Giant Polymer Compartments for Confined Reactions
Abstract
:1. Introduction
2. Polymers as Building Blocks of Micrometer-Sized Compartments
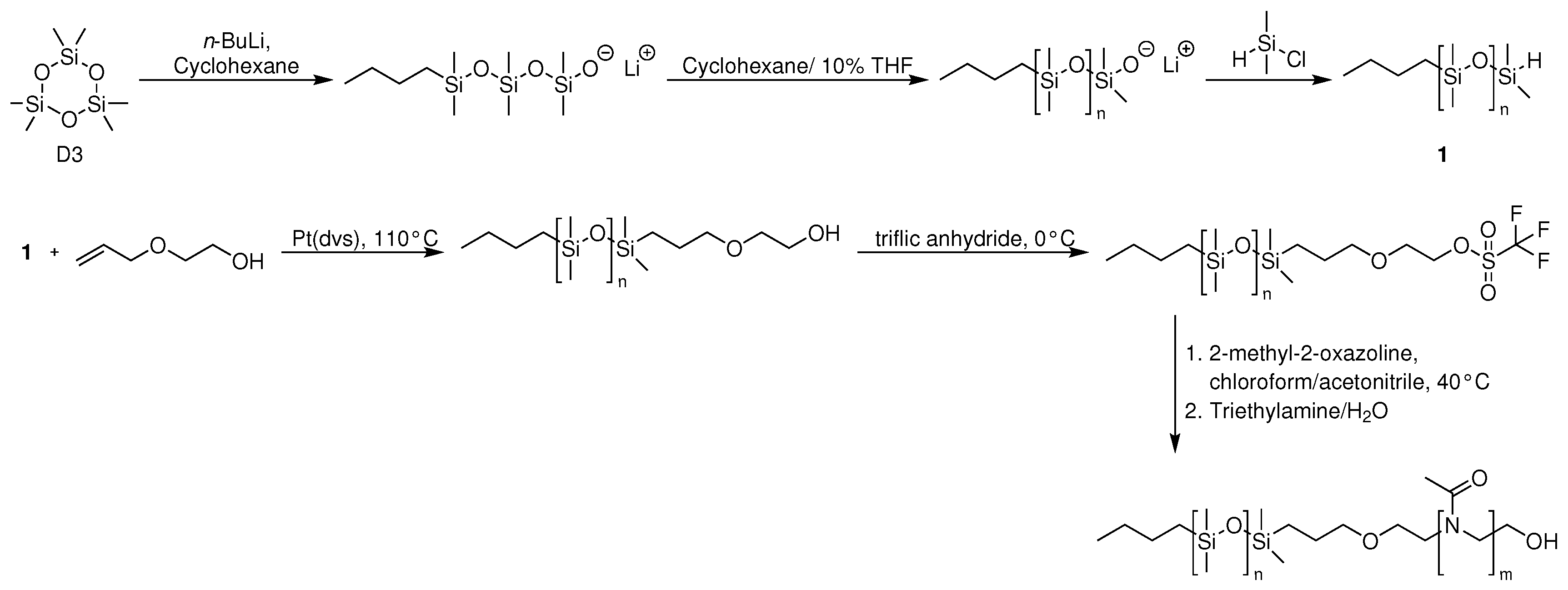
2.1. Amphiphilic Block Copolymers as Building Blocks for Generation of GUVs
2.2. Polymers as Building Blocks for Generation of Polymer Capsules
3. Technologies for Engineering Polymer Single and Multicompartments in Combination with Biomolecules
3.1. Polymer GUVs
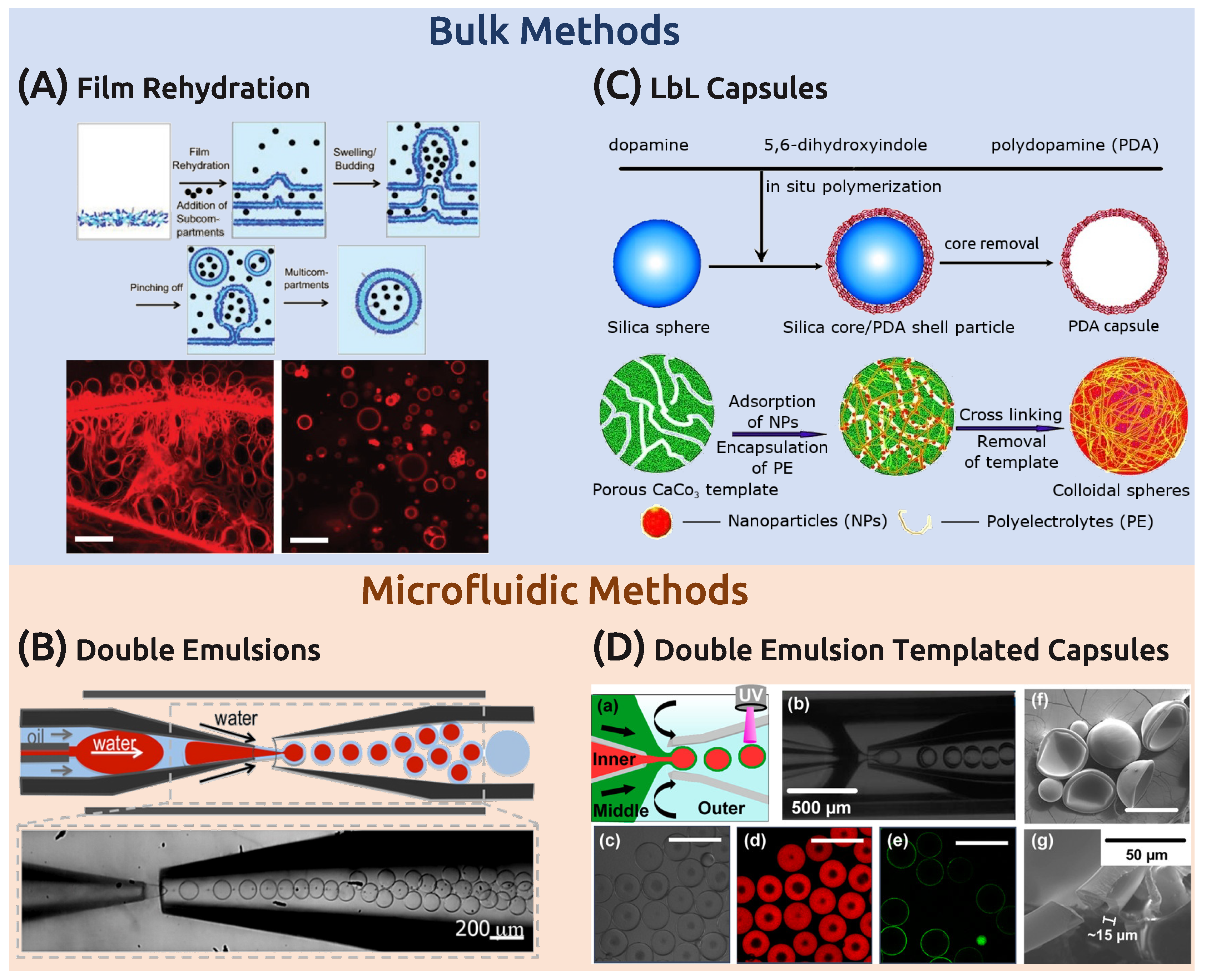
3.1.1. Bulk Techniques
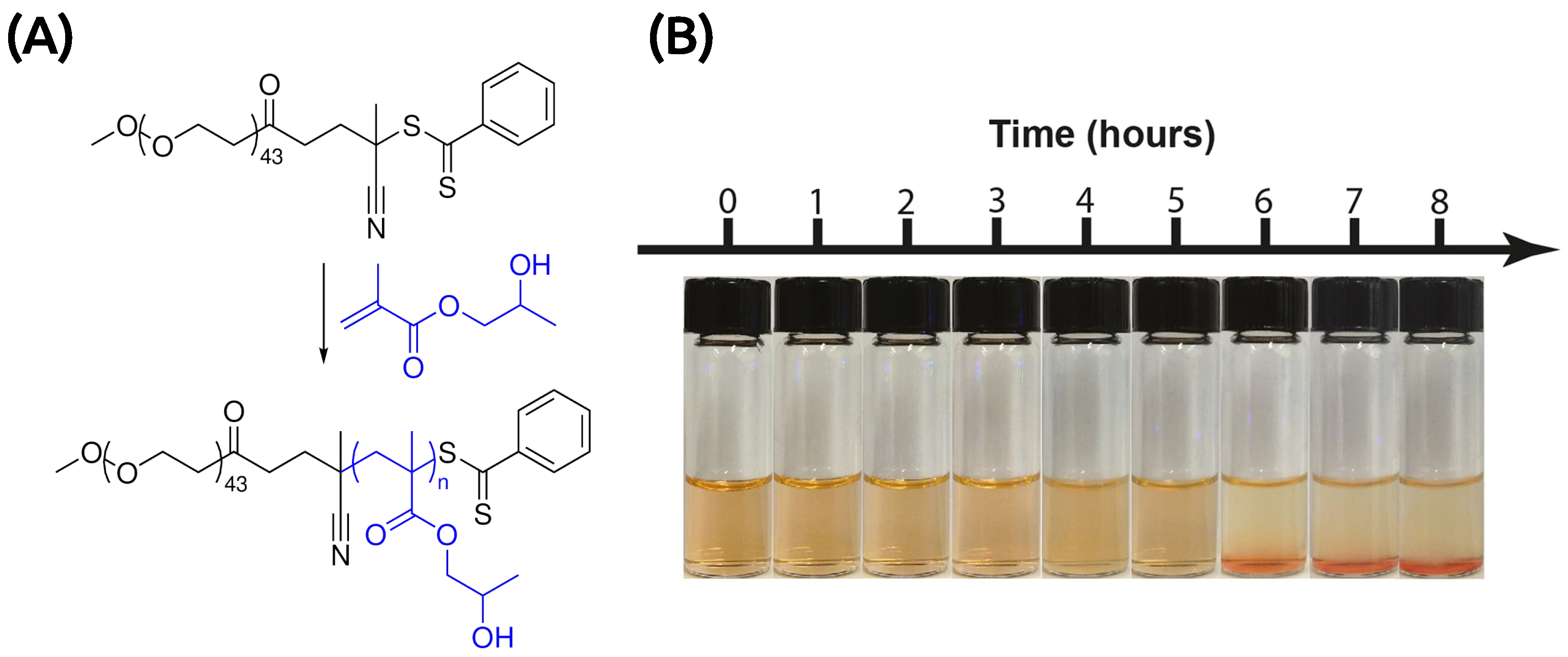
3.1.2. Microfluidics
3.2. Polymer Capsules
3.3. Building Multicompartments
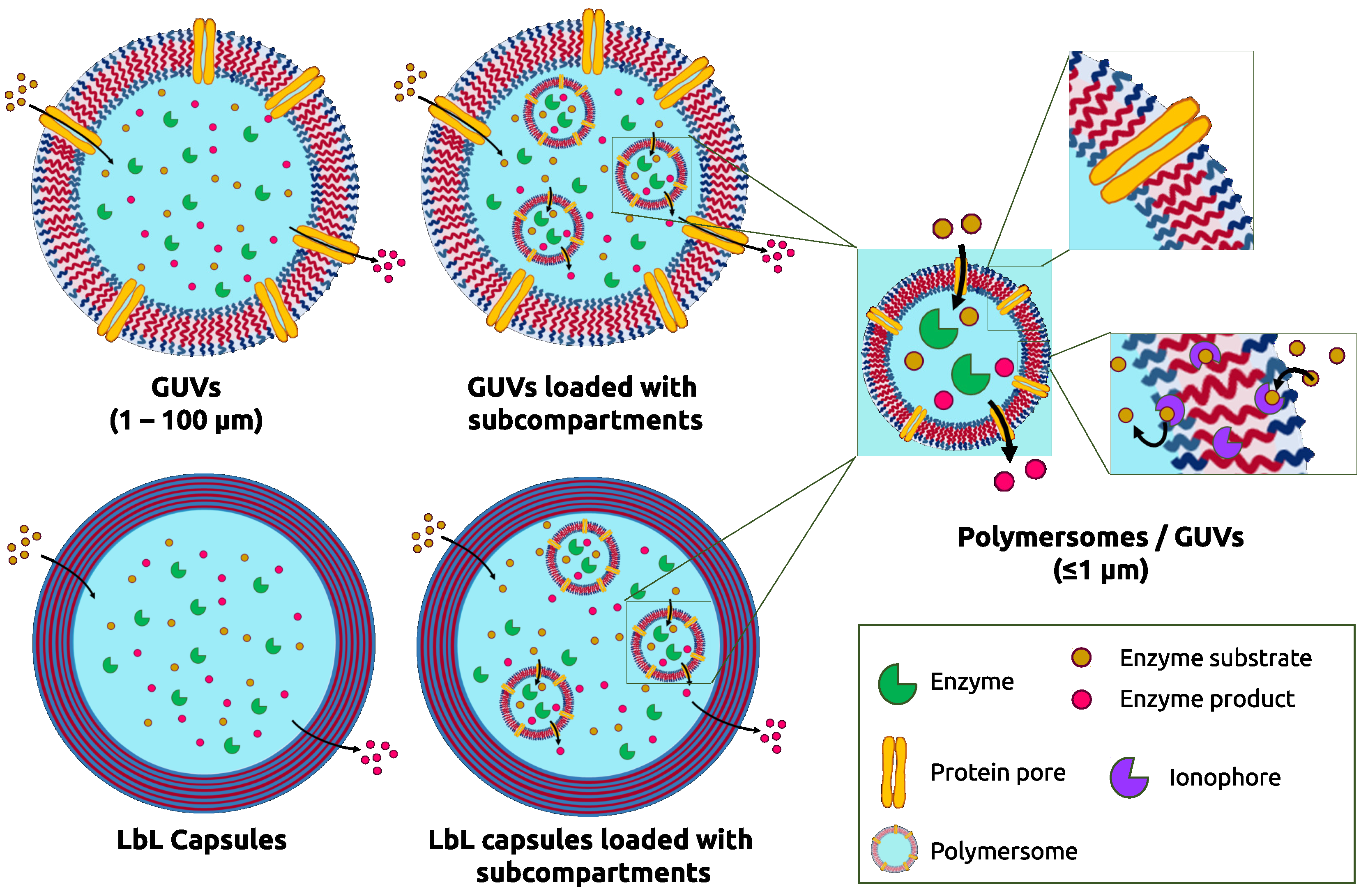
3.3.1. Loading Polymeric GUVS with Subcompartments
3.3.2. Layer-by-Layer Multicompartments
4. Vesicular Compartments for In Situ Reactions
4.1. Reactions inside Single Compartments
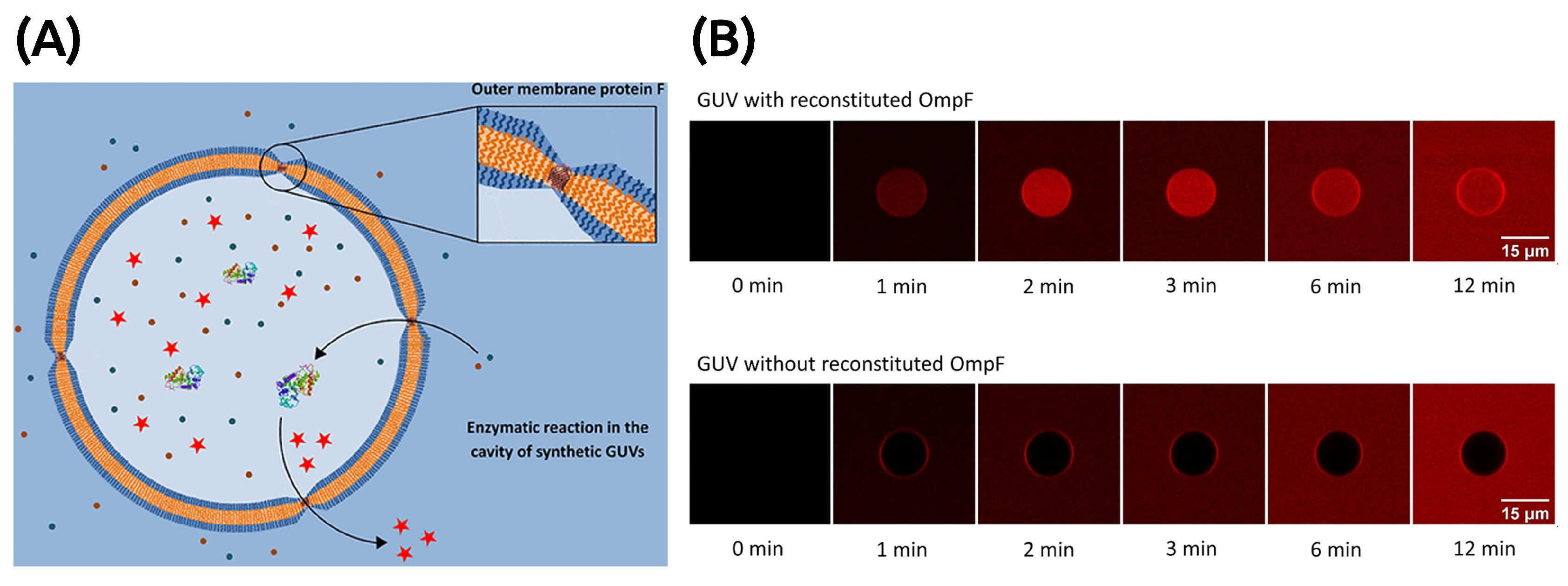
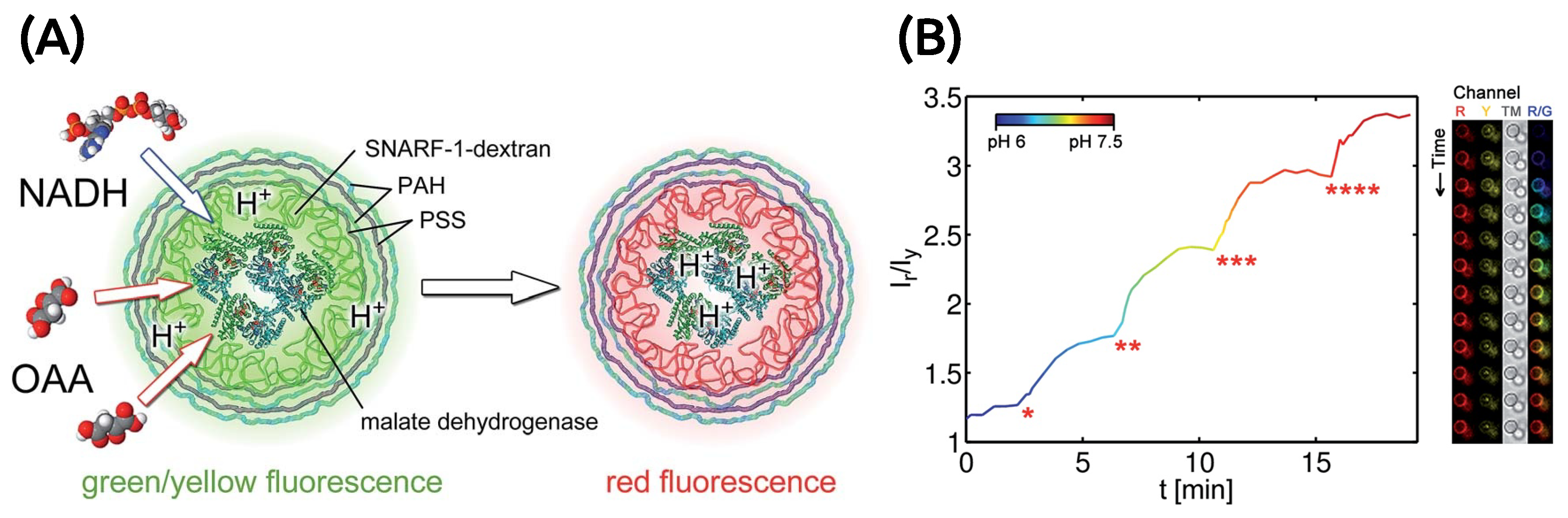
4.1.1. GUVs
4.1.2. Layer-by-Layer Microcapsules
4.2. Reactions inside Multicompartmentalized Structures
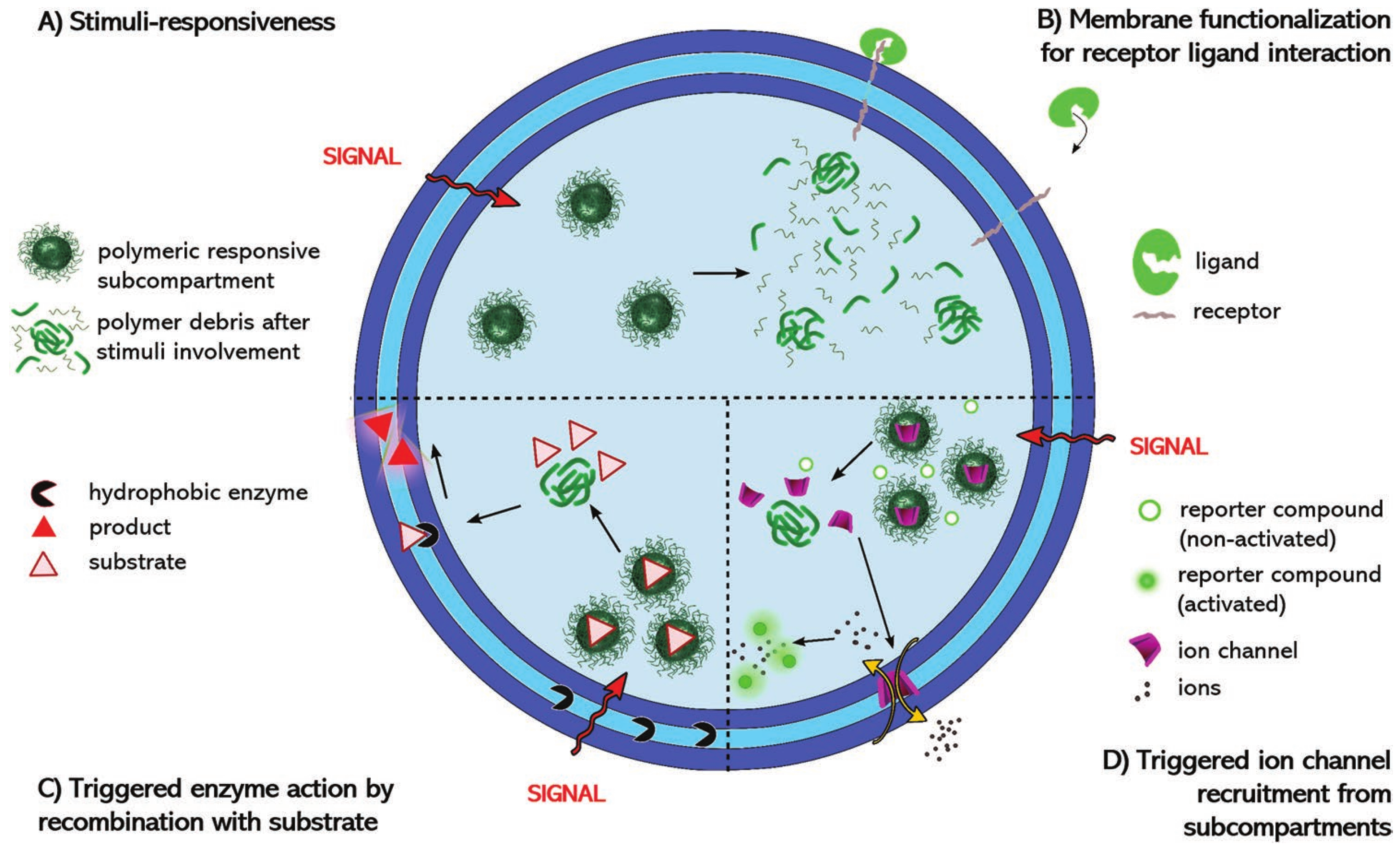
4.2.1. GUV Multicompartments
4.2.2. Layer-by-Layer Polymer Capsules as Multicompartments
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATRP | Atom transfer radical polymerization |
| CRP | Controlled radical polymerization |
| CTA | Chain transfer agent |
| CuAAC | Copper-catalyzed azide-alkyne cycloaddition |
| Cy5-IgG | Cyanine-5 conjugated immunoglobulin G proteins |
| DNA | Deoxyribonucleic acid |
| DPPC | Dipalmitoylphosphatidylcholine |
| E. coli | Escherichia coli |
| FITC | Fluorescein isothiocyanate |
| gA | Gramicidine |
| GFP | Green fluorescent protein |
| GOx | Glucose oxidase |
| GUV | Giant unilamellar vesicle |
| HRP | Horseradish peroxidase |
| ITO | Indium tin oxide |
| LbL | Layer-by-layer |
| MOXA | 2-methyl-2-oxazoline |
| NAD | Nicotinamide adenine dinucleotide |
| NADH | Nicotinamide adenine dinucleotide (reduced) |
| n-BuLi | n-butyllithium |
| NR | Nanorod |
| OAA | Oxaloacetic acid |
| OmpF | Outer membrane protein F |
| OmpF-M | Outer membrane protein double mutant |
| PA444 | Poly(4”-acryloyloxybutyl 2,5-bis(4’-butyloxybenzoyloxy)benzoate) |
| PAA | Poly(acrylic acid) |
| PAH | Poly(allylamine hydrochloride) |
| PBD | Polybutadiene |
| PDA | Polydopamine |
| PDEAEMA | Poly(2-(diethylamino)ethyl methacrylate) |
| PDMS | Poly(dimethyl sulfoxide) |
| PDPA | Poly(2-(diisopropylamino)-ethyl methacrylate) |
| PEE | Poly(ethyl ethylene) |
| PEG/PEO | Poly(ethylene glycol)/poly(ethylene oxide) |
| PEGDA | Poly(ethylene glycol) diacrylate |
| PFPA | Poly(pentafluorophenyl acrylate) |
| PGA | Poly(L-glutamic acid) |
| PHPMA | Poly(2-hydroxypropyl methacrylate) |
| PIAT | Poly(L-isocyanoalanine(2-thiophen-3-yl-ethyl)amide) |
| PISA | Polymerization induced self-assembly |
| PLA | Poly(lactic acid) |
| PMA | Poly(methyl acrylate) |
| PMAA | Poly(methacrylic acid) |
| PMOXA | Poly(2-methyl-2-oxazoline) |
| PnBA | Poly(n-butyl acrylate) |
| PNIPAM | Poly(N-isopropylacrylamide) |
| POEGMA | Poly(oligo(ethylene glycol) methacrylate) |
| PPS | Poly(propylene sulfide) |
| PS | Polystyrene |
| PSBA | Poly(styrene boronic acid) |
| PSS | Poly(styrene sulfonate) |
| PtBA | Poly(tert-butyl acrylate) |
| PTMC | Poly(trimethylene carbonate) |
| PVP | Polyvinylpyrrolidone |
| RAFT | Reversible addition fragmentation chain transfer |
| SNARF-1 | Seminaphtharhodafluor |
| TA | Tannic acid |
| THF | Tetrahydrofuran |
References
- Chen, A.H.; Silver, P.A. Designing biological compartmentalization. Trends Cell Biol. 2012, 22, 662–670. [Google Scholar] [CrossRef]
- Palivan, C.G.; Fischer-Onaca, O.; Delcea, M.; Itel, F.; Meier, W. Protein–polymer nanoreactors for medical applications. Chem. Soc. Rev. 2012, 41, 2800–2823. [Google Scholar] [CrossRef]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S. Vesicles and Liposomes: A Self-Assembly Principle Beyond Lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Messager, L.; Gaitzsch, J.; Chierico, L.; Battaglia, G. Novel aspects of encapsulation and delivery using polymersomes. Curr. Opin. Pharmacol. 2014, 18, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Rigo, S.; Gunkel-Grabole, G.; Meier, W.; Palivan, C.G. Surfaces with Dual Functionality through Specific Coimmobilization of Self-Assembled Polymeric Nanostructures. Langmuir 2019, 35, 4557–4565. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Rigo, S.; Di Leone, S.; Belluati, A.; Constable, E.C.; Housecroft, C.E.; Palivan, C.G. Brushing the surface: Cascade reactions between immobilized nanoreactors. Nanoscale 2020, 12, 1551–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulygin, O.; Price, A.D.; Chong, S.F.; Staedler, B.; Zelikin, A.N.; Caruso, F. Subcompartmentalized Polymer Hydrogel Capsules withSelectively Degradable Carriers and Subunits. Small 2010, 6, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Lomas, H.; Johnston, A.P.R.; Such, G.K.; Zhu, Z.; Liang, K.; Koeverden, M.P.V.; Alongkornchotikul, S.; Caruso, F. Polymersome-Loaded Capsules for Controlled Release of DNA. Small 2011, 7, 2109–2119. [Google Scholar] [CrossRef] [Green Version]
- Stadler, B.; Chandrawati, R.; Price, A.D.; Chong, S.F.; Breheney, K.; Postma, A.; Connal, L.A.; Zelikin, A.N.; Caruso, F. A Microreactor with Thousands of Subcompartments: Enzyme-Loaded Liposomes within Polymer Capsules. Angew. Chem. 2009, 48, 4359–4362. [Google Scholar] [CrossRef]
- Tanner, P.; Balasubramanian, V.; Palivan, C.G. Aiding nature’s organelles: Artificial peroxisomes play their role. Nano Lett. 2013, 13, 2875–2883. [Google Scholar] [CrossRef]
- Thamboo, S.; Najer, A.; Belluati, A.; von Planta, C.; Wu, D.; Craciun, I.; Meier, W.; Palivan, C.G. Mimicking Cellular Signaling Pathways within Synthetic Multicompartment Vesicles with Triggered Enzyme Activity and Induced Ion Channel Recruitment. Adv. Funct. Mater. 2019, 29, 1904267. [Google Scholar] [CrossRef]
- Peters, R.J.R.W.; Marguet, M.; Marais, S.; Fraaije, M.W.; van Hest, J.C.M.; Lecommandoux, S. Cascade Reactions in Multicompartmentalized Polymersomes. Angew. Chem. Int. Ed. 2014, 53, 146–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itel, F.; Chami, M.; Najer, A.; Lorcher, S.; Wu, D.L.; Dinu, I.A.; Meier, W. Molecular Organization and Dynamics in Polymersome Membranes: A Lateral Diffusion Study. Macromolecules 2014, 47, 7588–7596. [Google Scholar] [CrossRef]
- Belluati, A.; Mikhalevich, V.; Yorulmaz Avsar, S.; Daubian, D.; Craciun, I.; Chami, M.; Meier, W.P.; Palivan, C.G. How Do the Properties of Amphiphilic Polymer Membranes Influence the Functional Insertion of Peptide Pores? Biomacromolecules 2020, 21, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Lomora, M.; Garni, M.; Itel, F.; Tanner, P.; Spulber, M.; Palivan, C.G. Polymersomes with engineered ion selective permeability as stimuli-responsive nanocompartments with preserved architecture. Biomaterials 2015, 53, 406–414. [Google Scholar] [CrossRef]
- Baumann, P.; Spulber, M.; Fischer, O.; Car, A.; Meier, W. Investigation of Horseradish Peroxidase Kinetics in an “Organelle-Like” Environment. Small 2017, 13, 1603943. [Google Scholar] [CrossRef]
- Garni, M.; Einfalt, T.; Goers, R.; Palivan, C.G.; Meier, W. Live Follow-Up of Enzymatic Reactions Inside the Cavities of Synthetic Giant Unilamellar Vesicles Equipped with Membrane Proteins Mimicking Cell Architecture. ACS Synth. Biol. 2018, 7, 2116–2125. [Google Scholar] [CrossRef]
- Garni, M.; Thamboo, S.; Schoenenberger, C.A.; Palivan, C.G. Biopores/membrane proteins in synthetic polymer membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 619–638. [Google Scholar] [CrossRef]
- Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.A.; Meier, W.P.; Palivan, C.G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-polymer Platforms Into Biomimetic Interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom Transfer Radical Polymerization: Billion Times More Active Catalysts and New Initiation Systems. Macromol. Rapid Commun. 2019, 40, 1800616. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H. Synthesis of Block Copolymers. In Block Copolymers I; Abetz, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–124. [Google Scholar]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster, S.; Krämer, E. Synthesis of PB-PEO and PI-PEO Block Copolymers with Alkyllithium Initiators and the Phosphazene Base t-BuP4. Macromolecules 1999, 32, 2783–2785. [Google Scholar] [CrossRef]
- Nuss, H.; Chevallard, C.; Guenoun, P.; Malloggi, F. Microfluidic trap-and-release system for lab-on-a-chip-based studies on giant vesicles. Lab Chip 2012, 12, 5257–5261. [Google Scholar] [CrossRef] [PubMed]
- Christian, D.A.; Tian, A.; Ellenbroek, W.G.; Levental, I.; Rajagopal, K.; Janmey, P.A.; Liu, A.J.; Baumgart, T.; Discher, D.E. Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Nat. Mater. 2009, 8, 843–849. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Bates, F.S. Synthesis and Characterization of Model Polyalkane-Poly(ethylene oxide) Block Copolymers. Macromolecules 1996, 29, 6994–7002. [Google Scholar] [CrossRef]
- Wu, D.; Spulber, M.; Itel, F.; Chami, M.; Pfohl, T.; Palivan, C.G.; Meier, W. Effect of Molecular Parameters on the Architecture and Membrane Properties of 3D Assemblies of Amphiphilic Copolymers. Macromolecules 2014, 47, 5060–5069. [Google Scholar] [CrossRef]
- Mabrouk, E.; Cuvelier, D.; Pontani, L.L.; Xu, B.; Lévy, D.; Keller, P.; Brochard-Wyart, F.; Nassoy, P.; Li, M.H. Formation and material properties of giant liquid crystal polymersomes. Soft Matter 2009, 5, 1870–1878. [Google Scholar] [CrossRef]
- Kubilis, A.; Abdulkarim, A.; Eissa, A.M.; Cameron, N.R. Giant Polymersome Protocells Dock with Virus Particle Mimics via Multivalent Glycan-Lectin Interactions. Sci. Rep. 2016, 6, 32414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertsen, A.N.; Szymański, J.K.; Pérez-Mercader, J. Emergent Properties of Giant Vesicles Formed by a Polymerization-Induced Self-Assembly (PISA) Reaction. Sci. Rep. 2017, 7, 41534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeuwissen, S.A.; Bruekers, S.M.C.; Chen, Y.; Pochan, D.J.; van Hest, J.C.M. Spontaneous shape changes in polymersomes via polymer/polymer segregation. Polym. Chem. 2014, 5, 489–501. [Google Scholar] [CrossRef]
- Fauquignon, M.; Ibarboure, E.; Carlotti, S.; Brûlet, A.; Schmutz, M.; Le Meins, J.F. Large and Giant Unilamellar Vesicle(s) Obtained by Self-Assembly of Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers, Membrane Properties and Preliminary Investigation of Their Ability to Form Hybrid Polymer/Lipid Vesicles. Polymers 2019, 11, 2013. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V.; Agrahari, V. Advances and applications of block-copolymer-based nanoformulations. Drug Discov. Today 2018, 23, 1139–1151. [Google Scholar] [CrossRef]
- Qi, Y.; Li, B.; Wang, Y.; Huang, Y. Synthesis and sequence-controlled self-assembly of amphiphilic triblock copolymers based on functional poly(ethylene glycol). Polym. Chem. 2017, 8, 6964–6971. [Google Scholar] [CrossRef]
- Discher, D.E.; Ahmed, F. POLYMERSOMES. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef]
- Garni, M.; Wehr, R.; Avsar, S.Y.; John, C.; Palivan, C.; Meier, W. Polymer membranes as templates for bio-applications ranging from artificial cells to active surfaces. Eur. Polym. J. 2019, 112, 346–364. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Cui, J.; Van Koeverden, M.P.; Müllner, M.; Kempe, K.; Caruso, F. Emerging methods for the fabrication of polymer capsules. Adv. Colloid Interface Sci. 2014, 207, 14–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the layer-by-layer assembly of multilayered polymer composites: Strategy, structural control and applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-By-Layer-Assembled Capsules and Films for Therapeutic Delivery. Small 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, G.B.; Antipov, A.A.; Voigt, A.; Donath, E.; Möhwald, H. pH-Controlled Macromolecule Encapsulation in and Release from Polyelectrolyte Multilayer Nanocapsules. Macromol. Rapid Commun. 2001, 22, 44–46. [Google Scholar] [CrossRef]
- Georgieva, R.; Moya, S.; Hin, M.; Mitlöhner, R.; Donath, E.; Kiesewetter, H.; Möhwald, H.; Bäumler, H. Permeation of Macromolecules into Polyelectrolyte Microcapsules. Biomacromolecules 2002, 3, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. Chembiochem 2010, 11, 848–865. [Google Scholar] [CrossRef]
- Lim, S.; de Hoog, H.P.; Parikh, A.; Nallani, M.; Liedberg, B. Hybrid, Nanoscale Phospholipid/Block Copolymer Vesicles. Polymers 2013, 5, 1102–1114. [Google Scholar] [CrossRef] [Green Version]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of Unilamellar Vesicles Using an Inverted Emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Itel, F.; Najer, A.; Palivan, C.G.; Meier, W. Dynamics of Membrane Proteins within Synthetic Polymer Membranes with Large Hydrophobic Mismatch. Nano Lett. 2015, 15, 3871–3878. [Google Scholar] [CrossRef]
- Lomora, M.; Itel, F.; Dinu, I.A.; Palivan, C.G. Selective ion-permeable membranes by insertion of biopores into polymersomes. Phys. Chem. Chem. Phys. 2015, 17, 15538–15546. [Google Scholar] [CrossRef] [Green Version]
- Picker, A.; Nuss, H.; Guenoun, P.; Chevallard, C. Polymer vesicles as microreactors for bioinspired calcium carbonate precipitation. Langmuir 2011, 27, 3213–3218. [Google Scholar] [CrossRef] [PubMed]
- Kita-Tokarczyk, K.; Grumelard, J.; Haefele, T.; Meier, W. Block copolymer vesicles—Using concepts from polymer chemistry to mimic biomembranes. Polymer 2005, 46, 3540–3563. [Google Scholar] [CrossRef] [Green Version]
- Fetsch, C.; Gaitzsch, J.; Messager, L.; Battaglia, G.; Luxenhofer, R. Self-Assembly of Amphiphilic Block Copolypeptoids – Micelles, Worms and Polymersomes. Sci. Rep. 2016, 6, 33491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, M.E.; Prud’homme, R.K.; Robb, I.; Adamson, D.H. Formation and characterization of polymersomes made by a solvent injection method. Polym. Adv. Technol. 2007, 18, 427–432. [Google Scholar] [CrossRef]
- Daubian, D.; Gaitzsch, J.; Meier, W. Synthesis and complex self-assembly of amphiphilic block copolymers with a branched hydrophobic poly(2-oxazoline) into multicompartment micelles, pseudo-vesicles and yolk/shell nanoparticles. Polym. Chem. 2020, 11, 1237–1248. [Google Scholar] [CrossRef] [Green Version]
- Dionzou, M.; Morère, A.; Roux, C.; Lonetti, B.; Marty, J.D.; Mingotaud, C.; Joseph, P.; Goudounèche, D.; Payré, B.; Léonetti, M.; et al. Comparison of methods for the fabrication and the characterization of polymer self-assemblies: What are the important parameters? Soft Matter 2016, 12, 2166–2176. [Google Scholar] [CrossRef]
- Pearson, R.T.; Warren, N.J.; Lewis, A.L.; Armes, S.P.; Battaglia, G. Effect of pH and Temperature on PMPC–PDPA Copolymer Self-Assembly. Macromolecules 2013, 46, 1400–1407. [Google Scholar] [CrossRef]
- Fernyhough, C.; Ryana, A.J.; Battaglia, G. pH controlled assembly of a polybutadiene–poly(methacrylic acid) copolymer in water: Packing considerations and kinetic limitations. Soft Matter 2009, 5, 1674–1682. [Google Scholar] [CrossRef]
- Lorenceau, E.; Utada, A.S.; Link, D.R.; Cristobal, G.; Joanicot, M.; Weitz, D.A. Generation of Polymerosomes from Double-Emulsions. Langmuir 2005, 21, 9183–9186. [Google Scholar] [CrossRef]
- Shum, H.C.; Lee, D.; Yoon, I.; Kodger, T.; Weitz, D.A. Double Emulsion Templated Monodisperse Phospholipid Vesicles. Langmuir 2008, 24, 7651–7653. [Google Scholar] [CrossRef]
- Shum, H.C.; Kim, J.W.; Weitz, D.A. Microfluidic Fabrication of Monodisperse Biocompatible and Biodegradable Polymersomes with Controlled Permeability. J. Am. Chem. Soc. 2008, 130, 9543–9549. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Caspi, Y.; Meijering, A.E.C.; Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, D.F.; Arriaga, L.R.; Eggersdorfer, M.; Ziblat, R.; Marques, M.d.F.V.; Reynaud, F.; Koehler, S.A.; Weitz, D.A. Microfluidic Fabrication of Pluronic Vesicles with Controlled Permeability. Langmuir 2016, 32, 5350–5355. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Kim, S.; Horsfall, L.; Abbaspourrad, A.; Rosser, S.J.; David, A.; Weitz, J.C. Protein Expression, Aggregation, and Triggered Release from Polymersomes as Artificial Cell-like Structures. Angew. Chem. 2012, 51, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, J.; Möhwald, H. Templating Assembly of Multifunctional Hybrid Colloidal Spheres. Adv. Mater. 2012, 24, 2663–2667. [Google Scholar] [CrossRef]
- Postma, A.; Yan, Y.; Wang, Y.; Zelikin, A.N.; Tjipto, E.; Caruso, F. Self-Polymerization of Dopamine as a Versatile and Robust Technique to Prepare Polymer Capsules. Chem. Mater. 2009, 21, 3042–3044. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, W.; Abbaspourrad, A.; Ahn, J.; Bader, A.; Bose, S.; Vegas, A.; Lin, J.; Tao, J.; Hang, T.; et al. Microfluidic Fabrication of Colloidal Nanomaterials-Encapsulated Microcapsules for Biomolecular Sensing. Nano Lett. 2017, 17, 2015–2020. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lomora, M.; Sarasua, J.; Palivan, C.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: Current strategies to control membrane permeability. Prog. Mater. Sci. 2017, 90, 325–357. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Sukhorukov, G.B. Co-encapsulation of enzyme and sensitive dye as a tool for fabrication of microcapsule based sensor for urea measuring. Phys. Chem. Chem. Phys. 2011, 13, 11110–11117. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Anastasova, S.; Pavlov, A.M.; Vadgama, P.; Skirtach, A.G.; Sukhorukov, G.B. Chemosensors and biosensors based on polyelectrolyte microcapsules containing fluorescent dyes and enzymes. Anal. Bioanal. Chem. 2013, 405, 1559–1568. [Google Scholar] [CrossRef]
- Hosta-Rigau, L.; York-Duran, M.J.; Zhang, Y.; Goldie, K.N.; Städler, B. Confined Multiple Enzymatic (Cascade) Reactions within Poly(dopamine)-based Capsosomes. ACS Appl. Mater. Interfaces 2014, 6, 12771–12779. [Google Scholar] [CrossRef]
- Chen, H.; Man, J.; Li, Z.; Li, J. Microfluidic Generation of High-Viscosity Droplets by Surface-Controlled Breakup of Segment Flow. ACS Appl. Mater. Interfaces 2017, 9, 21059–21064. [Google Scholar] [CrossRef] [PubMed]
- Kisak, E.T.; Coldren, B.; Zasadzinski, J.A. Nanocompartments Enclosing Vesicles, Colloids, and Macromolecules via Interdigitated Lipid Bilayers. Langmuir 2002, 18, 284–288. [Google Scholar] [CrossRef]
- Fu, Z.; Ochsner, M.A.; de Hoog, H.P.M.; Tomczak, N.; Nallani, M. Multicompartmentalized polymersomes for selective encapsulation of biomacromolecules. Chem. Commun. 2011, 47, 2862–2864. [Google Scholar] [CrossRef] [PubMed]
- Marguet, M.; Edembe, L.; Lecommandoux, S. Polymersomes in Polymersomes: Multiple Loading and Permeability Control. Angew. Chem. Int. Ed. 2012, 51, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shum, H.C.; Kim, J.W.; Cho, J.C.; Weitz, D.A. Multiple Polymersomes for Programmed Release of Multiple Components. J. Am. Chem. Soc. 2011, 133, 15165–15171. [Google Scholar] [CrossRef] [PubMed]
- Kreft, O.; Prevot, M.; Möhwald, H.; Sukhorukov, G.B. Shell-in-Shell Microcapsules: A Novel Tool for Integrated, Spatially Confined Enzymatic Reactions. Angew. Chem. 2007, 46, 5605–5608. [Google Scholar] [CrossRef]
- Hosta-Rigau, L.; Chung, S.F.; Postma, A.; Chandrawati, R.; Caruso, B.S.F. Capsosomes with “Free-Floating” Liposomal Subcompartments. Angew. Chem. 2011, 23, 4082–4087. [Google Scholar] [CrossRef]
- Schattling, P.; Dreier, C.; Städler, B. Janus subcompartmentalized microreactors. Soft Matter 2015, 11, 5327–5335. [Google Scholar] [CrossRef] [Green Version]
- Küchler, A.; Yoshimoto, M.; Luginbühl, S.; Mavelli, F.; Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 2016, 11, 409. [Google Scholar] [CrossRef]
- Jeong, S.; Nguyen, H.T.; Kim, C.H.; Ly, M.N.; Shin, K. Toward Artificial Cells: Novel Advances in Energy Conversion and Cellular Motility. Adv. Funct. Mater. 2020, 30, 1907182. [Google Scholar] [CrossRef]
- Sauer, M.; Haefele, T.; Graff, A.; Nardin, C.; Meier, W. Ion-carrier controlled precipitation of calcium phosphate in giant ABA triblock copolymer vesicles. Chem. Commun. 2001, 2452–2453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harimech, P.K.; Hartmann, R.; Rejman, J.; del Pino, P.; Rivera-Gil, P.; Parak, W.J. Encapsulated enzymes with integrated fluorescence-control of enzymatic activity. J. Mater. Chem. B 2015, 3, 2801–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.; Wang, Y.; Zhu, H.; Zhou, L. Formulation of robust organic–inorganic hybrid magnetic microcapsules through hard-template mediated method for efficient enzyme immobilization. J. Mater. Chem. B 2015, 3, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Karamitros, C.S.; Yashchenok, A.M.; Möhwald, H.; Skirtach, A.G.; Konrad, M. Preserving Catalytic Activity and Enhancing Biochemical Stability of the Therapeutic Enzyme Asparaginase by Biocompatible Multilayered Polyelectrolyte Microcapsules. Biomacromolecules 2013, 14, 4398–4406. [Google Scholar] [CrossRef] [Green Version]
- Einfalt, T.; Witzigmann, D.; Edlinger, C.; Sieber, S.; Goers, R.; Najer, A.; Spulber, M.; Onaca-Fischer, O.; Huwyler, J.; Palivan, C.G. Biomimetic artificial organelles with in vitro and in vivo activity triggered by reduction in microenvironment. Nat. Commun. 2018, 9, 1127. [Google Scholar] [CrossRef] [Green Version]
- Marguet, M.; Bonduelle, C.; Lecommandoux, S. Multicompartmentalized polymeric systems: Towards biomimetic cellular structure and function. Chem. Soc. Rev. 2013, 42, 512–529. [Google Scholar] [CrossRef]
- Peyret, A.; Ibarboure, E.; Tron, A.; Beauté, L.; Rust, R.; Sandre, O.; McClenaghan, N.D.; Lecommandoux, S. Polymersome Popping by Light-Induced Osmotic Shock under Temporal, Spatial, and Spectral Control. Angew. Chem. Int. Ed. 2017, 56, 1566–1570. [Google Scholar] [CrossRef]
- Delcea, M.; Yashchenok, A.; Videnova, K.; Kreft, O.; Möhwald, H.; Skirtach, A.G. Multicompartmental micro-and nanocapsules: Hierarchy and applications in biosciences. Macromol. Biosci. 2010, 10, 465–474. [Google Scholar] [CrossRef]
- Xiong, R.; Soenen, S.J.; Braeckmans, K.; Skirtach, A.G. Towards theranostic multicompartment microcapsules: In-situ diagnostics and laser-induced treatment. Theranostics 2013, 3, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, E.C.; Angelini, A.; Hürlimann, D.; Meier, W.; Palivan, C.G. Giant Polymer Compartments for Confined Reactions. Chemistry 2020, 2, 470-489. https://doi.org/10.3390/chemistry2020028
dos Santos EC, Angelini A, Hürlimann D, Meier W, Palivan CG. Giant Polymer Compartments for Confined Reactions. Chemistry. 2020; 2(2):470-489. https://doi.org/10.3390/chemistry2020028
Chicago/Turabian Styledos Santos, Elena C., Alessandro Angelini, Dimitri Hürlimann, Wolfgang Meier, and Cornelia G. Palivan. 2020. "Giant Polymer Compartments for Confined Reactions" Chemistry 2, no. 2: 470-489. https://doi.org/10.3390/chemistry2020028
APA Styledos Santos, E. C., Angelini, A., Hürlimann, D., Meier, W., & Palivan, C. G. (2020). Giant Polymer Compartments for Confined Reactions. Chemistry, 2(2), 470-489. https://doi.org/10.3390/chemistry2020028





