Polymeric Bioinks for 3D Hepatic Printing
Abstract
1. Introduction
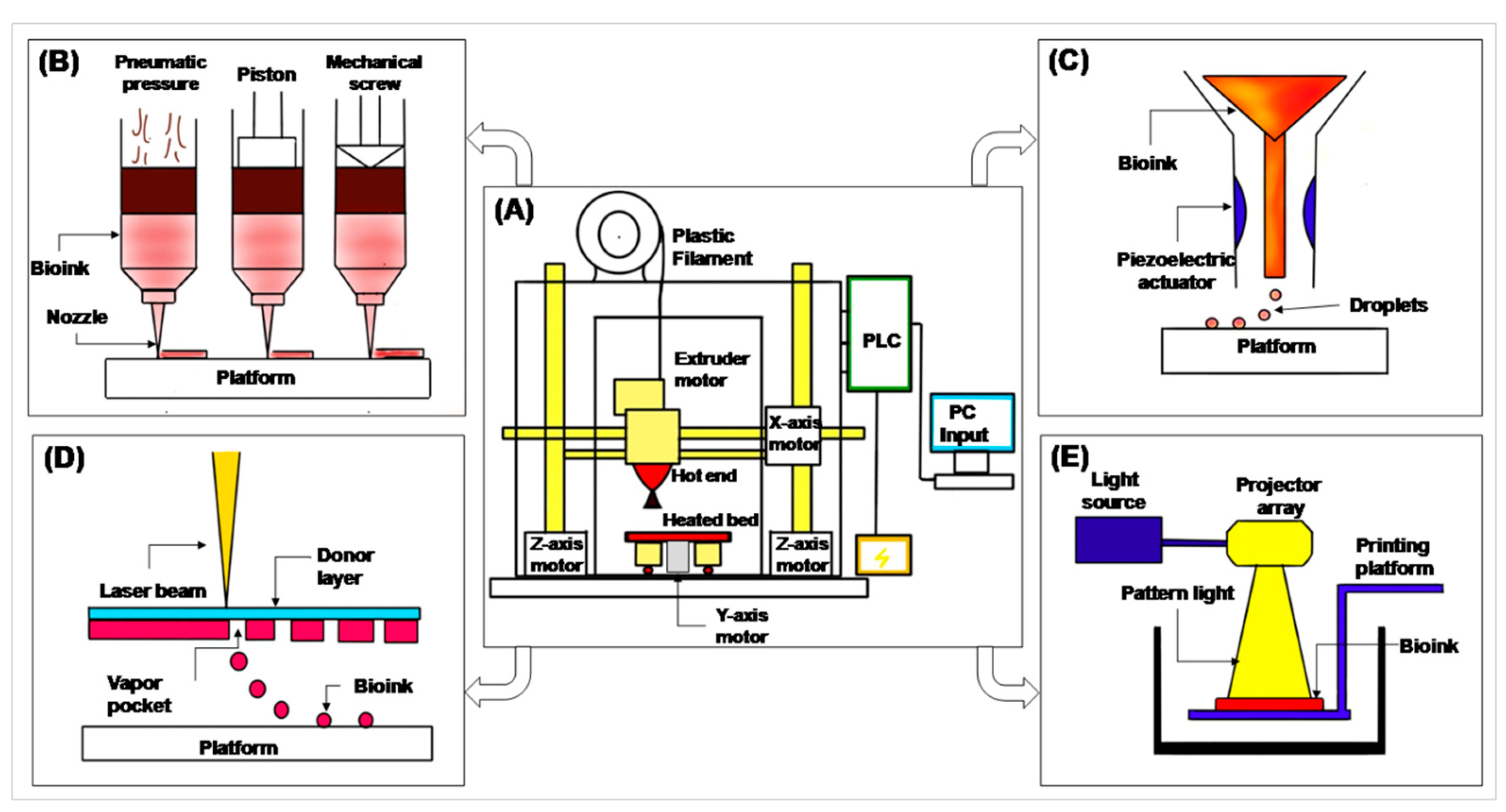
2. 3D Printing and Its Prerequisites
- Filament—a filament-shaped polymer of required material, in which a 3D model is printed.
- Extruder motor—has a heating coil which melts the filament for printing.
- Hot end—the end of the extruder motor which extrudes melted polymer on a heating bed. It is connected to the X- and Z-axis motor to print in the X- and Z-axis.
- Heated bed—a platform on which the 3D model is printed. It is connected to the Y-axis motor to move the platform in the Y-axis.
- Programmable Logic Control (PLC) and computer input—gives input to the PLC. The PLC reads that input and performs actions as per the given commands.
3. Polymers Used as Bionks in 3D Hepatic Printing
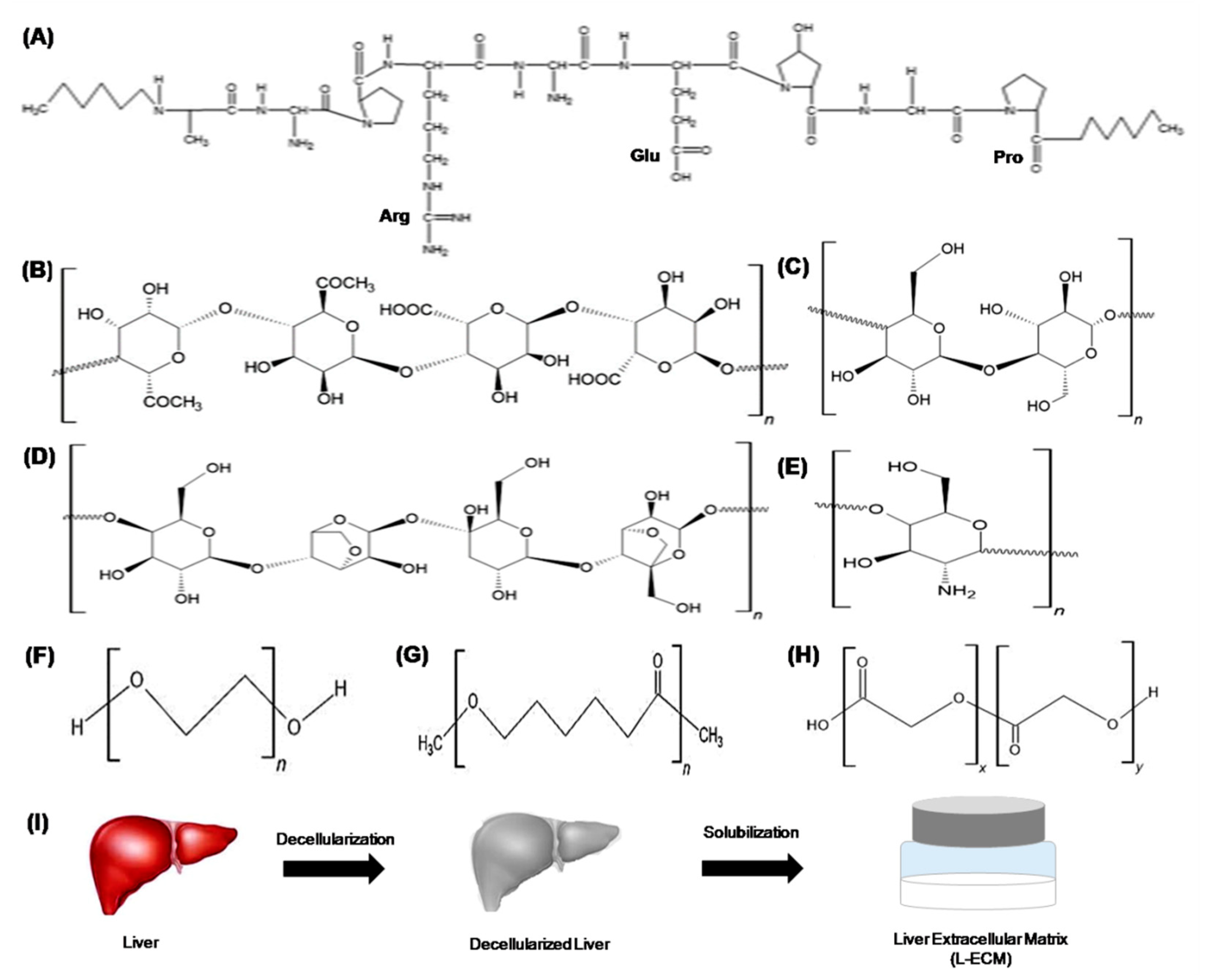
3.1. Natural Polymers
3.2. Synthetic Polymers
3.3. Decellularized Matrix
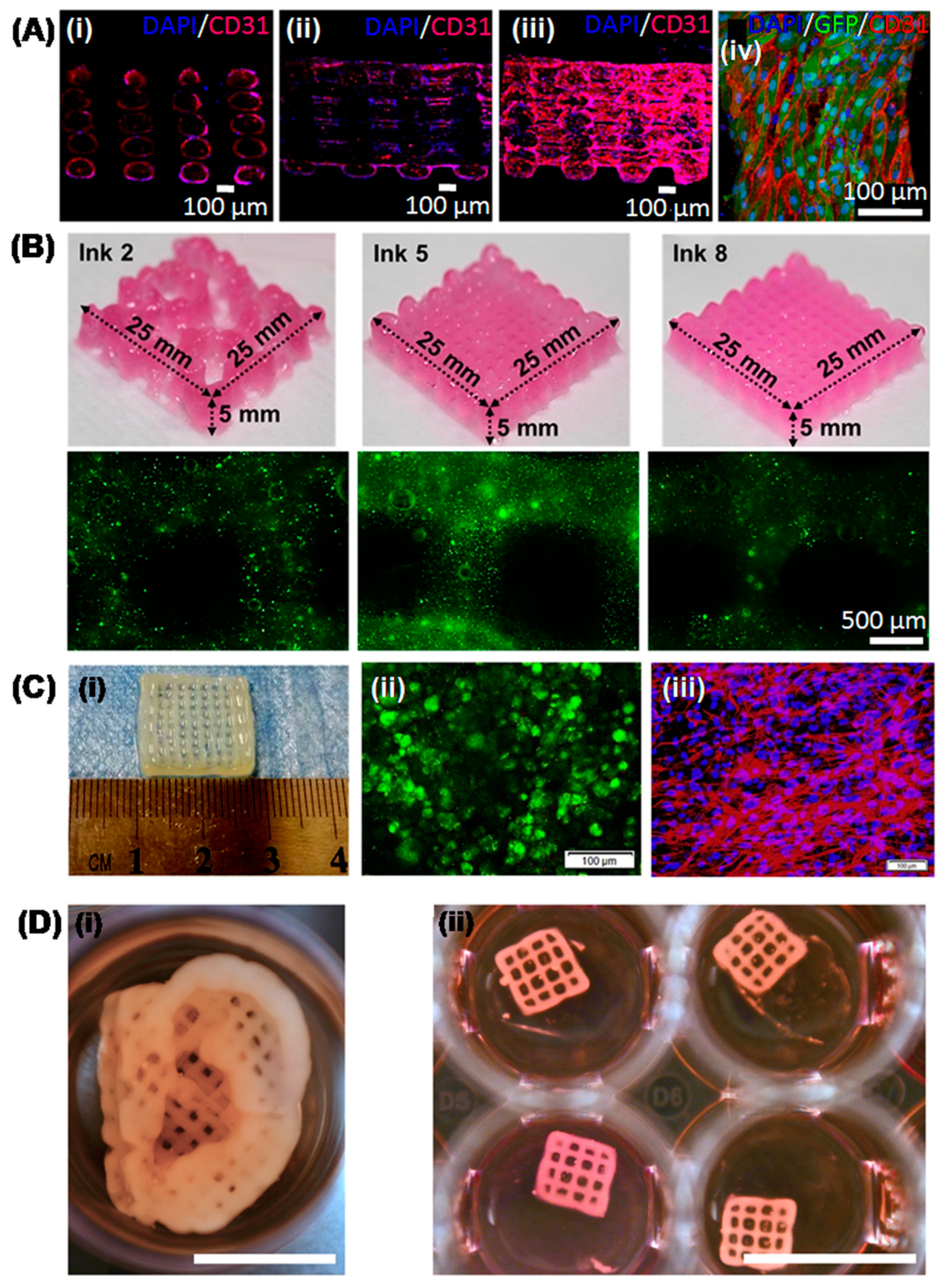
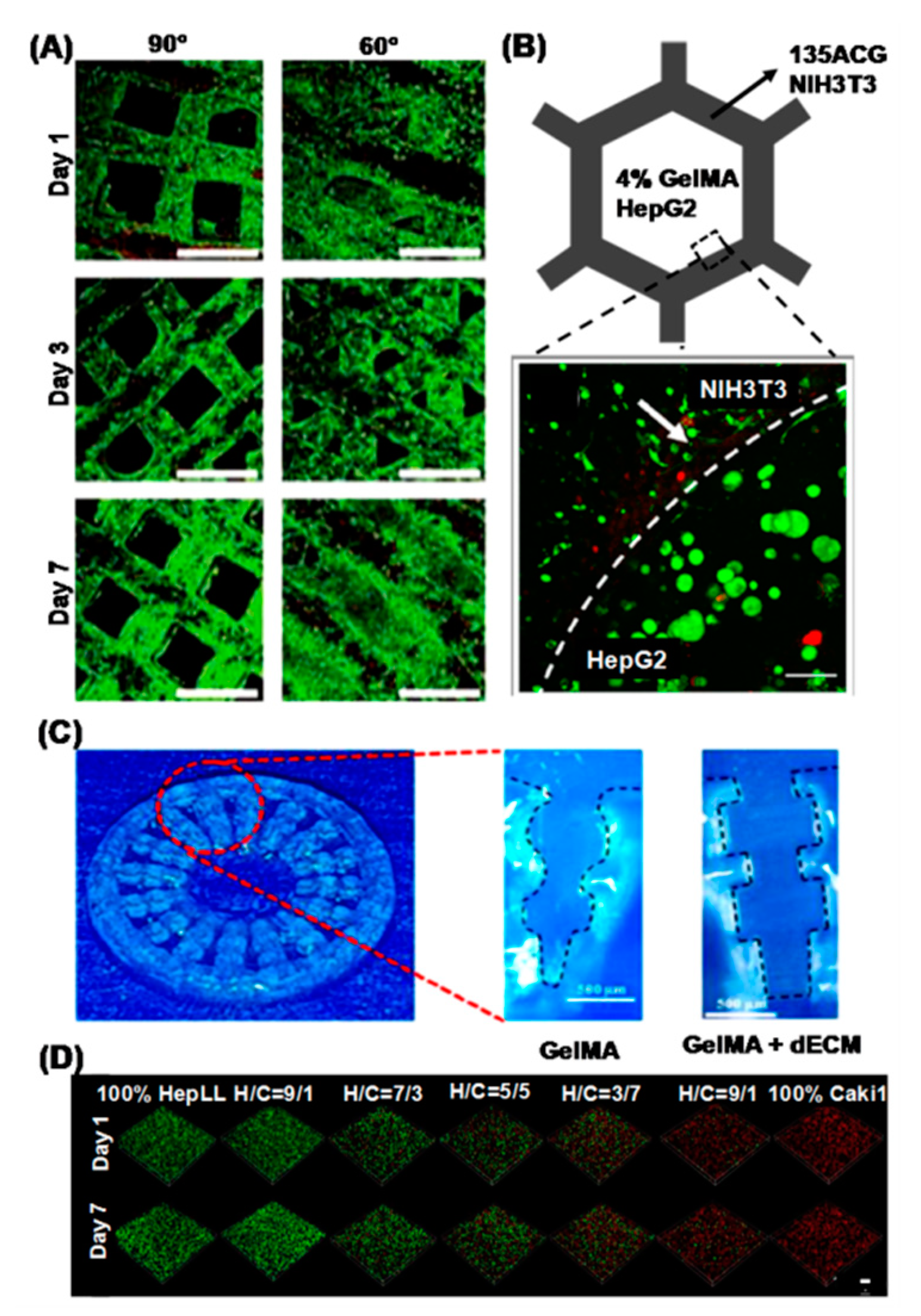
4. Recent Advances in 3D Printed Hepatic Structures with Polymeric Bioink
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeCluyse, E.L.; Witek, R.P.; Andersen, M.E.; Powers, M.J. Organotypic Liver Culture Models: Meeting Current Challenges in Toxicity Testing. Crit. Rev. Toxicol. 2012, 42, 501–548. [Google Scholar] [CrossRef]
- Wells, R.G. Cellular Sources of Extracellular Matrix in Hepatic Fibrosis. Clin. Liver Dis. 2008, 12, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Elvevold, K.; Smedsrød, B.; Martinez, I. The Liver Sinusoidal Endothelial Cell: A Cell Type of Controversial and Confusing Identity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G391–G400. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer Cells in the Pathogenesis of Liver Disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef]
- Bogert, P.T.; LaRusso, N.F. Cholangiocyte Biology. Curr. Opin. Gastroenterol. 2007, 23, 299–305. [Google Scholar] [CrossRef]
- Turner, R.; Lozoya, O.; Wang, Y.; Cardinale, V.; Gaudio, E.; Alpini, G.; Mendel, G.; Wauthier, E.; Barbier, C.; Alvaro, D.; et al. Human Hepatic Stem Cell and Maturational Liver Lineage Biology. Hepatology 2011, 53, 1035–1045. [Google Scholar] [CrossRef]
- Sarkar, J.; Kamble, S.C.; Patil, R.; Kumar, A.; Gosavi, S.W. Gelatin Interpenetration in Poly N-isopropylacrylamide Network Reduces the Compressive Modulus of the Scaffold: A Property Employed to Mimic Hepatic Matrix Stiffness. Biotechnol. Bioeng. 2020, 117, 567–579. [Google Scholar] [CrossRef]
- Wu, D.B.; Chen, E.Q.; Tang, H. Stem Cell Transplantation for the Treatment of End-Stage Liver Disease. World J. Hepatol. 2018, 10, 907–910. [Google Scholar] [CrossRef]
- Kaplowitz, N. Idiosyncratic Drug Hepatotoxicity. Nat. Rev. Drug Discov. 2005, 4, 489–499. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Hendriks, D.F.G.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4575330A, 11 March 1986. [Google Scholar]
- Kryou, C.; Leva, V.; Chatzipetrou, M.; Zergioti, I. Bioprinting for Liver Transplantation. Bioengineering 2019, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Munaz, A.; Vadivelu, R.K.; St. John, J.; Barton, M.; Kamble, H.; Nguyen, N.T. Three-Dimensional Printing of Biological Matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef]
- Loai, S.; Kingston, B.R.; Wang, Z.; Philpott, D.N.; Tao, M.; Chen, H.-L.M. Clinical Perspectives on 3D Bioprinting Paradigms for Regenerative Medicine. Regen. Med. Front. 2019, 1, e190004. [Google Scholar]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.; Lewis, J.A. In Vitro Human Tissues via Multi-Material 3-D Bioprinting. Altern. Lab. Anim. 2018, 46, 209–215. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing of Viable Mammalian Cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of Inkjet Printing to Tissue Engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing for High-Throughput Cell Patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Pepper, M.E.; Seshadri, V.; Burg, T.C.; Burg, K.J.L.; Groff, R.E. Characterizing the Effects of Cell Settling on Bioprinter Output. Biofabrication 2012, 4, 011001. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B.; Lopez, J.; Faucon, M.; Pippenger, B.; Bareille, R.; Rémy, M.; Bellance, S.; et al. High-Throughput Laser Printing of Cells and Biomaterials for Tissue Engineering. Acta Biomater. 2010, 6, 2494–2500. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef]
- Wang, X. Advanced Polymers for Three-Dimensional (3D) Organ Bioprinting. Micromachines 2019, 10, 814. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural Polymers for Organ 3D Bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-Ink Properties and Printability for Extrusion Printing Living Cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering Alginate as Bioink for Bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, W.; Lee, S.M.; Kim, J.B.; Jin, S.; Kang, H.W. Decellularized Extracellular Matrix-Based Bio-Ink with Enhanced 3D Printability and Mechanical Properties. Biofabrication 2020, 12, 025003. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.G.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P.G. Cardiac Tissue Engineering Using Tissue Printing Technology and Human Cardiac Progenitor Cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Piou, M.; Darling, E.; Cormier, D.; Sun, J.; Wan, J. Bio-Printing Cell-Laden Matrigel-Agarose Constructs. J. Biomater. Appl. 2016, 31, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, H.; Kim, Y.; Yoo, S.Y.; Chung, W.J.; Kim, G. Phage as Versatile Nanoink for Printing 3-D Cell-Laden Scaffolds. Acta Biomater. 2016, 29, 112–124. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Chen, Q.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Progress in Organ 3D Bioprinting. Int. J. Bioprinting 2018, 4, 128. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Causa, F.; Sarracino, F.; De Santis, R.; Netti, P.A.; Ambrosio, L.; Nicolais, L. Basic Structural Parameters for the Design of Composite Structures as Ligament Augmentation Devices. J. Appl. Biomater. Biomech. 2006, 4, 21–30. [Google Scholar] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The Bioink: A Comprehensive Review on Bioprintable Materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Yan, Y.; Zhang, R. Liver Tissue Responses To gelatin and Gelatin/Chitosan Gels. J. Biomed. Mater. Res.Part A 2008, 87, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial Application of Cardiac Progenitor Cells in a 3D-Printed Gelatin/Hyaluronic Acid Patch Preserves Cardiac Function after Myocardial Infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and Characterization of Photocrosslinkable Gelatin and Silk Fibroin Interpenetrating Polymer Network Hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Lee, H.; Cho, D.W. One-Step Fabrication of an Organ-on-a-Chip with Spatial Heterogeneity Using a 3D Bioprinting Technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of Complex Porous Tissue Engineering Scaffolds Using 3D Projection Stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef]
- Wang, X. Bioartificial Organ Manufacturing Technologies. Cell Transplant. 2019, 28, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Samitier, J.; Sánchez, S. Bioprinting of 3D Hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv. Healthc. Mater. 2016, 5, 2353–2362. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yi, H.G.; Kim, S.W.; Cho, D.W. 3D Cell Printed Tissue Analogues: A New Platform for Theranostics. Theranostics 2017, 7, 3118–3137. [Google Scholar] [CrossRef]
- Mao, B.; Divoux, T.; Snabre, P. Impact of Saccharides on the Drying Kinetics of Agarose Gels Measured by In-Situ Interferometry. Sci. Rep. 2017, 7, 41185. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Kreimendahl, F.; Köpf, M.; Thiebes, A.L.; Duarte Campos, D.F.; Blaeser, A.; Schmitz-Rode, T.; Apel, C.; Jockenhoevel, S.; Fischer, H. Three-Dimensional Printing and Angiogenesis: Tailored Agarose-Type i Collagen Blends Comprise Three-Dimensional Printability and Angiogenesis Potential for Tissue-Engineered Substitutes. Tissue Eng. Part C Methods 2017, 23, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-Alginate as Bioink for Three-Dimensional (3D) Cell Printing Based Cartilage Tissue Engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pascual, F.; Slatter, D.A. Collagen Cross-Linking: Insights on the Evolution of Metazoan Extracellular Matrix. Sci. Rep. 2016, 6, 37374. [Google Scholar] [CrossRef] [PubMed]
- Stratesteffen, H.; Köpf, M.; Kreimendahl, F.; Blaeser, A.; Jockenhoevel, S.; Fischer, H. GelMA-Collagen Blends Enable Drop-on-Demand 3D Printablility and Promote Angiogenesis. Biofabrication 2017, 9, 045002. [Google Scholar] [CrossRef]
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.M.; Sercombe, T.B.; Liew, L.J.; Dilley, R.J.; Doyle, B.J. Characterisation of Hyaluronic Acid Methylcellulose Hydrogels for 3D Bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399. [Google Scholar] [CrossRef]
- Martínez Ávila, H.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D Bioprinting of Human Chondrocyte-Laden Nanocellulose Hydrogels for Patient-Specific Auricular Cartilage Regeneration. Bioprinting 2016, 1–2, 22–35. [Google Scholar]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic Inks Based on Cellulose Nanofibrils and Cross-Linkable Xylans for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886. [Google Scholar] [CrossRef]
- Bedossa, P.; Paradis, V. Liver Extracellular Matrix in Health and Disease. J. Pathol. 2003, 200, 504–515. [Google Scholar] [CrossRef]
- Dzobo, K.; Motaung, K.S.C.M.; Adesida, A. Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628. [Google Scholar] [CrossRef]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-Laden 3D Bioprinting Hydrogel Matrix Depending on Different Compositions for Soft Tissue Engineering: Characterization and Evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring Mechanical Properties of Decellularized Extracellular Matrix Bioink by Vitamin B2-Induced Photo-Crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Pati, F.; Ha, D.H.; Jang, J.; Han, H.H.; Rhie, J.W.; Cho, D.W. Biomimetic 3D Tissue Printing for Soft Tissue Regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef]
- Gori, M.; Giannitelli, S.M.; Torre, M.; Mozetic, P.; Abbruzzese, F.; Trombetta, M.; Traversa, E.; Moroni, L.; Rainer, A. Biofabrication of Hepatic Constructs by 3D Bioprinting of a Cell-Laden Thermogel: An Effective Tool to Assess Drug-Induced Hepatotoxic Response. Adv. Healthc. Mater. 2020, 9, e2001163. [Google Scholar] [CrossRef]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-Printed Gelatin Scaffolds of Differing Pore Geometry Modulate Hepatocyte Function and Gene Expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.H.; Jang, J.; Kim, B.S.; Cho, D.W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.Y.; Wenger, A.C.; Tam, K.C.; Tang, X.S. 3D Bioprinting of Liver-Mimetic Construct with Alginate/Cellulose Nanocrystal Hybrid Bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Rijff, B.L.; Khang, G. A Building-Block Approach to 3D Printing a Multichannel, Organ-Regenerative Scaffold. J. Tissue Eng. Regen. Med. 2017, 11, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Sun, L.; Pang, Y.; Xu, G.; Jin, B.; Xu, H.; Lu, X.; Xu, Y.; Du, S.; Wang, Y.; et al. Three-Dimensional bio-Printing of Primary Human Hepatocellular Carcinoma for Personalized Medicine. Biomaterials 2021, 265, 120416. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; He, J.; Zhao, Y.; Liu, T.; Xie, F.; Yang, H.; Mao, Y.; Pang, Y.; Sun, W. Bioprinting of Patient-Derived In Vitro Intrahepatic Cholangiocarcinoma Tumor Model: Establishment, Evaluation and Anti-Cancer Drug Testing. Biofabrication 2020, 12, 045014. [Google Scholar] [CrossRef] [PubMed]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A Liver-on-a-Chip Platform with Bioprinted Hepatic Spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of Collagen Type I-Hyaluronan Hybrid Bioink for 3D Bioprinted Liver Microenvironments. Biofabrication 2019, 11, 015003. [Google Scholar] [CrossRef]
- Wu, Y.; Wenger, A.; Golzar, H.; Tang, X.S. 3D Bioprinting of Bicellular Liver Lobule-Mimetic Structures via Microextrusion of Cellulose Nanocrystal-Incorporated Shear-Thinning Bioink. Sci. Rep. 2020, 10, 20648. [Google Scholar] [CrossRef]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of Liver Microtissue with Liver Decellularized Extracellular Matrix (dECM) Bioink by Digital Light Processing (DLP) Bioprinting. Mater. Sci. Eng. C 2020, 109, 110625. [Google Scholar] [CrossRef]
- Lee, J.S.; Yoon, H.; Yoon, D.; Kim, G.H.; Yang, H.T.; Chun, W. Development of Hepatic Blocks Using Human Adipose Tissue-Derived Stem Cells Through Three-Dimensional Cell Printing Techniques. J. Mater. Chem. B 2017, 5, 1098–1107. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, K.; Yoon, S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Lee, S.B.; Ryu, K.Y.; Jeong, J.; Choi, D. Prolongation of Liver-Specific Function for Primary Hepatocytes Maintenance in 3D Printed Architectures. Organogenesis 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Kang, K.; Kim, Y.; Jeon, H.; Lee, S.B.; Kim, J.S.; Park, S.A.; Kim, W.D.; Yang, H.M.; Kim, S.J.; Jeong, J.; et al. Three-Dimensional Bioprinting of Hepatic Structures with Directly Converted Hepatocyte-Like Cells. Tissue Eng. Part A 2018, 24, 576–583. [Google Scholar] [CrossRef]
- Arai, K.; Yoshida, T.; Okabe, M.; Goto, M.; Mir, T.A.; Soko, C.; Tsukamoto, Y.; Akaike, T.; Nikaido, T.; Zhou, K.; et al. Fabrication of 3D-Culture Platform with Sandwich Architecture for Preserving Liver-Specific Functions of Hepatocytes Using 3D Bioprinter. J. Biomed. Mater. Res. Part A 2017, 105, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, K.; Jeong, J.; Paik, S.S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Park, J.; Choi, D. Three-Dimensional (3D) Printing of Mouse Primary Hepatocytes to Generate 3D Hepatic Structure. Ann. Surg. Treat. Res. 2017, 92, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kang, K.; Park, S.A.; Kim, W.D.; Paik, S.S.; Lee, S.H.; Jeong, J.; Choi, D. Generation of Multilayered 3D Structures of HepG2 Cells Using a Bio-Printing Technique. Gut Liver 2017, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D Cell Printed Construct Considering Angiogenesis for Liver Tissue Engineering. Biofabrication 2016, 8, 015007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, D.; Wu, G.; Wu, J.; Lu, S.; Lo, J.; He, Y.; Zhao, C.; Zhao, X.; Zhang, H.; et al. Metastasis-on-a-Chip Mimicking the Progression of Kidney Cancer in the Liver for Predicting Treatment Efficacy. Theranostics 2020, 10, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.C.; Al-Zeer, M.A.; Kurtz, A.; Hocke, A.C.; et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef]
- Norona, L.M.; Nguyen, D.G.; Gerber, D.A.; Presnell, S.C.; LeCluyse, E.L. Modeling Compound-Induced Fibrogenesis In Vitro Using Three-Dimensional Bioprinted Human Liver Tissues. Toxicol. Sci. 2016, 154, 354–367. [Google Scholar] [CrossRef]
| Polymer | Biomolecule Class | Cytoadherent | Aqueous Solubility | Biodegradable | Other Important Properties | References |
|---|---|---|---|---|---|---|
| Natural Polymers | ||||||
| Gelatin | Protein/Peptide | Yes | Soluble | Yes | Self-gelation at lower temperatures | [30,31,45,46,47,48,49,50,51,52,53] |
| Alginate | Polysaccharide | No | Soluble | Yes | Cationic gelation | [15,30,31,44,53,54,55,56,57,58,59] |
| Agarose | Polysaccharide | No | Soluble at high temperature | Yes | Provides exceptional mechanical support | [30,31,37,44,60,61] |
| Collagen | Protein | Yes | Soluble at low pH | Yes | High gelation time at 37 °C | [30,31,44,62,63,64,65] |
| Cellulose | Polysaccharide | No | Insoluble | No | Efficient for long-term application | [30,31,44,66,67,68,69] |
| Chitosan | Polysaccharide | No | Soluble at low pH | Yes | Poor gelation and mechanical strength | |
| Synthetic Polymers | ||||||
| PEG 1 | Polyether | No | Soluble | No | Effective control on mechanical strength | [30] |
| PCL 2 | Polyester | No | Insoluble | Yes | Produces stiff structures | [30] |
| PLGA 3 | Polyester | No | Degrades in water | Yes | -- | [30] |
| Decellularized Matrix | ||||||
| Liver dECM 4 | Proteins, polysaccharide, glycoproteins, proteoglycans | Yes | Soluble | Yes | Retains native chemical structure and microgeometry | [30,70,71] |
| S. No. | Ink Composition | Cell/s Used | Bioprinting Process | Printed Structure/s | Application | Major Finding/s | References |
|---|---|---|---|---|---|---|---|
| 1. | Liver dECM 1-gelatin | NIH3T3, HUVEC | Inkjet-based | 2D and 3D liver shaped structures | Artificial tissue/organ regeneration | dECM powder-based bioink with enhanced printability and mechanical properties | [34] |
| 2. | Pluronic F127- Alginate | HepG2/C3A | Extrusion-based | 3D squared structure | In vitromodel for drug screening | 3D hepatic model bioprinted without instructive signals | [78] |
| 3. | Gelatin | Huh7 | Extrusion-based | 3D mesh with different strut angles | In vitrohepatic model with enhanced functionality | Scaffolds with strut angle of 60° showed increased hepatic functions | [79] |
| 4. | PCL 2-Liver dECM; collagen | HepG2, BMMSCs | Extrusion-based | 2D and 3D patterns | Hepatic tissue engineering | Bioink printing structures with optimum strength and differentiation capacity | [80] |
| 5. | Alginate-cellulose nanocrystals | Fibroblasts, human hepatoma cells | Extrusion-based | 3D honeycomb structure | Hepatic tissue engineering | Novel cellulose-based bioink with excellent printability | [81] |
| 6. | PLGA 3 | Acellular | Extrusion-based | Single channel cubical; cylindrical; branched tri-channel hemisphere | Liver regenerative scaffolds | PLGA multichannel scaffolds using low-temperature deposition manufacturing device | [82] |
| 7. | Gelatin-alginate | Primary hepatocellular carcinoma cells | Extrusion-based | 3D cube | Personalized medicine | 3D printed primary cells cultured in vitro with preservation of tumerogenicity | [83] |
| 8. | gelatin-alginate-Matrigel™ | Primary intrahepatic cholangio-carcinoma cells | Extrusion-based | 3D cube | Personalized medicine | Patient-specific 3D bioprinted model for anticancer drug testing | [84] |
| 9. | GelMA 4 | HepG2/C3A spheroids | Inkjet-based | Liquid droplet | Organ-on-a-chip | Hepatic spheroid laden bioink printed directly in bioreactor culture chamber | [85] |
| 10. | Collagen I-hyaluronan | Lx2, primary fetal activated hepatic stellate cells | Extrusion-based | Four-spoke wheel structure | In vitro drug screening, disease modeling | Tunable bioink with ability to incorporate other components for additional functionality | [86] |
| 11. | Alginate-Cellulose nanocrystal-GelMA | NIH3T3, HepG2 | Extrusion-based | 3D honeycomb structure | Hepatic tissue engineering | Bicellular liver lobule-mimetic structures with precise positioning of the two cells | [87] |
| 12. | Liver dECM-GelMA | Human-induced hepatocytes | Digital light processing | Inner gear-like structure | Liver substitute | Novel bioink compatible with high resolution digital light processing printing | [88] |
| 13. | Collagen | Human adipose-derived stem cells (hASCs) | Extrusion-based | 3D cube | Bioartificial liver | hASC-induced hepatocyte-like cells interfere with liver regeneration | [89] |
| 14. | Alginate | Mouse primary hepatocytes, mesenchymal stem cells | Extrusion-based | 3D cube | Hepatic tissue engineering | Co-culture of hepatic and stem cells in 3D bioprinted construct | [90] |
| 15. | Alginate | Mouse-induced hepatocytes | Extrusion-based | 3D cube | Bioartificial organs | Mouse-induced hepatocytes as hepatic cell source | [91] |
| 16. | Galactosylated alginate | Mouse primary hepatocytes | Inkjet-based | Gel sheet | Hepatic tissue engineering | Controlled 3D geometrical arrangement of cells during printing | [92] |
| 17. | Alginate | Mouse primary hepatocytes | Extrusion-based | 3D cube | Hepatic tissue engineering | Long-term viability and functionality of primary hepatocytes | [93] |
| 18. | Alginate | HepG2 | Extrusion-based | 3D cube | Regenerative medicine | Improved hepatic functions of HepG2 | [94] |
| 19. | Atelocollagen | Rat primary hepatocytes, HUVEC, human lung fibroblast | Extrusion-based | 3D cube | Regenerative medicine | Co-culture of parenchymal and non-parenchymal cells, angiogenesis | [95] |
| 20. | Liver dECM-GelMA | HepLL, Caki-1 | Lithography | Microfluidic device | Tumor progression model | Metastasis-on-a-chip for migration of kidney cancer cells to liver | [96] |
| 21. | Human lung dECM-alginate-gelatin | HepaRG | Extrusion-based | 3D cube | Infection and transduction studies | Printed tissue model allowed for extensive transduction otherwise not achieved in spheroid models | [97] |
| 22. | NovoGel® | Primary cryopreserved human hepatocytes, hepatic stellate cells, HUVEC | Extrusion-based | Two-compartment planar geometry | In vitro hepatic model | Precise delivery of each cell type to designated locations, recapitulation of native tissue structure | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, J.; Kamble, S.C.; Kashikar, N.C. Polymeric Bioinks for 3D Hepatic Printing. Chemistry 2021, 3, 164-181. https://doi.org/10.3390/chemistry3010014
Sarkar J, Kamble SC, Kashikar NC. Polymeric Bioinks for 3D Hepatic Printing. Chemistry. 2021; 3(1):164-181. https://doi.org/10.3390/chemistry3010014
Chicago/Turabian StyleSarkar, Joyita, Swapnil C. Kamble, and Nilambari C. Kashikar. 2021. "Polymeric Bioinks for 3D Hepatic Printing" Chemistry 3, no. 1: 164-181. https://doi.org/10.3390/chemistry3010014
APA StyleSarkar, J., Kamble, S. C., & Kashikar, N. C. (2021). Polymeric Bioinks for 3D Hepatic Printing. Chemistry, 3(1), 164-181. https://doi.org/10.3390/chemistry3010014







