Enantiopure Cyclometalated Rh(III) and Ir(III) Complexes Displaying Rigid Configuration at Metal Center: Design, Structures, Chiroptical Properties and Role of the Iodide Ligand
Abstract
:1. Introduction
2. Results and Discussions
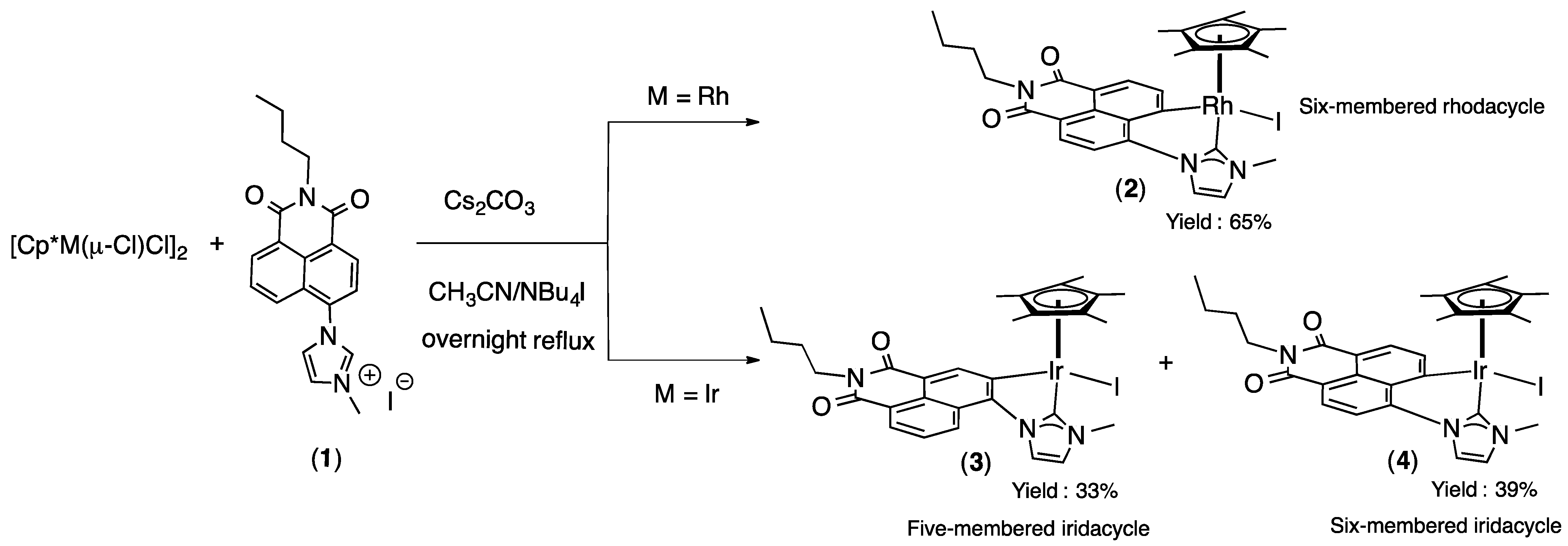
2.1. Synthesis and Characterization
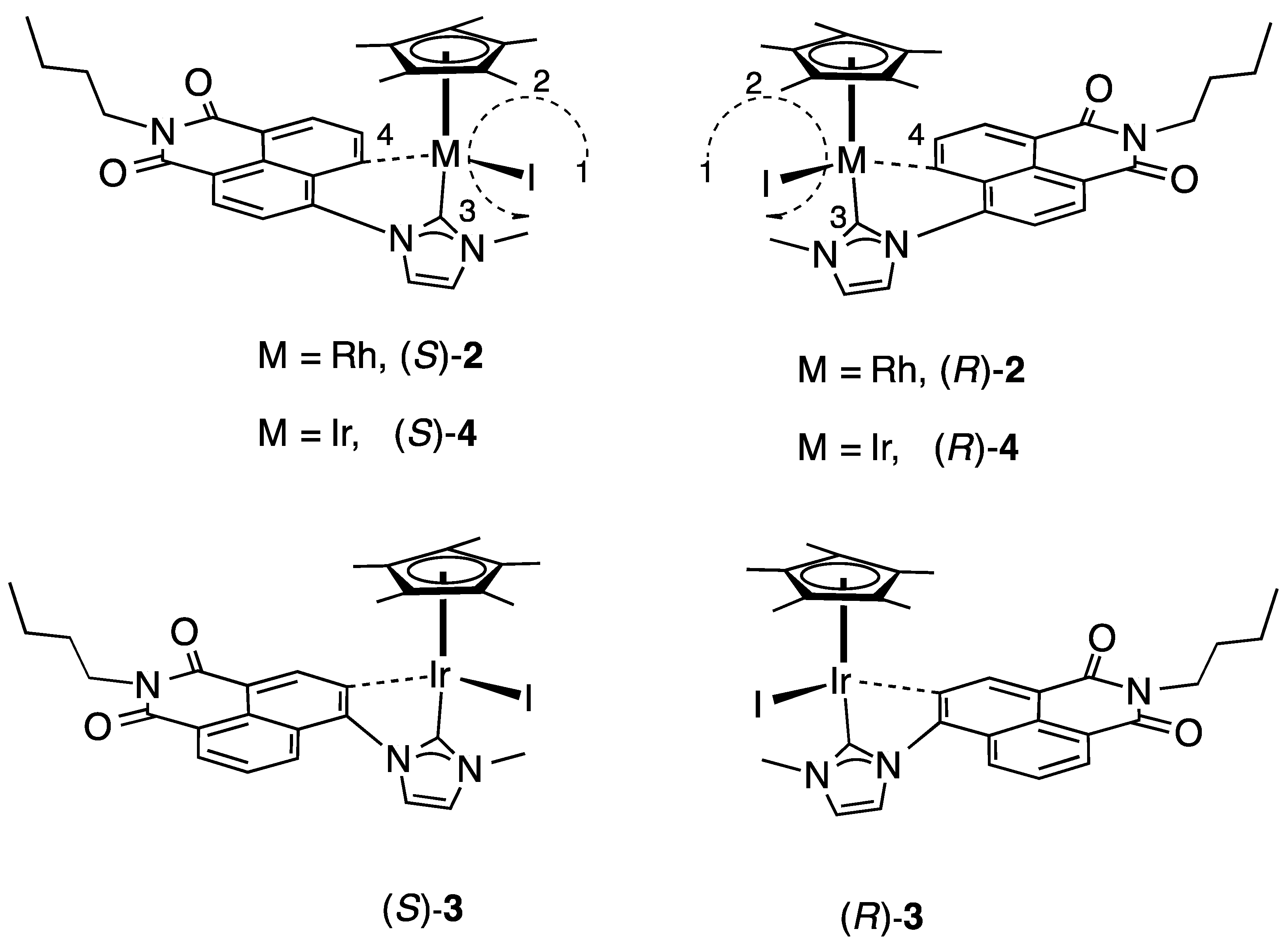
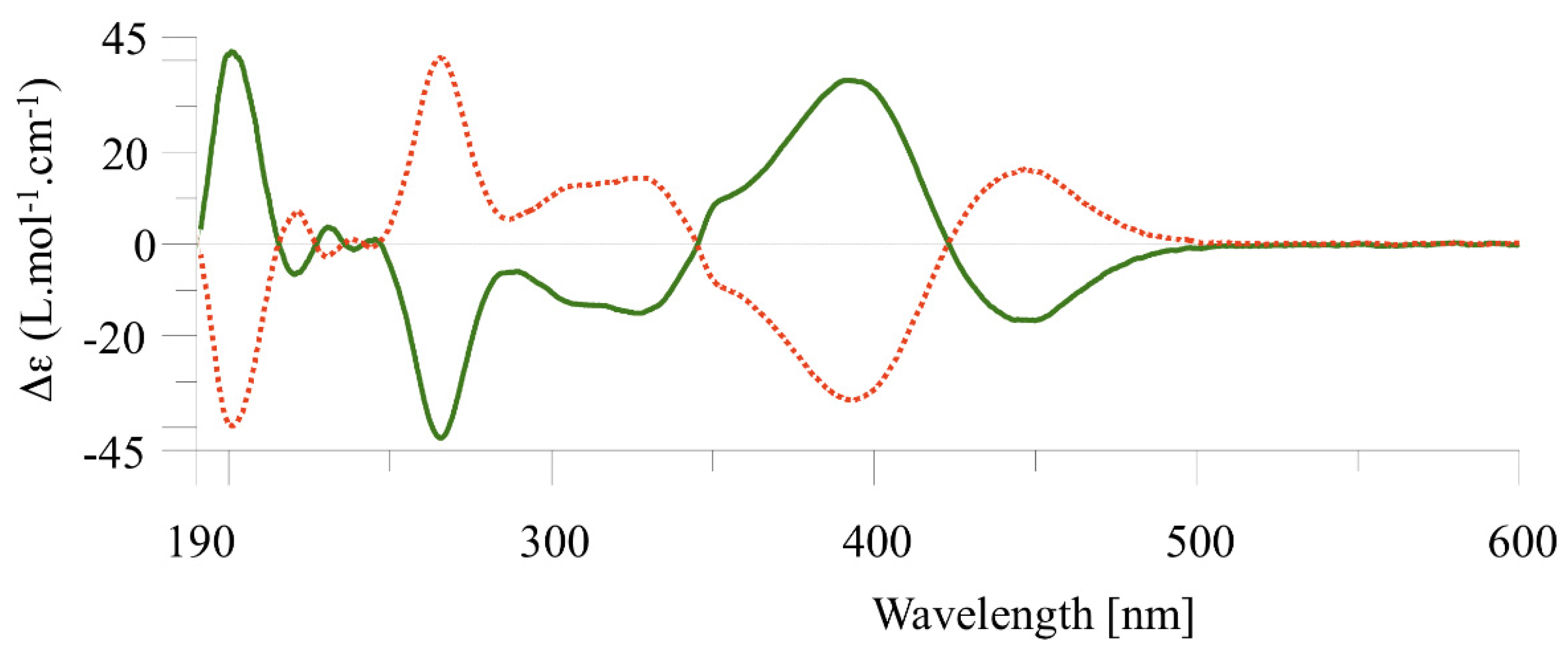
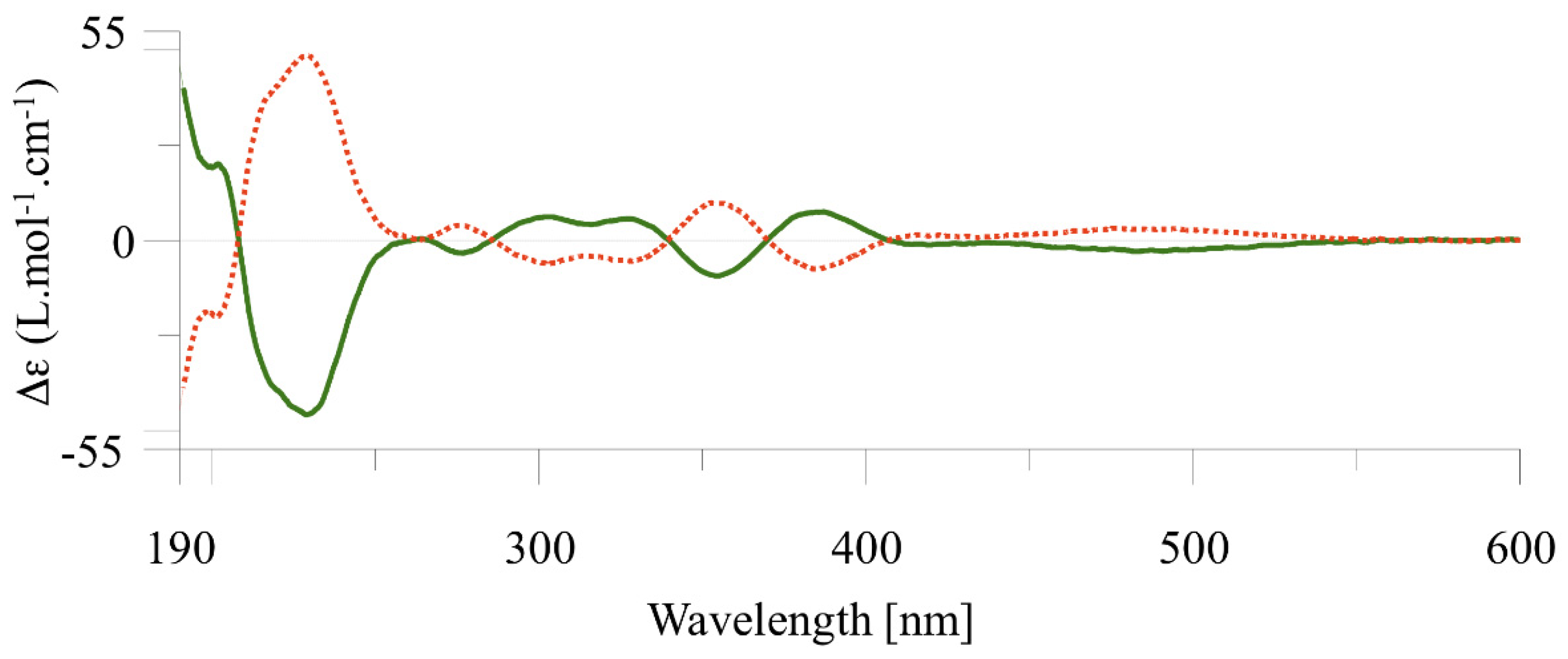
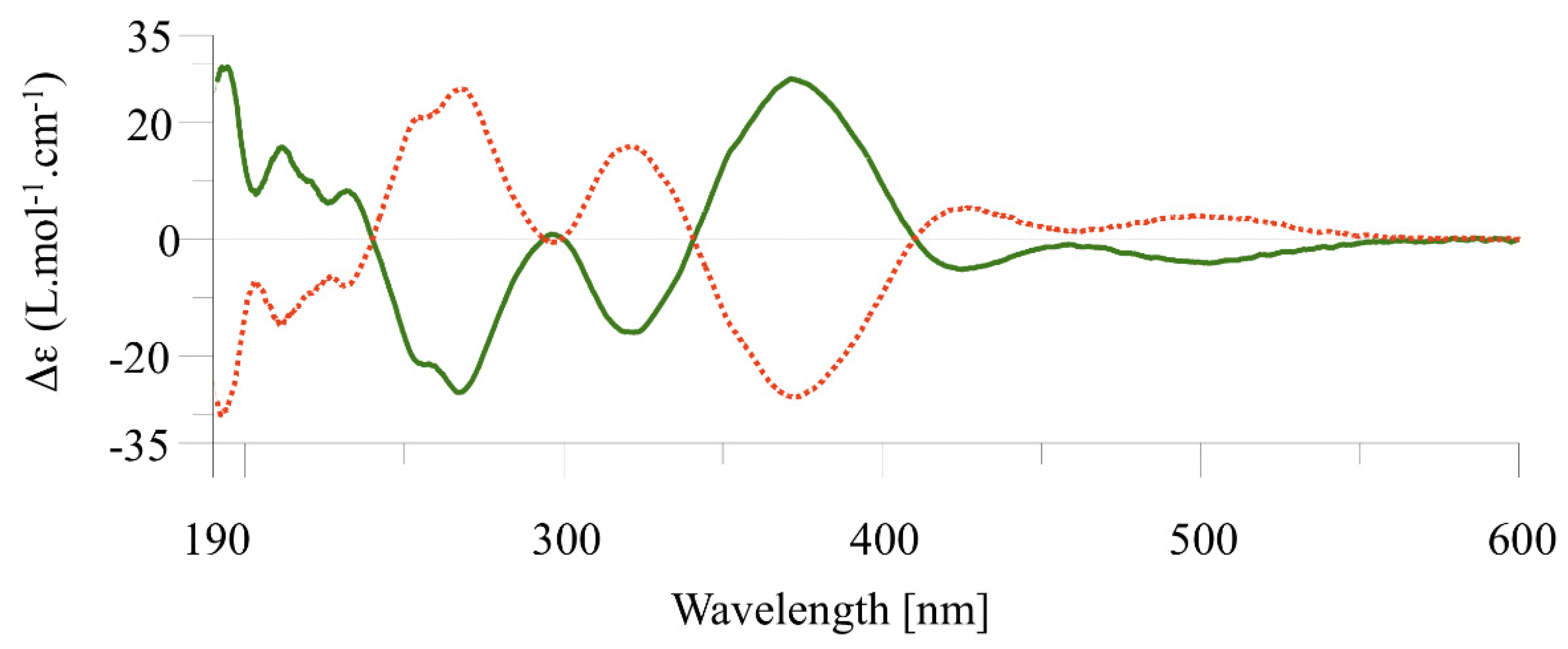
2.2. Chiral Resolution of Complexes [Cp*M(C^C:)I] (M = Rh, (2); M = Ir (3–4) and Chiroptical Properties
2.3. Molecular Structures of S-2 and S-4
2.4. Determination of the Enantiomerization Barriers and Configurational Stability
2.5. Discussion
3. Materials and Methods
General Synthetic Procedure
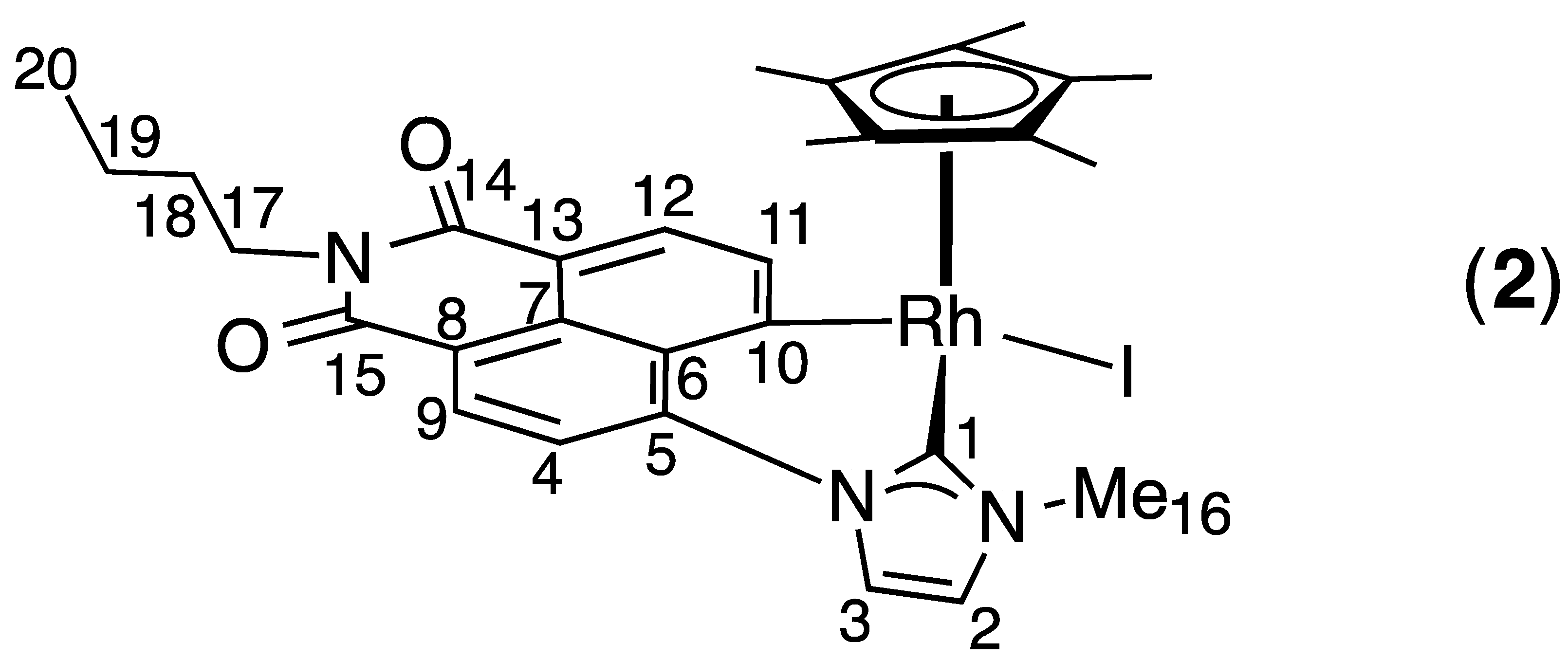
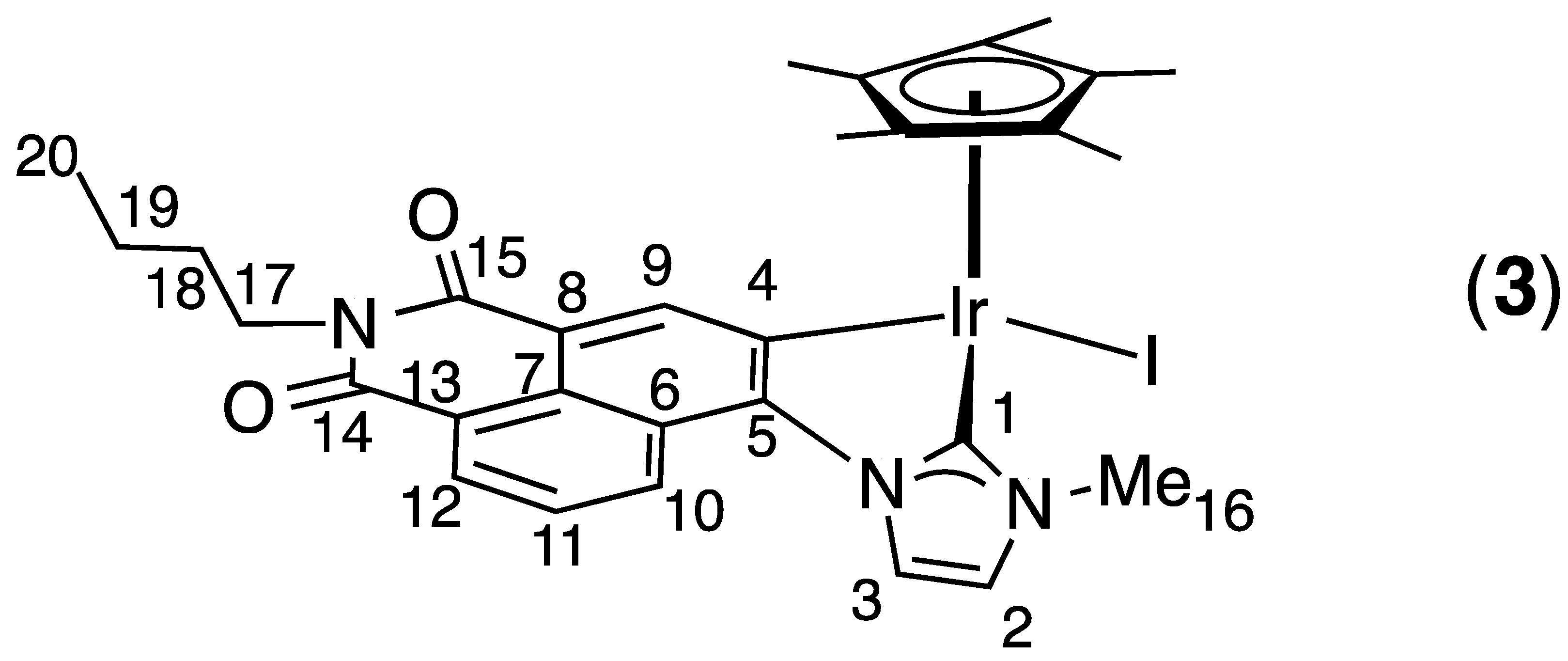
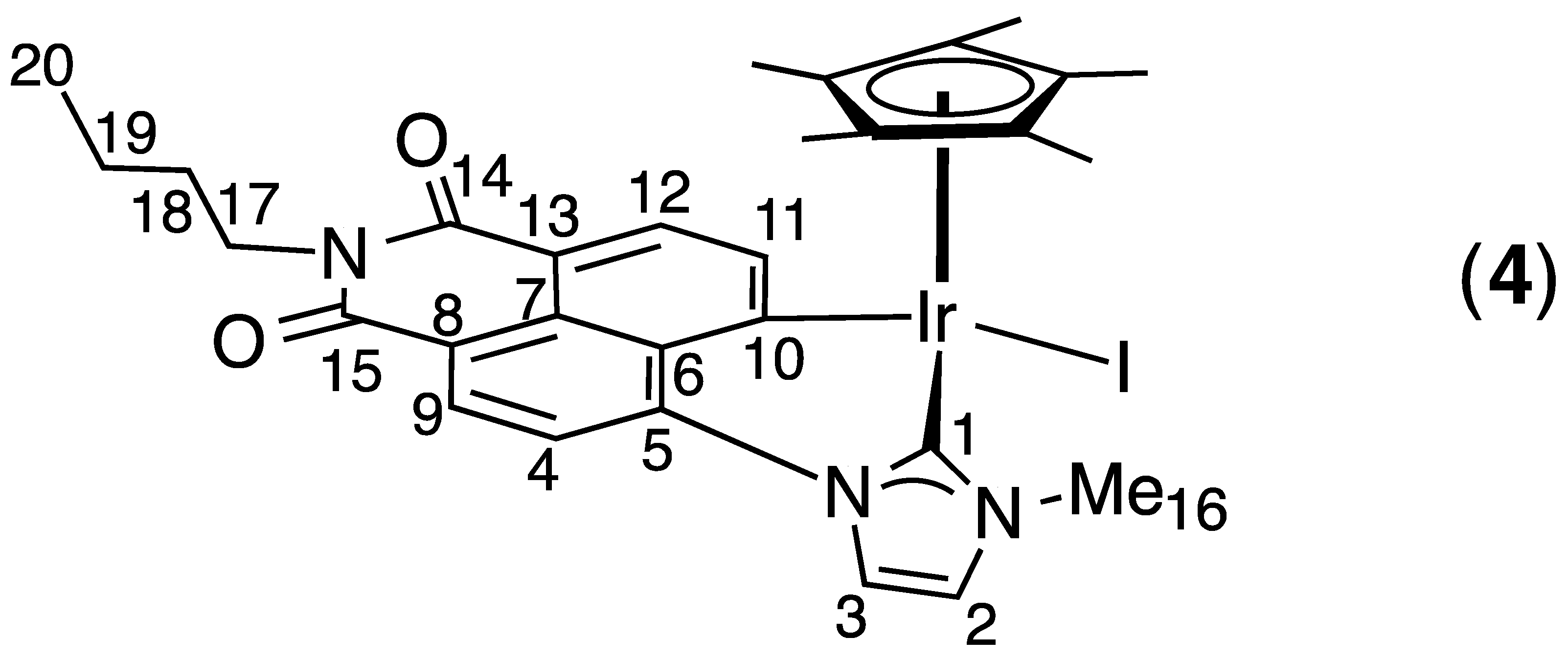
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- von Zelewsky, A. Stereochemistry of Coordination Compounds; Wiley: Chichester, UK, 1996; p. 254. [Google Scholar]
- Constable, E.C. Through a Glass Darkly-Some Thoughts on Symmetry and Chemistry. Symmetry 2021, 13, 1891. [Google Scholar] [CrossRef]
- Collet, A.; Crassous, J.; Dutasta, J.P.; Guy, L. Molécules Chirales: Stéreochimie et Propriétes; EDP Sciences: Les Ulis, France, 2006. [Google Scholar]
- Amouri, H.; Gruselle, M. Chirality in Transition Metal Chemistry: Molecules, Supramolecular Assemblies and Materials; Wiley: Chichester, UK, 2008. [Google Scholar]
- Brunner, H. Optical Activity at an Asymmetrical Manganese Atom. Angew. Chem. Int. Ed. 1969, 8, 382–383. [Google Scholar] [CrossRef]
- Brunner, H.; Tsuno, T. Ligand Dissociation: Planar or Pyramidal Intermediates? Acc. Chem. Res. 2009, 42, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, J.H.; Strouse, C.E.; Gladysz, J.A. Synthesis, Optical Resolution, and Absolute-Configuration of Pseudotetrahedral Organorhenium Complexes (Eta-C5H5)Re(NO)(PPh3)(X). Organometallics 1982, 1, 1204–1211. [Google Scholar] [CrossRef]
- Mukherjee, T.; Ghosh, S.K.; Wititsuwannakul, T.; Bhuvanesh, N.; Gladysz, J.A. Chiral-at-Metal Ruthenium Complexes with Guanidinobenzimidazole and Pentaphenylcyclopentadienyl Ligands: Synthesis, Resolution, and Preliminary Screening as Enantioselective Second Coordination Sphere Hydrogen Bond Donor Catalysts. Organometallics 2020, 39, 1163–1175. [Google Scholar] [CrossRef]
- Mamula, O.; von Zelewsky, A. Supramolecular coordination compounds with chiral pyridine and polypyridine ligands derived from terpenes. Coord. Chem. Rev. 2003, 242, 87–95. [Google Scholar] [CrossRef]
- Huang, X.; Meggers, E. Asymmetric photocatalysis with bis-cyclometalated rhodium complexes. Acc. Chem. Res. 2019, 52, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C. Stereogenic metal centres-from Werner to supramolecular chemistry. Chem. Soc. Rev. 2013, 42, 1637–1651. [Google Scholar] [CrossRef]
- Li, L.P.; Yao, S.Y.; Ou, Y.L.; Wei, L.Q.; Ye, B.H. Diastereoselective Synthesis and Photophysical Properties of Bis-Cyclometalated Ir(III) Stereoisomers with Dual Stereocenters. Organometallics 2017, 36, 3257–3265. [Google Scholar] [CrossRef]
- Li, L.P.; Peng, H.L.; Ye, B.H. Thermodynamic Resolution and Enantioselective Synthesis of C-2-Symmetric Bis-sulfoxides Based on Chiral Iridium(III) Complexes. Inorg. Chem. 2019, 58, 12245–12253. [Google Scholar] [CrossRef]
- Ehnbom, A.; Ghosh, S.K.; Lewis, K.G.; Gladysz, J.A. Octahedral Werner complexes with substituted ethylenediamine ligands: A stereochemical primer for a historic series of compounds now emerging as a modern family of catalysts. Chem. Soc. Rev. 2016, 45, 6799–6811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffner-Hamann, C.; von Zelewsky, A.; Barbieri, A.; Barigelletti, F.; Muller, G.; Riehl, J.P.; Neels, A. Diastereoselective formation of chiral tris-cyclometalated iridium (III) complexes: Characterization and photophysical properties. J. Am. Chem. Soc. 2004, 126, 9339–9348. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, F.J.; Westrol, M.S.; Oyler, K.D.; Byrne, N.; Kraml, C.; Zysman-Colman, E.; Lowry, M.S.; Bernhard, S. Synthesis, separation, and circularly polarized luminescence studies of enantiomers of iridium(III) luminophores. Inorg. Chem. 2008, 47, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Chepelin, O.; Ujma, J.; Wu, X.; Slawin, A.M.Z.; Pitak, M.B.; Coles, S.J.; Michel, J.; Jones, A.C.; Barran, P.E.; Lusby, P.J. Luminescent, Enantiopure, Phenylatopyridine Iridium-Based Coordination Capsules. J. Am. Chem. Soc. 2012, 134, 19334–19337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damas, A.; Moussa, J.; Rager, M.N.; Amouri, H. Chiral octahedral bimetallic assemblies with Δ-TRISPHAT as counter anion: Design, anion metathesis, and Cp*Ir as a probe for chiral recognition. Chirality 2010, 22, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Groue, A.; Montier-Sorkine, E.; Cheng, Y.P.; Rager, M.N.; Jean, M.; Vanthuyne, N.; Crassous, J.; Lopez, A.C.; Moncada, A.S.; Barbieri, A.; et al. Enantiopure, luminescent, cyclometalated Ir(III) complexes with N-heterocyclic carbene-naphthalimide chromophore: Design, vibrational circular dichroism and TD-DFT calculations. Dalton Trans. 2022, 51, 2750–2759. [Google Scholar] [CrossRef]
- Djukic, J.-P.; Hijazi, A.; Flack, H.D.; Bernardinelli, G. Non-racemic (scalemic) planar-chiral five-membered metallacycles: Routes, means, and pitfalls in their synthesis and characterization. Chem. Soc. Rev. 2008, 37, 406–425. [Google Scholar] [CrossRef]
- Amouri, H.; Thouvenot, R.; Gruselle, M. Differentiation of planar chiral enantiomers of [Cp*M(2-alkyl-phenoxo)][BF4] {M = Rh, Ir} by the trisphat anion. C. R. Chim. 2002, 5, 257–262. [Google Scholar] [CrossRef]
- Amouri, H.; Caspar, R.; Gruselle, M.; Guyard-Duhayon, C.; Boubekeur, K.; Lev, D.A.; Collins, L.S.B.; Grotjahn, D.B. Chiral Recognition and Resolution Mediated by p-p Interactions: Synthesis and X-ray Structure of trans-[(Sp,Sp)-bis(CpRu)-carbazolyl][D-Trisphat]. Organometallics 2004, 23, 4338–4341. [Google Scholar] [CrossRef]
- Dubarle-Offner, J.; Axet, M.R.; Chamoreau, L.M.; Amouri, H.; Cooksy, A.L. Enantiomerically Pure, Planar Chiral Cp*Ru Complexes: Synthesis, Molecular Structures, DFT and Coordination Properties. Organometallics 2012, 31, 4429–4434. [Google Scholar] [CrossRef]
- Moussa, J.; Chamoreau, L.M.; Amouri, H. Planar Chiral Iridium Complexes with the -TRISPHAT Anion: Toward the First Enantiopure o-Quinone Methide pi-Complex. Chirality 2013, 25, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Puig, E.; Gontard, G.; Rager, M.N.; Amouri, H. Optically active Pt-terpyridyl coordination assemblies derived from planar chiral metallothioligands. Inorg. Chim. Acta 2021, 517, 120208. [Google Scholar] [CrossRef]
- Brunner, H. Stability of the metal configuration in chiral-at-metal half-sandwich compounds. Eur. J. Inorg. Chem. 2001, 4, 905–912. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Fianchini, M.; Marchetti, F.; Skelton, B.W.; White, A.H. Syntheses, structures, and reactivity of new pentamethylcyclopentadienyl-rhodium(III) and -iridium(III) 4-acyl-5-pyrazolonate complexes. Inorg. Chem. 2005, 44, 7933–7942. [Google Scholar] [CrossRef] [PubMed]
- Mimassi, L.; Guyard-Duhayon, C.; Rager, M.N.; Amouri, H. Chiral recognition and resolution of the enantiomers of supramolecular triangular hosts: Synthesis, circular dichroism, NMR, and X-ray molecular structure of [Li subset of(R,R,R)-{Cp*Rh(5-chloro-2,3-dioxopyridine)}(3)][Delta-TRISPHAT]. Inorg. Chem. 2004, 43, 6644–6649. [Google Scholar] [CrossRef] [PubMed]
- Feghali, E.; Barloy, L.; Issenhuth, J.T.; Karmazin-Brelot, L.; Bailly, C.; Pfeffer, M. Cyclometalation of (2R,5R)-2,5-Diphenylpyrrolidine and 2-Phenyl-2imidazoline Ligands with Half-Sandwich Iridium(III) and Rhodium(III) Complexes. Organometallics 2013, 32, 6186–6194. [Google Scholar] [CrossRef]
- Corberan, R.; Sanau, M.; Peris, E. Highly stable Cp*-Ir(III) complexes with N-heterocyclic carbene ligands as C-H activation catalysts for the deuteration of organic molecules. J. Am. Chem. Soc. 2006, 128, 3974–3979. [Google Scholar] [CrossRef]
- Hellou, N.; Jahier-Diallo, C.; Basle, O.; Srebro-Hooper, M.; Toupet, L.; Roisnel, T.; Caytan, E.; Roussel, C.; Vanthuyne, N.; Autschbach, J.; et al. Electronic and chiroptical properties of chiral cycloiridiated complexes bearing helicenic NHC ligands. Chem. Commun. 2016, 52, 9243–9246. [Google Scholar] [CrossRef] [Green Version]
- Groue, A.; Tranchier, J.-P.; Rager, M.-N.; Gontard, G.; Jean, M.; Vanthuyne, N.; Pearce, H.R.; Cooksy, A.L.; Amouri, H. Unique Class of Enantiopure N-Heterocyclic Carbene Half-Sandwich Iridium(III) Complexes with Stable Configurations: Probing Five-Membered versus Six-Membered Iridacycles. Inorg. Chem. 2019, 58, 2930–2933. [Google Scholar] [CrossRef]
- Fagnou, K.; Lautens, M. Halide effects in transition metal catalysis. Angew. Chem. Int. Ed. 2002, 41, 26–47. [Google Scholar] [CrossRef]
- Stanley, K.; Baird, M.C. Demonstration of controlled asymmetric induction in organoiron chemistry. Suggestions concerning the specification of chirality in pseudotetrahedral metal complexes containing polyhapto ligands. J. Am. Chem. Soc. 1975, 97, 6598–6599. [Google Scholar] [CrossRef]
- Merrifield, J.H.; Fernandez, J.M.; Buhro, W.E.; Gladysz, J.A. Cleavage of the Rhenium Methyl Bond of (Eta-C5h5)Re(No)(Pph3)(Ch3) by Protic and Halogen Electrophiles-Stereochemistry at Rhenium. Inorg. Chem. 1984, 23, 4022–4029. [Google Scholar] [CrossRef]
- Brunner, H.; Kollnberger, A.; Burgemeister, T.; Zabel, M. Optically active transition metal complexes-Part 125. Preparation and epimerization of chiral-at-metal pentamethylcyclopentadienyl-rhodium(III) and iridium(III) half-sandwich complexes. Polyhedron 2000, 19, 1519–1526. [Google Scholar] [CrossRef]
- Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Fletcher, S.A.; Kisova, A.; Vrana, O.; Salassa, L.; Bruijnincx, P.C.A.; Clarkson, G.J.; Brabec, V.; et al. Organometallic half-sandwich iridium anticancer complexes. J. Med. Chem. 2011, 54, 3011–3026. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sadler, P.J. Organoiridium Complexes: Anticancer Agents and Catalysts. Acc. Chem. Res. 2014, 47, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Grotjahn, D.B.; Brown, D.B.; Martin, J.K.; Marelius, D.C.; Abadjian, M.-C.; Tran, H.N.; Kalyuzhny, G.; Vecchio, K.S.; Specht, Z.G.; Cortes-Llamas, S.A.; et al. Evolution of Iridium-Based Molecular Catalysts during Water Oxidation with Ceric Ammonium Nitrate. J. Am. Chem. Soc. 2011, 133, 19024–19027. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Steffen, A.; Wurthner, F. Near-IR Phosphorescent Ruthenium(II) and Iridium(III) Perylene Bisimide Metal Complexes. Angew. Chem. Int. Ed. 2015, 54, 1570–1573. [Google Scholar] [CrossRef]
- Specht, Z.G.; Cortes-Llamas, S.A.; Tran, H.N.; van Niekerk, C.J.; Rancudo, K.T.; Golen, J.A.; Moore, C.E.; Rheingold, A.L.; Dwyer, T.J.; Grotjahn, D.B. Enabling Bifunctionality and Hemilability of N-Heteroaryl NHC Complexes. Chem. Eur. J. 2011, 17, 6606–6609. [Google Scholar] [CrossRef]
- Gomez-Lopez, J.L.; Chavez, D.; Parra-Hake, M.; Royappa, A.T.; Rheingold, A.L.; Grotjahn, D.B.; Miranda-Soto, V. Synthesis and reactivity of bis(protic N-heterocyclic carbene)iridium(III) complexes. Organometallics 2016, 35, 3148–3153. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Thongpaen, J.; Schmid, T.E.; Toupet, L.; Dorcet, V.; Mauduit, M.; Basle, O. Directed ortho C-H borylation catalyzed using Cp*Rh(iii)-NHC complexes. Chem. Commun. 2018, 54, 8202–8205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, T.; Hashimoto, H.; Kinoshita, I.; Yano, S.; Nishioka, T. Unprecedented diastereoselective generation of chiral-at-metal, half sandwich Ir(III) and Rh(III) complexes via anomeric isomerism on “sugar-coated” N-heterocyclic carbene ligands. Dalton Trans. 2011, 40, 4826–4829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Banerjee, S.; Hughes, G.M.; Bridgewater, H.E.; Song, J.I.; Breeze, B.; Clarkson, G.J.; Coverdale, J.P.C.; Sanchez-Cano, C.; Ponte, F.; et al. Ligand-centred redox activation of inert organoiridium anticancer catalysts. Chem. Sci. 2020, 11, 5466–5480. [Google Scholar] [CrossRef] [PubMed]
- Lanoe, P.H.; Chan, J.; Gontard, G.; Monti, F.; Armaroli, N.; Barbieri, A.; Amouri, H. Deep-Red Phosphorescent Iridium(III) Complexes with Chromophoric N-Heterocyclic Carbene Ligands: Design, Photophysical Properties, and DFT Calculations. Eur. J. Inorg. Chem. 2016, 2016, 1631–1634. [Google Scholar] [CrossRef]
- Lanoe, P.H.; Najjari, B.; Hallez, F.; Gontard, G.; Amouri, H. N-Heterocyclic Carbene Coinage Metal Complexes Containing Naphthalimide Chromophore: Design, Structure, and Photophysical Properties. Inorganics 2017, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Lanoe, P.H.; Chan, J.; Groue, A.; Gontard, G.; Jutand, A.; Rager, M.N.; Armaroli, N.; Monti, F.; Barbieri, A.; Amouri, H. Cyclometalated N-heterocyclic carbene iridium(III) complexes with naphthalimide chromophores: A novel class of phosphorescent heteroleptic compounds. Dalton Trans. 2018, 47, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
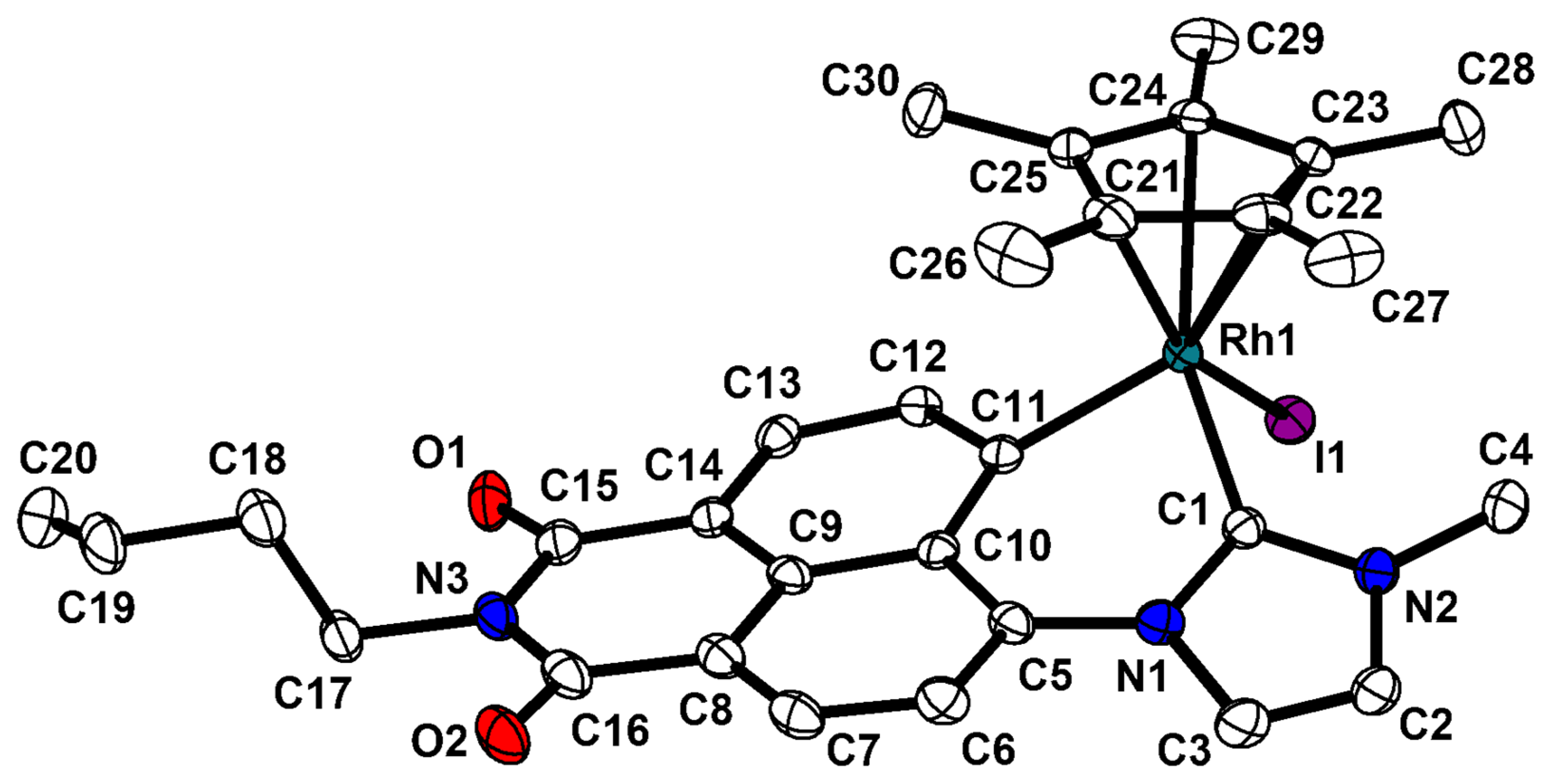
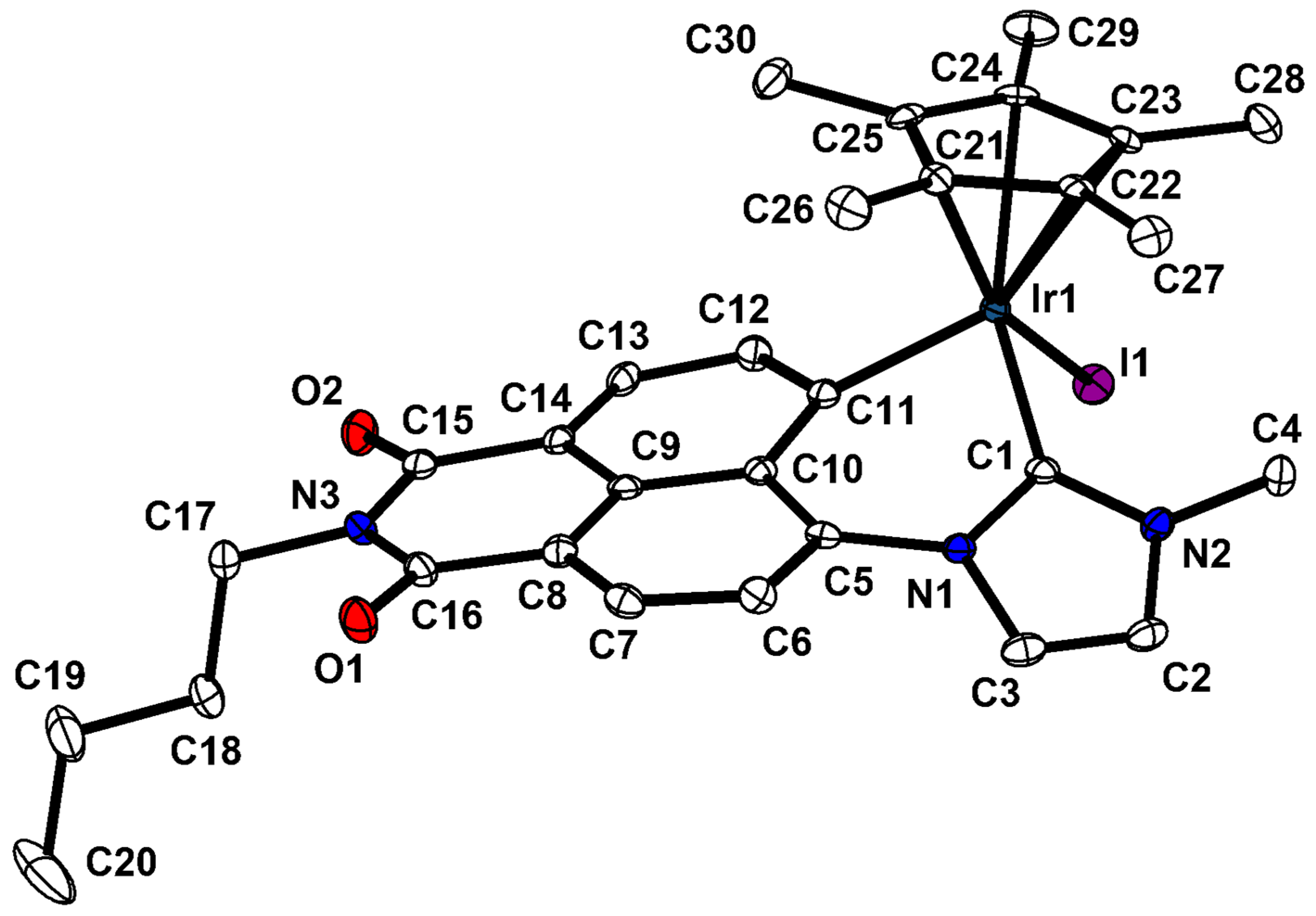
| Compound | (S)-[Cp*Rh(C^C:)I] (S)-2 | (S)-[Cp*Ir(C^C:)I] (S)-4 |
|---|---|---|
| Empirical formula | C30 H33 I RhN3 O2 | C30 H33 I Ir N3 O2 |
| Formula weight | 697.40 | 786.69 |
| Crystal system | Monoclinic | Orthorhombic |
| Space group | P 21 | P 21 21 21 |
| Unit cell dimensions | a = 17.2315(4) Å | a = 8.0943(2) Å |
| b = 10.6098(2) Å | b = 16.2456(4) Å | |
| c = 17.2604(4) Å | c = 21.3531(5) Å | |
| α = 90° | α = 90° | |
| β = 119.616(1)° | β = 90° | |
| γ = 90° | γ = 90° | |
| Volume | 2743.34(11) Å3 | 2807.86(12) Å3 |
| Z | 4 | 4 |
| Temperature | 200(1) K | 200(1) K |
| Wavelength | 1.54178 Å | 1.54178 Å |
| θ range for data collection | 5.10° to 66.62° | 4.96° to 66.64° |
| Reflections (all/independent) | 34,037/9689 | 15,376/4959 |
| R(int) | 2.54% | 2.28% |
| Data/parameters/restraints | 4959/340/0 | 9689/679/1 |
| R1 [I > 2σ(I)] | 2.08% | 1.79% |
| wR2 (all data) | 5.31% | 4.55% |
| Flack parameter | −0.015(2) | 0.001(3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groué, A.; Tranchier, J.-P.; Gontard, G.; Jean, M.; Vanthuyne, N.; Amouri, H. Enantiopure Cyclometalated Rh(III) and Ir(III) Complexes Displaying Rigid Configuration at Metal Center: Design, Structures, Chiroptical Properties and Role of the Iodide Ligand. Chemistry 2022, 4, 156-167. https://doi.org/10.3390/chemistry4010014
Groué A, Tranchier J-P, Gontard G, Jean M, Vanthuyne N, Amouri H. Enantiopure Cyclometalated Rh(III) and Ir(III) Complexes Displaying Rigid Configuration at Metal Center: Design, Structures, Chiroptical Properties and Role of the Iodide Ligand. Chemistry. 2022; 4(1):156-167. https://doi.org/10.3390/chemistry4010014
Chicago/Turabian StyleGroué, Antoine, Jean-Philippe Tranchier, Geoffrey Gontard, Marion Jean, Nicolas Vanthuyne, and Hani Amouri. 2022. "Enantiopure Cyclometalated Rh(III) and Ir(III) Complexes Displaying Rigid Configuration at Metal Center: Design, Structures, Chiroptical Properties and Role of the Iodide Ligand" Chemistry 4, no. 1: 156-167. https://doi.org/10.3390/chemistry4010014
APA StyleGroué, A., Tranchier, J.-P., Gontard, G., Jean, M., Vanthuyne, N., & Amouri, H. (2022). Enantiopure Cyclometalated Rh(III) and Ir(III) Complexes Displaying Rigid Configuration at Metal Center: Design, Structures, Chiroptical Properties and Role of the Iodide Ligand. Chemistry, 4(1), 156-167. https://doi.org/10.3390/chemistry4010014








