Discovery of Novel 1,2,4-Oxadiazole Derivatives as Potent Caspase-3 Activator for Cancer Treatment
Abstract
1. Introduction
2. Material and Methods
2.1. Dataset
2.2. 2D QSAR
2.3. Molecular Docking Analysis
3. Results and Discussion
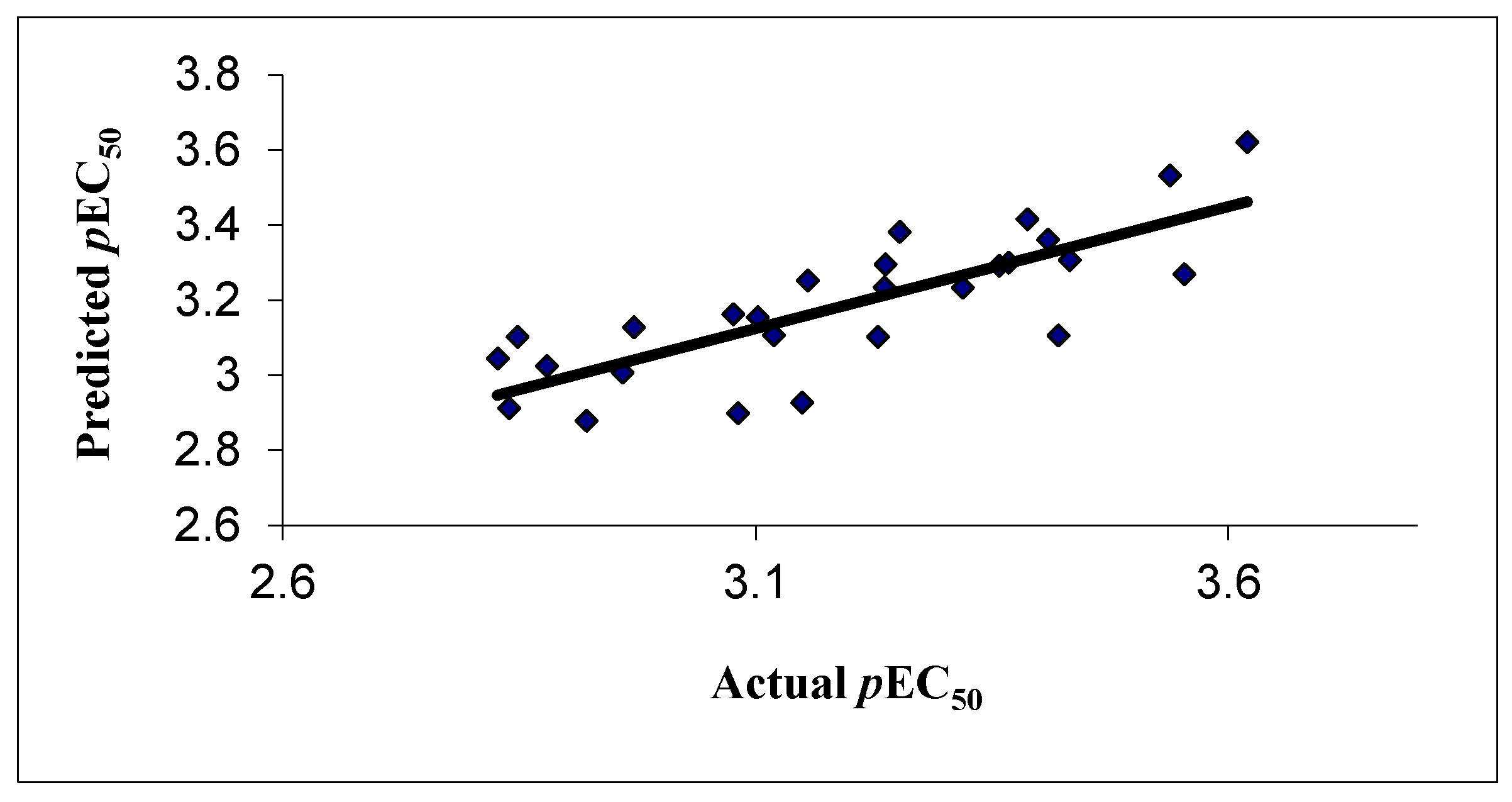
3.1. 2D QSAR Results
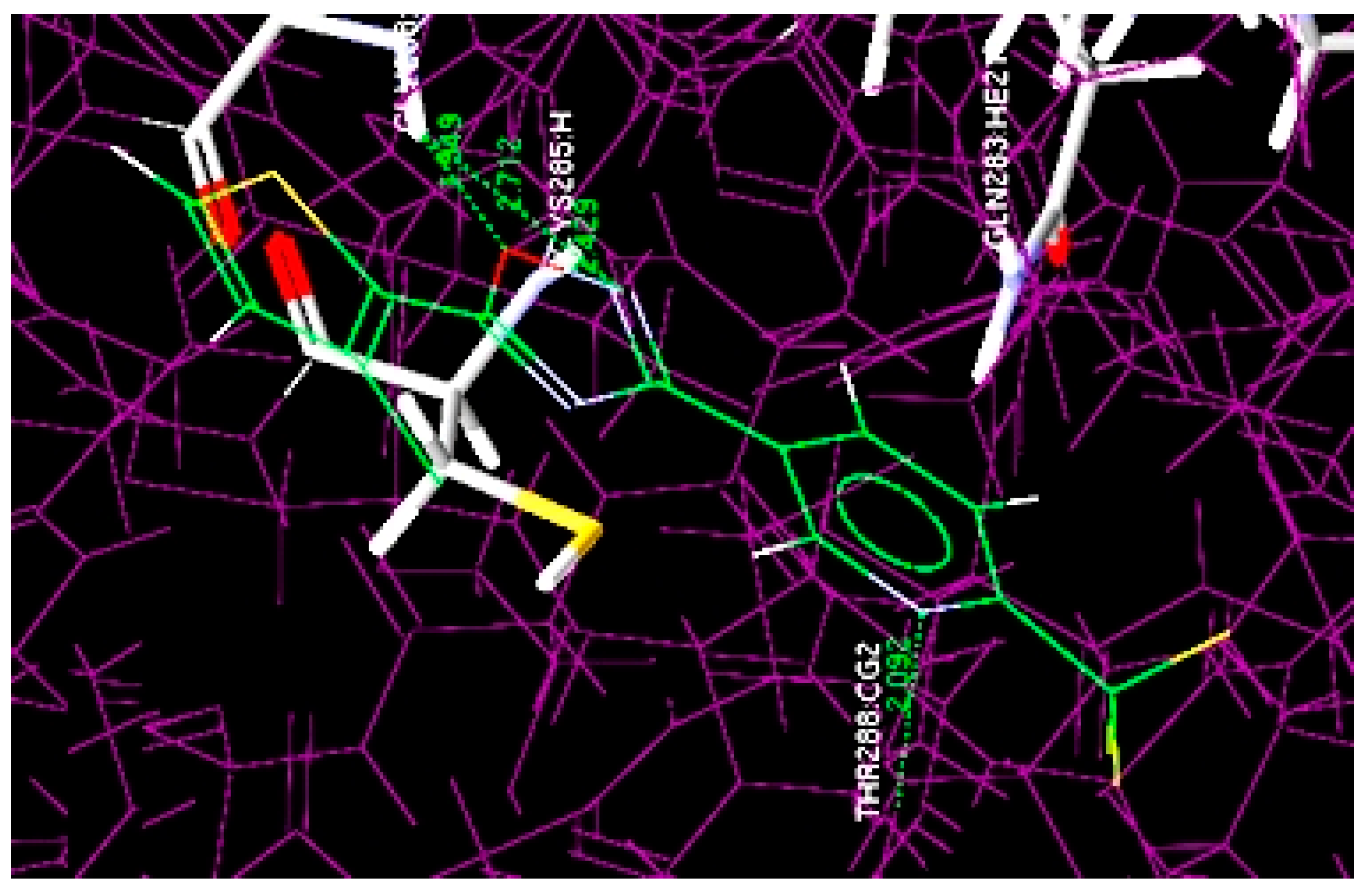
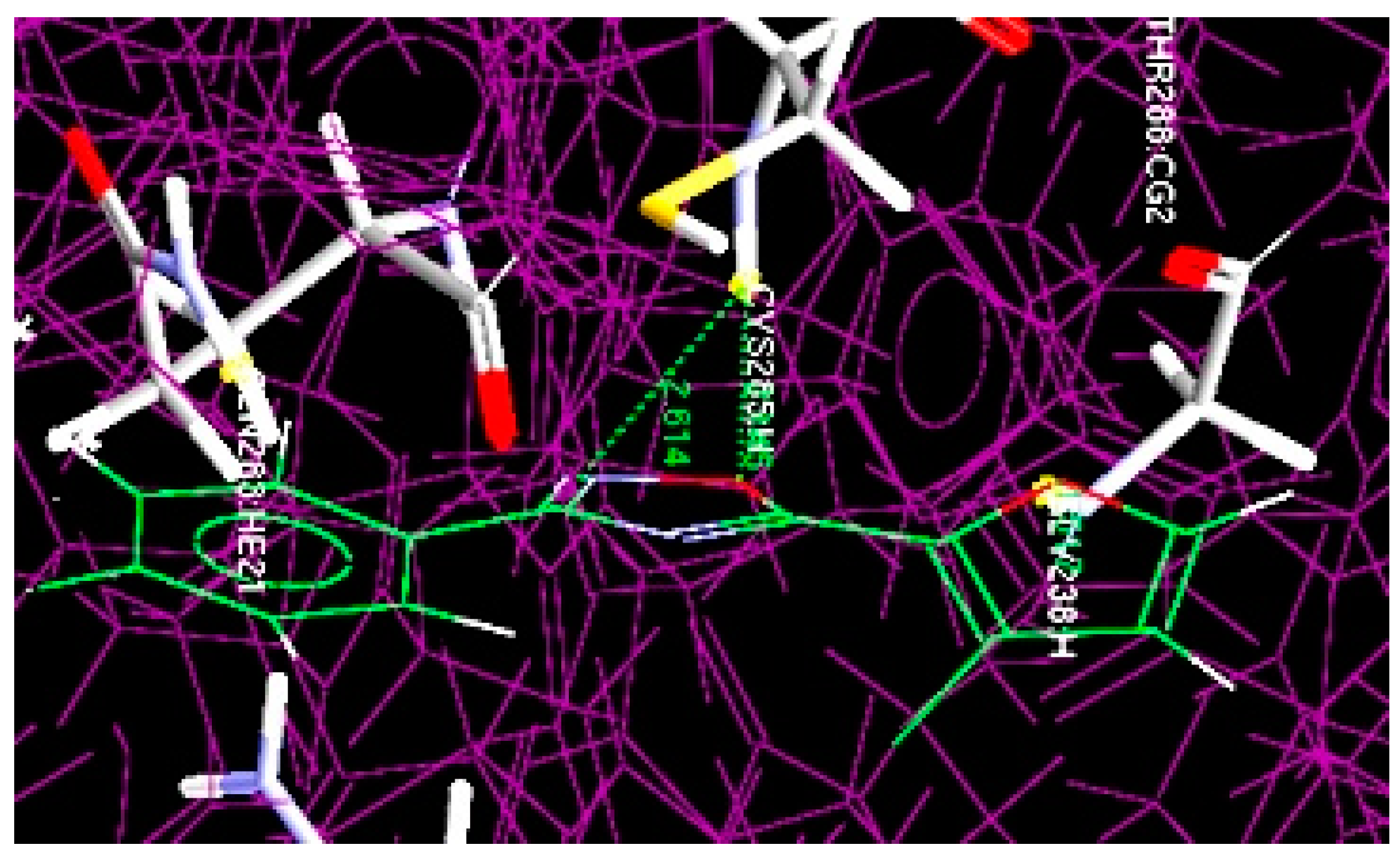
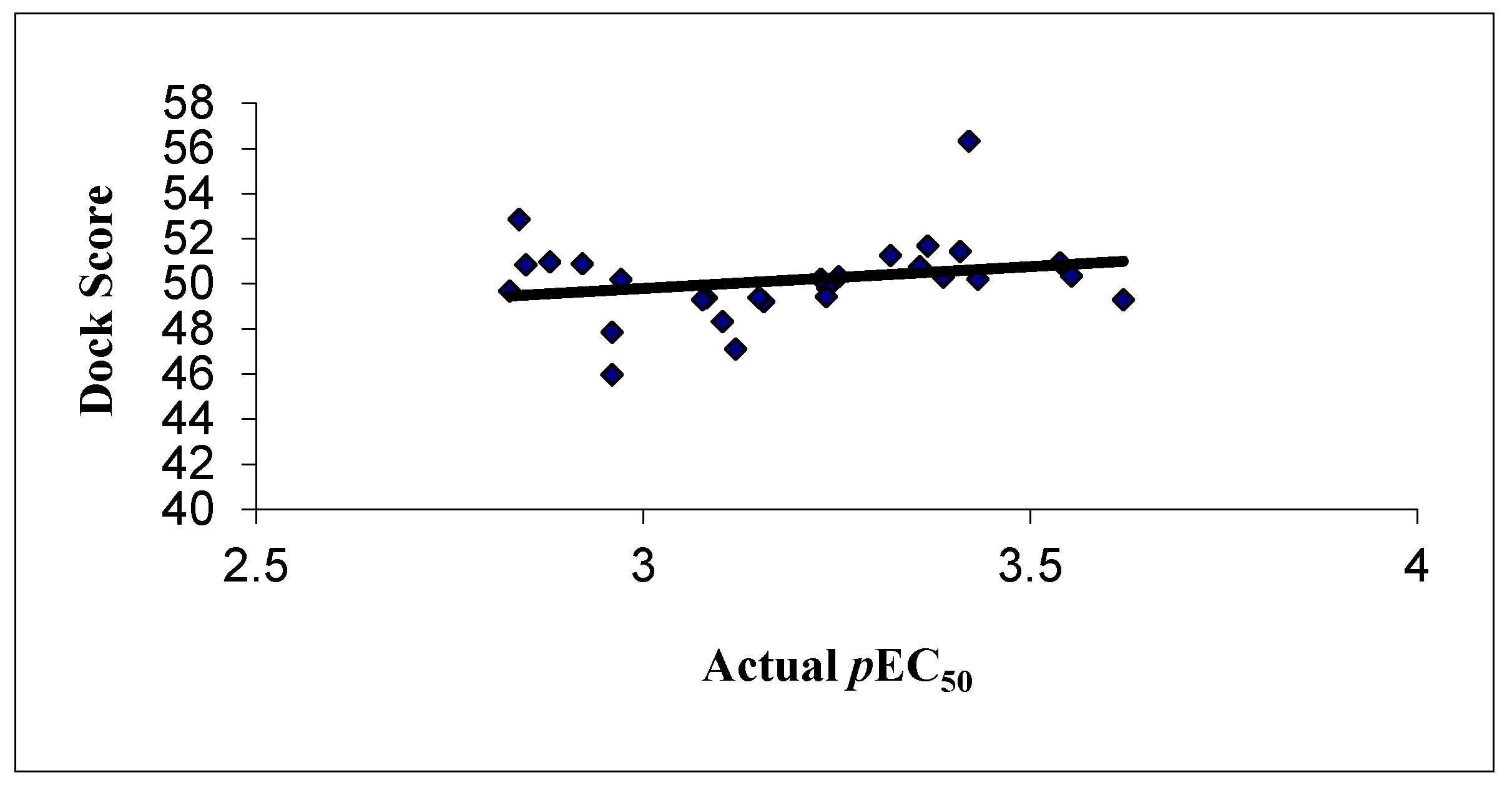
3.2. GOLD Docking Studies
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thun, M.J.; Henley, S.J.; Burns, D.; Jemal, A.; Shanks, T.G. Lung cancer death rates in lifelong nonsmokers. J. Natl. Cancer Inst. 2006, 98, 691–699. [Google Scholar] [CrossRef]
- Jain, S.; Pathak, K.; Vaidya, A. Molecular therapy using siRNA: Recent trends and advances of multi target inhibition of cancer growth. Int. J. Biol. Macromol. 2018, 116, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Jain, S.; Sahu, S.; Jain, P.K.; Pathak, K.; Pathak, D.; Kumar, R.; Jain, S.K. Anticancer agents based on vulnerable components in a signalling pathway. Mini Rev. Med. Chem. 2020, 20, 886–907. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Jain, A.K.; Agrawal, A.; Kashaw, S.K.; Jain, S.K.; Agrawal, R.K. Metabotropic Glutamate Receptors: A Review on Prospectives and Therapeutic Aspects. Mini Rev. Med. Chem. 2013, 12, 1967–1981. [Google Scholar] [CrossRef]
- Jain, S.; Vaidya, A.; Jain, A.K.; Agrawal, R.K.; Kashaw, S.K. Computational analysis of benzyl vinylogous derivatives as potent PDE3B inhibitors. Arab. J. Chem. 2017, 10, S109–S113. [Google Scholar] [CrossRef]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K. Recent Developments and Biological Activities of Thiazolidinone Derivatives: A Review. Bioorg. Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and Its Derivatives: A Review on Recent Progress in Biological Activities. Chem. Biol. Drug. Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Pattnaik, S.; Pathak, K.; Kumar, S.; Pathak, D.; Jain, S.K.; Vaidya, A. Anticancer Potential of Thiazole Derivatives: A Retrospective Review. Mini Rev. Med. Chem. 2017, 18, 640–655. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Jain, A.K.; Prashanthakumar, B.R.; Kashaw, S.K.; Agrawal, R.K. Computational Analysis of Quinoline Derivatives as Potent Topoisomerase-II Inhibitors. Med. Chem. Res. 2015, 24, 383–393. [Google Scholar] [CrossRef]
- Jain, S.; Chandra, V.; Pankaj Kumar, J.; Pathak, K.; Pathak, D.; Vaidya, A. Comprehensive review on current developments of quinoline-based anticancer agents. Arab. J. Chem. 2019, 12, 4920–4946. [Google Scholar] [CrossRef]
- Anjos, J.V.; Ricardo, A.W.; Filho, N.; Nascimento, S.C.; Srivastava, R.M.; Melo, S.; Sinou, D.J. Synthesis and cytotoxic profile of glycosyl-triazole linked to 1,2,4-oxadiazole moiety at C-5 through a straight-chain carbon and oxygen atoms. Eur. J. Med. Chem. 2009, 44, 3571–3576. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Jain, P.; Jain, P.; Tiwari, N.; Jain, R.; Jain, R.; Jain, A.K.; Agrawal, R.K. Synthesis and Biological Activities of Oxadiazole Derivatives: A Review. Mini Rev. Med. Chem. 2016, 16, 825–845. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Kaisbhatla, S.; Kuemmerle, J.; Kemnitzer, W.; Mason, K.O.; Qui, L.; Grundy, C.C.; Tseng, B.; Drew, J.; Cai, S.X. Discovery and Structure−Activity Relationship of 3-Aryl-5-aryl-1,2,4-oxadiazoles as a New Series of Apoptosis Inducers and Potential Anticancer Agents. J. Med. Chem. 2005, 48, 5215–5223. [Google Scholar] [CrossRef]
- Bhatiya, R.; Vaidya, A.; Kashaw, S.K.; Jain, A.K.; Agrawal, R.K. QSAR analysis of furanone derivatives as potential COX-2 inhibitors: kNN MFA approach. J. Saudi Chem. Soc. 2014, 18, 977–984. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Jain, S.; Jain, A.K.; Agrawal, R.K. Quantitative Structure-Activity Relationships: A Novel Approach of Drug Design and Discovery. J. Pharm. Sci. Pharmacol. 2014, 1, 219–232. [Google Scholar] [CrossRef]
- Agrawal, R.K.; Jain, A.K.; Veerasamy, R.; Vaidya, A.; Kashaw, S.; Mourya, V.K.; Agrawal, R.K. QSAR analysis of B-ring-modified diaryl ether derivatives as a InhA inhibitors. Med. Chem. Res. 2012, 21, 145–151. [Google Scholar]
- Vaidya, A.; Jain, A.K.; Kumar, P.; Kashaw, S.K.; Agrawal, R.K. Predicting anti-cancer activity of quinoline derivatives: CoMFA and CoMSIA approach. J. Enzyme Inhib. Med. Chem. 2011, 26, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Veerasamy, R.; Vaidya, A.; Mourya, V.; Agrawal, R.K. QSAR analysis of some novel sulfonamides incorporating 1,3,5-triazine derivatives as carbonic anhydrase inhibitors. Med. Chem. Res. 2010, 19, 1191–1202. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, A.K.; Kumar, B.R.P.; Sastry, G.N.; Kashaw, S.K.; Agrawal, R.K. CoMFA, CoMSIA, kNN MFA and Docking studies of 1.;2.;4-Oxadiazole derivatives as potent Caspase-3 activators. Arab. J. Chem. 2017, 10, S3936–S3946. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Kumar, B.R.P.; Singh, S.K.; Kashaw, S.K.; Agrawal, R.K. Synthesis of 1,2,4-oxadiazole derivatives: Anticancer and 3D QSAR studies. Mon. Chem. 2020, 151, 385–395. [Google Scholar] [CrossRef]
- Vaidya, A.; Pathak, D.; Shah, K. 1,3,4-oxadiazole and its Derivatives: A Review on Recent Progress in Anticancer Activities. Chem. Biol. Drug Des. 2020, 97, 572–591. [Google Scholar] [CrossRef] [PubMed]
- Kemnitzer, W.; Kuemmerle, J.; Zhang, H.Z.; Kaisbhatla, S.; Tseng, B.; Drew, J.; Cai, S.X. Discovery of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers. 2. Identification of more aqueous soluble analogs as potential anticancer agents. Bioorg. Med. Chem. Lett. 2009, 19, 4410–4415. [Google Scholar] [PubMed]
- Roy, K.; Das, R.N. A review on principles, theory and practices of 2D-QSAR. Curr. Drug. Metab. 2014, 15, 346–379. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, C.; Mishra, B.K. Quantitative Structure-Activity Relationships of Aquatic Narcosis: A Review. Curr. Comput. Aided Drug Des. 2018, 14, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Wood, D. Modern 2D QSAR for drug discovery. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 505–522. [Google Scholar] [CrossRef]

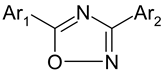
| S. No. | Compound | Ar1 | Ar2 | Experimental Activity pEC50 (nM) (DLD1) |
|---|---|---|---|---|
| 1 | 1d | 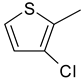 |  | 3.357 |
| 2 | 4a | 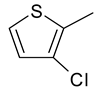 |  | 3.102 |
| 3 | 4b | 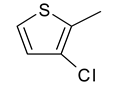 |  | 3.119 |
| 4 | 4c |  |  | 3.367 |
| 5 | 4d | 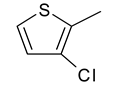 | 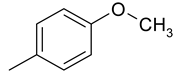 | 2.839 |
| 6 | 4e |  | 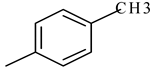 | 2.848 |
| 7 | 4g |  |  | 3.553 |
| 8 | 4h | 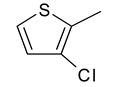 | 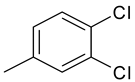 | 3.432 |
| 9 | 4i | 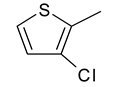 |  | 3.252 |
| 10 | 4j | 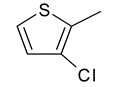 | 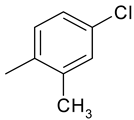 | 3.420 |
| 11 | 4k | 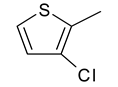 | 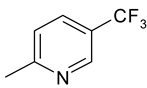 | 3.387 |
| 12 | 4l | 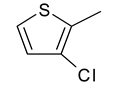 | 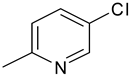 | 3.409 |
| 13 | 4m |  | 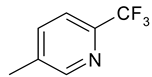 | 3.620 |
| 14 | 4n |  | 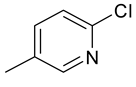 | 3.538 |
| 15 | 4o | 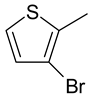 | 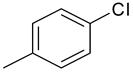 | 2.879 |
| 16 | 10a | 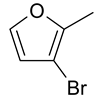 | 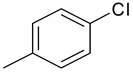 | 3.081 |
| 17 | 10b | 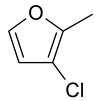 |  | 2.827 |
| 18 | 10d |  | 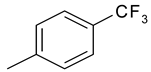 | 2.971 |
| 19 | 10e |  | 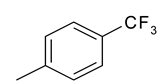 | 3.319 |
| 20 | 10f | 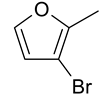 |  | 3.076 |
| 21 | 10g |  | 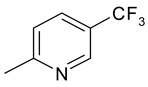 | 3.155 |
| 22 | 10h | 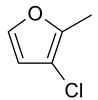 | 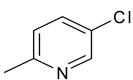 | 3.237 |
| 23 | 11a | 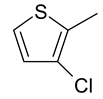 |  | 3.229 |
| 24 | 11b | 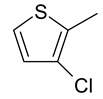 |  | 3.236 |
| 25 | 11c | 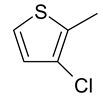 | 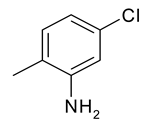 | 2.959 |
| 26 | 11d | 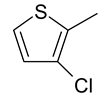 | 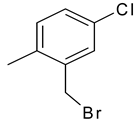 | 2.959 |
| 27 | 11e |  | 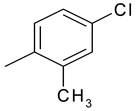 | 3.149 |
| 28 | 11f | 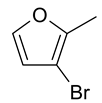 |  | 2.921 |
| Average | Maxima | Minima | Std. Deviation | |
|---|---|---|---|---|
| Training set | 3.174 | 3.620 | 2.827 | 0.228 |
| Test set | 3.234 | 3.553 | 2.848 | 0.264 |
| Model | r | r2 | q2 | SE (r2 se) | Pred_r2 | F-Value | Descriptors |
|---|---|---|---|---|---|---|---|
| 1 | 0.862 | 0.743 | 0.610 | 0.130 | 0.553 | 11.561 | IP, BC, DM, PSA |
| 2D QSAR | Docking | |||
|---|---|---|---|---|
| Comp. No. | Experimental pEC50 | [(SW) kNN MFA] Predicted pEC50 | [(SW) kNN MFA] Residual | GOLD Docking |
| 1d | 3.357 | 3.291 | 0.065 | 50.771 |
| 4a | 3.102 | 3.155 | −0.052 | 48.324 |
| 4b | 3.119 | 3.106 | 0.013 | 47.105 |
| 4c | 3.367 | 3.300 | 0.067 | 51.672 |
| 4d | 2.839 | 2.912 | −0.074 | 52.859 |
| 4e | 2.848 | 3.102 | −0.255 | 50.846 |
| 4g | 3.553 | 3.269 | 0.284 | 50.357 |
| 4h | 3.432 | 3.306 | 0.126 | 50.212 |
| 4i | 3.252 | 3.382 | −0.130 | 50.304 |
| 4j | 3.420 | 3.106 | 0.315 | 56.319 |
| 4k | 3.387 | 3.415 | −0.028 | 50.294 |
| 4l | 3.409 | 3.361 | 0.048 | 51.432 |
| 4m | 3.620 | 3.620 | −0.0002 | 49.303 |
| 4n | 3.538 | 3.532 | 0.005 | 50.930 |
| 4o | 2.879 | 3.025 | −0.146 | 50.968 |
| 10a | 3.081 | 2.899 | 0.182 | 49.383 |
| 10b | 2.827 | 3.045 | −0.218 | 49.680 |
| 10d | 2.971 | 3.128 | −0.157 | 50.205 |
| 10e | 3.319 | 3.233 | 0.086 | 51.265 |
| 10f | 3.076 | 3.162 | −0.086 | 49.302 |
| 10g | 3.155 | 3.253 | −0.098 | 49.203 |
| 10h | 3.237 | 3.295 | −0.058 | 49.839 |
| 11a | 3.229 | 3.102 | 0.127 | 50.212 |
| 11b | 3.236 | 3.234 | 0.003 | 49.423 |
| 11c | 2.959 | 3.007 | −0.049 | 45.962 |
| 11d | 2.959 | 3.007 | −0.049 | 47.860 |
| 11e | 3.149 | 2.927 | 0.222 | 49.377 |
| 11f | 2.921 | 2.879 | 0.042 | 50.891 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaidya, A. Discovery of Novel 1,2,4-Oxadiazole Derivatives as Potent Caspase-3 Activator for Cancer Treatment. Chemistry 2021, 3, 373-381. https://doi.org/10.3390/chemistry3010027
Vaidya A. Discovery of Novel 1,2,4-Oxadiazole Derivatives as Potent Caspase-3 Activator for Cancer Treatment. Chemistry. 2021; 3(1):373-381. https://doi.org/10.3390/chemistry3010027
Chicago/Turabian StyleVaidya, Ankur. 2021. "Discovery of Novel 1,2,4-Oxadiazole Derivatives as Potent Caspase-3 Activator for Cancer Treatment" Chemistry 3, no. 1: 373-381. https://doi.org/10.3390/chemistry3010027
APA StyleVaidya, A. (2021). Discovery of Novel 1,2,4-Oxadiazole Derivatives as Potent Caspase-3 Activator for Cancer Treatment. Chemistry, 3(1), 373-381. https://doi.org/10.3390/chemistry3010027





