Spectrophotometric Determination of Formation Constants of Iron(III) Complexes with Several Ligands
Abstract
1. Introduction
2. Results and Discussion
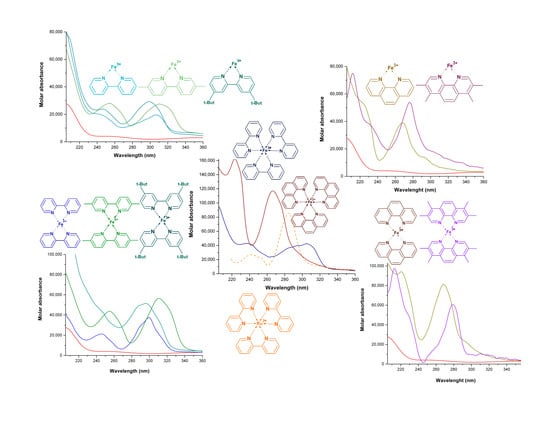
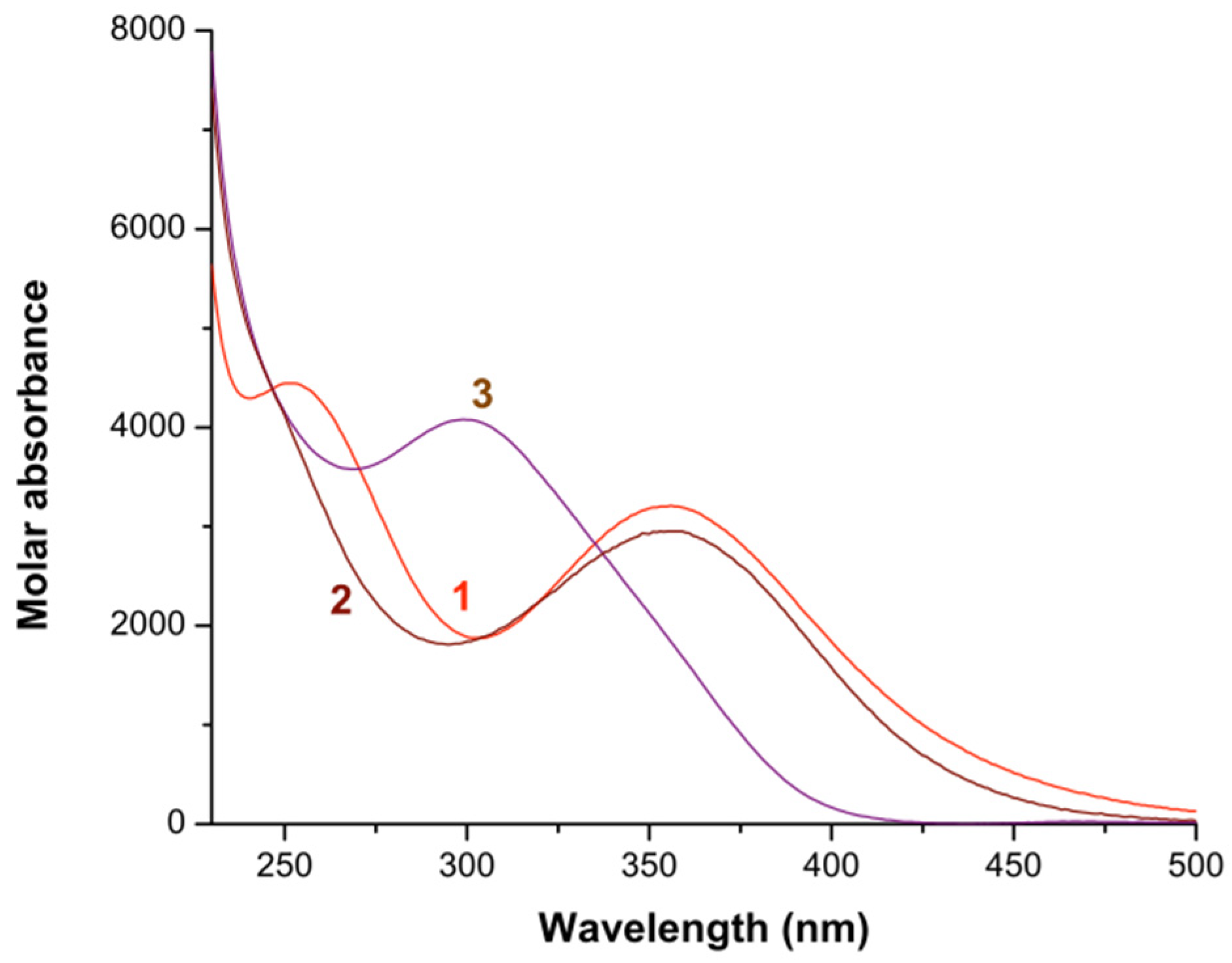
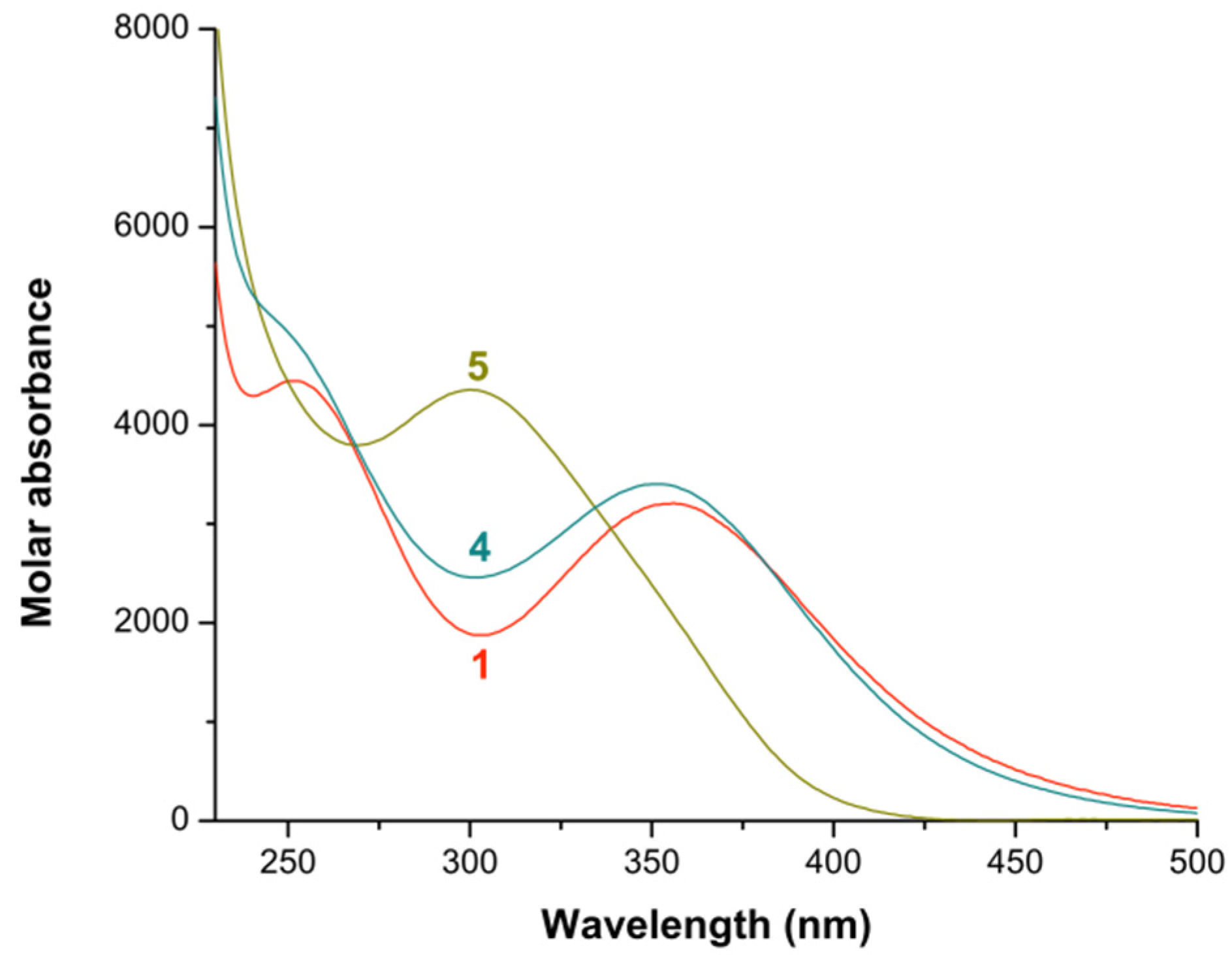
2.1. Formation Constants of Iron(III)-Diamine Ligands Systems
2.2. Formation Constants of Iron(III)-Heterocyclic Ligand Systems
2.3. Distribution Curves of the Iron(III) System with Diamines, Substituted and Unsubstituted Heterocyclic Ligands
2.4. Far Infrared Spectrum of the Complexes of iron(III) with Ethylenediamine, 1,3-Propanediamine, 1,4-Butanediamine, 2,2′-Bipyridyl, 5,5′-Dimethyl-2,2′-bipyridyl, 4,4′-Di-tert-butyl-2,2′-bipyridyl, 1,10-Phenanthroline or 3,4, 7,8-Tetramethyl-1,10-phenanthroline
3. Materials and Methods
3.1. Materials, Physical Measurements and Methods
3.2. Equilibrium Studies and Singular Electronic Spectrum Determination for the iron(III)-Diamine Complexes
3.3. Equilibrium Studies and Singular Electronic Spectrum Determination for iron(III)-Heterocyclic and iron(III)-Heterocyclic-Substituted Complexes
3.4. Synthesis of the Complexes: Fe(en)i, Fe(pn)i, Fe(bn)i, Fe(bipy)i, Fe(dmbipy)i, Fe(dtbbipy)i, Fe(phen)i, Fe(tmphen)i, and Its Far Infrared Spectrum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Yıldız, İ. 1.12 Fossil Fuels. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Oxford, UK, 2018; pp. 521–567. [Google Scholar]
- Santika, W.G.; Anisuzzaman, M.; Bahri, P.A.; Shafiullah, G.M.; Rupf, G.V.; Urmee, T. From goals to joules: A quantitative approach of interlinkages between energy and the Sustainable Development Goals. Energy Res. Soc. Sci. 2019, 50, 201–214. [Google Scholar] [CrossRef]
- Asim, N.; Sopian, K.; Ahmadi, S.; Saeedfar, K.; Alghoul, M.A.; Saadatian, O.; Zaidi, S.H. A review on the role of materials science in solar cells. Renew. Sustain. Energy Rev. 2012, 16, 5834–5847. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Yang, M. Progress in light harvesting and charge injection of dye-sensitized solar cells. Mater. Sci. Eng. B 2011, 176, 1142–1160. [Google Scholar] [CrossRef]
- Liska, P.; Vlachopoulos, N.; Nazeeruddin, M.K.; Comte, P.; Graetzel, M. cis-Diaquabis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) sensitizes wide band gap oxide semiconductors very efficiently over a broad spectral range in the visible. J. Am. Chem. Soc. 1988, 110, 3686–3687. [Google Scholar] [CrossRef]
- Van Houten, J.; Watts, R.J. Temperature dependence of the photophysical and photochemical properties of the tris(2,2′-bipyridyl)ruthenium(II) ion in aqueous solution. J. Am. Chem. Soc. 1976, 98, 4853–4858. [Google Scholar] [CrossRef]
- Lee, K.W.; Slinker, J.D.; Gorodetsky, A.A.; Flores-Torres, S.; Abruña, H.D.; Houston, P.L.; Malliaras, G.G. Photophysical properties of tris(bipyridyl)ruthenium(ii) thin films and devices. PCCP 2003, 5, 2706–2709. [Google Scholar] [CrossRef]
- Chábera, P.; Lindh, L.; Rosemann, N.W.; Prakash, O.; Uhlig, J.; Yartsev, A.; Wärnmark, K.; Sundström, V.; Persson, P. Photofunctionality of iron(III) N-heterocyclic carbenes and related d5 transition metal complexes. Coord. Chem. Rev. 2021, 426, 213517. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Zhou, S.; Zhang, X.; Bo, H. The antitumor effect and toxicity of a ruthenium(II) complex in vivo. Inorg. Chem. Commun. 2018, 87, 49–52. [Google Scholar] [CrossRef]
- Sigma-Aldrich: Analytical, Biology, Chemistry, Materials Science Products and Services. Available online: www.sigmaaldrich.com/ (accessed on 1 June 2022).
- Young, E.R.; Oldacre, A. Iron hits the mark. Science 2019, 363, 225. [Google Scholar] [CrossRef] [PubMed]
- Chabera, P.; Liu, Y.; Prakash, O.; Thyrhaug, E.; Nahhas, A.; Honarfar, A.; Essén, S.; Fredin, L.; Harlang, T.; Kjær, K.; et al. A low-spin Fe(III) complex with 100-ps ligand-to-metal charge transfer photoluminescence. Nature 2017, 543, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Wang, X.-L.; Qin, C.; Zhang, Z.-M.; Li, Y.-G.; Chen, W.-L.; Wang, E.-B. Polyoxometalate-assisted synthesis of transition-metal cubane clusters as artificial mimics of the oxygen-evolving center of photosystem II. Coord. Chem. Rev. 2016, 313, 94–110. [Google Scholar] [CrossRef]
- Kober, E.M.; Meyer, T.J. Concerning the absorption spectra of the ions M(bpy)32+ (M = Fe, Ru, Os; bpy = 2,2′-bipyridine). lnorg. Chem. 1982, 21, 3967–3977. [Google Scholar] [CrossRef]
- Bullock, R.M. Abundant Metals Give Precious Hydrogenation Performance. Science 2013, 342, 1054–1055. [Google Scholar] [CrossRef]
- Figgis, B.N.; Lewis, J.; Mabbs, F.E.; Webb, G.A. Magnetic properties of some iron(III) and ruthenium(III) low-spin complexes. J. Chem. Soc. A Inorg. Phys. Theor. 1966, 422–426. [Google Scholar] [CrossRef]
- Ojea, M.J.H.; Reta, D.; Rosado, L.; Zuazo, J.R.; Castro, G.; Tewary, S.; Gopalan, R.; Aromí, G.; Jimenez, E.; Sañudo, E.C. Characterization of a Robust CoII Fluorescent Complex Deposited Intact On HOPG. Chem. A Eur. J. 2014, 20. [Google Scholar] [CrossRef]
- Bräuer, B.; Zahn, D.; Ffer, T.; Salvan, G. Deposition of thin films of a transition metal complex by spin coating. Chem. Phys. Lett. 2006, 432, 226–229. [Google Scholar] [CrossRef]
- Gorelsky, S.I. 2.38—Semiempirical SCF MO Methods, Electronic Spectra, and Configurational Interaction. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Pergamon: Oxford, UK, 2003; pp. 467–489. [Google Scholar]
- Stephenson, N.A.; Bell, A.T. Effects of Methanol on the Thermodynamics of Iron(III) [Tetrakis(pentafluorophenyl)]porphyrin Chloride Dissociation and the Creation of Catalytically Active Species for the Epoxidation of Cyclooctene. lnorg. Chem. 2006, 45, 5591–5599. [Google Scholar] [CrossRef]
- Harris, D.C.; Bertolucci, M.D. Symmetry and Spectroscopy: An Introduction to Vibrational and Electronic Spectroscopy; Dover Publications: Mignola, NY, USA, 1978. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. HypSpec 2008 Software; Protonic Software: Leeds, UK; Florence, Italy, 2008. [Google Scholar]
- Ehnbom, A.; Ghosh, S.; Lewis, K.G.; Gladysz, J. Octahedral Werner complexes with substituted ethylenediamine ligands: A stereochemical primer for a historic series of compounds now emerging as a modern family of catalysts. Chem. Soc. Rev. 2016, 45, 6799–6811. [Google Scholar] [CrossRef]
- Tuck, D.G. A proposal for the use of a standard format for the publication of stability constant measurements. Pure Appl. Chem. 1989, 61, 1161. [Google Scholar] [CrossRef]
- Segoviano-Garfias, J.J.N.; Mendoza-Díaz, G.; Moreno-Esparza, R. A comparative study of the speciation in methanol solution and activity in the oxidative coupling of 2,6-di-tert-butylphenol presented by the complexes: Copper(II)-N,N′-di-tert-butylethylenediamine-halogen and some copper(II)-diamine-halogen complexes. Inorg. Chim. Acta 2014, 411, 148–157. [Google Scholar] [CrossRef]
- Paoletti, P. Formation of metal complexes with ethylenediamine: A critical survey of equilibrium constants, enthalpy and entropy values. Pure Appl. Chem. 1984, 56, 491. [Google Scholar] [CrossRef]
- Pecsok, R.; Bjerrum, J. Metal Ammine Formation in Solution XI. Stability of Ethylenediamine Complexes and the Coordination. Acta Chem. Scand 1957, 11. [Google Scholar] [CrossRef]
- Miessler, G.L.; Fischer, P.J.; Tarr, D.A. Inorganic Chemistry, 2nd ed.; Pearson Education: Upper Saddle River, NJ, USA, 2013. [Google Scholar]
- Segoviano-Garfias, J.J.N.; Mendoza-Díaz, G.; Moreno-Esparza, R. Spectrophotometric determination of the formation constants of the cupric halogen complexes with 1,3-propanediamine and 1,4-butanediamine in methanol solution and their activity on the oxidative coupling of the 2,6-di-tert-butylphenol. Inorg. Chim. Acta 2013, 400, 184–190. [Google Scholar] [CrossRef]
- Miessler, G.L.; Tarr, D.A. Inorganic Chemistry; Pearson Education: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Setyawati, I.A.; Rettig, S.J.; Orvig, C. Cationic iron(III) complex with a hexadentate N2,N’2′,O2-aminopyridylphenolate ligand. Can. J. Chem. 1999, 77, 2033–2038. [Google Scholar] [CrossRef]
- Janzen, D.E.; Patel, K.; VanDerveer, D.G.; Grant, G.J. π–π Interactions in diimine Pt(II) complexes with thiacrown ligands: The crystal structure of (1,4,7-trithiacyclononane)(3,4,7,8-tetramethyl-1, 10-phenanthroline)platinum(II) hexafluorophosphate. J. Chem. Crystallogr. 2006, 36, 83–91. [Google Scholar] [CrossRef]
- Amani, V.; Safari, N.; Khavasi, H.R.; Mirzaei, P. Iron(III) mixed-ligand complexes: Synthesis, characterization and crystal structure determination of iron(III) hetero-ligand complexes containing 1,10-phenanthroline, 2,2′-bipyridine, chloride and dimethyl sulfoxide, [Fe(phen)Cl3(DMSO)] and [Fe(bipy)Cl3(DMSO)]. Polyhedron 2007, 26, 4908–4914. [Google Scholar]
- Mukherjee, G.N.; Das, A. Mixed ligand complex formation of FeIII with boric acid and typical N-donor multidentate ligands. J. Chem. Sci. 2002, 114, 163–174. [Google Scholar] [CrossRef]
- Alexander, R.D.; Buisson, D.H.; Dudeney, A.W.L.; Irving, R.J. Spectrophotometric investigations of aqueous solutions at elevated temperatures. Effect of temperature on the stability constants of the tris complexes of 1,10-phenanthroline, 5-nitro-1,10-phenanthroline and 2,2[prime or minute]-bipyridyl with iron (II). J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1978, 74, 1081–1088. [Google Scholar] [CrossRef]
- Anderegg, G. Pyridinderivate als Komplexbildner III. Komplexbildung des dreiwertigen Eisen-Ions mit 1,10-Phenanthrolin und α,α′-Dipyridyl. Helv. Chim. Acta 1962, 45, 1643–1657. [Google Scholar] [CrossRef]
- Mayer, U. Solvent effects on ion-pair equilibria. Coord. Chem. Rev. 1976, 21, 159–179. [Google Scholar] [CrossRef]
- Sreekanth, A.; Fun, H.-K.; Kurup, M.R.P. Structural and spectral studies of an iron(III) complex [Fe(Pranthas)2][FeCl4] derived from 2-acetylpyridine-N(4), N(4)-(butane-1, 4-diyl) thiosemicarbazone (HPranthas). J. Mol. Struct. 2005, 737, 61–67. [Google Scholar] [CrossRef]
- Sreekanth, A.; Kurup, M.R.P. Synthesis, EPR and Mössbauer spectral studies of new iron(III) complexes with 2-benzoylpyridine-N(4), N(4)-(butane-1,4-diyl) thiosemicarbazone (HBpypTsc): X-ray structure of [Fe(BpypTsc)2]FeCl4·2H2O and the free ligand. Polyhedron 2004, 23, 969–978. [Google Scholar] [CrossRef]
- Valicsek, Z.; Horváth, O. Application of the electronic spectra of porphyrins for analytical purposes: The effects of metal ions and structural distortions. Microchem. J. 2013, 107, 47–62. [Google Scholar] [CrossRef]
- Zafar, M.N.; Nazar, M.F.; Sumrra, S.H.; Gul-E-Saba; Yasmin, A.; Atif, A.H. Development of some important nitrogen donor ligands for transition metal homogeneous catalysis. Russ. J. Coord. Chem. 2016, 42, 225–251. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- The Original Spectral Data of Tris (2,2′-bipyridyl) Ruthenium (II) Complex Was Obtained from the Website. Available online: https://omlc.org/spectra/PhotochemCAD/html/085.htmlandusedwithpermisionofProfessorScottPrahl (accessed on 1 December 2021).
- Duan, L.; Bozoglian, F.; Mandal, S.; Stewart, B.; Privalov, T.; Llobet, A.; Sun, L. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 2012, 4, 418–423. [Google Scholar] [CrossRef]
- Duan, L.; Xu, Y.; Zhang, P.; Wang, M.; Sun, L. Visible Light-Driven Water Oxidation by a Molecular Ruthenium Catalyst in Homogeneous System. Inorg. Chem. 2010, 49, 209–215. [Google Scholar] [CrossRef]
- Bozoglian, F.; Romain, S.; Ertem, M.Z.; Todorova, T.K.; Sens, C.; Mola, J.; Rodriguez, M.; Romero, I.; Benet-Buchholz, J.; Fontrodona, X. The Ru−Hbpp water oxidation catalyst. J. Am. Chem. Soc. 2009, 131, 15176–15187. [Google Scholar] [CrossRef]
- Pal, S. Syntheses, structures and properties of trans-dichlororuthenium(II) complexes with N4-donor Schiff bases. Polyhedron 2003, 22, 867–873. [Google Scholar] [CrossRef]
- Borgström, M.; Shaikh, N.; Johansson, O.; Anderlund, M.F.; Styring, S.; Åkermark, B.; Magnuson, A.; Hammarström, L. Light Induced Manganese Oxidation and Long-Lived Charge Separation in a Mn2II,II–RuII(bpy)3–Acceptor Triad. J. Am. Chem. Soc. 2005, 127, 17504–17515. [Google Scholar] [CrossRef] [PubMed]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Lever, A.B.P.; Mantovani, E. The far infrared and electronic spectra of mono(ethylenediamine) and related complexes of copper(II). An example of the trans effect, and the influence of axial interactions upon the in-plane bond strength. Inorg. Chim. Acta 1971, 5, 429–433. [Google Scholar] [CrossRef]
- Lever, A.B.P.; Mantovani, E. Isotopic Studies of the Metal–Ligand Bond. Part III. The Far Infrared Spectra of Some Tetragonal Diamine Complexes of Cobalt(II) and Nickel(II): Studies of the Metal–Nitrogen Bond, as a Function of Metal Ion and of Spin State. Can. J. Chem. 2011, 51, 1567–1581. [Google Scholar] [CrossRef]
- Sari, N.; Kahraman, E.; Sari, B.; Özgün, A. Synthesis of Some Polymer-Metal Complexes and Elucidation of their Structures. J. Macromol. Sci. 2006, 43. [Google Scholar] [CrossRef]
- Tsuchida, E.; Nishide, H. Polymer-metal complexes and their catalytic activity. In Molecular Properties; Springer: Berlin/Heidelberg, Germany, 1977; pp. 1–87. [Google Scholar]
- Singh, D.P.; Grover, V.; Kumar, R.; Jain, K. Template synthesis of macrocyclic complexes and their spectroscopic and antibacterial studies. J. Enzyme Inhib. Med. Chem. 2010, 25, 445–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, D.P.; Kumar, R.; Singh, J. Antibacterial activity and spectral studies of trivalent chromium, manganese, iron macrocyclic complexes derived from oxalyldihydrazide and glyoxal. J. Enzyme Inhib. Med. Chem. 2009, 24, 883–889. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsuda, Y.; Sakata, K.; Harada, K. Transition-metal Complexes of Pyrrole Pigments. IX. Divalent and Trivalent Iron Chelates of Dipyrromethenes. Bull. Chem. Soc. Jpn. 1974, 47, 458–462. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar, R.; Singh, J. Synthesis and spectroscopic studies of biologically active compounds derived from oxalyldihydrazide and benzil, and their Cr(III), Fe(III) and Mn(III) complexes. Eur. J. Med. Chem. 2009, 44, 1731–1736. [Google Scholar] [CrossRef]
- Likussar, W. Computer approach to the continuous variations method for spectrophotometric determination of extraction and formation constants. Anal. Chem. 1973, 45, 1926–1931. [Google Scholar] [CrossRef]
- Segoviano-Garfias, J.J.N.; Nájera-Lara, M.; Pérez-Arredondo, M.d.l.L.; López-Ramírez, V.; Rubio-Jimenez, C.A.; Ramírez-Vázquez, J.A.; Moreno-Esparza, R. Spectrophotometric determination of formation constants of copper(II) complexes with 2,2′-bipyridyl, 1,10-phenanthroline and their halides in methanol. J. Mol. Struct. 2017, 1147, 217–225. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segoviano-Garfias, J.J.N.; Zanor, G.A.; Ávila-Ramos, F.; Bivián-Castro, E.Y.; Rubio-Jiménez, C.A. Spectrophotometric Determination of Formation Constants of Iron(III) Complexes with Several Ligands. Chemistry 2022, 4, 701-716. https://doi.org/10.3390/chemistry4030050
Segoviano-Garfias JJN, Zanor GA, Ávila-Ramos F, Bivián-Castro EY, Rubio-Jiménez CA. Spectrophotometric Determination of Formation Constants of Iron(III) Complexes with Several Ligands. Chemistry. 2022; 4(3):701-716. https://doi.org/10.3390/chemistry4030050
Chicago/Turabian StyleSegoviano-Garfias, José J. N., Gabriela A. Zanor, Fidel Ávila-Ramos, Egla Yareth Bivián-Castro, and Carlos A. Rubio-Jiménez. 2022. "Spectrophotometric Determination of Formation Constants of Iron(III) Complexes with Several Ligands" Chemistry 4, no. 3: 701-716. https://doi.org/10.3390/chemistry4030050
APA StyleSegoviano-Garfias, J. J. N., Zanor, G. A., Ávila-Ramos, F., Bivián-Castro, E. Y., & Rubio-Jiménez, C. A. (2022). Spectrophotometric Determination of Formation Constants of Iron(III) Complexes with Several Ligands. Chemistry, 4(3), 701-716. https://doi.org/10.3390/chemistry4030050