Nanotheranostics in Periodontitis: Bridging Diagnosis and Therapy Through Smart Integrated Nanosystems
Abstract
1. Introduction
2. Pathophysiological Basis for Theranostic Targeting in Periodontitis
2.1. Overview of the Periodontium Architecture
2.2. Microbial and Molecular Mechanisms Involved in Periodontitis Pathogenesis
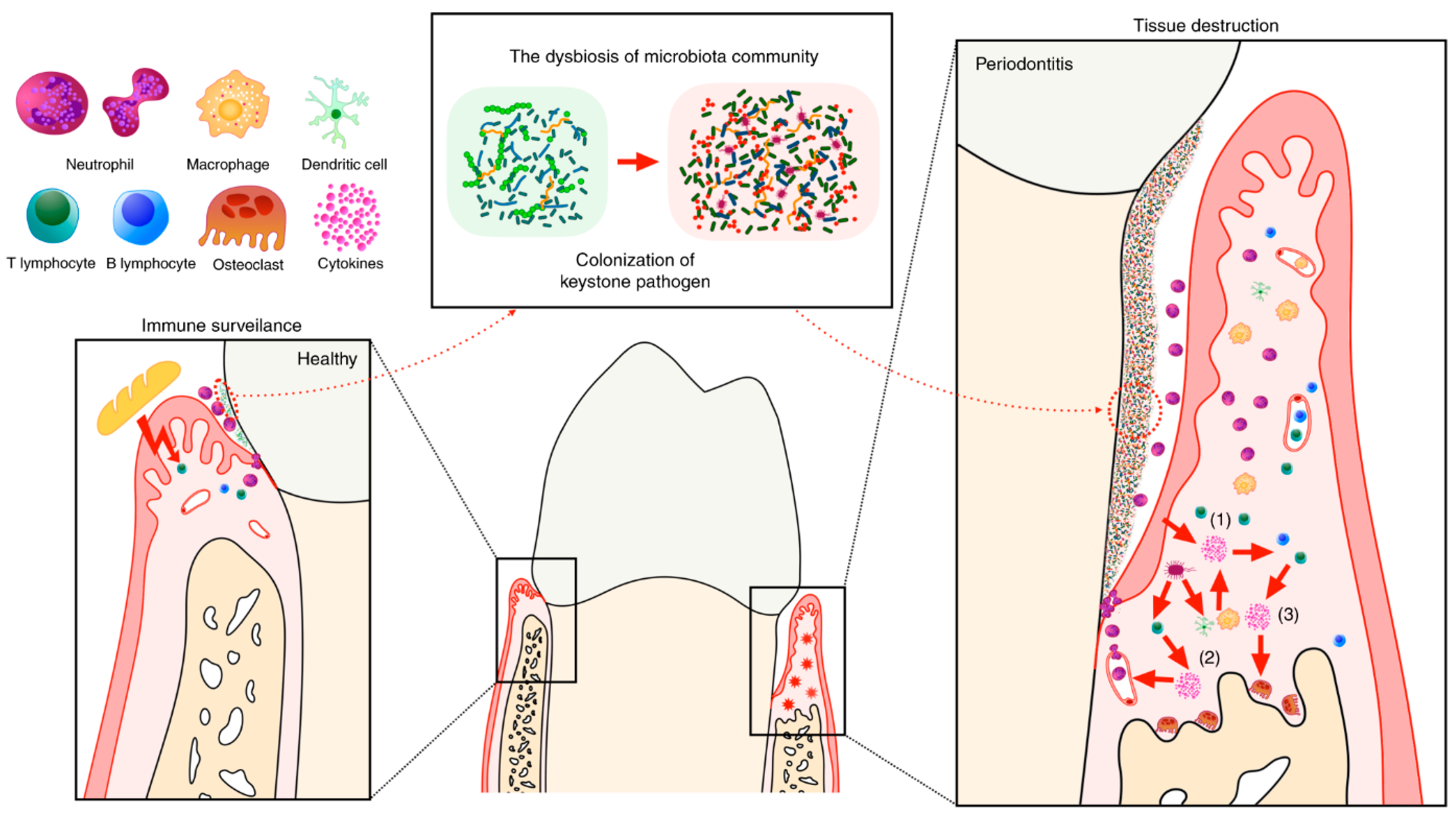
2.3. Potential Biomarkers as Diagnostic and Therapeutic Targets in Periodontitis
2.3.1. Salivary Fluid
2.3.2. Gingival Crevicular Fluid
2.3.3. Dental Plaque
2.3.4. Exhaled Breath Condensate
2.4. The Necessity for Dual Targeting of Chronic Inflammation and Microbial Invasion
3. Current Limitations in the Diagnosis and Treatment of Periodontitis
3.1. Diagnostic Limitations
3.2. Therapeutic Limitations
3.3. Challenges in Treatment Monitoring and Follow-Ups
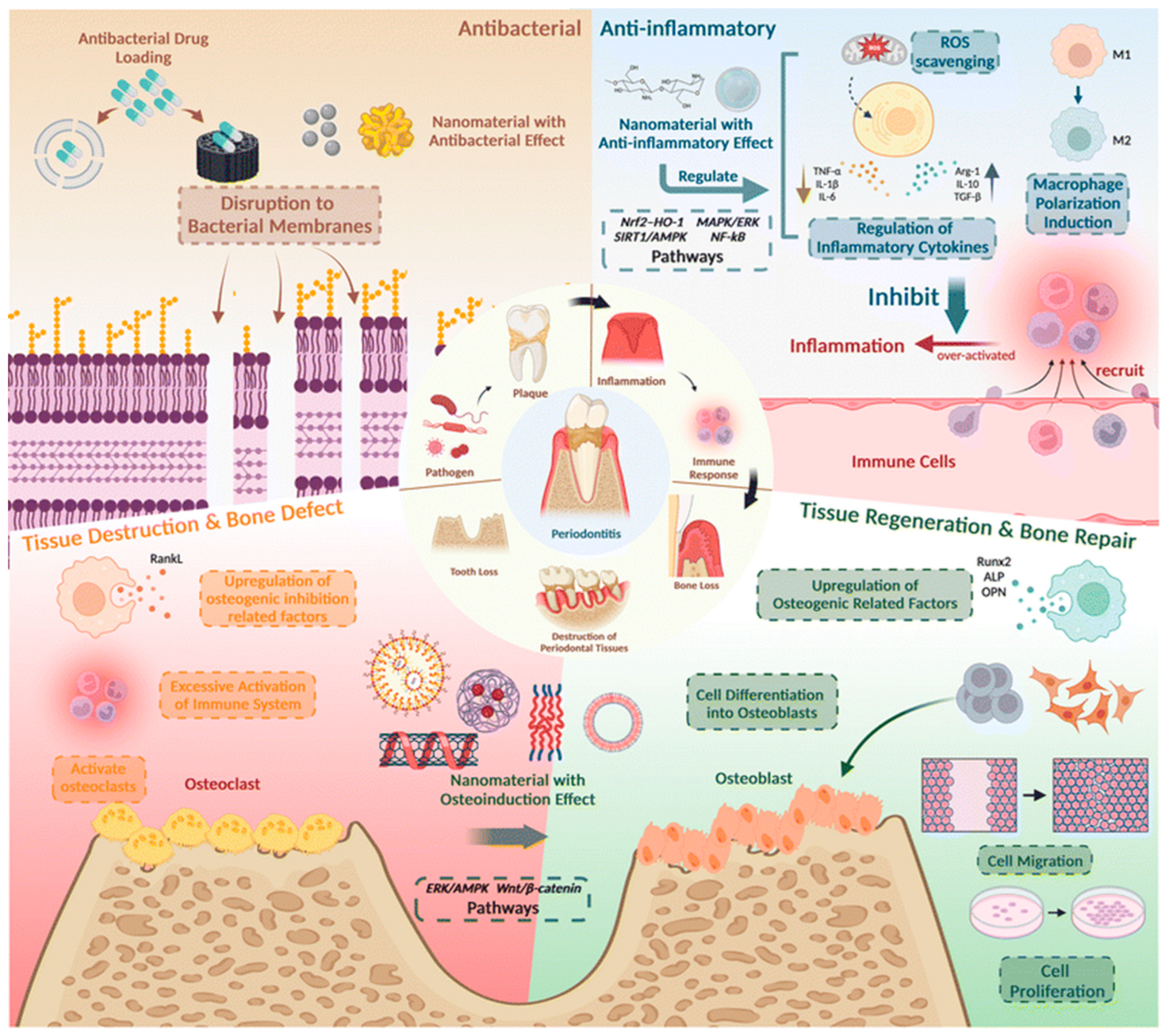
4. Nanomaterials for Periodontal Applications
4.1. Polymeric Nanomaterials
4.2. Lipid-Based Nanomaterials
4.3. Inorganic Nanomaterials
4.4. Carbon-Based Nanomaterials
4.5. Smart Nanocomposite Hybrid Systems for Periodontal Applications
5. Integrating Diagnostics and Therapeutics: A Promise for Nanotheranostics in Periodontitis
6. Translational and Clinical Considerations in Periodontitis Nanotheranostics
6.1. Design Complexity and Functional Compromises
6.2. Biological Complexity and In Vivo Relevance
6.3. Safety, Immunogenicity, and Long-Term Toxicity
6.4. Manufacturing, Scalability, and Quality Control
6.5. Regulatory and Ethical Considerations
6.6. Interdisciplinary Collaboration and Integration into Clinical Workflow
6.7. Cost, Accessibility, and Implementation Feasibility
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GCF | Gingival crevicular fluid |
| TLRs | Toll-like receptors |
| ROS | Reactive oxygen species |
| MMPs | Metalloproteinases |
| NETs | Neutrophil extracellular traps |
| RANKL | Receptor activator of NF-κB Ligand |
| EBC | Exhaled breath condensate |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| LDH | Lactate dehydrogenase |
| OPG | Osteoprotegerin |
| VOCs | Volatile organic compounds |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLA | Polylactic acid |
| PCL | Polycaprolactone |
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| SLNs | Solid lipid nanoparticles |
| NLCs | Nanostructured lipid carriers |
| LNCs | Lipid nanocapsules |
| MSNs | Mesoporous silica nanoparticles |
| ZIF-8 | Zeolitic imidazolate framework 8 |
| UCNPs | Upconversion nanoparticles |
| NPs | Nanoparticles |
| NIR | Near-infrared |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| GQDs | Graphene quantum dots |
| CDs | Carbon dots |
| MWCNTs | Multi-walled carbon nanotubes |
| CSHSs | Copper silicate nanozymes |
| QDs | Quantum dots |
References
- Wang, Y.; Zhuo, L.; Yang, S.; Dong, C.; Shu, P. Burden of Periodontal Diseases in Young Adults. Sci. Rep. 2025, 15, 6653. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Alves-Costa, S.; Romandini, M. Burden of Severe Periodontitis and Edentulism in 2021, with Projections up to 2050: The Global Burden of Disease 2021 Study. J. Periodontal Res. 2024, 59, 823–867. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N. Environmental Factors and Periodontal Microbiome. Periodontology 2021, 85, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontology 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Gasner, N.S.; Schure, R.S. Periodontal Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hooshiar, M.H.; Moghaddam, M.A.; Kiarashi, M.; Al-Hijazi, A.Y.; Hussein, A.F.; Alrikabi, H.; Salari, S.; Esmaelian, S.; Mesgari, H.; Yasamineh, S. Recent Advances in Nanomaterial-Based Biosensor for Periodontitis Detection. J. Biol. Eng. 2024, 18, 28. [Google Scholar] [CrossRef]
- Shi, R.; Zhu, Y.; Lu, W.; Zhai, R.; Zhou, M.; Shi, S.; Chen, Y. Nanomaterials: Innovative Approaches for Addressing Key Objectives in Periodontitis Treatment. RSC Adv. 2024, 14, 27904–27927. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Xiao, C.; Wang, H.; Dong, S. Nanoparticles in Periodontitis Therapy: A Review of the Current Situation. Int. J. Nanomed. 2024, 19, 6857–6893. [Google Scholar] [CrossRef]
- Sunila, B.S.; Shivakumar, G.C.; Abdul, N.S.; Sudhakar, N.; Franco, R.; Ronsivalle, V.; Cicciù, M.; Minervini, G. Conventional Periodontal Probing versus Salivary Biomarkers in Diagnosis and Evaluation of Chronic Periodontitis in Type 2 Diabetes Mellitus. Minerva Dent. Oral Sci. 2025, 74, 187–194. [Google Scholar] [CrossRef]
- Yussif, N.; Akarslan, Z. Pathogenesis of Periodontal Disease. In Periodontal Disease-Diagnostic and Adjunctive Non-Surgical Considerations; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-78984-460-3. [Google Scholar]
- Nanci, A.; Bosshardt, D.D. Structure of Periodontal Tissues in Health and Disease*. Periodontology 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Omi, M.; Mishina, Y. Roles of Osteoclasts in Alveolar Bone Remodeling. Genesis 2022, 60, e23490. [Google Scholar] [CrossRef]
- Vitkov, L.; Singh, J.; Schauer, C.; Minnich, B.; Krunić, J.; Oberthaler, H.; Gamsjaeger, S.; Herrmann, M.; Knopf, J.; Hannig, M. Breaking the Gingival Barrier in Periodontitis. Int. J. Mol. Sci. 2023, 24, 4544. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Gershovich, E. The Prevention of Periodontal Disease—An Overview. Periodontology 2020, 84, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Villoria, G.E.M.; Fischer, R.G.; Tinoco, E.M.B.; Meyle, J.; Loos, B.G. Periodontal Disease: A Systemic Condition. Periodontology 2024, 96, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Yekani, M.; Dastgir, M.; Fattahi, S.; Shahi, S.; Maleki Dizaj, S.; Memar, M.Y. Microbiological and Molecular Aspects of Periodontitis Pathogenesis: An Infection-Induced Inflammatory Condition. Front. Cell. Infect. Microbiol. 2025, 15, 1533658. [Google Scholar] [CrossRef]
- Ray, R.R. Periodontitis: An Oral Disease with Severe Consequences. Appl. Biochem. Biotechnol. 2023, 195, 17–32. [Google Scholar] [CrossRef]
- Chen, E.; Wang, T.; Tu, Y.; Sun, Z.; Ding, Y.; Gu, Z.; Xiao, S. ROS-Scavenging Biomaterials for Periodontitis. J. Mater. Chem. B 2023, 11, 482–499. [Google Scholar] [CrossRef]
- Kinane, D.F. Causation and Pathogenesis of Periodontal Disease. Periodontology 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Bartold, P.M.; Van Dyke, T.E. An Appraisal of the Role of Specific Bacteria in the Initial Pathogenesis of Periodontitis. J. Clin. Periodontol. 2019, 46, 6–11. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The Cytokine Network Involved in the Host Immune Response to Periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Lehner, M.P.; Kaufmann, M.E.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Oral Diagnostic Methods for the Detection of Periodontal Disease. Diagnostics 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Ali Alftaikhah, S.A.; Issrani, R.; Alnasser, M.; Almutairi, H.A.; Khattak, O.; Iqbal, A.; Prabhu, N. Salivary Biomarkers in Periodontitis: A Scoping Review. Cureus 2023, 15, e50207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Dong, Z.; Xia, X.; Li, X. Cathepsins in Oral Diseases: Mechanisms and Therapeutic Implications. Front. Immunol. 2023, 14, 1203071. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Shimada, Y.; Hanada, N.; Numabe, Y.; Kamoi, K.; Sato, T.; Gomi, K.; Arai, T.; Inagaki, K.; Fukuda, M.; et al. Salivary Biomarkers for Predicting the Progression of Chronic Periodontitis. Arch. Oral Biol. 2012, 57, 413–420. [Google Scholar] [CrossRef]
- Koppolu, P.; Sirisha, S.; Mishra, A.; Deshpande, K.; Lingam, A.S.; Alotaibi, D.H.; Saleh Alwahibi, M.; Penela, S. Alkaline Phosphatase and Acid Phosphatase Levels in Saliva and Serum of Patients with Healthy Periodontium, Gingivitis, and Periodontitis before and after Scaling with Root Planing: A Clinico-Biochemical Study. Saudi J. Biol. Sci. 2021, 28, 380–385. [Google Scholar] [CrossRef]
- Taymour, N.; Hassan, M.G.; AlGhamdi, M.A.; Omara, W.S. From Detection to Treatment: Nanomaterial-Based Biosensors Transforming Prosthetic Dentistry and Oral Health Care: A Scoping Review. Prosthesis 2025, 7, 51. [Google Scholar] [CrossRef]
- Alsaykhan, K.; Khan, N.S.; Aljumah, M.I.; Albughaylil, A.S. Comparative Evaluation of Salivary Enzyme in Patients With Gingivitis and Periodontitis: A Clinical-Biochemical Study. Cureus 2022, 14, e20991. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, N.H.; Al-Marzooq, F.; Al-Nuaimi, A.S.; Hachim, M.Y.; Hamoudi, R. Salivary microRNA 155, 146a/b and 203: A Pilot Study for Potentially Non-Invasive Diagnostic Biomarkers of Periodontitis and Diabetes Mellitus. PLoS ONE 2020, 15, e0237004. [Google Scholar] [CrossRef]
- Wu, P.; Feng, J.; Wang, W. Expression of miR-155 and miR-146a in the Saliva of Patients with Periodontitis and Its Clinical Value. Am. J. Transl. Res. 2021, 13, 6670–6677. [Google Scholar]
- Mata-Monterde, M.; Serrano-Valcarce, A.; Almiñana-Pastor, P.J.; Micó-Martínez, P.; López-Roldán, A. miRNAs as Epigenetic Biomarkers in the Study of the Bidirectional Relationship between Type 2 Diabetes Mellitus and Periodontitis: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10723. [Google Scholar] [CrossRef]
- Subbarao, K.C.; Nattuthurai, G.S.; Sundararajan, S.K.; Sujith, I.; Joseph, J.; Syedshah, Y.P. Gingival Crevicular Fluid: An Overview. J. Pharm. Bioallied Sci. 2019, 11, S135. [Google Scholar] [CrossRef] [PubMed]
- Sirisereephap, K.; Maekawa, T.; Tamura, H.; Hiyoshi, T.; Domon, H.; Isono, T.; Terao, Y.; Maeda, T.; Tabeta, K. Osteoimmunology in Periodontitis: Local Proteins and Compounds to Alleviate Periodontitis. Int. J. Mol. Sci. 2022, 23, 5540. [Google Scholar] [CrossRef]
- Alwandawi, T.K. Immunodiagnostic Potential of the RANK/RANKL/OPG Ratio in Gingival Crevicular Fluid for Periodontitis. Folia Med. 2025, 67, e144949. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental Plaque as a Biofilm and a Microbial Community-Implications for Health and Disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Proctor, G.; Zaric, S. Diagnostic Accuracy of Microbiome-Derived Biomarkers in Periodontitis: Systematic Review and Meta-Analysis. J. Periodontal Res. 2025, 60, 748–761. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Cui, L.; Huang, S. Metagenomic and Metatranscriptomic Insight Into Oral Biofilms in Periodontitis and Related Systemic Diseases. Front. Microbiol. 2021, 12, 728585. [Google Scholar] [CrossRef]
- Kametani, M.; Nagasawa, Y.; Usuda, M.; Kaneki, A.; Ogawa, M.; Shojima, K.; Yamazaki, H.; Tokumoto, K.; Matsuoka, D.; Suehara, K.; et al. Relationship Between the Presence of Red Complex Species and the Distribution of Other Oral Bacteria, Including Major Periodontal Pathogens in Older Japanese Individuals. Int. J. Mol. Sci. 2024, 25, 12243. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, B.; An, Y.; Zhou, Z.; Xiong, P.; Li, X.; Mi, Y.; He, T.; Chen, F.; Wu, B. Gingipain from Porphyromonas Gingivalis Causes Insulin Resistance by Degrading Insulin Receptors through Direct Proteolytic Effects. Int. J. Oral Sci. 2024, 16, 53. [Google Scholar] [CrossRef]
- Foroughi, M.; Torabinejad, M.; Angelov, N.; Ojcius, D.M.; Parang, K.; Ravnan, M.; Lam, J. Bridging Oral and Systemic Health: Exploring Pathogenesis, Biomarkers, and Diagnostic Innovations in Periodontal Disease. Infection 2025, 1–26. [Google Scholar] [CrossRef]
- Ghelli, F.; Panizzolo, M.; Garzaro, G.; Squillacioti, G.; Bellisario, V.; Colombi, N.; Bergamaschi, E.; Guseva Canu, I.; Bono, R. Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9820. [Google Scholar] [CrossRef]
- Ghasemirad, M.; Tavakol, O.; Etesami, S.; Nikeghbal, K.; Nikeghbal, D.; Diznab, F.A.; Anzabi, R.M. A State of Art Review: Volatile Organic Compounds and Periodontitis: Volatile Organic Compounds and Periodontitis. Galen Med. J. 2024, 13, e3730. [Google Scholar] [CrossRef]
- Haiek, M.; Dvoyris, V.; Broza, Y.Y.; Haick, H.; Weiss, E.; Houri-Haddad, Y. Bacterial Volatile Organic Compounds as Potential Caries and Periodontitis Disease Biomarkers. Int. J. Mol. Sci. 2025, 26, 3591. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Hong, J.-Y. Oral Microbiome as a Co-Mediator of Halitosis and Periodontitis: A Narrative Review. Front. Oral Health 2023, 4, 1229145. [Google Scholar] [CrossRef] [PubMed]
- Constantin, V.; Luchian, I.; Goriuc, A.; Budala, D.G.; Bida, F.C.; Cojocaru, C.; Butnaru, O.-M.; Virvescu, D.I. Salivary Biomarkers Identification: Advances in Standard and Emerging Technologies. Oral 2025, 5, 26. [Google Scholar] [CrossRef]
- Campos, J.V.; Pontes, J.T.C.; Canales, C.S.C.; Roque-Borda, C.A.; Pavan, F.R. Advancing Nanotechnology: Targeting Biofilm-Forming Bacteria with Antimicrobial Peptides. BME Front. 2025, 6, 0104. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Awoyemi, R.F.; Faderin, E.; Akobundu, U.U.; Ajayi, A.S.; Chukwu, J.U.; Lekan, O.K.; Asiriuwa, O.D.; Maliki, M.; Ikhuoria, E.U. Protein-Based Nanoparticles for Antimicrobial and Cancer Therapy: Implications for Public Health. RSC Adv. 2025, 15, 14966–15016. [Google Scholar] [CrossRef]
- Fidyawati, D.; Masulili, S.L.C.; Iskandar, H.B.; Suhartanto, H.; Kiswanjaya, B.; Li, X. Clinical and Radiographic Parameters for Early Periodontitis Diagnosis: A Comparative Study. Dent. J. 2024, 12, 407. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Ong, M.M.A. Minimally Invasive Periodontology: A Treatment Philosophy and Suggested Approach. Int. J. Dent. 2021, 1, 2810264. [Google Scholar] [CrossRef]
- de Molon, R.S.; Rodrigues, J.V.S.; Deroide, M.B.; da Silva Barbirato, D.; Garcia, V.G.; Theodoro, L.H. The Efficacy of Topical or Systemic Antibiotics as Adjuvants to Non-Surgical Periodontal Treatment in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2024, 13, 4763. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, Drug Resistance and Alternatives to Conventional Approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Shi, L.; Liu, Y.; Shen, J. New Advances in Periodontal Functional Materials Based on Antibacterial, Anti-Inflammatory, and Tissue Regeneration Strategies. Adv. Healthc. Mater. 2025, 14, 2403206. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Chen, J.-Y.; Suo, W.-H.; Shao, W.-R.; Huang, C.-Y.; Li, M.-T.; Li, Y.-Y.; Li, Y.-H.; Liang, E.-L.; Chen, Y.-H.; et al. Unlocking the Future of Periodontal Regeneration: An Interdisciplinary Approach to Tissue Engineering and Advanced Therapeutics. Biomedicines 2024, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Li, X.; Liu, S.; Du, J.; Xu, J.; Hu, J.; Liu, Y. Challenges and Tissue Engineering Strategies of Periodontal-Guided Tissue Regeneration. Tissue Eng. Part C Methods 2022, 28, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Budală, D.G.; Luchian, I.; Tatarciuc, M.; Butnaru, O.; Armencia, A.O.; Virvescu, D.I.; Scutariu, M.M.; Rusu, D. Are Local Drug Delivery Systems a Challenge in Clinical Periodontology? J. Clin. Med. 2023, 12, 4137. [Google Scholar] [CrossRef]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced Drug Delivery Approaches against Periodontitis. Drug Deliv. 2016, 23, 363–377. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef]
- Uskoković, V.; Pejčić, A.; Koliqi, R.; Anđelković, Z. Polymeric Nanotechnologies for the Treatment of Periodontitis: A Chronological Review. Int. J. Pharm. 2022, 625, 122065. [Google Scholar] [CrossRef]
- D’Amico, E.; Aceto, G.M.; Petrini, M.; Cinquini, C.; D’Ercole, S.; Iezzi, G.; Pierfelice, T.V. How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy? Int. J. Mol. Sci. 2025, 26, 592. [Google Scholar] [CrossRef]
- Mutreja, I.; Kumar, D.; Kaushik, A.; Mishra, Y.K. Lipid Nanoparticle-Based Formulations for High-Performance Dentistry Applications. J. Mater. Chem. B 2023, 11, 5990–6023. [Google Scholar] [CrossRef]
- Lima de Sousa, T.; Dourado, D.; Rodrigues, J.S.; de Souza Rebouças, J.; Montes, M.A.J.R.; Formiga, F.R. Treatment of Periodontal Disease: Does Drug Delivery Matter? Front. Bioeng. Biotechnol. 2024, 12, 1427758. [Google Scholar] [CrossRef]
- Attri, N.; Das, S.; Banerjee, J.; Shamsuddin, S.H.; Dash, S.K.; Pramanik, A. Liposomes to Cubosomes: The Evolution of Lipidic Nanocarriers and Their Cutting-Edge Biomedical Applications. ACS Appl. Bio Mater. 2024, 7, 2677–2694. [Google Scholar] [CrossRef]
- Malode, T.D.; Pillare, S.B.; Shahi, A.K.; Lade, S.N.; Taksande, J.B.; Umekar, M.J. Cubosomes as Versatile Nanocarriers: Insights into Composition, Mechanisms, and Therapeutic Applications. Biomed. Mater. Devices 2025, 1–22. [Google Scholar] [CrossRef]
- Hedayatipanah, M.; Gholami, L.; Farmany, A.; Alikhani, M.Y.; Hooshyarfard, A.; Hashemiyan, F.S. Green Synthesis of Silver Nanoparticles and Evaluation of Their Effects on the Porphyromonas Gingivalis Bacterial Biofilm Formation. Clin. Exp. Dent. Res. 2024, 10, e887. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Masoumi, S.M.; Amini, S.; Goudarzi, M.; Tafreshi, S.M.; Bagheri, A.; Yasamineh, S.; alwan, M.; Arellano, M.T.C.; Gholizadeh, O. Recent Advances in Metal Nanoparticles to Treat Periodontitis. J. Nanobiotechnol. 2023, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Kim, M.; Lee, A.; Ji, S.; Jang, M.; Yim, S.; Song, W.; Lee, S.S.; Yoon, D.H.; An, K.-S. Nanometric Lamination of Zinc Oxide Nanofilms with Gold Nanoparticles for Self-Perceived Periodontal Disease Sensors. Compos. Part B Eng. 2022, 230, 109490. [Google Scholar] [CrossRef]
- Tong, F.; Wang, P.; Chen, Z.; Liu, Y.; Wang, L.; Guo, J.; Li, Z.; Cai, H.; Wei, J. Combined Ferromagnetic Nanoparticles for Effective Periodontal Biofilm Eradication in Rat Model. Int. J. Nanomed. 2023, 18, 2371–2388. [Google Scholar] [CrossRef]
- Nawaz, M.Z.; Alghamdi, H.A.; Zahoor, M.; Rashid, F.; Alshahrani, A.A.; Alghamdi, N.S.; Pugazhendhi, A.; Zhu, D. Synthesis of Novel Metal Silica Nanoparticles Exhibiting Antimicrobial Potential and Applications to Combat Periodontitis. Environ. Res. 2024, 241, 117415. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Li, M.; Boccaccini, A.R. Strontium and Zinc Co-Doped Mesoporous Bioactive Glass Nanoparticles for Potential Use in Bone Tissue Engineering Applications. Nanomaterials 2024, 14, 575. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, H.; Cheng, L.; Wang, Y.; Liu, F.; Wang, S. The Application of Mesoporous Silica Nanoparticles as a Drug Delivery Vehicle in Oral Disease Treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1124411. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Carbon-Based Nanomaterials. Int. J. Mol. Sci. 2021, 22, 7726. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Bai, H.; Niu, Y.; Wu, Y. Characterization and Application of in Situ Curcumin/ZNP Hydrogels for Periodontitis Treatment. BMC Oral Health 2024, 24, 395. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Qu, X.; Li, Y.; Zhou, C.; Xie, D.; Dong, J.; Ji, L.; Xu, J.; Zhou, J. Highly-Sensitive Ultra-Thin Dental Patches Assisted with Artificial-Intelligence Recognition for Mapping Hidden Periodontitis Lesions. Sens. Actuators B Chem. 2025, 435, 137648. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Li, P.; Tang, F.K.; He, J.; Sun, H.; Wang, X.; Wang, M.; Lan, X.; Wang, X.; et al. Berberine-Functionalized Bismuth-Doped Carbon Dots in a Pathogen-Responsive Hydrogel System: A Multifaceted Approach to Combating Periodontal Diseases. ACS Nano 2025, 19, 17554–17577. [Google Scholar] [CrossRef]
- Di Cristo, F.; Valentino, A.; De Luca, I.; Peluso, G.; Bonadies, I.; Calarco, A.; Di Salle, A. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules 2022, 27, 2205. [Google Scholar] [CrossRef]
- Milosevic, M.; Stojanovic, D.B.; Simic, V.; Grkovic, M.; Bjelovic, M.; Uskokovic, P.S.; Kojic, M. Preparation and Modeling of Three-layered PCL/PLGA/PCL Fibrous Scaffolds for Prolonged Drug Release. Sci. Rep. 2020, 10, 11126. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeei, S.; Mansurian, M.; Asare-Addo, K.; Nokhodchi, A. Metronidazole- and Amoxicillin-Loaded PLGA and PCL Nanofibers as Potential Drug Delivery Systems for the Treatment of Periodontitis: In Vitro and In Vivo Evaluations. Biomedicines 2021, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Chegini, Z.; Ghaznavi-Rad, E.; Zare, E.N.; Hosseini, S.M. PLGA-Based Nanoplatforms in Drug Delivery for Inhibition and Destruction of Microbial Biofilm. Front. Cell. Infect. Microbiol. 2022, 12, 926363. [Google Scholar] [CrossRef]
- Yürük, G.; Damla Demir, Y.; Vural, Ş.; Seda Kehr, N. Polymeric Biomaterials for Periodontal Tissue Engineering and Periodontitis. RSC Appl. Polym. 2024, 2, 534–556. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Osorio, R.; Bueno, J.; Vallecillo, C.; Vallecillo-Rivas, M.; Sanz, M. Next-Generation Antibacterial Nanopolymers for Treating Oral Chronic Inflammatory Diseases of Bacterial Origin. Int. Endod. J. 2024, 57, 787–803. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, W.; Liu, H.; Li, R.; Chang, L.; Kan, S.; Hao, M.; Wang, D. The Applications of Polysaccharides in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 970041. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U. The Role of Polysaccharide-Based Biodegradable Soft Polymers in the Healthcare Sector. Adv. Ind. Eng. Polym. Res. 2025, 8, 132–156. [Google Scholar] [CrossRef]
- Sadighi Shamami, M.; Ekhlaspour, M.; Sulaiman, J.M.A.; Abdul Kareem, R.; Mahmood Ahmed Alsultany, N.; Nasiri, K.; Shenasa, N. The Current Advancements in Chitosan Nanoparticles in the Management of Non-Surgical Periodontitis Treatment. Nanotoxicology 2025, 19, 290–324. [Google Scholar] [CrossRef]
- Bajpai, D.; Ramamurthy, J. Role of Alginate-Based Scaffolds for Periodontal Regeneration of Intrabony Defects: A Systematic Review. World J. Dent. 2024, 15, 181–187. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, Z.; Fei, T. Innovative Nanomaterials for Periodontal Disease Management: Antibacterial ZnO Nanoparticle-Sodium Alginate-Gelatin Composite. Iran. J. Chem. Chem. Eng. 2025, 44, 29–43. [Google Scholar] [CrossRef]
- Li, Y.; Xing, Z.; Wang, S.; Wang, Y.; Wang, Z.; Dong, L. Disruption of Biofilms in Periodontal Disease through the Induction of Phase Transition by Cationic Dextrans. Acta Biomater. 2023, 158, 759–768. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amaral, M.H.; Conceição, J.; Sousa Lobo, J.M. Nanotechnological Carriers for Cancer Chemotherapy: The State of the Art. Colloids Surf. B Biointerfaces 2015, 126, 631–648. [Google Scholar] [CrossRef]
- Murgia, D.; Angellotti, G.; D’Agostino, F.; De Caro, V. Bioadhesive Matrix Tablets Loaded with Lipophilic Nanoparticles as Vehicles for Drugs for Periodontitis Treatment: Development and Characterization. Polymers 2019, 11, 1801. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Ashfaq, R.; Tóth, N.; Kovács, A.; Berkó, S.; Katona, G.; Ambrus, R.; Polgár, T.F.; Szécsényi, M.; Burián, K.; Budai-Szűcs, M. Hydrogel–Nanolipid Formulations for the Complex Anti-Inflammatory and Antimicrobial Therapy of Periodontitis. Pharmaceutics 2025, 17, 620. [Google Scholar] [CrossRef]
- Yaghmur, A.; Mu, H. Recent Advances in Drug Delivery Applications of Cubosomes, Hexosomes, and Solid Lipid Nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef]
- Silveira, G.R.C.; Ganzaroli, V.F.; Toro, L.F.; da Costa, L.L.; Pereira, R.I.L.; da Silva, A.B.; Ferreira, I.R.S.; de Mello-Neto, J.M.; Garcia, V.G.; Theodoro, L.H.; et al. Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis. Biomedicines 2025, 13, 1643. [Google Scholar] [CrossRef]
- Wang, M.; Skripka, A.; Zhang, Y.; Cheng, T.; Ng, M.; Wong, S.Y.; Zhao, Y.; Sun, X.; Li, X.; Bhakoo, K.K.; et al. Theranostic Nanocapsules: Heating, Imaging, and Luminescence Nanothermometry. Chem. Mater. 2024, 36, 3285–3295. [Google Scholar] [CrossRef]
- Monika; Sharma, S.; Shrivastva, M.; Kumar, S.; Rabbani, S.A.; Garg, A. Novel In-Situ NanoEmulGel (NEG) of Azithromycin with Eugenol for the Treatment of Periodontitis: Formulation Development and Characterization. J. Clust. Sci. 2022, 33, 2589–2600. [Google Scholar] [CrossRef]
- Kumari, D.; Karmakar, V.; Sisinthy, S.P.; Pandey, M.; Jain, N.; Gorain, B. Nanoemulsion and Nanoemulgel-Based Carriers as Advanced Delivery Tools for the Treatment of Oral Diseases. Drug Deliv. Transl. Res. 2025, 15, 1139–1155. [Google Scholar] [CrossRef]
- Hu, Q.-Y.; Hu, J.; Li, H.; Fang, X.; Sun, Z.-J.; Xu, Z.; Zhang, L. Anti-Inflammatory and Antioxidant Effects of Rhein Loaded Nanomicelles in Periodontitis. Colloids Surf. Physicochem. Eng. Asp. 2022, 654, 130164. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Zhang, S. Sustained Release of Azithromycin from Lipid Liquid-Crystalline Nanoparticles Laden in Situ Gel for the Treatment of Periodontitis: In Vitro and Efficacy Study. J. Biomater. Appl. 2022, 37, 482–492. [Google Scholar] [CrossRef]
- Tang, M.; Li, J.; Wang, G.; Wang, Y.; Peng, C.; Chang, X.; Tao, Y.; Guo, J.; Gui, S. Cubic Liquid Crystals Containing Propolis Flavonoids as in Situ Thermo-Sensitive Hydrogel Depots for Periodontitis Treatment: Preparation, Pharmacodynamics and Therapeutic Mechanisms. Eur. J. Pharm. Sci. 2024, 196, 106762. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Cieciórski, P.; Barcińska, E.; Jaśkiewicz, M.; Narajczyk, M.; Bauer, M.; Kamysz, W.; Megiel, E.; Inkielewicz-Stepniak, I. Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: Their Potential Use in Treating Periodontitis. Int. J. Nanomed. 2022, 17, 495–517. [Google Scholar] [CrossRef]
- Hernández-Venegas, P.A.; Martínez-Martínez, R.E.; Zaragoza-Contreras, E.A.; Domínguez-Pérez, R.A.; Reyes-López, S.Y.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Molina-Frechero, N.; Espinosa-Cristóbal, L.F. Bactericidal Activity of Silver Nanoparticles on Oral Biofilms Related to Patients with and without Periodontal Disease. J. Funct. Biomater. 2023, 14, 311. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Liu, X.; Liu, L.; Jiang, S.; Zhang, Z.; Li, Y.; Pan, S. Activated Silver Nanoparticle-Based Platform for Specific Capture of Porphyromonas Gingivalis in Human Saliva. Sens. Actuators B Chem. 2024, 403, 135171. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Song, Y.; Liu, J.; Lan, S.; Chen, B.; Li, Y.; Han, J. Nanoparticle-Mediated Photothermal and Photodynamic Antibacterial Therapy for the Treatment of Periodontitis. Colloids Surf. Physicochem. Eng. Asp. 2025, 708, 135988. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Mao, J.; Mao, L.; Li, W.; Liu, Z.; Shin, A.; Wu, J.; Hou, L.; Li, D.; et al. ZIF-8-Based Nanoparticles for Inflammation Treatment and Oxidative Stress Reduction in Periodontitis. ACS Appl. Mater. Interfaces 2024, 16, 36077–36094. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, Z.; Ma, A.; Shi, E.; Li, Z.; Liang, Z.; Qian, Z.; Yang, L.; Wang, Y.; Cao, M.; et al. A Robust ROS Generation Nanoplatform Combating Periodontitis via Sonodynamic/Chemodynamic Combination Therapy. Chem. Eng. J. 2023, 451, 138782. [Google Scholar] [CrossRef]
- Al-Timimi, Z. Illuminating the Path: The Role of Photodynamic Therapy in Comprehensive Periodontal Treatment. Ir. J. Med. Sci. 1971 2025, 194, 1083–1096. [Google Scholar] [CrossRef]
- Palomar, Q.; Svärd, A.; Zeng, S.; Hu, Q.; Liu, F.; Aili, D.; Zhang, Z. Detection of Gingipain Activity Using Solid State Nanopore Sensors. Sens. Actuators B Chem. 2022, 368, 132209. [Google Scholar] [CrossRef]
- Zong, B.; Li, X.; Xu, Q.; Wang, D.; Gao, P.; Zhou, Q. Enhanced Eradication of Enterococcus Faecalis Biofilms by Quaternized Chitosan-Coated Upconversion Nanoparticles for Photodynamic Therapy in Persistent Endodontic Infections. Front. Microbiol. 2022, 13, 909492. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, C.; Sun, C.; Bai, H.; Xie, J.; Gu, Y.; Li, M.; Jiang, J.; Le, A.; Qiu, J.; et al. Carvacrol Combined with NIR Light-Responsive Nano-Drug Delivery System with Specific Anti-Bacteria, Anti-Inflammation, and Immunomodulation for Periodontitis. Nano Res. 2023, 16, 7199–7215. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, J.; Zhou, L.; Han, W.; Ye, J.; Hu, D.; Xie, W.; Qiu, J.; Chen, R.; Wang, X. Near Infrared Responsive Nitric Oxide and Carbon Monoxide Nanoplatform for Synergistic Photodynamic Therapy against Periodontitis. Chem. Eng. J. 2024, 480, 147850. [Google Scholar] [CrossRef]
- He, W.; You, M.; Li, Z.; Cao, L.; Xu, F.; Li, F.; Li, A. Upconversion Nanoparticles-Based Lateral Flow Immunoassay for Point-of-Care Diagnosis of Periodontitis. Sens. Actuators B Chem. 2021, 334, 129673. [Google Scholar] [CrossRef]
- Hou, J.; Tamura, Y.; Lu, H.-Y.; Takahashi, Y.; Kasugai, S.; Nakata, H.; Kuroda, S. An In Vitro Evaluation of Selenium Nanoparticles on Osteoblastic Differentiation and Antimicrobial Properties against Porphyromonas Gingivalis. Nanomaterials 2022, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Hamman, N.; Ramburrun, P.; Dube, A. Selenium Nanoparticle Activity against S. Mutans Biofilms as a Potential Treatment Alternative for Periodontitis. Pharmaceutics 2024, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.-P.; Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Ortega-Maldonado, M.; Ximenez-Fyvie, L.-A. Antibacterial Properties In Vitro of Magnesium Oxide Nanoparticles for Dental Applications. Nanomaterials 2023, 13, 502. [Google Scholar] [CrossRef]
- Koga, A.; Thongsiri, C.; Kudo, D.; Phuong, D.N.D.; Iwamoto, Y.; Fujii, W.; Nagai-Yoshioka, Y.; Yamasaki, R.; Ariyoshi, W. Mechanisms Underlying the Suppression of IL-1β Expression by Magnesium Hydroxide Nanoparticles. Biomedicines 2023, 11, 1291. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, S.; Gu, D.; Zhu, B.; Liu, H.; Wu, W.; Wu, J.; Wei, H.; Miao, L. Cerium Oxide Nanozyme Attenuates Periodontal Bone Destruction by Inhibiting the ROS–NFκB Pathway. Nanoscale 2022, 14, 2628–2637. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium Oxide Nanoparticles Loaded Nanofibrous Membranes Promote Bone Regeneration for Periodontal Tissue Engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef]
- Chatzimentor, I.; Tsamesidis, I.; Ioannou, M.-E.; Pouroutzidou, G.K.; Beketova, A.; Giourieva, V.; Papi, R.; Kontonasaki, E. Study of Biological Behavior and Antimicrobial Properties of Cerium Oxide Nanoparticles. Pharmaceutics 2023, 15, 2509. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Yin, I.X.; Lung, C.Y.K.; Niu, J.Y.; Mei, M.L.; Chu, C.H. In Vitro Studies of Graphene for Management of Dental Caries and Periodontal Disease: A Concise Review. Pharmaceutics 2022, 14, 1997. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Ma, X.; Wei, Z.; Ye, H.; Zou, L.; Liu, D.; Qu, S.; Liu, Z.; Li, D.; et al. Artesunate Carbon Dots for Attenuating Periodontal Inflammation and Promoting Bone Regeneration Activation of AMPK. Colloids Surf. B Biointerfaces 2025, 254, 114873. [Google Scholar] [CrossRef]
- Pop, D.; Buzatu, R.; Moacă, E.-A.; Watz, C.G.; Cîntă Pînzaru, S.; Barbu Tudoran, L.; Nekvapil, F.; Avram, Ș.; Dehelean, C.A.; Crețu, M.O.; et al. Development and Characterization of Fe3O4@Carbon Nanoparticles and Their Biological Screening Related to Oral Administration. Materials 2021, 14, 3556. [Google Scholar] [CrossRef]
- Valdivieso, M.C.; Ortiz, L.; Castillo, J.J. Myeloperoxidase as a Biomarker in Periodontal Disease: Electrochemical Detection Using Printed Screen Graphene Electrodes. Odontology 2025, 113, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ramos-López, C.; García-Rodrigo, L.; Sánchez-Tirado, E.; González-Cortés, A.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanocellulose-Modified Electrodes for Simultaneous Biosensing of Microbiome-Related Oral Diseases Biomarkers. Microchim. Acta 2025, 192, 141. [Google Scholar] [CrossRef]
- Ramos-López, C.; García-Rodrigo, L.; Sánchez-Tirado, E.; Agüí, L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Cerium Dioxide-Based Nanostructures as Signal Nanolabels for Current Detection in the Immunosensing Determination of Salivary Myeloperoxidase. Microchem. J. 2024, 201, 110505. [Google Scholar] [CrossRef]
- Han, M.; Tang, K.; Chen, Z. Preparation of NIR Responsive resveratrol@Au Nanocages Using for Regulating Oxidative Stress Microenvironment of Periodontitis. Chem. Eng. J. 2025, 504, 158774. [Google Scholar] [CrossRef]
- Liu, X.; Ding, Z.; Xu, C.; Zhang, J.; Liu, Y.; Chen, T.; Dai, S.; Bao, X.; Hu, M.; Liu, Z. In Situ Valence Engineering of Copper Silicate Nanozymes with Enhanced Peroxidase-Like Catalytic Activity for Oral Disease Detection. Adv. Sci. 2025, 12, e03237. [Google Scholar] [CrossRef]
- Li, S.; Fan, Z.; Zheng, K.; Wu, Y.; Zhong, G.; Xu, X. Engineered Probiotics with Low Oxygen Targeting Porphyromonas Gingivalis and Gingival Fibroblasts for the Treatment of Periodontitis. ACS Biomater. Sci. Eng. 2025, 11, 2753–2767. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, M.; He, P.; Liu, F.; Wang, X.; Zhou, C.; Li, Y.; Yang, S. Genetically Engineered Cell Membrane-Coated Nanoparticles for Antibacterial and Immunoregulatory Dual-Function Treatment of Ligature-Induced Periodontitis. Front. Bioeng. Biotechnol. 2023, 11, 1113367. [Google Scholar] [CrossRef]
- Mlachkova, A.; Dosseva-Panova, V.; Maynalovska, H.; Pashova-Tasseva, Z. Nanoparticles as Strategies for Modulating the Host’s Response in Periodontitis Treatment. Nanomaterials 2025, 15, 476. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; Cadenas De Llano-Pérula, M. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [Google Scholar] [CrossRef]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical Translation of Nanomedicines: Challenges, Opportunities, and Keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef]
- Wang, C.; Xu, T.; Seneviratne, C.J.; Ong, L.J.Y.; Zhou, Y. Modelling Periodontitis in Vitro: Engineering Strategies and Biofilm Model Development. Front. Biomater. Sci. 2024, 3, 1380153. [Google Scholar] [CrossRef]
- Gawne, P.J.; Ferreira, M.; Papaluca, M.; Grimm, J.; Decuzzi, P. New Opportunities and Old Challenges in the Clinical Translation of Nanotheranostics. Nat. Rev. Mater. 2023, 8, 783–798. [Google Scholar] [CrossRef]
- Jürgensen, N.; Petersen, P.E.; Ogawa, H.; Matsumoto, S. Translating Science into Action: Periodontal Health through Public Health Approaches. Periodontology 2012, 60, 173–187. [Google Scholar] [CrossRef]
- Frantsve-Hawley, J.; Kumar, S.S.; Rindal, D.B.; Weyant, R.J. Implementation Science and Periodontal Practice: Translation of Evidence into Periodontology. Periodontology 2020, 84, 188–201. [Google Scholar] [CrossRef]

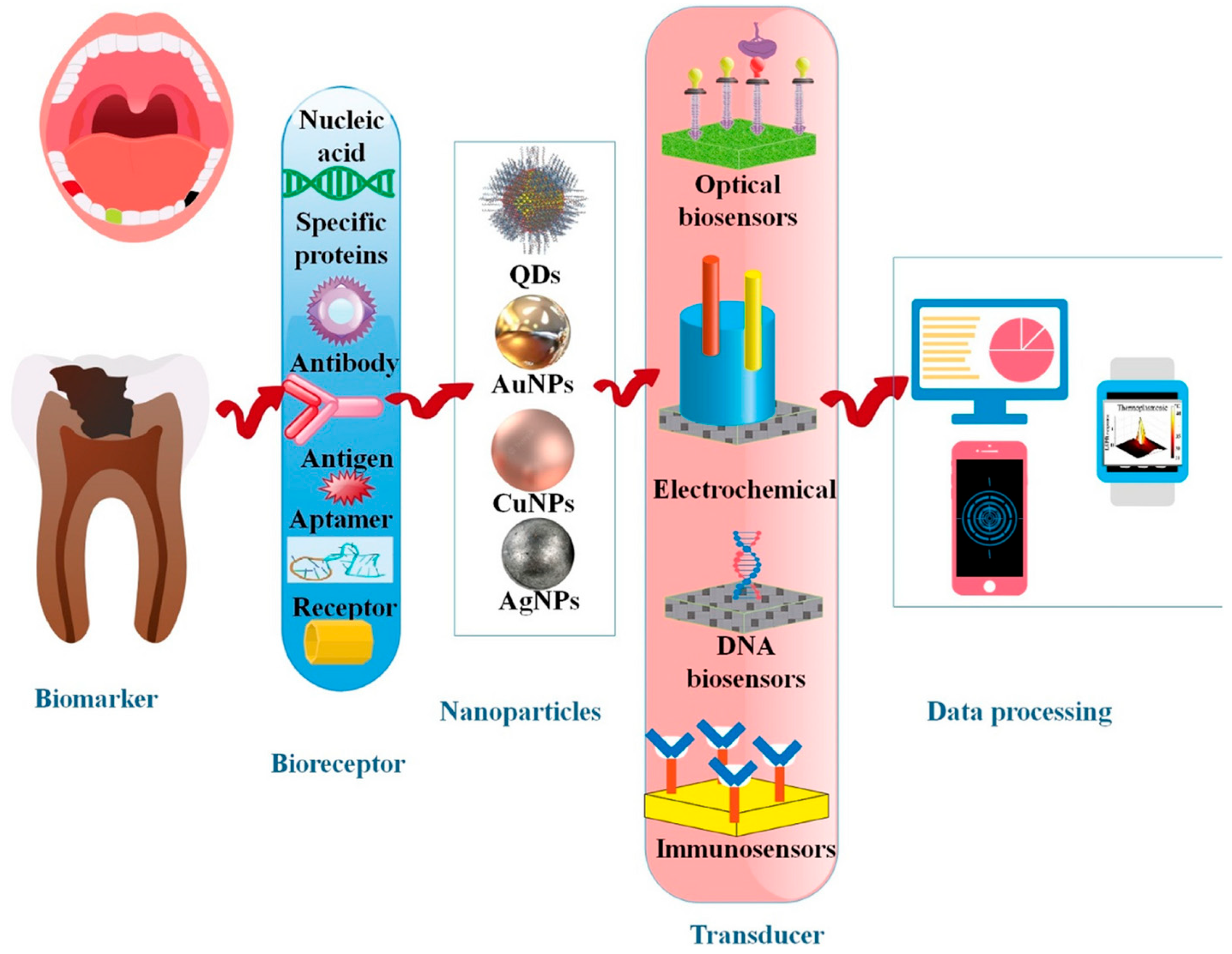
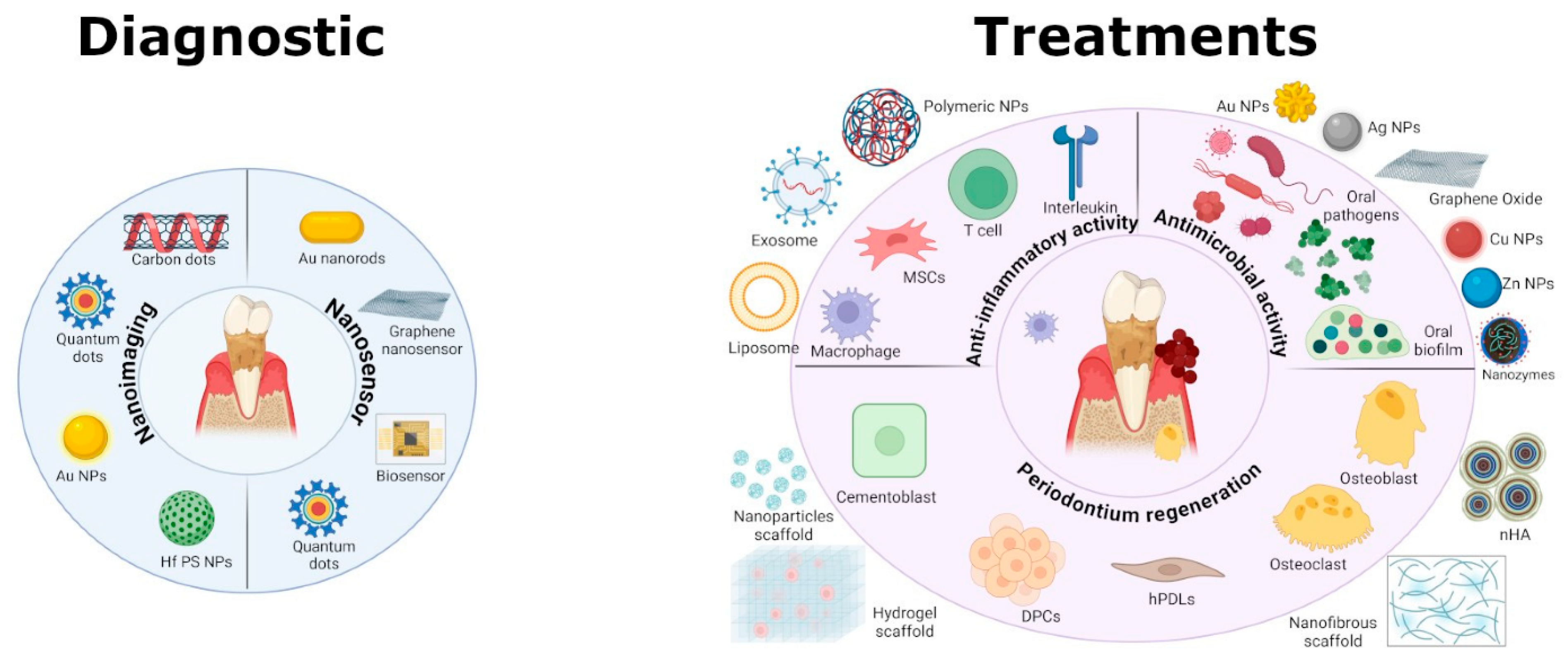
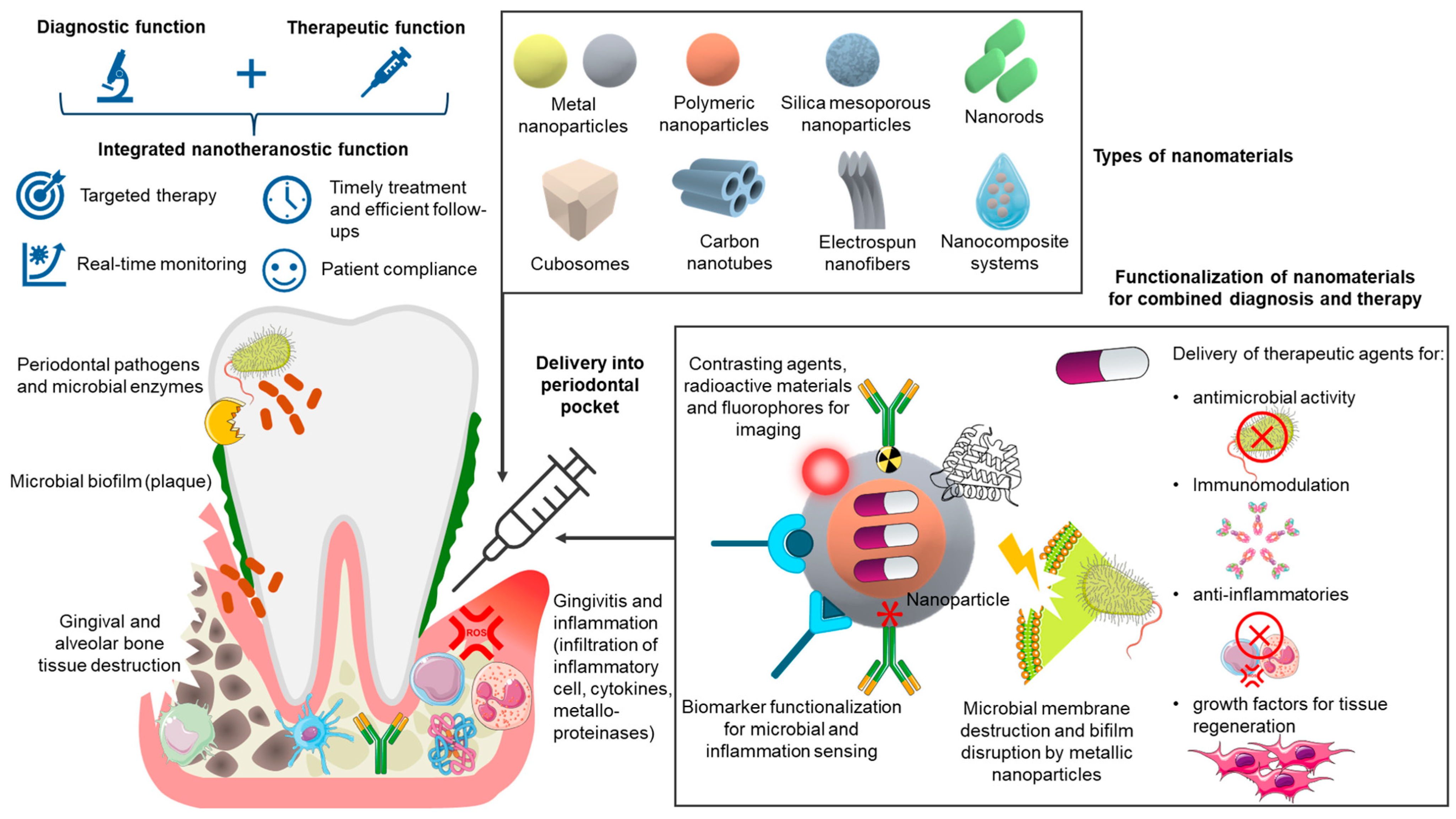
| Nanomaterial Type | Key Properties | Diagnostic Functions | Therapeutic Functions | References |
|---|---|---|---|---|
| Polymeric nanoparticles (e.g., PLGA, chitosan). | Biodegradable, tunable size/charge, controlled release. | Encapsulation of fluorescent dyes, radiocontrast or antibodies and peptides as diagnostic probes for localized sensing and imaging. | Sustained release of antimicrobials, anti-inflammatories, and regenerative growth factors. | [6,7,8,59,60] |
| Lipid-based nanomaterials (e.g., liposomes, solid lipid nanoparticles, nanostructured lipid carriers). | Amphiphilic bilayer, biomimetic lipid layers, biocompatible, versatile encapsulation for hydrophilic and lipophilic molecules. | Integration of fluorescent/luminescent markers or contrasting agents for imaging. | Delivery of antibiotics, anti-inflammatory agents, and regenerative molecules. | [61,62,63,64] |
| Metal/ metal Oxide nanoparticles and nanofilms (e.g., Ag, Au, ZnO, TiO2). | High surface reactivity, optical/electrical properties, antimicrobial activity. | Biosensing of pathogens and biomarkers (AgNP-based colorimetric detection, AuNP-based plasmonic sensors) and volatile sulfur compounds. | Broad-spectrum antimicrobial activity and biofilm inhibiting and penetrating activity, ROS modulation, and anti-inflammatory effects, and periodontal bone regeneration. | [6,8,28,65,66,67] |
| Magnetic Nanoparticles (e.g., Fe3O4). | Superparamagnetic, high surface area, easily functionalized. | MRI contrast, magnetic biosensing of bacterial virulence factors. | Magnetically guided local drug delivery, hyperthermia for bacterial eradication. | [8,28,68] |
| Silica-Based Nanoparticles (e.g., mesoporous silica NPs). | High surface area, tunable porosity, modifiable chemistry. | Loading of biosensors or imaging probes. | Controlled and stimuli-responsive drug release, potential biofilm penetration, periodontal bone regeneration. | [69,70,71,72] |
| Carbon-Based Nanomaterials (e.g., graphene oxide, CNTs, carbon dots). | High mechanical strength, conductivity, large surface area. | Electrochemical biosensors for bacterial/inflammatory biomarkers. | Antimicrobial activity, anti-inflammatory modulation, scaffold reinforcement. | [6,28,73] |
| Nanocomposite systems (e.g., nanoparticle-loaded hydrogels or polymeric scaffolds). | Injectable, stimuli-responsive, ECM-mimicking. | Embedded carbon-based biosensors for microbial enzyme-responsive activity, and colorimetric detection of volatile sulfur compounds. | Localized drug release, tissue regeneration, antimicrobial/anti-inflammatory delivery, sustained release of nanoparticles, and metal oxide nanozymes. | [74,75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramburrun, P.; Varughese, T.P.K.; Choonara, Y.E. Nanotheranostics in Periodontitis: Bridging Diagnosis and Therapy Through Smart Integrated Nanosystems. J. Nanotheranostics 2025, 6, 31. https://doi.org/10.3390/jnt6040031
Ramburrun P, Varughese TPK, Choonara YE. Nanotheranostics in Periodontitis: Bridging Diagnosis and Therapy Through Smart Integrated Nanosystems. Journal of Nanotheranostics. 2025; 6(4):31. https://doi.org/10.3390/jnt6040031
Chicago/Turabian StyleRamburrun, Poornima, Theresa P. K. Varughese, and Yahya E. Choonara. 2025. "Nanotheranostics in Periodontitis: Bridging Diagnosis and Therapy Through Smart Integrated Nanosystems" Journal of Nanotheranostics 6, no. 4: 31. https://doi.org/10.3390/jnt6040031
APA StyleRamburrun, P., Varughese, T. P. K., & Choonara, Y. E. (2025). Nanotheranostics in Periodontitis: Bridging Diagnosis and Therapy Through Smart Integrated Nanosystems. Journal of Nanotheranostics, 6(4), 31. https://doi.org/10.3390/jnt6040031







