Coordination Polymers Constructed from Semi-Rigid N,N′-Bis(3-pyridyl)terephthalamide and Dicarboxylic Acids: Effect of Ligand Isomerism, Flexibility, and Identity
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Materials
2.3. Preparations
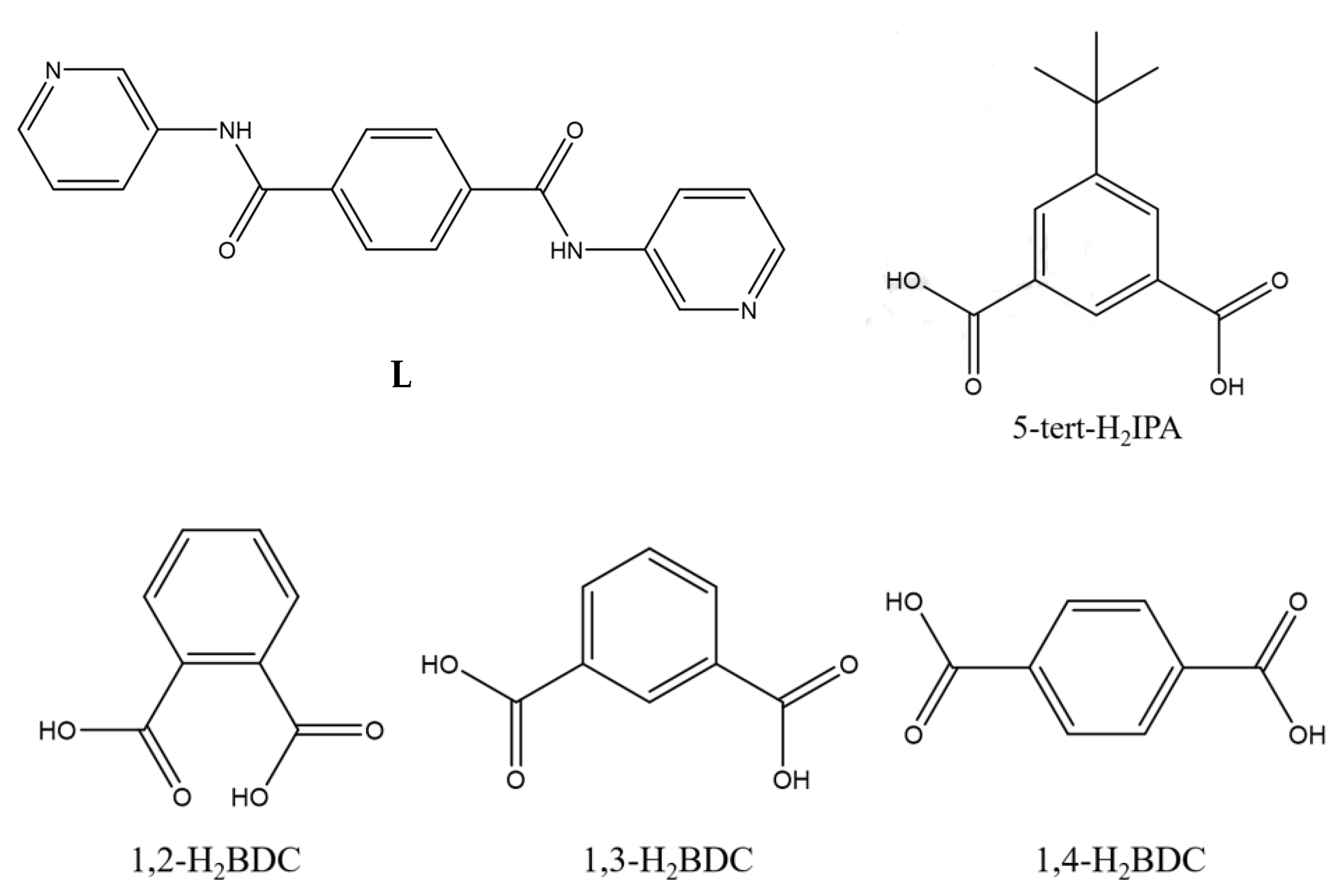
2.3.1. [Cd(L)0.5(1,2-BDC)(H2O)]n, 1
2.3.2. {[Cd(L)1.5(1,3-BDC)(H2O)]·5H2O}n, 2a, and {[Cd(1,3-BDC)(H2O)3]·2H2O}n, 2b
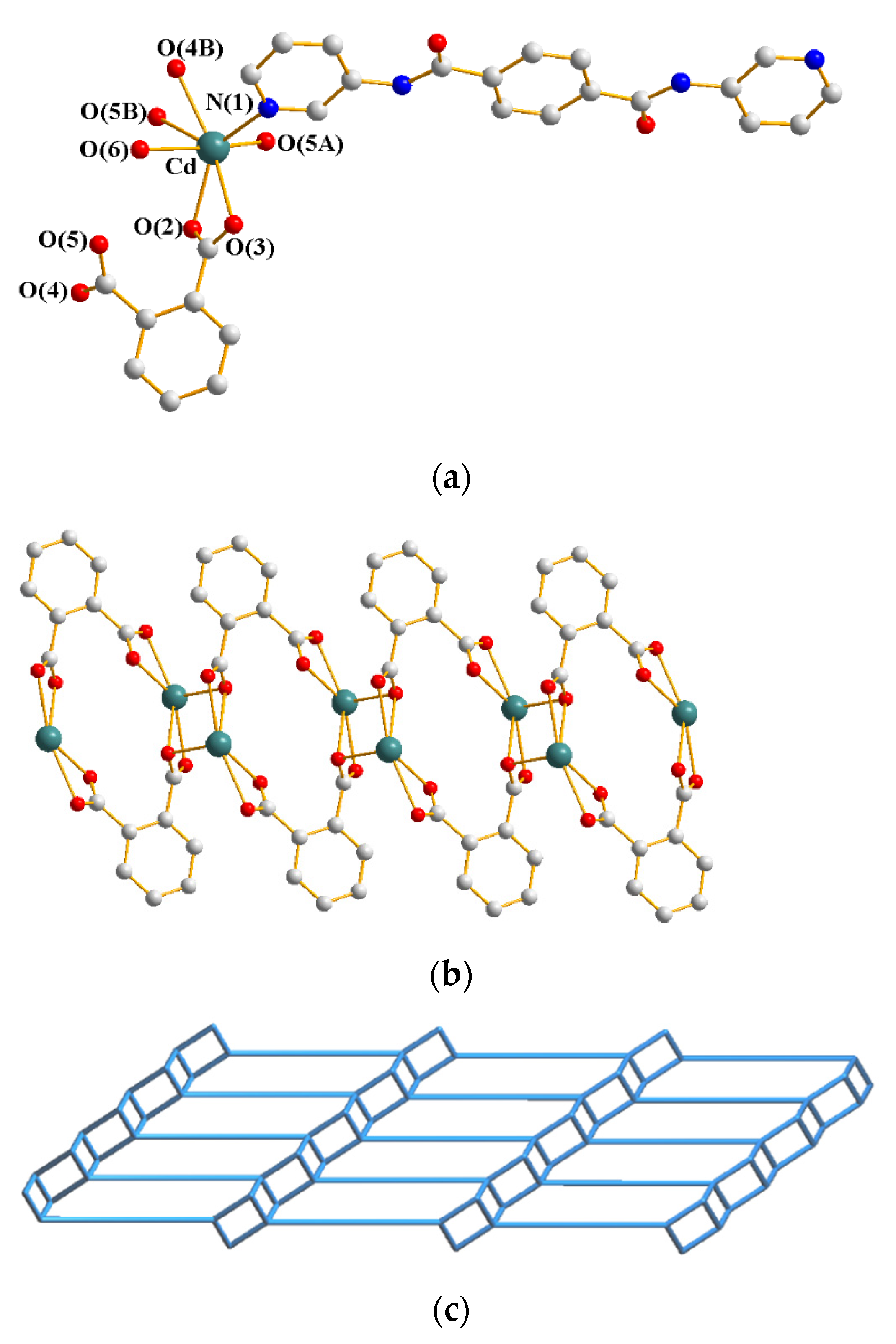
2.3.3. {[Cd(L)0.5(1,4-BDC)(H2O)2]·H2O}n, 3
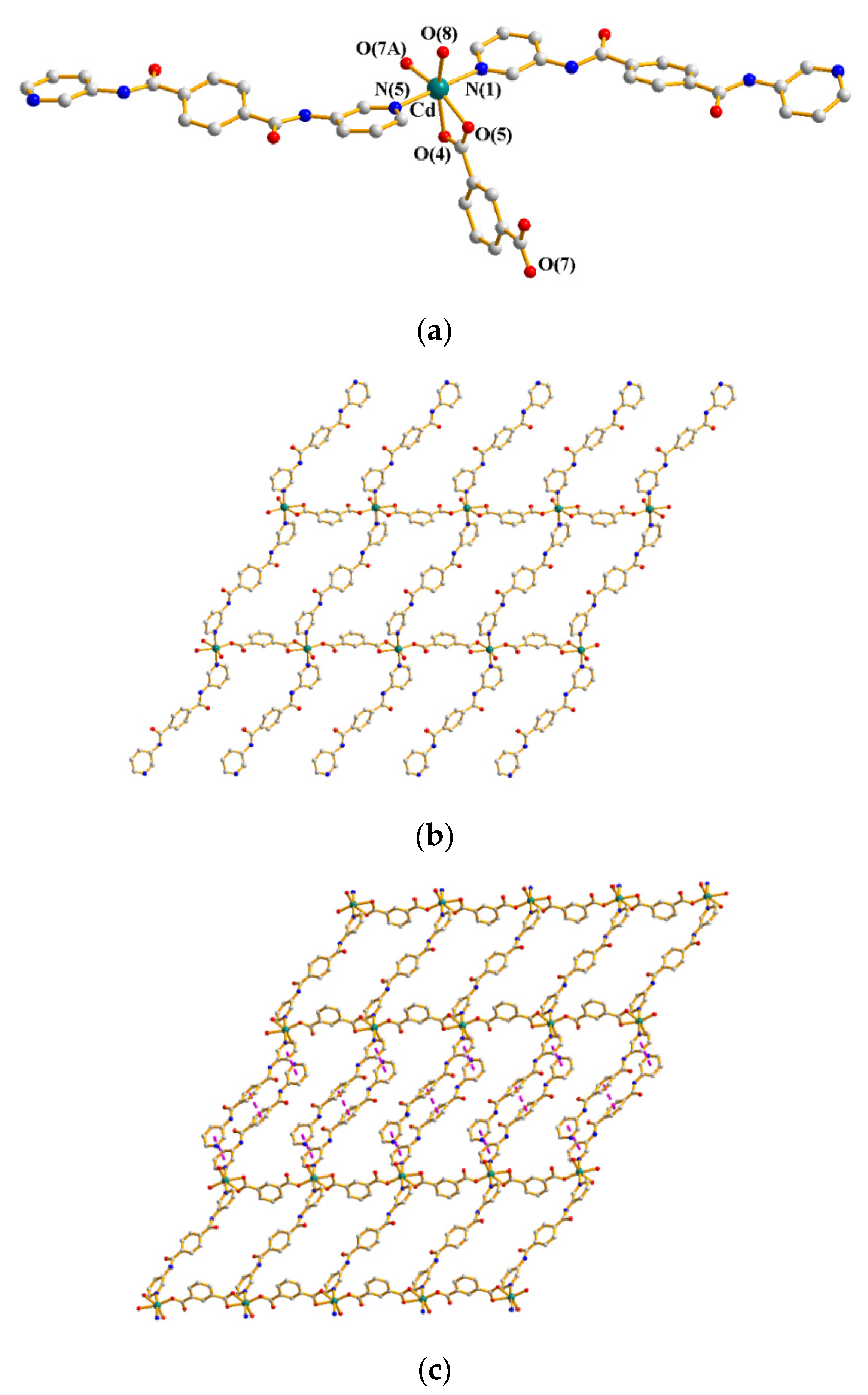
2.3.4. [Cu(L)0.5(5-tert-IPA)]n, 4
2.4. X-ray Crystallography
3. Results
3.1. Structure of 1
3.2. Structures of 2a and 2b
3.3. Structure of 3
3.4. Structure of 4
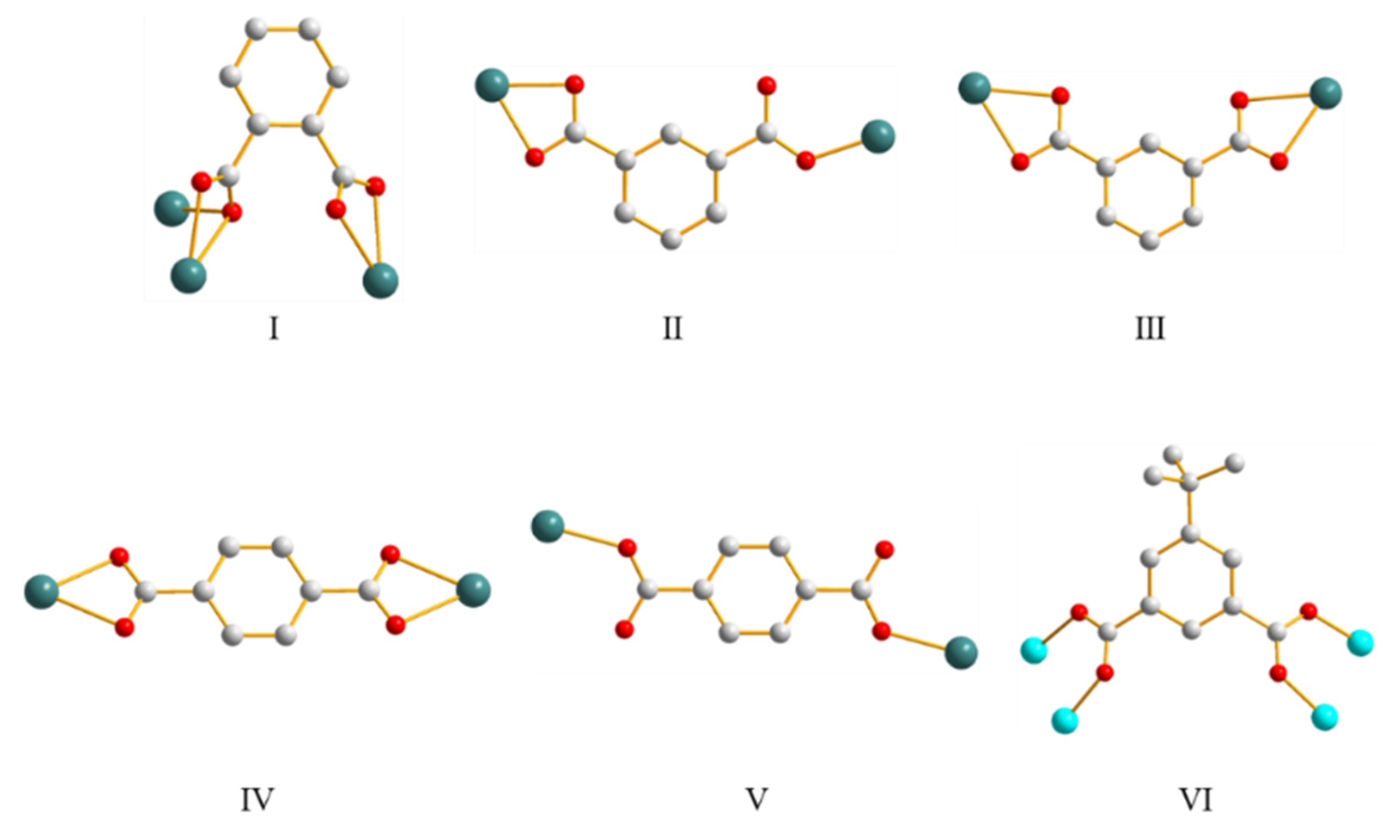
3.5. Ligand Conformation and Bonding Mode
3.6. Ligand Flexibility and Isomeric Effect
3.7. Thermal Properties
3.8. Luminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, K.B.; Chen, J.-D. Crystal engineering of coordination polymers containing flexible bis-pyridyl-bis-amide ligands. CrystEngComm 2015, 17, 4611–4626. [Google Scholar] [CrossRef]
- Hsu, Y.-F.; Hu, H.-L.; Wu, C.-J.; Yeh, C.-W.; Proserpio, D.M.; Chen, J.-D. Ligand isomerism-controlled structural diversity of cadmium(II) perchlorate coordination polymers containing dipyridyladipoamide ligands. CrystEngComm 2009, 11, 168–176. [Google Scholar] [CrossRef]
- Cheng, P.-C.; Kuo, P.-T.; Liao, Y.-H.; Xie, M.-Y.; Hsu, W.; Chen, J.-D. Ligand-isomerism controlled structural diversity of Zn(II) and Cd(II) coordination polymers from mixed dipyridyladipoamide and benzenedicarboxylate ligands. Cryst. Growth Des. 2013, 13, 623–632. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Hsu, W.; He, H.-Y.; Hyde, S.-T.; Proserpio, D.M.; Chen, J.-D. Structural directing roles of isomeric phenylenediacetate ligands in the formation of coordination networks based on flexible N,N-di(3-pyridyl)suberoamide. CrystEngComm 2015, 17, 90–97. [Google Scholar] [CrossRef] [Green Version]
- He, H.-Y.; Hsu, C.-H.; Chang, H.-Y.; Yang, X.-K.; Chhetri, P.M.; Proserpio, D.M.; Chen, J.-D. Self-catenated coordination polymers involving bis-pyridyl-bis-amide. Cryst. Growth. Des. 2017, 17, 1991–1998. [Google Scholar] [CrossRef]
- Chen, C.-J.; Lee, W.-T.; Hu, J.-H.; Chhetri, P.M.; Chen, J.-D. Structural Diversity and Modification in Ni(II) Coordination Polymers: Peculiar Phenomenon of Reversible Structural Transformation between 1D Ladder and 2D Layer. CrystEngComm 2020, 22, 7565–7574. [Google Scholar] [CrossRef]
- Rajput, L.; Singha, S.; Biradha, K. Comparative Structural Studies on Homologues of Amides and Reverse Amides: Unprecedented 4-fold Interpenetrated Quartz Network, New β-Sheet, and Two-Dimensional Layers. Cryst. Growth Des. 2007, 7, 2788–2795. [Google Scholar] [CrossRef]
- Bruker AXS. APEX2, V2008.6; SAD ABS V2008/1; SAINT+ V7.60A.; SHELXTL V6.14; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Zhang, F.; Bei, F.-L.; Cao, J.-M. Solvothermal Synthesis and Crystal Structure of Two CdII Coordination Polymers of Benzenedicarboxylic. J. Chem. Cryst. 2008, 38, 561–565. [Google Scholar] [CrossRef]
- Ahmed, I.; Yunus, U.; Nadeem, M.; Bhatti, M.H.; Mehmood, M. Post synthetically modified compounds of Cd-MOF by L-amino acids for luminescent applications. J. Solid State Chem. 2020, 287, 121320. [Google Scholar] [CrossRef]
- Chang, M.-N.; Yang, X.-K.; Chhetri, P.-M.; Chen, J.-D. Metal and Ligand Effects on the Construction of Divalent Coordination Polymers Based on bis-Pyridyl-bis-amide and Polycarboxylate Ligands. Polymers 2017, 9, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Yan, Z.-H.; Blatov, V.A.; Wang, L.; Sun, D.-F. Syntheses, topological structures, and photoluminescences of six new Zn(II) coordination polymers based on mixed tripodal imidazole ligand and varied polycarboxylates. Cryst. Growth Des. 2013, 13, 1277–1289. [Google Scholar] [CrossRef]




| 1 | 2b | 3 | 4 | |
|---|---|---|---|---|
| Formula | C17H13CdN2O6 | C8 H14 Cd O9 | C17H17CdN2O8 | C21H19CuN2O5 |
| Formula weight | 453.69 | 366.59 | 489.72 | 442.92 |
| Crystal system | Monoclinic | Triclinic | Triclinic | Monoclinic |
| Space group | P21/c | Pī | Pī | C2/c |
| a, Å | 14.6784(10) | 10.2076(3) | 6.34570(10) | 21.600(3) |
| b, Å | 5.8992(3) | 11.1103(3) | 9.4314(2) | 10.6511(15) |
| c, Å | 19.8917(11) | 12.6791(3) | 16.7255(3) | 17.041(2) |
| α,° | 90 | 102.1594(10) | 100.8415(12) | 90 |
| β, ° | 107.498(4) | 102.2797(10) | 95.6385(12) | 100.715 |
| γ, ° | 90 | 103.5718(10) | 104.0750(11) | 90 |
| V, Å3 | 1642.74(17) | 1313.41(6) | 942.66(3) | 3852.2(9) |
| Z | 4 | 4 | 2 | 8 |
| Dcalc, Mg/m3 | 1.834 | 1.854 | 1.725 | 1.527 |
| F(000) | 900 | 728 | 490 | 1824 |
| µ(Mo Kα), mm−1 | 1.368 | 1.697 | 1.206 | 1.170 |
| Range(2θ) for data collection, deg | 2.91 to 52.00 | 3.42 to 56.63 | 4.70 to 56.63 | 3.84 to 56.52 |
| Independent reflections | 3173 [R(int) = 0.0794] | 6502 [R(int) = 0.0306] | 4703 [R(int) = 0.0344] | 4756 [R(int) = 0.0847] |
| Data/restraints/parameters | 3173/0/241 | 6502/0/348 | 4703/0/269 | 4756/492/285 |
| quality-of-fit indicator c | 1.004 | 1.003 | 1.034 | 1.059 |
| Final R indices [I > 2σ(I)] a,b | R1 = 0.0436, wR2 = 0.0561 | R1 = 0.0305, wR2 = 0.0965 | R1 = 0.0303, wR2 = 0.0596 | R1 = 0.0589, wR2 = 0.1310 |
| R indices (all data) | R1 = 0.0886, wR2=0.0638 | R1 = 0.0331, wR2 = 0.0994 | R1 = 0.0418, wR2=0.0633 | R1 = 0.0950, wR2=0.1486 |
| Complex | Weight Loss of Solvent, T, °C (Observed/Calc),% | Weight Loss of Ligand, T, °C (Observed/Calc),% |
|---|---|---|
| 1 | 100–280 (4.76/3.97) | 280–800 (81.83/71.42) |
| 3 | 40–95 (4.12/3.68) 100–350(8.21/7.35) | 360–706 (66.41/66.19) |
| 4 | 250–800 (81.98/85.79) |
| Ligand. | λex (nm) | λem (nm) | Complex | λex (nm) | λem (nm) |
|---|---|---|---|---|---|
| L | 276 | 431 | 1 | 318 | 416 |
| 1,2-H2BDC | 283 | 340 | 3 | 334 | 434 |
| 1,4-H2BDC | 277/331 | 384 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-J.; Chen, C.-L.; Liu, Y.-H.; Lee, W.-T.; Hu, J.-H.; Chhetri, P.M.; Chen, J.-D. Coordination Polymers Constructed from Semi-Rigid N,N′-Bis(3-pyridyl)terephthalamide and Dicarboxylic Acids: Effect of Ligand Isomerism, Flexibility, and Identity. Chemistry 2021, 3, 1-12. https://doi.org/10.3390/chemistry3010001
Chen C-J, Chen C-L, Liu Y-H, Lee W-T, Hu J-H, Chhetri PM, Chen J-D. Coordination Polymers Constructed from Semi-Rigid N,N′-Bis(3-pyridyl)terephthalamide and Dicarboxylic Acids: Effect of Ligand Isomerism, Flexibility, and Identity. Chemistry. 2021; 3(1):1-12. https://doi.org/10.3390/chemistry3010001
Chicago/Turabian StyleChen, Chia-Jou, Chia-Ling Chen, Yu-Hsiang Liu, Wei-Te Lee, Ji-Hong Hu, Pradhumna Mahat Chhetri, and Jhy-Der Chen. 2021. "Coordination Polymers Constructed from Semi-Rigid N,N′-Bis(3-pyridyl)terephthalamide and Dicarboxylic Acids: Effect of Ligand Isomerism, Flexibility, and Identity" Chemistry 3, no. 1: 1-12. https://doi.org/10.3390/chemistry3010001
APA StyleChen, C.-J., Chen, C.-L., Liu, Y.-H., Lee, W.-T., Hu, J.-H., Chhetri, P. M., & Chen, J.-D. (2021). Coordination Polymers Constructed from Semi-Rigid N,N′-Bis(3-pyridyl)terephthalamide and Dicarboxylic Acids: Effect of Ligand Isomerism, Flexibility, and Identity. Chemistry, 3(1), 1-12. https://doi.org/10.3390/chemistry3010001






