Abstract
Viral diseases remain a persistent threat to global health, agriculture, and biodiversity, as demonstrated by recent pandemics. The high mutation rates, diversity, and intricate replication mechanisms within a host can often challenge conventional detection and therapeutic approaches. The emergence of novel viruses underscores the critical importance of innovative and multidisciplinary strategies to outpace these diseases. In this context, nanotechnology has emerged as a transformative frontier, offering unique tools to address the limitations of traditional virology. This review examines the latest nanotechnological innovations designed to combat viral diseases. Like the development of advanced nanoplatforms, metallic and polymeric nanostructures, and carbon-based materials, and evaluating their roles in viral theranostics. This article provides critical biomedical insights into the function and relationship of nanomaterials, mechanisms of action, and their interaction with biological systems. This work aims to provide a valuable resource for guiding future research toward the clinical translation of nanomaterial-based strategies for the prevention, diagnosis, and treatment of viral infections.
1. Introduction
Viral diseases are pathologies caused by submicroscopic biological entities containing nucleic acids, called viruses, which can only reproduce by infecting a host cell, as they lack self-sufficient or self-replicating cellular machinery. When a virus infects a host cell, it uses the host machinery to create copies of itself, which can damage or destroy the infected cell and cause disease symptoms [1].
To date, there are more than 16,215 classified virus species, being the most abundant biological entities in the environment, with a complex array of billions of viruses that differ in their genome architecture and size, virion structure, genomic expression, and replication strategies [2]. It is reasonable to assume that many of these viruses can infect most organisms, including bacteria, blue-green algae, fungi, plants, insects, vertebrates, and even other viruses. Many viral infections cause serious diseases in livestock and crop plants, which have a significant impact on agriculture, leading to significant socioeconomic consequences [3,4]. In addition, various viral diseases also pose a risk to healthcare systems at risk, and many others have even put global public health at risk throughout history [5,6].
As shown in Table 1, some of the most important epidemics and pandemics in history have been caused by viral diseases, and their outbreaks still threaten humanity because there is no way to predict when or where the next major new viral pathogen will emerge, nor is there a way to reliably predict the ultimate significance of a virus upon its first appearance [7]. So that, in a multidisciplinary effort, several sciences are involved in the research and development of strategies to prevent pandemics caused by viruses by combining knowledge of health, technology, environment, and human behavior. In this sense, nanotechnology has emerged as a multidisciplinary science that offers promising tools for combating viral diseases by novel strategies such as improving drug delivery, enhancing antiviral therapies, and developing new diagnostic and therapeutic approaches [8,9,10].

Table 1.
Major pandemics caused by viruses throughout history.
In this review, we will summarize the recent advances in nanotechnology to combat viral diseases, first trying to understand the nature and evolution of viruses and the subsequent efforts to develop new strategies for combat and detection of viruses.
2. Viruses: Definition and Evolutionary Patterns
Even if the definition of a virus is open to discussion, the more commonly used definition refers to viruses as small infectious, intracellular parasites that infect all living organisms. This definition embraces the fact that viruses cannot replicate by themselves, and they utilize the host machinery for replication and expression of their genetic material [11]. This particularity implies that the selection of pressures, inherent to the host’s environment and genetic machinery, promotes the viruses to mutate and adapt to their genomes to increase viability in a more diverse number of hosts.
The adaptation to the host has been observed in some viruses of the Flaviviridae family, or the African swine fever virus [12,13]. In the case of the African swine fever virus (ASFV), it results in a severe hemorrhagic disease affecting domestic pigs and wild boars. Genomic analysis of relative synonymous codon usage reveals Codon usage bias variation within clades and among ASFVs and their host. With highly adapted to Ornithodoros moubata, as a possible result of the long-term co-evolution, adaptation and host interactions.
However, other selection pressures may act on viral and host genomes, and when studied, one must remember that genomes are the essential part of an organism, and at the same time are shaped by the evolutionary relationships of selective forces and the genetic drift. Additional mutations can be the result of structural and functional changes in proteins, and in some genome characteristics [14]. Many organisms have imprints of unique patterns in the distribution and arrangement of oligonucleotides in their genome, referred to as the genomic signature. Reflecting the diverse selection pressures influencing their evolution and differing between organisms [15,16].
New studies try to understand the genomic signatures to capture all possible evolutionary features and traits, acting as a selection pressure on viral species, comparing genomes, and identifying on the genomic level the relationship between virus and host. A recent study analyzed the genome sequences of 2768 eukaryotic viruses from 105 different viral families. Demonstrating that viruses have specific genomic signatures, and these are conserved among the viral families, with the existence of families with distinct signatures. However, as highlighted, these signatures are divergent from those present in their hosts. With a clear clue that evolutionary selection pressures are first imposed on the viruses, and later act on viral genomes to shape and preserve the genomic signatures [14].
The virus genome is plastic and with diverse ranges and sizes, from three orders of magnitude, some are less than 2 kilobases (kb) in some of the smallest RNA and DNA viruses to more than 2 megabases (Mb) in some pandoraviruses. This is an example of how diverse viruses can be, some representing the simplest protein codon replication and others surpassing some prokaryotic genome size and complexity [11].
The virus can also be segmented into a single viral particle or a multipartite virus; these segmented viruses have the capability of reassortment between genomic components during mixed infections. The evolutionary origin of viruses, monopartite or segmented/multipartite viral genomes, even when segmented/multipartite genomes showed some advantages, such as better virion stability [17,18].
One of the greatest advantages of this kind of virus are that the genome integrity is not mandatory either at the individual cell level or during transmission, deep-sequencing analysis of twenty FBNSV lineages demonstrated that the genome plasticity to the host and revealed that nanoviruses are capable of adjusting gene expression at the population level in changing environments, as well as modulating the copy number but not the sequence of each of their genes [19,20].
The multipartitism in viruses is an example of how evolution can produce phenotype novelty and adaptation to an ecological niche at the same time. Among the theories arising from the emergence of this novelty in viruses, the most accepted are those related to selection for cheats, which avoid producing a shared gene product but still benefit from gene products produced by other genomes. This can be key for the evolution of both multipartite and segmented viruses [14,18]. This theory is consistent with empirical patterns across a significant number of viruses [18]. The diversity of niches, genome structure, patterns, and adaptations to hosts is a key signature of the evolution and persistence of viruses.
3. Viral Diseases in Changing Environments
Viruses can colonize a big part of the known organisms, from archaea to humans, and are present in different and challenging environments [21,22]. However, most of the known viral diversity has been described over the last two decades, following advances in metagenomics and metatranscriptomics that enable the characterization of a wide variety of viruses associated with novel environmental and host habitats [21,23].
This novel understanding of viruses has been essential for identifying viruses capable of infecting humans. From the 14,691 species of viruses classified by the International Committee on Taxonomy of Viruses (ICTV) and documented in the Virus Metadata Resource spreadsheet (VMR_MSL39_v4 20241106), https://ictv.global/taxonomy (accessed on 26 September 2025) only 3.5% are considered human viruses; however, even these are still under classification and pending sequencing and genomic analysis. Many human viruses have a zoonotic origin. As climate variation increases contact between human populations and wildlife, the potential for zoonotic diseases also increases, as seen in the recent Rift Valley fever (RVF) outbreak in Rwanda in 2022, which resulted in 173 human cases, including 22 deaths, and was detected through molecular techniques [24]. Environmental fragmentation has been predicted to produce around 15,000 novel viral events by 2070, particularly in highly populated regions with tropical weather [25].
Some of the more studied reservoirs of viruses in mammals are Bats (order Chiroptera), which, in fact, have been proven to host more zoonotic viruses per species than any other mammalian order. This particularity is linked to hypotheses that include the evolution of flight, unique immune system adaptations, and a strong coevolution between bats and viruses [26,27]. Phylogenomic analysis of 60 Old World Myotis genomes reveals a high rate of introgression and hybridization within these species′ genomes. Interestingly, the higher rates of introgression were enriched in regions containing an antiviral pathway gene, suggesting a selection force that led to the immune-related genes being located in highly recombining genomic regions. This can be related to antiviral immune responses in bats [26].
Bats, as a reservoir of viruses, can also provide clues to the mechanisms of resistance, as is the case with the deadly Marburg virus disease (MVD) in humans. This virus can be harbored in Egyptian rousette bats asymptomatically, maybe as a relationship of specific host immunity–pathogen. Transcriptional responses revealed that bats employ a strategy that limits the induction of pro-inflammatory genes [28]. Subsequent studies on pro-inflammatory responses have shown that after these responses are diminished, the virus replicates and presents severe liver pathology, suggesting that bats balance immunoprotective tolerance with pro-inflammatory responses [29].
Some of these findings have shed light on environmental stress and its effect on bats′ immunity, as well as the possibility of transmission to an unprotected host and human populations [29,30]. The climate-induced competition for space and natural resources has led to the proliferation of high-density livestock operations. Places that have served as amplifying reservoirs for viral diseases [31]. The case of the avian influenza (HPAI) H5N1 virus is remembered as an outbreak that not only affected the health of avian populations but also caused an outbreak in humans across 60 countries in Asia, Europe, and Africa. To this day, this disease is still monitored in developing countries [31,32]. The severity of these waves of virus outbreaks can be attributed to the intensification of poultry production in China for ducks and chickens, and it is now also detected in cow production in Western countries [32,33].
While viruses affecting human health are drawing attention, environmental changes have been affecting the defenses of other organisms and disrupting outbreaks in animal and plant populations. In the case of plants, the temperature rise can lead to higher plant diseases, related to increased plant susceptibility and changes in pathogen presence [34]. In the case of the pathogen relocation, one of those cases is the bluetongue disease (BT), a viral disease with a small presence in Latin America, but as the bioclimatic factors have improved, the development of Culicoides, an important vector for the virus in the Ecuador region, has led to new outbreaks and reports in Ecuador and Andean countries [35].
In the relationship between plant hosts and climate change, associated with warm temperatures, the plant response is modulated by factors such as hormones and defense molecules, including reactive oxygen species (ROS) [36]. Across the multiple defenses that plants have developed as defense, the viral infection in plants is countered by plant RNA silencing, which recognizes and degrades the viral double-stranded genetic material through the activity of DICER-like (DCL) proteins [37]. The symptoms of viral diseases in plants can also be attenuated or suppressed at high temperatures and exacerbated by low temperatures; this particularity can pose a challenge in the future as the Earth’s temperature rises and winters become colder [38]. As global temperatures change and new viruses emerge, the problems humanity faces are the high rates of adaptation of these organisms and the connectivity of modern society that enables them to spread more rapidly [39,40]. Therefore, understanding the evasion mechanisms and developing improved detection methods is crucial for controlling viruses.
4. Viral Mechanisms of Evasion of Challenges Associated with Their Detection
Once the virus is inside the host, a cascade of responses is disrupted through the interplay of positive and negative regulation, as well as the pattern recognition receptor (PRR)-initiated immune responses. The first steps are essential not only for the importance of this first line of defense but also for correct immune activation. However, even when the defense is a complex network that evolves from host–virus relationships, viruses have developed strategies to subvert the host’s antiviral response and establish an infection, such as the expression of genes encoding immunomodulatory proteins that can deceive the host’s immune system. Some of the mechanisms of immune evasion described are focused on enhancing their replication. This is because the viral genome encodes numerous immunomodulatory proteins, which are essential for invading and disrupting the host’s immune system, thereby ensuring viral persistence [41,42]. One trait of interest is the molecular mimicry, which refers to the viral production of proteins that mimic the structure of some host proteins. The mimicked proteins served as a protective mechanism, limiting the targeting of the epitopes by the host and blocking the immune response, because the viral mimicry interactions have a similar binding strength and conformation to the endogenous interactions [43,44].
Other strategies include the use of viral proteases to cleave target proteins or promote the degradation of immune signaling molecules. Additionally, deubiquitinase enzymes are utilized to remove polyubiquitin chains, thereby preventing the activation of immune responses. Considering that signaling molecule complexes are essential for the transduction of immune signals, viral interference and interactions with these complexes, such as TRAF3, TANK, and TBK1, enable the virus to proliferate within the host [45,46].
In the same order of ideas, the virus can regulate the expression of immune proteins, as is the case with Stimulator of Interferon Genes (STING), a membrane signaling protein located in the endoplasmic reticulum with a size of 379 aa with a TM portion at the N-terminus, which regulates its cellular localization and homodimerization. STING plays a critical role in the defense against DNA viruses; however, some viruses have developed strategies to antagonize STING signaling [47,48]. In the case of the HSV ICP27 protein, it translocates to the cytoplasm after infection. When the protein interacts with STING, it inhibits the activation of IRF3. Protein γ34.5, produced by the HSV, downregulates STING trafficking from the ER to the Golgi by interacting with the N-terminus of STING. Others described proteins from HSV-1, such as UL46, and abundant HSV tegument proteins that interact with STING to prevent its activation. And the VP1-2 protein deubiquitinates STING, inhibiting its downstream signaling [49,50,51]. These exemplify the numerous strategies developed by a virus to evade the host immune response. For that complex landscape of potential virus outbreaks the humanity must be capable of detecting them in the population. Nowadays, a lot of strategies focus on the development of accessible and fast technology, such as the offer of nanotechnology or biosensors.
5. Macromolecular Features of Viruses of Biomedical Interest
Viruses of biomedical interest exhibit diverse macromolecular features that are crucial for their structure, replication, and pathogenicity [52]. These features primarily involve the viral capsid, a protein shell that encapsulates the viral genome, and in some cases, an envelope derived from host cell membranes. The viral capsid is the protein shell that encapsulates and protects the genetic material of a virus. This structure is composed of multiple protein subunits called capsomeres, which self-assemble to form a symmetrical or asymmetrical shape specific to each virus type [53].
Capsids serve several critical functions, including shielding the viral genome from environmental factors, facilitating viral entry into host cells, and assisting in the assembly and release of new virus particles [54]. The structural biology of capsids ranges from simple icosahedral symmetry to complex asymmetric arrangements, with some viruses employing helical or pleomorphic structures. This architectural variability directly influences capsid functionality and stability, determining factors such as genome packaging efficiency, resistance to environmental stressors, and the ability to undergo conformational changes during cell entry [55]. Moreover, capsid architecture plays a crucial role in immune evasion strategies. Some viruses utilize intricate surface patterns to shield antigenic epitopes, while others incorporate dynamic structural elements that can rapidly mutate to evade host immune recognition.
Viral surface proteins are specialized molecular structures located on the outer layer of a virus particle. Viral surface proteins display crucial roles in the virus’s life cycle, including host cell recognition, attachment, and entry. The properties of viral proteins arise from their glycoprotein nature, where the presence of carbohydrate-type components can confer protection towards proteolytic degradation and host immune evasion [56]. Viral surface proteins can take various forms, such as spikes, knobs, or fibers, depending on the type of virus. Understanding the macromolecular aspect of viruses is important, as viral surface proteins can undergo mutations, allowing them to adapt to new and challenging host environments [57].
Viral genomes exhibit remarkable diversity in structure, size, and organization, reflecting the vast array of viral pathogens that exist in nature. The genetic material of viruses can be either DNA or RNA, single-stranded or double-stranded, linear or circular, and segmented or non-segmented [58]. Genome sizes range from just a few kilobases in small viruses to over a megabase in giant viruses. The organization of viral genomes is often highly compact, with overlapping genes and regulatory elements to maximize coding capacity within size constraints. Replication mechanisms vary depending on the genome type but generally involve virus-encoded enzymes such as RNA-dependent RNA polymerases or reverse transcriptases [59]. Key enzymes, such as DNA polymerases, helicases, and integrases, are also involved in genome replication and integration. Viral genomes serve as invaluable resources in the development of antiviral therapies, vaccines, and diagnostic tools.
The viral envelope is a critical structural component of many viruses, serving as the outermost layer that encapsulates the viral nucleocapsid. Composed primarily of a lipid bilayer derived from host cell membranes, the envelope incorporates viral glycoproteins that play essential roles in host cell recognition and entry [60]. These envelope proteins, such as hemagglutinin in influenza viruses or spike proteins in coronaviruses, often form distinctive surface projections visible under electron microscopy [61]. The formation of the viral envelope typically occurs during the budding process, where the virus acquires its lipid coat from the host cell membrane. Enveloped viruses can be broadly categorized based on their genome type (DNA or RNA) and include important human pathogens like influenza, HIV, and herpesviruses [62]. The envelope’s role in pathogenesis is multifaceted, facilitating viral attachment to host cells, mediating membrane fusion for viral entry, and, in some cases, helping the virus evade host immune responses.
6. Nanotechnology: Historical Background, Synthesis Methods, and Classification of Nanomaterials
Nanotechnology, a discipline that emerged in the latter half of the 20th century, has significantly transformed agricultural, industrial, and medical practices. Richard Feynman initially introduced the concept in his 1959 lecture “There’s Plenty of Room at the Bottom,” although the term itself was later coined by Norio Taniguchi in 1974 [63]. In subsequent decades, researchers made considerable advancements in understanding and manipulating matter at the nanoscale. The invention of the scanning tunneling microscope in 1981 by Gerd Binnig and Heinrich Rohrer at IBM Zurich represented a pivotal milestone, allowing scientists to visualize individual atoms for the first time [64]. This breakthrough was succeeded by the development of atomic force microscopy in 1986, further enhancing the toolkit for nanoscale research. The discovery of fullerenes in 1985 and carbon nanotubes in 1991 opened new avenues for materials science and engineering at the nanoscale [65]. As the field advanced, interdisciplinary collaborations among physicists, chemists, biologists, and engineers led to innovative applications in various domains, including electronics, medicine, and energy. The establishment of national nanotechnology initiatives in various countries during the late 1990s and early 2000s further accelerated the research and development of materials with nanometric arrangements [66].
The synthesis of nanomaterials can be achieved through both top-down and bottom-up methodologies [67]. Top-down techniques encompass lithography and mechanical milling, whereas bottom-up strategies include chemical vapor deposition, sol–gel processing, and self-assembly. The top-down approach involves reducing bulk materials to nanoscale dimensions through physical or chemical processes. This method is based on the principles of mechanical engineering and materials science, frequently employing techniques such as lithography, etching, or milling [68]. The primary advantage of top-down synthesis is its capacity to produce large quantities of nanomaterials with precise control over size and shape. However, it often encounters issues related to surface imperfections and structural defects, which may compromise the material’s properties.
Conversely, bottom-up synthesis constructs nanomaterials from atomic or molecular precursors through chemical reactions or self-assembly processes [69]. Contrary to top-down techniques, bottom-up approaches are grounded in the principles of supramolecular chemistry and molecular self-organization. Examples include chemical vapor deposition, sol–gel processing, and template-directed synthesis. Regarding their advantages, it has been noted that bottom-up methods are particularly effective in producing nanomaterials with high purity, homogeneity, and fewer defects, together with high tailoring and structure capacity [70]. However, bottom-up routes also possess several disadvantages, including challenges in scaling up production and achieving uniform size distribution, especially for complex nanostructures.
Once the nanomaterials are synthesized, they are classified based on their dimensionality into 0D, 1D, 2D, and 3D structures, reflecting the number of dimensions that exceed the nanoscale range (1–100 nm). The three spatial dimensions are designated as the X-, Y-, and Z-axis, representing the horizontal, vertical, and depth dimensions, respectively [71]. Considering this, zero-dimensional (0D) nanomaterials, such as quantum dots, are confined in all three spatial dimensions, where their electron movement is restricted in all directions, resulting in discrete energy levels and unique optical and electronic properties [72]. On the other hand, 1D nanomaterials, including nanowires and nanotubes, exhibit confinement in two dimensions, allowing for electron movement along their length [73]. Moreover, 2D nanomaterials, such as graphene and nanosheets, are confined in one dimension, allowing electron movement in a plane. Finally, 3D nanostructures, such as nanoparticle assemblies, have no confinement at the nanoscale but maintain nanoscale features in their overall structure [74].
Related to the biomedical field, particularly in the treatment and diagnosis of viral diseases, the size-dependent emission spectra of 0D nanomaterials enable the precise detection of viral particles in diagnostic assays, where their tunable fluorescence capacity allows for the simultaneous multiplexed imaging of different viral components [75]. In treatment, nanomaterials can serve as carriers for antiviral drugs, thereby enhancing cellular uptake and facilitating real-time monitoring of drug delivery [76]. Similar events have been observed during the evaluation of 1D nanomaterials, where their high surface-to-volume ratio increases the number of binding sites for viral particles. Compared to 0D nanomaterials, the unique features of 1D nanomaterials can be leveraged to serve as scaffolds for antiviral drug delivery, allowing sustained release and improved efficacy [77]. Graphene-based biosensors are representative examples of 2D nanomaterials, offering rapid, label-free detection of viral antigens or nucleic acids with high sensitivity [78]. This can be achieved due to their large surface area, which enables the efficient immobilization of biomolecules for virus capture.
7. Treatment of Viral Diseases Utilizing Nanomaterials
Nanotechnology holds a promising future in the fight against viral diseases due to its unique ability to interact with biological systems at the molecular and cellular levels, enabling unprecedented control and precision in the prevention, diagnosis, and treatment of infections [79]. On a therapeutic level, nanoparticles can be designed to deliver antiviral drugs or vaccines directly to infected cells, increasing their efficacy and reducing side effects. While in the diagnostic field, nanosensors enable highly sensitive and rapid detection of viruses, even in the early stages of infection, which is crucial for containing outbreaks [80,81].
Many of the nanotechnological developments aimed at treating viral diseases have been achieved thanks to the development of nanomaterials with unique properties derived from their nanoscale structure, such as enhanced mechanical strength, high electrical or thermal conductivity, and controlled chemical reactivity. Nanomaterials possess several key properties that give them superior therapeutic potential compared to conventional approaches, making them highly effective in controlling viral diseases. Their nanometric size and high specific surface area enable them to interact more effectively with the biological membranes of host cells, resulting in specific uptake, cellular absorption, and even interaction with intracellular structures or the virus itself [82].
Furthermore, poorly water-soluble and unstable drugs can be packaged or complexed with nanomaterials to improve their solubility and stability under physiological conditions. They can also be designed to specifically target viral particles or infected cells, potentially increasing treatment efficacy and reducing side effects and systemic toxicity, potentially overcoming some of the common limitations of current treatments [83,84,85]. On the other hand, some nanomaterials act as “nanodecoys” that mimic cellular receptors and capture viruses, or as metallic nanoparticles (such as silver or gold) capable of blocking essential viral proteins, thereby inhibiting their entry into the host cell [86,87]. Others, such as magnetic nanoparticles, can be guided by external fields to achieve targeted delivery [88]. Likewise, materials such as mesoporous silica or biodegradable polymers offer high biocompatibility, reduce inflammation, and allow for prolonged and stable drug release [89,90]. Finally, their high loading capacity enables the simultaneous administration of multiple antiviral agents, generating therapeutic synergies that increase efficacy against various pathogens [91,92]. Therefore, nanomaterials represent a promising tool in the fight against viral diseases, opening new possibilities for both prevention and treatment. Several nanomaterial-based strategies for treating and controlling viral diseases are described below.
7.1. Metallic Nanoparticles (MNPs)
Despite the rapid advancement of nanotechnology and the development of new nanomaterials, the versatility of metallic nanoparticles continues to position them as a key element for the research and development of discoveries. These types of nanomaterials have been widely utilized in the development of strategies for preventing and treating viral diseases. Gold and silver nanoparticles exhibit promising antiviral potential due to their unique physicochemical properties and antiviral effects resulting from ligand-receptor chemical interactions. They can inhibit viral replication, disrupt viral envelopes, and enhance drug delivery, making them a viable alternative for combating viral infections [67,93,94]. These metallic nanoparticles have been shown with effective potentials for the prevention, diagnosis, treatment and mitigation of several virulent viral diseases including the human immunodeficiency virus (HIV), herpes simplex virus (HSV), hepatitis C virus (HCV), severe acute respiratory syndrome CoV 1 (SARS-CoV-1), Middle East respiratory syndrome CoV (MERS-CoV) and more recently SARS-CoV-2, the cause of the global pandemic.
Metallic nanoparticles (MNPs) have demonstrated different mechanisms of action, such as binding of nanoparticles to surface moieties of viral particles like spike glycoproteins, that disrupt viral attachment and uncoating in host cells; generation of reactive oxygen species (ROS) that denature viral macromolecules such as nucleic acids, capsid proteins, and/or lipid envelopes; inactivation of viral glycoproteins by the disruption of the disulfide bonds of viral proteins; but also other less specific ones such as photothermal effects; photocatalysis; intercalation/inhibition of viral DNA/RNA; apoptotic-inclined interactions and disruptive mimicry of viral structures due to morphological similarities [94,95].
Gold nanoparticles (AuNPs) have been used as a class of functional nanomaterials with enhanced antiviral properties, offering a versatile and promising approach for combating viral diseases. AuNPs can bind directly to viral particles or infected cells, blocking their adhesion and entry into host cells and thus inhibiting viral replication. They have proven effective against the influenza virus, herpes simplex virus, and human immunodeficiency virus [96,97,98,99]. AuNPs can also serve as carriers for antiviral drugs, enhancing their solubility, stability, and targeted release to the site of infection, thereby allowing for higher drug concentrations at the therapeutic target. Furthermore, when combined with existing antivirals or used to deliver new agents, AuNPs can help overcome drug resistance problems common in some viral infections. They can also be designed to present viral antigens, strengthening the body’s immune response and promoting the development of more effective vaccines [100,101,102,103,104].
Similarly, silver nanoparticles (AgNPs) have also been shown to be effective in treating viral diseases, and it is hypothesized that their antiviral mechanism of action may be similar to that of AuNPs. This means that AgNPs can directly act on viruses and inhibit their initial interaction with the host cell by blocking crucial steps of viral replication [105,106,107]. AgNPs have demonstrated broad antiviral and preventative potential against a wide range of viruses, such as respiratory syncytial virus (RSV), influenza virus (A, H1N1, H3N2), hepatitis virus, human parainfluenza virus, herpes virus (HSV-1 and HSV-2), poliovirus (PV), dengue virus (DENV), HIV, and coronaviruses (porcine epidemic diarrhea virus (PEDV) and feline coronavirus (FCoV), as well as against oncogenic viruses such as Kaposi’s sarcoma-associated herpesvirus and Epstein–Barr virus, through mechanisms such as reactive oxygen species (ROS) generation and autophagy [67,93,105,106,107].
The efficacy of AGNPs can be enhanced by controlled-release systems, such as mucoadhesive hydrogels containing tannic acid, which prolong contact and provide additional antiviral activity [108]. Furthermore, it has been demonstrated that pretreatment of respiratory syncytial virus (RSV) with curcumin-functionalized AgNPs exhibits significant inhibitory effects on viral titer, decreasing viral titers by approximately two orders of magnitude to non-toxic concentrations in host cells, while also directly inactivating the virus [109]. They also modulate the immune response by reducing the production of pro-inflammatory cytokines and chemokines and by stimulating the activation of cytotoxic T lymphocytes, NK cells, and B/plasma cells. Thanks to this combination of physical, chemical, and immunological effects, AgNPs are emerging as highly versatile, broad-spectrum antiviral agents [110,111]. The growing scientific interest in MNPs, particularly those of gold and silver, is due to their remarkable versatility and wide range of applications through diverse mechanisms, which are strongly influenced by physicochemical parameters such as size, shape, concentration, and surface functionalization. These parameters determine both efficacy and safety. While these properties position them as promising candidates for antiviral applications, further research is required to optimize their design, clarify their in vivo mechanisms, and ensure biocompatibility for clinical use.
7.2. Metal Oxide Nanoparticles (MO-NPs)
Another group of nanomaterials widely used to combat viral diseases is metal oxide nanoparticles (MONPs). MONPs exhibit a wide range of antiviral mechanisms like those of metal nanoparticles, which combine physical, chemical, and, in some cases, photochemical effects. MONPs are reported to act as antiviral agents by attaching to the surface of the virus, thereby preventing the interaction between binding sites on the virus’s exterior and specific receptors on the host cell surface, and thus inhibiting virus entry into the cell [112,113]. For example, in a study carried out to evaluate the antiviral efficacy of cuprous oxide nanoparticles (CuONPs) against hepatitis C virus (HCV), the results showed that CuONPs, at non-cytotoxic concentrations, significantly inhibited viral infectivity by blocking both viral binding and entry into liver cells, without affecting viral replication [114]. Zinc oxide nanoparticles (ZnONPs) have also been shown to have negatively charged surfaces that can interact with herpes simplex virus type 2, thus preventing its entry into host cells. Because the virus bound to ZnONPs cannot infect cells, dendritic cells in the vaginal lining produce antibodies that identify and destroy infected cells, thus hindering the spread of infection [115].
Another of the most relevant mechanisms of MONPs is the generation of reactive oxygen species (ROS), such as hydrogen peroxide, hydroxyl and superoxide radicals, which trigger processes such as lipid peroxidation that alter lipids, proteins, carbohydrates, and viral nucleic acids, ultimately leading to viral destruction. Research has shown that semiconductor oxides such as ZnO, TiO2, and SnO2 can absorb UV-vis light, dissociate water molecules, and release metallic ions, generating ROS and causing direct damage to nucleic acids, proteins, and viral membranes [116,117,118]. Other studies have shown that this oxidative effect can also induce conformational alterations in viral glycoproteins, such as the SARS-CoV-2 spike protein, hindering their binding to host cell receptors [119].
In addition, MONPs can serve as a versatile platform for delivering therapeutic agents, thanks to their ability to function with bioactive molecules attached to the nanoparticle surface. This has been exemplified by iron oxide nanoparticles (IONPs), which were employed as delivery systems carrying a DNAzyme targeting the hepatitis C virus (HCV) NS3 gene, a critical component for viral replication that encodes an essential helix and protease. Furthermore, in vivo studies on mice have demonstrated that IONPs accumulate in hepatocytes and macrophages within the liver, suggesting their potential utility as a targeted therapeutic tool for the treatment of hepatitis C [120]. Like MNPs, MONPs represent a promising therapeutic platform for treating viral diseases, thanks to their ability to combine direct antiviral effects with additional properties such as controlled drug release, targeting, photocatalysis, and immune response modulation. However, the clinical translation of these nanoparticles requires overcoming challenges related to their biocompatibility, long-term toxicity, biodistribution, and potential generation of viral resistance.
7.3. Carbon-Based Nanomaterials (CB-NMs)
CB-NMs have emerged as promising tools in the development of innovative antiviral strategies due to their physical and chemical properties. CBNMs encompass a range of nanoscale structures composed primarily of carbon atoms, including fullerenes, carbon nanotubes (CNTs), graphene oxide (GO), and carbon quantum dots (CDs), each of which possesses unique properties and distinct applications as therapeutic tools for combating viral diseases.
7.3.1. Fullerenes
Fullerenes and their derivatives were the first carbon-based nanomaterials to be discovered in 1985, and, since then, they have also been the first carbon-based nanomaterials to be tested as antiviral treatments. Fullerene molecules are spherical or ellipsoidal in shape, featuring a hollow cage structure that enables them to interact with key viral proteins. Additionally, they possess unique electronic characteristics, photophysical properties, and excellent biocompatibility [121]. The antiviral mechanisms of fullerenes include their use as a scaffold to create multivalent compounds, such as glycodendrofullerenes, to block cell-surface receptors and inhibit the entry of viruses into host cells [122]. But they also have demonstrated that they can allosterically inhibit essential enzymes such as HIV-1 protease and reverse transcriptase due to the hydrophobic surface and suitable size of the carbon cage [123,124,125]. For a long time, several research groups have been working on tailoring the synthesis of fullerene derivatives to inhibit the activity of enzymes from hepatitis C virus (HCV), respiratory syncytial virus (RSV), influenza virus (H1N1), herpes simplex virus (HSV), human cytomegalovirus, Zika, and Dengue viruses [126,127,128,129].
Fullerene derivatives, particularly those with strong scavenging capabilities for reactive oxygen species (ROS), have also shown promise in antiviral therapy and as drug delivery systems [130,131]. They can act as antioxidants, scavenging ROS generated during viral infections and even disrupting viral replication by targeting viral enzymes or interfering with viral maturation. A key property of fullerene is its ability to generate singlet oxygen, a highly reactive form of molecular oxygen that enhances its reactivity and capacity to oxidize various biological molecules. Singlet oxygen is an oxidizing radical characterized by a longer lifetime compared to other radicals; furthermore, singlet oxygen has a lower reduction potential and is more specific. Therefore, several research groups have explored the potential of using photoactivated fullerene and its derivatives as antiviral agents [132,133].
7.3.2. CNTs
CNTs are cylindrical molecules composed of carbon atoms, with a structure similar to a rolled sheet of graphene, that can be classified according to the number of walls that compose them: single-walled carbon nanotubes (SWCNTs) or multi-walled carbon nanotubes (MWCNTs) [134]. These nanomaterials have been utilized for the treatment of viral diseases due to their ability to interact physically with immune system components and soluble plasma proteins, making them effective in treating several pathogenic viruses, including HIV-1, dengue virus, influenza virus, and coronaviruses [135,136]. Some research has shown that, due to its geometric complementarity and strong Van der Waals interactions with the active site of HIV-1 protease, CNTs can bind to it with high affinity, thereby interfering with its function [137].
CNTs can also be functionalized using covalent or noncovalent strategies to act as carriers, inhibitors, or adjuvants in antiviral therapies. Covalent functionalization encompasses processes such as carboxylation, amination, amide coupling, or 1,3-dipolar cycloaddition, which enable the direct attachment of siRNA, oligonucleotides, or drugs to the nanotube structure. Non-covalent functionalization relies on π–π interactions or coating with polymers and lipids, preserving the CNT structure and facilitating the adsorption of biomolecules [138]. CNTs have been used as an antigen delivery tool against dengue. For this purpose, CNTs were covalently functionalized with the recombinant dengue serotype 3 virus envelope, thereby enhancing the antigenicity of a previously poor immunogenic antigen and improving cellular and neutralizing antibody responses against dengue envelope proteins. In this way, specific immune responses were induced, and the expression of a tetravalent vaccine was enhanced in both in vitro and in vivo models, with potential applications for other dengue serotypes [139].
CNTs have also been functionalized as nonviral molecular transporters for the delivery of siRNA into human T cells and primary cells, achieving effective silencing of the CXCR4 and CD4 receptors, which are required for HIV viral entry. Moreover, the functionalized CNTs were made water-soluble by the strong adsorption of phospholipids grafted onto amine-terminated polyethylene glycol, resulting in a nanotube suspension that remained stable as a solution. Therefore, the development of transporter vehicles for a wide range of cell types should facilitate the manipulation of genes and the investigation of cell functions in cell culture, with potential applications extending to in vivo settings [140]. On the other hand, other studies have evaluated the use of porphyrin-functionalized CNTs as light-activated antiviral agents in photodynamic therapies. This approach leverages the high absorption capacity of porphyrins in the near-infrared (NIR) range, generating reactive oxygen species (ROS) that significantly reduce the influenza A virus’s ability to infect mammalian cells [141].
7.3.3. GO
Graphene is a single layer of carbon atoms with sp2 hybridized orbitals forming a honeycomb lattice. In contrast, graphene oxide (GO) is a layered carbon structure with oxygen-containing functional groups (hydroxyl, epoxide, ketone, and carboxyl) attached to both sides of the layer as well as the edges of the plane. Both graphene and graphene oxide have attracted significant interest as nanomaterials with antiviral potential due to their high surface-to-volume ratios, their unique mechanical, electrical, and physicochemical properties, their ability to form a molecular barrier, and their superior mechanical strength [142,143]. Several studies have shown that these nanostructures can inactivate viruses through various mechanisms. One of the most significant effects is the direct interaction between the surface of the nanomaterial and the viral particles. In a recent study, the antiviral activity of GO against pseudorabies virus (PRV) and porcine epidemic diarrhea virus (PEDV) was evaluated. The results showed that GO significantly reduced viral infectivity at non-cytotoxic concentrations, which is attributed to GO’s single-layer lamellar structure, where its sharp edges physically disrupt the virus’s structure, and to its electrostatic interactions, where the negative charge allows destructive interactions through direct contact with the positively charged viral envelope before host entry.
Furthermore, it was observed that functionalization with nonionic polymers maintained antiviral activity, while conjugation with cationic polymers abolished it [144]. Similarly, thermally synthesized polysulfated dendritic polyglycerol-functionalized reduced graphene has demonstrated antiviral performance against various viruses, including African swine fever virus, orthopoxvirus, and herpesvirus strains. In these studies, it was confirmed that the large surface area of graphene scaffolds provides the highest ligand contact area for the adsorption of negatively charged sulfates, which can interact with the positively charged residues of enveloped viruses, showing signs of strong inhibitory results [145,146,147]. GO, used without additional markers or functionalization, has also been tested as a nanomaterial to inhibit the viral activity of two enteric viruses: the endemic gastrointestinal avian Influenza A virus (H9N2) and Enterovirus 71 (EV71), a virus responsible for hand, foot, and mouth disease. Herein, the results showed that GO sheets, on their own and without the need for additional markers, can bind strongly to different types of viruses thanks to their large surface area and electrostatic interactions, subsequently causing the structural destruction of viral particles and reducing their infectivity. Nevertheless, the antiviral effect of GO was found to be highly temperature-dependent, as only weak antiviral activity was detected at 25 °C and 37 °C; however, at 56 °C, GO eliminated the infectivity of both H9N2 and EV71 viruses [148].
7.3.4. CQDs
As mentioned above, another option for carbon-based nanomaterials is the use of carbon dots (CDs). CDs have a very high surface-to-volume ratio, with diameters of around 10 nm, are hydrophobic, environmentally inert, non-toxic in vitro and in vivo, and exhibit bright fluorescence, simple synthesis routes, and photocatalytic functions [149,150]. CDs include different types of nanomaterials such as amorphous carbon nanoparticles, partially graphitized core–shell carbon nanoparticles, amorphous fluorescent polymeric nanoparticles, and graphene quantum dots [125]. Antiviral mechanisms of CDs have been investigated, and it has been demonstrated that they can significantly inhibit the replication of the porcine reproductive and respiratory syndrome virus (PRRSV) and pseudorabies virus (PRV) by activating interferon production and the expression of interferon-stimulated genes [151].
8. Diagnosis of Viral Diseases Utilizing Nanomaterials
Nanotechnology has enabled significant advances in medicine, specifically in diagnostic and imaging methods, as well as in targeted therapies for the treatment of chronic degenerative diseases [152]. In the case of diagnosis, this process is essential, as it provides information that allows for proper treatment of the disease. Especially in viral diseases, early diagnosis is important to prevent the spread of the virus and stem the outbreak [153]. Although there are currently a large number of methods for virus detection, which are based on cell cultures, serological methods using specific viral antigens, enzyme-linked immunosorbent assay (ELISA), immunofluorescence, immunoperoxidase, hemagglutination, and even molecular methods such as polymerase chain reaction and gene sequencing, these methods are somewhat imprecise and take a long time [154,155]. These limitations have thus accelerated the development of new diagnostic strategies based on nanomaterials, due to their unique physicochemical and optical characteristics, to improve testing times, sensitivity, and accuracy, and make them easier to perform [156]. Among the strategies employed are the use of metallic nanoparticulate systems (gold nanoparticles, silver nanoparticles, platinum nanoparticles, magnetic nanoparticles, copper nanoparticles, silica nanoparticles), carbon-based nanomaterials (carbon nanotubes, carbon dots, and graphene oxide), and polymeric nanoparticles (micelles, dendrimers, polyplexes, and nanospheres/nanocapsules) (Figure 1) [157,158,159].
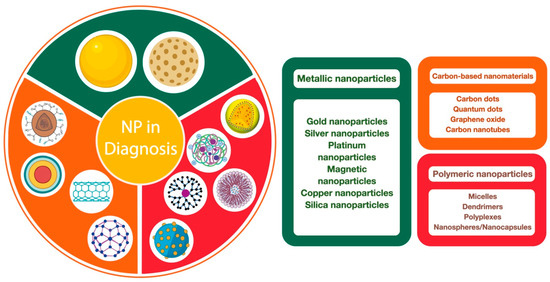
Figure 1.
Types of nanoparticulated systems used in the diagnosis of viral infections.
Among the different nanoparticulate systems that have been developed for the diagnosis of viral diseases, metallic or metallic oxide nanoparticles are the most reported due to their greater sensitivity and selectivity, due to their special structure and their considerable mechanical, thermal, optical, and electrical properties [160]. In fact, at very low concentrations of metal or metal oxide nanoparticles, they have significant potential to detect biomarkers associated with viral infections [161]. In this sense, their use in electrochemical biosensors or optical devices has enabled improved detection capacity, as they can bind to these materials through chemical bonding or physical adsorption [161]. Some examples of metallic nanoparticles or metallic oxides are gold, silver, platinum, palladium, copper, zinc oxide, cadmium sulfide, copper oxide, and magnetic nanoparticles [162,163].
Virus detection methods involve measuring electrochemical, optical, or magnetic changes (Figure 2). However, in the case of metallic nanoparticles, optical detection is the most important due to their surface plasmonic resonance state [164]. Optical detection systems include fluorescence, colorimetry (aggregation or color change), surface-enhanced Raman spectroscopy, and Förster resonance energy transfer [165].
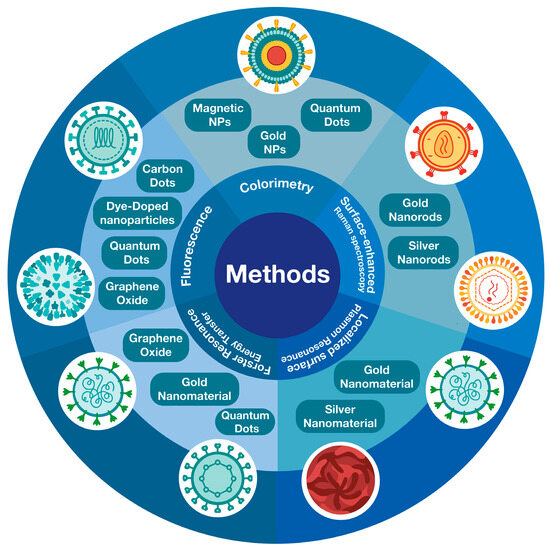
Figure 2.
Types of nanomaterials used by optical methods for virus detection.
Compared to other nanomaterials, metallic nanoparticles are the most frequently used for detecting different types of viruses, due to their simplicity in synthesis, characterization, surface modification, excellent stability, and biocompatibility, as well as high absorption coefficients [158]. AuNPs were undoubtedly the first nanomaterials developed for virus detection [153]. One of the first reports of their use dates back to the late 1990s when AuNPs were used to detect the human papillomavirus [166]. Considering their photochemical and physical characteristics, such as shape and size, they are ideally suited for use in optical methods for virus detection. Actually, the morphology and shape (spheres, rods, prisms, tetrapods, cubes, shells, and hollow structures) of these nanostructures play an important role because they exhibit different excitation/emission ranges [158]. Furthermore, they easily form active and robust conjugates with target biomolecules, such as proteins and DNA [153]. Likewise, their surfaces can be modified with antigens and antibodies through electrostatic interactions. Therefore, detection methods for these types of nanomaterials include probes that utilize plasmonic resonance shifting, surface-enhanced Raman spectroscopy, and naked-eye monitoring through color change [167].
Like AuNPs, AgNPs have been used in the biomedical field due to their diverse applications, excellent chemical stability, photostability, non-toxicity, biocompatibility, and substantial thermal and electrical conductivities [168]. AgNPs are characterized by a highly reactive surface, which is why their optical and catalytic properties are excellent despite their colloidal nature; that is, they cause considerable changes in biological, physical, and chemical activities [169,170]. For this reason, they have been considered promising nanomaterials for the early diagnosis of viral diseases. These types of nanomaterials exhibit lower refractive indices compared to other metallic nanoparticles; however, when bound to biomolecules, they exhibit an increase in the local refractive index and a shift in Ag extinction, resulting in the effective detection of the target molecule [171]. Optical detection methods for these types of nanomaterials include surface plasmon resonance and Raman spectroscopy [172]. Furthermore, AgNPs exhibit greater fluorescence capacity compared to AuNPs or copper nanoparticles (CuNPs), and their fluorescence is even enhanced when the DNA hairpin structure contains guanine-rich molecules at the two terminals [173,174]. Therefore, they are highly sensitive and selective in distinguishing between tiny nucleotides [175].
Unlike AuNPs and AgNPs, CuNPs have not been extensively studied for virus detection, but they are known to exhibit good biocompatibility, low toxicity and oxidation resistance, are easy to synthesize and inexpensive, and possess excellent antiviral, antifungal, and antibacterial activity. However, the use of CuNPs for detecting respiratory syncytial virus using the surface plasmon resonance method has been reported [176]. These nanomaterials demonstrated superior detection and quantification capabilities compared to AuNPs and AgNPs [176].
Platinum nanoparticles (PtNPs) have gained much attention for their several applications in the biomedical field due to their excellent biocompatibility, high surface-to-mass ratio, small particle size, high surface reactivity, and good electrocatalytic properties [177], so they have been used as nanoenzymes due to their similar behavior to superoxide dismutase and catalase [178,179]. PtNPs are commonly used as catalysts due to their excellent stability in acidic electrolytes. Still, they are also characterized by exhibiting good catalytic properties, making them very effective in detecting viruses by electrochemical methods [157]. As for silica nanoparticles (SiNPs), these, like other metallic nanoparticles, have applications in biomedical sciences due to their biocompatibility, large specific and reactive surface area, pore volume, adjustable particle size, and simple and economical synthesis Biomolecules such as peptides, DNA, antigens, and antibodies have been described to bind to SiNPs, and thus have been promisingly used for virus detection in biosensors and immunosensors [180].
In the case of metal oxide nanoparticles, ZnONPs have proven useful in signal transduction in biological sensors due to their high isoelectric point and biocompatibility. The large surface area of ZnONPs provides signal amplification for target biomarker detection, thus increasing sensitivity and selectivity. Furthermore, their ionic and semiconducting properties enable them to achieve maximum sensitivity for detecting target biomarkers at very low concentrations [161]. Remarkably, ZnONPs can be produced using current techniques that are scalable in volume and have a low production cost [161]. Meanwhile, cadmium sulfide nanoparticles (CdSNPs) have demonstrated excellent photodynamic behavior and desirable bioactivity, making them an interesting strategy for photocatalysis [181]. Indeed, photoelectrically active CdS nanorods modified with beta-cyclodextrin have been reported to exhibit higher sensitivity in detecting human immunodeficiency virus (HIV) DNA in human serum samples compared to AuNPs and carbon dots, as well as to fluorometric methods [182].
Finally, magnetic nanoparticles are composed primarily of chemical compounds such as iron oxide, cobalt oxide, and nickel oxide. These types of nanomaterials possess superior properties compared to others due to their high specific surface area and favorable magnetic characteristics, which generally depend on the type of material and size. Magnetic nanoparticles have emerged as a viable strategy in disease diagnosis, owing to their unique optical properties, as well as their magnetoelectric, chemical, mechanical, and thermal properties [183]. Although virus detection methods using metal nanoparticles are simple and rapid compared to conventional methods, they require several processing steps, such as synthesis, binding, washing, and elution, which could be complex in clinical practice [184]. Some examples of metallic nanoparticles in the diagnosis of viral infections are shown in Table 2 [158,161,163,165,173,185,186].

Table 2.
Examples of metallic nanoparticles in the diagnosis of viral infections.
Perhaps after metal nanoparticles, carbon-based nanomaterials are the second most widely used nanomaterials for the detection of viral diseases. The main carbon nanostructures are based on their dimensions (zero, one, and two dimensions) and are widely used in sensing. Carbon dots and graphene quantum dots are examples of zero-dimensional carbon nanostructures. Carbon nanotubes are considered one-dimensional nanostructures, and graphene and its derivatives are two-dimensional carbon nanostructures [187]. Their electronic characteristics, fluorescent, photoluminescent, chemiluminescent, and electro chemiluminescent properties give them significant potential for disease detection. Furthermore, they are relatively simple to synthesize, the synthesis methods are cost-effective, the materials used are inexpensive, they are water-soluble, have low toxicity, good chemical stability, and are easily functionalized. The detection methods for this type of nanomaterial are based on: fluorescence quenching, static quenching, dynamic quenching, energy transfer, internal filter effect, photoinduced electron transfer, and fluorescence resonance energy transfer [188]. SARS-CoV-2, dengue, Ebola, hepatitis, human immunodeficiency virus (HIV), influenza (H5N1 and H1N1), Zika, and adenovirus are some of the viruses that can be detected by carbon nanomaterials [187].
Carbon nanotubes (CNTs) are one-dimensional hollow tubular structures composed of sp2-hybridized carbon sheets. They can be composed of single-walled (SWCNTs), with diameters ranging from 0.7 to 1.4 nm and lengths ranging from a few hundred nm to a few μm, and multi-walled (MWCNTs), which can have diameters up to 100 nm [189]. Furthermore, the large surface area of these carbon nanomaterials makes them optimal candidates for chemical functionalization with aptamers, polymers/biopolymers, proteins, nucleic acids, among others, making them biocompatible, less toxic, and more sensitive for virus detection [190]. CNTs are interesting options for electrochemical sensing applications. However, CNTs have been reported to exhibit excellent luminous intensity and important characteristics ideal for their use in optical biosensing. Furthermore, semiconductor CNTs can act as fluorophore quenching agents and exhibit a distinctive near-infrared (NIR, wavelength~0.8–2 μm) photoluminescence that emits from bandgap fluorescence [191].
Carbon dots (CDs) are zero-dimensional carbon nanomaterials (less than 10 nm in size), which have been categorized into: carbon quantum dots (CQDs), graphene quantum dots (GQDs), carbon nanodots (CNDs), and carbonized polymer dots (CPDs) [192]. Unlike other carbon nanomaterials, CDs have modifiable surfaces because they generally contain functional groups such as amino (-NH2), hydroxyl (-OH), carboxyl (-COOH), sulfhydryl (-SH), and aldehyde (-CHO) groups. Furthermore, they have low toxicity, good solubility in different solvents and conductivity, and excellent chemical and mechanical properties [192]. CDs are characterized by remarkable optical properties, as well as fluorescence, chemiluminescence, electrochemiluminescence, phosphorescence, and photoluminescence, and have therefore been used for biodetection [187]. Although optical detection and biosensing methods used for CDs have been widely used, electrochemical methods have generated greater interest due to their faster response, high sensitivity and accuracy, affordability for in situ detection, and easy manufacturing [192].
Graphene consists of a two-dimensional monoatomic sheet of carbon arranged in a honeycomb lattice. It is the most widely used carbon nanomaterial for virus detection [190]. This nanomaterial features excellent thermal and electrical conductivity, optical absorption, good mechanical strength, flexibility, and biocompatibility, as well as a large surface area, making it very easy to interact with biomolecules [193]. Graphene can be found in the form of graphene oxide (GO), reduced graphene oxide (rGO), graphene sheets, and layered/multilayer graphenes [194]. However, GO and rGO have been the most studied due to their similarity to graphene in terms of properties such as flexibility and low cytotoxicity. Graphene and its derivatives can adsorb molecules through non-covalent interactions such as London forces, polarization, hydrophobic effects, and electrostatic attraction forces, so hybrid structures can be formed with some materials such as nanoparticles, quantum dots, nanoclusters, polymers, and various biomolecules, making them more efficient in virus detection [195]. Some examples of carbon nanomaterials in the diagnosis of viral infections are shown in Table 3 [187,190,195].

Table 3.
Diagnosis of viral infections used carbon-based nanomaterials.
Polymer-based nanomaterials are not only used for the development of drug delivery systems but also for the detection of viruses. These nanomaterials are considered inexpensive and simple to synthesize, as well as bioinert and biocompatible. Furthermore, they are thermally stable and easily coordinate with metals to form porous and structurally detailed structures, thus favoring increased fluorescence and a viable option for receptor immobilization, as they can act as substrates. In this sense, these types of nanomaterials are the first choice for virus detection using optical methods [196]. For example, hyperbranched polymers, such as dendrimers, can enhance conjugation between the target and the sensing platform [197]. Likewise, the conjugation of polymeric nanomaterials with metallic or carbon nanomaterials enhances detection sensitivity and has been reported to suppress background noise [190]. Polymer dots have the potential to replace cadmium-based quantum dots in virus detection and imaging due to their exceptional fluorescent characteristics and biocompatibility [198].
9. Conclusions
With over 16,215 classified virus species, the rapid mutation rates and diverse replication mechanisms of these pathogens necessitate the development of novel approaches that can effectively diagnose and treat viral diseases. Nanotechnology has emerged as a transformative frontier in this context, providing unique tools that enhance the efficacy of traditional therapeutic and diagnostic methods. The exploration of various nanomaterials demonstrates their potential advantages, including improved drug delivery, enhanced bioavailability, and increased sensitivity in diagnostic assays. The advantages of these nanomaterials include their high surface area-to-volume ratios, tunable properties, and ability to interact with biological systems at the molecular level.
Nanotechnology has adapted to be a great strategy to detect viruses, among the reasons for this include the sensitivity of some nano biosensors that are capable of single-molecule-level detection and have shown accurate detection of target substances and avoiding false positives for noise interference. Another real advantage is biocompatibility with almost all living organisms, which leads to minimal toxicity and an immune response to living organisms.
But even with a range of advantages, there is also a group of limitations, like those related to fabrication, the challenges of the nature of the sample, and the correct substrate functionalization. Luckily, in the past years, these disadvantages have been improved to make it possible to achieve better reproducibility, detection limit, and selectivity of the nano biosensors. The next frontier of the development of nanobiosensors is in the design of disposable and self-contained materials and the need for autonomous systems with reproducible results.
Among the perspectives for the development of nano biosensors, the interplay with artificial intelligence is now a primary focus; in some cases, the AI and ML biosensors are applied for detecting viruses like COVID-19. With the application of artificial neural networks and a simple statistical test to identify SARS-CoV-2 positive patients, artificial intelligence in combination with nanomaterials can transform health prophylaxis, with effective, efficient clinical management on viruses’ future outbreaks.
From the retrieved evidence, it was noted that Au- and AgNPs have exhibited promising antiviral effects, while carbon-based materials such as GO and carbon-based nanomaterials offer unique optical properties that enhance virus detection. As environmental factors continue to change and new viral threats emerge, the need for multidisciplinary collaboration becomes increasingly critical. Future research should focus on optimizing the design and biocompatibility of nanomaterials, ensuring their safety for clinical use while maximizing their therapeutic potential.
Author Contributions
Conceptualization, M.C.-T. and J.L.M.-M.; validation, M.C.-T., B.S.-C., K.S.G.-S. and D.H.-P.; investigation, M.C.-T., B.S.-C., D.H.-P. and J.L.M.-M.; writing—original draft preparation, M.C.-T., B.S.-C., D.H.-P. and J.L.M.-M.; writing—review and editing, M.C.-T., E.R.L.-M., B.S.-C., D.H.-P. and J.L.M.-M.; visualization, K.S.G.-S., D.H.-P. and J.L.M.-M.; supervision, D.H.-P. and J.L.M.-M.; funding acquisition; J.L.M.-M.; project administration, D.H.-P. and J.L.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kotra, L.P. Viral Disease. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–3. [Google Scholar]
- Mougari, S.; Sahmi-Bounsiar, D.; Levasseur, A.; Colson, P.; La Scola, B. Virophages of Giant Viruses: An Update at Eleven. Viruses 2019, 11, 733. [Google Scholar] [CrossRef]
- Furuse, Y.; Oshitani, H. Viruses That Can and Cannot Coexist With Humans and the Future of SARS-CoV-2. Front. Microbiol. 2020, 11, 583252. [Google Scholar] [CrossRef]
- He, M.; He, C.Q.; Ding, N.Z. Human Viruses: An Ever-Increasing List. Virology 2025, 604, 110445. [Google Scholar] [CrossRef]
- Parvez, M.K.; Parveen, S. Evolution and Emergence of Pathogenic Viruses: Past, Present, and Future. Intervirology 2017, 60, 1–7. [Google Scholar] [CrossRef]
- Alizadeh, H.; Sharifi, A.; Damanbagh, S.; Nazarnia, H.; Nazarnia, M. Impacts of the COVID-19 Pandemic on the Social Sphere and Lessons for Crisis Management: A Literature Review. Nat. Hazards 2023, 117, 2139–2164. [Google Scholar] [CrossRef]
- Murphy, F.A.; Nathanson, N. The Emergence of New Virus Diseases: An Overview. Semin. Virol. 1994, 5, 87–102. [Google Scholar] [CrossRef]
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The Role of Nanotechnology in the Treatment of Viral Infections. Ther. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology Approaches for Global Infectious Diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, M.; Vora, A. Nanotechnology-Based Antiviral Therapeutics. Drug Deliv. Transl. Res. 2020, 11, 748–787. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. The Logic of Virus Evolution. Cell Host. Microbe. 2022, 30, 917–929. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, C.; Zhang, J.; Zhang, K.; Deng, D.; Zheng, W.; Chen, N.; Meurens, F.; Zhu, J. Systematic Analysis of the Codon Usage Patterns of African Swine Fever Virus Genome Coding Sequences Reveals Its Host Adaptation Phenotype. Microb. Genom. 2024, 10, 001186. [Google Scholar] [CrossRef]
- Mifsud, J.C.O.; Lytras, S.; Oliver, M.R.; Toon, K.; Costa, V.A.; Holmes, E.C.; Grove, J. Mapping Glycoprotein Structure Reveals Flaviviridae Evolutionary History. Nature 2024, 633, 695–703. [Google Scholar] [CrossRef]
- Holmudden, M.; Gustafsson, J.; Bertrand, Y.J.K.; Schliep, A.; Norberg, P. Evolution Shapes and Conserves Genomic Signatures in Viruses. Commun. Biol. 2024, 7, 1412. [Google Scholar] [CrossRef]
- Fan, Z.; Whitaker, V.M. Genomic Signatures of Strawberry Domestication and Diversification. Plant Cell 2024, 36, 1622–1636. [Google Scholar] [CrossRef]
- Brownstein, C.D.; MacGuigan, D.J.; Kim, D.; Orr, O.; Yang, L.; David, S.R.; Kreiser, B.; Near, T.J. The Genomic Signatures of Evolutionary Stasis. Evolution 2024, 78, 821–834. [Google Scholar] [CrossRef]
- Michalakis, Y.; Blanc, S. Aspects of the Lifestyle of Multipartite Viruses Apply to Monopartite Segmented and Perhaps Nonsegmented Viruses. npj Viruses 2024, 2, 31. [Google Scholar] [CrossRef]
- Leeks, A.; Young, P.G.; Turner, P.E.; Wild, G.; West, S.A. Cheating Leads to the Evolution of Multipartite Viruses. PLoS Biol. 2023, 21, e3002092. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, J.; Torralba, B.; Yvon, M.; Zeddam, J.L.; Blanc, S.; Michalakis, Y. Nonconcomitant Host-to-Host Transmission of Multipartite Virus Genome Segments May Lead to Complete Genome Reconstitution. Proc. Natl. Acad. Sci. USA 2022, 119, e2201453119. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Di Mattia, J.; Ravel, S.; Zeddam, J.L.; Vitalis, R.; Michalakis, Y.; Blanc, S. Gene Copy Number Variations at the Within-Host Population Level Modulate Gene Expression in a Multipartite Virus. Virus Evol. 2022, 8, veac107. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, S.; Borrel, G.; Krupovic, M.; Gribaldo, S. A Compendium of Viruses from Methanogenic Archaea Reveals Their Diversity and Adaptations to the Gut Environment. Nat. Microbiol. 2023, 8, 2170–2182. [Google Scholar] [CrossRef]
- Minch, B.; Moniruzzaman, M. Expansion of the Genomic and Functional Diversity of Global Ocean Giant Viruses. npj Viruses 2025, 3, 32. [Google Scholar] [CrossRef]
- Neri, U.; Wolf, Y.I.; Roux, S.; Camargo, A.P.; Lee, B.; Kazlauskas, D.; Chen, I.M.A.; Ivanova, N.; Zeigler Allen, L.; Paez-Espino, D.; et al. Expansion of the Global RNA Virome Reveals Diverse Clades of Bacteriophages. Cell 2022, 185, 4023–4037.e18. [Google Scholar] [CrossRef] [PubMed]
- Remera, E.; Rwagasore, E.; Muvunyi, C.M.; Ahmed, A. Emergence of the First Molecularly Confirmed Outbreak of Rift Valley Fever Among Humans in Rwanda, Calls for Institutionalizing the One Health Strategy. IJID One Health 2024, 4, 100035. [Google Scholar] [CrossRef]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Foley, N.M.; Harris, A.J.; Bredemeyer, K.R.; Ruedi, M.; Puechmaille, S.J.; Teeling, E.C.; Criscitiello, M.F.; Murphy, W.J. Karyotypic Stasis and Swarming Influenced the Evolution of Viral Tolerance in a Species-Rich Bat Radiation. Cell Genom. 2024, 4, 100482. [Google Scholar] [CrossRef] [PubMed]
- Wynne, J.W.; Wang, L.F. Bats and Viruses: Friend or Foe? PLoS Pathog. 2013, 9, e1003651. [Google Scholar] [CrossRef]
- Guito, J.C.; Prescott, J.B.; Arnold, C.E.; Amman, B.R.; Schuh, A.J.; Spengler, J.R.; Sealy, T.K.; Harmon, J.R.; Coleman-McCray, J.D.; Kulcsar, K.A.; et al. Asymptomatic Infection of Marburg Virus Reservoir Bats Is Explained by a Strategy of Immunoprotective Disease Tolerance. Curr. Biol. 2021, 31, 257–270.e5. [Google Scholar] [CrossRef]
- Guito, J.C.; Kirejczyk, S.G.M.; Schuh, A.J.; Albariño, C.G.; Sealy, T.K.; Chakrabarti, A.K.; Kainulainen, M.H.; Welch, S.R.; Montgomery, J.M.; Spengler, J.R.; et al. Coordinated Inflammatory Responses Dictate Marburg Virus Control by Reservoir Bats. Nat. Commun. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Gibb, R.; Franklinos, L.H.V.; Redding, D.W.; Jones, K.E. Ecosystem Perspectives Are Needed to Manage Zoonotic Risks in a Changing Climate. BMJ 2020, 371, m3389. [Google Scholar] [CrossRef]
- Ahmed, W.; Liu, Y.; Smith, W.; Ingall, W.; Belby, M.; Bivins, A.; Bertsch, P.; Williams, D.T.; Richards, K.; Simpson, S. Leveraging Wastewater Surveillance to Detect Viral Diseases in Livestock Settings. Sci. Total Environ. 2024, 931, 172593. [Google Scholar] [CrossRef]
- Peña-Mosca, F.; Frye, E.A.; MacLachlan, M.J.; Pabilonia, K.L.; Sibley, S.D.; Rettler, H.; Fojtik, A.; Harden, M.V.; Anderson, T.K.; Bowman, A.S.; et al. The Impact of Highly Pathogenic Avian Influenza H5N1 Virus Infection on Dairy Cows. Nat. Commun. 2025, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Xiao, X.; Robinson, T.P. Intensifying Poultry Production Systems and the Emergence of Avian Influenza in China: A “One Health/Ecohealth” Epitome. Arch. Public Health 2017, 75, 1–7. [Google Scholar] [CrossRef]
- Roussin-Léveillée, C.; Rossi, C.A.M.; Castroverde, C.D.M.; Moffett, P. The Plant Disease Triangle Facing Climate Change: A Molecular Perspective. Trends Plant Sci. 2024, 29, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Barrera, M.; Jarrín, D.; Cevallos, V.; Polanco, J.; Silva, M.; Ponce, P.; Lana, R.; Sotomayor, D.; Barrera, R.; et al. Linking Vector Favourable Environmental Conditions with Serological Evidence of Widespread Bluetongue Virus Exposure in Livestock in Ecuador. Sci. Rep. 2025, 15, 14382. [Google Scholar] [CrossRef]
- Danve, C.; Castroverde, M.; Dina, D.; Van Zanten, M. Temperature Regulation of Plant Hormone Signaling During Stress and Development. J. Exp. Bot. 2021, 72, 7436–7458. [Google Scholar] [CrossRef]
- Liu, S.; Han, Y.; Li, W.X.; Ding, S.W. Infection Defects of RNA and DNA Viruses Induced by Antiviral RNA Interference. Microbiol. Mol. Biol. Rev. 2023, 87, e00035-22. [Google Scholar] [CrossRef]
- Carisse, O.; Vincent, S.; Lafond-Lapalme, J.; Fall, M.L.; Van der Heyden, H. Quantitative Insights into Grapevine Anthracnose (Elsinoë ampelina) Epidemiology: Impact of Temperature and Leaf Age on Incubation, Lesion Development, and Sporulation. Plant Dis. 2024, 108, 2838–2844. [Google Scholar] [CrossRef]
- Yamaji, R.; Zhang, W.; Kamata, A.; Ujie, M.; Kondo, H.; Imai, M.; Takashima, E.; Ito, K.; Kawaoka, Y. Pandemic Risk Characterisation of Zoonotic Influenza A Viruses Using the Tool for Influenza Pandemic Risk Assessment (TIPRA). Lancet. Microbe. 2025, 6, e100973. [Google Scholar] [CrossRef]
- de Souza, W.M.; Weaver, S.C. Effects of Climate Change and Human Activities on Vector-Borne Diseases. Nat. Rev. Microbiol. 2024, 22, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Chathuranga, K.; Weerawardhana, A.; Dodantenna, N.; Lee, J.S. Regulation of Antiviral Innate Immune Signaling and Viral Evasion Following Viral Genome Sensing. Exp. Mol. Med. 2021, 53, 1647–1668. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, Z.; Li, X.; Yang, M.; Fang, Q.; Li, Z.; Wang, C.; Chen, T.; Cao, X. E3 Ligase HECTD3 Promotes RNA Virus Replication and Virus-Induced Inflammation via K33-Linked Polyubiquitination of PKR. Cell Death Dis. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Mihalič, F.; Simonetti, L.; Giudice, G.; Davey, N.E.; Linding, R.; Lenassi, M.; Zagar, L.; Zupan, B.; Pugh, M.; Jerala, R.; et al. Large-Scale Phage-Based Screening Reveals Extensive Pan-Viral Mimicry of Host Short Linear Motifs. Nat. Commun. 2023, 14, 1–20. [Google Scholar] [CrossRef]
- Maguire, C.; Wang, C.; Ramasamy, A.; Fonken, C.; Morse, B.; Lopez, N.; Wylie, D.; Melamed, E. Molecular Mimicry as a Mechanism of Viral Immune Evasion and Autoimmunity. Nat. Commun. 2024, 15, 9403. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhuang, M.W.; Han, L.; Zhang, J.; Nan, M.L.; Zhan, P.; Kang, D.; Liu, X.; Gao, C.; Wang, P.H. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Membrane (M) Protein Inhibits Type I and III Interferon Production by Targeting RIG-I/MDA-5 Signaling. Signal Transduct. Target. Ther. 2020, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Ma, C.; Xu, G.; Li, Y.; Wang, Y.; Zhang, X.; Chen, X.; Li, J.; Huang, A.; Wang, Y. Hepatitis B Surface Antigen Hijacks TANK-Binding Kinase 1 to Suppress Type I Interferon and Induce Early Autophagy. Cell Death Dis. 2025, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Lanng, K.R.B.; Lauridsen, E.L.; Jakobsen, M.R. The Balance of STING Signaling Orchestrates Immunity in Cancer. Nat. Immunol. 2024, 25, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Dogrammatzis, C.; Saud, R.; Waisner, H.; Lasnier, S.; Suma, S.M.; Grieshaber, B.; Kalamvoki, M. Tracing the STING Exocytosis Pathway During Herpes Viruses Infection. mBio 2024, 15, e00373-24. [Google Scholar] [CrossRef]
- Bodda, C.; Reinert, L.S.; Fruhwürth, S.; Richardo, T.; Sun, C.; Zhang, B.; Kalamvoki, M.; Pohlmann, A.; Mogensen, T.H.; Bergström, P.; et al. HSV1 VP1-2 Deubiquitinates STING to Block Type I Interferon Expression and Promote Brain Infection. J. Exp. Med. 2020, 217, e20191422. [Google Scholar] [CrossRef]
- Deschamps, T.; Kalamvoki, M. Evasion of the STING DNA-Sensing Pathway by VP11/12 of Herpes Simplex Virus 1. J. Virol. 2017, 91, e00535-17. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Ma, Y.; Cao, Y.; He, B. Herpes Simplex Virus 1 γ134.5 Protein Inhibits STING Activation That Restricts Viral Replication. J. Virol. 2018, 92, e01015-18. [Google Scholar] [CrossRef]
- Na, E.J.; Chae, S.B.; Oh, B.; Jeong, C.G.; Park, S.C.; Oem, J.K. A Novel Approach Using IFNAR1 KO Mice for Assessing Akabane Virus Pathogenicity and Vaccine Efficacy. Vaccine 2025, 53, 127094. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Agarkova, I.V.; Dunigan, D.D.; Shao, Q.; Fang, Q. Emerging Structure of Chlorovirus PBCV-1. Virology 2025, 608, 110552. [Google Scholar] [CrossRef]
- Landeras-Bueno, S.; Hariharan, C.; Avalos, R.D.; Kago, G.; Nguyen, H.T.; Sotomayor, M.; Gronenborn, A.M.; Salgado, E.N. Structural Stabilization of the Intrinsically Disordered SARS-CoV-2 N by Binding to RNA Sequences Engineered from the Viral Genome Fragment. Nat. Commun. 2025, 16, 6521. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like Particles: Fundamentals and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 8579. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, J.; Ma, S.; Song, H.; Feng, D.; Yan, G.Q.; Zhang, Y.; Lu, H. Ultrafast Tyrosinase-Mediated Biotinylation of Living Cell Surface Analysis Reveals Novel Cell Surface Proteins Responsible for Influenza A Virus Entry. J. Am. Chem. Soc. 2025, 147, 12360–12369. [Google Scholar] [CrossRef]
- Sequeira, D.P.; Suomalainen, M.; Freitag, P.C.; Plückthun, A.; Klingenbrunner, M.; Fischer, L.; Hemmi, S.; Münz, C.; Volle, R.; Greber, U.F. Activated Blood-Derived Human Primary T Cells Support Replication of HAdV C5 and Virus Transmission to Polarized Human Primary Epithelial Cells. J. Virol. 2025, 99, e01825-24. [Google Scholar] [CrossRef]
- Zwi-Dantsis, L.; Mohamed, S.; Massaro, G.; Moeendarbary, E. Adeno-Associated Virus Vectors: Principles, Practices, and Prospects in Gene Therapy. Viruses 2025, 17, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, K.; Zhou, S.; Wang, Y.; Li, X.; Zhang, H.; Chen, Y.; Li, Z.; Wang, N.; Liu, J. G3BP1 Regulates the Cell Cycle by Promoting IFNβ Production to Promote PCV2 Replication and Promotes Nuclear Transfer of Viral Proteins by Direct Binding. Int. J. Mol. Sci. 2025, 26, 1083. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, N.H.; Joyoti, S.A.; Zaker, B.B.; Hossain, M.S.; Islam, M.R.; Rahman, M.S.; Al-Mamun, M.; Islam, M.T. Molecular Activity of Bioactive Phytocompounds for Inhibiting Host Cell Attachment and Membrane Fusion Interacting with West Nile Virus Envelope Glycoprotein. PLoS ONE 2025, 20, e0321902. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.R.; Qin, H.; Zhang, Y.F.; Li, Y.; Wang, Y.; Liu, H.; Chen, J.; Li, L.; Zhang, G. Optimizing Encephalomyocarditis Virus VP1 Protein Assembly on Pseudorabies Virus Envelope via US9 Protein Anchoring. Virulence 2025, 16, 2445235. [Google Scholar] [CrossRef]
- Dewangan, N.; Jana, I.D.; Yadav, S.; Sardar, A.; Mallick, A.I.; Mondal, A.; Tarafdar, P.K. Design of Flavonoid-Based Lipid Domains as Fusion Inhibitors to Efficiently Block Coronavirus and Other Enveloped Virus Infection. Small 2025, 21, 2410727. [Google Scholar] [CrossRef]
- Pisano, R.; Durlo, A. Nanosciences and Nanotechnologies: A Scientific–Historical Introductory Review. In History of Nanotechnology; Springer: Cham, Switzerland, 2025; pp. 3–90. [Google Scholar]
- Pisano, R.; Durlo, A. Physics Scanning Devices and Nanoscale Techniques: An Historical Perspective. In History of Nanotechnology; Springer: Cham, Switzerland, 2025; pp. 475–524. [Google Scholar]
- Hargittai, I. Forty Years of the Discovery of Buckminsterfullerene. Struct. Chem. 2025, 36, 779–782. [Google Scholar] [CrossRef]
- Eldin, J.; Ibrahim, F.M.; Khatoon, U.T.; Velidandi, A. An Overview on the Role of Government Initiatives in Nanotechnology Innovation for Sustainable Economic Development and Research Progress. Sustainability 2025, 17, 1250. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Yasin, H.K.A.; Samdani, N.; Satpute, N.N.; Yadav, S.; Pratap, A.P. Silver Nanoparticles (AgNPs) as Potential Antiviral Agents: Synthesis, Biophysical Properties, Safety, Challenges and Future Directions─Update Review. Molecules 2025, 30, 2004. [Google Scholar] [CrossRef]
- Birusanti, A.B.; Espenti, C.S.; Surendra, T.V.; Srinivas, B.; Srinivasulu, M.; Peddulaiah, K.; Rao, K.M.; Han, S.S. Synthesis and Characterization of Multi-Responsive Iron Oxide Nanoparticles: Evaluation of Antibacterial Properties and Photocatalytic Activity. J. Mol. Liq. 2025, 417, 126619. [Google Scholar] [CrossRef]
- Bhandari, K.; Nautiyal, N.; Bhandari, G.; Dhasmana, A.; Gupta, S.; Gangola, S.; Kumar, S.; Khan, A.A.; Fatima, S.; Kumar, P. A Comparative Investigation of Ultrasonication and Magnetic Stirring Methods for Green Synthesis of Zinc Oxide Nanoparticles Using Punica granatum Peels. Sci. Rep. 2025, 15, 24869. [Google Scholar] [CrossRef]
- Xi, M.; Cheng, Y.; Meng, F.; Yao, X.; Nawaz, M.A.; Saif, M.; Li, Z.; Reina, T.R. Densely Embedded Homogeneous FeOx Nanoparticles in N-Doped Carbon Frameworks: A 3D Scaffold Catalyst for High-Temperature Fischer-Tropsch Synthesis. Chem. Eng. J. 2025, 503, 158333. [Google Scholar] [CrossRef]
- Kong, N.; Ariga, K. Confined Nanoarchitectonics for Nano-reactors: In Situ Characterization and Tracking Systems at the Nanoscale. Nanoscale Horiz. 2025, 10, 1882–1904. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, L.; Zhou, C.; Zhao, Y.; Huang, H.; Wu, J. Low-Dimensional Antimicrobial Nanomaterials in Anti-infection Treatment and Wound Healing. Chin. Chem. Lett. 2025, 36, 110028. [Google Scholar] [CrossRef]
- Sun, B.; Zhong, W.; Liu, H.; Ai, X.; Han, S.; Chen, Y. Controlling Rhodium-Based Nanomaterials for High-Efficiency Energy-Related Electrocatalysis. Energy Chem. 2025, 7, 100148. [Google Scholar] [CrossRef]
- Xu, Z.; Tong, C.; Zhang, T.; Xu, Z.; Ma, Y.; Niu, Z.; Chen, J.; Zhang, M.; Shi, F. Rapid Detection of Brucellosis by Two-Signal Vertical Flow Immunofiltration Based on Three-Dimensional Flower-Like Au@Mn3O4 Nanozymes. ACS Appl. Nano Mater. 2025, 8, 6014–6024. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.; Kang, D.; Lee, S.H.; Cho, A.Y.; Lee, H.; Kwon, J.H.; Shin, Y.; Kim, Y.P.; Lee, J. Near-Infrared Long-Lived Luminescent Nanoparticle-Based Time-Gated Imaging for Background-Free Detection of Avian Influenza Virus. ACS Sens. 2025, 10, 1312–1320. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, C.; Andrews, G.; Wang, J.; Ge, Y. Recent Advances in Carbon Quantum Dots for Virus Detection, as Well as Inhibition and Treatment of Viral Infection. Nano Converg. 2022, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Mishra, V.; Mishra, Y.; Ranjan, A.; Aljabali, A.A.A.; El-Tanani, M.; Alfagih, I.M.; Tambuwala, M.M. Development and Evaluation of a Protease Inhibitor Antiretroviral Drug-Loaded Carbon Nanotube Delivery System for Enhanced Efficacy in HIV Treatment. Int. J. Pharm. 2024, 650, 123678. [Google Scholar] [CrossRef] [PubMed]
- Kanagavalli, P.; Elkaffas, R.A.; Eissa, S. Ultrasensitive Electrochemical Biosensor for the Simultaneous Detection of the Two Monkeypox Virus Antigens M1R and A29 Using Reduced Graphene Oxide-ZIF-8 Nanocomposite. Chem. Eng. J. 2025, 516, 164015. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Wang, X.; Lv, C.; Zhou, Q.; Jiang, G.; Yan, B.; Chen, L. Beyond the Promise: Exploring the Complex Interactions of Nanoparticles Within Biological Systems. J. Hazard. Mater. 2024, 468, 133800. [Google Scholar] [CrossRef]
- Ghorai, S.; Shand, H.; Patra, S.; Panda, K.; Santiago, M.J.; Rahman, M.S.; Chinnapaiyan, S.; Unwalla, H.J. Nanomedicine for the Treatment of Viral Diseases: Smaller Solution to Bigger Problems. Pharmaceuticals 2024, 16, 407. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, G.; Rodriguez, W.R. Micro- and Nanotechnology for Viral Detection. Anal. Bioanal. Chem. 2009, 393, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Cavalli, R. Nanoparticulate Delivery Systems for Antiviral Drugs. Antivir. Chem. Chemother. 2010, 21, 53–70. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez-Ribot, J.L. Nanotechnology as an Alternative to Reduce the Spread of COVID-19. Challenges 2020, 11, 15. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef] [PubMed]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in Therapeutics: A Focus on Nanoparticles as a Drug Delivery System. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef]
- Rao, L.; Tian, R.; Chen, X. Cell-Membrane-Mimicking Nanodecoys Against Infectious Diseases. ACS Nano 2020, 14, 2569–2574. [Google Scholar] [CrossRef]
- Imran, M.; Jha, L.A.; Hasan, N.; Shrestha, J.; Pangeni, R.; Parvez, N.; Mohammed, Y.; Jha, S.K.; Paudel, K.R. “Nanodecoys”—Future of Drug Delivery by Encapsulating Nanoparticles in Natural Cell Membranes. Int. J. Pharm. 2022, 621, 121790. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic Nanoparticles for Drug Delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous Silica Nanoparticles in Medicine—Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and Their Loading Strategies. Adv. Healthc. Mater. 2019, 8, 1801002. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral Potential of Nanoparticles—Can Nanoparticles Fight Against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ayipo, Y.O.; Bakare, A.A.; Badeggi, U.M.; Jimoh, A.A.; Lawal, A.; Mordi, M.N. Recent Advances on Therapeutic Potentials of Gold and Silver Nanobiomaterials for Human Viral Diseases. Curr. Res. Chem. Biol. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and Metal Oxide-Based Antiviral Nanoparticles: Properties, Mechanisms of Action, and Applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Paradowska, E.; Studzińska, M.; Jabłońska, A.; Lozovski, V.; Rusinchuk, N.; Mukha, I.; Vitiuk, N.; Leśnikowski, Z.J. Antiviral Effect of Nonfunctionalized Gold Nanoparticles Against Herpes Simplex Virus Type-1 (HSV-1) and Possible Contribution of Near-Field Interaction Mechanism. Molecules 2021, 26, 5960. [Google Scholar] [CrossRef]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of Influenza Virus Infection by Multivalent Sialic-Acid-Functionalized Gold Nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Andresen, H.; Mager, M.; Grießner, M.; Charchar, P.; Todorova, N.; Bell, N.; Theocharidis, G.; Bertazzo, S.; Yarovsky, I.; Stevens, M.M. Single-Step Homogeneous Immunoassays Utilizing Epitope-Tagged Gold Nanoparticles: On the Mechanism, Feasibility, and Limitations. Chem. Mater. 2014, 26, 4696–4704. [Google Scholar] [CrossRef]
- Bowman, M.C.; Ballard, T.E.; Ackerson, C.J.; Feldheim, D.L.; Margolis, D.M.; Melander, C. Inhibition of HIV Fusion with Multivalent Gold Nanoparticles. J. Am. Chem. Soc. 2008, 130, 6896–6897. [Google Scholar] [CrossRef]
- Rawat, P.; Gupta, S. Dual Engineered Gold Nanoparticle Based Synergistic Prophylaxis Delivery System for HIV/AIDS. Med. Hypotheses 2021, 150, 110576. [Google Scholar] [CrossRef]
- Garrido, C.; Simpson, C.A.; Dahl, N.P.; Lee, B.; Moyo, V.; Kavanaugh, J.S.; Cronican, J.J.; Elmer, J.J.; Weiss, R.A.; Becker, M.L. Gold Nanoparticles to Improve HIV Drug Delivery. Future Med. Chem. 2015, 7, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Tessaro, L.; Conte-Junior, C.A. Gold Nanoparticles for Treatment of Infectious Diseases. In Gold Nanoparticles for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2024; pp. 277–303. [Google Scholar]
- Farfán-Castro, S.; García-Soto, M.J.; Comas-García, M.; Arévalo-Villalobos, J.I.; Palestino, G.; González-Ortega, O.; Rosales-Mendoza, S. Synthesis and Immunogenicity Assessment of a Gold Nanoparticle Conjugate for the Delivery of a Peptide from SARS-CoV-2. Nanomedicine 2021, 34, 102372. [Google Scholar] [CrossRef]
- Salazar, V.A.; Comenge, J.; Suárez-López, R.; Burger, J.A.; Sanders, R.W.; Bastús, N.G.; Jaime, C.; Joseph-Munne, J.; Puntes, V. Gold Nanoparticle Virus-like Particles Presenting SARS-CoV-2 Spike Protein: Synthesis, Biophysical Properties and Immunogenicity in BALB/c Mice. Vaccines 2024, 12, 829. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A Preliminary Assessment of Silver Nanoparticle Inhibition of Monkeypox Virus Plaque Formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar] [CrossRef]
- Lu, L.; Sun, R.W.Y.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.K.; Che, C.M. Silver Nanoparticles Inhibit Hepatitis B Virus Replication. Antivir. Ther. 2008, 13, 252–262. [Google Scholar] [CrossRef]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin Modified Silver Nanoparticles for Highly Efficient Inhibition of Respiratory Syncytial Virus Infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R. The Potential Anti-infective Applications of Metal Oxide Nanoparticles: A Systematic Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1592. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral Activity of Cuprous Oxide Nanoparticles Against Hepatitis C Virus In Vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Antoine, T.E.; Hadigal, S.R.; Yakoub, A.M.; Mishra, Y.K.; Bhattacharya, P.; Haddad, C.; Valyi-Nagy, T.; Adelung, R.; Prabhakar, B.S.; Shukla, D. Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents Against Genital Herpes. J. Immunol. 2016, 196, 4566–4575. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Rios-Ibarra, C.P.; Salinas-Santander, M.; Orozco-Nunnelly, D.A.; Bravo-Madrigal, J. Nanoparticle-based Antiviral Strategies to Combat the Influenza Virus (Review). Biomed. Rep. 2024, 20, 1–8. [Google Scholar] [CrossRef]
- Patoo, T.S.; Khanday, F.; Qurashi, A. Prospectus of Advanced Nanomaterials for Antiviral Properties. Mater. Adv. 2022, 3, 2960–2970. [Google Scholar] [CrossRef]
- Greijer, B.; Nefedova, A.; Agback, T.; Agback, P.; Kisand, V.; Rausalu, K.; Vanetsev, A.; Seisenbaeva, G.A.; Ivask, A.; Kessler, V.G. Molecular Mechanisms Behind the Anti Corona Virus Activity of Small Metal Oxide Nanoparticles. Nanoscale 2025, 17, 3728–3738. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.R.; Jang, H.; Kim, K.S.; Lee, B.; Kim, K.B.; Kim, Y.K.; Yeo, W.S.; Lee, Y.; Kim, D.E.; Min, D.H. Functional Delivery of DNAzyme with Iron Oxide Nanoparticles for Hepatitis C Virus Gene Knockdown. Biomaterials 2012, 33, 2754–2761. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.Y.; Li, X.Q.; Chen, W.G.; Deng, L.L.; Tan, Y.Z.; Zhang, Q.; Xie, S.Y.; Zheng, L.S. Progress in Antiviral Fullerene Research. Nanomaterials 2022, 12, 2547. [Google Scholar] [CrossRef]
- Muñoz, A.; Sigwalt, D.; Illescas, B.M.; Luczkowiak, J.; Rodríguez-Pérez, L.; Nierengarten, I.; Holler, M.; Remy, J.S.; Buffet, K.; Vincent, S.P.; et al. Synthesis of Giant Globular Multivalent Glycofullerenes as Potent Inhibitors in a Model of Ebola Virus Infection. Nat. Chem. 2015, 8, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Shimotohno, K.; Ikegami, N.; Nishikawa, D.; Okuda, K.; Takahashi, K.; Nakamura, S.; Mochizuki, M. Human Immunodeficiency Virus-Reverse Transcriptase Inhibition and Hepatitis C Virus RNA-Dependent RNA Polymerase Inhibition Activities of Fullerene Derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 1107–1109. [Google Scholar] [CrossRef]
- Yasuno, T.; Ohe, T.; Kataoka, H.; Umeda, S.; Nakamura, S.; Mashino, T. Fullerene Derivatives as Dual Inhibitors of HIV-1 Reverse Transcriptase and Protease. Bioorg. Med. Chem. Lett. 2021, 31, 127675. [Google Scholar] [CrossRef]
- Innocenzi, P.; Stagi, L. Carbon-based Antiviral Nanomaterials: Graphene, C-dots, and Fullerenes. A Perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, N.E.; Klimova, R.R.; Tulenev, Y.A.; Chichev, E.V.; Kornev, A.B.; Troshin, P.A.; Kushch, A.A. Carboxylic Fullerene C60 Derivatives: Efficient Microbicides Against Herpes Simplex Virus and Cytomegalovirus Infections In Vitro. Mendeleev Commun. 2012, 22, 254–256. [Google Scholar] [CrossRef]
- Ramos-Soriano, J.; Reina, J.J.; Illescas, B.M.; De La Cruz, N.; Rodríguez-Pérez, L.; Lasala, F.; Rojo, J.; Delgado, R.; Martín, N. Synthesis of Highly Efficient Multivalent Disaccharide/[60]Fullerene Nanoballs for Emergent Viruses. J. Am. Chem. Soc. 2019, 141, 15403–15412. [Google Scholar] [CrossRef] [PubMed]
- Falynskova, I.N.; Ionova, K.S.; Dedova, A.V.; Leneva, I.A.; Makhmudova, N.R.; Rasnetsov, L.D. Antiviral Activity of Fullerene-(tris-aminocaproic acid) Hydrate Against Respiratory Syncytial Virus in HEp-2 Cell Culture. Pharm. Chem. J. 2014, 48, 85–88. [Google Scholar] [CrossRef]
- Kataoka, H.; Ohe, T.; Takahashi, K.; Nakamura, S.; Mashino, T. Novel Fullerene Derivatives as Dual Inhibitors of Hepatitis C Virus NS5B Polymerase and NS3/4A Protease. Bioorg. Med. Chem. Lett. 2016, 26, 4565–4567. [Google Scholar] [CrossRef]
- Da Ros, T.; Spalluto, G.; Prato, M. Biological Applications of Fullerene Derivatives: A Brief Overview. Croat. Chem. Acta 2001, 74, 743–755. [Google Scholar]
- Bosi, S.; Da Ros, T.; Spalluto, G.; Prato, M. Fullerene Derivatives: An Attractive Tool for Biological Applications. Eur. J. Med. Chem. 2003, 38, 913–923. [Google Scholar] [CrossRef]
- Käsermann, F.; Kempf, C. Photodynamic Inactivation of Enveloped Viruses by Buckminsterfullerene. Antiviral Res. 1997, 34, 65–70. [Google Scholar] [CrossRef]
- Käsermann, F.; Kempf, C. Buckminsterfullerene and Photodynamic Inactivation of Viruses. Rev. Med. Virol. 1998, 8, 143–151. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Pondman, K.M.; Salvador-Morales, C.; Paudyal, B.; Sim, R.B.; Kishore, U. Interactions of the Innate Immune System with Carbon Nanotubes. Nanoscale Horiz. 2017, 2, 174–186. [Google Scholar] [CrossRef]
- Nunes, E.; Lima, C.; Luiza, A.; Octaviano, M.; Roberto, J.; Piqueira, C.; Sobhie Diaz, R.; Justo, F.; Justo, J.F. Coronavirus and Carbon Nanotubes: Seeking Immunological Relationships to Discover Immunotherapeutic Possibilities. Int. J. Nanomed. 2022, 17, 751–781. [Google Scholar] [CrossRef]
- Meher, B.R.; Wang, Y. Binding of Single Walled Carbon Nanotube to WT and Mutant HIV-1 Proteases: Analysis of Flap Dynamics and Binding Mechanism. J. Mol. Graph. Model. 2012, 38, 430–445. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized Carbon Nanotubes: Synthesis, Properties and Applications in Water Purification, Drug Delivery, and Material and Biomedical Sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Versiani, A.F.; Astigarraga, R.G.; Rocha, E.S.O.; da Silva, J.B.; Barboza, A.P.M.; Fernandes, L.L.; Ladeira, L.O.; Guatimosim, C.; Ladeira, L.O.; da Fonseca, F.G. Multi-walled Carbon Nanotubes Functionalized with Recombinant Dengue Virus 3 Envelope Proteins Induce Significant and Specific Immune Responses in Mice. J. Nanobiotechnol. 2017, 15, 26. [Google Scholar] [CrossRef]
- Liu, Z.; Winters, M.; Holodniy, M.; Dai, H. siRNA Delivery into Human T Cells and Primary Cells with Carbon-Nanotube Transporters. Angew. Chem. Int. Ed. 2007, 46, 2023–2027. [Google Scholar] [CrossRef]
- Banerjee, I.; Douaisi, M.P.; Mondal, D.; Kane, R.S. Light-activated Nanotube–Porphyrin Conjugates as Effective Antiviral Agents. Nanotechnology 2012, 23, 105101. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Nayak, V.; Singh, K.R.; Jain, B.; Baid, M.; Alexis, F.; Singh, A.K. Potentialities of Graphene and Its Allied Derivatives to Combat Against SARS-CoV-2 Infection. Mater. Today Adv. 2022, 13, 100208. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21578–21579. [Google Scholar] [CrossRef]
- Sametband, M.; Kalt, I.; Gedanken, A.; Sarid, R. Herpes Simplex Virus Type-1 Attachment Inhibition by Functionalized Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Ziem, B.; Thien, H.; Achazi, K.; Yue, C.; Stern, D.; Silberreis, K.; Gholami, M.F.; Beckert, F.; Gröger, D.; Mülhaupt, R.; et al. Highly Efficient Multivalent 2D Nanosystems for Inhibition of Orthopoxvirus Particles. Adv. Healthc. Mater. 2016, 5, 2922–2930. [Google Scholar] [CrossRef]
- Ziem, B.; Azab, W.; Gholami, M.F.; Rabe, J.P.; Osterrieder, N.; Haag, R. Size-dependent Inhibition of Herpesvirus Cellular Entry by Polyvalent Nanoarchitectures. Nanoscale 2017, 9, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, X.; Zhu, G. Virus Capture and Destruction by Label-Free Graphene Oxide for Detection and Disinfection Applications. Small 2015, 11, 1171–1176. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Corpino, R.; Salis, M.; Mocci, F.; Thakkar, S.V.; Olla, C.; Ricci, P.C. On the Emission Properties of Carbon Dots: Reviewing Data and Discussing Models. C 2019, 5, 60. [Google Scholar] [CrossRef]
- Dong, X.; Moyer, M.M.; Yang, F.; Sun, Y.P.; Yang, L. Carbon Dots’ Antiviral Functions Against Noroviruses. Sci. Rep. 2017, 7, 519. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon Dots as Inhibitors of Virus by Activation of Type I Interferon Response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical Field: A Brief Review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Sharifi, E.; Yousefiasl, S.; Trovato, M.; Sartorius, R.; Esmaeili, Y.; Saghazadeh, A.; Goodarzi, P.; Kumar, A.; Roviello, G.; Moghaddam, F.A.; et al. Nanostructures for Prevention, Diagnosis, and Treatment of Viral Respiratory Infections: From Influenza Virus to SARS-CoV-2 Variants. J. Nanobiotechnol. 2023, 21, 199. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based Biosensors for Detection of Pathogenic Virus. TrAC Trends Anal. Chem. 2017, 97, 445–457. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, C.; He, Q.; Chen, J.; Yu, D.; Li, J.; Zhai, S.; Qin, Z.; Du, K.; Chu, Z. Current and Perspective Diagnostic Techniques for COVID-19. ACS Infect. Dis. 2020, 6, 1998–2016. [Google Scholar] [CrossRef] [PubMed]
- Thwala, L.N.; Ndlovu, S.C.; Mpofu, K.T.; Lugongolo, M.Y.; Mthunzi-Kufa, P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials 2023, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.; Madhivanan, K.; Atchudan, R.; Arya, S.; Sundramoorthy, A.K. Nanoparticle Electrochemical Biosensors for Virus Detection. Clin. Chim. Acta 2025, 566, 120054. [Google Scholar] [CrossRef]
- Draz, M.S.; Shafiee, H. Applications of Gold Nanoparticles in Virus Detection. Theranostics 2018, 8, 1985–2017. [Google Scholar] [CrossRef]
- Lai, H.; Huang, R.; Weng, X.; Huang, B.; Yao, J.; Pian, Y. Classification and Applications of Nanomaterials in Vitro Diagnosis. Heliyon 2024, 10, e32314. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Najafi Moghadam, P.; Ehsanimehr, S.; Fareghi, A.R. Preparation of a New Magnetic Nanocomposite for the Removal of Dye Pollutions from Aqueous Solutions: Synthesis and Characterization. Mater. Chem. Horizons 2022, 1, 23–34. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Sajadi, S.M.; Seidi, F.; Habibzadeh, S.; Munir, M.T.; Abida, O.; Rabiee, N.; Bagherzadeh, M.; Iravani, S.; Saeb, M.R. Metal Nanoparticles-assisted Early Diagnosis of Diseases. OpenNano 2022, 8, 100104. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles′ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G. A Proposed Insight into the Anti-viral Potential of Metallic Nanoparticles Against Novel Coronavirus Disease-19 (COVID-19). Bull. Natl. Res. Cent. 2021, 45, 36. [Google Scholar] [CrossRef]
- Heydari-Bafrooei, E.; Ensafi, A.A. Nanomaterials-based Biosensing Strategies for Biomarkers Diagnosis, A Review. Biosens. Bioelectron. X 2023, 13, 100245. [Google Scholar] [CrossRef]
- Nautiyal, D.; Jain, U. Emerging Trends in Fluorescent and Quenching Nanomaterials for Viral Detection: Innovations in Biological and Chemical Sensing. Talanta Open 2025, 11, 100430. [Google Scholar] [CrossRef]
- Zehbe, I.; Hacker, G.W.; Su, H.; Hauser-Kronberger, C.; Hainfeld, J.F.; Tubbs, R. Sensitive In Situ Hybridization with Catalyzed Reporter Deposition, Streptavidin-Nanogold, and Silver Acetate Autometallography: Detection of Single-copy Human Papillomavirus. Am. J. Pathol. 1997, 150, 1553–1561. [Google Scholar] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications Towards Drugs. J. Environ. Anal. Toxicol. 2016, 6, 384. [Google Scholar] [CrossRef]
- Bagheri, M.; Validi, M.; Gholipour, A.; Makvandi, P.; Sharifi, E. Chitosan Nanofiber Biocomposites for Potential Wound Healing Applications: Antioxidant Activity with Synergic Antibacterial Effect. Bioeng. Transl. Med. 2022, 7, e10254. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Morey, J.; Torres, M.; Seqqat, R.; de las, N.; Piña, M. Silver Nanoparticles as a Potential Treatment Against SARS-CoV-2: A Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1707. [Google Scholar] [CrossRef]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C.B. Silver Nanoparticle in Biosensor and Bioimaging: Clinical Perspectives. Biotechnol. Appl. Biochem. 2021, 68, 1236–1242. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Li, D.; Chen, H.; Gao, X.; Mei, X.; Yang, L. Development of General Methods for Detection of Virus by Engineering Fluorescent Silver Nanoclusters. ACS Sens. 2021, 6, 613–627. [Google Scholar] [CrossRef]
- Yang, J.; Jin, R. New Advances in Atomically Precise Silver Nanoclusters. ACS Mater. Lett. 2019, 1, 482–489. [Google Scholar] [CrossRef]
- Cao, Q.; Teng, Y.; Yang, X.; Wang, J.; Wang, E. A Label-free Fluorescent Molecular Beacon Based on DNA-Ag Nanoclusters for the Construction of Versatile Biosensors. Biosens. Bioelectron. 2015, 74, 318–321. [Google Scholar] [CrossRef]
- Valdez, J.; Bawage, S.; Gomez, I.; Singh, S.R. Facile and Rapid Detection of Respiratory Syncytial Virus Using Metallic Nanoparticles. J. Nanobiotechnol. 2016, 14, 13. [Google Scholar] [CrossRef]
- Pawar, A.A.; Sahoo, J.; Verma, A.; Lodh, A.; Lakkakula, J. Usage of Platinum Nanoparticles for Anticancer Therapy over Last Decade: A Review. Part. Part. Syst. Charact. 2021, 38, 2100115. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Cassani, M.C.; Rubini, K.; Fini, M.; Pagani, S.; Bigi, A. Platinum Nanoparticles Supported on Functionalized Hydroxyapatite: Anti-oxidant Properties and Bone Cells Response. Ceram. Int. 2020, 46, 19574–19582. [Google Scholar] [CrossRef]
- Gutiérrez de la Rosa, S.Y.; Muñiz Diaz, R.; Villalobos Gutiérrez, P.T.; Patakfalvi, R.; Gutiérrez Coronado, Ó. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Wolff, C.M.; Frischmann, P.D.; Schulze, M.; Bohn, B.J.; Wein, R.; Livadas, P.; Carlson, M.T.; Jäckel, F.; Feldmann, J.; Würthner, F. All-in-one Visible-light-driven Water Splitting by Combining Nanoparticulate and Molecular Co-catalysts on CdS Nanorods. Nat. Energy 2018, 3, 862–869. [Google Scholar] [CrossRef]
- Qaddare, S.H.; Salimi, A. Amplified Fluorescent Sensing of DNA Using Luminescent Carbon Dots and AuNPs/GO as a Sensing Platform: A Novel Coupling of FRET and DNA Hybridization for Homogeneous HIV-1 Gene Detection at Femtomolar Level. Biosens. Bioelectron. 2017, 89, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Ahmad, M.S.; Shah, M.I.A.; Ateeq, M. Magnetic Nanoparticles: Drug Delivery and Bioimaging Applications. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 189–213. [Google Scholar]
- Zhao, Z.; Cui, H.; Song, W.; Ru, X.; Zhou, W.; Yu, X. A Simple Magnetic Nanoparticles-based Viral RNA Extraction Method for Efficient Detection of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Khizar, S.; Al-Dossary, A.A.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Contribution of Magnetic Particles in Molecular Diagnosis of Human Viruses. Talanta 2022, 241, 123243. [Google Scholar] [CrossRef]
- Jat, S.K.; Gandhi, H.A.; Bhattacharya, J.; Sharma, M.K. Magnetic Nanoparticles: An Emerging Nano-based Tool to Fight Against Viral Infections. Mater. Adv. 2021, 2, 4479–4496. [Google Scholar] [CrossRef]
- Ehtesabi, H. Application of Carbon Nanomaterials in Human Virus Detection. J. Sci. Adv. Mater. Devices 2020, 5, 436–450. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, Mechanisms, and Application of Carbon Quantum Dots in Sensors: A Review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Mondal, S. Carbon Nanotube-reinforced Polymer Nanocomposite for Biomedical Applications. In Green Biocomposites for Biomedical Engineering; Hoque, M.E., Sharif, A., Jawaid, M., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 265–283. [Google Scholar]
- Pirzada, M.; Altintas, Z. Nanomaterials for Virus Sensing and Tracking. Chem. Soc. Rev. 2022, 51, 5805–5841. [Google Scholar] [CrossRef]
- Zhu, Z. An Overview of Carbon Nanotubes and Graphene for Biosensing Applications. Nano-Micro Lett. 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, C.; Wu, L.; Tan, W.; Shang, Z.; Tian, Y.; Ma, J. Recent Advances in Carbon Dots for Electrochemical Sensing and Biosensing: A Systematic Review. Microchem. J. 2024, 207, 111687. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene Based Sensors and Biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Dutta, R.; Rajendran, K.; Jana, S.K.; Saleena, L.M.; Ghorai, S. Use of Graphene and Its Derivatives for the Detection of Dengue Virus. Biosensors 2023, 13, 349. [Google Scholar] [CrossRef]
- Vermisoglou, E.; Panáček, D.; Jayaramulu, K.; Pykal, M.; Frébort, I.; Kolář, M.; Hajdúch, M.; Zbořil, R.; Otyepka, M. Human Virus Detection with Graphene-based Materials. Biosens. Bioelectron. 2020, 166, 112436. [Google Scholar] [CrossRef]
- Altintas, Z.; Gittens, M.; Guerreiro, A.; Thompson, K.A.; Walker, J.; Piletsky, S.; Tothill, I.E. Detection of Waterborne Viruses Using High Affinity Molecularly Imprinted Polymers. Anal. Chem. 2015, 87, 6801–6807. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Anas, N.A.A.; Ramdzan, N.S.M.; Mahdi, M.A. Optical and Structural Properties of Cadmium Sulphide Quantum Dots Based Thin Films as Potential Sensing Material for Dengue Virus E-protein. Results Phys. 2018, 11, 734–739. [Google Scholar] [CrossRef]
- Wang, C.Z.; Han, H.H.; Tang, X.Y.; Zhou, D.M.; Wu, C.; Chen, G.R.; He, X.P.; Tian, H. Sialylglycan-Assembled Supra-Dots for Ratiometric Probing and Blocking of Human-Infecting Influenza Viruses. ACS Appl. Mater. Interfaces 2017, 9, 25164–25170. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).