Interactions of Small-Molecule Guests with Interior and Exterior Surfaces of a Coordination Cage Host
Abstract
1. Introduction
2. Materials and Methods
3. Results
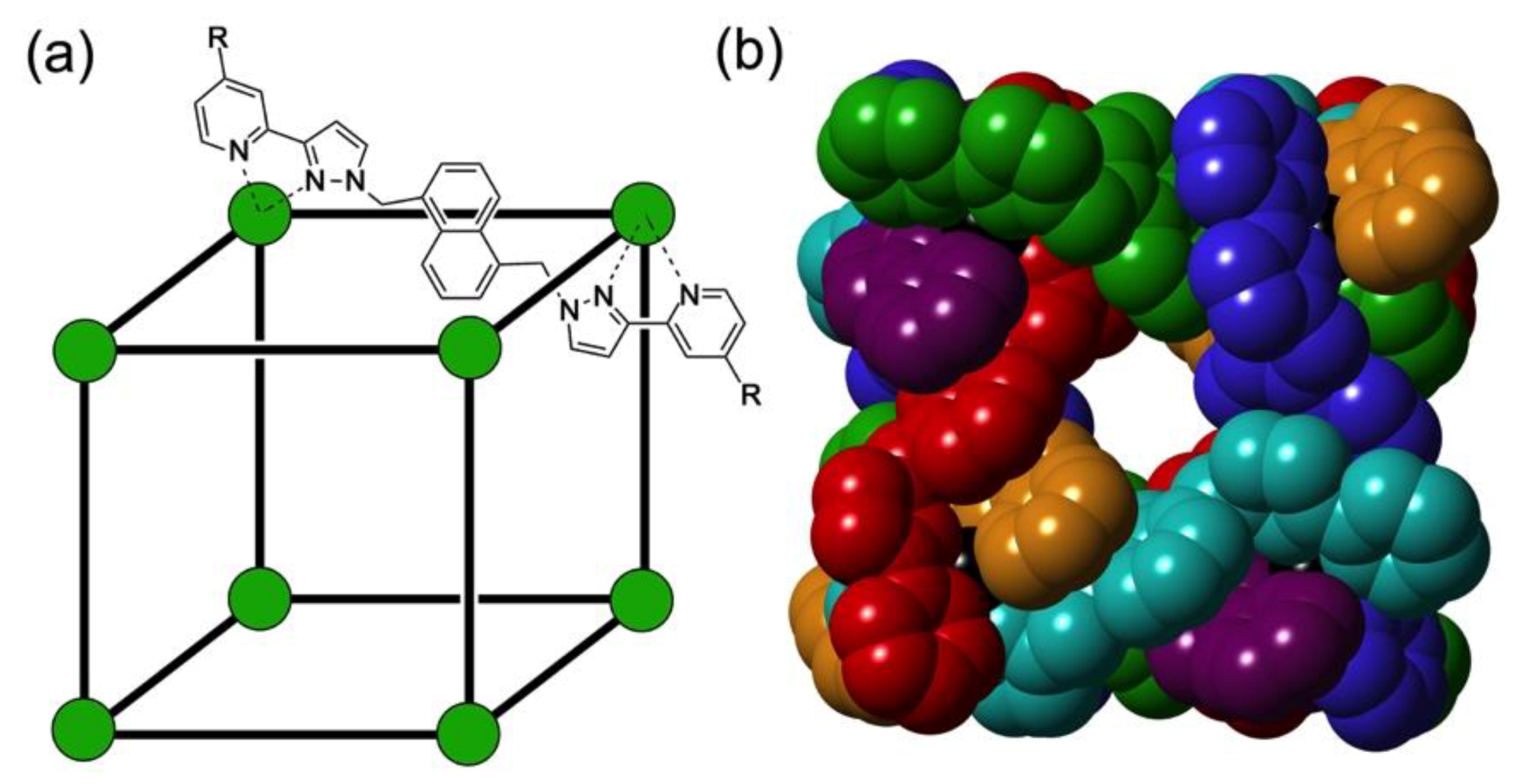
3.1. The Cage Hosts
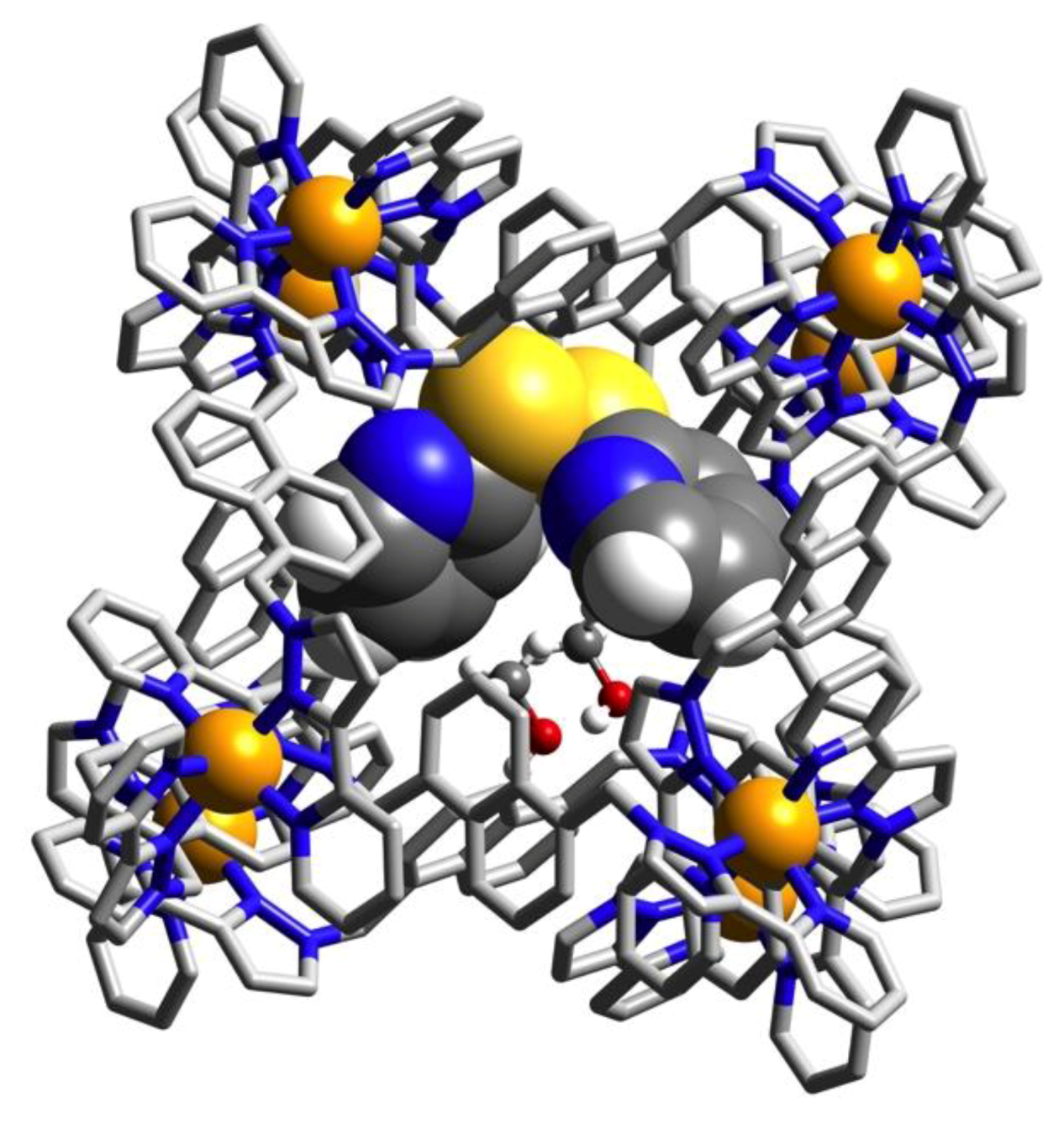
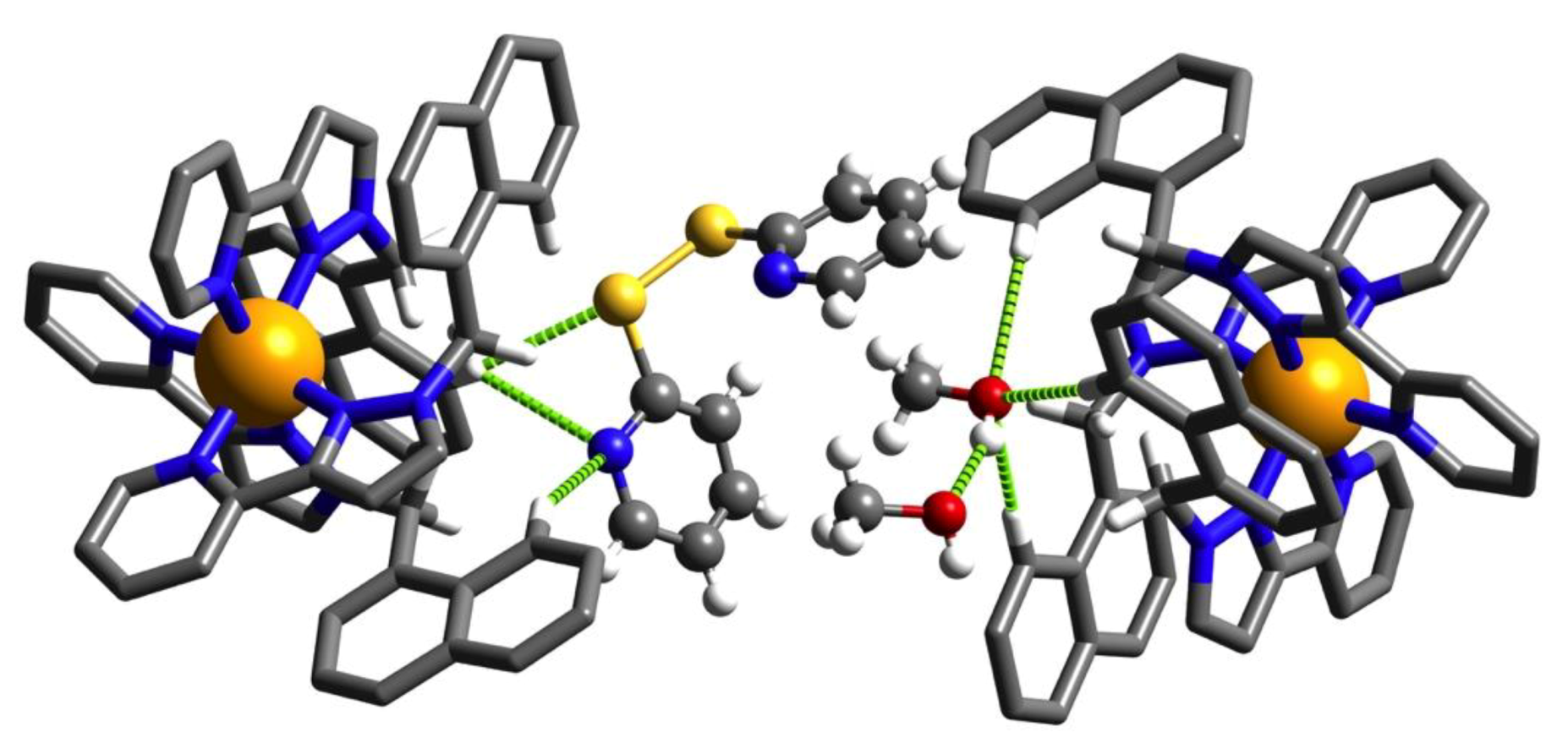
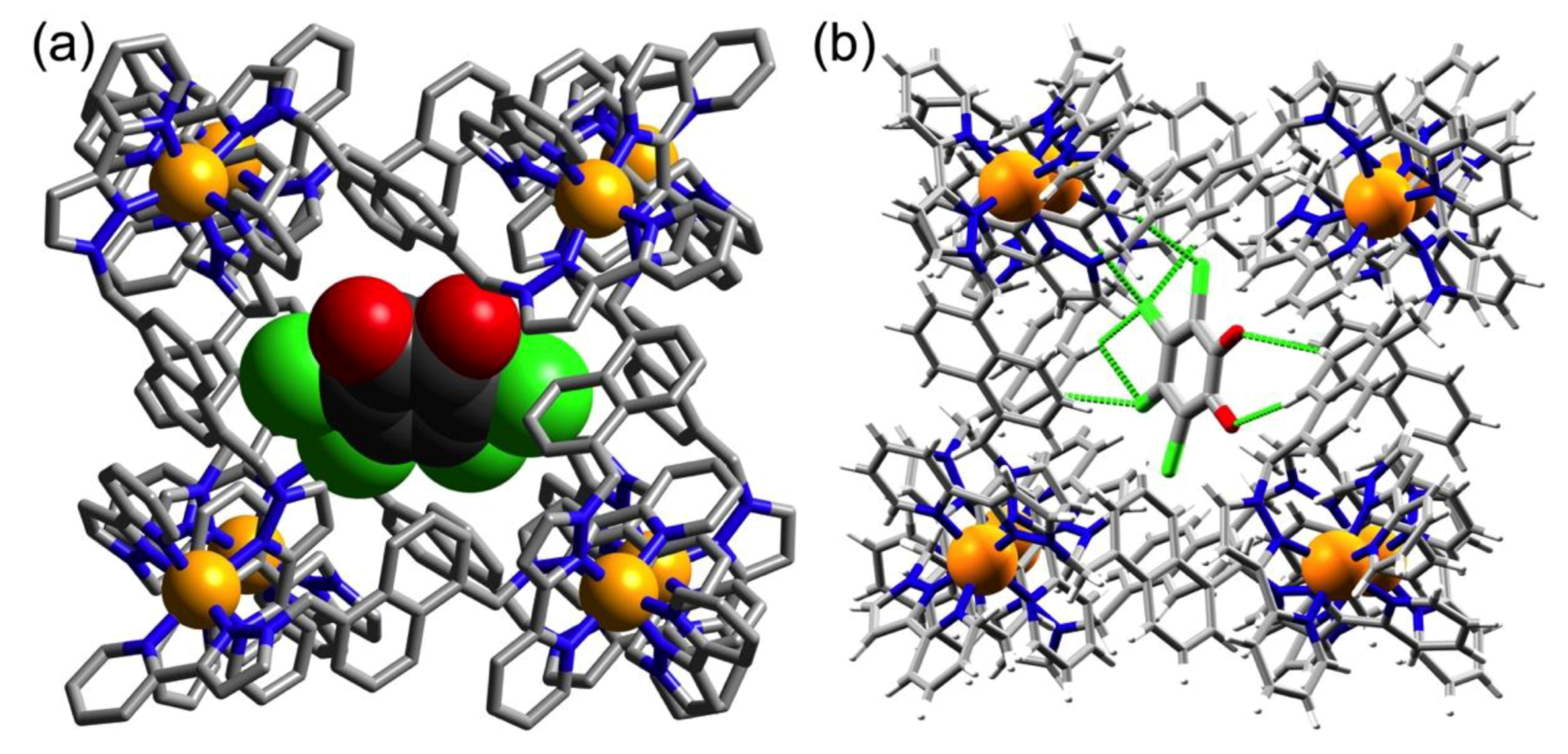
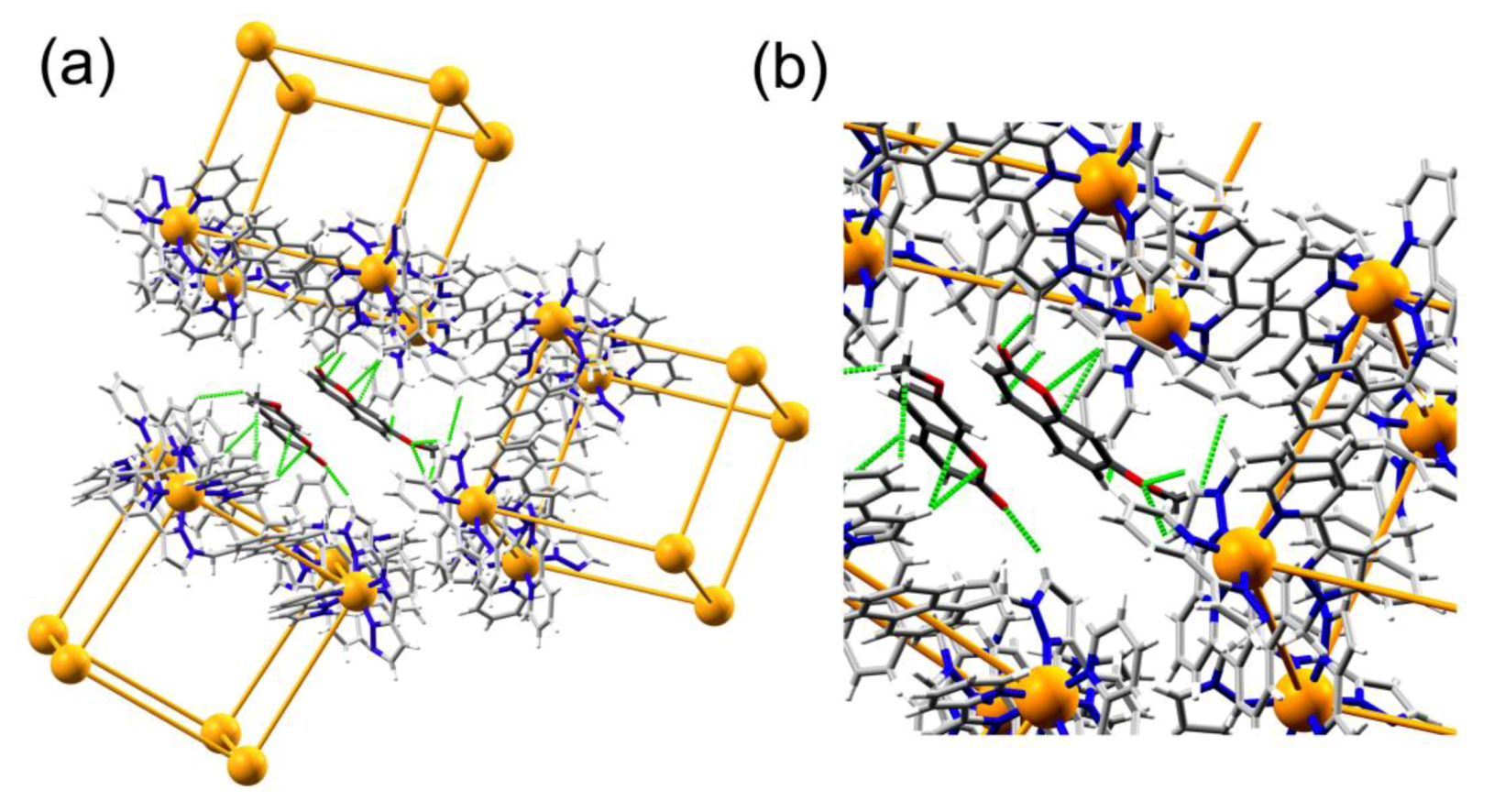
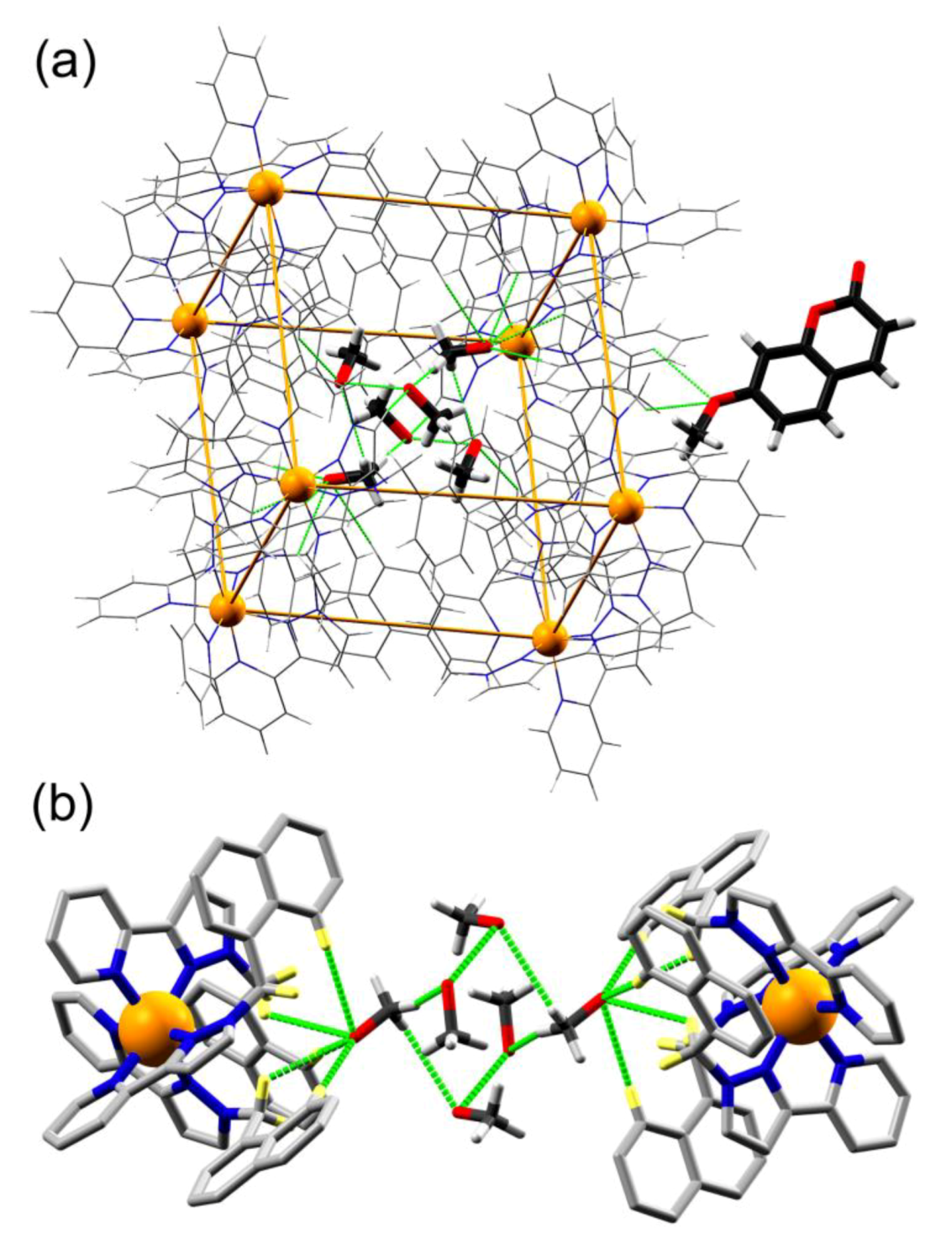
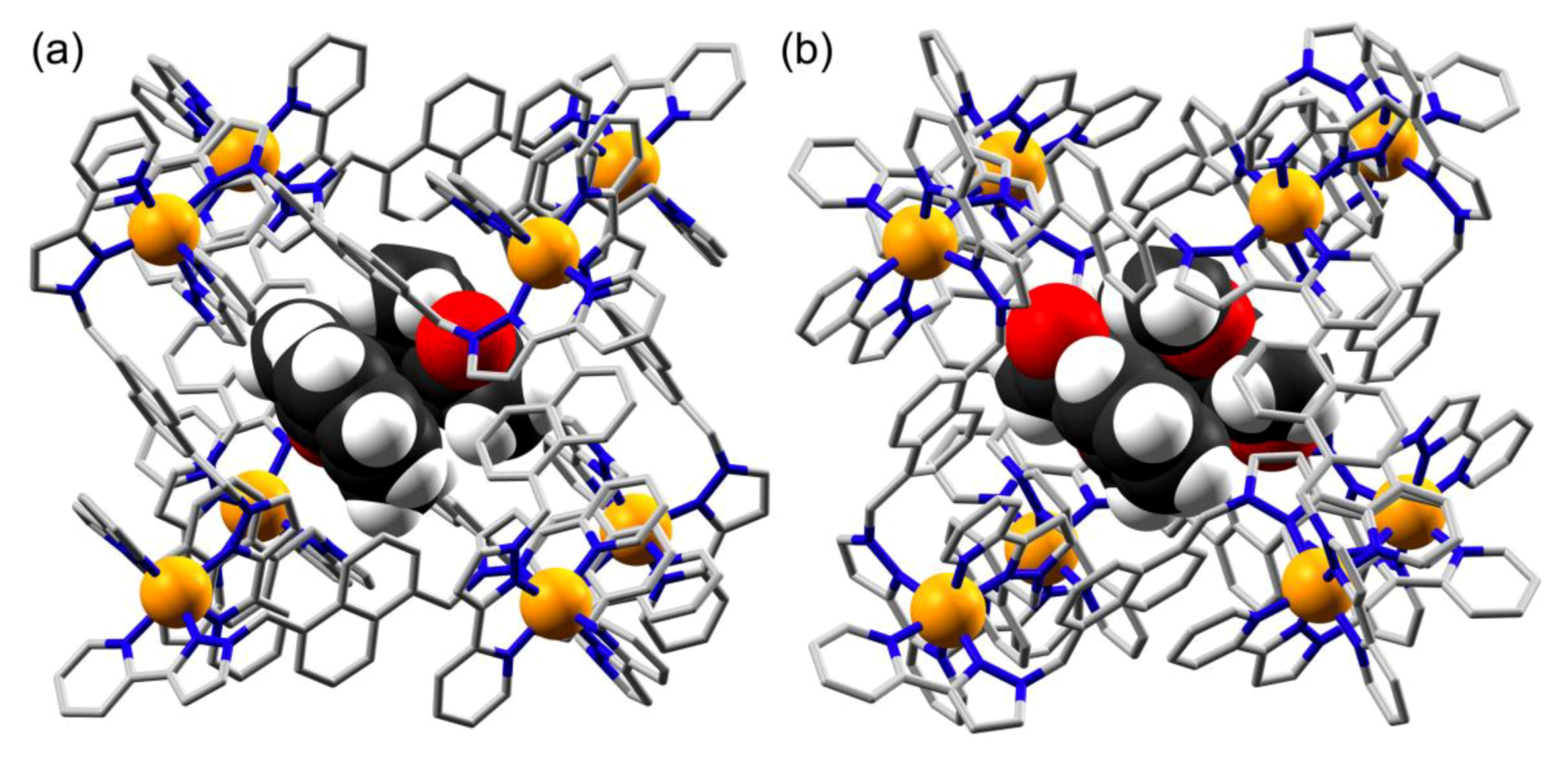
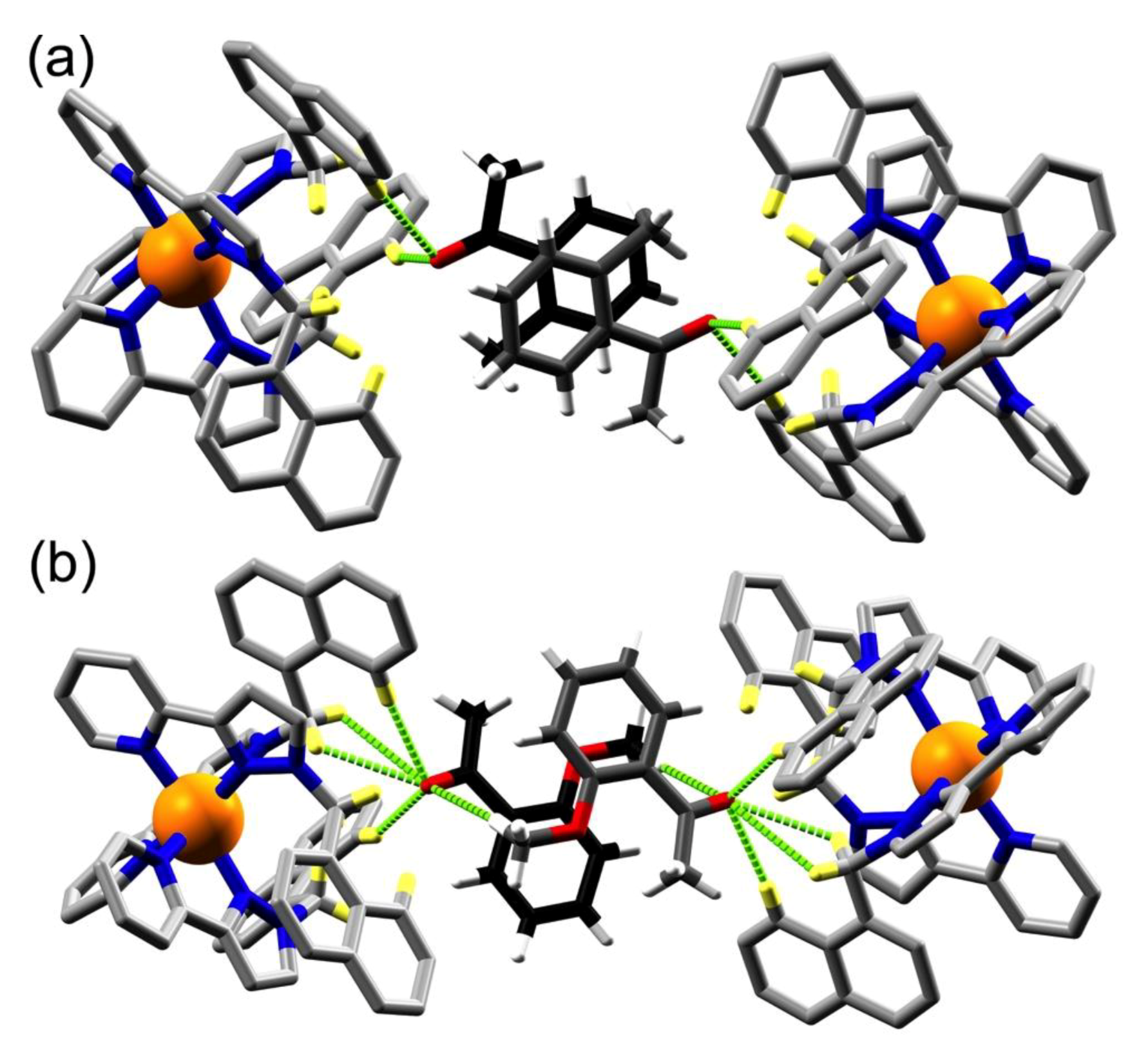
3.2. Structures of Cage/Guest Complexes
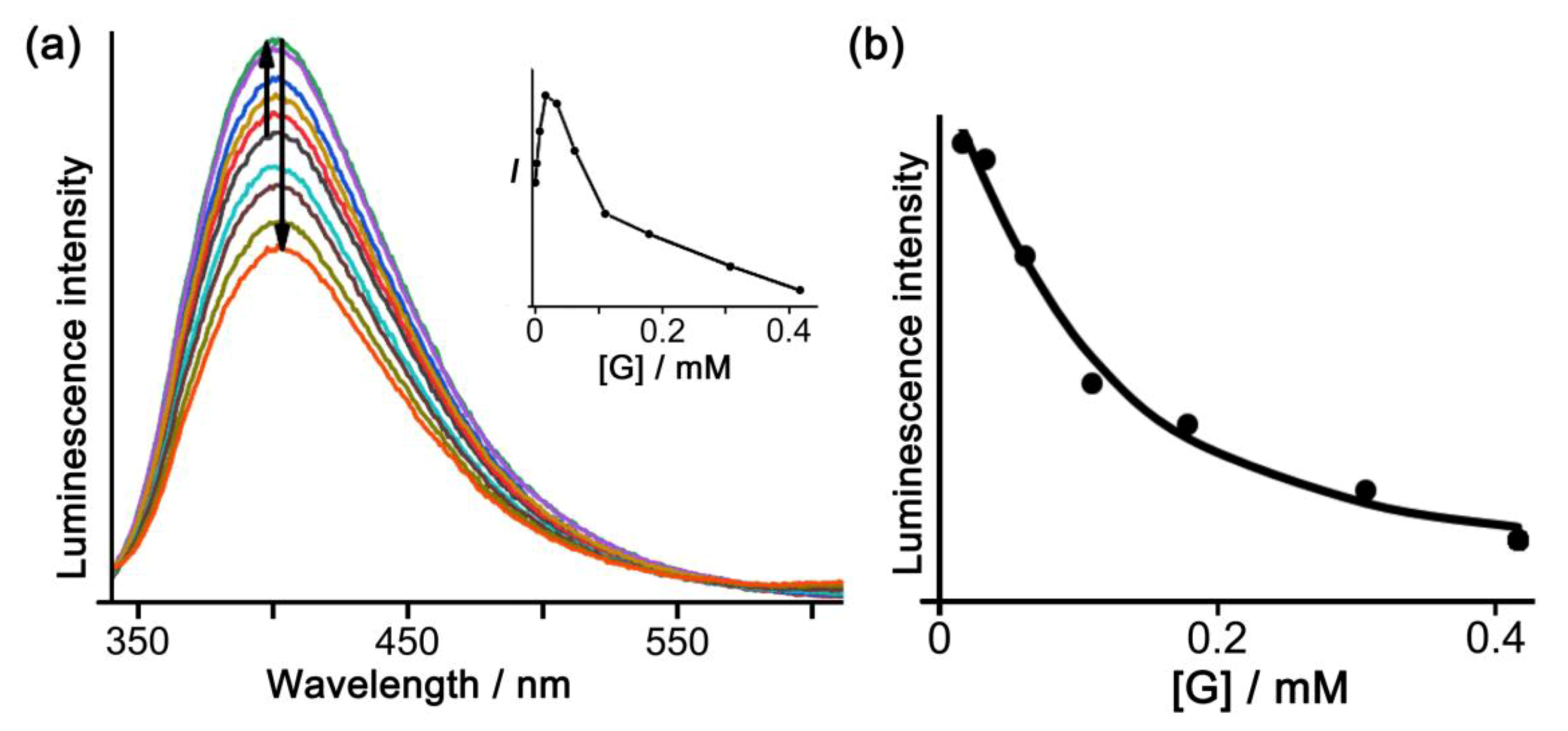
3.3. Cage/Guest Interactions in Solution Studied by Fluorescence Titrations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Ronson, T.K.; Nitschke, J.R. Functional Capsules via Subcomponent Self-Assembly. Acc. Chem. Res. 2018, 51, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional molecular flasks: New properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Yusubov, M.S.; Verpoort, F. Self-assembled metal–organic polyhedra: An overview of various applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P. Recent Developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Otte, M. Size-Selective Molecular Flasks. ACS Catal. 2016, 6, 6491–6510. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Zhao, L.; Duan, C. Photochemical Properties of Host–Guest Supramolecular Systems with Structurally Confined Metal–Organic Capsules. Acc. Chem. Res. 2018, 52, 100–109. [Google Scholar] [CrossRef]
- Catti, L.; Zhang, Q.; Tiefenbacher, K. Advantages of catalysis in self-assembled molecular capsules. Chem. Eur. J. 2016, 22, 9060–9066. [Google Scholar] [CrossRef]
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular Catalysis in Metal–Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef]
- Gao, W.-X.; Zhang, H.-N.; Jin, G.-X. Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages. Coord. Chem. Rev. 2019, 386, 69–84. [Google Scholar] [CrossRef]
- Hong, C.M.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Self-Assembled Tetrahedral Hosts as Supramolecular Catalysts. Acc. Chem. Res. 2018, 51, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Grommet, A.B.; Nitschke, J.R. Directed Phase Transfer of an FeII4L4 Cage and Encapsulated Cargo. J. Am. Chem. Soc. 2017, 139, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Garci, A.; Mbakidi, J.-P.; Chaleix, V.; Sol, V.; Orhan, E.; Therrien, B. Tunable Arene Ruthenium Metallaprisms to Transport, Shield, and Release Porphin in Cancer Cells. Organometallics 2015, 34, 4138–4146. [Google Scholar] [CrossRef]
- Mihara, N.; Ronson, T.K.; Nitschke, J.R. Different Modes of Anion Response Cause Circulatory Phase Transfer of a Coordination Cage with Controlled Directionality. Angew. Chem. Int. Ed. 2019, 58, 12497–12501. [Google Scholar] [CrossRef]
- Grancha, T.; Carne-Sanchez, A.; Hernández-López, L.; Albalad, J.; Imaz, I.; Juanhuix, J.; Maspoch, D. Phase Transfer of Rhodium(II)-Based Metal–Organic Polyhedra Bearing Coordinatively Bound Cargo Enables Molecular Separation. J. Am. Chem. Soc. 2019, 141, 18349–18355. [Google Scholar] [CrossRef]
- Saha, M.L.; Yan, X.; Stang, P. Photophysical Properties of Organoplatinum(II) Compounds and Derived Self-Assembled Metallacycles and Metallacages: Fluorescence and its Applications. Acc. Chem. Res. 2016, 49, 2527–2539. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Zi, M.; Yuan, L. Recent advances of application of porous molecular cages for enantioselective recognition and separation. J. Sep. Sci. 2019, 43, 134–149. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Lawrence, A.L.; Lusby, P.J. High Activity and Efficient Turnover by a Simple, Self-Assembled “Artificial Diels–Alderase”. J. Am. Chem. Soc. 2018, 140, 2862–2868. [Google Scholar] [CrossRef]
- Custelcean, R.; Bonnesen, P.V.; Duncan, N.C.; Zhang, X.; Watson, L.A.; Van Berkel, G.; Parson, W.B.; Hay, B.P. Urea-Functionalized M4L6 Cage Receptors: Anion-Templated Self-Assembly and Selective Guest Exchange in Aqueous Solutions. J. Am. Chem. Soc. 2012, 134, 8525–8534. [Google Scholar] [CrossRef]
- Whitehead, M.; Turega, S.; Stephenson, A.; Hunter, C.A.; Ward, M.D. Quantification of solvent effects on molecular recognition in polyhedral coordination cage hosts. Chem. Sci. 2013, 4, 2744. [Google Scholar] [CrossRef]
- Turega, S.; Cullen, W.; Whitehead, M.; Hunter, C.A.; Ward, M.D. Mapping the Internal Recognition Surface of an Octanuclear Coordination Cage Using Guest Libraries. J. Am. Chem. Soc. 2014, 136, 8475–8483. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.; Turega, S.; Hunter, C.A.; Ward, M.D. Virtual screening for high affinity guests for synthetic supramolecular receptors. Chem. Sci. 2015, 6, 2790–2794. [Google Scholar] [CrossRef] [PubMed]
- Metherell, A.; Cullen, W.; Williams, N.H.; Ward, M.D. Binding of Hydrophobic Guests in a Coordination Cage Cavity is Driven by Liberation of “High-Energy” Water. Chem. Eur. J. 2017, 24, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Leenders, S.H.A.M.; Becker, R.; Kumpulainen, T.; De Bruin, B.; Sawada, T.; Kato, T.; Fujita, M.; Reek, J.N.H. Selective Co-Encapsulation Inside an M6L4 Cage. Chem. Eur. J. 2016, 22, 15468–15474. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, H.; Murase, T.; Resnati, G.; Metrangolo, P.; Fujita, M. Halogen-bond-assisted guest inclusion in a synthetic cavity. Angew. Chem. Int. Ed. 2015, 54, 8411–8414. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, F.J.; Von Krbek, L.; Nitschke, J.R. Strategies for binding multiple guests in metal–organic cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Ibukuro, F.; Kusukawa, T.; Fujita, M. A Thermally Switchable Molecular Lock. Guest-Templated Synthesis of a Kinetically Stable Nanosized Cage. J. Am. Chem. Soc. 1998, 120, 8561–8562. [Google Scholar] [CrossRef]
- Johnson, D.W.; Raymond, K.N. The role of guest molecules in the self-assembly of metal—ligand clusters. Supramol. Chem. 2001, 13, 639–659. [Google Scholar] [CrossRef]
- Ward, M.D.; Hunter, C.A.; Williams, N.H. Coordination Cages Based on Bis(pyrazolylpyridine) Ligands: Structures, Dynamic Behavior, Guest Binding, and Catalysis. Acc. Chem. Res. 2018, 51, 2073–2082. [Google Scholar] [CrossRef]
- Cullen, W.; Metherell, A.J.; Wragg, A.B.; Taylor, C.G.P.; Williams, N.H.; Ward, M.D.; Metherell, A. Catalysis in a Cationic Coordination Cage Using a Cavity-Bound Guest and Surface-Bound Anions: Inhibition, Activation, and Autocatalysis. J. Am. Chem. Soc. 2018, 140, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, F.J.; Wu, W.; Ronson, T.K.; Frantz, D.E. Peripheral Templation Generates an MII 6L4 Guest-Binding Capsule. Angew. Chem. Int. Ed. 2016, 55, 7958–7962. [Google Scholar] [CrossRef] [PubMed]
- Sgarlata, C.; Mugridge, J.S.; Pluth, M.D.; Tiedemann, B.E.F.; Zito, V.; Arena, G.; Raymond, K.N. External and Internal Guest Binding of a Highly Charged Supramolecular Host in Water: Deconvoluting the Very Different Thermodynamics. J. Am. Chem. Soc. 2010, 132, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.; Misuraca, M.C.; Hunter, C.A.; Williams, N.H.; Ward, M.D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 2016, 8, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.G.P.; Metherell, A.J.; Argent, S.P.; Ashour, F.M.; Williams, N.H.; Ward, M.D. Coordination-Cage-Catalysed Hydrolysis of Organophosphates: Cavity- or Surface-Based? Chem. Eur. J. 2020, 26, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Mozaceanu, C.; Taylor, C.G.P.; Piper, J.R.; Argent, S.P.; Ward, M.D. Catalysis of an Aldol Condensation Using a Coordination Cage. Chemistry 2020, 2, 22–32. [Google Scholar] [CrossRef]
- Taylor, C.G.P.; Argent, S.P.; Ludden, M.D.; Piper, J.R.; Mozaceanu, C.; Barnett, S.A.; Ward, M.D. One Guest or Two? A Crystallographic and Solution Study of Guest Binding in a Cubic Coordination Cage. Chem. Eur. J. 2020, 26, 3054–3064. [Google Scholar] [CrossRef]
- Tidmarsh, I.S.; Faust, T.; Adams, H.; Harding, L.P.; Russo, L.; Clegg, W.; Ward, M.D. Octanuclear Cubic Coordination Cages. J. Am. Chem. Soc. 2008, 130, 15167–15175. [Google Scholar] [CrossRef]
- Piper, J.R.; Cletheroe, L.; Taylor, C.G.P.; Metherell, A.; Weinstein, J.A.; Sazanovich, I.V.; Ward, M.D. Photoinduced energy- and electron-transfer from a photoactive coordination cage to bound guests. Chem. Commun. 2017, 53, 408–411. [Google Scholar] [CrossRef]
- Train, J.S.; Wragg, A.B.; Auty, A.J.; Metherell, A.; Chekulaev, D.; Taylor, C.G.P.; Argent, S.P.; Weinstein, J.A.; Ward, M.D. Photophysics of Cage/Guest Assemblies: Photoinduced Electron Transfer between a Coordination Cage Containing Osmium(II) Luminophores, and Electron-Deficient Bound Guests in the Central Cavity. Inorg. Chem. 2019, 58, 2386–2396. [Google Scholar] [CrossRef]
- Spartan ′18, Version 1; Wavefunction Inc.: Irvine, CA, USA, 2018.
- Allan, D.R.; Nowell, H.; Barnett, S.A.; Warren, M.R.; Wilcox, A.; Christensen, J.; Saunders, L.K.; Peach, A.; Hooper, M.T.; Zaja, L.; et al. A Novel Dual Air-Bearing Fixed-χ Diffractometer for Small-Molecule Single-Crystal X-ray Diffraction on Beamline I19 at Diamond Light Source. Crystals 2017, 7, 336. [Google Scholar] [CrossRef]
- Johnson, N.T.; Waddell, P.; Clegg, W.; Probert, M. Remote Access Revolution: Chemical Crystallographers Enter a New Era at Diamond Light Source Beamline I19. Crystals 2017, 7, 360. [Google Scholar] [CrossRef]
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Hitora, Y.; Takada, K.; Matsunaga, S.; Rissanen, K.; Fujita, M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 2013, 495, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Khutia, A.; Xing, H.; Inokuma, Y.; Fujita, M. The crystalline sponge method updated. IUCrJ 2016, 3, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Isono, H.; Yasui, M.; Iwasaki, F.; Kamigata, N. Solid state optical activity of dichalcogenides: Isolation by chiral crystallization and determination of absolute configuration. Org. Lett. 2001, 3, 3639–3641. [Google Scholar] [CrossRef]
- Metherell, A.J.; Ward, M.D. Geometric isomerism in coordination cages based on tris-chelate vertices: A tool to control both assembly and host/guest chemistry. Dalton Trans. 2016, 45, 16096–16111. [Google Scholar] [CrossRef]
- Rebek, J.J. Molecular Behavior in Small Spaces. Acc. Chem. Res. 2009, 42, 1660–1668. [Google Scholar] [CrossRef]
- Mecozzi, S.; Rebek, J.J. The 55 % Solution: A Formula for Molecular Recognition in the Liquid State. Chem. Eur. J. 1998, 4, 1016–1022. [Google Scholar] [CrossRef]
- Puttreddy, R.; Beyeh, N.K.; Kalenius, E.; Ras, R.H.A.; Rissanen, K. 2-Methylresorcinarene: A very high packing coefficient in a mono-anion based dimeric capsule and the X-ray crystal structure of the tetra-anion. Chem. Commun. 2016, 52, 8115–8118. [Google Scholar] [CrossRef]
- Roos, G.; De Proft, F.; Geerlings, P. Electron Capture by the Thiyl Radical and Disulfide Bond: Ligand Effects on the Reduction Potential. Chem. Eur. J. 2013, 19, 5050–5060. [Google Scholar] [CrossRef]
- Taylor, C.G.P.; Piper, J.R.; Ward, M.D. Binding of chemical warfare agent simulants as guests in a coordination cage: Contributions to binding and a fluorescence-based response. Chem. Commun. 2016, 52, 6225–6228. [Google Scholar] [CrossRef] [PubMed]

| Guest | Di(2-pyridyl)disulfide | 3,4,5,6-Tetrachloro-1,2-benzoquinone |
|---|---|---|
| Empirical formula | C378.65H417.32B16Co8F64N73.08 O37.25S1.08 | C377H416B16Cl2Co8F64N72O39 |
| Formula weight | 8483.10 | 8511.09 |
| T/K | 100(1) | 100(1) |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c |
| Crystal size/mm3 | 0.1 × 0.1 × 0.1 | 0.1 × 0.1 × 0.1 |
| a/Å | 32.81236(7) | 32.85880(17) |
| b/Å | 30.21497(6) | 30.06718(13) |
| c/Å | 40.09564(8) | 40.1053(2) |
| β/degrees | 96.33120(19) | 96.0585(5) |
| V/Å3 | 39509.35(10) | 39401.6(3) |
| Z | 4 | 4 |
| ρcalc/g cm−3 | 1.426 | 1.435 |
| μ/mm−1 | 0.410 | 0.415 |
| Radiation | Synchrotron (λ = 0.6889) | Synchrotron (λ = 0.6889) |
| Reflections collected | 348244 | 341329 |
| Data/restraints/parameters | 62856/6404/2425 | 62724/5560/2167 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0672, wR2 = 0.2167 | R1 = 0.0829, wR2 = 0.2810 |
| Final R indexes (all data) | R1 = 0.0926, wR2 = 0.2358 | R1 = 0.1178, wR2 = 0.3061 |
| 7-Methoxycoumarin | 2-Methylacetophenone | 2-Methoxyacetophenone |
| C376H392B16Co8F64N72O33 | C407.8H417.66B16Co8F64N72 O31.03 | C395.5H411B16Co8F64N72O35 |
| 8307.99 | 8684.25 | 8593.34 |
| 100(1) | 100(1) | 100(1) |
| Monoclinic | Monoclinic | Monoclinic |
| C2/c | C2/c | C2/c |
| 0.15 × 0.12 × 0.1 | 0.04 × 0.03 × 0.03 | 0.04 × 0.04 × 0.04 |
| 33.04596(13) | 32.56923(10) | 32.8649(2) |
| 30.13654(16) | 30.16165(10) | 29.89225(18) |
| 39.97965(19) | 40.83634(17) | 40.5389(4) |
| 96.4337(4) | 95.9061(3) | 95.9164(7) |
| 39564.3(2) | 39902.31(19) | 39613.5(4) |
| 4 | 4 | 4 |
| 1.395 | 1.446 | 1.441 |
| 0.398 | 0.398 | 0.401 |
| Synchrotron (λ = 0.6889) | Synchrotron (λ = 0.6889) | Synchrotron (λ = 0.6889) |
| 344542 | 346719 | 337691 |
| 62978/6651/2517 | 63534/7141/2727 | 63039/6014/2426 |
| R1 = 0.0719, wR2 = 0.2392 | R1 = 0.0827, wR2 = 0.2704 | R1 = 0.0853, wR2 = 0.2817 |
| R1 = 0.0971, wR2 = 0.2596 | R1 = 0.1201, wR2 = 0.2981 | R1 = 0.1351, wR2 = 0.3169 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, C.G.P.; Train, J.S.; Ward, M.D. Interactions of Small-Molecule Guests with Interior and Exterior Surfaces of a Coordination Cage Host. Chemistry 2020, 2, 510-524. https://doi.org/10.3390/chemistry2020031
Taylor CGP, Train JS, Ward MD. Interactions of Small-Molecule Guests with Interior and Exterior Surfaces of a Coordination Cage Host. Chemistry. 2020; 2(2):510-524. https://doi.org/10.3390/chemistry2020031
Chicago/Turabian StyleTaylor, Christopher G. P., Jennifer S. Train, and Michael D. Ward. 2020. "Interactions of Small-Molecule Guests with Interior and Exterior Surfaces of a Coordination Cage Host" Chemistry 2, no. 2: 510-524. https://doi.org/10.3390/chemistry2020031
APA StyleTaylor, C. G. P., Train, J. S., & Ward, M. D. (2020). Interactions of Small-Molecule Guests with Interior and Exterior Surfaces of a Coordination Cage Host. Chemistry, 2(2), 510-524. https://doi.org/10.3390/chemistry2020031







