Theranostic Nanoplatforms in Nuclear Medicine: Current Advances, Emerging Trends, and Perspectives for Personalized Oncology
Abstract
1. Introduction
1.1. Background: From Monotherapy to Integrated Theranostics
1.2. The Convergence of Nanotechnology and Nuclear Medicine
1.3. Advances in Nanoplatform Design and Their Pharmacokinetic and Functional Benefits
1.4. Scope of This Review
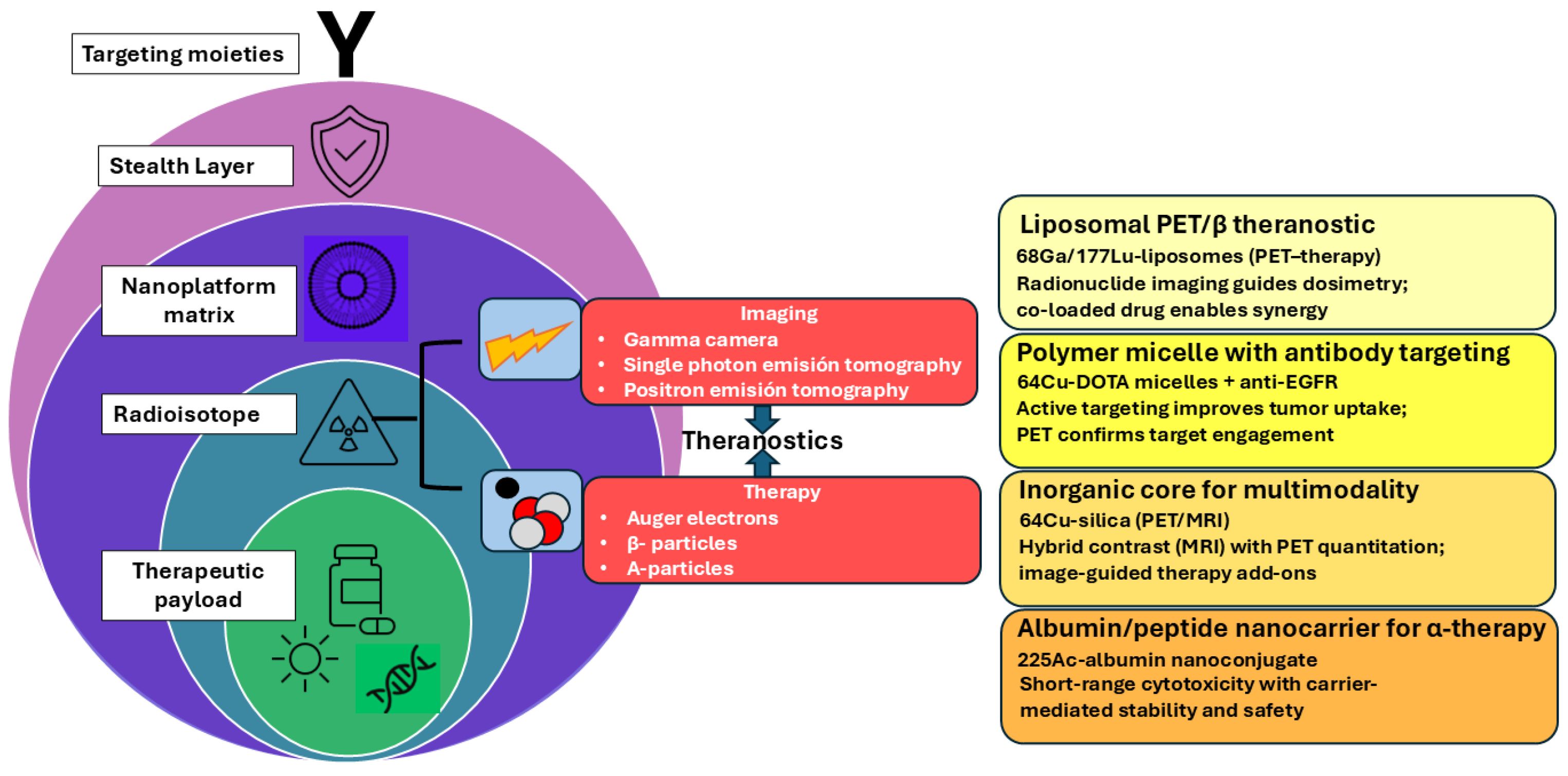
2. Theranostic Principles and the Role of Nanoplatforms
2.1. Definition and Conceptual Evolution of Theranostics
2.2. Main Categories of Nanoplatforms Used in Nuclear Theranostics
2.3. Organic Nanoplatforms
2.4. Inorganic Nanoplatforms
2.5. Hybrid and Multifunctional Nanostructures
2.6. Rationale for Combining Diagnostic and Therapeutic Modalities
3. Radioisotope Selection, Radiolabelling Strategies and Stability Concerns
3.1. Radioisotopes for Diagnostic
3.2. Radioisotopes for Therapy
3.3. Radioisotope Pairing Strategies
3.4. Radiolabeling Strategies
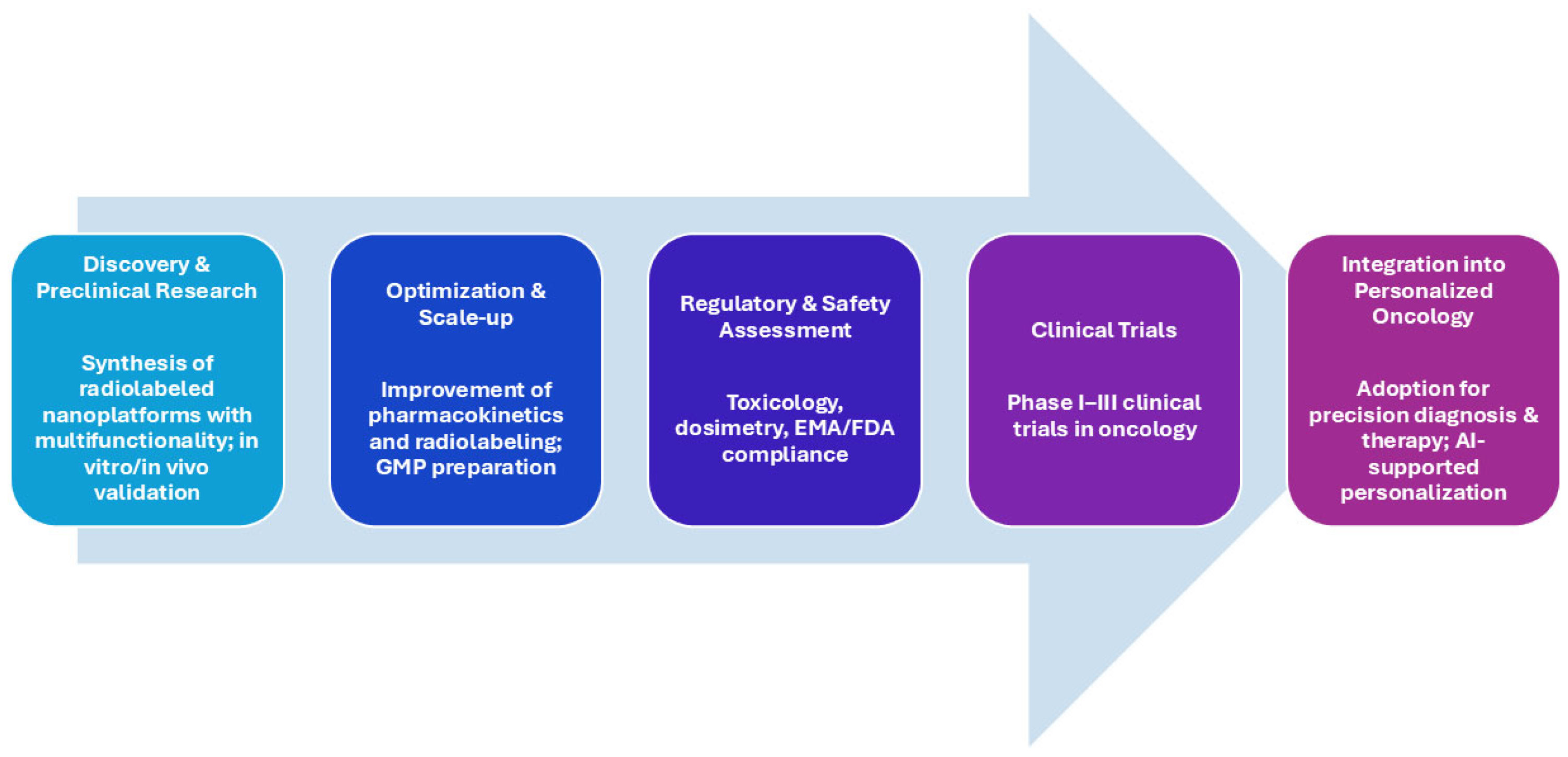
3.5. Regulatory Perspectives and GMP Considerations
3.6. Clinical Trials and Challenges in Nanotheranostics Translation
4. Final Remarks and Conclusion
Funding
Conflicts of Interest
Abbreviations
| ATMP | Advanced Therapy Medicinal Product |
| AuNPs | Gold nanoparticles |
| BFCAs | Bifunctional Chelating Agents |
| CT | Computed Tomography |
| DAPTA | D-Ala-peptide T-amide |
| DFO | Deferoxamine |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DOTAGA | 2-[1,4,7,10-Tetraazacyclododecane-4,7,10-tris(t-butyl aceta-te)]-pentanedioic acid-1t-butyl ester |
| DOX | Doxorubicin |
| DTPA | diethylenetriaminepentaacetic acid |
| EC | Electron Capture |
| EGFR | Epidermal Growth Factor Receptor |
| EMA | European Medicines Agency |
| EPR | Enhanced Permeability and Retention |
| FDA | Food and Drug Administration |
| Fn | Ferritin nanocages |
| FR | Folate Receptor |
| GLP | Good Laboratory Practice |
| GMP | Good Manufacturing Practices |
| GRPR | Gastrin-Releasing Peptide Receptor |
| HBED | N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| IAEA | International Atomic Energy Agency |
| IMPs | Investigational Medicinal Products |
| LET | Linear energy transfer |
| MOFs | Metal–Organic Frameworks |
| MRI | Magnetic Resonance Imaging |
| MSNs | Mesoporous silica nanoparticles |
| NODAGA | 2-[4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl]-5-(2,5-dioxopyrrolidin-1-yl)oxy-5-oxopentanoic acid |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| NP | Nanoparticles |
| PAMAM | Poly(amidoamine) |
| PCL | Polycaprolactone |
| PEG | Polyethylene glycol |
| PET | Positron Emission Tomography |
| PLGA | Poly(lactic-co-glycolic acid) |
| PNPs | Polymeric nanoparticles |
| PSMA | Prostate Specific Membrane Antigen |
| QDs | Quantum Dots |
| RES | Reticuloendothelial System |
| RGD | Arginylglycylaspartic acid |
| SPECT | Single Photon Emission Computed Tomography |
| SPIONs | Iron oxide nanoparticles |
| WHO | World Health Organization |
References
- James, M.L.; Gambhir, S.S. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.A.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in nuclear medicine practice. OncoTargets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef]
- Dash, A.; Chakraborty, S.; Pillai, M.R.A.; Knapp, F.F. Peptide Receptor Radionuclide Therapy: An Overview. Cancer Biotherapy Radiopharm. 2015, 30, 47–71. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R. THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy-The Bad Berka Experience. Theranostics 2012, 2, 437–447. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Gao, X.; Chen, X.; Li, L.; Li, G.; Liu, C.; Miao, Y.; Wang, R.; Hu, K. Radiopharmaceuticals and their applications in medicine. Signal Transduct. Target. Ther. 2025, 10, 1–51. [Google Scholar] [CrossRef]
- Fani, M.; André, J.P.; Maecke, H.R. 68Ga-PET: A powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 53–63. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [ 177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian, J. Pharm. Health Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef]
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2011, 41, 2656–2672. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Lankoff, A.M.; Czerwińska, M.; Kruszewski, M. Advances in Nanotheranostic Systems for Concurrent Cancer Imaging and Therapy: An Overview of the Last 5 Years. Molecules 2024, 29, 5985. [Google Scholar] [CrossRef]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef]
- Lankoff, A.; Czerwińska, M.; Kruszewski, M. Nanoparticle-Based Radioconjugates for Targeted Imaging and Therapy of Prostate Cancer. Molecules 2023, 28, 4122. [Google Scholar] [CrossRef]
- Pérez-Medina, C.; Abdel-Atti, D.; Tang, J.; Zhao, Y.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.M.; Reiner, T. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun. 2016, 7, 11838. [Google Scholar] [CrossRef]
- Zhang, C.; Jugold, M.; Woenne, E.C.; Lammers, T.; Morgenstern, B.; Mueller, M.M.; Zentgraf, H.; Bock, M.; Eisenhut, M.; Semmler, W.; et al. Specific Targeting of Tumor Angiogenesis by RGD-Conjugated Ultrasmall Superparamagnetic Iron Oxide Particles Using a Clinical 1.5-T Magnetic Resonance Scanner. Cancer Res. 2007, 67, 1555–1562. [Google Scholar] [CrossRef][Green Version]
- Kotb, S.; Detappe, A.; Lux, F.; Appaix, F.; Barbier, E.L.; Tran, V.-L.; Plissonneau, M.; Gehan, H.; Lefranc, F.; Rodriguez-Lafrasse, C.; et al. Gadolinium-Based Nanoparticles and Radiation Therapy for Multiple Brain Melanoma Metastases: Proof of Concept before Phase I Trial. Theranostics 2016, 6, 418–427. [Google Scholar] [CrossRef]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef]
- Boros, E.; Gale, E.M.; Caravan, P. MR imaging probes: Design and applications. Dalton Trans. 2014, 44, 4804–4818. [Google Scholar] [CrossRef]
- Van Der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Silberstein, E.B. Radioiodine: The Classic Theranostic Agent. Semin. Nucl. Med. 2012, 42, 164–170. [Google Scholar] [CrossRef]
- Kassis, A.I.; Korideck, H.; Wang, K.; Pospisil, P.; Adelstein, S.J. Novel Prodrugs for Targeting Diagnostic and Therapeutic Radionuclides to Solid Tumors. Molecules 2008, 13, 391–404. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Galliford, C.V.; Low, P.S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 2015, 14, 203–219. [Google Scholar] [CrossRef]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Louie, A. Multimodality Imaging Probes: Design and Challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of Pegylated Liposomal Doxorubicin. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Phillips, W.T.; Goins, B.A.; Bao, A. Radioactive liposomes. WIREs Nanomed. Nanobiotechnology 2008, 1, 69–83. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Franco, M.S.; Gomes, E.R.; Roque, M.C.; Oliveira, M.C. Triggered Drug Release from Liposomes: Exploiting the Outer and Inner Tumor Environment. Front. Oncol. 2021, 11, 623760. [Google Scholar] [CrossRef]
- Konda, S.D.; Aref, M.; Brechbiel, M.; Wiener, E.C. Development of a Tumor-Targeting MR Contrast Agent Using the High-Affinity Folate Receptor. Investig. Radiol. 2000, 35, 50–57. [Google Scholar] [CrossRef]
- Patri, A.K.; Majoros, I.J.; Baker, J.R. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Biol. 2002, 6, 466–471. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmár, M.; Vlk, M.; İlem-Özdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled nanomaterials for biomedical applications: Radiopharmacy in the era of nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tamura, H.; Selvamurugan, N. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef]
- Pérez-Campaña, C.; Gómez-Vallejo, V.; Puigivila, M.; Martín, A.; Calvo-Fernández, T.; Moya, S.E.; Ziolo, R.F.; Reese, T.; Llop, J. Biodistribution of Different Sized Nanoparticles Assessed by Positron Emission Tomography: A General Strategy for Direct Activation of Metal Oxide Particles. ACS Nano 2013, 7, 3498–3505. [Google Scholar] [CrossRef]
- Guo, J.; Hong, H.; Chen, G.; Shi, S.; Zheng, Q.; Zhang, Y.; Theuer, C.P.; Barnhart, T.E.; Cai, W.; Gong, S. Image-guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles. Biomaterials 2013, 34, 8323–8332. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, H.; Fales, A.M.; Register, J.K.; Vo-Dinh, T. Multifunctional gold nanostars for molecular imaging and cancer therapy. Front. Chem. 2015, 3, 51. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Radiolabeled Iron Oxide Nanomaterials for Multimodal Nuclear Imaging and Positive Contrast Magnetic Resonance Imaging (MRI): A Review. ACS Appl. Nano Mater. 2023, 6, 20523–20538. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Prokopiou, D.; Theodosiou, M.; Efthimiadou, E.; Koźmiński, P.; Xanthopoulos, S.; Avgoustakis, K.; Bouziotis, P. 177Lu-Labeled Iron Oxide Nanoparticles Functionalized with Doxorubicin and Bevacizumab as Nanobrachytherapy Agents against Breast Cancer. Molecules 2024, 29, 1030. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, Y.; Luehmann, H.; Yang, X.; Detering, L.; You, M.; Zhang, C.; Zhang, L.; Li, Z.-Y.; Ren, Q.; et al. 64Cu-Doped PdCu@Au Tripods: A Multifunctional Nanomaterial for Positron Emission Tomography and Image-Guided Photothermal Cancer Treatment. ACS Nano 2016, 10, 3121–3131. [Google Scholar] [CrossRef]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramírez, F.d.M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Luna-Gutiérrez, M.; Isaac-Olivé, K. Fluorescent, Plasmonic, and Radiotherapeutic Properties of the 177Lu–Dendrimer-AuNP–Folate–Bombesin Nanoprobe Located Inside Cancer Cells. Mol. Imaging 2017, 16, 1536012117704768. [Google Scholar] [CrossRef]
- Tian, Q.; Hu, J.; Zhu, Y.; Zou, R.; Chen, Z.; Yang, S.; Li, R.; Su, Q.; Han, Y.; Liu, X. Sub-10 nm Fe3O4@Cu2–xS Core–Shell Nanoparticles for Dual-Modal Imaging and Photothermal Therapy. J. Am. Chem. Soc. 2013, 135, 8571–8577. [Google Scholar] [CrossRef]
- Wu, Q.; Hou, Y.; Han, G.; Liu, X.; Tang, X.; Li, H.; Song, X.; Zhang, G. Mixed Shell Mesoporous Silica Nanoparticles for Controlled Drug Encapsulation and Delivery. Nanomedicine 2017, 12, 2699–2711. [Google Scholar] [CrossRef]
- Shi, Z.; Qing, S.; Luo, Z.; Liu, Y.; Li, J. Thermal physical and magnetic properties of water-based yolk-shell Fe3O4@C nanofluids. Inorg. Chem. Commun. 2023, 151, 110562. [Google Scholar] [CrossRef]
- Liang, R.; Li, F.; Chen, X.; Tan, F.; Lan, T.; Yang, J.; Liao, J.; Yang, Y.; Liu, N. Multimodal Imaging-Guided Strategy for Developing 177Lu-Labeled Metal–Organic Framework Nanomedicine with Potential in Cancer Therapy. ACS Appl. Mater. Interfaces 2023, 15, 45713–45724. [Google Scholar] [CrossRef]
- Krasnovskaya, O.O.; Abramchuck, D.; Erofeev, A.; Gorelkin, P.; Kuznetsov, A.; Shemukhin, A.; Beloglazkina, E.K. Recent Advances in 64Cu/67Cu-Based Radiopharmaceuticals. Int. J. Mol. Sci. 2023, 24, 9154. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Radionuclide-Activated Nanomaterials and Their Biomedical Applications. Angew. Chem. 2019, 58, 13232–13252. [Google Scholar] [CrossRef]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Cañellas, C.O.; Salgueiro, M.J.; Zubillaga, M. Radiofármacos: Del Laboratorio al Paciente, 1st ed.; CJP Ediciones: Ciudad Autó-noma de Buenos Aires, Argentina, 2017. [Google Scholar]
- Roy, I.; Krishnan, S.; Kabashin, A.V.; Zavestovskaya, I.N.; Prasad, P.N. Transforming Nuclear Medicine with Nanoradiopharmaceuticals. ACS Nano 2022, 16, 5036–5061. [Google Scholar] [CrossRef]
- Maecke, H.R. Radiolabeled Peptides in Nuclear Oncology: Influence of Peptide Structure and Labeling Strategy on Pharmacology. In Molecular Imaging: An Essential Tool in Preclinical Research, Diagnostic Imaging, and Therapy; Springer: Berlin, Germany, 2005; pp. 43–72. [Google Scholar]
- Krutzek, F.; Donat, C.K.; Stadlbauer, S. Chelator impact: Investigating the pharmacokinetic behavior of copper-64 labeled PD-L1 radioligands. EJNMMI Radiopharm. Chem. 2024, 9, 14. [Google Scholar] [CrossRef]
- Xu, J.; Cai, F.; Luo, Z.; Fan, W.; Dai, J.; Cui, J.; Li, S.; Geng, C.; Zheng, Q.; Wang, Z.; et al. Design, synthesis, and preclinical evaluation of a novel bifunctional macrocyclic chelator for theranostics of cancers. Eur. J. Nucl. Med. 2022, 49, 2618–2633. [Google Scholar] [CrossRef]
- Von Witting, E.; Garousi, J.; Lindbo, S.; Vorobyeva, A.; Altai, M.; Oroujeni, M.; Mitran, B.; Orlova, A.; Hober, S.; Tolmachev, V. Selection of the optimal macrocyclic chelators for labeling with 111In and 68Ga improves contrast of HER2 imaging using engineered scaffold protein ADAPT6. Eur. J. Pharm. Biopharm. 2019, 140, 109–120. [Google Scholar] [CrossRef]
- Phua, V.J.X.; Yang, C.-T.; Xia, B.; Yan, S.X.; Liu, J.; Aw, S.E.; He, T.; Ng, D.C.E. Nanomaterial Probes for Nuclear Imaging. Nanomaterials 2022, 12, 582. [Google Scholar] [CrossRef]
- Goel, S.; Chen, F.; Ehlerding, E.B.; Cai, W. Intrinsically Radiolabeled Nanoparticles: An Emerging Paradigm. Small 2014, 10, 3825–3830. [Google Scholar] [CrossRef]
- Garcés, M.; Magnani, N.D.; Pecorelli, A.; Calabró, V.; Marchini, T.; Cáceres, L.; Pambianchi, E.; Galdoporpora, J.; Vico, T.; Salgueiro, J.; et al. Alterations in oxygen metabolism are associated to lung toxicity triggered by silver nanoparticles exposure. Free. Radic. Biol. Med. 2021, 166, 324–336. [Google Scholar] [CrossRef]
- Garcés, M.; Marchini, T.; Cáceres, L.; Calabró, V.; Mebert, A.M.; Tuttolomondo, M.V.; Vico, T.; Vanasco, V.; Tesan, F.; Salgueiro, J.; et al. Oxidative metabolism in the cardiorespiratory system after an acute exposure to nickel-doped nanoparticles in mice. Toxicology 2021, 464, 153020. [Google Scholar] [CrossRef]
- Fuentes, P.; Bernabeu, E.; Bertera, F.; Garces, M.; Oppezzo, J.; Zubillaga, M.; Evelson, P.; Salgueiro, M.J.; Moretton, M.A.; Höcht, C.; et al. Dual strategy to improve the oral bioavailability of efavirenz employing nanomicelles and curcumin as a bio-enhancer. Int. J. Pharm. 2023, 651, 123734. [Google Scholar] [CrossRef]
- Galdopórpora, J.M.; Martinena, C.; Bernabeu, E.; Riedel, J.; Palmas, L.; Castangia, I.; Manca, M.L.; Garcés, M.; Lázaro-Martinez, J.; Salgueiro, M.J.; et al. Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics 2022, 14, 959. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.L.; Salgueiro, J.; Bernabeu, E.; Gonzalez, L.; Manca, M.L.; Amiano, N.; Valenti, D.; Manconi, M.; García, V.; et al. Pulmonary delivery of rifampicin-loaded soluplus micelles against Mycobacterium tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 53, 101170. [Google Scholar] [CrossRef]
- Giménez, V.M.M.; Moretton, M.A.; Chiappetta, D.A.; Salgueiro, M.J.; Fornés, M.W.; Manucha, W. Polymeric Nanomicelles Loaded with Anandamide and Their Renal Effects as a Therapeutic Alternative for Hypertension Treatment by Passive Targeting. Pharmaceutics 2023, 15, 176. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Dash, A.; Cai, W. Radiolabeled inorganic nanoparticles for positron emission tomography imaging of cancer: An overview. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 181–204. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Ehlerding, E.B.; Huang, P.; Cai, W. Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications. Accounts Chem. Res. 2018, 51, 778–788. [Google Scholar] [CrossRef]
- Cheng, L.; Shen, S.; Jiang, D.; Jin, Q.; Ellison, P.A.; Ehlerding, E.B.; Goel, S.; Song, G.; Huang, P.; Barnhart, T.E.; et al. Chelator-Free Labeling of Metal Oxide Nanostructures with Zirconium-89 for Positron Emission Tomography Imaging. ACS Nano 2017, 11, 12193–12201. [Google Scholar] [CrossRef]
- Shaffer, T.M.; Wall, M.A.; Harmsen, S.; Longo, V.A.; Drain, C.M.; Kircher, M.F.; Grimm, J. Silica Nanoparticles as Substrates for Chelator-free Labeling of Oxophilic Radioisotopes. Nano Lett. 2015, 15, 864–868. [Google Scholar] [CrossRef]
- Shi, S.; Xu, C.; Yang, K.; Goel, S.; Valdovinos, H.F.; Luo, H.; Ehlerding, E.B.; England, C.G.; Cheng, L.; Chen, F.; et al. Chelator-Free Radiolabeling of Nanographene: Breaking the Stereotype of Chelation. Angew. Chem. Int. Ed. Engl. 2017, 56, 2889–2892. [Google Scholar] [CrossRef]
- Tian, L.; Yi, X.; Dong, Z.; Xu, J.; Liang, C.; Chao, Y.; Wang, Y.; Yang, K.; Liu, Z. Calcium Bisphosphonate Nanoparticles with Chelator-Free Radiolabeling to Deplete Tumor-Associated Macrophages for Enhanced Cancer Radioisotope Therapy. ACS Nano 2018, 12, 11541–11551. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, C.; Zhou, M.; Shi, S.; Chow, D.S.-L.; Li, C. Integrin αvβ3-Targeted [64Cu]CuS Nanoparticles for PET/CT Imaging and Photothermal Ablation Therapy. Bioconjugate Chem. 2018, 29, 4062–4071. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef]
- Khorshidi, A. Gold nanoparticles production using reactor and cyclotron based methods in assessment of 196,198Au production yields by 197Au neutron absorption for therapeutic purposes. Mater. Sci. Eng. C 2016, 68, 449–454. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Mashel, T.V.; Gerasimova, E.N.; Peltek, O.O.; Alexandre, N.; Bruyere, S.; Kondratenko, Y.A.; et al. Radiolabeling Strategies of Micron- and Submicron-Sized Core–Shell Carriers for In Vivo Studies. ACS Appl. Mater. Interfaces 2020, 12, 31137–31147. [Google Scholar] [CrossRef]
- Zhai, D.; Wang, Y.; Yu, S.; Zhou, J.; Song, J.; Hao, S.; Chen, X. Design and evaluation of 32 P-labeled hydroxyapatite nanoparticles for bone tumor therapy. Drug Deliv. 2023, 30, 2168791. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, D.; Guleria, A.; Chakravarty, R. Production, purification and formulation of nanoradiopharmaceutical with 211At: An emerging candidate for targeted alpha therapy. Nucl. Med. Biol. 2024, 138–139, 108947. [Google Scholar] [CrossRef]
- Kukleva, E.; Suchánková, P.; Štamberg, K.; Vlk, M.; Šlouf, M.; Kozempel, J. Surface protolytic property characterization of hydroxyapatite and titanium dioxide nanoparticles. RSC Adv. 2019, 9, 21989–21995. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Nykl, E.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Hydroxyapatite and Titanium Dioxide Nanoparticles: Radiolabelling and In Vitro Stability of Prospective Theranostic Nanocarriers for 223Ra and 99mTc. Nanomaterials 2020, 10, 1632. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Determination, Modeling and Evaluation of Kinetics of 223Ra Sorption on Hydroxyapatite and Titanium Dioxide Nanoparticles. Materials 2020, 13, 1915. [Google Scholar] [CrossRef]
- Li, R.G.; Napoli, E.; Jorstad, I.S.; Bønsdorff, T.B.; Juzeniene, A.; Bruland, Ø.S.; Larsen, R.H.; Westrøm, S. Calcium Carbonate Microparticles as Carriers of 224 Ra: Impact of Specific Activity in Mice with Intraperitoneal Ovarian Cancer. Curr. Radiopharm. 2021, 14, 145–153. [Google Scholar] [CrossRef]
- Korde, A.; Mikolajczak, R.; Kolenc, P.; Bouziotis, P.; Westin, H.; Lauritzen, M.; Koole, M.; Herth, M.M.; Bardiès, M.; Martins, A.F.; et al. Practical considerations for navigating the regulatory landscape of non-clinical studies for clinical translation of radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2022, 7, 18. [Google Scholar] [CrossRef]
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal L 311, 28/11/2001 P. 0067-0128; OPOCE. 2001. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32001L0083:EN:HTML (accessed on 13 July 2025).
- Directive 2013/59/Euratom: BSS for protection against the dangers arising from exposure to ionising radia-tionEUR-Lex-32013L0059-EN-EUR-Lex. 2013. Available online: https://eur-lex.europa.eu/eli/dir/2013/59/oj (accessed on 13 July 2025).
- Anonymous. ICH M3 (R2) Non-clinical safety studies for the conduct of human clinical trials pharmaceuticals. EMA/CPMP/ICH/286/1995, European Medicines Agency. 2009. Available online: https://www.ema.europa.eu/en/ich-m3-r2-non-clinical-safety-studies-conduct-human-clinical-trials-pharmaceuticals (accessed on 13 July 2025).
- Anonymous. ICH S9 Non-clinical evaluation for anticancer pharmaceuticals. EMA/CHMP/ICH/646107/2008, European Medicines Agency. 2010. Available online: https://www.ema.europa.eu/en/ich-s9-nonclinical-evaluation-anticancer-pharmaceuticals (accessed on 13 July 2025).
- EMA. Radiopharmaceuticals. European Medicines Agency. 2018. Available online: https://www.ema.europa.eu/en/radiopharmaceuticals (accessed on 13 July 2025).
- World Health Organization. Annex 2 International Atomic Energy Agency and World Health Organization Guideline on Good Manufacturing Practices for Radiopharmaceutical Product. WHO Technical Report Series, No. 1025. 2020. Available online: https://cdn.who.int/media/docs/default-source/medicines/who-technical-report-series-who-expert-committee-on-specifications-for-pharmaceutical-preparations/trs1025-annex2.pdf?sfvrsn=7aceb0c1_6 (accessed on 13 July 2025).
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Drug Products, Including Biological Products, that Contain Nanomaterials: Guidance for Industry. 2020. Available online: https://www.gmp-compliance.org/files/guidemgr/19640025_FNL.pdf (accessed on 15 August 2025).
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability and Labeling Documentation: Guidance for Industry. U.S. Food and Drug Administration. 2018. Available online: https://www.fda.gov/media/70837/download (accessed on 12 August 2025).
| Radioisotope | Decay Mode | T1/2 | Energy of the Main Photon in keV (Abundance%) | Diagnostic Method |
|---|---|---|---|---|
| 99mTc | γ | 6 h | 140 (89) | SPECT |
| 131I | β− | 8 d | 364 (81) | SPECT |
| 123I | EC | 13.2 h | 159 (83) | SPECT |
| 67Ga | EC | 78.3 h | 93 (37); 185 (20); 300 (17); 395 (5) | SPECT |
| 111In | EC | 2.8 d | 171 (90); 245 (94) | SPECT |
| 11C | Β+ | 20 min | 511 | PET |
| 18F | β+ | 110 min | 511 | PET |
| 68Ga | β+ | 68 min | 511 | PET |
| Radioisotope | T1/2 | Emitted Particle (Energy MeV) | Max Range in Soft Tissue |
|---|---|---|---|
| 131I | 8 d | β− (0.606) | 2.3 mm |
| 223Ra | 11.43 d | 4α 2β− (5.64, 5.715) | <100 µm |
| 90Y | 64.1 h | β− (2.27) | 11.3 mm |
| 177Lu | 6.65 d | β− γ (0.497) | 1.8 mm |
| 188Re | 0.7 d | β− (2.12) | 10 mm |
| 225Ac | 10 d | 4α 2β− (6.83) | 47-85 μm |
| Diagnostic/Therapeutic Pair | Chelator | Nanotheragnostic Suitability | Clinical Application |
|---|---|---|---|
| [68Ga]/[177Lu]-DOTA-TATE | DOTA | High: stable coordination, mild labeling conditions | NETs (neuroendocrine tumors) |
| [68Ga]/[177Lu]-PSMA-617 | DOTA/DOTAGA | High: widely adapted to nanocarriers | Prostate cancer |
| [64Cu]/[67Cu]-Chelate | NOTA/SarAr | Moderate–High: versatile chelation, redox sensitivity requires stabilization | Experimental-solid tumors |
| [89Zr]/[90Y]-Chelate | DFO (for 89Zr), DOTA (for 90Y) | Moderate: DFO less stable long-term, 90Y well adapted | Antibody labeling/ solid tumors |
| [123I]/[131I]-MIBG | Direct iodination | Low for nanoplatforms-instability in vivo without encapsulation | Neuroblastoma, pheochromocytoma |
| [68Ga]/[225Ac]-PSMA | DOTA/Macropa | High: α-emitter integration into nanoparticles for targeted delivery | mCRPC, α-therapy under investigation |
| Technological Innovation Level | Radionuclide Type | Regulatory Classification | Non-Clinical Requirements | GMP Considerations |
|---|---|---|---|---|
| Incremental (e.g., liposomes + 99mTc) | Conventional (99mTc, 111In, 131I) | IMP/Radiopharmaceutical | Reduced studies if vector known | Standard GMP processes apply |
| Intermediate (e.g., new polymers + 177Lu) | Emerging therapeutic (177Lu, 90Y) | Radiotherapeutic | Toxicology (S9), biodistribution, dosimetry | Process validation, radiochemical stability |
| Disruptive (e.g., hybrid NP + 225Ac) | High-risk a-emitter (225Ac, 213Bi, 223Ra) | Advanced Radiotherapeutic/ATMP | Full toxicology, genotoxicity, organ dosimetry | Custom GMP: shielding, purity, retention |
| Increasing Regulatory Complexity | Greater innovation and risk demand more stringent regulatory oversight and tailored GMP solutions. | |||
| Nanoplatform (Trade/Code) | Composition/Radionuclide | Indication | Clinical Phase | Trial ID/Reference |
|---|---|---|---|---|
| Cornell dots | Silica NP + PEG, cRGDY peptide/124I | Imaging of metastatic melanoma | Phase I | NCT01266096 |
| AGuIX | Gadolinium-based NP/radiosensitizer | Brain metastases | Phase I | NCT03818386 |
| 177Lu-PSMA-I&T NP | Polymer-based NP/177Lu | Prostate cancer | Phase I/II | NCT04647526 |
| 188Re-BMEDA-liposome | Liposome + BMEDA/188Re | Advanced or metastatic solid tumors | Phase I | NCT02271516 |
| 64Cu SPION | Superparamagnetic iron oxide nanoparticles/64Cu | Refractory myeloma with extramedullary disease | Phase I | NCT05666700 |
| 64Cu-NOTA-PSMAi-PEG-Cy5.5-C’ dots | Ultrasmall silica C’ dots/64Cu | Prostate cancer (diagnostics and guided surgery) | Phase I | NCT04167969 |
| 89Zr-DFO-PSMAi-PEG-Cy5.5-C’ dots | Ultrasmall silica C’ dots/89Zr | Prostate cancer (diagnostics and guided surgery) | Phase I | NCT04167969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgueiro, M.J.; Zubillaga, M. Theranostic Nanoplatforms in Nuclear Medicine: Current Advances, Emerging Trends, and Perspectives for Personalized Oncology. J. Nanotheranostics 2025, 6, 27. https://doi.org/10.3390/jnt6040027
Salgueiro MJ, Zubillaga M. Theranostic Nanoplatforms in Nuclear Medicine: Current Advances, Emerging Trends, and Perspectives for Personalized Oncology. Journal of Nanotheranostics. 2025; 6(4):27. https://doi.org/10.3390/jnt6040027
Chicago/Turabian StyleSalgueiro, María Jimena, and Marcela Zubillaga. 2025. "Theranostic Nanoplatforms in Nuclear Medicine: Current Advances, Emerging Trends, and Perspectives for Personalized Oncology" Journal of Nanotheranostics 6, no. 4: 27. https://doi.org/10.3390/jnt6040027
APA StyleSalgueiro, M. J., & Zubillaga, M. (2025). Theranostic Nanoplatforms in Nuclear Medicine: Current Advances, Emerging Trends, and Perspectives for Personalized Oncology. Journal of Nanotheranostics, 6(4), 27. https://doi.org/10.3390/jnt6040027






