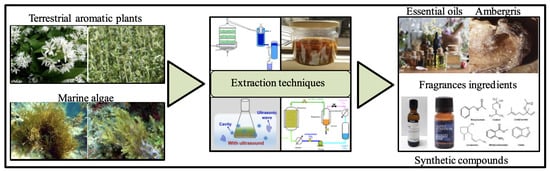
Occurrence of Marine Ingredients in Fragrance: Update on the State of Knowledge
Abstract
1. Introduction
2. General Aspects of Fragrance Classes
- -
- Total synthesis, which consists of producing totally artificial “new” molecules from simple chemical reagents;
- -
- Hemisynthesis uses a molecule derived from natural products and has a chemical structure close to the one to be reproduced. Thus, the basic reagent (called the precursor) is slightly transformed to improve its scent qualities.
- -
- -
- The total synthetic materials, conducting a diverse chemical function such as aldehyde methyl ionone, ketone, heterocycle and so forth;
- -
- The isolates separated from natural products. This is the case of many compounds serving as the raw material in (or produced) hemisynthesis. We can cite the example of coumarin isolated by Vogel in 1820 from the tonka bean (Dipteryx odorata) [40], which was the first synthetic fragrance compound released on the market in 1866 [41], followed by salicylaldehyde (1876) and vanillin. The interesting odoriferous qualities of coumarin and its derivatives have led to more and more extensive and up-to-date research [42,43,44].
3. Aroma Properties of Fragrance
4. Essential Oils as Natural Sources of Fragrance Compounds
5. Chemical Composition of Natural and Synthetic Fragrances
6. Marine Fragrance Chemistry
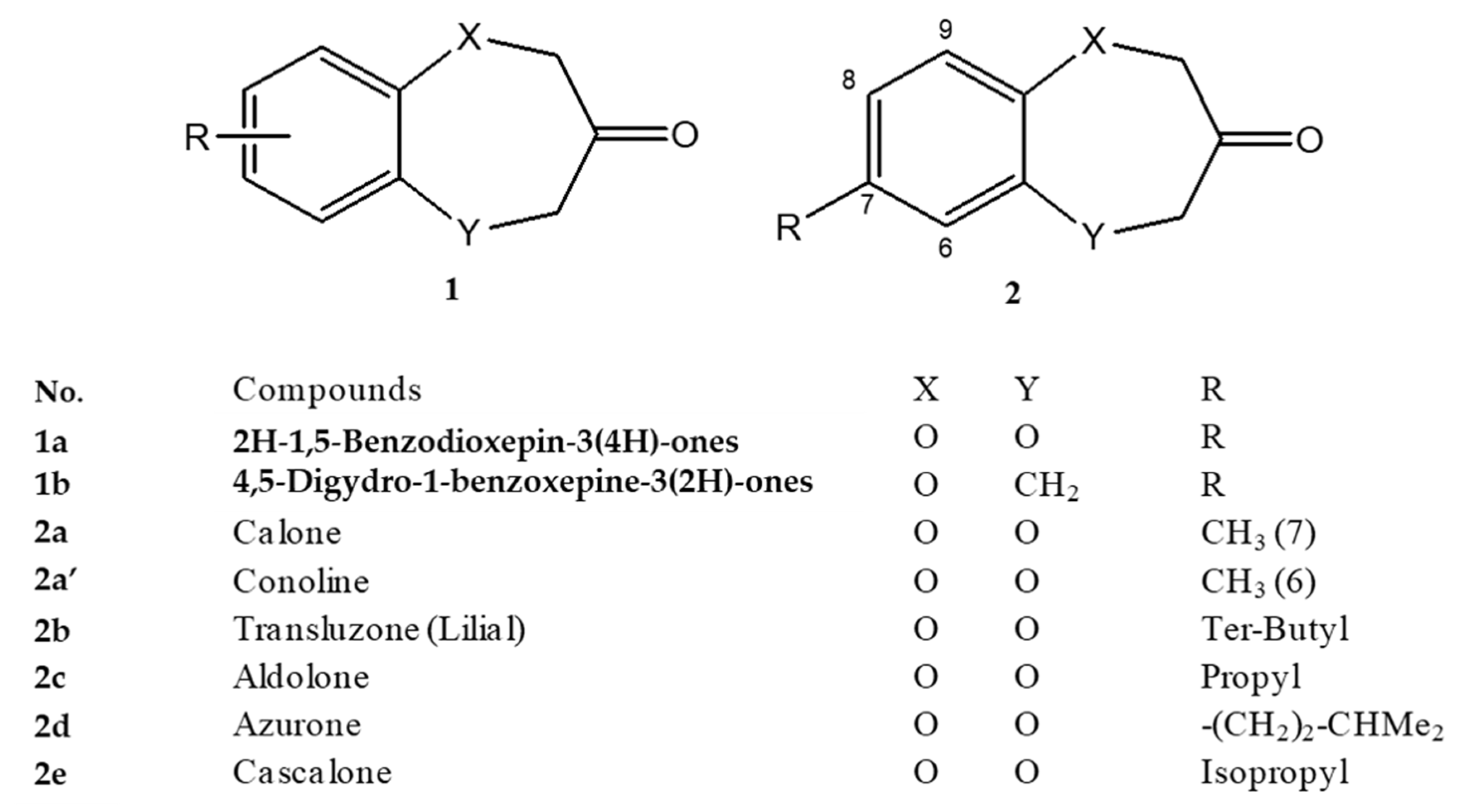
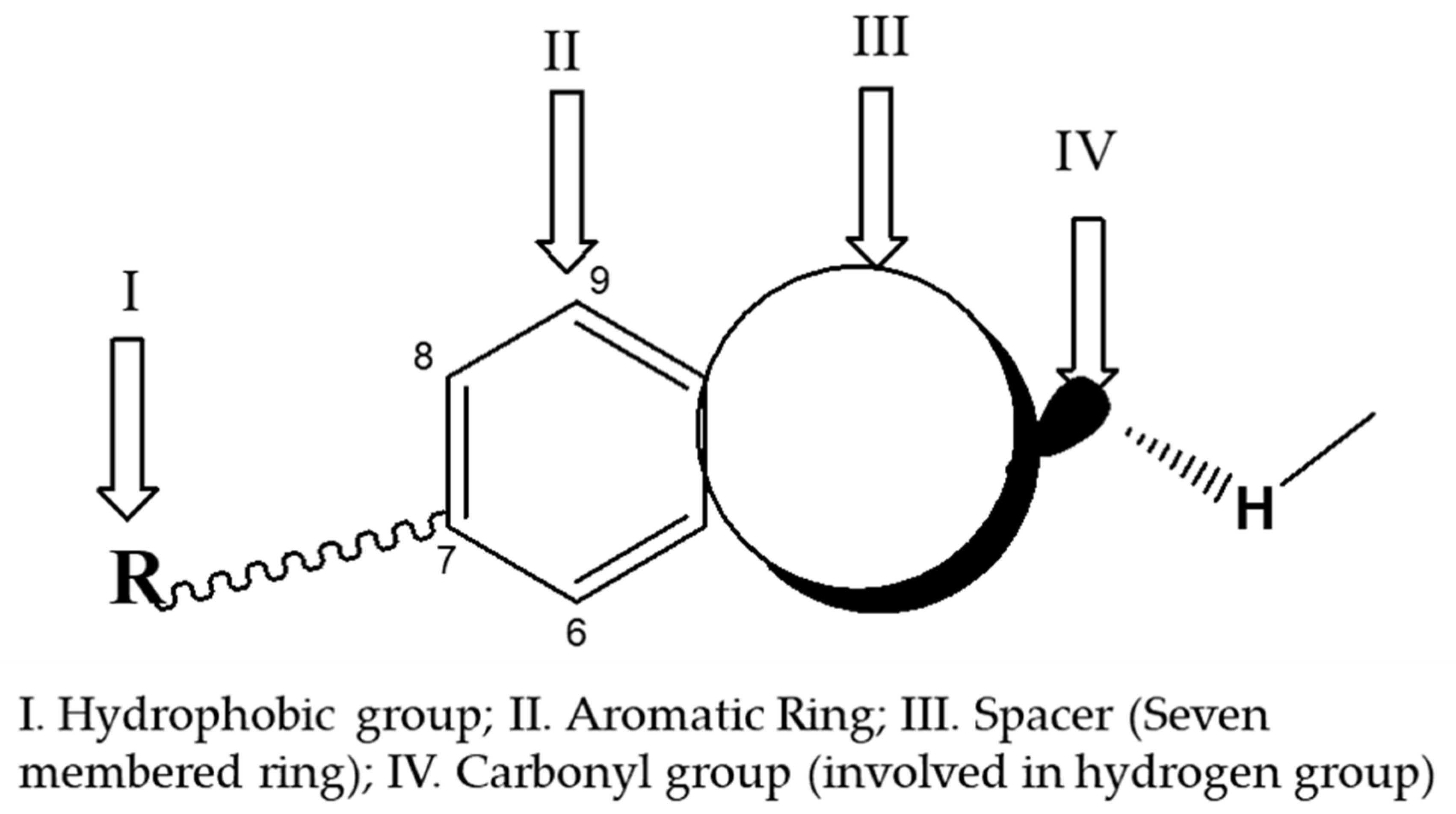
6.1. Synthetic Ingredients of Marine Fragrance
6.2. Other Synthetic Marine Fragrance Ingredients
6.3. Natural Ingredients of Marine Fragrance
6.3.1. Fragrance of Marine Animals
6.3.2. Fragrance of Marine Flora
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodrigues, A.; Nogueira, I.; Faria, R. Perfume and Flavor Engineering: A Chemical Engineering Perspective. Molecules 2021, 26, 3095. [Google Scholar] [CrossRef]
- Qader, M.M.; Perera, K.D.S.P. Chemistry of Perfumes. Tri-Annu. Publ. Inst. Chem. Ceylon 2020, 37, 26–29. [Google Scholar]
- Fortineau, A.-D. Chemistry Perfumes Your Daily Life. J. Chem. Educ. 2004, 81, 45–50. [Google Scholar] [CrossRef]
- Mori, K.; Takahashi, Y.K.; Igarashi, K.M.; Yamaguchi, M. Maps of Odorant Molecular Features in the Mammalian Olfactory Bulb. Physiol. Rev. 2006, 86, 409–433. [Google Scholar] [CrossRef]
- Touhara, K.; Vosshall, L.B. Sensing Odorants and Pheromones with Chemosensory Receptors. Annu. Rev. Physiol. 2009, 71, 307–332. [Google Scholar] [CrossRef]
- Wagner, Z.; Bendová, M.; Rotrekl, J.; Orvalho, S. Densities, Vapor Pressures, and Surface Tensions of Selected Terpenes. J. Solut. Chem. 2019, 48, 1147–1166. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Pokorný, V.; Štejfa, V.; Ferreira, O.; Pinho, S.P.; Růžička, K.; Fulem, M. Vapor Pressure and Thermophysical Properties of Eugenol and (+)-Carvone. Fluid Phase Equilibria 2019, 499, 112248. [Google Scholar] [CrossRef]
- Nelson, C.R. Investigation of Vaporization Enthalpies and Vapor Pressures of Organic Compounds by Correlation Gas Chromatography. Ph.D. Thesis, University of Missouri, St. Louis, MO, USA, 2018. [Google Scholar]
- Soria-Gómez, E. Special Issue Olfaction: From Genes to Behavior. Genes 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Zak, J.D.; Reddy, G.; Vergassola, M.; Murthy, V.N. Antagonistic Odor Interactions in Olfactory Sensory Neurons are Widespread in Freely Breathing Mice. Nat. Commun. 2020, 11, 3350. [Google Scholar] [CrossRef]
- Shilling, A.J.; Von Salm, J.L.; Sanchez, A.R.; Kee, Y.; Amsler, C.D.; McClintock, J.B.; Baker, B.J.; Anverenes, B.-E. New Polyhalogenated Monoterpenes from the Antarctic Red Alga Plocamium cartilagineum. Mar. Drugs 2019, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-C.; Lin, W.-S.; Peng, B.-R.; Chang, Y.-C.; Fang, L.-S.; Li, G.-Q.; Hwang, T.-L.; Wen, Z.-H.; Sung, P.-J. New Furanocembranoids from Briareum violaceum. Mar. Drugs 2019, 17, 214. [Google Scholar] [CrossRef]
- Avila, C. Terpenoids in Marine Heterobranch Molluscs. Mar. Drugs 2020, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Marquet, N.; Hubbard, P.C.; da Silva, J.P.; Afonso, J.; Canário, A.V.M. Chemicals Released by Male Sea Cucumber Mediate Aggregation and Spawning Behaviours. Sci. Rep. 2018, 8, 239. [Google Scholar] [CrossRef]
- Li, K.; Buchinger, T.J.; Li, W. Discovery and Characterization of Natural Products that Act as Pheromones in Fish. Nat. Prod. Rep. 2018, 35, 501–513. [Google Scholar] [CrossRef]
- Smith, C.U.M. Biology of Sensory Systems, 2nd ed.; John Wiley & Sons: Chichester, UK, 2008; pp. 203–243. [Google Scholar]
- Hay, M.E. Marine Chemical Ecology: Chemical Signals and Cues Structure Marine Populations, Communities, and Ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef]
- Say, T.E.; Degnan, S.M. Molecular and Behavioural Evidence that Interdependent Photo—and Chemosensory Systems Regulate Larval Settlement in a Marine Sponge. Mol. Ecol. 2019, 29, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Mollo, E.; Fontana, A.; Roussis, V.; Polese, G.; Amodeo, P.; Ghiselin, M.T. Sensing Marine Biomolecules: Smell, Taste, and the Evolutionary Transition from Aquatic to Terrestrial Life. Front. Chem. 2014, 2, 92. [Google Scholar] [CrossRef]
- Heinrichs, M.E.; Mori, C.; Dlugosch, L. Complex Interactions between Aquatic Organisms and Their Chemical Environment Elucidated from Different Perspectives. In Youmares 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 279–297. [Google Scholar]
- Staehr, P.; Thomsen, M.; Wernberg, T.; Staehr, P.; Schiel, D. Ecological Interactions between Marine Plants and Alien Species. In Marine Macrophytes as Foundation Species; Ólafsson, E., Ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2016; pp. 226–258. [Google Scholar] [CrossRef]
- Valdez, S.R.; Zhang, Y.S.; Van der Heide, T.; Vanderklift, M.A.; Tarquinio, F.; Orth, R.J.; Silliman, B.R. Positive Ecological Interactions and the Success of Seagrass Restoration. Front. Mar. Sci. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Bressman, N.R.; Simms, M.; Perlman, B.M.; Ashley-Ross, M.A. Where Do Fish Go When Stranded on Land? Terrestrial Orientation of the Mangrove Rivulus Kryptolebias Marmoratus. J. Fish Biol. 2018, 95, 335–344. [Google Scholar] [CrossRef]
- Collins, S.; Boyd, P.W.; Doblin, M.A. Evolution, Microbes, and Changing Ocean Conditions. Annu. Rev. Mar. Sci. 2020, 12, 181–208. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Chibucos, M.; Munro, J.B.; Daugherty, S.; Coelho, M.M.; Silva, J.C. Signature of Adaptive Evolution in Olfactory Receptor Genes in Cory’s Shearwater Supports Molecular Basis for Smell in Procellariform Sea-Birds. Sci. Rep. 2020, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Sell, C.S. Fundamentals of Fragrance Chemistry; Wiley-VCH: Weinheim, Germany, 2019; pp. 243–326. [Google Scholar]
- Niu, Y.; Liu, Y.; Xiao, Z. Evaluation of Perceptual Interactions between Ester Aroma Components in Langjiu by GC-MS, GC-O, Sensory Analysis, and Vector Model. Foods 2020, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.; Plainfossé, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of Natural Fragrance Ingredients: History Overview and Future Trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Fernandez, X.; Antoniotti, S.; Bussotti, E.; Hurel, M.P. Parfum, Chimie et Création. Actual. Chim. 2008, 323–324, 42–51. [Google Scholar]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Available online: https://www.doterra.com/US/en/blog/science-research-news-cold-press (accessed on 8 July 2019).
- Jugreet, B.S.; Mahomoodally, M.F.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar]
- Kowalski, R.; Kowalska, G.; Jankowska, M.; Nawrocka, A.; Kałwa, K.; Pankiewicz, U.; Włodarczyk-Stasiak, M. Secretory Structures and Essential Oil Composition of Selected Industrial Species of Lamiaceae. Acta Sci. Pol. Hortorum Cultus 2019, 18, 53–69. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef]
- El Sawi, S.A.; Ibrahim, M.E.; Gad El-Rokiek, K.; Amin Saad El-Din, S. Allelopathic Potential of Essential Oils Isolated from Peels of Three Citrus Species. Ann. Agric. Sci. 2019, 64, 89–94. [Google Scholar] [CrossRef]
- Bai, L.; Wang, W.; Hua, J.; Guo, Z.; Luo, S. Defensive Functions of Volatile Organic Compounds and Essential Oils from Northern White-Cedar in China. BMC Plant Biol. 2020, 20, 500. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials Preparation, Properties and Uses; Wiley-VCH Verlag: Weinheim, Germany, 2016; pp. 177–238. [Google Scholar]
- Masango, P. Towards Understanding Steam Distillation of Essential Oils by Differential Quantification of Principal Components Using Capillary Gas Chromatography. Ph.D. Thesis, University of Surrey, Guildford, UK, 2001. [Google Scholar]
- Vogel, A. Darstellung von Benzoesaure aus der Tonka-Boline und aus den MeliIoten-oder Steinklee-Blumen. Ann. Phys. 1820, 64, 161–166. [Google Scholar] [CrossRef]
- Frater, G.; Bajgrowicz, J.A.; Kraft, P. Fragrance Chemistry. Tetrahedron 1998, 54, 7633–7703. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef]
- Zeydi, M.M.; Kalantarian, S.J.; Kazeminejad, Z. Overview on Developed Synthesis Procedures of Coumarin Heterocycles. J. Iran. Chem. Soc. 2020, 17, 3031–3094. [Google Scholar] [CrossRef]
- Stepanyuk, A.; Kirschning, A. Synthetic Terpenoids in the World of Fragrances: Iso E Super® is the Showcase. Beilstein J. Org. Chem. 2019, 15, 2590–2602. [Google Scholar] [CrossRef]
- Sommer, C. The Role of Musk and Musk Compounds in the Fragrance Industry. Handb. Env. Chem. 2004, 3, 1–16. [Google Scholar]
- Zhang, T.; Jin, W.; Yang, S.; Li, Y.; Zhang, M.; Shi, M.; Guo, X.; Li, D.; Zhang, B.; Liu, S.; et al. Study of Compositions of Musks in Different Types Secreted by Forest Musk Deer (Moschus berezovskii). PLoS ONE 2021, 16, e0245677. [Google Scholar] [CrossRef]
- David, O.R.P. A Chemical History of Polycyclic Musks. Chem. Eur. J. 2020, 26, 7537–7555. [Google Scholar] [CrossRef]
- Morin, É.; Sosoe, J.; Raymond, M.; Amorelli, B.; Boden, R.M.; Collins, S.K. Synthesis of a Renewable Macrocyclic Musk: Evaluation of Batch, Microwave, and Continuous Flow Strategies. Org. Process. Res. Dev. 2019, 23, 283–287. [Google Scholar] [CrossRef]
- Hong, J.-H.; Lee, J.-Y.; Ha, H.-J.; Lee, J.-H.; Oh, S.-R.; Lee, Y.-M.; Lee, M.-Y.; Zoh, K.-D. Occurrence and Sources of Synthetic Musk Fragrances in the Sewage Treatment Plants and the Han River, Korea. Water 2021, 13, 392. [Google Scholar] [CrossRef]
- Wise, P.M.; Olsson, M.; Cain, W.S. Quantification of Odor Quality. Chem. Senses 2000, 25, 429–443. [Google Scholar] [CrossRef]
- Thiboud, M. Empirical Classification of Odours. In Perfumes: Art, Science, and Technology; Müller, P.M., Lamparsky, D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 253–286. [Google Scholar]
- Bae, J.; Yi, J.-Y.; Moon, C. Odor Quality Profile is Partially Influenced by Verbal Cues. PLoS ONE 2019, 14, e0226385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mi, S.; Liu, R.B.; Sang, Y.X.; Wang, X.H. Evaluation of Volatile Compounds during the Fermentation Process of Yogurts by Streptococcus thermophilus Based on Odor Activity Value and Heat Map Analysis. Int. J. Anal. Chem. 2020, 2020, 3242854. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zhao, L.; Wang, H.-Y.; Zhi, R.-C.; Shi, B.-L.; Xie, N. Determination of Recognition Threshold and Just Noticeable Difference in the Sensory Perception of Pungency of Zanthoxylum bangeanum. Int. J. Food Prop. 2015, 19, 1044–1052. [Google Scholar] [CrossRef]
- Piornos, J.A.; Delgado, R.C.J.; de La Burgade, L.; Methven, D.; Balagiannis, P.; Koussissi, E.; Brouwer, E.; Parker, J.K. Orthonasal and Retronasal Detection Thresholds of 26 Aroma Compounds in a Model Alcohol-Free Beer: Effect of Threshold Calculation Method. Food Res. Int. 2019, 123, 317–326. [Google Scholar] [CrossRef]
- Caul, J.F. The Profile Method of Flavor Analysis. Adv. Food Res. 1957, 7, 1–40. [Google Scholar] [CrossRef]
- Guld, Z.; Sárdy, D.N.; Gere, A.; Rácz, A. Comparison of Sensory Evaluation Techniques for Hungarian Wines. J. Chemom. 2020, 34, e3219. [Google Scholar] [CrossRef]
- Keane, P. The Flavor Profile. In Manual on Descriptive Analysis Testing for Sensory Evaluation; Hootman, R., Ed.; ASTM International: Philadelphia, PA, USA, 2008; pp. 5–14. [Google Scholar]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Reinvestigation on Odour Thresholds of Key Food Aroma Compounds and Development of an Aroma Language Based on Odour Qualities of Defined Aqueous Odorant Solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Kim, M.; Drake, S.; Drake, M. Evaluation of Key Flavor Compounds in Reduced-and Full-Fat Cheddar Cheeses Using Sensory Studies on Model Systems. J. Sens. Stud. 2011, 26, 278–290. [Google Scholar] [CrossRef]
- Leksrisompong, P.; Barbano, D.M.; Foegeding, A.E.; Gerard, P.; Drake, M. The Roles of Fat and pH on the Detection Thresholds and Partition Coefficients of Three Compounds: Diacetyl, δ-Decalactone and Furaneol. J. Sens. Stud. 2010, 25, 347–370. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Deutsche Forschungsanstalt für Lebensmittelchemie: Garching, Germany, 1998; pp. 1–63. [Google Scholar]
- Fazzalari, F.A. Compilation of Odor and Taste Threshold Values Data. In American Society of Testing and Materials; The Society: Philadelphia, PA, USA, 1978; pp. 1–497. [Google Scholar]
- Yuan, F.; He, F.; Qian, Y.L.; Zheng, J.; Qian, M.C. Aroma Stability of Lemon-Flavored Hard Iced Tea Assessed by Chirality and Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2016, 64, 5717–5723. [Google Scholar] [CrossRef]
- Kamadia, V.; Yoon, Y.; Schilling, M.W.; Marshall, D.L. Relationships between Odorant Concentration and Aroma Intensity. J. Food Sci. 2006, 71, S193–S197. [Google Scholar] [CrossRef]
- Chen, Q.-C.; Zhu, Y.; Yan, H.; Chen, M.; Xie, D.-C.; Wang, M.-Q.; Ni, D.-J.; Lin, Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules 2020, 25, 6050. [Google Scholar] [CrossRef]
- Gong, X.; Han, Y.; Zhu, J.; Hong, L.; Zhu, D.; Liu, J.; Zhang, X.; Niu, Y.; Xiao, Z. Identification of the Aroma-Active Compounds in Longjing Tea Characterized by Odor Activity Value, Gas Chromatography-Olfactometry, and Aroma Recombination. Int. J. Food Prop. 2017, 20, S1107–S1121. [Google Scholar] [CrossRef]
- McDaniel, M.R.; Miranda-Lopwz, R.; Wason, B.T.; Michaels, N.J.; Libbey, L.M. Pinot Noir Aroma: A Sensory/Gas Chromatographic Approach. Dev. Food Sci. 1990, 24, 23–36. [Google Scholar]
- Shu, C.; She, Y.; Xiao, Z.; Xu, L.; Niu, Y.; Zhu, J. Investigations on the Aroma Active Compounds in Fresh and Aged Longjing Tea by SPME/GC-MS/GC-O/OAV. Food Ind. 2016, 37, 279–285. [Google Scholar]
- Zhu, Y.; Chen, J.; Chen, X.; Chen, D.; Deng, S. Use of Relative Odor Activity Value (ROAV) to Link Aroma Pro-Files to Volatile Compounds: Application to Fresh and Dried Eel (Muraenesox cinereus). Int. J. Food Prop. 2020, 23, 2257–2270. [Google Scholar] [CrossRef]
- Tunick, M.H. Analyzing Volatile Compounds in Dairy Products. J. Sci. Food Agric. 2014, 94, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Kong, J.; Xiao, Z.; Chen, F.; Ma, N.; Zhu, J. Characterization of Odor-Active Compounds of Various Chinese “Wuliangye” Liquors by Gas Chromatography–Olfactometry, Gas Chromatography–Mass Spectrometry and Sensory Evaluation. Int. J. Food Prop. 2017, 20, S735–S745. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Yan, L.; Chen, H.; Shao, H.; Meng, T. Assessment of Odor Activity Value Coefficient and Odor Contribution Based on Binary Interaction Effects in Waste Disposal Plant. Atmos. Environ. 2015, 103, 231–237. [Google Scholar] [CrossRef]
- Aravisini, L.; Guichard, E. Interactions between Aroma Compounds and Food Matrix. In Flavour: From Food to Perception; Guichard, E., Salles, C., Morzel, M., Le Bon, A.M., Eds.; John Wiley & Sons, Ltd., Blackwell: Oxford, UK, 2016; pp. 208–234. [Google Scholar]
- Babita, S.; Sellam, P.; Jayoti, M.; Puja, R. Floral Essential Oils: Importance and Uses for Mankind. HortFlora. Res. Spectr. 2014, 3, 7–13. [Google Scholar]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils and Science, Technology and Applications; Hüsnü Can Baser, K., Buchbauer, G., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 39–81. [Google Scholar]
- Mollova, S.; Fidan, H.; Antonova, D.; Bozhilov, D.; Stanev, S.; Kostova, I.; Stoyanova, A. Chemical composition and antimicrobial and antioxidant activity of Helichrysum italicum (Roth) G. Don subspecies essential oils. Turk. J. Agric. For. 2020, 44, 371–378. [Google Scholar] [CrossRef]
- Kesdek, M.; Kordali, S.; Bozhüyük, A.U.; Güdek, M. Larvicidal Effect of Achillea biebersteinii Afan. (Asteraceae) Essential Oil Against Larvae of Pine Processionary Moth, Thaumetopoea pityocampa (Denis & Schiffermüller, 1775) (Lepidoptera: Notodontidae). Turk. J. Agric. For. 2020, 44, 451–460. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Green, I.R. Frankincense (Boswellia) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–440. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2015; pp. 619–659. [Google Scholar]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential Oils Used in Aromatherapy: A Systemic Review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Kumar, Y.; Prakash, O.; Tripathi, H.; Tandon, S.; Gupta, M.M.; Rahman, L.U.; Lal, R.K.; Semwal, M.; Darokar, M.P.; Khan, F. AromaDb: A Database of Medicinal and Aromatic Plant’s Aroma Molecules with Phytochemistry and Therapeutic Potentials. Front. Plant Sci. 2018, 9, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation, Potential of Essential Oils; El-Shemy, H., Ed.; IntechOpen: London, UK, 2018; pp. 107–127. [Google Scholar]
- Lingan, K. A Review on Major Constituents of Various Essential Oils and Its Application. Transl. Med. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Bayala, B.; Bassolé, I.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.-M.; Simpore, J. Anticancer Activity of Essential Oils and Their Chemical Components—A Review. Am. J. Cancer Res. 2014, 4, 591–607. [Google Scholar]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in Biosynthesis, Regulation, and Metabolic Engineering of Plant Specialized Terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Dehsheikh, A.B.; Sourestani, M.M.; Dehsheikh, P.B.; Mottaghipisheh, J.; Vitalini, S.; Iriti, M. Monoterpenes: Essential Oil Components with Valuable Features. Mini Rev. Med. Chem. 2020, 20, 958–974. [Google Scholar] [CrossRef]
- Fotsing, F.; Stephane, Y.; Kezetas, B.; Jules, J. Terpenoids as Important Bioactive Constituents of Essential Oils. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; de Oliveira, M.S., da Costa, W.A., Silva, S.G., Eds.; IntechOpen: London, UK, 2020; pp. 1–33. [Google Scholar]
- Loza-Tavera, H. Monoterpenes in Essential Oils. In Chemicals via Higher Plant Bioengineering, Advances in Experimental Medicine and Biology; Shahidi, F., Kolodziejczyk, P., Whitaker, J.R., Munguia, A.L., Fuller, G., Eds.; Springer: Boston, MA, USA, 1999; pp. 49–62. [Google Scholar]
- Hajdari, A.; Mustafa, B.; Hyseni, L.; Bajrami, A.; Mustafa, G.; Quave, C.L.; Nebija, D. Phytochemical Study of Eight Medicinal Plants of the Lamiaceae Family Traditionally Used as Tea in the Sharri Mountains Region of the Balkans. Sci. World J. 2020, 2020, 4182064. [Google Scholar] [CrossRef][Green Version]
- Santos Sánchez, N.F.; Salas-Coronado, R.; Hernandez-Carlos, B.; Villanueva, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; pp. 1–16. [Google Scholar]
- Amaya Olivas, N.; Villalba Bejarano, C.; Ayala Soto, G.; Zermeño Ortega, M.; Sandoval Salas, F.; Sánchez Chávez, E.; Hernández Ochoa, L. Bioactive Compounds and Antioxidant Activity of Essential Oils of Origanum dictamnus from Mexico. AIMS Agric. Food 2020, 5, 387–394. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Elik, G.; Kılıç, G.; Kanbolat, Ş.; Şener, S.Ö.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological Activity, and Volatile and Phenolic Compounds from Five Lamiaceae Species. Flavour Fragr. J. 2021, 36, 223–232. [Google Scholar]
- Czerny, M.; Brueckner, R.; Kirchhoff, E.; Schmitt, R.; Buettner, A. The Influence of Molecular Structure on Odor Qualities and Odor Detection Thresholds of Volatile Alkylated Phenols. Chem. Senses 2011, 36, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kottho, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Wüst, M. Volatile Phenols—Important Contributors to the Aroma of Plant-Derived Foods. Molecules 2020, 25, 4529. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Molecular Basis of Mammalian Odor Discrimination: A Status Report. J. Agric. Food Chem. 2018, 66, 13346–13366. [Google Scholar] [CrossRef]
- Maga, J.A.; Katz, I. The Role of Sulfur Compounds in Food Flavor Part I: Thiazoles, C R C Critical Reviews in Food Science and Nutrition. J. Agric. Food Chem. 1975, 6, 153–176. [Google Scholar]
- McGorrin, R.J. Sensory-Directed Flavor Analysis; Marsili, R., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 223–267. [Google Scholar]
- Zhu, Y.-L.; Zheng, G.-D.; Gao, D.; Chen, T.-B.; Wu, F.-K.; Niu, M.-J.; Zou, K.-H. Odor Composition Analysis and Odor Indicator Selection during Sewage Sludge Composting. J. Air Waste Manag. Assoc. 2016, 66, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, C.; Lecocq, S.; Collin, S. Polyfunctional Thiols and Drinkability of Beer. In Proceedings of the 29th European Brewery Convention Congress, Dublin, Ireland, 17–22 May 2003. [Google Scholar]
- Iranshahi, M.A. Review of Volatile Sulfur Containing Compounds from Terrestrial Plants: Biosynthesis, Distribution and Analytical Methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Kasaian, J.; Asili, J.; Iranshahi, M. Sulphur-Containing Compounds in the Essential Oil of Ferula alliacea Roots and Their Mass Spectral Fragmentation Patterns. Pharm. Biol. 2016, 54, 2264–2268. [Google Scholar] [CrossRef] [PubMed]
- Goeke, A. Sulfur-Containing Odorants in Fragrance Chemistry. Sulfur Rep. 2002, 23, 243–278. [Google Scholar] [CrossRef]
- Varlet, V.; Fernandez, X. Review. Sulfur-containing Volatile Compounds in Seafood: Occurrence, Odorant Properties and Mechanisms of Formation. Food Sci. Technol. Int. 2010, 16, 463–503. [Google Scholar] [CrossRef] [PubMed]
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors. In Volatile Sulfur Compounds in Food; Qian, M., Fan, X., Mahattanatawee, K., Eds.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2011; pp. 1–29. [Google Scholar]
- Vermeulen, C.; Gijs, L.; Collin, S. Sensorial Contribution and Formation Pathways of Thiols in Foods: A Review. Food Rev. Int. 2005, 21, 69–137. [Google Scholar] [CrossRef]
- Lin, J.; Jella, P.; Rouseff, R.L. Gas Chromatography-Olfactometry and Chemiluminescence Characterization of Grapefruit Juice Volatile Sulfur Compounds. In Heteroatomic Aroma Compounds; Reineccius, G.A., Reineccius, T.A., Eds.; American Chemical Society: Washington, DC, USA, 2002; pp. 102–112. [Google Scholar]
- Rouseff, R.; Jabalpurwala, F.; Gurbuz, O. Analysis of Grapefruit Sulphur Volatiles using SPME and Pulsed Flame Photometric Detection. Food Chem. 2010, 120, 296–303. [Google Scholar]
- Cannon, R.J.; Ho, C.T. Volatile Sulfur Compounds in Tropical Fruits. J. Food Drug Anal. 2018, 26, 445–468. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Volatile Compounds of Fresh and Processed Garlic (Review). Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef]
- Abad, P.; Arroyo-Manzanares, N.; Gil Martínez, L.; García-Campaña, A.M. Use of Onion Extract as a Dairy Cattle Feed Supplement: Monitoring Propyl Propane Thiosulfonate as a Marker of Its Effect on Milk Attributes. J. Agric. Food Chem. 2017, 65, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maro-to-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals 2021, 14, 21. [Google Scholar] [CrossRef]
- Eib, S.; Schneider, D.J.; Hensel, O.; Seuß-Baum, I. Relationship between Mustard Pungency and Allyl-Isothiocyanate Content: A Comparison of Sensory and Chemical Evaluations. J. Food Sci. 2020, 85, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Pegiou, E.; Mumm, R.; Acharya, P.; de Vos, R.C.H.; Hall, R.D. Green and White Asparagus (Asparagus officinalis): A Source of Developmental, Chemical and Urinary Intrigue. Metabolites 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhang, Y.; Zou, L.; Sun, J.; Song, X.; Mao, J.; Wu, Y. Simultaneous Hydrolysis and Extraction Increased Erucin Yield from Broccoli Seeds. ACS Omega 2021, 6, 6385–6392. [Google Scholar] [CrossRef]
- Wermter, N.S.; Rohn, S.; Hanschen, F.S. Seasonal Variation of Glucosinolate Hydrolysis Products in Commercial White and Red Cabbages (Brassica oleracea var. capitata). Foods 2020, 9, 1682. [Google Scholar] [CrossRef]
- Romeilah, R.M.; Fayed, S.A.; Mahmoud, G.I. Chemical Compositions, Antiviral and Antioxidant Activities of Seven Essential Oils. J. Appl. Sci. Res. 2010, 6, 50–62. [Google Scholar]
- Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The Chemical Compositions of the Volatile Oils of Garlic (Allium sativum) and Wild Garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Chizzola, R.; Ramadan, A.A.; Edris, A.E. Chemical Composition and Antimicrobial Activity of Garlic Essential Oils Evaluated in Organic Solvent, Emulsifying, and Self-Microemulsifying Water Based Delivery Systems. Food Chem. 2017, 221, 196–204. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; My, T.T.A.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Loan, H.T.P.; Triet, N.T.; Van Anh, T.T.; Quy, P.T.; Tat, P.V.; et al. Investigation into SARS-CoV-2 Resistance of Compounds in Garlic Essential Oil. ACS Omega 2020, 5, 8312–8320. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; Peris, D.; Belloch, C.; Flores, M. Debaryomyces Hansenii Metabolism of Sulfur Amino Acids as Precursors of Volatile Sulfur Compounds of Interest in Meat Products. J. Agric. Food Chem. 2019, 67, 9335–9343. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Rauhut, D. Recent Developments on the Origin and Nature of Reductive Sulfurous Off-Odours in Wine. Fermentation 2018, 4, 62. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Hui, T.; Fang, F.; Zhang, D. New Insight into the Formation Mechanism of 2-Furfurylthiol in the Glucose-Cysteine Reaction with Ribose. Food Res. Int. 2021, 143, 110295. [Google Scholar] [CrossRef]
- Janes, J.F.; Marr, I.M.; Unwin, N.; Banthorpe, D.V.; Yusuf, A. Reaction of Monoterpenoids with Hydrogen Sulfide to form Thiols and Epi-Sulfides of Potential Organoleptic Significance. Flavour Fragr. J. 1993, 8, 289–294. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Demole, E.; Enggist, P.; Ohloff, G. 1-p-Menthene-8-thiol: A Powerful Flavor Impact Constituent of Grapefruit Juice (Citrus Parodisi MACFAYDEN). Helv. Chim. Acta 2004, 65, 1785–1794. [Google Scholar] [CrossRef]
- Schoenauer, S.; Schieberle, P. Structure–Odor Activity Studies on Monoterpenoid Mercaptans Synthesized by Changing the Structural Motifs of the Key Food Odorant 1-p-Menthene-8-Thiol. J. Agric. Food Chem. 2016, 64, 3849–3861. [Google Scholar] [CrossRef]
- Ravi, R.; Prakash, M.; Bhat, K.K. Aroma Characterization of Coriander (Coriandrum sativum L.) Oil Samples. Eur. Food Res. Technol. 2007, 225, 367–374. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Q.; Xiao, Z.; Mao, H.; Zhang, J. Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail. Molecules 2020, 25, 1083. [Google Scholar] [CrossRef] [PubMed]
- Zeller, A.; Rychlik, M. Impact of Estragole and Other Odorants on the Flavour of Anise and Tarragon. Flavour Fragr. J. 2007, 22, 105–113. [Google Scholar] [CrossRef]
- Schieberle, P.; Buettner, A. Influence of the Chain Length on the Aroma Properties of Homologous Epoxyal-Dehydes, Ketones, and Alcohols. In Aroma Active Compounds in Foods: Chemistry and Sensory Properties; Takeoka, G.R., Güntert, M., Engel, K.-H., Eds.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2001; Volume 794, pp. 109–118. [Google Scholar]
- Güntert, M.; Krammer, G.; Lambrecht, S.; Sommer, S.H.; Surburg, H.; Werkhoff, P. Flavor Chemistry of Peppermint Oil (Mentha piperita L.). In Araoma Active Compounds: Chemistry and Sensory Properties; Takeoka, G.R., Güntert, M., Engel, K.-H., Eds.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2001; Volume 794, pp. 119–137. [Google Scholar]
- Moreno, J.A.; Zea, L.; Moyano, L.; Medina, M. Aroma Compounds as Markers of the Changes in Sherry Wines Subjected to Biological Ageing. Food Control 2005, 16, 333–338. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldra, F. Handbook of Food Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; Volume 1, pp. 47–64. [Google Scholar]
- Takeoka, G. Volatile Constituents of Asafoetida. In Araoma Active Compounds: Chemistry and Sensory Properties; Takeoka, G.R., Güntert, M., Engel, K.-H., Eds.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2001; Volume 794, pp. 33–44. [Google Scholar]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, W. The Volatile Constituents in the Peel and Pulp of Green Thai Mango, Khieo Sawoei Cultivar (Mangifera indica L.). Food Sci. Technol. Res. 2001, 7, 72–77. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, X.; Xiao, Z.; Niu, Y. Characterization of the Differences in the Aroma of Cherry Wines from Different Price Segments Using Gas Chromatography–Mass Spectrometry, Odor Activity Values, Sensory Analysis, and Aroma Reconstitution. Food Sci. Biotechnol. 2017, 26, 331–338. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners: Utrecht, The Netherlands, 2011; pp. 1–486. [Google Scholar]
- Peng, Y.; Bishop, K.S.; Quek, S.Y. Compositional Analysis and Aroma Evaluation of Feijoa Essential Oils from New Zealand Grown Cultivars. Molecules 2019, 24, 2053. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of Volatile Compounds to Mango (Mangifera indica L.) Aroma. Flvour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Plotto, A.; Margaria, C.A.; Goodner, K.L.; Goodrich, R.; Baldwin, E.A. Odour and Flavour Thresholds for Key Aroma Components in an Orange Juice Matrix: Terpenes and Aldehydes. Flavour Fragr. J. 2004, 19, 491–498. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.; Lei, L.; Lin, L.; Wang, D.; Wu, J. Identification and Aroma Impact of Volatile Terpenes in Moutai Liquor. Int. J. Food Prop. 2016, 19, 1335–1352. [Google Scholar] [CrossRef]
- Ferreira, V.; Ardanuy, M.; Lopez, R.; Cacho, J.F. Relationship between Flavor Dilution Values and Odor Unit Values in Hydroalcoholic Solutions: Role of Volatility and a Practical Rule for Its Estimation. J. Agric. Food Chem. 1998, 46, 4341–4346. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.; Cortes, B.; Medina, M. Discrimination of the Aroma Fraction of Sherry Wines Obtained by Oxidative and Biological Ageing. Food Chem. 2001, 75, 79–84. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical Study of Aromatic Series in Sherry Wines Subjected to Biological Aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Seideneck, R.; Schieberle, P. Comparison of the Key Aroma Compounds in Hand-Squeezed and Unpasteurised, Commercial NFC Juices Prepared from Brazilian Pera Rio Oranges. Eur. Food Res. Technol. 2011, 232, 995–1005. [Google Scholar] [CrossRef]
- Cometto–Muñiz, J.E.; Cain, W.S.; Abraham, M.H.; Gola, J.M. Chemosensory Detectability of 1-Butanol and 2-Heptanone Singly and in Binary Mixtures. Physiol. Behav. 1999, 67, 269–276. [Google Scholar] [CrossRef]
- Ong, P.K.; Acree, T.E.; Lavin, E.H. Characterization of Volatiles in Rambutan Fruit (Nephelium lappaceum L.). J. Agric. Food Chem. 1998, 46, 611–615. [Google Scholar] [CrossRef]
- D’Acampora Zellner, B.; Lo Presti, M.; Barata, L.E.S.; Dugo, P.; Dugo, G.; Mondello, L. Evaluation of Leaf-Derived Extracts as an Environmentally Sustainable Source of Essential Oils by Using Gas Chromatography—Mass Spectrometry and Enantioselective Gas Chromatography—Olfactometry. Anal. Chem. 2006, 78, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Boonbumrung, S.; Tamura, H.; Mookdasanit, J.; Nakamoto, H.; Ishihara, M.; Yoshizawa, T.; Varanyanond, W. Characteristic Aroma Components of the Volatile Oil of Yellow Keaw Mango Fruits Determined by Limited Odor Unit Method. Food Sci. Technol. Res. 2001, 7, 200–206. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Identification of Potent Flavor Compounds formed in an Aqueous Lemon Oil/Citric Acid Emulsion. J. Agric. Food Chem. 1998, 36, 797–800. [Google Scholar] [CrossRef]
- Dharmawan, J.; Kasapis, S.; Sriramula, P.; Lear, M.J.; Curran, P. Evaluation of Aroma-Active Compounds in Pontianak Orange Peel Oil (Citrus nobilis Lour. Var. microcarpa Hassk.) by Gas Chromatography-Olfactometry, Aroma Reconstitution, and Omission Test. J. Agric. Food Chem. 2009, 57, 239–244. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Voirol, E.; Daget, N. Comparative Study of Nasal and Retronasal Olfactory Perception. Lebensm. Wiss. Technol. 1986, 19, 316–319. [Google Scholar]
- Randebrock, R.E. Geruch und konstitution. Eine Beweisführung für die Molekulartheorie des Geruches. Teil IV: Olfaktometerversuche zur Molekulartheorie des Geruches. Parfuem Kosmet 1986, 67, 10–24. [Google Scholar]
- Buhr, K.; Köhlnhofer, B.; Heilig, A.; Hinrichs, J.; Schieberle, P. Behaviour of Selected Flavour Compounds in Dairy Matrices: Stability and Release. In Expression of Multidisciplinary Flavour Scienc; Blank, I., Wüst, H., Yeretzian, C., Eds.; ZHAW Zürcher College for Applied Sciences: Winterthur, Switzerland, 2010; pp. 165–168. [Google Scholar]
- Langridge, P.; Chalmers, K. The Principle: Identification and Application of Molecular Markers. In Molecular Marker Systems in Plant Breeding and Crop Improvement; Springer: Heidelberg, Germany, 2004; pp. 3–22. [Google Scholar]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Osako, K.; Ohshima, T. Identification and Characterization of Headspace Volatiles of Fish Miso, a Japanese Fish Meat Based Fermenented Paste, with Special Emphasis on Effect of Fish Species and Meat Washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Averbeck, M.; Schieberle, P. Influence of Different Storage Conditions on Changes in the Key Aroma Compounds of Orange Juice Reconstituted from Concentrate. Eur. Food Res. Technol. 2011, 232, 129–142. [Google Scholar] [CrossRef]
- Molhave, L.; Kjaergaard, S.K.; Hempeljorgensen, A.; Juto, J.E.; Andersson, K.; Stridh, G.; Falk, J. The Eye Irritation and Odor Potencies of Four Terpenes Which Are Major Constituents of the Emissions of VOCs from Nordic Soft Woods. Indoor Air 2000, 10, 315–318. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Boidron, J.N.; Terrier, A. Aroma of Muscat Grape Varieties. J. Agric. Food Chem. 1975, 23, 1042–1047. [Google Scholar] [CrossRef]
- Elss, S.; Kleinhenz, S.; Schreier, P. Odor and Taste Thresholds of Potential Carry-Over/Off-Flavor Compounds in Orange and Apple Juice. LWT-Food Sci. Technol. 2007, 40, 1826–1831. [Google Scholar] [CrossRef]
- Padrayuttawat, A.; Yoshizawa, T.; Tamura, H.; Tokunaga, T. Optical Isomers and Odor Thresholds of Vola-Tile Constituents in Citrus Sudachi. Food Sci. Technol. Int. 1997, 3, 402–408. [Google Scholar]
- Christoph, N. Die Anwendung der Gaschromatographischen Sniffing—Technik zur Bestimmung von Geruchsschwellen und Aromawerten. Ph.D. Thesis, Universität München, München, Germany, 1983. [Google Scholar]
- Nagata, Y.; Takeuchi, N. Measurement of Odor Threshold by Triangle Odor Bag Method. Odor Meas. Rev. 2003, 118, 118–127. [Google Scholar]
- Kirsch, F.; Buettner, A. Odor Qualities and Thresholds of Physiological Metabolites of 1,8-Cineole as an Example for Structure—Activity Relationships Considering Chirality Aspects. Chem. Biodivers. 2013, 10, 1683–1695. [Google Scholar] [CrossRef]
- Farina, L.; Boido, E.; Carrau, F.; Versini, G.; Dellacassa, E. Terpene Compounds as Possible Precursors of 1,8-Cineole in Red Grapes and Wines. J. Agric. Food Chem. 2005, 53, 1633–1636. [Google Scholar] [CrossRef]
- Tamura, H.; Yang, R.-H.; Sugisawa, H. Aroma Profiles of Peel Oils of Acid Citrus. ACS Symp. Ser. 1993, 525, 121–136. [Google Scholar] [CrossRef]
- Yang, R.; Sugisawa, H.; Nakatani, H.; Tamura, H.; Takagi, N. Comparison of Odor Quality in Peel Oils of Acid Citrus. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 16–24. [Google Scholar] [CrossRef]
- Matsushita, H.; Arito, H.; Suzuki, Y.; Soda, R. Determination of Threshold Values for Olfactory Perception of primary Substances. Ind. Health 1967, 5, 221–237. [Google Scholar] [CrossRef]
- Usami, A.; Ono, T.; Marumoto, S.; Miyazawa, M. Comparison of Volatile Compounds with Characteristic Odor in Flowers and Leaves of Nojigiku (Chrysanthemum japonense). J. Oleo Sci. 2013, 62, 631–636. [Google Scholar] [CrossRef]
- Tempelaar, H.C.G. Over den invloed van licht op reukstoffen. Ph.D. Thesis, Unversity of Utrecht, Utrecht, Germany, 1913. [Google Scholar]
- Buchbauer, G.; Jirovetz, L.; Jager, W.; Planck, C.; Dietrich, H. Fragrance Compounds and Essential Oils with Sedative Effects upon Inhalation. J. Pharm. Sci. 1993, 82, 660–664. [Google Scholar] [CrossRef]
- Sugisawa, H.; Takeda, M.; Yang, R.H.; Takagi, N. The Comparison of Odor Quality of Volatiles in Peel Oils of Five Kinds of Navel Oranges. Nippon Shokuhin Kogyo Gakkaishi 1991, 38, 668–674. [Google Scholar] [CrossRef]
- Chaves, M.; Zea, L.; Moyano, L.; Medina, M. Changes in Color and Odorant Compounds during Oxidative Aging of Pedro Ximinez Sweet Wines. J. Agric. Food Chem. 2007, 55, 3592–3598. [Google Scholar] [CrossRef]
- Tominaga, T.; Niclass, Y.; Frerot, E.; Dubourdieu, D. Stereoisomeric Distribution of 3-Mercaptohexan-1-ol and 3-Mercaptohexyl Acetate in Dry and Sweet White Wines Made from Vitis Vinifera (Var. Sauvignon Blanc and Semillon). J. Agric. Food Chem. 2006, 54, 7251–7255. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the Aroma-Active Compounds in Pink Guava (Psidium guajava, L.) by Application of the Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2008, 56, 4120–4127. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Dietrich, A.; Hener, U.; Mosandl, A. Stereoisomeric Flavour Compounds LXX, 1-p-Menthene-8-thiol: Separation and Sensory Evaluation of the Enantiomers by Enantioselective Gas Chromatography/Olfactometry. Phytochem. Anal. 1995, 6, 255–257. [Google Scholar] [CrossRef]
- Huber, U.A. Erfassung des Geruchs von Riechstoffen. Seifen Öle Fette Wachse 1984, 110, 448–451. [Google Scholar]
- Sell, C.S. Chemistry and the Sense of Smell; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 188–209. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: London, UK, 2009; pp. 227–370. [Google Scholar]
- Bitar, A.; Ghaddar, T.; Malek, A.; Haddad, T.; Toufeili, I. Sensory Thresholds of Selected Phenolic Constituents from Thyme and Their Antioxidant Potential in Sunflower Oil. J. Am. Oil Chem. Soc. 2008, 85, 641–646. [Google Scholar] [CrossRef]
- Baldus, C. Odour Threshold Study. Ph.D. Thesis, University of Würzburg, Würzburg, Germany, 1936. [Google Scholar]
- Beereboom, J.J.; Cameron, D.P.; Stephens, C.R. Pfizer. U.S. Patent 3799892, Prior, 15 August 1966. [Google Scholar]
- Kraft, P.; Eichenberger, W. Conception, Characterization and Correlation of New Marine Odorants. Eur. J. Org. Chem. 2003, 19, 3681–3874. [Google Scholar] [CrossRef]
- Hügel, H.M.; Drevermann, B.; Lingham, A.R.; Marriott, P.J. Marine Fragrance Chemistry. In Current Topics in Flavor and Fragrance Research; Kraft, P., Swift, K.A.D., Eds.; Verlag Helvetiva Chimica Acta: Zürich, Switzerland; Wiley-VCH: Weinheim, Germany, 2008; pp. 199–209. [Google Scholar]
- Helbig, C. Discover Why the Perfume Ingredient Calone Is So Popular. Available online: https://www.liveabout.com/what-is-calone-346209 (accessed on 8 May 2019).
- Behnke, M. Olfactory Chemistry: Calone- The Smell of the Sea (and Watermelon). Available online: http://colognoisseur.com/olfactory-chemistry-calone-the-smell-of-the-sea-and-watermelon/ (accessed on 14 August 2015).
- Fragrantica. Calone. Available online: https://www.fragrantica.com/notes/Calone-423.html (accessed on 8 May 2019).
- Gaudin, J.M.; Blanc, P.A. 7-Propyl-benzodioxepin-3-one and Its Use in Perfumery. Firmenich SA EP 902024 A1, 17 March 1999. [Google Scholar]
- Kraft, P. 1,2-Substituted 2,3-dihydro-1H-5,9-dioxacyclohepta[f]inden-7-ones and 7-substituted benzo[b][1,4]dioxepin-3-ones. Givaudan SA EP 1136481 B1, 13 November 2002. [Google Scholar]
- Gaudin, J.M.; Nikolaenko, O.; de Saint Laumer, J.Y.; Winter, B.; Blanc, P.A. Structure–Activity Relationship in the Domain of Odorants Having Marine Notes. Helv. Chim. Acta 2007, 90, 1245–1265. [Google Scholar] [CrossRef]
- Kraft, P.; Schär, M. Dérivé de 2H-benzo[b][1,4]dioxepin-3 (4h) -one derivative and its use as fragrance. Givaudan SA WO 2010/121981 Al, 19 April 2010. [Google Scholar]
- Kraft, P.; Popaj, K.; Müller, P.; Schär, M. ‘Vanilla Oceanics’: Synthesis and Olfactory Properties of (1′E)-7-(Prop-1′-enyl)-2H-benzo[b][1,4]dioxepin-3(4H)-ones and Homologues. Synthesis 2010, 17, 3029–3036. [Google Scholar] [CrossRef]
- Kozlov, N.G.; Basalaeva, L.I.; Vyglazov, O.G.; Chuiko, A. Synthesis of Calone Derivatives. Chem. Nat. Compd. 2011, 47, 391–394. [Google Scholar] [CrossRef]
- Kowalewski, J.; Ray, A. Predicting Human Olfactory Perception from Activities of Odorant Receptors. iScience 2020, 23, 101361. [Google Scholar] [CrossRef]
- Hauser, N.; Kraft, P.; Carreira, E.M. The Serendipitous Discovery of a Rose Odorant. Chimia 2020, 74, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Armanino, N.; Charpentier, J.; Flachsmann, F.; Goeke, A.; Liniger, M.; Kraft, P. What’s Hot, What’s Not: The Trends of the Past 20 Years in the Chemistry of Odorants. Angew. Chem. Int. Ed. Engl. 2020, 59, 16310–16344. [Google Scholar] [CrossRef]
- Drevermann, B.; Lingham, A.; Hugel, H.; Marriott, P. Synthesis of Benzodioxepinone Analogues via a Novel Synthetic Route with Qualitative Olfactory Evaluation. Helv. Chim. Acta 2007, 90, 1006–1027. [Google Scholar] [CrossRef]
- Plummer, C.M.; Kraft, P.; Froese, J.; Hudlicky, T.; Rook, T.J.; Jones, O.A.H.; Hugel, H.M. Synthesis and Olfactory Properties of 2-Substituted and 2,3-Annulated 1,4-Dioxepan-6-ones. Asian J. Org. Chem. 2015, 4, 1075–1084. [Google Scholar] [CrossRef]
- Rowe, D.J. Chemistry and Technology of Flavours and Fragrances; Wiley-Blackwell: Poole, UK, 2005; pp. 85–197. [Google Scholar]
- Drevermann, B.; Lingham, A.R.; Hügel, H.M.; Marriott, P.J. Synthesis and Qualitative Olfactory Evaluation of Benzodioxepine Analogues. Helv. Chim. Acta 2007, 90, 854–862. [Google Scholar] [CrossRef]
- Li, H.; Spannenberg, A.; Neumann, H.; Beller, M.; Wu, X.F. Regioselective Synthesis of 2,3-dihydrobenzodioxepinones from Epoxides and 2-bromophenols via Palladium-Catalyzed Carbonylation. Chem. Commun. 2014, 50, 2114–2116. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, J.M.; de Saint Laumer, J.Y. Structure–Activity Relationships in the Domain of Odorants Having Marine Notes. Eur. J. Org. Chem. 2015, 3, 1–12. [Google Scholar] [CrossRef]
- Huboux, A.; Gaudin, J.M.; Millet, P.; Robvieux, F. Benzodioxole Derivatives as Watery Odorants. Int. Pat. Appl. Firmenich WO 2012045646A1, 12 April 2012. [Google Scholar]
- Oertling, H. 4-Alkyl substituted pyridines as olfactory substances. Eur. Pat. Appl. Symrise EP 2100589A1, 16 September 2009. [Google Scholar]
- Liniger, M. Aldehydes for Use as Odorants. Int. Pat. Appl. Givaudan WO 2019092056A1, 16 May 2019. [Google Scholar]
- Müller, U. 6-Methoxy-2,6-dimethyloctanal and Its Use as a Fragrance Ingredient. Eur. Pat. Appl. (to Givaudan), EP 1764355A1, 21 March 2007. [Google Scholar]
- Dannenfeldt, K.H. Ambergris: The Search for its Origin. J. Hist. Sci. Soc. 1982, 73, 382–387. [Google Scholar] [CrossRef]
- Ohloff, G.; Vial, C.; Wolf, H.R.; Job, K.; Jégou, E.; Polonsky, J.; Lederer, E. Stereochemistry-Odor Relationships in Enantiomeric Ambergris Fragrances. Helv. Chim. Acta 1980, 63, 1932–1946. [Google Scholar] [CrossRef]
- Rowland, S.J.; Sutton, P.A. Chromatographic and Spectral Studies of Jetsam and Archived Ambergris. Nat. Prod. Res. 2017, 31, 1752–1757. [Google Scholar] [CrossRef]
- Rowland, S.J.; Sutton, P.A.; Belt, S.T.; Fitzsimmons-Thoss, V.; Scarlett, A.G. Further Spectral and Chromatographic Studies of Ambergris. Nat. Prod. Res. 2018, 32, 2603–2609. [Google Scholar] [CrossRef]
- Rowland, S.J.; Sutton, P.A.; Wolff, G.A. Biosynthesis of Ambrein in Ambergris: Evidence from Isotopic Data and Identification of Possible Intermediates. Nat. Prod. Res. 2019, 35, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.J.; Sutton, P.A.; Knowles, T.M. The Age of Ambergris. Nat. Prod. Res. 2019, 33, 3134–3142. [Google Scholar] [CrossRef]
- Coste-Manière, I.C.; Zahra, J.P.; Waegell, B. Synthesis of Ambergris Fragrance Chemicals from Sclareol, Involving Palladium Catalysed Key Steps. Tetrahedron Lett. 1988, 29, 1017–1020. [Google Scholar] [CrossRef]
- Neu, T.R.; Lawrence, J.R. Investigation of Microbial Biofilm Structure by Laser Scanning Microscopy. In Productive Biofilms. Advances in Biochemical Engineering/Biotechnology; Muffler, K., Ulber, R., Eds.; Springer International Publishing: Cham, Switzerland, 2014; Volume 146, pp. 1–52. [Google Scholar]
- Zinkel, D.; Toda, J.; Rowe, J. Occurrence of Anticopalic acid in Pinus Monticola. Phytochemistry 1971, 10, 1161–1163. [Google Scholar] [CrossRef]
- Cheng, L.P.; Xu, L.; Mao, H.F.; Wang, G.L. Study of Structural and Electronic Origin of Ambergris Odor of Some Compounds. J. Mol. Model 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Mookherjee, B.D.; Wilson, R.A. Tobacco Constituents—Their Importance in Flavor and Fragrance Chemistry. Perfum. Flavor 1990, 15, 27–49. [Google Scholar]
- Winter, B. Spirocyclic Ethers Related to Ambrox®: Synthesis and Structure-Odor Relationships. Helv. Chim. Acta 2004, 87, 1616–1627. [Google Scholar] [CrossRef]
- Ncube, E.N.; Steenkamp, L.; Dubery, I.A. Ambrafuran (AmbroxTM) Synthesis from Natural Plant Product Precursors. Molecules 2020, 25, 3851. [Google Scholar] [CrossRef]
- Kozioł, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gaweł, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- Kutney, J.P.; Chen, Y.H. The Chemistry of Thujone. XVII. The Synthesis of Ambergris Fragrances and Related Analogues. Can. J. Chem. 1994, 72, 1570–1581. [Google Scholar] [CrossRef]
- Barrero, A.F.; Altarejos, J.; Alvarez-Manzaneda, E.J.; Ramos, J.M.; Salido, S. Synthesis of (±)-Ambrox from (E)-Nerolidol and β-Ionone via Allylic Alcohol [2,3] Sigmatropic Rearrangement. J. Org. Chem. 1996, 61, 2215–2218. [Google Scholar] [CrossRef]
- Aoki, T.; Ataka, Y. Process for Production of (+)-3a,6,6,9a-Tetramethyldecahydro-naphtho [2,1-b]furan-2(1H)-one. U.S. Patent 8,153,826 B2, 10 April 2012. [Google Scholar]
- Serra, S. Recent Developments in the Synthesis of the Flavors and Fragrances of Terpenoid Origin, Studies in Natural Products Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2015; Volume 46, pp. 201–226. [Google Scholar]
- Benites, J.; Lopez, J.; Farias, J.G.; Cortes, M. The Preparation of Oxygenated Derivatives of Ambrox and Isoambrox from Drimenol. J. Chil. Chem. Soc. 2006, 51, 979–981. [Google Scholar] [CrossRef]
- Castro, J.M.; Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. Synthesis of Ambrox® from Labdanolic Acid. Tetrahedron 2002, 58, 5941–5949. [Google Scholar] [CrossRef]
- Bolster, M.G.; Jansen, B.J.; De Groot, A. The Synthesis of Ambrox®-Like Compounds Starting From (+)-Larixol. Tetrahedron 2001, 57, 5663–5679. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, G.; Xian, M.; Xie, C. Improved cis-Abienol Production through Increasing Precursor Supply in Escherichia coli. Sci. Rep. 2020, 10, 16791. [Google Scholar] [CrossRef]
- Zerbe, P.; Bohlmann, J. Enzymes for Synthetic Biology of Ambroxide-Related Diterpenoid Fragrance Compounds. Adv. Biochem. Eng. Biotechnol. 2015, 148, 427–447. [Google Scholar]
- Martins, M.P.; Ouazzani, J.; Arcile, G.; Jeller, A.H.; de Lima, J.P.; Seleghim, M.H.; Oliveira, A.L.; Debonsi, H.M.; Venâncio, T.; Yokoya, N.S.; et al. Biohydroxylation of (−)-ambrox®, (−)-sclareol, and (+)-sclareolide by whole Cells of Brazilian Marine-derived Fungi. Mar. Biotechnol. 2015, 17, 211–218. [Google Scholar] [CrossRef]
- Shen, Y.C.; Cheng, S.Y.; Kuo, Y.H.; Hwang, T.L.; Chiang, M.Y.; Khalil, A.T. Chemical Transformation and Biological Activities of Ambrein, A Major Product of Ambergris from Physeter macrocephalus (Sperm Whale). J. Nat. Prod. 2007, 70, 147–153. [Google Scholar] [CrossRef]
- Al-Khalil, S. A review on Ambergris Perspective and Modern Chemical Composition and Pharmacology. Acad. J. Plants 2020, 8, 96–101. [Google Scholar]
- Sandroni, P. Aphrodisiacs Past and Present: A Historical Review. Clin. Auton. Res. 2001, 11, 303–307. [Google Scholar] [CrossRef]
- Taha, S.A. Studies on the Mode of Action of Ambrein as a New Antinociceptive Compound. Jpn. J. Pharmacol. 1992, 60, 67–71. [Google Scholar] [CrossRef]
- Gode, P.K. History of Ambergris in India between about A.D. 700 and 1900. Chymia 1949, 2, 51–56. [Google Scholar] [CrossRef]
- Fahlbusch, K.G.; Hammerschmidt, F.J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; Volume 15, pp. 73–198. [Google Scholar]
- Bouchet, P. The Magnitude of Marine Biodiversity. In The Exploration of Marine Biodiversity: Scientific and Technological Challenges; Duarte, C.M., Ed.; Fundación BBVA: Bilbao, Spain, 2006; pp. 31–62. [Google Scholar]
- May, R.M. Tropical Arthropod Species, More or Less? Science 2010, 329, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, H.; Reimer, J.D.; Brandt, M.I.; Dumais, P.; Jażdżewska, A.M.; Jeffery, N.W.; Thielen, P.M.; Costello, M.J. Global marine Biodiversity in the Context of Achieving the Aichi Targets: Ways Forward and Addressing Data Gaps. Peer J. 2019, 7, e7221. [Google Scholar] [CrossRef] [PubMed]
- El Hattab, M. Algae Essential Oils: Chemistry, Ecology, and Biological Activities. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; de Oliveira, M.S., Silva, S., Da Costa, W.A., Eds.; IntechOpen: London, UK, 2020; pp. 1–20. [Google Scholar]
- Moore, R.E. Volatile Compounds from Marine Algae. Acc. Chem. Res. 1977, 10, 40–47. [Google Scholar] [CrossRef]
- El Hattab, M.; Culioli, G.; Piovetti, L.; Chitour, S.E.; Valls, R. Comparison of Various Extraction Methods for Identification and Determination of Volatile Metabolites from the Brown Alga Dictyopteris membranacea. J. Chromatogr. A 2007, 1143, 1–7. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.; Silva, G.; Pereira, L. Sea-weed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A Review on Phytoconstituents of Marine Brown Algae. Future J. Pharm. Sci. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated Compounds from Marine Algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Tang, K. Chemical Diversity and Biochemical Transformation of Biogenic Organic Sulfur in the Ocean. Front. Mar. Sci. 2020, 7, 68. [Google Scholar] [CrossRef]
- Francioso, A.; Conrado, A.B.; Mosca, L.; Fontana, M. Chemistry and Biochemistry of Sulfur Natural Compounds: Key Intermediates of Metabolism and Redox Biology. Oxid. Med. Cell Longev. 2020, 2020, 1–27. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Brito-Romano, O.; Robaina, R.R. Production of Volatiles by the Red Seaweed Gelidium arbuscula (Rhodophyta): Emission of Ethylene and Dimethyl Sulfide. J. Phycol. 2013, 49, 661–669. [Google Scholar] [CrossRef]
- Challenger, F.; Simpson, M.I. Studies on Biological Methylation. Part XII. A Precursor of the Dimethyl Sulphide Evolved by Polysiphonia fastigiata. Dimethyl-2-carboxyethyl Sulphonium Hydroxide and its Salts. J. Chem. Soc. 1948, 159, 1–7. [Google Scholar]
- Flodin, C.; Whitfield, F. 4-Hydroxybenzoic acid: A likely precursor of 2,4,6-tribromo phenol in Ulva lactuca. Phytochemistry 1999, 51, 249–255. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jurin, M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Update on Monoterpenes from Red Macroalgae: Isolation, Analysis, and Bioactivity. Mar. Drugs 2019, 17, 537. [Google Scholar] [CrossRef]
- Cikoš, A.M.; Jurin, M.; Coz-Rakovac, R.; Gašo-Sokač, D.; Jokic, S.; Jerkovic, I. Update on Sesquiterpenes from Red Macroalgae of the Laurencia Genus and their Biological Activities (2015–2020). Algal. Res. 2021, 56, 102330. [Google Scholar] [CrossRef]
- Blunt, J.; Carroll, A.; Copp, B.; Davis, R.; Keyzers, R.; Prinsep, M. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Boland, W. The Chemistry of Gamete Attraction: Chemical Structures, Biosynthesis, and (a)Biotic Degradation of Algal Pheromones. Proc. Natl. Acad. Sci. USA 1995, 92, 37–43. [Google Scholar] [CrossRef]
- Zatelli, G.A.; Philippus, A.C.; Falkenberg, M. An Overview of Odoriferous Marine Seaweeds of the Dictyopteris genus: Insights into their Chemical Diversity, Biological Potential and Ecological Roles. Rev. Bras. Farmacogn. 2018, 28, 243–260. [Google Scholar] [CrossRef]
- Maier, I.; Muller, D.G. Sexual Pheromones in Algae. Biol. Bull. 1986, 170, 145–175. [Google Scholar] [CrossRef]
- Riad, N.; Reda Zahi, M.R.; Trovato, E.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Chemical Screening and Antibacterial Activity of Essential Oil and Volatile Fraction of Dictyopteris polypodioides. Microchem. J. 2020, 152, 104415. [Google Scholar] [CrossRef]
- Baldovini, N.; Chaintreau, A. Identification of Key Odorants in Complex Mixtures Occurring in Nature. Nat. Prod. Rep. 2020, 37, 1589–1626. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Pettus, J.A.; Dictyopterene, A. An Odoriferous Constituent from Algae of the Genus Dictyopteris. Tetrahedron Lett. 1968, 9, 4787–4790. [Google Scholar] [CrossRef]
- Ohloff, G.; Pickenhagen, W. Synthese von (±)-dictyopterene. A HeIv. Chim. Acta. 1969, 52, 880–886. [Google Scholar] [CrossRef]
- Stratmann, K.; Boland, W.; Müller, D.G. Biosynthesis of Pheromones in Female Gametes of Marine Brown Algae (Phaeophyceae). Tetrahedron 1993, 49, 3755–3766. [Google Scholar] [CrossRef]
- Schnitzler, I.; Boland, W.; Hay, M.E. Organic Sulfur Compounds from Dictyopteris spp. Deter feeding by an Herbivorous Amphipod (Ampithoe longimana) but not by an Herbivorous Sea Urchin (Arbacia punctulata). J. Chem. Ecol. 1998, 24, 1715–1732. [Google Scholar] [CrossRef]
- Dimou, M.; Ioannou, E.; Daskalaki, M.G.; Tziveleka, L.A.; Kampranis, S.C.; Roussis, V. Disulfides with Anti-Inflammatory Activity from the Brown Alga Dictyopteris membranacea. J. Nat. Prod. 2016, 79, 584–589. [Google Scholar] [CrossRef]
- Roller, P.; Kalfred, A.; Moore, R.E. Isolation of S-(3-oxoundecyl)thioacetate, bis-(3-oxoundecyl) disulphide, (−)-3-hexyl-4,5-dithiacycloheptanone, and S-(trans-3-oxoundec-4-enyl) thioacetate from Dictyopteris. Chem. Commun. 1971, 273, 503–504. [Google Scholar] [CrossRef]
- Wratten, S.J.; Faulkner, D.J. Cyclic Polysulfides from the Red Alga Chondria Californica. J. Org. Chem. 1976, 41, 2465–2467. [Google Scholar] [CrossRef]
- Hay, M.E.; Duffy, J.E.; Fenical, W.; Gustaíson, K. Chemical Defense in the Seaweed Dictyopteris delicatula: Differential Effects Against Reef Fishes and Amphipods. Mar. Ecol. Prog. Ser. 1988, 48, 185–192. [Google Scholar] [CrossRef]
- Oigman, S.S.; Fernandes, Y.F.M.; Teles, D.; Maia, L.F.; Epifanio, R.A.; Rezende, C.M. Brazilian Gorgonians: A Source of Odoriferous Compounds? Rev. Bras. Farmacogn. 2015, 25, 612–618. [Google Scholar] [CrossRef][Green Version]
- Triqui, R. Sensory and Flavor Profiles as a Means of Assessing Freshness of Hake (Merluccius merluccius) during Ice Storage. Eur. Food. Res. Technol. 2006, 222, 41–47. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Let, M.B.; Meyer, A.S.; Jacobsen, C. Modeling the Sensory Impact of Defined Combinations of Volatile Lipid Oxidation Products on Fishy and Metallic Off-Flavors. J. Agric. Food Chem. 2004, 52, 1635–1641. [Google Scholar] [CrossRef]
- Hammer, M.; Schieberle, P. Model Studies on the Key Aroma Compounds Formed by an Oxidative Degradation of ω-3 Fatty Acids Initiated by either Copper(II) Ions or Lipoxygenase. J. Agric. Food Chem. 2013, 61, 10891–10900. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulou, E.A.; Dekker, H.L.; Steemers, L.; van Maarseveen, J.H.; de Koster, C.G.; Haring, M.A.; Schuurink, R.C.; Allmann, S. Identification and Characterization of (3Z):(2E)-Hexenal Isomerases from Cu-cumber. Front. Plant Sci. 2017, 8, 1342. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, T.; Matsui, K.; Akakabe, Y.; Kawai, T.; Ishihara, M. Preparation of Dictyopterene B Isomers and Fragrance Compositions Containing Them Jpn Kokai Tokkyo Koho JP 003137819. Apud Chem Abstr. 2003, 138, 369034. [Google Scholar]
- Milchakova, N. Marine Plants of the Black Sea. An Illustrated Field Guide; Digit Print Press: Sevastopol, Ukraine, 2011; pp. 37–120. [Google Scholar]
- Ramesh, C.H.; Koushik, S.; Shunmugaraj, T.; Murthy, M.V.R. A Red Alga Portieria hornemannii (Lyngb.) P. C. Silva 1987 (Gigartinales, Rhizophyllidaceae): A Source of Fragrance Ingredient for Perfume Industry. Indian J. Mar. Sci. 2020, 49, 898–902. [Google Scholar]
- Allen, J.A.; Duke, N.C. Bruguiera Gymnorrhiza (Large-Leafed Mangrove), ver. 2.1. In Species Profiles for Pacific Island Agroforestry; Elevitch, C.R., Ed.; Permanent Agriculture Resources (PAR): Hōlualoa, HI, USA, 2006; pp. 1–14. [Google Scholar]
- Adkar, P.P.; Bhaskar, V.H. Pandanus odoratissimus (Kewda): A review on ethnopharmacology, phytochemistry, and nutritional aspects. Advan. Pharmacol. Sci. 2014, 120895, 1–19. [Google Scholar] [CrossRef]
- Uppala, L. A Review on Active Ingredients from Marine Sources used in Cosmetics. SOJ Pharm. Pharm. Sci. 2015, 2, 1–3. [Google Scholar] [CrossRef][Green Version]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Phycocosmetics and Other Marine Cosmetics, Specific Cosmetics Formulated Using Marine Resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
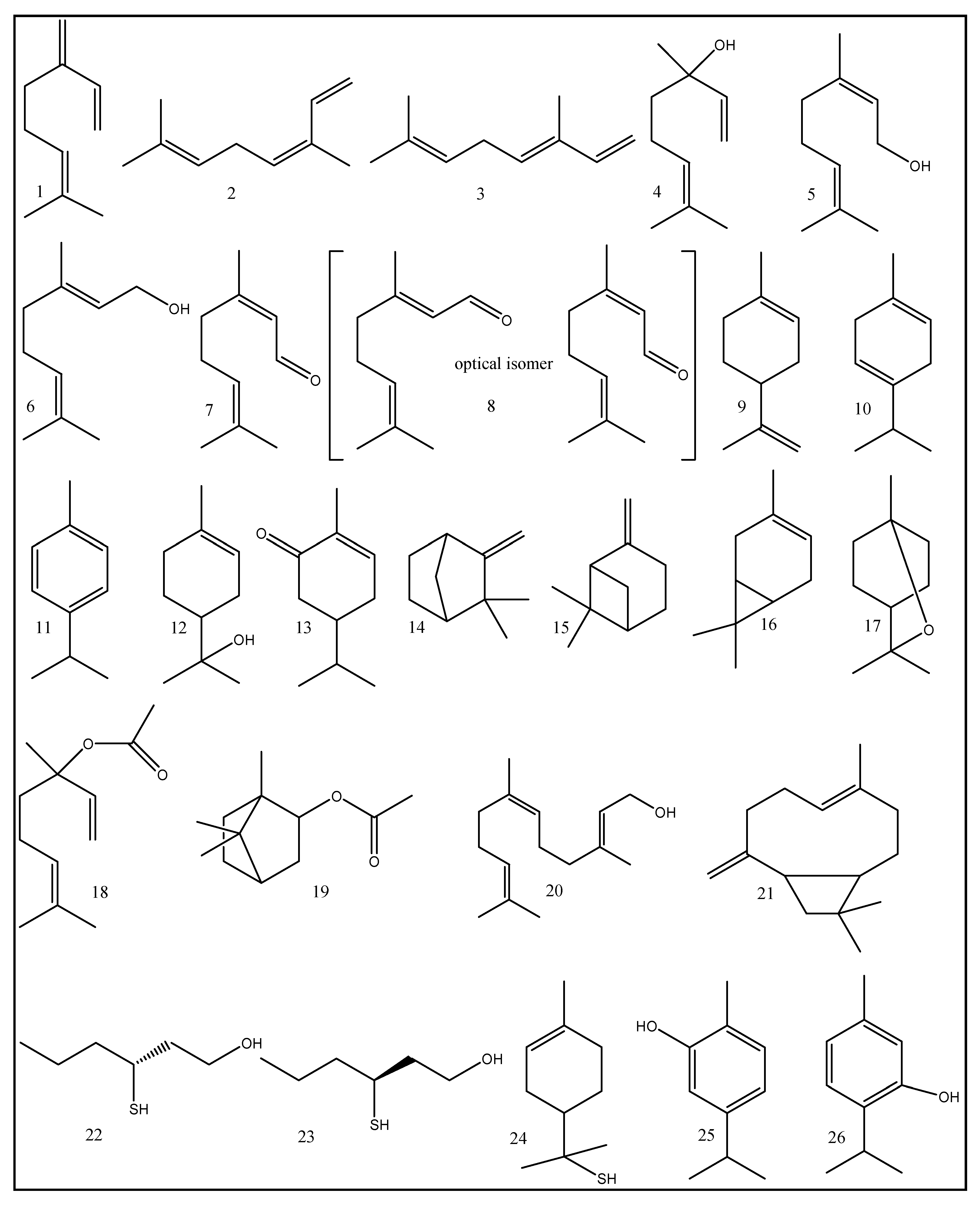

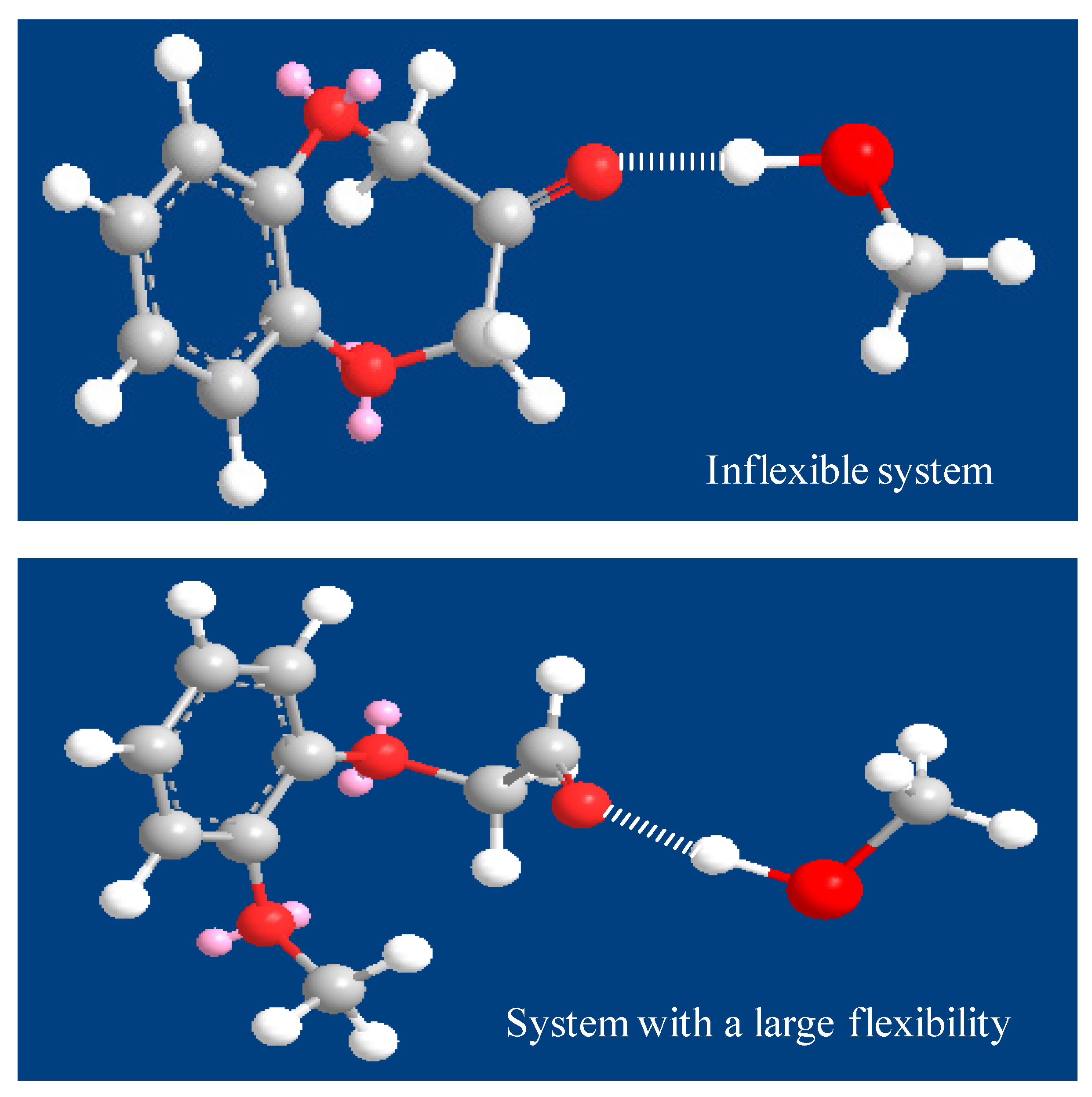

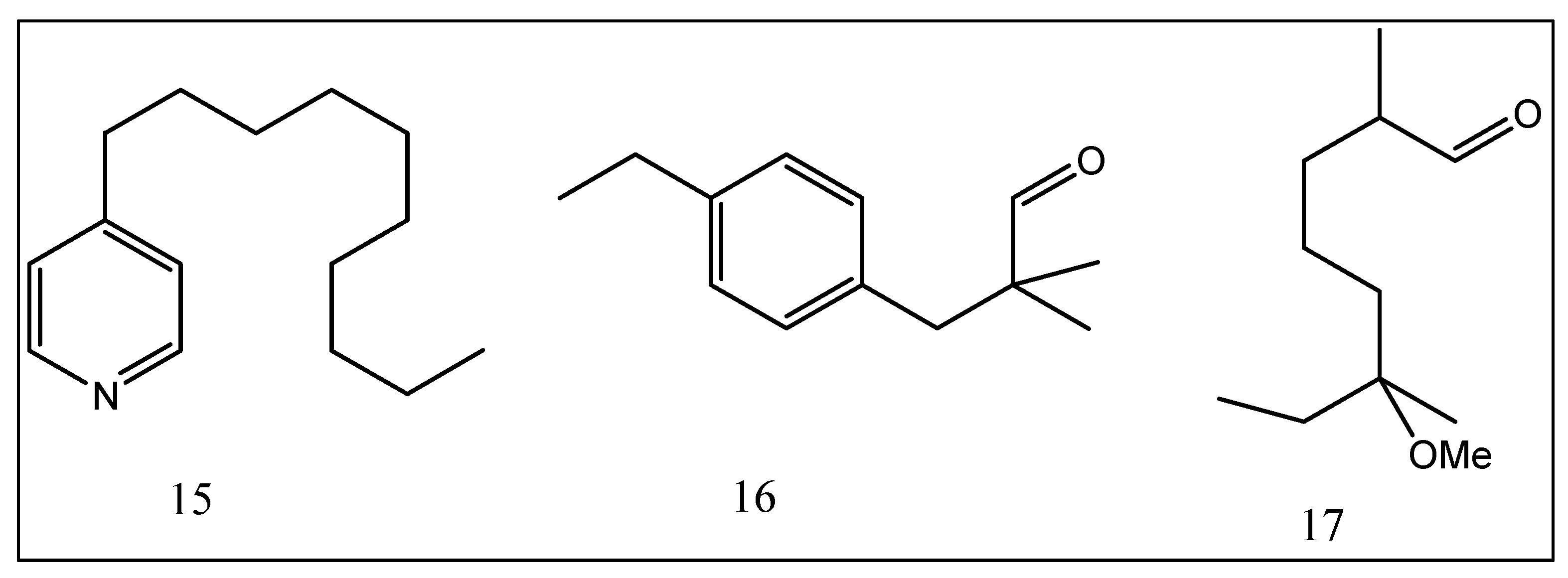
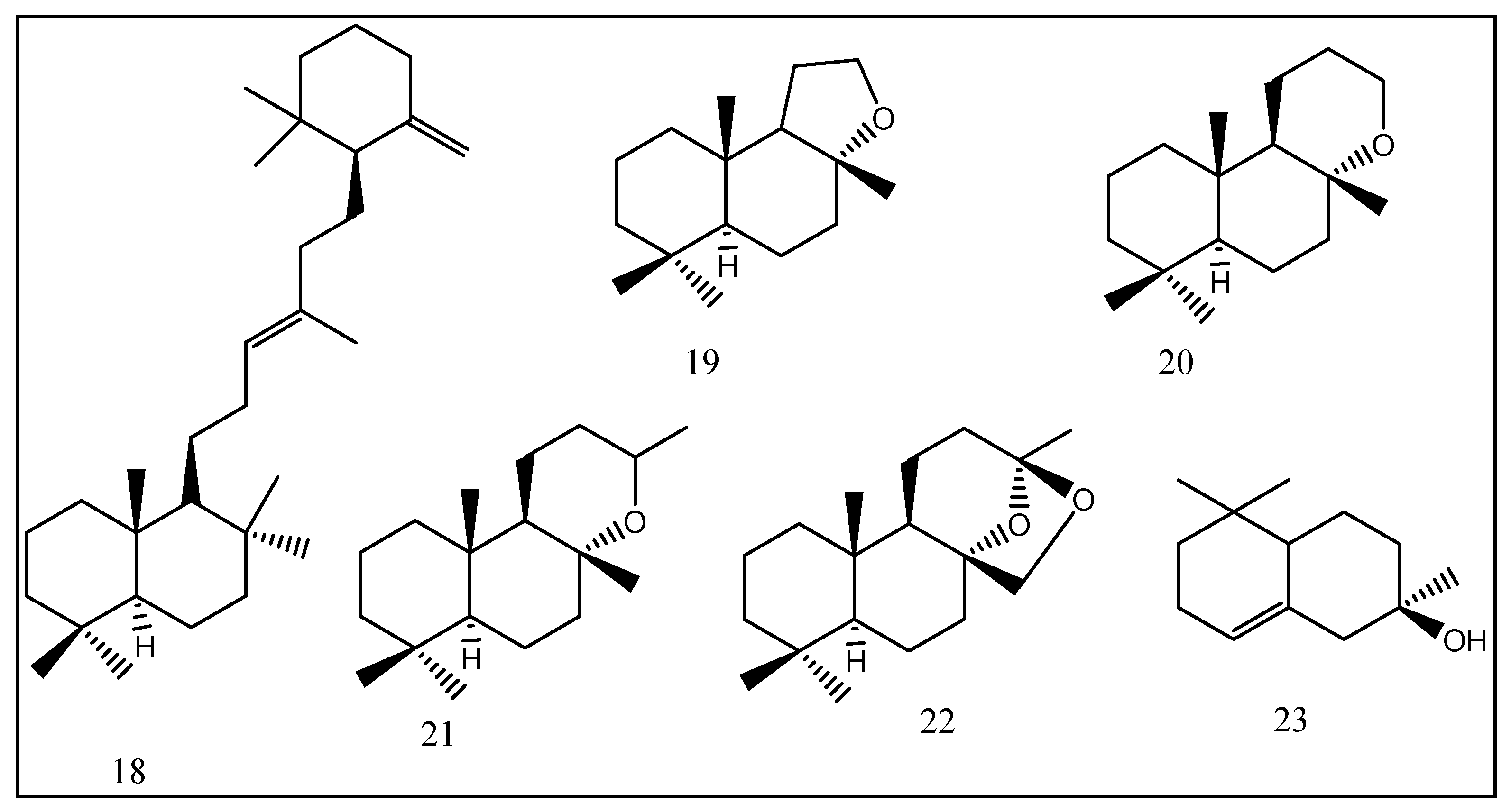
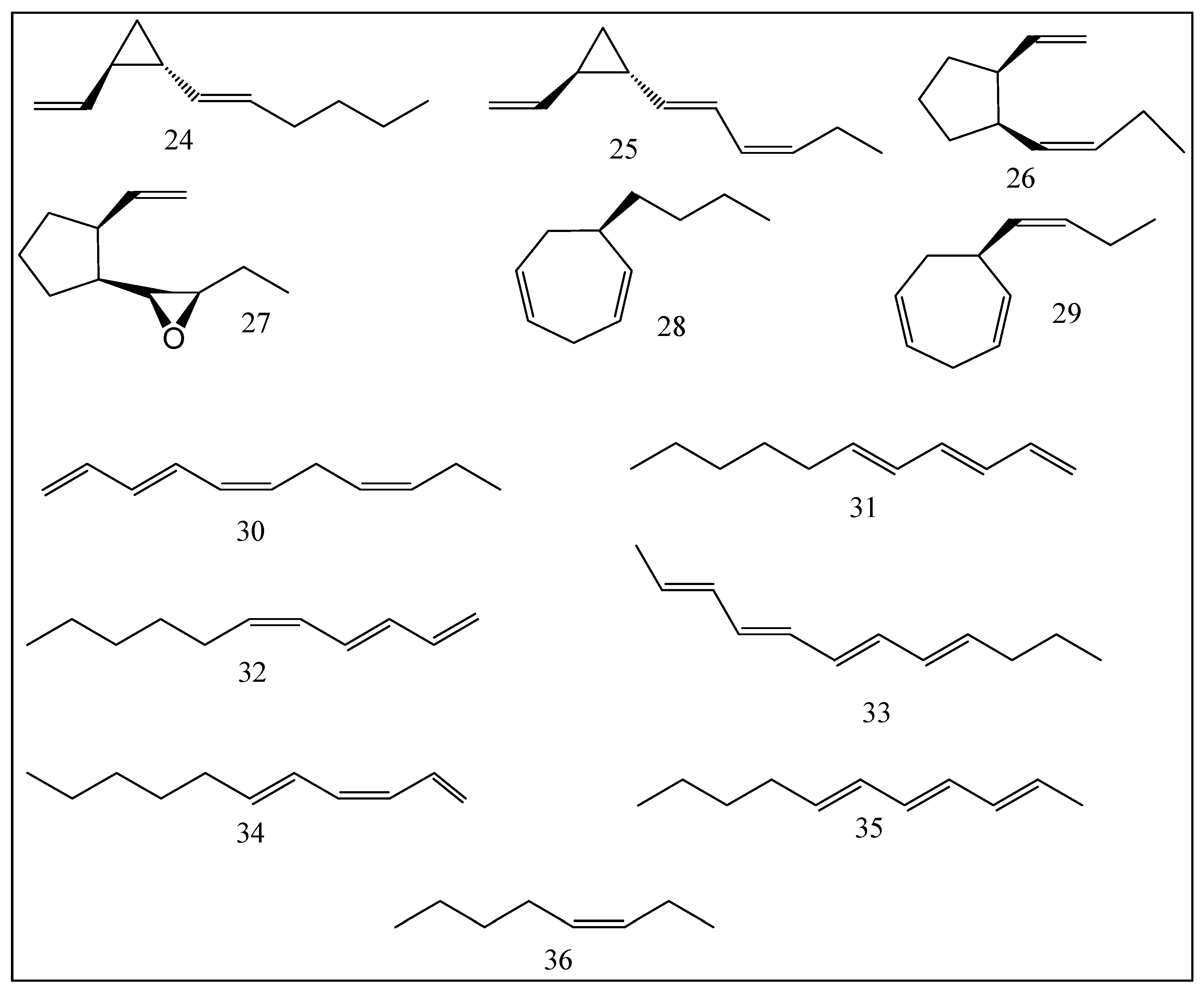
| No. | Compounds | Odor Quality | Threshold Range Reported in Literature (ng/L) | AI | (OAV) | ||
|---|---|---|---|---|---|---|---|
| Water | Air | Other Medium | |||||
| 1 | β-Myrcene | (pleasant floral) [133], (green, woody) [134] | 0.013–0.015 [60,135] | 0.041–0.15 [136,137] | 1.0 [138] | 4.2 [134] | 42 [139] 36 [139] |
| 2 | (Z)-β-Ocimene | (warm floral, herbal, sweet) [134] | 0.034–0.055 [140,141] | 0.001 [137] | - | 3.6 [134] | 16 [142,143] |
| 3 | (E)-β-Ocimene | sweet and herbal [142] | 0.034 [141] | 0.0187 [137] | - | 1.7 [144] | |
| 4 | Linalool | (green, floral, sweet) [134], (grassy, pleasant,citrus) [133] | 0.006 [141,145] | - | 0.067–0.113 [146] | 11 [147] | ˂1 [139] 8 [139] |
| 5 | Nerol | (sweet, citrus) [134] | 0.049 [148] | 0.5 [149,150] | 0.8 [134] | ˂1 [142,143] | |
| 6 | Geraniol | (floral, fruity) [144], (fresh, rose-like) [133] | 0.0011–0.006 [60,151] | 0.6 [152] | 0.005 [153] | 8 [147] | ˂1 [142,143] |
| 7 | Neral | (Citric, green) [154] | 0.053 [155] | 0.0088 [156] | - | - | 1330 [157] |
| 8 | Citral | Lemony [158] | 0.04–0.12 [159] | 0.00015 [160] | 0.656–1.23 [149] | ||
| 9 | Limonene | (citrus, sweet) [134], (Strong odor of orange) [158] | 0.2 [161] | 0.0539 [162] | 14.7 [161] | 4.7 [134] | 228 [139] 134 [139] |
| 10 | γ-Terpinene | (Woody, lemon, tropical, herbal) [163] | 1 [145] | 55 [162] | 2.39–3.26 [146] | 2.0 [134] | ˂1 [142,143] |
| 11 | p-Cymene | (Fresh, citrus, terpene, woody, spice) [163] | 0.00501 [164] | 7.2 [152] | 66 [138] | - | - |
| 12 | α-Terpineol | (Sweet, lilac odor) [133] (fresh and minty) [144] | 1.2 [165] | 0.86 [166] | 0.46 [167] | 10 [147] | - |
| 13 | Carvone | (Minty herbaceous) [154] | 0.027 [145] | 0.0002 [162] | 0.4–0.6 [168] | 1620 [157] | |
| 14 | Camphene | (woody, herbal) [134] | 1.86 [169] | [0–26] [170] | 1.0 [134] | ˂1 [142,143] | |
| 15 | β-Pinene | (dry woody, green) [134] | 0.14 [145] | 0.18 [133] | 37.2–38.7 [146] | 1.3 [134] | ˂1 [142,143] |
| 16 | ∆3-Carene | (sweet, fruity) [134] (woody turepentine) [133] | 0.77 [145] | 9.3 [152] | - | 4.5 [134] | 124 [133,134] |
| 17 | 1,8-Cineole | (Eucalyptus-like, fresh/pungent) [171] | 0.0011 [172] | - | 0.0013 [60] | - | - |
| 18 | Linalyl acetate | (Floral, sweet citrus) [156] | 1 [173,174] | 4.0–6.0 [175] | 10 [172] | - | - |
| 19 | Bornylacetate | (Woody, camphor, mentholic, spicy) [143,176] | 1.38 [173,174] | 0.44 [177] | 0.08 [178] | 0.1 [134] | - |
| 20 | Farnesol | (Flowery, weak-citrus odor) [158] | 1 [179] | - | 5 [180] | 5 [147] | - |
| 21 | β-Caryophyllene | (sweet, woody, spice) [134] | 0.064 [145] | - | 8 [147] | 8 [149] | 14 [142,143] |
| 22 | (3R)-(−)-3-Mercaptohexan-1-ol | fruitier, with a zesty aroma reminiscent of grapefruit [181] | - | 0.08 [182] | - | - | - |
| 23 | (3S)-(+)-3-Mercaptohexan-1-ol | passion fruit [182] | - | 0.07 [181] | - | - | - |
| 24 | p-Menthene-8-thiol | grapefruit-like [134] | 0.000002 [183] | 0.00000 [184] | - | - | - |
| 25 | Carvacrol | medicinal, tarry [185] | 2.29 [145] | 0.18 [186] | 30.97 [187] | - | - |
| 26 | Thymol | Sweet, herbal [185] | 1.7 [145] | 0.1 [188] | 124 [187] | - | - |
| No. | Compounds | Olfactory Profile |
|---|---|---|
| 9 | 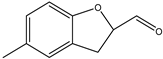 | Aldehydic, green, marine tenacious, powerful |
| 10 | 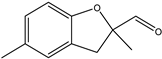 | Phenolic, crab, oakmoss, slightly watery |
| 11 | 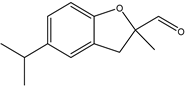 | Watermelon, aldehydic, Aldolone, cyclosal, green, oyster, ozone, watery |
| 12 |  | Aquozone, marine, fruity, green, rubbery |
| 13 | 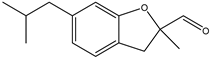 | Watery, fatty, floral, aldehydic |
| 14 |  | Watery, floral, marine, watermelon |
| No. | Odorant Compounds | Olfactory Profile | Odor Threshold (ng/L in Air) |
|---|---|---|---|
| 46 | (Z)-4-heptenal | fishy | 0.8 |
| 47 | Trans-4,5-epoxy-(E,Z)-2,7-decadienal | metallic, pungent | 0.01 |
| 48 | (Z,Z)-2,5-octadienal | sweet, melon-like | 0.08 |
| 49 | (E,Z)-2,5-octadienal | sweet, melon-like | 0.7 |
| 50 | (E,E,Z)-2,4,7-decatrienal | fatty, pungent | 0.8 |
| 51 | (E,E,E)-2,4,7-decatrienal | fatty, cucumber-like | 0.2 |
| 52 | (E,Z)-2,6-nonadienal | cucumber-like | 0.01 |
| 53 | (Z,Z)-3,6-nonadienal | fresh, watermelon-like | - |
| 54 | (Z,Z)-2,5-octadienal | sweet, melon-like | 0.08 |
| 55 | (Z)-1,5-octadien-3-one | geranium-like | 9 |
| 56 | (E,E,E)-2,4,7-decatrienal | fatty, cucumber-like | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riad, N.; Zahi, M.R.; Bouzidi, N.; Daghbouche, Y.; Touafek, O.; El Hattab, M. Occurrence of Marine Ingredients in Fragrance: Update on the State of Knowledge. Chemistry 2021, 3, 1437-1463. https://doi.org/10.3390/chemistry3040103
Riad N, Zahi MR, Bouzidi N, Daghbouche Y, Touafek O, El Hattab M. Occurrence of Marine Ingredients in Fragrance: Update on the State of Knowledge. Chemistry. 2021; 3(4):1437-1463. https://doi.org/10.3390/chemistry3040103
Chicago/Turabian StyleRiad, Nacera, Mohamed Reda Zahi, Naima Bouzidi, Yasmina Daghbouche, Ouassila Touafek, and Mohamed El Hattab. 2021. "Occurrence of Marine Ingredients in Fragrance: Update on the State of Knowledge" Chemistry 3, no. 4: 1437-1463. https://doi.org/10.3390/chemistry3040103
APA StyleRiad, N., Zahi, M. R., Bouzidi, N., Daghbouche, Y., Touafek, O., & El Hattab, M. (2021). Occurrence of Marine Ingredients in Fragrance: Update on the State of Knowledge. Chemistry, 3(4), 1437-1463. https://doi.org/10.3390/chemistry3040103