Cluster-Based Coordination Polymers of Mn/Fe-Oxo Pivalates and Isobutyrates †
Abstract
1. Introduction
2. Oxo-Trinuclear Mn/Fe-Based CCPs
3. Oxo-Tetranuclear Mn/Fe-Based CCPs
4. Oxo-Hexanuclear Mn/Fe CCPs
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Metal cluster-based functional porous coordination polymers. Coord. Chem. Rev. 2015, 293–294, 263–278. [Google Scholar] [CrossRef]
- Kang, X.-M.; Tang, M.-H.; Yang, G.-L.; Zhao, B. Cluster/cage-based coordination polymers with tetrazole derivatives. Coord. Chem. Rev. 2020, 422, 213424. [Google Scholar] [CrossRef]
- Andruh, M. Oligonuclear complexes as tectons in crystal engineering: Structural diversity and magnetic properties. Chem. Commun. 2007, 2565–2577. [Google Scholar] [CrossRef]
- Roubeau, O.; Clérac, R. Rational assembly of high-spin polynuclear magnetic complexes into coordination networks: The case of a [Mn4] single-molecule magnet building block. Eur. J. Inorg. Chem. 2008, 4325–4342. [Google Scholar] [CrossRef]
- Jeon, I.R.; Clerac, R. Controlled association of single-molecule magnets (SMMs) into coordination networks: Towards a new generation of magnetic materials. Dalton Trans. 2012, 41, 9569–9586. [Google Scholar] [CrossRef]
- Shieh, M.; Liu, Y.-H.; Lia, Y.-H.; Lina, R.Y. Metal carbonyl cluster-based coordination polymers: Diverse syntheses, versatile network structures, and special properties. Cryst. Eng. Comm. 2019, 21, 7341–7364. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 1–471. [Google Scholar]
- Robson, R. A net-based approach to coordination polymers. J. Chem. Soc. Dalton Trans. 2000, 3735–3744. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortes, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Perry, J.J.; Permana, J.A.; Zaworotko, M.J. Design and synthesis of metal–organic frameworks using metal–organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Baca, S.G. The design of coordination networks from polynuclear Mn(II, III) and Fe(III) oxo-carboxylate clusters. In Advances in Chemistry Research; Taylor, J.C., Ed.; Nova Science Publishers: New York, NY, USA, 2018; Volume 43, pp. 81–130. [Google Scholar]
- Fang, X.; Kögerler, P. A polyoxometalate-based manganese carboxylate cluster. Chem. Commun. 2008, 3396–3398. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.G.F.; Andruh, M. Molecule-based magnetic materials constructed from paramagnetic organic ligands and two different metal ions. Coord. Chem. Rev. 2021, 427, 213611. [Google Scholar] [CrossRef]
- Ratera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef]
- Kahn, O. Chemistry and physics of supramolecular magnetic materials. Acc. Chem. Res. 2000, 33, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Mccleverty, J.A.; Ward, M.D. The role of bridging ligands in controlling electronic and magnetic properties in polynuclear complexes. Acc. Chem. Res. 1998, 31, 842–851. [Google Scholar] [CrossRef]
- Farrusseng, D. (Ed.) Metal–Organic Frameworks. Applications from Catalysis to Gas Storage; Wiley-VCH Verlag & Co. KgaA: Weinheim, Germany, 2011; p. 392. [Google Scholar]
- Winpenny, R.E.P. (Ed.) Single-Molecule Magnets and Related Phenomena; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2006; Volume 122. [Google Scholar]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Gatteschi, D.; Sessoli, R.; Cornia, A. Single-molecule magnets based on iron(III) oxo clusters. Chem. Commun. 2000, 725–732. [Google Scholar] [CrossRef]
- Moushi, E.E.; Stamatatos, T.C.; Wernsdorfer, W.; Nastopoulos, V.; Christou, G.; Tasiopoulos, A.J. A Family of 3D coordination polymers composed of Mn19 magnetic units. Angew. Chem. 2006, 118, 7886–7889. [Google Scholar] [CrossRef]
- Moushi, E.E.; Stamatatos, T.C.; Wernsdorfer, W.; Nastopoulos, V.; Christou, G.; Tasiopoulos, A.J. A Mn17 octahedron with a giant ground-state spin: Occurrence in discrete form and as multidimensional coordination polymers. Inorg. Chem. 2009, 48, 5049–5051. [Google Scholar] [CrossRef]
- Moushi, E.E.; Stamatatos, T.C.; Wernsdorfer, W.; Nastopoulos, V.; Christou, G.; Tasiopoulos, A.J. 1-D coordination polymers consisting of a high-spin Mn17 octahedral unit. Polyhedron 2009, 28, 1814–1817. [Google Scholar] [CrossRef]
- Hessel, L.W.; Romers, C. The crystal structure of “anhydrous manganic acetate”. Recl. Trav. Chim. Pays-Bas 1969, 88, 545–552. [Google Scholar] [CrossRef]
- Albores, P.; Rentschler, E. Structural and magnetic characterization of a μ-1,5-dicyanamide-bridged iron basic carboxylate [Fe3O(O2C(CH3)3)6] 1D chain. Inorg. Chem. 2008, 47, 7960–7962. [Google Scholar] [CrossRef]
- Long, D.L.; Kögerler, P.; Farrugia, L.J.; Cronin, L. Linking chiral clusters with molybdate building blocks: From homochiral helical supramolecular arrays to coordination helices. Chem. Asian J. 2006, 1, 352–357. [Google Scholar] [CrossRef]
- Wu, Q.L.; Han, S.D.; Wang, Q.L.; Zhao, J.P.; Ma, F.; Jiang, X.; Liu, F.C.; Bu, X.H. Divalent metal ions modulated strong frustrated M(II)–Fe(III)3O (M = Fe, Mn, Mg) chains with metamagnetism only in a mixed valence iron complex. Chem. Commun. 2015, 51, 15336–15339. [Google Scholar] [CrossRef] [PubMed]
- Baca, S.G.; Malaestean, I.L.; Keene, T.D.; Adams, H.; Ward, M.D.; Hauser, J.; Neels, A.; Decurtins, S. One-dimensional manganese coordination polymers composed of polynuclear cluster blocks and polypyridyl linkers: Structures and properties. Inorg. Chem. 2008, 47, 11108–11119. [Google Scholar] [CrossRef]
- Dulcevscaia, G.M.; Filippova, I.G.; Speldrich, M.; Leusen, J.; Kravtsov, V.C.; Baca, S.G.; Kögerler, P.; Liu, S.X.; Decurtins, S. Cluster-based networks: 1D and 2D coordination polymers based on {MnFe2(μ3-O)}-type clusters. Inorg. Chem. 2012, 51, 5110–5117. [Google Scholar] [CrossRef] [PubMed]
- Polunin, R.A.; Kiskin, M.A.; Cador, O.; Kolotilov, S.V. Coordination polymers based on trinuclear heterometallic pivalates and polypyridines: Synthesis, structure, sorption and magnetic properties. Inorg. Chim. Acta 2012, 380, 201–210. [Google Scholar] [CrossRef]
- Lytvynenko, A.S.; Kiskin, M.A.; Dorofeeva, V.N.; Mishura, A.M.; Titov, V.E.; Kolotilov, S.V.; Eremenko, I.L.; Novotortsev, V.M. Redox-active porous coordination polymer based on trinuclear pivalate: Temperature-dependent crystal rearrangement and redox-behavior. J. Solid State Chem. 2015, 223, 122–130. [Google Scholar] [CrossRef]
- Sotnik, S.A.; Polunin, R.A.; Kiskin, M.A.; Kirillov, A.M.; Dorofeeva, V.N.; Gavrilenko, K.S.; Eremenko, I.L.; Novotortsev, V.M.; Kolotilov, S.V. Heterometallic coordination polymers assembled from trigonal trinuclear Fe2Ni-pivalate blocks and polypyridine spacers: Topological diversity, sorption, and catalytic properties. Inorg. Chem. 2015, 54, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Lytvynenko, A.S.; Polunin, R.A.; Kiskin, M.A.; Mishura, A.M.; Titov, V.E.; Kolotilov, S.V.; Novotortsev, V.M.; Eremenko, I.L. Electrochemical and electrocatalytic characteristics of coordination polymers based on trinuclear pivalates and heterocyclic bridging ligands. Theor. Exp. Chem. 2015, 51, 54–61. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Tong, M.L.; Xue, W.; Zhang, W.X.; Chen, X.M.; Grandjean, F.; Long, G.J. A “Star” antiferromagnet: A polymeric iron(III) acetate that exhibits both spin frustration and long-range magnetic ordering. Angew. Chem. Int. Ed. 2007, 46, 6076–6080. [Google Scholar] [CrossRef]
- Lytvynenko, A.S.; Kolotilov, S.V.; Cador, O.; Gavrilenko, K.S.; Golhen, S.; Ouahab, L.; Pavlishchuk, V.V. Porous 2D coordination polymeric formate built up by Mn(II) linking of Fe3O units: Influence of guest molecules on magnetic properties. Dalton Trans. 2009, 3503–3509. [Google Scholar] [CrossRef]
- Polunin, R.A.; Kolotilov, S.V.; Kiskin, M.A.; Cador, O.; Mikhalyova, E.A.; Lytvynenko, A.S.; Golhen, S.; Ouahab, L.; Ovcharenko, V.I.; Eremenko, I.L.; et al. Topology control of porous coordination polymers by building block symmetry. Eur. J. Inorg. Chem. 2010, 5055–5057. [Google Scholar] [CrossRef]
- Dorofeeva, V.N.; Kolotilov, S.V.; Kiskin, M.A.; Polunin, R.A.; Dobrokhotova, Z.V.; Cador, O.; Golhen, S.; Ouahab, L.; Eremenko, I.; Novotortsev, V. 2D Porous honeycomb polymers versus discrete nanocubes from trigonal trinuclear complexes and ligands with variable topology. Chem. Eur. J. 2012, 18, 5006–5012. [Google Scholar] [CrossRef] [PubMed]
- Botezat, O.; van Leusen, J.; Kravtsov, V.C.; Filippova, I.G.; Hauser, J.; Speldrich, M.; Hermann, R.P.; Krämer, K.W.; Liu, S.-X.; Decurtins, S.; et al. Interpenetrated (8,3)-c and (10,3)-b metal–organic frameworks based on {FeIII3} and {FeIII2CoII} pivalate spin clusters. Cryst. Growth. Des. 2014, 14, 4721–4728. [Google Scholar] [CrossRef]
- Zhao, J.P.; Han, S.D.; Jiang, X.; Xu, J.; Chang, Z.; Bu, X.H. A three dimensional magnetically frustrated metal–organic framework via the vertices augmentation of underlying net. Chem. Commun. 2015, 51, 4627–4630. [Google Scholar] [CrossRef] [PubMed]
- Speldrich, M.; van Leusen, J.; Kögerler, P. CONDON 3.0: An updated software package for magnetochemical analysis - all the way to polynuclear actinide complexes. J. Comput. Chem. 2018, 39, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- van Leusen, J.; Speldrich, M.; Schilder, H.; Kögerler, P. Comprehensive insight into molecular magnetism via CONDON: Full vs. Effective Models. Coord. Chem. Rev. 2015, 289–290, 137–148. [Google Scholar] [CrossRef]
- Baca, S.G.; Filippova, I.G.; Keene, T.D.; Botezat, O.; Malaestean, I.L.; Stoeckli-Evans, H.; Kravtsov, V.C.; Chumacov, I.; Liu, S.-X.; Decurtins, S. Iron(III)-pivalate-based complexes with tetranuclear {Fe4(μ3-O)2}8+ cores and N-donor ligands: Formation of cluster and polymeric architectures. Eur. J. Inorg. Chem. 2011, 3, 356–367. [Google Scholar] [CrossRef]
- Wang, S.; Tsai, H.L.; Folting, K.; Martin, J.M.; Hendrickson, D.N.; Christou, G. Covalent linkage of [Mn4O2(O2CPh)6(dbm)2] into a dimer and a one-dimensional polymer (dbmH = dibenzoylmethane). Chem. Commun. 1994, 671–673. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, X.; Ma, C.; Chen, H.; Chen, C.; Liu, Q.; Zhang, C.; Liao, D.; Li, L. Aggregation of tetranuclear Mn building blocks with alkali ion. Syntheses, crystal structures and chemical behaviors of monomer, dimer and polymer containing [Mn4(µ3-O)2]8+ units. Dalton Trans. 2007, 680–688. [Google Scholar] [CrossRef]
- Nakata, K.; Miyasaka, H.; Sugimoto, K.; Ishii, T.; Sugiura, K.; Yamashita, M. Construction of a one-dimensional chain composed of Mn6 clusters and 4,4′-bipyridine linkers: The first step for creation of “Nano-Dots-Wires”. Chem. Lett. 2002, 31, 658–659. [Google Scholar] [CrossRef]
- Ma, C.-B.; Hu, M.Q.; Chen, H.; Chen, C.N.; Liu, Q.T. Aggregation of hexanuclear, mixed-valence manganese oxide clusters linked by propionato ligands to form a one-dimensional polymer [Mn6O2(O2CEt)10(H2O)4]n. Eur. J. Inorg. Chem. 2008, 5274–5280. [Google Scholar] [CrossRef]
- Moushi, E.E.; Tasiopoulos, A.J.; Manos, M.J. Synthesis and structural characterization of a metal cluster and a coordination polymer based on the [Mn6(μ4-O)2]10+ unit. Bioinorg. Chem. Appl. 2010, 367128. [Google Scholar] [CrossRef]
- Malaestean, I.L.; Kravtsov, V.C.; Speldrich, M.; Dulcevscaia, G.; Simonov, Y.A.; Lipkowski, J.; Ellern, A.; Baca, S.G.; Kögerler, P. One-dimensional coordination polymers from hexanuclear manganese carboxylate clusters featuring a {MnII4MnIII2(μ4-O)2} core and spacer linkers. Inorg. Chem. 2010, 49, 7764–7772. [Google Scholar] [CrossRef]
- Darii, M.; Filippova, I.G.; Hauser, J.; Decurtins, S.; Liu, S.-X.; Kravtsov, V.C.; Baca, S.G. Incorporation of hexanuclear Mn(II,III) carboxylate clusters with a {Mn6O2} core in polymeric structures. Crystals 2018, 8, 100. [Google Scholar] [CrossRef]
- Ovcharenko, V.; Fursova, E.; Romanenko, G.; Ikorskii, V. Synthesis and structure of heterospin compounds based on the [Mn6(O)2Piv10]-cluster unit and nitroxide. Inorg. Chem. 2004, 43, 3332–3334. [Google Scholar] [CrossRef] [PubMed]
- Baca, S.G.; Secker, T.; Mikosch, A.; Speldrich, M.; van Leusen, J.; Ellern, A.; Kögerler, P. Avoiding magnetochemical overparametrization, exemplified by one-dimensional chains of hexanuclear iron(III) pivalate clusters. Inorg. Chem. 2013, 52, 4154–4156. [Google Scholar] [CrossRef] [PubMed]
- Fursova, E.Y.; Ovcharenko, V.I.; Bogomyakov, A.S.; Romanenko, G.V. Structure of the complex of hexanuclear manganese pivalate with isonicotinamide. J. Struct. Chem. 2013, 54, 164–167. [Google Scholar] [CrossRef]
- Malaestean, I.L.; Ellern, A.; van Leusen, J.; Kravtsov, V.C.; Kögerler, P.; Baca, S.G. Cluster-based networks: Assembly of a (4,4) layer and a rare T-shaped bilayer from [MnIII2MnII4O2(RCOO)10] coordination clusters. Cryst. Eng. Comm. 2014, 16, 6523–6525. [Google Scholar] [CrossRef]
- Kar, P.; Haldar, R.; Gómez-García, C.J.; Ghosh, A. Antiferromagnetic porous metal–organic framework containing mixed-valence [MnII4MnIII2(μ4-O)2]10+ units with catecholase activity and selective gas adsorption. Inorg. Chem. 2012, 51, 4265–4273. [Google Scholar] [CrossRef]
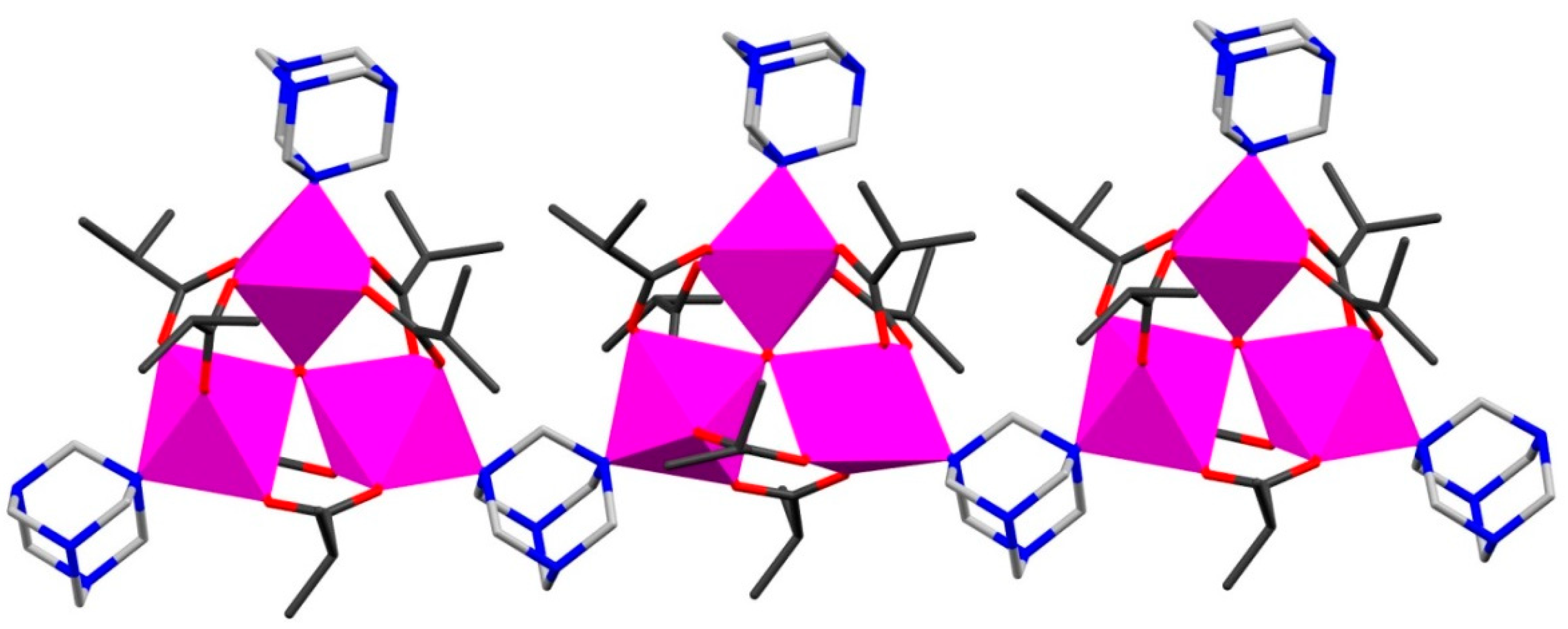
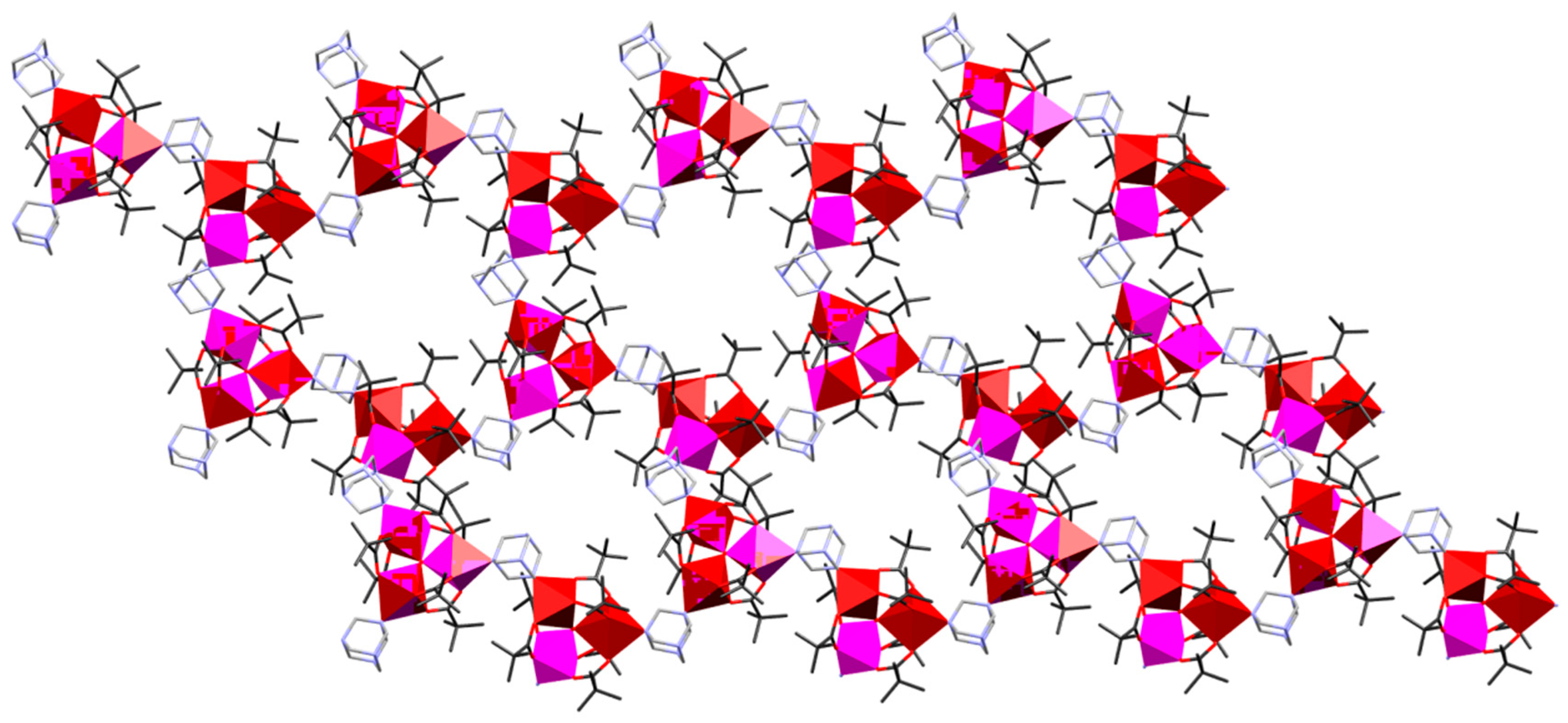
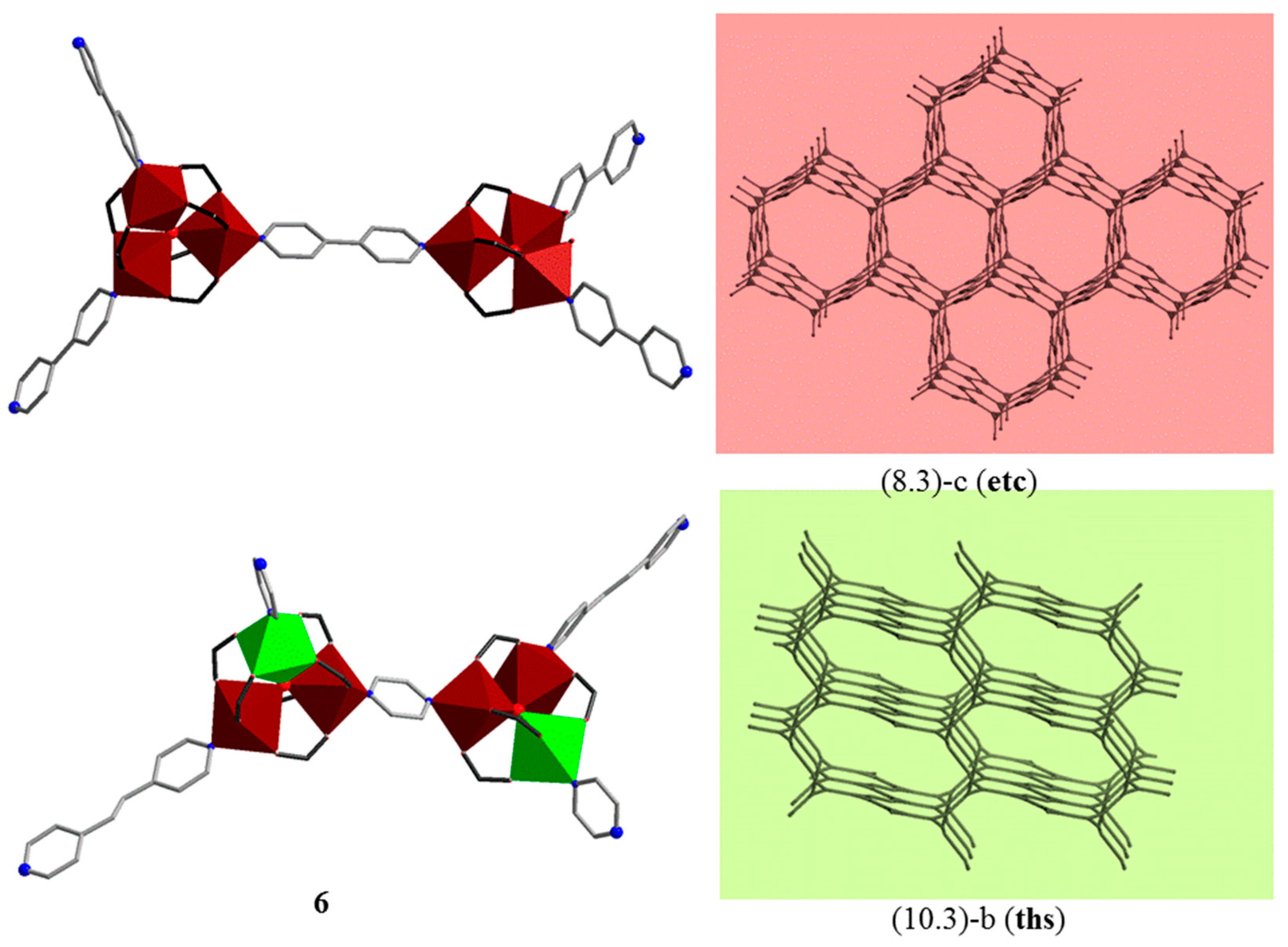
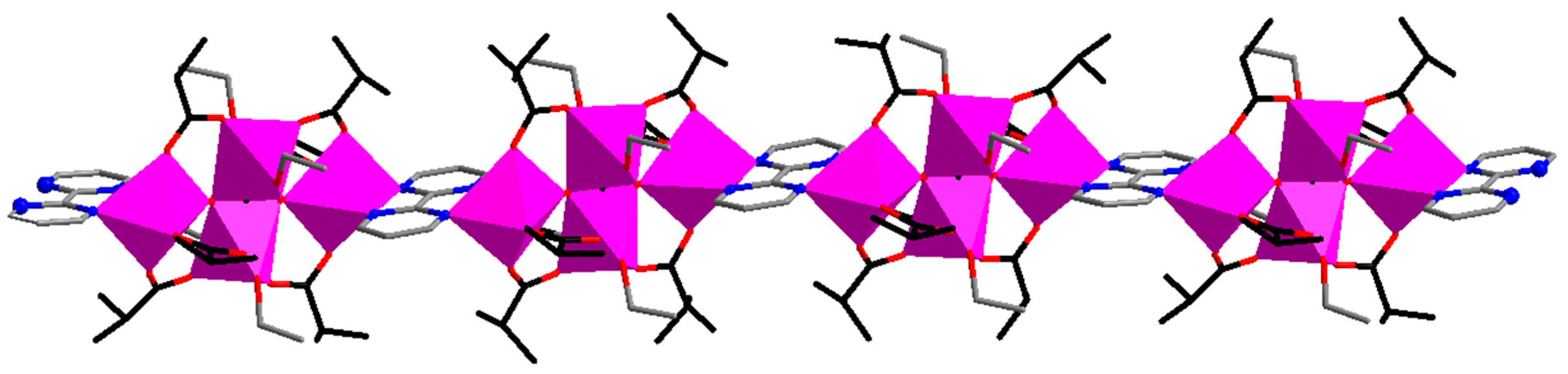
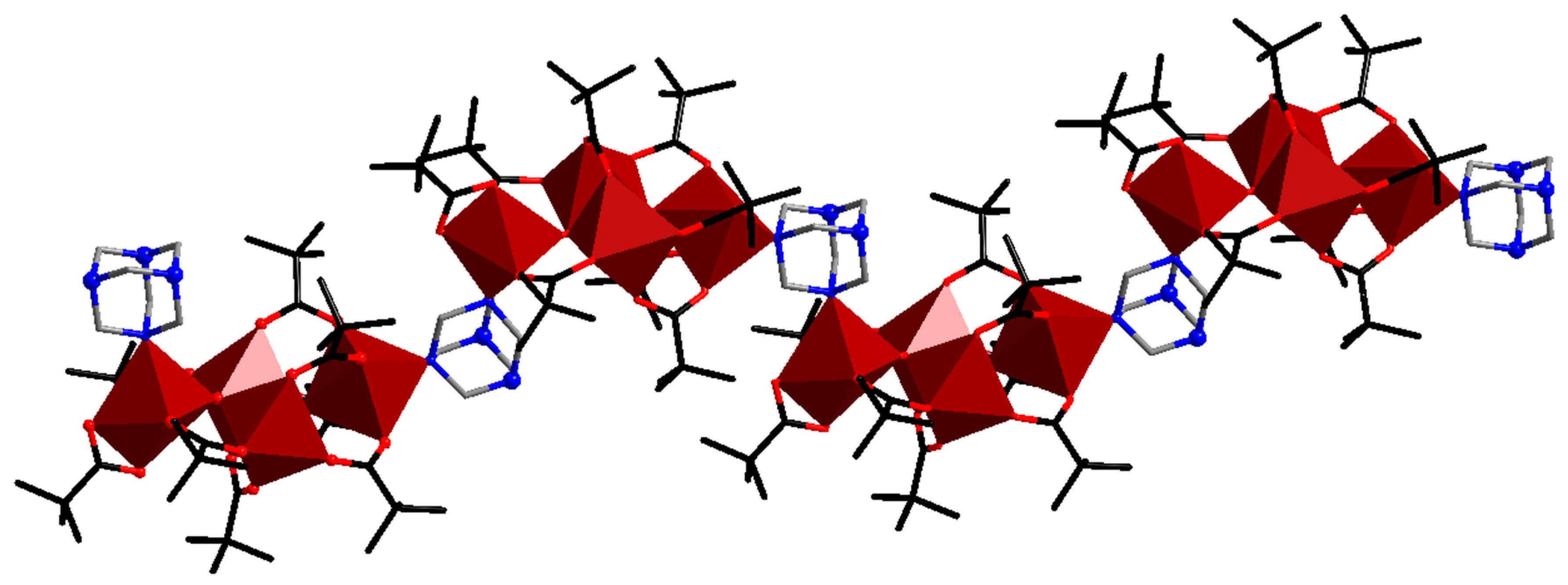


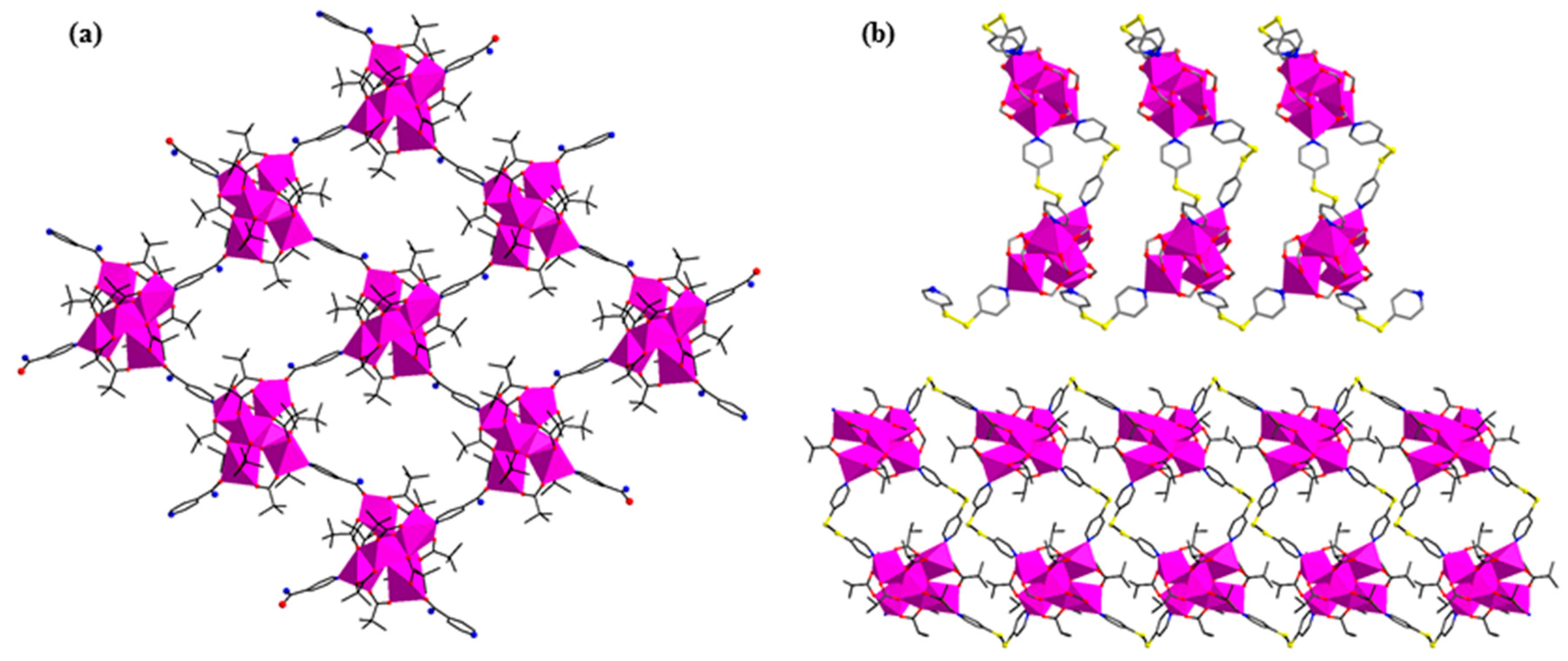
| Code | Formulae | Oxo Building Block | Linker 1 | Dimensionality | Refs |
|---|---|---|---|---|---|
| 1 | {[Mn3O(is)6(hmta)2]·EtOH}n | {MnIII2MnIIO} | hmta | 1D | [29] |
| 2 | {[Fe2MnO(piv)6(hmta)2]·0.5(MeCN)}n | {FeIII2MnIIO} | hmta | 1D | [30] |
| 3 | {[Fe2MnO(piv)6(hmta)2]·Hpiv·n-hexane}n | {FeIII2MnIIO} | hmta | 1D | [30] |
| 4 | {[Fe2MnO(piv)6(hmta)1.5]·toluene}n | {FeIII2MnIIO} | hmta | 2D | [30] |
| 5 | {[Fe3O(piv)6(4,4′-bpy)1.5](OH)·0.75(dcm)·8(H2O)}n | {FeIII3O} | 4,4′-bpy | 3D | [39] |
| 6 | [Fe2CoO(piv)6(bpe)0.5(pyz)]n | {FeIII2CoIIO} | bpe, pyz | 3D | [39] |
| 7 | [Mn4O2(is)6(bpm)(EtOH)4]n | {MnIII2MnII2O2} | bpm | 1D | [29] |
| 8 | [Fe4O2(piv)8(hmta)]n | {FeIII4O2} | hmta | 1D | [43] |
| 9 | {[Mn6O2(piv)10(Hpiv)(EtOH)(na)]·EtOH·H2O}n | {MnIII2MnII4O2} | na | 1D | [49] |
| 10 | [Mn6O2(piv)10(Hpiv)2(en)]n | {MnIII2MnII4O2} | en | 1D | [50] |
| 11 | {[Mn6O2(is)10(pyz)3]·2(H2O)}n | {MnIII2MnII4O2} | pyz | 1D | [49] |
| 12 | [Mn6O2(is)10(pyz)(MeOH)2]n | {MnIII2MnII4O2} | pyz | 1D | [50] |
| 13 | {[Mn6O2(is)10(pyz)1.5(H2O)]·0.5(H2O)}n | {MnIII2MnII4O2} | pyz | 1D | [50] |
| 14 | {[Mn6O2(is)10(His)(EtOH)(bpea)]·His}n | {MnIII2MnII4O2} | bpea | 1D | [49] |
| 15 | [Fe6O2(O2CH2)(piv)12(diox)]n | {FeIII6O2} | diox | 1D | [52] |
| 16 | [Fe6O2(O2CH2)(piv)12(4,4′-bpy)]n | {FeIII6O2} | 4,4′-bpy | 1D | [52] |
| 17 | [Mn6O2(piv)10(ina)2]n | {MnIII2MnII4O2} | ina | 2D | [54] |
| 18 | {[Mn6O2(piv)10(adt-4)2]·2(thf)}n | {MnIII2MnII4O2} | adt-4 | 2D | [54] |
| 19 | {[Mn6O2(is)10(adt-4)2]·thf·3(EtOH)}n | {MnIII2MnII4O2} | adt-4 | 2D | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baca, S.; Kögerler, P. Cluster-Based Coordination Polymers of Mn/Fe-Oxo Pivalates and Isobutyrates. Chemistry 2021, 3, 314-326. https://doi.org/10.3390/chemistry3010023
Baca S, Kögerler P. Cluster-Based Coordination Polymers of Mn/Fe-Oxo Pivalates and Isobutyrates. Chemistry. 2021; 3(1):314-326. https://doi.org/10.3390/chemistry3010023
Chicago/Turabian StyleBaca, Svetlana, and Paul Kögerler. 2021. "Cluster-Based Coordination Polymers of Mn/Fe-Oxo Pivalates and Isobutyrates" Chemistry 3, no. 1: 314-326. https://doi.org/10.3390/chemistry3010023
APA StyleBaca, S., & Kögerler, P. (2021). Cluster-Based Coordination Polymers of Mn/Fe-Oxo Pivalates and Isobutyrates. Chemistry, 3(1), 314-326. https://doi.org/10.3390/chemistry3010023





