Equilibrium Swelling of Thermo-Responsive Gels in Mixtures of Solvents
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Swelling of TR Gels in Water
3.2. Swelling of TR Gels in Binary Mixtures
3.2.1. Equilibrium Swelling at Temperatures Far below
3.2.2. Equilibrium Swelling at Temperatures Far above
3.2.3. Equilibrium Swelling at Temperatures in the Vicinity of VPTT
4. Results and Discussion
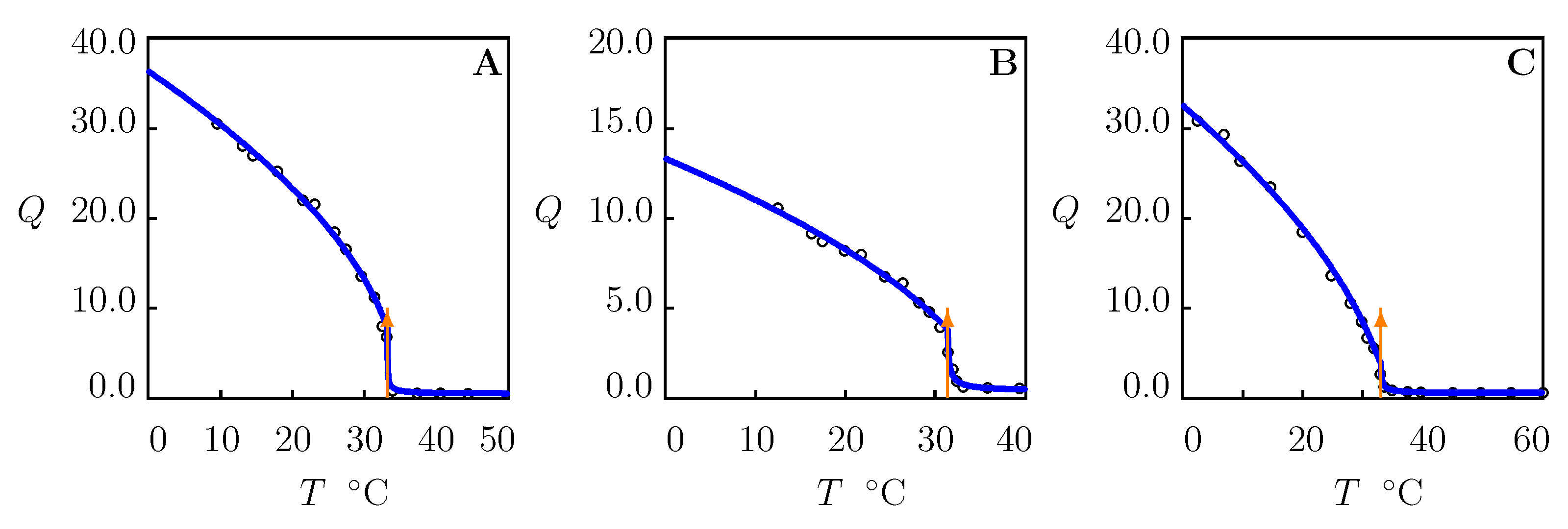
4.1. Swelling of PNIPAAm Gels in Water at Various Temperatures
- (I)
- The critical temperatures of PNIPAAm gels and microgels coincide practically ( C). According to Equation (15), this is caused by the fact that the parameter of these gels adopts similar values (in the range between 0.4 and 0.55 depending on the preparation conditions).
- (II)
- The coefficients and (that describe the kinetics of aggregation of hydrophobic segments above ) accept similar values for macroscopic gels and microgels.
- (III)
- The degrees of swelling in the reference state for microgels are substantially lower than that for the macroscopic gel.
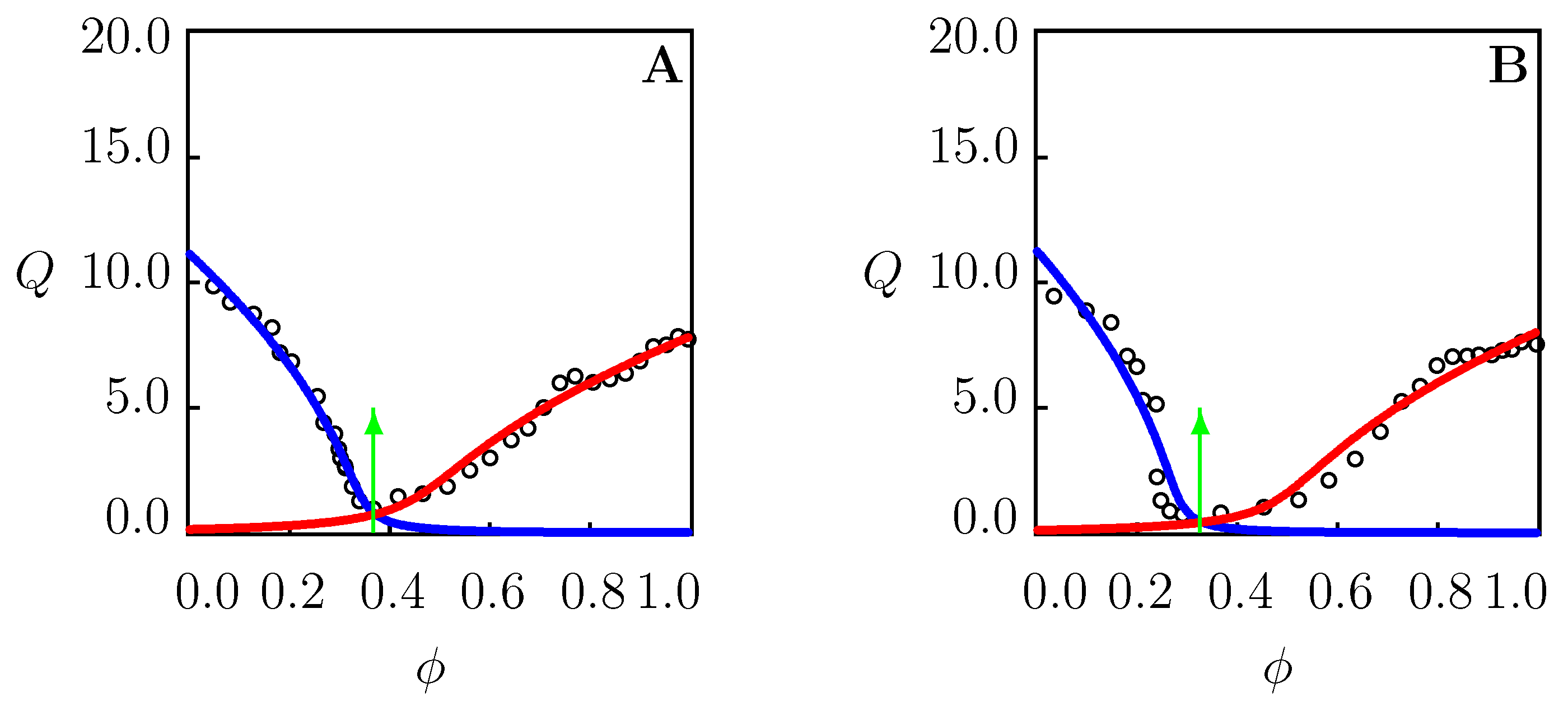
4.2. Swelling of PNIPAAm Gels in Binary Mixtures Far below
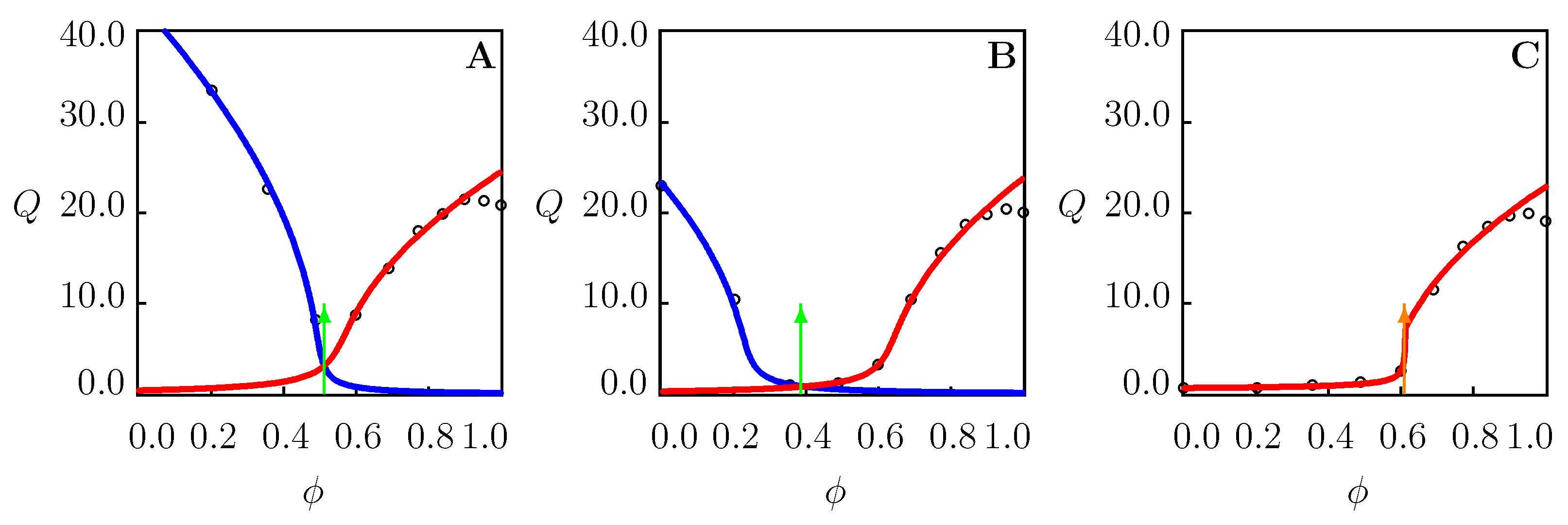
4.3. Swelling of PNIPAAm Gels in Binary Mixtures Far above VPTT
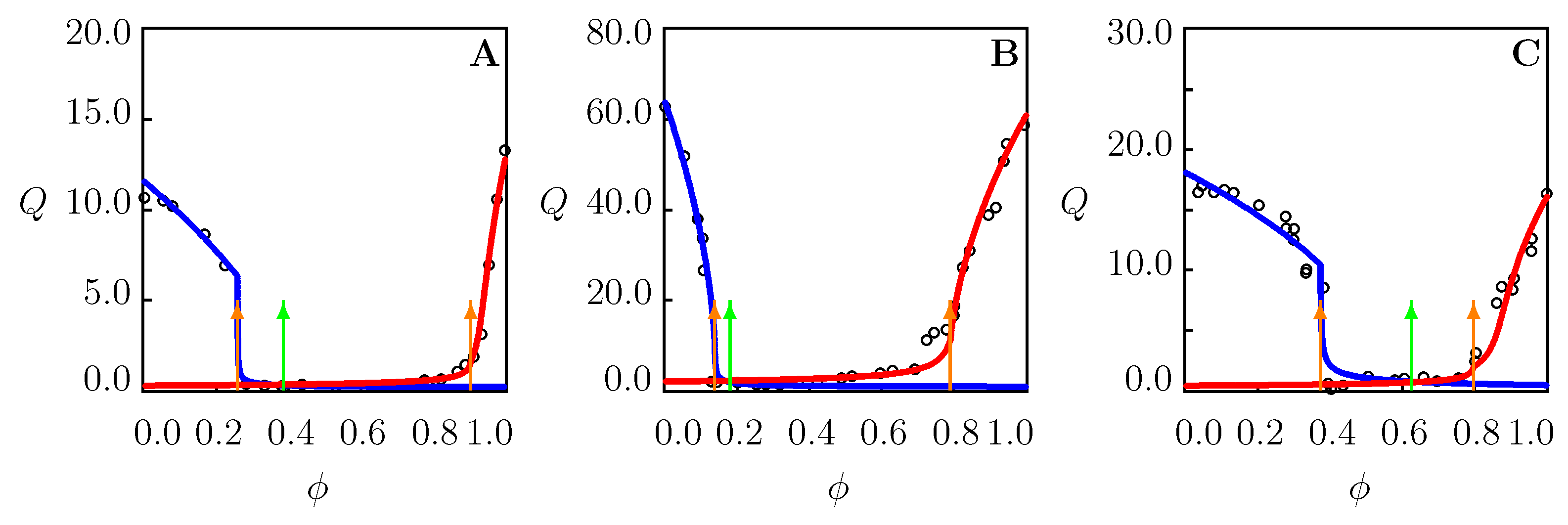
4.4. Swelling of PNIPAAm Gels in Binary Mixtures in the Vicinity of
4.5. Discussion
- Induced by aggregation of hydrophobic side groups and formation of hydrophobic clusters serving as physical cross-links between chains;
- Driven by replacement of water with cosolvent molecules in cage-like structures surrounding hydrophobic side chains;
- Caused by replacement of water (as the main element of hydration shells) with cosolvent molecules.
- One volume phase transition point for temperature-induced transformations of PNIPAAm gels in water (Figure 3). An increase in temperature weakens hydration shells supporting cage-like structures and destabilize the cages around hydrophobic side groups. The latter induces breakage of cages, release of hydrophobic groups, and their aggregation into clusters from which solvent molecules are expelled.
- One volume phase transition point for PNIPAAm gels in binary mixtures at temperatures T far below the critical temperature in water (Figure 1, Figure 2A, Figure 4A,B, Figure 5A,B, Figure 6A,B and Figure 7A). Structural transformation in TR gels demonstrating the re-entrance phenomenon is driven by replacement of water with cosolvent molecules in cage-like structures surrounding hydrophobic side groups (the phase transition point coincides with the point of minimum on swelling diagrams with decreasing and increasing branches).
- Three volume phase transition points for PNIPAAm gels in binary mixtures at temperatures T close to the critical temperature in water (Figure 2B and Figure 8A–C). Two phase transition points on a swelling diagram, and , correspond to aggregation of hydrophobic segments in TR gels whose cage-like structures are formed by water () and cosolvent () molecules. The third transition point reflects replacement of water molecules (as the main element of hydration shells) with cosolvent molecules in the collapsed gel.
- One volume phase transition point for PNIPAAm gels in binary mixtures at temperatures T exceeding the critical temperature in water (Figure 2C, Figure 5C, Figure 6C and Figure 7B). The structural transformation is driven by breakage of cage-like structures formed by cosolvent molecules and the aggregation of released hydrophobic side groups.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Dong, D.; Yao, M.; Yu, Q.; Sun, X.; Guo, Q.; Zhang, H.; Yao, F.; Li, J. Freezing-tolerant supramolecular organohydrogel with high toughness, thermoplasticity, and healable and adhesive properties. ACS Appl. Mater. Interfaces 2019, 11, 21184–21193. [Google Scholar] [CrossRef]
- Liu, B.; Li, F.; Niu, P.; Li, H. Tough adhesion of freezing- and drying-tolerant transparent nanocomposite organohydrogels. ACS Appl. Mater. Interfaces 2021, 13, 21822–21830. [Google Scholar] [CrossRef] [PubMed]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.; Suo, Z.; Vlassak, J.J. Highly stretchable and tough hydrogels below water freezing temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, D.; Wang, J.; Li, T.; Zhou, X.; Gan, T.; Handschuh-Wang, S.; Zhou, X. Rational fabrication of anti-freezing, non-drying tough organohydrogels by one-pot solvent displacement. Angew. Chem. Int. Ed. 2018, 57, 6568–6571. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Jiao, S.; Wang, C.; Jia, Y.; Dai, K.; Zheng, G.; Liu, C.; Wan, P.; Shen, C. Environment tolerant conductive nanocomposite organohydrogels as flexible strain sensors and power sources for sustainable electronics. Adv. Funct. Mater. 2021, 31, 2101696. [Google Scholar] [CrossRef]
- Shang, H.; Le, X.; Si, M.; Wu, S.; Peng, Y.; Shan, F.; Wu, S.; Chen, T. Biomimetic organohydrogel actuator with high response speed and synergistic fluorescent variation. Chem. Eng. J. 2022, 429, 132290. [Google Scholar] [CrossRef]
- Xu, Y.; Rong, Q.; Zhao, T.; Liu, M. Anti-freezing multiphase gel materials: Bioinspired design strategies and applications. Giant 2020, 2, 100014. [Google Scholar] [CrossRef]
- Zhuo, Y.; Chen, J.; Xiao, S.; Li, T.; Wang, F.; He, J.; Zhang, Z. Gels as emerging anti-icing materials: A mini review. Mater. Horiz. 2021, 8, 3266–3280. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, J. Bioinspired organohydrogels with heterostructures: Fabrications, performances, and applications. Adv. Colloid Interface Sci. 2021, 292, 102408. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lin, X.; Li, P.; Liu, F.-Q.; Guo, H.; Li, W.-H. Recent advances of organogels: From fabrications and functions to applications. Prog. Org. Coat. 2021, 159, 106417. [Google Scholar] [CrossRef]
- Castro, G.R.; Knubovets, T. Homogeneous biocatalysis in organic solvents and water-organic mixtures. Crit. Rev. Biotechnol. 2003, 23, 195–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Sagaama, A.; Issaoui, N.; Malyar, Y.N.; Vasilieva, N.Y.; Borovkova, V.S. Theoretical and experimental study of guar gum sulfation. J. Mol. Model. 2021, 27, 5. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Dusek, K.; Duskova-Smrckova, M. Volume phase transition in gels: Its discovery and development. Gels 2020, 6, 22. [Google Scholar] [CrossRef]
- Okay, O. Re-entrant conformation transition in hydrogels. Gels 2021, 7, 98. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Asano, M.; Winnik, F.M.; Yamashita, T.; Horie, K. Fluorescence studies of dansyl-labeled poly(N-isopropylacrylamide) gels and polymers in mixed water/methanol solutions. Macromolecules 1995, 28, 5861–5866. [Google Scholar] [CrossRef]
- Katayama, S.; Hirokawa, Y.; Tanaka, T. Reentrant phase transition in acrylamide-derivative copolymer gels. Macromolecules 1984, 17, 2641–2643. [Google Scholar] [CrossRef]
- Amiya, T.; Hirokawa, Y.; Hirose, Y.; Li, Y.; Tanaka, T. Reentrant phase transition of N-isopropylacrylamide gels in mixed solvents. J. Chem. Phys. 1987, 86, 2375–2379. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ringsdorf, H.; Venzmer, J. Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide). Macromolecules 1990, 23, 2415–2416. [Google Scholar] [CrossRef]
- Schild, H.G.; Muthukumar, M.; Tirrell, D.A. Cononsolvency in mixed aqueous solutions of poly(N-isopropylacrylamide). Macromolecules 1991, 24, 948–952. [Google Scholar] [CrossRef]
- Hirotsu, S. Critical points of the volume phase transition in N-isopropylacrylamide gels. J. Chem. Phys. 1988, 88, 427–431. [Google Scholar] [CrossRef]
- Mukae, K.; Sakurai, M.; Sawamura, S.; Makino, K.; Kim, S.W.; Shirahama, K. Swelling of poly (N-isopropylacrylamide) gels in water-alcohol (C1–C4) mixed solvents. J. Phys. Chem. 1993, 97, 737–741. [Google Scholar] [CrossRef]
- Miki, H.; Suzuki, A.; Yagihara, S.; Tokita, M. Reentrant swelling behavior of poly(N-isopropylacrylamide) gel. Trans. Mater. Res. Soc. Jpn. 2007, 32, 839–842. [Google Scholar] [CrossRef]
- Walter, J.; Sehrt, J.; Vrabec, J.; Hasse, H. Molecular dynamics and experimental study of conformation change of poly(N-isopropylacrylamide) hydrogels in mixtures of water and methanol. J. Phys. Chem. B 2012, 116, 5251–5259. [Google Scholar] [CrossRef]
- Kojima, H.; Tanaka, F.; Scherzinger, C.; Richtering, W. Temperature dependent phase behavior of PNIPAM microgels in mixed water/methanol solvents. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1100–1111. [Google Scholar] [CrossRef]
- Bischofberger, I.; Calzolari, D.C.E.; Trappe, V. Co-nonsolvency of PNiPAM at the transition between solvation mechanisms. Soft Matter 2014, 10, 8288–8295. [Google Scholar] [CrossRef] [Green Version]
- Nothdurft, K.; Muller, D.H.; Brands, T.; Bardow, A.; Richtering, W. Enrichment of methanol inside pNIPAM gels in the cononsolvency-induced collapse. Phys. Chem. Chem. Phys. 2019, 21, 22811–22818. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.V.; Molina, M.; Barbero, C.A. Poly(N-isopropylacrylamide) cross-linked gels as intrinsic amphiphilic materials: Swelling properties used to build novel interphases. J. Phys. Chem. B 2018, 122, 9038–9048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.W.; Napper, D.H. Coil-to-globule type transitions and swelling of poly(N-isopropylacrylamide) and poly(acrylamide) at latex interfaces in alcohol-water mixtures. J. Colloid Interface Sci. 1996, 177, 343–352. [Google Scholar] [CrossRef]
- Lopez-Leon, T.; Bastos-Gonzalez, D.; Ortega-Vinuesa, J.L.; Elaissari, A. Salt effects in the cononsolvency of poly(N-isopropylacrylamide) microgels. ChemPhysChem 2010, 11, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Backes, S.; Krause, P.; Tabaka, W.; Witt, M.U.; Mukherji, D.; Kremer, K.; von Klitzing, R. Poly(N-isopropylacrylamide) microgels under alcoholic intoxication: When a LCST polymer shows swelling with increasing temperature. ACS Macro Lett. 2017, 6, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Mukae, K.; Sakurai, M.; Sawamura, S.; Makino, K.; Kim, S.W.; Ueda, I.; Shirahama, K. Swelling of poly(N-isopropylacrylamide) gels in water-aprotic solvent mixtures. Colloid Polym. Sci. 1994, 272, 655–663. [Google Scholar] [CrossRef]
- Pagonis, K.; Bokias, G. Temperature- and solvent-sensitive hydrogels based on N-isopropylacrylamide and N,N-dimethylacrylamide. Polym. Bull. 2007, 58, 289–294. [Google Scholar] [CrossRef]
- Zhu, P.W.; Napper, D.H. Volume phase transitions of poly(N-isopropylacrylamide) latex particles in mixed water-N,N-dimethylformamide solutions. Chem. Phys. Lett. 1996, 256, 51–56. [Google Scholar] [CrossRef]
- Tokuyama, H.; Ishihara, N.; Sakohara, S. Porous poly(N-isopropylacrylamide) gels polymerized in mixed solvents of water and N,N-dimethylformamide. Polym. Bull. 2008, 61, 399–405. [Google Scholar] [CrossRef]
- Zhu, P.-W.; Chen, L. Effects of cosolvent partitioning on conformational transitions and chain flexibility of thermoresponsive microgels. Phys. Rev. E 2019, 99, 022501. [Google Scholar] [CrossRef]
- Ishidao, T.; Hashimoto, Y.; Iwai, Y.; Arai, Y. Solvent concentrations of dimethylsulfoxide–water and 1-propanol–water solutions inside and outside poly(N-isopropylacrylamide) gel. Colloid Polym. Sci. 1994, 272, 1313–1316. [Google Scholar] [CrossRef]
- Ishidao, T.; Akagi, M.; Sugimoto, H.; Iwai, Y.; Arai, Y. Swelling behaviors of poly(N-isopropylacrylamide) gel in polyethylene glycol–water mixtures. Macromolecules 1993, 26, 7361–7362. [Google Scholar] [CrossRef]
- Scherzinger, C.; Schwarz, A.; Bardow, A.; Leonhard, K.; Richtering, W. Cononsolvency of poly-N-isopropyl acrylamide (PNIPAM): Microgels versus linear chains and macrogels. Curr. Opin. Colloid Interface Sci. 2014, 19, 84–94. [Google Scholar] [CrossRef]
- Kojima, H. Studies on the phase transition of hydrogels and aqueous solutions of thermosensitive polymers. Polym. J. 2018, 50, 411–418. [Google Scholar] [CrossRef]
- Mukherji, D.; Marques, C.M.; Kremer, K. Collapse in two good solvents, swelling in two poor solvents: Defying the laws of polymer solubility? J. Phys. Condens. Matter 2018, 30, 024002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherji, D.; Marques, C.M.; Kremer, K. Smart responsive polymers: Fundamentals and design principles. Annu. Rev. Condens. Matter Phys. 2020, 11, 271–299. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Ropero, F.; Hajari, T.; Van Der Vegt, N.F.A. Mechanism of polymer collapse in miscible good solvents. J. Phys. Chem. B 2015, 119, 15780–15788. [Google Scholar] [CrossRef]
- Brochard, F.; De Gennes, P.G. Collapse of one polymer coil in a mixture of solvents. Ferroelectrics 1980, 30, 33–47. [Google Scholar] [CrossRef]
- Tanaka, F.; Koga, T.; Winnik, F.M. Temperature-responsive polymers in mixed solvents: Competitive hydrogen bonds cause cononsolvency. Phys. Rev. Lett. 2008, 101, 028302. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, F.; Koga, T.; Kojima, H.; Xue, N.; Winnik, F.M. Preferential adsorption and co-nonsolvency of thermoresponsive polymers in mixed solvents of water/methanol. Macromolecules 2011, 44, 2978–2989. [Google Scholar] [CrossRef]
- Mukherji, D.; Marques, C.M.; Kremer, K. Polymer collapse in miscible good solvents is a generic phenomenon driven by preferential adsorption. Nat. Commun. 2014, 5, 4882. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, N.; Liu, B.; Bai, J.; Gong, P.; Ru, G.; Feng, J. Preferential adsorption of the additive is not a prerequisite for cononsolvency in water-rich mixtures. Phys. Chem. Chem. Phys. 2017, 19, 30097–30106. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Van Der Vegt, N.F.A. Does preferential adsorption drive cononsolvency? Macromolecules 2019, 52, 4131–4138. [Google Scholar] [CrossRef]
- Zuo, T.; Ma, C.; Jiao, G.; Han, Z.; Xiao, S.; Liang, H.; Hong, L.; Bowron, D.; Soper, A.; Han, C.C.; et al. Water/cosolvent attraction induced phase separation: A molecular picture of cononsolvency. Macromolecules 2019, 52, 457–464. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Nayar, D.; Dalgicdir, C.; Van Der Vegt, N.F.A. An interplay of excluded-volume and polymer-(co)solvent attractive interactions regulates polymer collapse in mixed solvents. J. Chem. Phys. 2021, 154, 134903. [Google Scholar] [CrossRef] [PubMed]
- Dudowicz, J.; Freed, K.F.; Douglas, J.F. Communication: Cosolvency and cononsolvency explained in terms of a Flory-Huggins type theory. J. Chem. Phys. 2015, 143, 131101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, E.E.; Van Der Vegt, N.F.A. Molecular scale solvation in complex solutions. J. Am. Chem. Soc. 2019, 141, 12948–12956. [Google Scholar] [CrossRef]
- Van Der Vegt, N.F.A. Length-scale effects in hydrophobic polymer collapse transitions. J. Phys. Chem. B 2021, 125, 5191–5199. [Google Scholar] [CrossRef]
- Budkov, Y.A.; Kiselev, M.G. Flory-type theories of polymer chains under different external stimuli. J. Phys. Condens. Matter 2018, 30, 043001. [Google Scholar] [CrossRef]
- Kojima, H.; Tanaka, F. Reentrant volume phase transition of crosslinked poly(N-isopropylacrylamide) gels in mixed solvents of water/methanol. Soft Matter 2012, 8, 3010–3020. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Moskalets, A.P.; Dubovik, A.S.; Plashchina, I.G.; Khokhlov, A.R. Energetics and mechanisms of poly(N-isopropylacrylamide) phase transitions in water-methanol solutions. Macromolecules 2020, 53, 10765–10772. [Google Scholar] [CrossRef]
- Zhang, X.; Zong, J.; Meng, D. A unified understanding of the cononsolvency of polymers in binary solvent mixtures. Soft Matter 2020, 16, 7789–7796. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Nakamura, T.; Miyamoto, K.; Tokita, M.; Komai, T. Multiple volume phase transition of nonionic thermosensitive gel. J. Chem. Phys. 1995, 103, 6241–6247. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, Z.; Cai, Y.; Zhou, J.; Hua, W.; Chen, L.; Wang, L.; Zhang, J.; Han, D.; Liu, M.; et al. Adaptive and freeze-tolerant heteronetwork organohydrogels with enhanced mechanical stability over a wide temperature range. Nat. Commun. 2017, 8, 15911. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Perez, M.; Maroto-Centeno, J.A.; Forcada, J.; Hidalgo-Alvarez, R. Gel swelling theories: The classical formalism and recent approaches. Soft Matter 2011, 7, 10536–10547. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-ionic thermoresponsive polymers in water. Adv. Polym. Sci. 2011, 242, 29–89. [Google Scholar]
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Zaccarelli, E.; Chiessi, E. On the Molecular Origin of the Cooperative Coil-to-globule Transition of Poly(N-isopropylacrylamide) in Water. Phys. Chem. Chem. Phys. 2018, 20, 9997–10010. [Google Scholar] [CrossRef] [Green Version]
- Kurzbach, D.; Junk, M.J.N.; Hinderberger, D. Nanoscale inhomogeneities in thermoresponsive polymers. Macromol. Rapid Commun. 2013, 34, 119–134. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Drozdov, A.D. Self-oscillations of hydrogels driven by chemical reactions. Int. J. Appl. Mech. 2014, 6, 1450023. [Google Scholar] [CrossRef]
- Drozdov, A.D. Mechanical behavior of temperature-sensitive gels under equilibrium and transient swelling. Int. J. Eng. Sci. 2018, 128, 79–100. [Google Scholar] [CrossRef]
- Mochizuki, K.; Ben-Amotz, D. Hydration-shell transformation of thermosensitive aqueous polymers. J. Phys. Chem. Lett. 2017, 8, 1360–1364. [Google Scholar] [CrossRef]
- Drozdov, A.D.; deClaville Christiansen, J. Equilibrium swelling of thermo-responsive copolymer microgels. RSC Adv. 2020, 10, 42718–42732. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.D. Equilibrium swelling of biocompatible thermo-responsive copolymer gels. Gels 2021, 7, 40. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdov, A.D.; de Claville Christiansen, J. Equilibrium Swelling of Thermo-Responsive Gels in Mixtures of Solvents. Chemistry 2022, 4, 681-700. https://doi.org/10.3390/chemistry4030049
Drozdov AD, de Claville Christiansen J. Equilibrium Swelling of Thermo-Responsive Gels in Mixtures of Solvents. Chemistry. 2022; 4(3):681-700. https://doi.org/10.3390/chemistry4030049
Chicago/Turabian StyleDrozdov, Aleksey D., and Jesper de Claville Christiansen. 2022. "Equilibrium Swelling of Thermo-Responsive Gels in Mixtures of Solvents" Chemistry 4, no. 3: 681-700. https://doi.org/10.3390/chemistry4030049
APA StyleDrozdov, A. D., & de Claville Christiansen, J. (2022). Equilibrium Swelling of Thermo-Responsive Gels in Mixtures of Solvents. Chemistry, 4(3), 681-700. https://doi.org/10.3390/chemistry4030049







