0 |
| TS23i | 17.7 | −47.1 | 34.3 |
| TS23o | 19.2 | −48.2 | 36.3 |
| 16 | −24.9 | −52.1 | −6.6 |
| 4 | −21.4 | −54.4 | −2.2 |
| 17 | −12.0 | −51.2 | 6.1 |
| 6 | −11.1 | −51.4 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Unveiling the Chemistry of Higher-Order Cycloaddition Reactions within the Molecular Electron Density Theory. Chemistry 2022, 4, 735-752. https://doi.org/10.3390/chemistry4030052
Domingo LR, Ríos-Gutiérrez M, Pérez P. Unveiling the Chemistry of Higher-Order Cycloaddition Reactions within the Molecular Electron Density Theory. Chemistry. 2022; 4(3):735-752. https://doi.org/10.3390/chemistry4030052
Chicago/Turabian StyleDomingo, Luis R., Mar Ríos-Gutiérrez, and Patricia Pérez. 2022. "Unveiling the Chemistry of Higher-Order Cycloaddition Reactions within the Molecular Electron Density Theory" Chemistry 4, no. 3: 735-752. https://doi.org/10.3390/chemistry4030052
APA StyleDomingo, L. R., Ríos-Gutiérrez, M., & Pérez, P. (2022). Unveiling the Chemistry of Higher-Order Cycloaddition Reactions within the Molecular Electron Density Theory. Chemistry, 4(3), 735-752. https://doi.org/10.3390/chemistry4030052