Long-Range Supramolecular Synthon Isomerism: Insight from a Case Study of Vinylic Tellurium Trihalides Cl(Ph)C=C(Ph)TeX3 (X = Cl, I)
Abstract
:1. Introduction
2. Materials and Methods
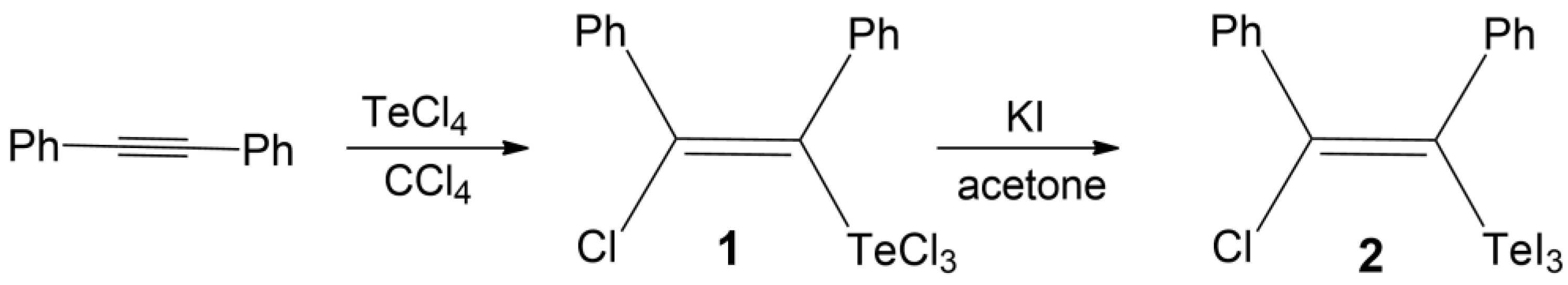
2.1. Preparation of Trichloro (Z)-2-Chloro-l,2-diphenylvinyl-tellurium(IV) Z-Cl(Ph)C=C(Ph)TeCl3 (1α and 1β)
2.2. Preparation of Triiodo [(Z)-2-Chloro-l,2-diphenylvinyl-tellurium(IV) Cl(Ph)C=C(Ph)TeI3 (2)
2.3. X-ray Crystallography
2.3.1. Single-Crystal XRD
2.3.2. PXRD
2.4. Intermolecular Energy Computations
3. Results and Discussion
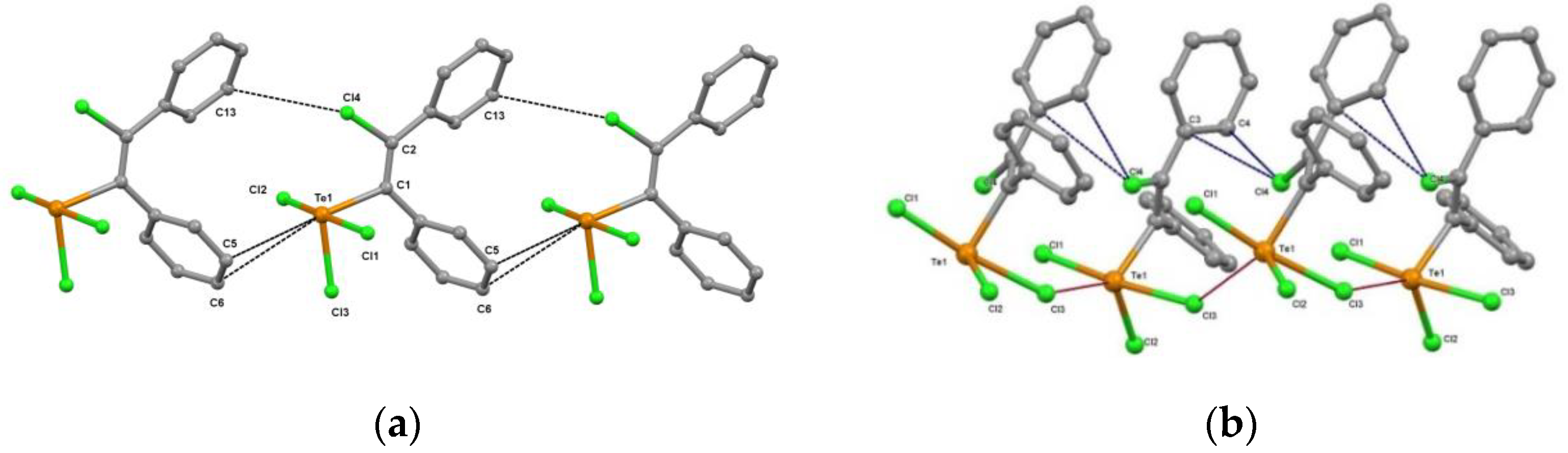
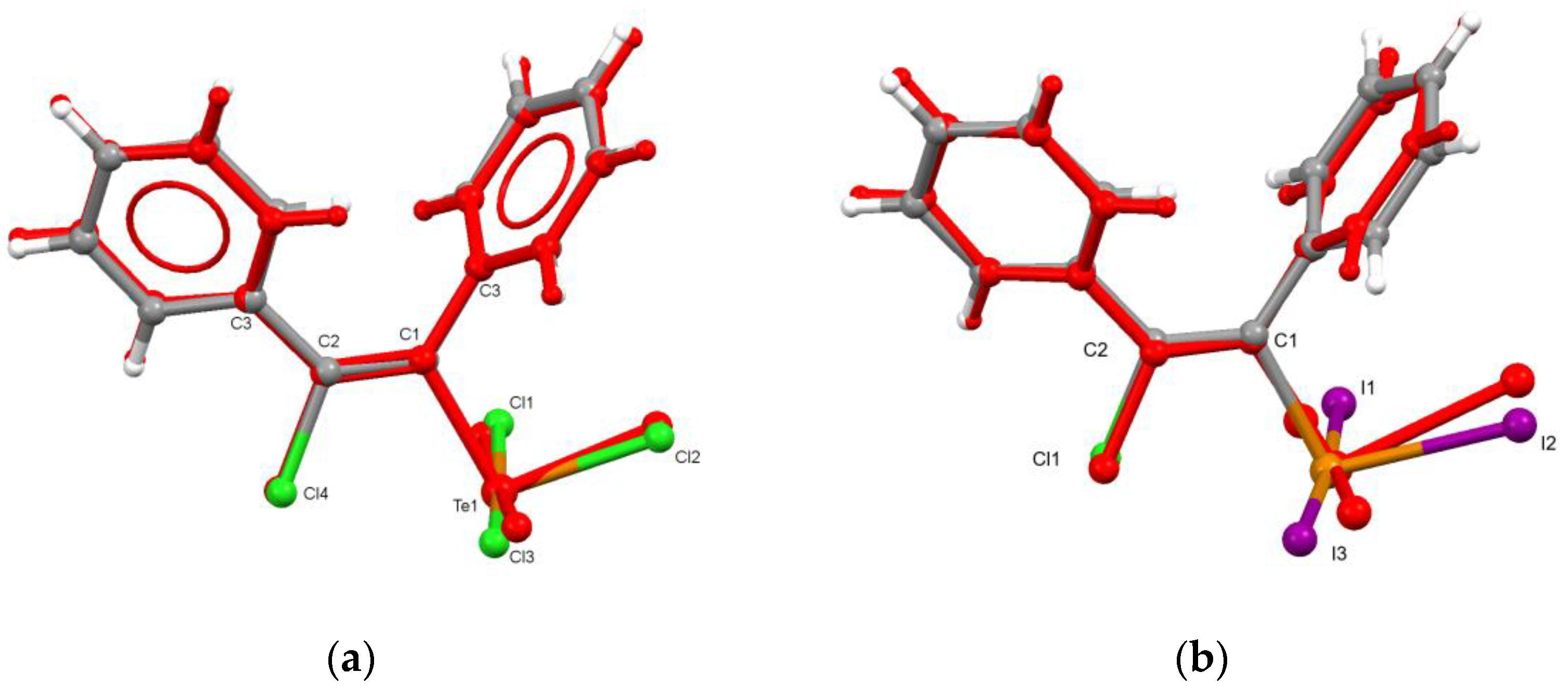
3.1. Preparation and Crystal Structure of New Polymorph of Ph(Cl)C=C(Ph)TeCl3 (1β)
3.2. Preparation and Crystal Structure of Ph(Cl)C=C(Ph)TeI3
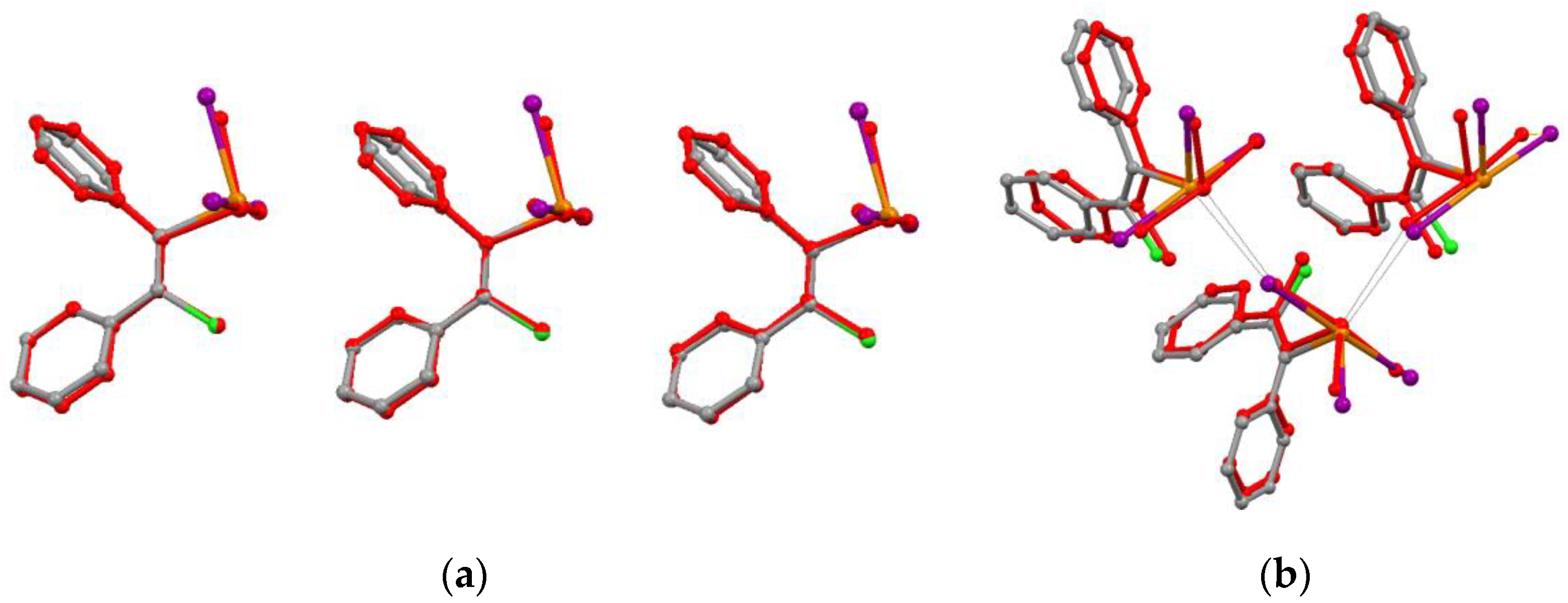
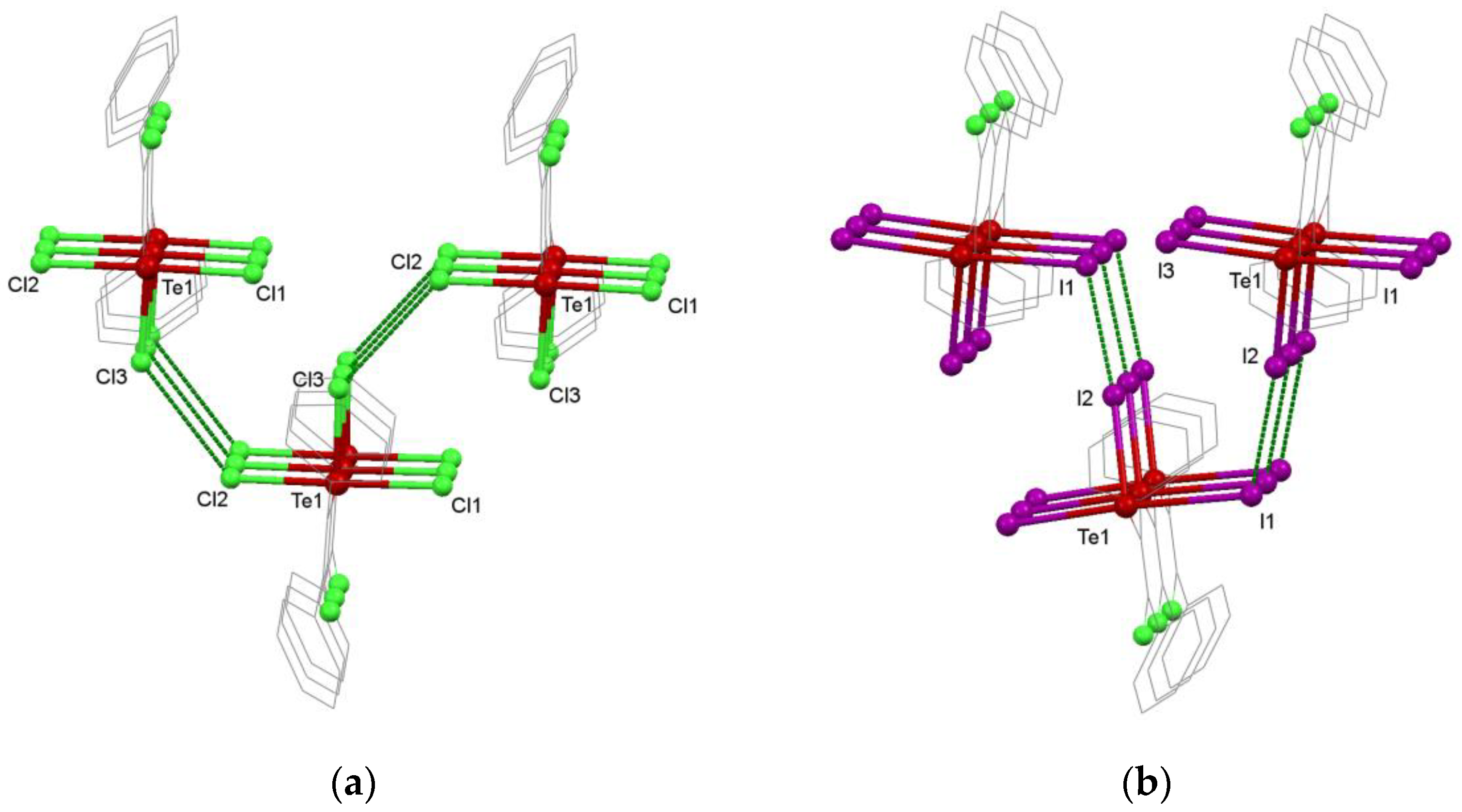
3.3. Supramolecular Organization Isomerism
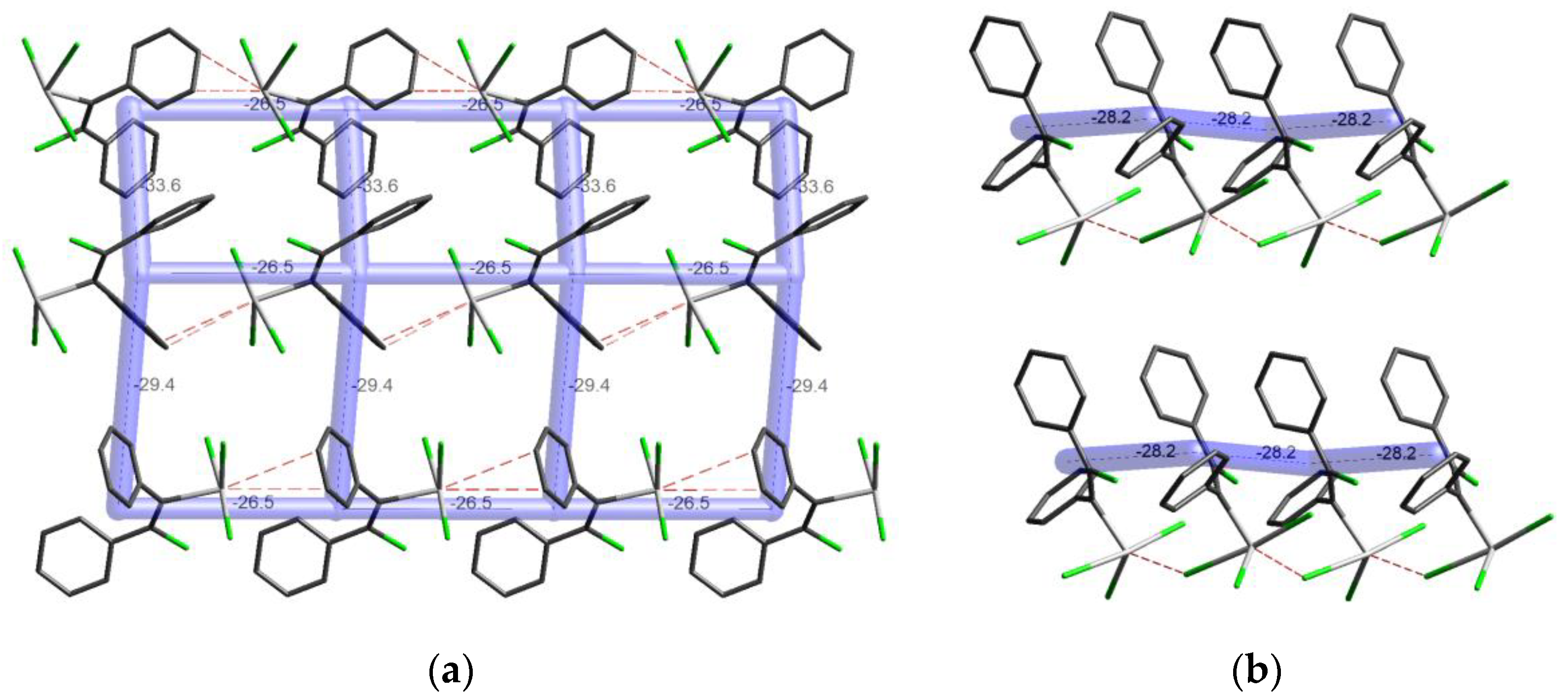
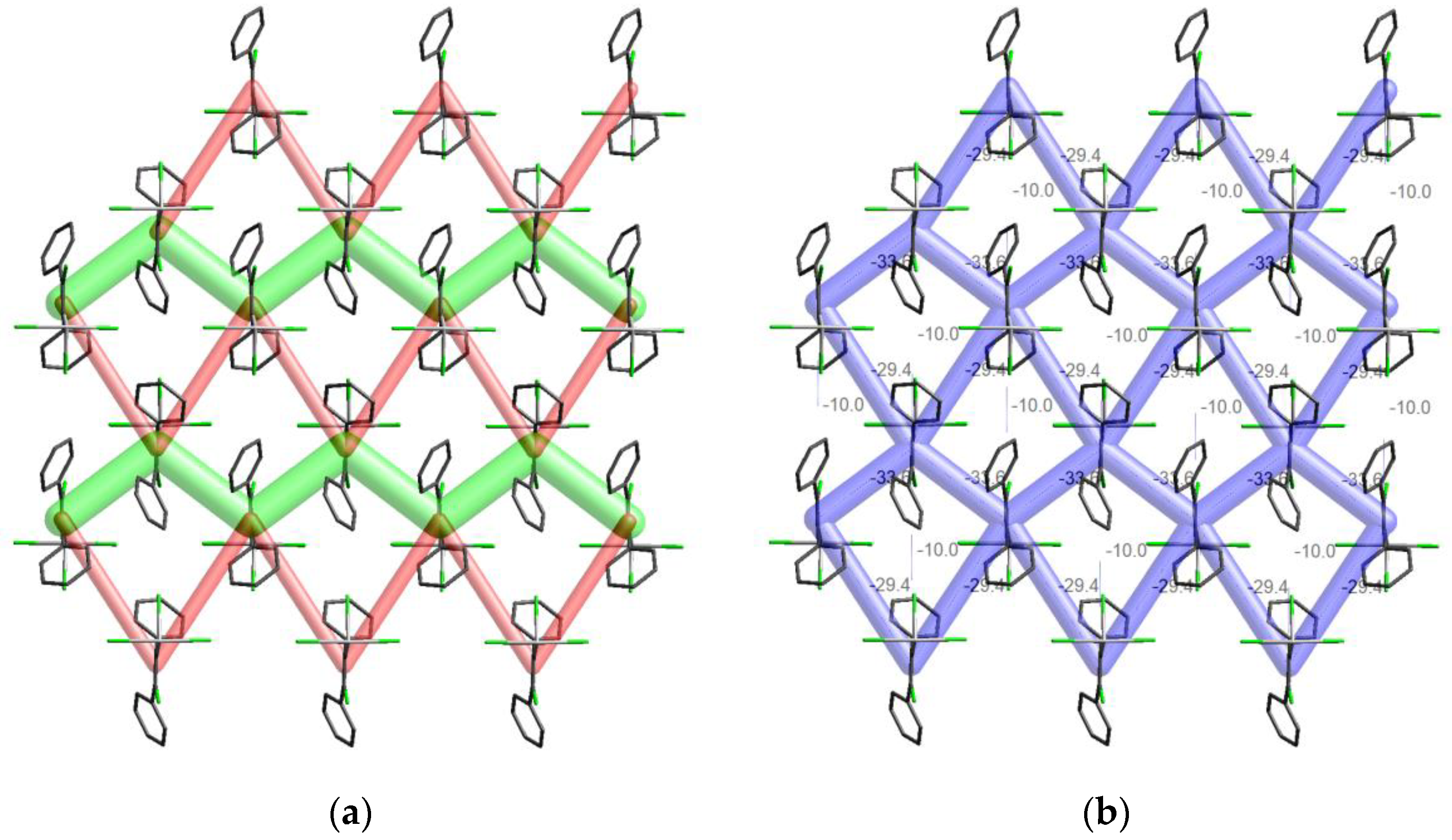
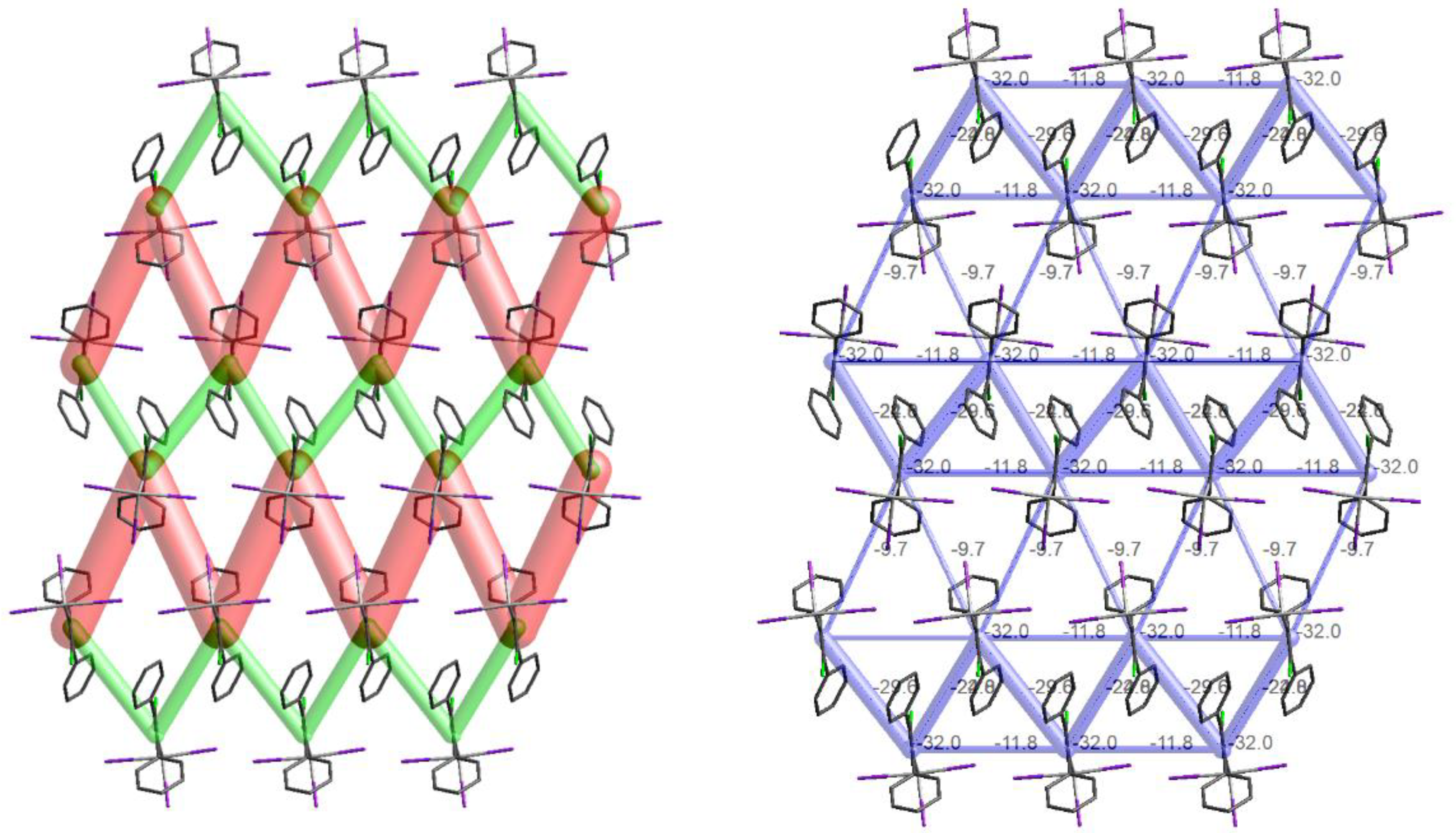
3.4. Energy Frameworks of Ph(Cl)C=C(Ph)TeX3 (X = Cl, I)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonnenberg, K.; Mann, L.; Redeker, F.A.; Schmidt, B.; Riedel, S. Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine. Angew. Chem. Int. Ed. Engl. 2020, 59, 5464–5493. [Google Scholar] [CrossRef]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen Bonding: An Overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef] [Green Version]
- Bulfield, D.; Engelage, E.; Mancheski, L.; Stoesser, J.; Huber, S.M. Crystal Engineering with Multipoint Halogen Bonding: Double Two-Point Donors and Acceptors at Work. Chemistry 2020, 26, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biot, N.; Bonifazi, D. Concurring Chalcogen- and Halogen-Bonding Interactions in Supramolecular Polymers for Crystal Engineering Applications. Chemistry 2020, 26, 2904–2913. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, K.; Wasilewska, A.; Wicher, B.; Gdaniec, M.; Połoński, T. Supramolecular Synthesis Based on a Combination of Se⋯N Secondary Bonding Interactions with Hydrogen and Halogen Bonds. Cryst. Growth Des. 2016, 16, 1282–1293. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Baldrighi, M.; Desper, J.; Metrangolo, P.; Resnati, G. Supramolecular hierarchy among halogen-bond donors. Chemistry 2013, 19, 16240–16247. [Google Scholar] [CrossRef] [Green Version]
- Torubaev, Y.V.; Dolgushin, F.M.; Skabitsky, I.V.; Popova, A.E. Isomorphic substitution in molecular crystals and geometry of hypervalent tellurium: Comments inspired by a case study of RMeTeI2 and [RMe2Te]+I− (R. = Ph, Fc). New J. Chem. 2019, 43, 12225–12232. [Google Scholar] [CrossRef]
- Chivers, T.; Laitinen, R.S. Tellurium: A maverick among the chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Zukerman-Schpector, J.; Haiduc, I. Tellurium⋯π-aryl interactions: A new bonding motif for supramolecular self-assembly and crystal engineering. CrystEngComm 2002, 4, 178–193. [Google Scholar] [CrossRef]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen Bond: A Sister Noncovalent Bond to Halogen Bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Torubaev, Y.; Pasynskii, A.; Mathur, P. Organotellurium halides: New ligands for transition metal complexes. Coord. Chem. Rev. 2012, 256, 709–721. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Lyssenko, K.A.; Popova, A.E. Halogen and Hydrogen Bonds in Co-crystalline Ferrocenium Organotellurium Halide Salts. Russ. J. Coord. Chem. 2019, 45, 788–794. [Google Scholar] [CrossRef]
- Petragnani, N.; Mendes, S.R.; Silveira, C.C. Tellurium tetrachloride: An improved method of preparation. Tetrahedron Lett. 2008, 49, 2371–2372. [Google Scholar] [CrossRef]
- Zukerman-Schpector, J.; Camillo, R.L.; Comasseto, J.V.; Santos, R.A.; Caracelli, I. Trichloro[(Z)-2-chloro-1,2-diphenylvinyl]tellurium(IV). Acta Crystallogr. Sect. C 1999, 55, 1577–1579. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPASandTOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.P.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Accurate Lattice Energies for Molecular Crystals from Experimental Crystal Structures. J. Chem. Theory Comput. 2018, 14, 1614–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, R.L.O.R.; Zukerman-Schpector, J.; Caracelli, I.; Comasseto, J.V. Revisiting the addition reaction of TeCl4 to alkynes: The crystal structure and docking studies of 1-chloro-2-trichlorotelluro-3-phenyl-propen-2-ol. J. Organomet. Chem. 2006, 691, 4807–4815. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Polymorphism in cocrystals: A review and assessment of its significance. CrystEngComm 2014, 16, 3451–3465. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Skabitsky, I.V.; Anisimov, A.A.; Ananyev, I.V. Long-range supramolecular synthon polymorphism: A case study of two new polymorphic cocrystals of Ph2Te2–1,4-C6F4I2. CrystEngComm 2022, 24, 1442–1452. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. Organic Chemistry Crytallography; Consultants Bureau: New York, NY, USA, 1961. [Google Scholar]
- Perlstein, J. Molecular Self-Assemblies. 4. Using Kitaigorodskii’s Aufbau Principle for Quantitatively Predicting the Packing Geometry of Semiflexible Organic Molecules in Translation Monolayer Aggregates. J. Am. Chem. Soc. 2002, 116, 11420–11432. [Google Scholar] [CrossRef]
- Torubaev, Y.; Skabitsky, I.; Lyssenko, K.A. Stages of Kitaigorodsky Aufbau Principle Detached in the Cocrystals of Cp2MX2 (M = Ti, Zr; X = Cl, Br, I) with σ- and π-Hole Donors. Cryst. Growth Des. 2022, 22, 1244–1252. [Google Scholar] [CrossRef]
- Dunitz, J.D. Intermolecular atom-atom bonds in crystals? IUCrJ 2015, 2, 157–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torubaev, Y.V.; Skabitsky, I.V.; Saratov, G.A.; Barzilovich, P.Y. Halogen vs. ionic bonding: An unusual isomorphism between the neutral (C5Me5)2Fe/C2I2 cocrystal and ionic [(C5Me5)2Fe]Br3 crystal. Mendeleev Commun. 2021, 31, 58–61. [Google Scholar] [CrossRef]
- Gavezzotti, A. Pillars of crystal engineering: Crystal energies and symmetry operators. CrystEngComm 2018, 20, 2511–2518. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Rai, D.K.; Skabitsky, I.V.; Pakhira, S.; Dmitrienko, A. Energy framework approach to the supramolecular reactions: Interplay of the secondary bonding interaction in Ph2E2 (E = Se, Te)/p-I-C6F4I2 co-crystals. New J. Chem. 2019, 43, 7941–7949. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Bernstein, J. Disappearing Polymorphs. Acc. Chem. Res. 2002, 28, 193–200. [Google Scholar] [CrossRef]
- Bucar, D.K.; Lancaster, R.W.; Bernstein, J. Disappearing polymorphs revisited. Angew. Chem. Int. Ed. Engl. 2015, 54, 6972–6993. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torubaev, Y.V.; Samigullina, A.S. Long-Range Supramolecular Synthon Isomerism: Insight from a Case Study of Vinylic Tellurium Trihalides Cl(Ph)C=C(Ph)TeX3 (X = Cl, I). Chemistry 2022, 4, 196-205. https://doi.org/10.3390/chemistry4010017
Torubaev YV, Samigullina AS. Long-Range Supramolecular Synthon Isomerism: Insight from a Case Study of Vinylic Tellurium Trihalides Cl(Ph)C=C(Ph)TeX3 (X = Cl, I). Chemistry. 2022; 4(1):196-205. https://doi.org/10.3390/chemistry4010017
Chicago/Turabian StyleTorubaev, Yury V., and Aida S. Samigullina. 2022. "Long-Range Supramolecular Synthon Isomerism: Insight from a Case Study of Vinylic Tellurium Trihalides Cl(Ph)C=C(Ph)TeX3 (X = Cl, I)" Chemistry 4, no. 1: 196-205. https://doi.org/10.3390/chemistry4010017
APA StyleTorubaev, Y. V., & Samigullina, A. S. (2022). Long-Range Supramolecular Synthon Isomerism: Insight from a Case Study of Vinylic Tellurium Trihalides Cl(Ph)C=C(Ph)TeX3 (X = Cl, I). Chemistry, 4(1), 196-205. https://doi.org/10.3390/chemistry4010017





