Synthesis and Characterization of Multifunctional Mesoporous Silica Nanoparticles Containing Gold and Gadolinium as a Theranostic System
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Synthesis of Mesoporous Silica Nanoparticles
2.3. Synthesis of Gold Nanoparticles
2.4. Synthesis of Nanocomposites
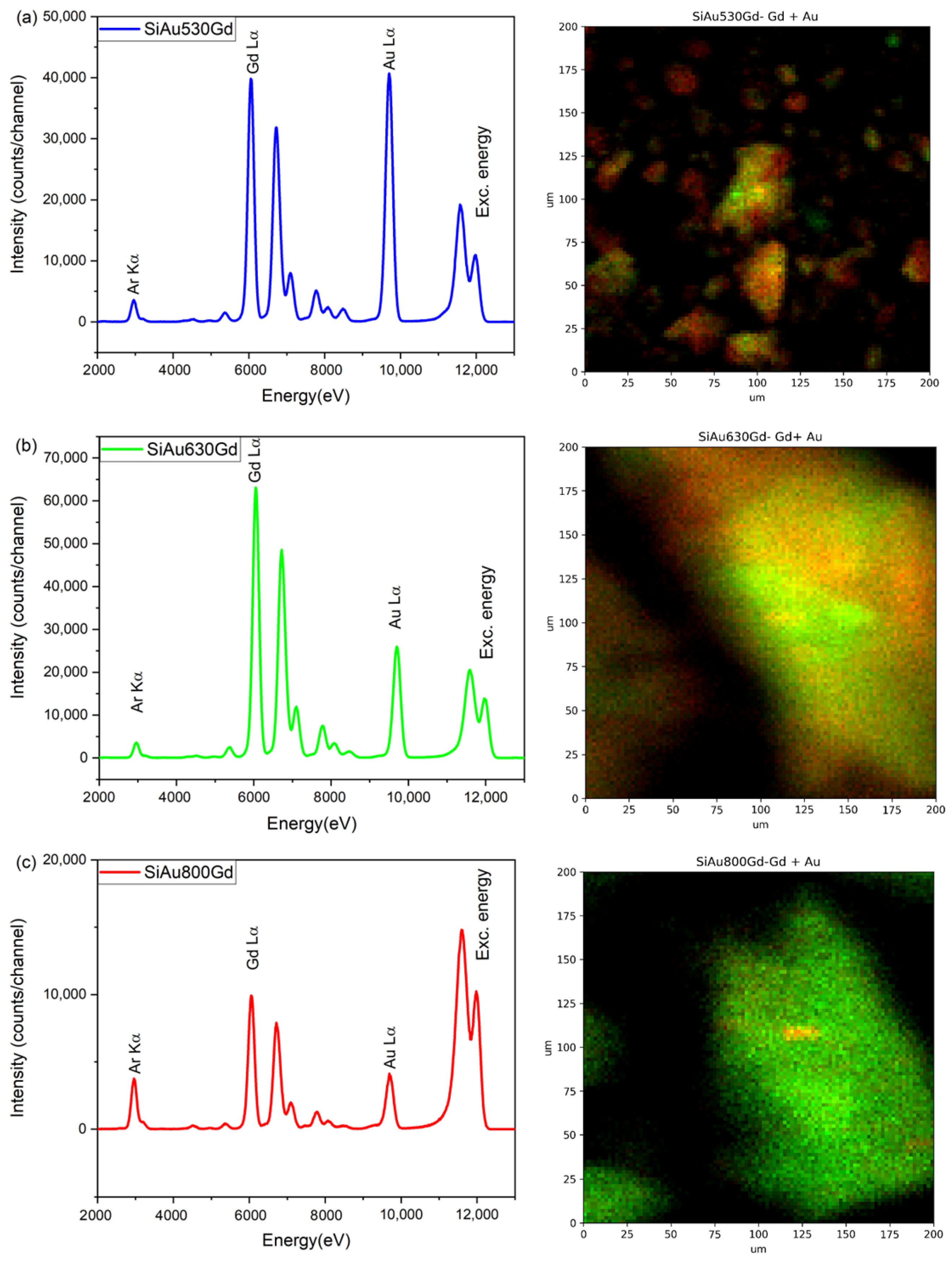
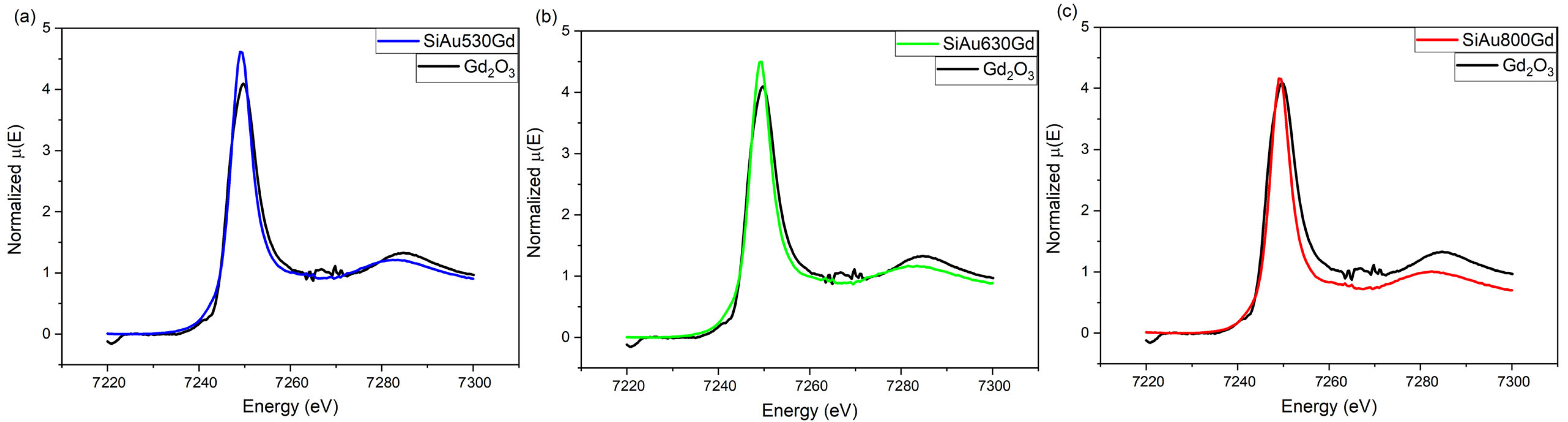
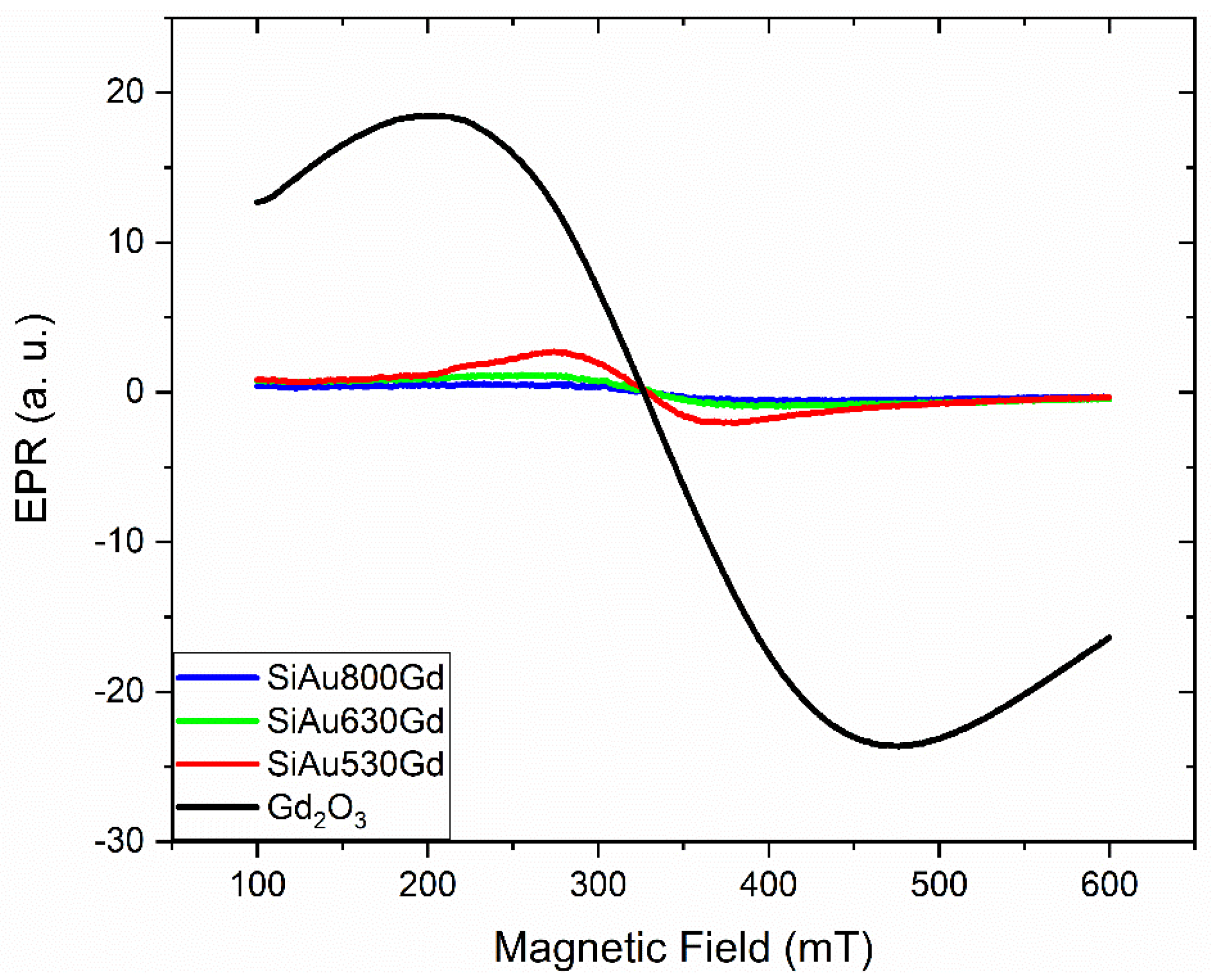
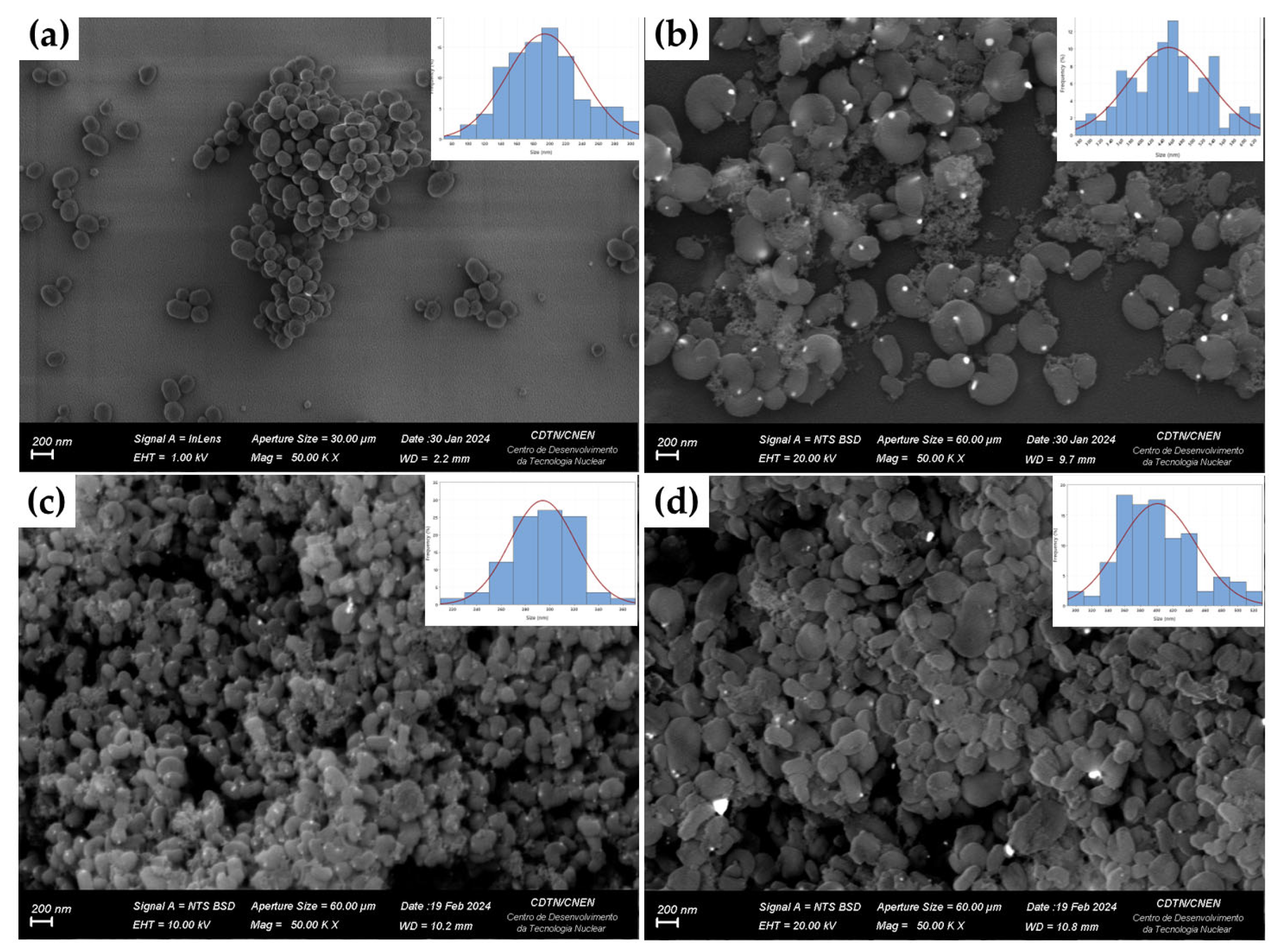
2.5. Characterization
2.6. Cell Viability Assay
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freitas, L.B.d.O.; Corgosinho, L.d.M.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; de Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Oliveira, A.F.; de Sousa, E.M.B. Synthesis and characterization of MSN/Fe3O4/Gd2O3 nanocomposite as theranostic systems. J. Nanoparticle Res. 2023, 25, 115. [Google Scholar] [CrossRef]
- Pires, I.C.B.; Shuchi, S.I.; Tostes, B.d.V.A.; Santos, D.K.D.D.N.; Burnett, W.L.; Leonce, B.C.; Harvey, O.R.; Coffer, J.L.; Filho, I.A.d.S.; de Athayde-Filho, P.F.; et al. Theranostics Using MCM-41-Based Mesoporous Silica Nanoparticles: Integrating Magnetic Resonance Imaging and Novel Chemotherapy for Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 8097. [Google Scholar] [CrossRef]
- Trayford, C.; van Rijt, S. In situ modified mesoporous silica nanoparticles: Synthesis, properties and theranostic applications. Biomater. Sci. 2024, 12, 5450–5467. [Google Scholar] [CrossRef]
- Sarnaik, S.; Bhatane, D.; Pamshong, S.R.; Alexander, A. Cutting-edge advancements in anticancer drug delivery and scope for theranostics using biocompatible multifunctional mesoporous silica nanoparticles. J. Drug Deliv. Sci. Technol. 2024, 94, 105504. [Google Scholar] [CrossRef]
- Gupta, Y.D.; Mackeyev, Y.; Krishnan, S.; Bhandary, S. Mesoporous silica nanotechnology: Promising advances in augmenting cancer theranostics. Cancer Nanotechnol. 2024, 15, 1–44. [Google Scholar] [CrossRef]
- Souza, K.C.; Mohallem, N.D.S.; Sousa, E.M.B. Mesoporous silica-magnetite nanocomposite: Facile synthesis route for application in hyperthermia. J. Sol-Gel Sci. Technol. 2009, 53, 418–427. [Google Scholar] [CrossRef]
- de Andrade, G.F.; Soares, D.C.F.; Almeida, R.K.d.S.; Sousa, E.M.B. Mesoporous Silica SBA-16 Functionalized with Alkoxysilane Groups: Preparation, Characterization, and Release Profile Study. J. Nanomater. 2012, 2012, 816496. [Google Scholar] [CrossRef]
- Andrade, G.F.; Soares, D.C.F.; dos Santos, R.G.; Sousa, E.M.B. Mesoporous silica SBA-16 nanoparticles: Synthesis, physicochemical characterization, release profile, and in vitro cytocompatibility studies. Microporous Mesoporous Mater. 2013, 168, 102–110. [Google Scholar] [CrossRef]
- Garrido, M.D.; Puchol, N.; El Haskouri, J.; Sánchez-Royo, J.F.; Folgado, J.V.; Marrachelli, V.G.; Terol, I.P.; Ros-Lis, J.V.; Marcos, M.D.; Ruíz, R.; et al. High content and dispersion of Gd in bimodal porous silica: T2 contrast agents under ultra-high magnetic fields. Microporous Mesoporous Mater. 2022, 336, 111863. [Google Scholar] [CrossRef]
- Fiedler, R.; Beizinger, B.; Walther, P.; Lindén, M. Synthesis of highly monodisperse superparamagnetic iron oxide core@mesoporous silica shell particles with independently tunable size, and porosity. Microporous Mesoporous Mater. 2022, 340, 112027. [Google Scholar] [CrossRef]
- Manzano, M.; Aina, V.; Areán, C.O.; Balas, F.; Cauda, V.; Colilla, M.; Delgado, M.R.; Vallet-Regí, M. Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chem. Eng. J. 2008, 137, 30–37. [Google Scholar] [CrossRef]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- de Sousa, A.; Maria, D.A.; de Sousa, R.G.; de Sousa, E.M.B. Synthesis and characterization of mesoporous silica/poly(N-isopropylacrylamide) functional hybrid useful for drug delivery. J. Mater. Sci. 2009, 45, 1478–1486. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- dos Apostolos, R.C.R.; Andrada, A.d.S.; Oliveira, A.F.; Neto, E.S.F.; de Sousa, E.M.B. pH-Sensitive Hybrid System Based on Eu3+/Gd3+ Co-Doped Hydroxyapatite and Mesoporous Silica Designed for Theranostic Applications. Polymers 2023, 15, 2681. [Google Scholar] [CrossRef] [PubMed]
- Meireles, I.B.d.C.J.; Oliveira, A.F.; Rodrigues, M.A.; de Sousa, E.M.B. A platform of gold nanoparticles coated with silica as controlled drug delivery for application in cancer treatment. J. Mater. Sci. 2024, 59, 22181–22205. [Google Scholar] [CrossRef]
- Ballem, M.A.; Söderlind, F.; Nordblad, P.; Käll, P.-O.; Odén, M. Growth of Gd2O3 nanoparticles inside mesoporous silica frameworks. Microporous Mesoporous Mater. 2012, 168, 221–224. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Zhang, M.; Li, G.; Hao, L. Gadolinium-Coated Mesoporous Silica Nanoparticle for Magnetic Resonance Imaging. Front. Chem. 2022, 10, 837032. [Google Scholar] [CrossRef]
- Croissant, J.G.; Guardado-Alvarez, T.M. Photocracking Silica: Tuning the Plasmonic Photothermal Degradation of Mesoporous Silica Encapsulating Gold Nanoparticles for Cargo Release. Inorganics 2019, 7, 72. [Google Scholar] [CrossRef]
- Meireles, I.B.d.C.J.; Cipreste, M.F.; Gastelois, P.L.; Macedo, W.A.d.A.; Gomes, D.A.; de Sousa, E.M.B. Synthesis and characterization of gold nanorods coated by mesoporous silica MCM-41 as a platform for bioapplication in photohyperthermia. Nanotechnology 2021, 32, 505720. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Wang, J.; Li, L.; Tang, F. Preparation of silver nanoparticles decorated mesoporous silica nanorods with photothermal antibacterial property. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129242. [Google Scholar] [CrossRef]
- Hammond-Pereira, E.; Bryant, K.; Graham, T.R.; Yang, C.; Mergelsberg, S.; Wu, D.; Saunders, S.R. Mesoporous silica-encapsulated gold core–shell nanoparticles for active solvent-free benzyl alcohol oxidation. React. Chem. Eng. 2020, 5, 1939–1949. [Google Scholar] [CrossRef]
- Trayford, C.; Crosbie, D.; Rademakers, T.; van Blitterswijk, C.; Nuijts, R.; Ferrari, S.; Habibovic, P.; LaPointe, V.; Dickman, M.; van Rijt, S. Mesoporous Silica-Coated Gold Nanoparticles for Multimodal Imaging and Reactive Oxygen Species Sensing of Stem Cells. ACS Appl. Nano Mater. 2022, 5, 3237–3251. [Google Scholar] [CrossRef]
- Song, J.-T.; Zhang, X.-S.; Qin, M.-Y.; Zhao, Y.-D. One-pot two-step synthesis of core–shell mesoporous silica-coated gold nanoparticles. Dalton Trans. 2015, 44, 7752–7756. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nagatsuka, M.; Akino, K.; Yamauchi, N.; Nakashima, K.; Inose, T.; Nishidate, C.; Sato, K.; Gonda, K.; Kobayashi, Y. Development of methods for fabricating nanoparticles composed of magnetite, gold, and silica toward diagnostic imaging. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128773. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Subedi, S.; Lee, C.L.; Wang, Q.; Jiang, Y.; Li, J.; Di, Y.; Fu, D. Emerging inorganic nanomaterials for pancreatic cancer diagnosis and treatment. Cancer Treat. Rev. 2012, 38, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- He, Z.; Zhang, C.Y.; Lei, Y.; Song, G.; Yao, Y. Plasmonic nanomaterials: A versatile phototheranostic platform of cancers. Mater. Today 2022, 62, 168–189. [Google Scholar] [CrossRef]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic Nanoshells: From Probe Design to Imaging and Treatment of Cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef]
- Scaletti, F.; Kim, C.S.; Messori, L.; Rotello, V.M. Rapid purification of gold nanorods for biomedical applications. MethodsX 2014, 1, 118–123. [Google Scholar] [CrossRef]
- Guo, T.; Lin, Y.; Li, Z.; Chen, S.; Huang, G.; Lin, H.; Wang, J.; Liu, G.; Yang, H.-H. Gadolinium oxysulfide-coated gold nanorods with improved stability and dual-modal magnetic resonance/photoacoustic imaging contrast enhancement for cancer theranostics. Nanoscale 2016, 9, 56–61. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Jiang, G.-L.; Qian, H.; Wang, L.-J.; Yang, H.-J.; Fu, X.-L.; Wu, K.-L.; Zang, Z.; Zhao, S. Escalated hyperfractionated accelerated radiation therapy for locally advanced non-small cell lung cancer: A clinical phase II trial. Radiother. Oncol. 2004, 71, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2022, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Santos, H.A.; Kumar, N.; Murzin, D.Y.; Salonen, J.; Laaksonen, T.; Peltonen, L.; Hirvonen, J.; Lehto, V.-P. Cytotoxicity study of ordered mesoporous silica MCM-41 and SBA-15 microparticles on Caco-2 cells. Eur. J. Pharm. Biopharm. 2010, 74, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.P.; Mahajan, R. Gold Polymer Nanomaterials: A Promising Approach for Enhanced Biomolecular Imaging. Nanotheranostics 2024, 8, 64–89. [Google Scholar] [CrossRef]
- Rotz, M.W.; Culver, K.S.B.; Parigi, G.; MacRenaris, K.W.; Luchinat, C.; Odom, T.W.; Meade, T.J. High Relaxivity Gd(III)–DNA Gold Nanostars: Investigation of Shape Effects on Proton Relaxation. ACS Nano 2015, 9, 3385–3396. [Google Scholar] [CrossRef]
- Barbezan, A.B.; Rosero, W.A.A.; Perez, D.V.; Rigo, M.E.Z.; da Silva, G.D.; Rodrigues, A.A.; de Almeida, L.F.; da Silva, F.F.A.; Rivera, A.G.; da Silva, N.G.; et al. Radioactive gold nanoparticles coated with BSA: A promising approach for prostate cancer treatment. Nanotheranostics 2024, 8, 112–126. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Chinchulkar, S.A.; Patra, P.; Dehariya, D.; Appidi, T.; Rengan, A.K. Gold nanoparticle–based biosensing applications and fundamentals of sensor technology: Principles and novel designs. In Fundamentals of Sensor Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 669–723. [Google Scholar]
- Neves, M.; Kling, A.; Oliveira, A. Radionuclides used for therapy and suggestion for new candidates. J. Radioanal. Nucl. Chem. 2005, 266, 377–384. [Google Scholar] [CrossRef]
- Kadria-Vili, Y.; Neumann, O.; Zhao, Y.; Nordlander, P.; Martinez, G.V.; Bankson, J.A.; Halas, N.J. Gd 2 O 3 -mesoporous silica/gold nanoshells: A potential dual T1/T2 contrast agent for MRI-guided localized near-IR photothermal therapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2123527119. [Google Scholar] [CrossRef]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct. Target. Ther. 2023, 8, 1–28. [Google Scholar] [CrossRef]
- Weinmann, H.J.; Brasch, R.C.; Press, W.R.; Wesbey, G.E. Characteristics of gadolinium-DTPA complex: A potential NMR contrast agent. Am. J. Roentgenol. 1984, 142, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.F.G.C.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-P.; Bottle, S.E.; Zhuo, R.-X.; Wei, L.; Liu, M.-L.; Li, L.-Y. Evaluation of Dendritic Gadolinium Complexes as MRI Contrast Agents. J. Bioact. Compat. Polym. 2004, 19, 453–465. [Google Scholar] [CrossRef]
- Cipreste, M.F.; Peres, A.M.; Cotta, A.A.; Aragón, F.H.; Antunes, A.d.M.; Leal, A.S.; Macedo, W.A.; de Sousa, E.M. Synthesis and characterization of 159 Gd-doped hydroxyapatite nanorods for bioapplications as theranostic systems. Mater. Chem. Phys. 2016, 181, 301–311. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Hung, Y.; Su, J.-K.; Lee, R.; Chang, C.; Lin, M.-L.; Mou, C.-Y. Gadolinium(III)-Incorporated Nanosized Mesoporous Silica as Potential Magnetic Resonance Imaging Contrast Agents. J. Phys. Chem. B 2004, 108, 15608–15611. [Google Scholar] [CrossRef]
- Moralles, M.; Pascholati, P.; Vanin, V.; Helene, O. Decay of 159Gd. Appl. Radiat. Isot. 1995, 46, 133–138. [Google Scholar] [CrossRef]
- Musa, A.S.; Hadi, M.F.R.A.; Ashour, N.I.; Hashikin, N.A.A. Theranostic Investigation of Gadolinium-159 for Hepatocellular Carcinoma: Monte Carlo Simulation Study. Appl. Sci. 2022, 12, 12396. [Google Scholar] [CrossRef]
- Musa, A.S.; Hadi, M.F.R.A.; Hashikin, N.A.A.; Ashour, N.I.; Ying, C.K. Dosimetric assessment of Gadolinium-159 for hepatic radioembolization: Tomographic images and Monte Carlo simulation. Appl. Radiat. Isot. 2023, 199, 110916. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, M.; Pekar, J.; Chettle, D.; McNeill, F.; Scott, A.; Sykes, J.; Prato, F.; Moran, G. An investigation of the toxicity of gadolinium based MRI contrast agents using neutron activation analysis. Magn. Reson. Imaging 2003, 21, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Yamano, T.; Kishimoto, H.; Venkatachalam, N.; Hyodo, H.; Soga, K. Cytotoxic aspects of gadolinium oxide nanostructures for up-conversion and NIR bioimaging. Acta Biomater. 2013, 9, 4734–4743. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, L.; Song, S.; Cao, R.; Liu, H.; Cui, C.; Li, X.; Bie, M.; Li, L. A novel one-step synthesis of Gd3+-incorporated mesoporous SiO2 nanoparticles for use as an efficient MRI contrast agent. Contrast Media Mol. Imaging 2010, 6, 110–118. [Google Scholar] [CrossRef]
- Fedorenko, S.; Stepanov, A.; Zairov, R.; Kaman, O.; Nizameev, I.; Kholin, K.; Ismaev, I.; Voloshina, A.; Sapunova, A.; Kadirov, M.; et al. One-pot embedding of iron oxides and Gd(III) complexes into silica nanoparticles—Morphology and aggregation effects on MRI dual contrasting ability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 60–67. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, H.; Xian, T.; Ma, J.; Zhang, H.; Feng, W.; Wei, Z.; Jiang, J. Morphology-controlled synthesis of orthorhombic LuFeO 3 particles via a hydrothermal route. J. Alloys Compd. 2014, 617, 855–862. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N. Gadolinium loaded nanoparticles in theranostic magnetic resonance imaging. Biomaterials 2012, 33, 5363–5375. [Google Scholar] [CrossRef]
- Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium Chelate Coated Gold Nanoparticles As Contrast Agents for Both X-ray Computed Tomography and Magnetic Resonance Imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Perry, H.L.; Botnar, R.M.; Wilton-Ely, J.D.E.T. Gold nanomaterials functionalised with gadolinium chelates and their application in multimodal imaging and therapy. Chem. Commun. 2020, 56, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Lelievre, E.; Chateau, A.; Berquand, A.; Laurent, G.; Carl, P.; Roux, S.; Chazee, L.; Bazzi, R.; Eghiaian, F.; et al. The detrimental invasiveness of glioma cells controlled by gadolinium chelate-coated gold nanoparticles. Nanoscale 2021, 13, 9236–9251. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Chateau, A.; Jubréaux, J.; Devy, J.; Paquot, H.; Laurent, G.; Bazzi, R.; Roux, S.; Richet, N.; Reinhard-Ruch, A.; et al. Radiosensitization with Gadolinium Chelate-Coated Gold Nanoparticles Prevents Aggressiveness and Invasiveness in Glioblastoma. Int. J. Nanomed. 2023, 18, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Jabariyan, S.; Zanjanchi, M.A. A simple and fast sonication procedure to remove surfactant templates from mesoporous MCM-41. Ultrason. Sonochemistry 2012, 19, 1087–1093. [Google Scholar] [CrossRef]
- Menezes, M.A.d.B.C.; Sabino, C.d.V.S.; Jaćimović, R. k0-Neutron Activation Analysis at CDTN, Brazil: 27 years of history, development and main achievements. J. Radioanal. Nucl. Chem. 2023, 332, 3457–3468. [Google Scholar] [CrossRef]
- Akpotu, S.O.; Moodley, B. Synthesis and characterization of citric acid grafted MCM-41 and its adsorption of cationic dyes. J. Environ. Chem. Eng. 2016, 4, 4503–4513. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Souza, M.J.; Araujo, A.S.; Pedrosa, A.M.; Marinkovic, B.A.; Jardim, P.M.; Morgado, E. Textural features of highly ordered Al-MCM-41 molecular sieve studied by X-ray diffraction, nitrogen adsorption and transmission electron microscopy. Mater. Lett. 2006, 60, 2682–2685. [Google Scholar] [CrossRef]
- Ni, K.; Zhao, Z.; Zhang, Z.; Zhou, Z.; Yang, L.; Wang, L.; Ai, H.; Gao, J. Geometrically confined ultrasmall gadolinium oxide nanoparticles boost the T1 contrast ability. Nanoscale 2016, 8, 3768–3774. [Google Scholar] [CrossRef]
- Hormes, J. X-ray absorption near edge structure (XANES) measurements of ceria-based solid electrolytes. Solid State Ionics 2000, 136-137, 945–954. [Google Scholar] [CrossRef]
- Szyczewski, A.; Krzyminiewski, R.; Lis, S.; Pietrzak, J.; Elbanowski, M. EPR study of selected gadolinium complexes: β-diketonates and polycarboxylates. Radiat. Phys. Chem. 1995, 45, 935–938. [Google Scholar] [CrossRef]
- Kliava, J.; Edelman, I.S.; Potseluyko, A.M.; Petrakovskaja, E.A.; Berger, R.; Bruckental, I.; Yeshurun, Y.; Malakhovskii, A.V.; Zarubina, T.V. Magnetic and optical properties and electron paramagnetic resonance of gadolinium-containing oxide glasses. J. Phys. Condens. Matter 2003, 15, 6671–6681. [Google Scholar] [CrossRef]
- Trudel, S. Unexpected magnetism in gold nanostructures: Making gold even more attractive. Gold Bull. 2011, 44, 3–13. [Google Scholar] [CrossRef]
- Hori, H.; Teranishi, T.; Nakae, Y.; Seino, Y.; Miyake, M.; Yamada, S. Anomalous magnetic polarization effect of Pd and Au nano-particles. Phys. Lett. A 1999, 263, 406–410. [Google Scholar] [CrossRef]





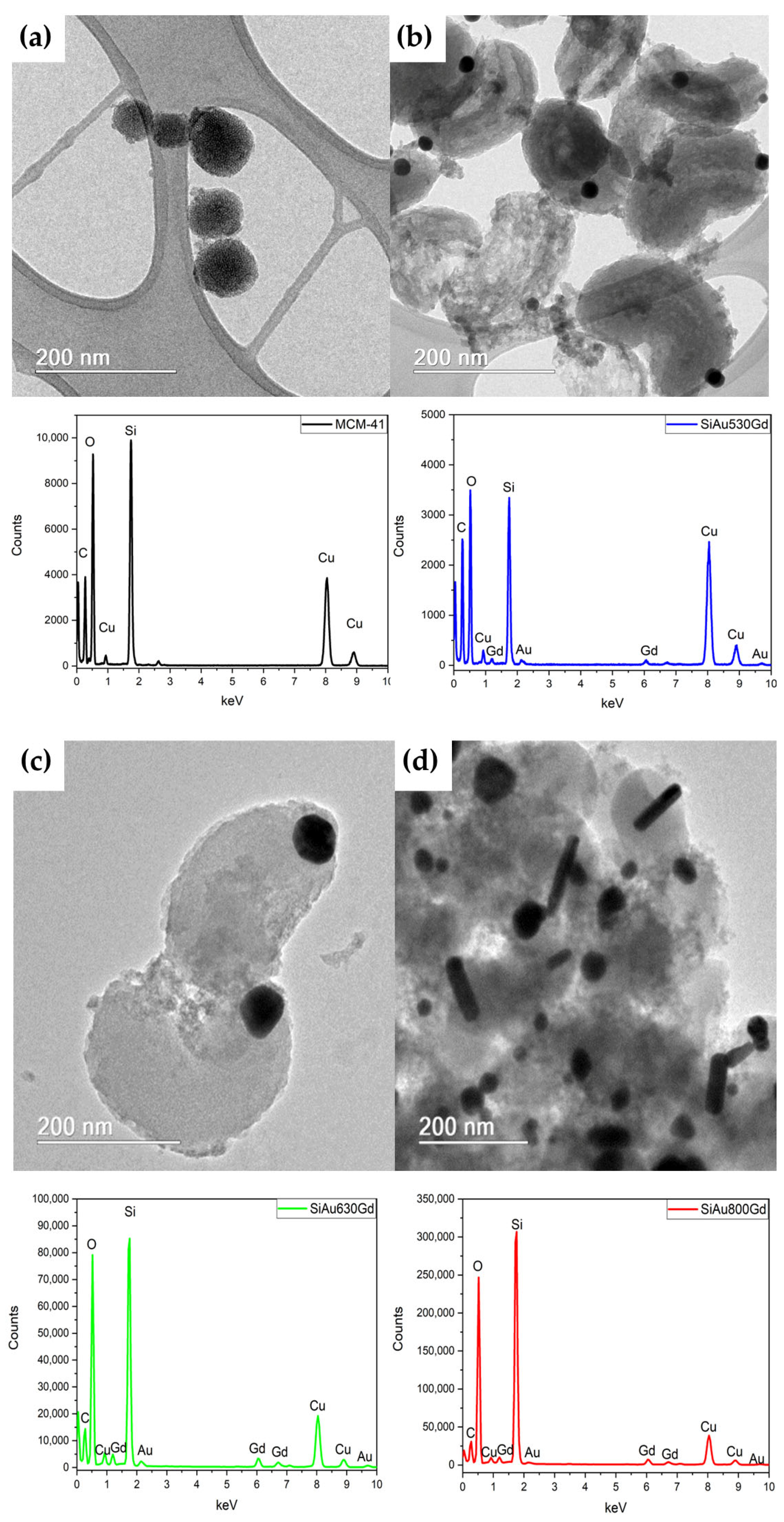



| Specific Area (m 2·g−1) | Pore Diameter (nm) | Pore Volume (cm3·nm−1·g−1) | |
|---|---|---|---|
| MCM-41 | 1162.7 | 2.6 | 0.8 |
| Si530Gd | 1006.6 | 3.5 | 0.9 |
| Si630Gd | 1039.9 | 3.5 | 0.9 |
| Si800Gd | 992.8 | 4.1 | 1.0 |
| 2Ɵ | d(100) (nm) | a0 (nm) | wt | |
|---|---|---|---|---|
| MCM-41 | 2.47° | 3.6 | 4.1 | 1.5 |
| SiAu530Gd | 2.39° | 3.7 | 4.3 | 0.8 |
| SiAu630Gd | 2.39° | 3.7 | 4.3 | 0.8 |
| SiAu800Gd | 2.33° | 3.8 | 4.4 | 0.3 |
| Si (% Mass) | O (% Mass) | Au (% Mass) | Gd (% Mass) | |
|---|---|---|---|---|
| MCM-41 | 46.0 ± 1.2 | 53.9 ± 1.2 | - | - |
| SiAu530Gd | 33.0 ± 0.1 | 57.5 ± 0.1 | 2.9 ± 0.3 | 6.6 ± 0.4 |
| SiAu630Gd | 32.8 ± 0.1 | 58.5 ± 0.1 | 1.7 ± 0.5 | 7.0 ± 0.1 |
| SAu800Gd | 29.2 ± 0.2 | 63.2± 0.1 | 1.6 ± 0.2 | 6.1 ± 0.3 |
| Theoretical percentile | 46.9/ 40.5 | 53.1/ 47.5 | 2.9 | 9.2 |
| Si (% Mass) | O (% Mass) | Au (% Mass) | Gd (% Mass) | |
|---|---|---|---|---|
| MCM-41 | 43.4 ± 0.2 | 55.7 ± 0.2 | - | - |
| SiAu530Gd | 37.8 ± 0.2 | 53.9 ± 0.2 | 3.1 ± 0.1 | 5.2 ± 0.1 |
| SiAu630Gd | 39.8 ± 0.2 | 49.2 ± 0.2 | 2.2 ± 0.1 | 8.3 ± 0.1 |
| SAu800Gd | 45.1 ± 0.1 | 48.4 ± 0.1 | 0.8 ± 0.1 | 5.3 ± 0.1 |
| Theoretical percentile | 46.9/ 40.5 | 53.1/ 47.5 | 2.9 | 9.2 |
| Size (nm) | PDI | Zeta (ζ) Potential (mV) | |
|---|---|---|---|
| MCM-41 | 208.1 ± 3.8 | 0.2 | −30.8 ± 1.4 |
| SiAu530Gd | 431.2 ± 1.4 | 0.2 | −9.5 ± 0.3 |
| SiAu630Gd | 285.9 ± 9.2 | 0.3 | −10.7 ± 0.3 |
| SiAu800Gd | 421.5 ± 15.4 | 0.4 | −9.0 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.F.; Meireles, I.B.d.C.J.; Menezes, M.A.B.C.; Krambrock, K.; Sousa, E.M.B.d. Synthesis and Characterization of Multifunctional Mesoporous Silica Nanoparticles Containing Gold and Gadolinium as a Theranostic System. J. Nanotheranostics 2025, 6, 26. https://doi.org/10.3390/jnt6040026
Oliveira AF, Meireles IBdCJ, Menezes MABC, Krambrock K, Sousa EMBd. Synthesis and Characterization of Multifunctional Mesoporous Silica Nanoparticles Containing Gold and Gadolinium as a Theranostic System. Journal of Nanotheranostics. 2025; 6(4):26. https://doi.org/10.3390/jnt6040026
Chicago/Turabian StyleOliveira, André Felipe, Isabela Barreto da Costa Januário Meireles, Maria Angela Barros Correia Menezes, Klaus Krambrock, and Edésia Martins Barros de Sousa. 2025. "Synthesis and Characterization of Multifunctional Mesoporous Silica Nanoparticles Containing Gold and Gadolinium as a Theranostic System" Journal of Nanotheranostics 6, no. 4: 26. https://doi.org/10.3390/jnt6040026
APA StyleOliveira, A. F., Meireles, I. B. d. C. J., Menezes, M. A. B. C., Krambrock, K., & Sousa, E. M. B. d. (2025). Synthesis and Characterization of Multifunctional Mesoporous Silica Nanoparticles Containing Gold and Gadolinium as a Theranostic System. Journal of Nanotheranostics, 6(4), 26. https://doi.org/10.3390/jnt6040026







