A Sustainable Synthetic Approach to the Indaceno[1,2-b:5,6-b′]dithiophene (IDT) Core through Cascade Cyclization–Deprotection Reactions
Abstract
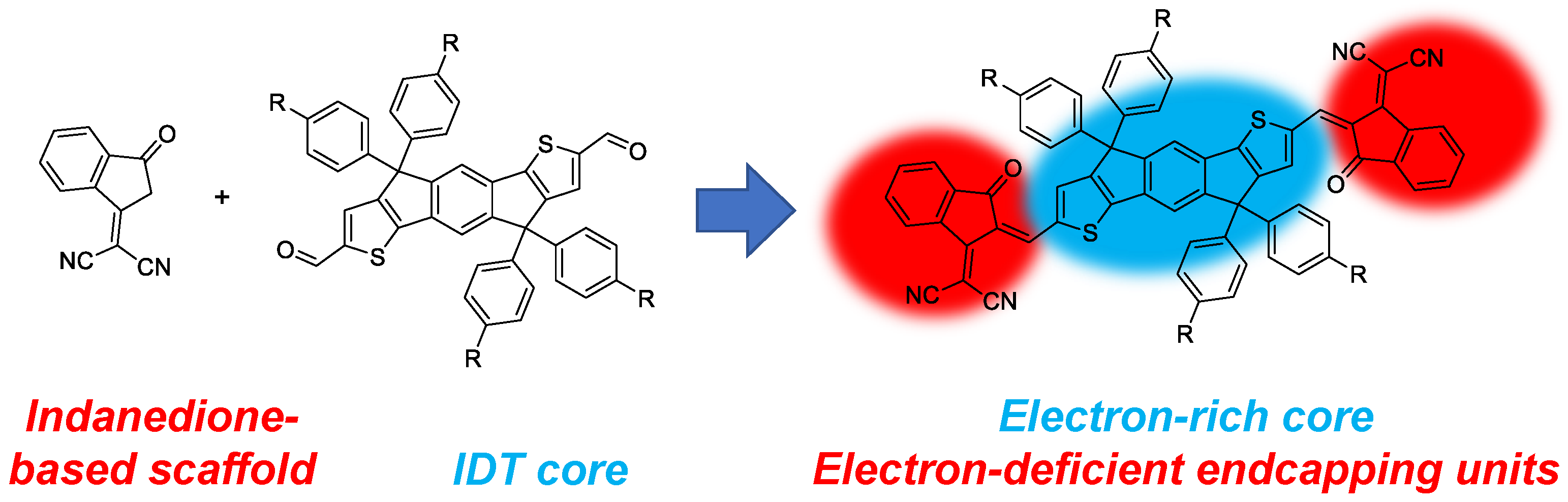
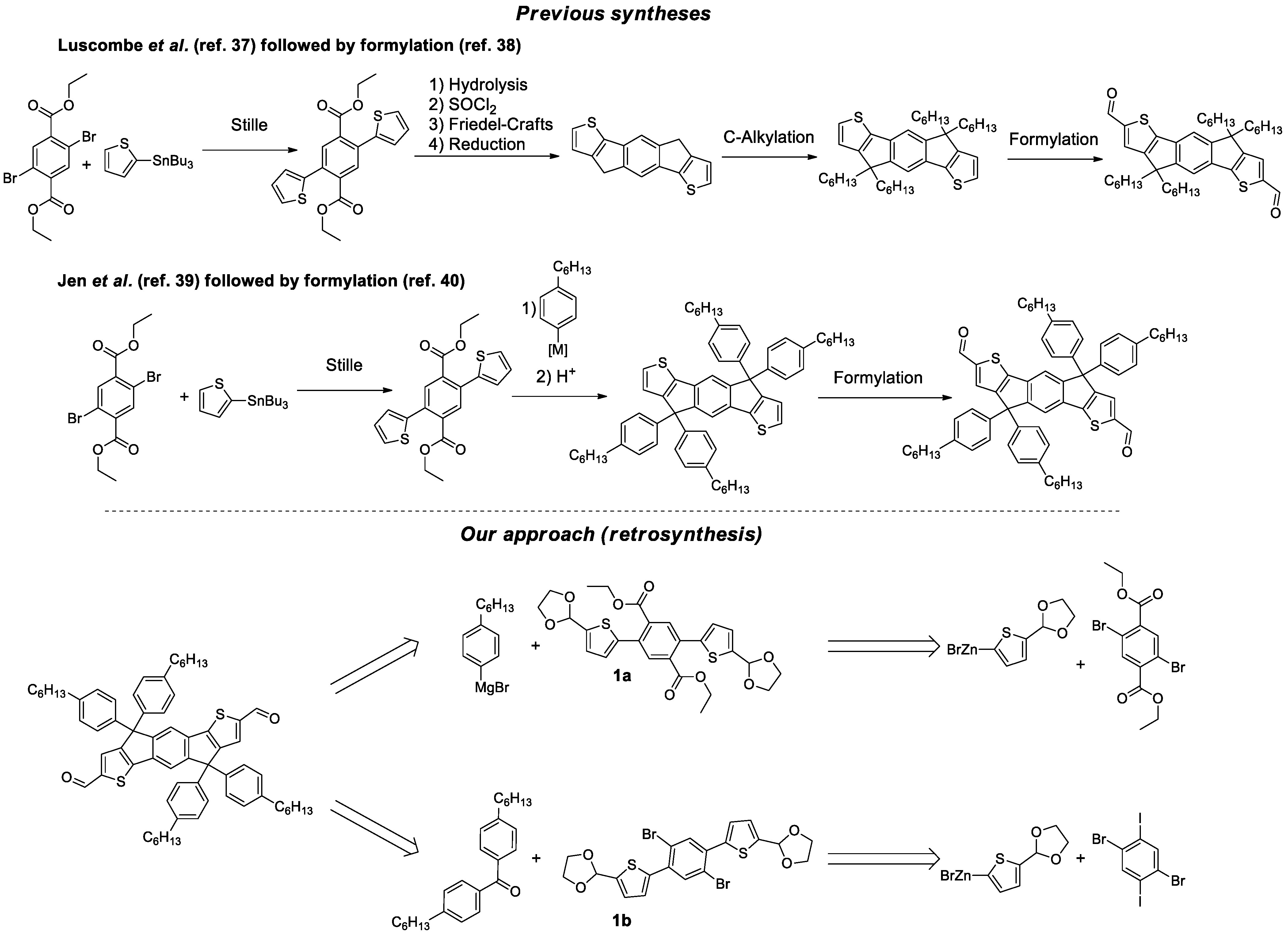
:1. Introduction
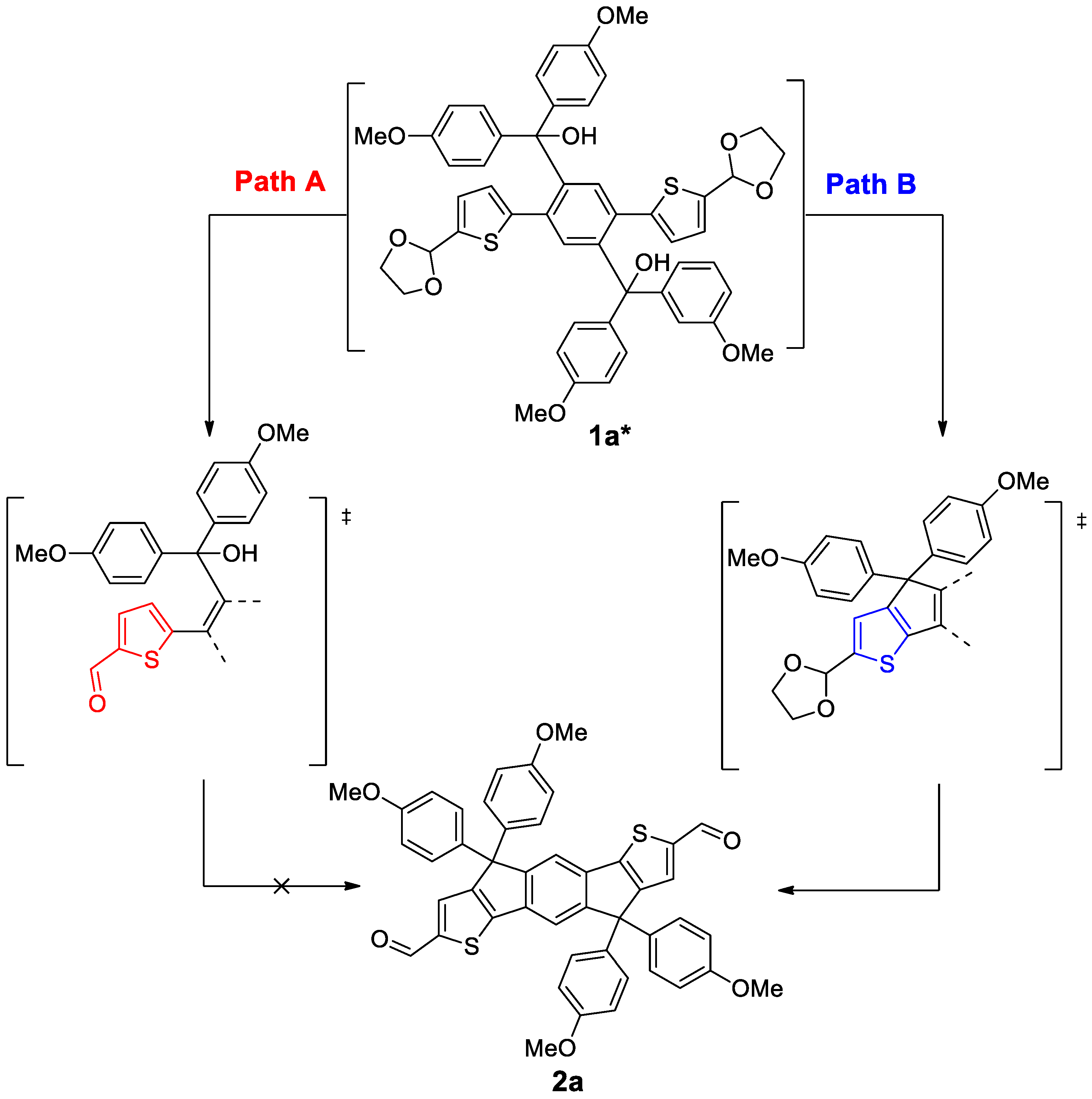
2. Results and Discussion
3. Experimental Section
3.1. General Experimental
3.2. Synthesis of New Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dauzon, E.; Sallenave, X.; Plesse, C.; Goubard, F.; Amassian, A.; Anthopoulos, T.D. Pushing the Limits of Flexibility and Stretchability of Solar Cells: A Review. Adv. Mater. 2021, 33, 2101469. [Google Scholar] [CrossRef]
- Seri, M.; Mercuri, F.; Ruani, G.; Feng, Y.; Li, M.; Xu, Z.-X.; Muccini, M. Toward Real Setting Applications of Organic and Perovskite Solar Cells: A Comparative Review. Energy Technol. 2021, 9, 2000901. [Google Scholar] [CrossRef]
- Yao, J.; Qiu, B.; Zhang, Z.-G.; Xue, L.; Wang, R.; Zhang, C.; Chen, S.; Zhou, Q.; Sun, C.; Yang, C.; et al. Cathode engineering with perylene-diimide interlayer enabling over 17% efficiency single-junction organic solar cells. Nat. Commun. 2020, 11, 2726. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, X.; Zhang, Y.; Zhou, H. Role of interface properties in organic solar cells: From substrate engineering to bulk-heterojunction interfacial morphology. Mater. Chem. Front. 2020, 4, 2863–2880. [Google Scholar] [CrossRef]
- Xu, X.; Li, D.; Yuan, J.; Zhou, Y.; Zou, Y. Recent advances in stability of organic solar cells. EnergyChem 2021, 3, 100046. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Kolhe, N.B.; West, S.M.; Tran, D.K.; Ding, X.; Kuzuhara, D.; Yoshimoto, N.; Koganezawa, T.; Jenekhe, S.A. Designing High Performance Nonfullerene Electron Acceptors with Rylene Imides for Efficient Organic Photovoltaics. Chem. Mater. 2020, 32, 195–204. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, Q.; Zhou, J.; Lai, H.; Liu, T.; Li, D.; Chen, W.; Xie, Z.; He, F. Multiple Fused Ring-Based Near-Infrared Nonfullerene Acceptors with an Interpenetrated Charge-Transfer Network. Chem. Mater. 2019, 31, 1664–1671. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Ye, L.; Weng, K.; Fu, H.; Ryu, H.S.; Wei, D.; Sun, X.; Woo, H.Y.; Sun, Y. Asymmetric A–D–π–A-type nonfullerene small molecule acceptors for efficient organic solar cells. J. Mater. Chem. A 2019, 7, 19348–19354. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, H.; Hou, J.; Zhu, J.; Zhang, J.; Li, W.; Yu, R.; Gao, B.; Zhang, S.; Hou, J. Over 14% Efficiency in Organic Solar Cells Enabled by Chlorinated Nonfullerene Small-Molecule Acceptors. Adv. Mater. 2018, 30, 1800613. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zhou, X.; Jia, Q.-Q.; Feng, S.; Jiang, P.; Xu, X.; Ma, W.; Li, H.-B.; Bo, Z. Nonfullerene Acceptors with Enhanced Solubility and Ordered Packing for High-Efficiency Polymer Solar Cells. ACS Energy Lett. 2018, 3, 1832–1839. [Google Scholar] [CrossRef]
- Forti, G.; Nitti, A.; Osw, P.; Bianchi, G.; Po, R.; Pasini, D. Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells. Int. J. Mol. Sci. 2020, 21, 8085. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, Q.; Zhao, F.; Huo, L.; Mai, J.; Lu, X.; Su, C.-J.; Li, T.; Wang, J.; Zhu, J.; et al. A Facile Planar Fused-Ring Electron Acceptor for As-Cast Polymer Solar Cells with 8.71% Efficiency. J. Am. Chem. Soc. 2016, 138, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Klopp, J.M.; Pasini, D.; Frechet, J.M.J.; Willson, C.G.; Byers, J.D. Microlithographic Assessment of a Novel Family of Transparent and Etch Resistant Chemically Amplified 193 nm Resists Based on Cyclopolymers. Chem. Mater. 2001, 13, 4147–4153. [Google Scholar] [CrossRef]
- Corsini, F.; Nitti, A.; Tatsi, E.; Mattioli, G.; Botta, C.; Pasini, D.; Griffini, G. Large-Area Semi-Transparent Luminescent Solar Concentrators Based on Large Stokes Shift Aggregation-Induced Fluorinated Emitters Obtained Through a Sustainable Synthetic Approach. Adv. Opt. Mater. 2021, 9, 2100182. [Google Scholar] [CrossRef]
- Chen, H.; Wadsworth, A.; Ma, C.; Nanni, A.; Zhang, W.; Nikolka, M.; Luci, A.M.T.; Perdigão, L.M.A.; Thorley, K.J.; Cendra, C.; et al. The Effect of Ring Expansion in Thienobenzo[b]indacenodithiophene Polymers for Organic Field-Effect Transistors. J. Am. Chem. Soc. 2019, 141, 18806–18813. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiao, J.; Wu, L.; Zhang, W.; Ge, Z.; Tan, S. Synthesis and photovoltaic properties of small molecule electron acceptors with twin spiro-type core structure. Dyes Pigment. 2019, 168, 197–204. [Google Scholar] [CrossRef]
- Nitti, A.; Bianchi, G.; Po, R.; Pasini, D. Scalable Synthesis of Naphthothiophene and Benzodithiophene Scaffolds as π-Conjugated Synthons for Organic Materials. Synthesis 2019, 51, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Nitti, A.; Bianchi, G.; Po, R.; Pasini, D. Direct Arylation Strategies in the Synthesis of pi-Extended Monomers for Organic Polymeric Solar Cells. Molecules 2017, 22, 21. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Dai, S.; Wu, Y.; Zhang, Q.; Wang, J.; Jiang, L.; Ling, Q.; Wei, Z.; Ma, W.; You, W.; et al. Single-Junction Binary-Blend Nonfullerene Polymer Solar Cells with 12.1% Efficiency. Adv. Mater. 2017, 29, 1700144. [Google Scholar] [CrossRef] [Green Version]
- Nitti, A.; Forti, G.; Bianchi, G.; Botta, C.; Tinti, F.; Gazzano, M.; Camaioni, N.; Po, R.; Pasini, D. Anthradithiophene-based organic semiconductors through regiodirected double annulations. J. Mater. Chem. C 2021, 9, 9302–9308. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Gao, B.; Qin, Y.; Zhang, S.; Yang, B.; He, C.; Xu, B.; Hou, J. Fine-Tuned Photoactive and Interconnection Layers for Achieving over 13% Efficiency in a Fullerene-Free Tandem Organic Solar Cell. J. Am. Chem. Soc. 2017, 139, 7302–7309. [Google Scholar] [CrossRef]
- Xie, D.; Liu, T.; Gao, W.; Zhong, C.; Huo, L.; Luo, Z.; Wu, K.; Xiong, W.; Liu, F.; Sun, Y.; et al. A Novel Thiophene-Fused Ending Group Enabling an Excellent Small Molecule Acceptor for High-Performance Fullerene-Free Polymer Solar Cells with 11.8% Efficiency. Sol. RRL 2017, 1, 1700044. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, W.; Wang, X.; Wu, Y.; Zhang, Q.; Yan, C.; Ma, W.; You, W.; Zhan, X. Enhancing Performance of Nonfullerene Acceptors via Side-Chain Conjugation Strategy. Adv. Mater. 2017, 29, 1702125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhan, X. Fused-Ring Electron Acceptors for Photovoltaics and Beyond. Acc. Chem. Res. 2021, 54, 132–143. [Google Scholar] [CrossRef]
- Nitti, A.; Bianchi, G.; Po, R.; Porta, A.; Galbiati, A.; Pasini, D. Weiss-Cook Condensations for the Synthesis of Bridged Bithiophene Monomers and Polymers. ChemistrySelect 2019, 4, 12569–12572. [Google Scholar] [CrossRef]
- Pasini, D.; Klopp, J.M.; Frechet, J.M.J. Design, Synthesis, and Characterization of Carbon-Rich Cyclopolymers for 193nm Microlithography. Chem. Mater. 2001, 13, 4136–4146. [Google Scholar] [CrossRef]
- Nitti, A.; Osw, P.; Calcagno, G.; Botta, C.; Etkind, S.I.; Bianchi, G.; Po, R.; Swager, T.M.; Pasini, D. One-Pot Regiodirected Annulations for the Rapid Synthesis of π-Extended Oligomers. Org. Lett. 2020, 22, 3263–3267. [Google Scholar] [CrossRef] [PubMed]
- Penconi, M.; Bianchi, G.; Nitti, A.; Savoini, A.; Carbonera, C.; Pasini, D.; Po, R.; Luzzati, S. A Donor Polymer with a Good Compromise between Efficiency and Sustainability for Organic Solar Cells. Adv. Energy Sustain. Res. 2021, 2, 2100069. [Google Scholar] [CrossRef]
- Osw, P.; Nitti, A.; Abdullah, M.N.; Etkind, S.I.; Mwaura, J.; Galbiati, A.; Pasini, D. Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as p-Type Organic Materials for Bulk-Heterojunction Solar Cells. Polymers 2020, 12, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitti, A.; Osw, P.; Abdullah, M.N.; Galbiati, A.; Pasini, D. Scalable Synthesis of Naphthothiophene-based D-π-D Extended Oligomers through Cascade Direct Arylation Processes. Synlett 2018, 29, 2577–2581. [Google Scholar]
- Nitti, A.; Bianchi, G.; Po, R.; Swager, T.M.; Pasini, D. Domino Direct Arylation and Cross-Aldol for Rapid Construction of Extended Polycyclic π-Scaffolds. J. Am. Chem. Soc. 2017, 139, 8788–8791. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tatum, W.K.; Onorato, J.W.; Barajas, S.D.; Yang, Y.Y.; Luscombe, C.K. An indacenodithiophene-based semiconducting polymer with high ductility for stretchable organic electronics. Polym. Chem. 2017, 8, 5185–5193. [Google Scholar] [CrossRef]
- Wang, C.; Du, T.; Liang, Z.; Zhu, J.; Ren, J.; Deng, Y. Synthesis of low-bandgap small molecules by extending the π-conjugation of the termini in quinoidal compounds. J. Mater. Chem. C 2021, 9, 2054–2062. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Yip, H.-L.; Chen, K.-S.; Zeigler, D.F.; Sun, Y.; Jen, A.K.-Y. Indacenodithiophene and Quinoxaline-Based Conjugated Polymers for Highly Efficient Polymer Solar Cells. Chem. Mater. 2011, 23, 2289–2291. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wu, F.-P.; Zhou, Y.; Jiang, Z.-Q.; Song, B.; Xia, Y.; Zhang, Z.-G.; Gao, F.; Inganäs, O.; et al. Non-fullerene acceptor with low energy loss and high external quantum efficiency: Towards high performance polymer solar cells. J. Mater. Chem. A 2016, 4, 5890–5897. [Google Scholar] [CrossRef]
- Terenti, N.; Petronela Crisan, A.; Jungsuttiwong, S.; Hadade, N.D.; Pop, A.; Grosu, I.; Roncali, J. Effect of the mode of fixation of the thienyl rings on the electronic properties of electron acceptors based on indacenodithiophene (IDT). Dyes Pigment. 2021, 187, 109116. [Google Scholar] [CrossRef]
- Li, J.-N.; Cui, M.; Dong, J.; Jing, W.; Bao, J.; Liu, Z.; Ma, Z.; Gao, X. Voltage loss analysis of novel non-fullerene acceptors with chlorinated non-conjugated thienyl chains. Dyes Pigment. 2021, 188, 109162. [Google Scholar] [CrossRef]
- Ming, S.; Liu, Y.; Feng, S.; Jiang, P.; Zhang, C.; Li, M.; Song, J.; Bo, Z. Fused-ring acceptor with a spiro-bridged ladder-type core for organic solar cells. Dyes Pigment. 2019, 163, 153–158. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Sanzone, A.; Calascibetta, A.; Ghiglietti, E.; Ceriani, C.; Mattioli, G.; Mattiello, S.; Sassi, M.; Beverina, L. Suzuki-Miyaura Micellar One-Pot Synthesis of Symmetrical and Unsymmetrical 4,7-Diaryl-5,6-difluoro-2,1,3-benzothiadiazole Luminescent Derivatives in Water and under Air. J. Org. Chem. 2018, 83, 15029–15042. [Google Scholar] [CrossRef] [PubMed]
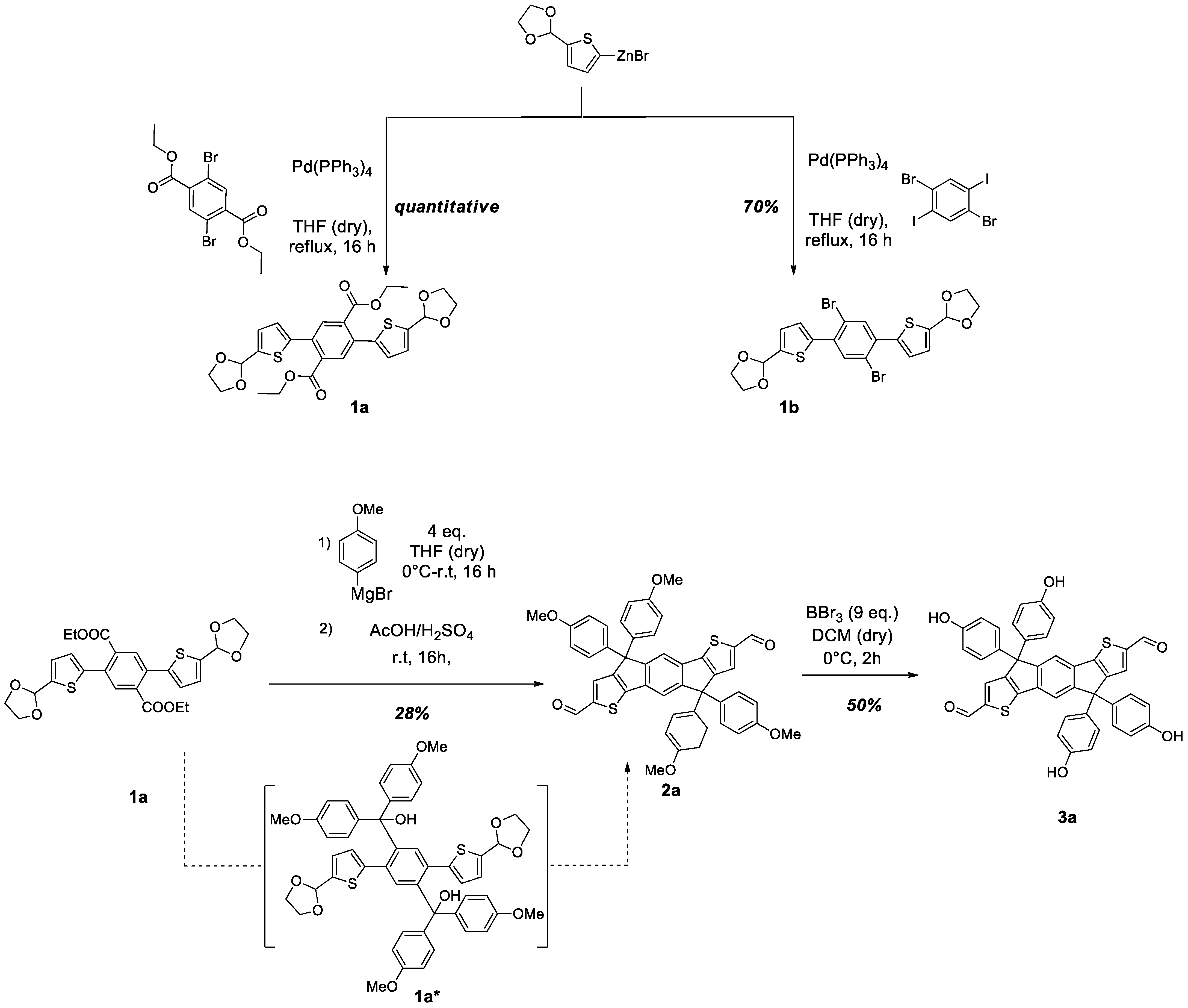
| Entry | H+ Source | Conditions | Yield 1 |
|---|---|---|---|
| 1 | HCl/AcOH | Reflux, 3 h | 7% |
| 2 | H2SO4/AcOH | Reflux, 3 h | 7% |
| 3 | H2SO4/AcOH | r.t., 16 h | 28% |
| 4 | SiO2/H2O | r.t., 16 h | - |
| 5 | Amberlyst | r.t., 16 h | - |
| 6 | BF3·Et2O | 0 °C to r.t., 2 h | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forti, G.; Nitti, A.; Bianchi, G.; Po, R.; Pasini, D. A Sustainable Synthetic Approach to the Indaceno[1,2-b:5,6-b′]dithiophene (IDT) Core through Cascade Cyclization–Deprotection Reactions. Chemistry 2022, 4, 206-215. https://doi.org/10.3390/chemistry4010018
Forti G, Nitti A, Bianchi G, Po R, Pasini D. A Sustainable Synthetic Approach to the Indaceno[1,2-b:5,6-b′]dithiophene (IDT) Core through Cascade Cyclization–Deprotection Reactions. Chemistry. 2022; 4(1):206-215. https://doi.org/10.3390/chemistry4010018
Chicago/Turabian StyleForti, Giacomo, Andrea Nitti, Gabriele Bianchi, Riccardo Po, and Dario Pasini. 2022. "A Sustainable Synthetic Approach to the Indaceno[1,2-b:5,6-b′]dithiophene (IDT) Core through Cascade Cyclization–Deprotection Reactions" Chemistry 4, no. 1: 206-215. https://doi.org/10.3390/chemistry4010018
APA StyleForti, G., Nitti, A., Bianchi, G., Po, R., & Pasini, D. (2022). A Sustainable Synthetic Approach to the Indaceno[1,2-b:5,6-b′]dithiophene (IDT) Core through Cascade Cyclization–Deprotection Reactions. Chemistry, 4(1), 206-215. https://doi.org/10.3390/chemistry4010018







