Abstract
Background and Aim: Colorectal cancer remains a leading cause of cancer-related mortality, with growing interest in nanotechnology-driven immunotherapeutics. Gold nanoparticles (AuNPs) offer a promising platform due to their biocompatibility, functional versatility, and immunomodulatory potential. Carcinoembryonic antigens (CEAs), highly expressed in colorectal tumors, provide an ideal target for antigen-specific immune activation. The aim of this study is to evaluate the immunogenicity, biodistribution, and therapeutic efficacy of a CEA-functionalized gold nanoparticle (CEA-AuNP) construct in a mouse model of colorectal cancer following oral administration via a customized capsular delivery system. Methods: A 30-day oral administration study was performed in BALB/c mice (n = 30), who received increasing doses of CEA-AuNPs (5–50 mg/kg/day). Histological, hyperspectral imaging, and ELISA-based cytokine analyses were conducted to assess organ integrity, nanoparticle accumulation, and immune modulation. Results: CEA-AuNPs demonstrated a favorable safety profile and dose-dependent accumulation in reticuloendothelial tissues, particularly the spleen and liver. Cytokine profiling revealed enhanced IL-10 responses in the spleen, indicating anti-inflammatory immune modulation, with localized pro-inflammatory signals observed in hepatic tissue at higher doses. No signs of systemic toxicity or significant off-target effects were detected. Conclusions: The oral administration of CEA-AuNPs in healthy mice induced tissue-specific immune responses and exhibited a dose-dependent biodistribution pattern. These results support the further development of CEA-AuNPs as a nanovaccine platform for colorectal cancer immunoprophylaxis.
1. Introduction
Colorectal cancer remains one of the most prevalent and lethal malignancies worldwide, with only limited success gained from conventional therapies, particularly in metastatic settings [1]. While often effective in early-stage disease, these therapies are often associated with significant limitations, such as adverse effects, limited efficacy in metastatic disease, and tumor immune evasion [2]. Consequently, the development of innovative immunoprophylactic strategies capable of inducing a potent, tumor-specific immune response has become a major focus of contemporary oncological research [3].
Increasing attention has turned to nanovaccine platforms, which could prime the immune system against early tumor emergence [4]. Tumor-associated antigens (TAAs) represent the molecular basis for these approaches, with the carcinoembryonic antigen (CEA) being a particularly attractive target [5]. CEA is a glycoprotein overexpressed in a wide range of epithelial malignancies, including colorectal cancer while showing restricted expression in normal adult tissues [6]. This expression profile, combined with its immunogenic properties, supports the rationale for CEA-based vaccination platforms aimed at both treatment and disease prevention.
However, effective antigen delivery remains a major challenge in the development of cancer vaccines. Traditional formulations are often limited by rapid antigen degradation, inefficient uptake by antigen-presenting cells (APCs), and insufficient trafficking to secondary lymphoid organs, resulting in suboptimal immune activation [7]. Addressing these limitations requires innovative delivery systems that preserve antigen structures, enhance cellular internalization, and sustain immunostimulatory signaling.
Nanotechnology offers a compelling solution, with gold nanoparticles (AuNPs) standing out as highly versatile carriers for vaccine design. AuNPs possess a favorable safety profile, tunable size, surface chemistry, and unique optical properties that allow for functionalization with a wide range of biomolecules [8]. When administered orally, these constructs may access gut-associated lymphoid tissue and reticuloendothelial organs, initiating mucosal and systemic immune priming. When conjugated with tumor antigens such as CEA, these nanoconstructs can improve antigen stability, facilitate multivalent presentation to immune cells, and enhance the magnitude and duration of the immune response [9]. Moreover, their compatibility with oral delivery systems may enable mucosal priming and systemic immunity, offering a minimally invasive strategy for cancer prevention [10]. However, the preclinical evaluation of such constructs in healthy models is essential to establish safety, biodistribution, and immunomodulatory potential prior to advancing into tumor-bearing systems.
The aim of this study is to evaluate the translational potential of a CEA-functionalized gold nanoparticle (CEA-AuNP) platform for oral cancer immunoprophylaxis. Based on previously published in vitro results [11]—which demonstrated effective CEA loading, acceptable cytocompatibility, and macrophage internalization of the CEA-AuNP construct—we proceeded to perform in vivo evaluations. Specifically, we sought to characterize the in vivo safety profile, biodistribution, and immunomodulatory effects of orally administered CEA-AuNPs in a healthy murine model. This study further assesses the cytokine modulation and histological changes associated with nanoparticle exposure, establishing the foundation for future therapeutic development against colorectal cancer.
2. Materials and Methods
2.1. Synthesis and Characterization of CEA-AuNPs
Gold nanoparticles were synthesized using the Turkevich method. Briefly, 40 mg of HAuCl4·3H2O was dissolved in bidistilled water, brought to a boil, and reduced by adding 5 mL of a 20 mg/mL sodium citrate solution under vigorous stirring. The reaction proceeded for 2 h before cooling to room temperature. Synthesized AuNPs were characterized via UV-VIS spectroscopy, which showed a distinct surface plasmon resonance (SPR) peak at 522 nm, indicative of spherical nanoparticles. Dynamic light scattering (DLS) confirmed a hydrodynamic diameter of ~20 nm, and atomic force microscopy (AFM) verified morphological uniformity.
CEA functionalization was performed using thiol–gold chemistry. The CEA peptide (MyBiosource.com) was first reduced using 100 mM DTT to expose free thiol groups before being incubated with the AuNP colloid at room temperature for 2 h at pH 7. Purification was achieved through ultracentrifugation (>10,000 rpm, 15 min) and redispersion in distilled water. Successful conjugation was verified by a redshift and broadening of the UV-VIS spectrum (510–700 nm plateau), a DLS-confirmed size increase to ~80 nm, and AFM spectral fingerprint changes. This confirmed peptide binding and colloidal stability supports its suitability for cellular and immunological studies. All preparations were handled under aseptic conditions, using sterile materials and buffers to minimize microbial contamination.
2.2. The Study Design for In Vivo Experiments
All in vivo experiments were conducted on healthy BALB/c mice without the chemical (e.g., AOM/DSS) or genetic induction of cancer or inflammation. The purpose was to evaluate the safety, biodistribution, and immunogenic potential of CEA-functionalized AuNPs in a prophylactic context. The 30-day oral administration period was chosen based on OECD TG 407 guidelines for subacute toxicity studies in rodents [12] and supported by previous reports on prolonged oral exposure to gold nanomaterials. This timeline also mimics cumulative antigen presentation similar to prophylactic vaccination models. We conducted a subacute toxicity and biodistribution study on 30 female BALB/c mice, aged 6–8 weeks, with an initial body weight of 18–22 g, divided into five groups (n = 6 per group). Animals received the daily oral administration of CEA-AuNP formulations via gavage for 30 consecutive days; Phosphate-Buffered Saline (PBS) for the control group; and escalating doses of 5 mg, 10 mg, 20 mg, and 50 mg/kg /day for the test groups. Nanoparticle suspensions were freshly prepared from a stock solution at 50 µg/mL prior to each administration. Animals were housed under standard conditions with ad libitum access to food and water. All procedures were approved by the Veterinary Sanitary Directorate of Cluj County (Approval No. 287/19 January 2022).
The dose range of 5–50 mg/kg/day was selected based on previous in vitro cytocompatibility assays and is consistent with doses reported in similar murine models investigating gold nanoparticle biodistribution and immunological safety [8,9,10]. These studies have shown that oral doses up to 50 mg/kg are generally well tolerated and elicit dose-dependent immune responses without overt systemic toxicity. This range allowed us to explore both low- and high-end exposure scenarios relevant to prophylactic applications.
2.3. Tissue Collection and Processing
Following euthanasia, tissues, including the liver, spleen, mesenteric lymph nodes, and small intestine, were immediately collected for histological and optical analyses. For a detailed comparative evaluation, samples from the control group and the highest-dose group were prioritized.
For hyperspectral dark-field microscopy, tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned at 5 μm thickness using a rotary microtome. The sections intended for optical and hyperspectral analysis were left unstained to preserve the intrinsic light-scattering properties of gold nanoparticles. Unstained sections were mounted on standard glass slides using a DPX mounting medium and allowed to dry overnight before imaging. Serial sections from the same block were stained with hematoxylin-eosin (H&E) and Congo red (with and without potassium permanganate pretreatment) for parallel histopathological evaluations.
2.4. Hyperspectral Dark-Field Microscopy
The biodistribution of CEA-AuNPs was assessed using hyperspectral dark-field microscopy. Optical images and spectral data were acquired using a CytoViva hyperspectral imaging system (CytoViva Inc., Auburn, AL, USA) mounted on an Olympus BX43 light microscope equipped with an automated x-y motorized stage. Images were captured using a DageXL camera (Dage-MTI, Michigan City, IN, USA) and processed with Exponent 7 and ENVI 4.8 software for the spectral mapping of gold nanoparticle accumulation.
2.5. Cytokine Quantification
Cytokine levels (IL-4, IL-1β, IL-10, IL-12, TNF-α, and IFN-γ) were assessed using commercial ELISA kits (Elabscience Biotechnology Inc., Houston, TX, USA) according to the manufacturer’s instructions. For each group, spleen and liver samples from six animals (n = 6) were collected and pooled to form a composite sample. Organ homogenates were prepared by mechanical disruption in PBS supplemented with protease inhibitors, followed by centrifugation to isolate the supernatant. To obtain sufficient protein quantities for reliable cytokine quantification, each cytokine was measured once per pooled group lysate for each organ (n = 1). This approach, while limiting statistical interpretation, allowed exploratory comparisons and has been previously used in preliminary or resource-limited studies [13]. Due to the absence of replicate measurements, data are presented as absolute values for descriptive purposes only. No statistical analysis was carried out. The results should be interpreted as preliminary trends pending confirmation with appropriate replicates.
2.6. Statistical Analysis
Cytokine measurements were performed as single determinations on pooled samples from each group and organ. Given the absence of technical or biological replicates, no statistical analyses (e.g., ANOVA, t-tests) were applied to cytokine data. Instead, the results are presented descriptively to illustrate the potential trends observed between groups. These findings should be interpreted as exploratory and hypothesis-generating, not as statistically validated outcomes.
3. Results
The results presented below derive from a subacute toxicity study in a murine model (n = 30) in which CEA-AuNPs were administered orally and daily for 30 days in single doses ranging from 5 to 50 mg/kg. The analysis focused on the evaluation of histopathologic changes, including tissue distribution and immunologic response associated with the sustained administration of the test compound.
The experimental groups were systematically organized, with each cohort receiving a specific treatment regimen as outlined in Table 1. The dosages were calibrated according to the assigned group, ensuring a controlled and reproducible exposure protocol.

Table 1.
The composition of the experimental groups.
3.1. Macroscopic Findings
The administration of CEA-AuNPs at subacute doses over a 30-day period to 30 laboratory mice revealed observable differences between control and high-dose treated groups, most notably in the spleen. Due to limitations in sample availability, spleen measurements were recorded in full only for the control group and the high-dose group (50 mg/kg). The maximum spleen length observed in the control group was 20.78 mm, while the corresponding value in the 50 mg/kg group reached 31.25 mm. Although these findings are consistent with nanoparticle-induced splenomegaly, a complete statistical comparison could not be made. This observation is reported descriptively and should be interpreted with caution.
The current study design did not include intermediate dose groups for macroscopic evaluation, which limits the ability to comprehensively assess the dose–response relationship. It is important to note that this experiment was conceived as an exploratory investigation intended to screen for potential significant alterations at the highest dose level. The observations reported provide qualitative insights that will inform the design of future studies involving multiple dose groups and sufficient replicates for statistical evaluation.
3.2. Histopathological Findings
In order to assess dose-related tissue responses to CEA-AuNP exposure, histological evaluation was performed on tissue sections from all study groups and compared against the standard reference histology for each organ. This approach allowed for the identification of both subtle and overt pathological changes relative to normal tissue architecture. No significant histopathological alterations were observed in the low-dose groups when compared to the expected normal histological structure. These low-dose images are presented as a visual baseline for comparison in the absence of significant alterations, ensuring the interpretability of dose-dependent tissue responses.
The analysis was organized by organ system and stratified by the administered dose: 10 mg/kg (low), 20 mg/kg (intermediate), and 50 mg/kg (high). The representative images provided are intended to illustrate the absence or presence of pathological changes relative to expected normal histology, as determined during the microscopic assessment.
In the spleen, both the 10 mg/kg and 20 mg/kg groups preserved normal histological architecture (Figure 1A). Active extramedullary hematopoiesis was present, involving erythroid, myeloid, and megakaryocytic precursors, which is consistent with physiological activity in murine spleen tissue. In contrast, the 50 mg/kg group exhibited multifocal amyloid deposits in the perifollicular zones (Figure 1B). These deposits led to the compression and focal atrophy of adjacent splenic tissue, suggesting a dose-related accumulation of proteinaceous material potentially associated with nanoparticle clearance or chronic immune activation.
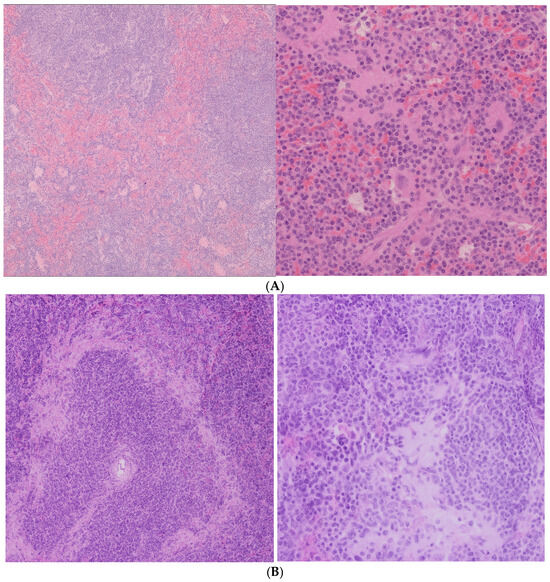
Figure 1.
(A) Spleen histological analysis. (Left): 10 mg AuNP/kg/day, no significant changes (HE ob ×10). (Right): 20 mg AuNP/kg/day, no significant changes. Foci of extramedullary (regular) hematopoiesis of the erythroid and myeloid lines, in mixture with megakaryocytes, HE, obj ×20. (B) Spleen Histological Analysis—50 mg CEA-AuNP/kg/day. Multifocal amyloidosis, predominantly in the perifollicular areas, with moderate compression atrophy of peripheral zonal tissue. HE, obj ×10 (Left) and ×40. (Right).
In the liver, the 10 mg/kg and 20 mg/kg groups exhibited preserved hepatic architecture (Figure 2A). Occasional extramedullary hematopoietic foci were noted, which is a common finding in murine livers. At the highest dose (50 mg/kg), eosinophilic amyloid-like deposits were identified in the space of Disse, accompanied by local hepatocellular atrophy. These findings suggest hepatic stress or altered protein turnover at higher exposure levels. This may be evidence of focal hepatocellular alterations, accompanied by mild periportal inflammatory infiltration, suggestive of early hepatic stress or immune activation.
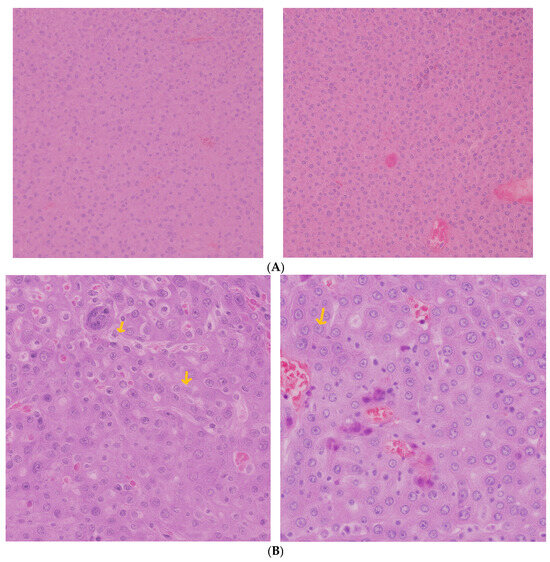
Figure 2.
(A) Liver histological analysis—(Left): 10 mgCEA-AuNP/kg/day, no significant changes. HE, obj ×10. (Right): 20 mg CEA-AuNP/kg/day, no significant changes. Foci of extramedullary changes HE, obj ×20. (B) Liver histological analysis—50 mg CEA-AuNP/kg/day, hepatic amyloidosis, accumulation of eosinophilic extracellular material in the space of Disse (arrows), with moderate compression atrophy of the parenchyma. HE, obj ×40.
The myocardium showed no histological abnormalities across all dose groups (Figure 3A,B). Myocardial fibers remained intact, with no evidence of inflammation, fibrosis, or degenerative changes, indicating minimal cardiotoxicity following the systemic administration of CEA-AuNPs.
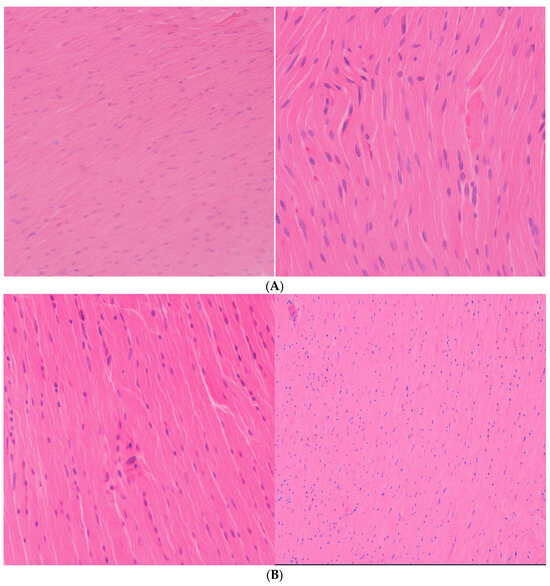
Figure 3.
(A) Myocardium: 10 mg AuNP/kg/day, no significant changes. HE, obj ×20 (Left image); HE, obj ×40 (Right image). (B) Myocardium histological analysis: (Left): 20 mg CEA-AuNP/kg/day, no changes. HE, obj ×20. (Right): 50 mg CEA-AuNP/kg/day, no significant changes. HE, obj ×20.
Histological sections of the stomach, including the transition zone between the glandular and fermentative mucosa, were unremarkable across all tested doses (Figure 4A,B). Only at the low dose (10 mg/kg) were a few superficial yeast cells observed in the mucosa, which were not associated with inflammation or tissue damage and were considered incidental findings.
Figure 4.
(A) Stomach (transition area between fermentative and glandular segments) 10 mg CEA-AuNP/kg/day, no significant changes. Rare yeast cells are present in the superficial gastric mucosa. HE, obj ×10 (left image); HE, obj ×40 (Right image). (B) Stomach (glandular-fermentative transition zone). (Left): 20 mg CEA-AuNP/kg/day, no changes. HE, obj ×10; (Right): 50 mg CEA-AuNP/kg/day, no significant changes. HE. obj ×10.
Pulmonary tissue appeared to be normal across all dose groups (Figure 5). No morphological changes, inflammatory infiltrates, or alveolar damage were noted, indicating the favorable pulmonary biocompatibility of the nanostructured compound.
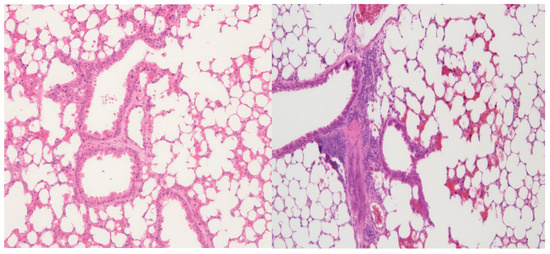
Figure 5.
Lung histological analysis: (Left)—10 mg CEA-AuNP/kg/day, no significant changes. HE, obj ×10. (Right): 50 mg AuNP/kg/day, no significant changes. HE, obj ×20.
In the kidneys (Figure 6), the 10 mg/kg group showed no structural abnormalities upon the histological examination of the renal cortex. The intermediate dose (20 mg/kg) revealed the focal accumulation of brown, basophilic cytoplasmic granules in tubular epithelial cells. These were presumed to be residual nanoparticle aggregates or lipofuscin pigments, although they were not associated with degenerative changes. The highest dose (50 mg/kg) did not demonstrate additional pathological alterations.
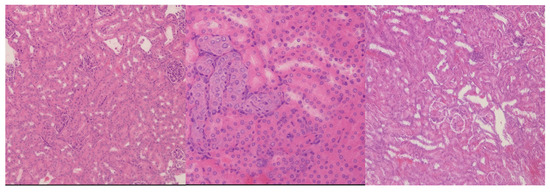
Figure 6.
Renal cortex histological analysis. (Left): 10 mg CEA-AuNP/kg/day, no significant changes. HE, obj ×10. (Center): 20 mg CEA-AuNP/kg/day—multifocal, basophilic renal epithelial cells with a brown granular appearance (possible lipofuscin), HE, obj ×40. (Right): 50 mg CEA-AuNP/kg/day: no significant changes. HE, obj ×10.
Finally, the cerebral cortex retained its normal architecture at all dose levels, with no evidence of neuronal degeneration, gliosis, or inflammatory cell infiltration (Figure 7). These findings suggest that the CEA-AuNP construct does not cross the blood–brain barrier at cytotoxic levels or exert neurotoxic effects under the tested conditions.
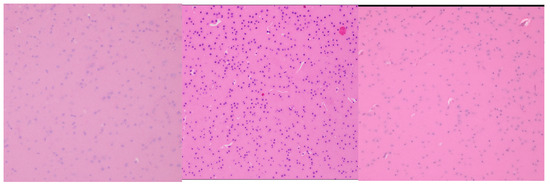
Figure 7.
Cerebral cortex histological analysis. (Left): 10 mg CEA-AuNP/kg/day, no significant changes. HE, obj ×20. (Center): 20 mg CEA-AuNP/kg/day, no significant changes HE, obj ×40. (Right): 50 mg CEA-AuNP/kg/day: no significant changes. HE, obj ×10.
These features are consistent with chronic immune stimulation, potentially reflecting the spleen’s role in nanoparticle clearance and immune modulation. These findings highlight a tissue-specific response to high-dose exposure, with pronounced effects in organs involved in immune processing and detoxification.
3.3. Hyperspectral Dark-Field Microscopy
Hyperspectral dark-field microscopy enabled the high-resolution spectral mapping of gold-based nanomaterials across multiple tissue types. Characteristic white light reflectance signatures, attributable to the optical properties of the nanoparticles, were detected with varying intensities depending on the organ type and dosage.
For visualization purposes, hyperspectral images from the control at 5 mg/kg/day and 50 mg/kg/day groups are presented in Figure 8 and Figure 9. These groups were selected to represent baseline (control), low-dose, and high-dose conditions, which showed the most distinct spectral signal contrasts. While intermediate dose groups (10 mg and 20 mg/kg/day) were also analyzed, the reflectance signals in those tissues were modest and spatially inconsistent. As such, they were excluded from the figure panels to maintain visual clarity and prevent misinterpretation. Nevertheless, the complete dataset supports a dose-dependent biodistribution profile, as corroborated by qualitative spectral analysis.
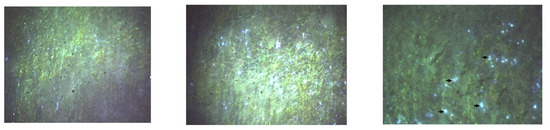
Figure 8.
Hyperspectral microscopy images (Cytoviva) from the splenic level. (Left): Control. (Center): The group with 5 mg CEA-AuNP/kg/day. (Right): Numerous white light reflectance spots are observed for the 50 mg CEA-AuNP/kG body/day batch (black arrow).
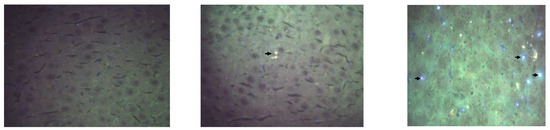
Figure 9.
Hyperspectral microscopy images (Cytoviva) of the liver. (Left): Control. (Center): The group receiving 5 mg CEA-AuNP/kg/day. (Right): Numerous white light reflectance spots are observed for the 50 mg CEA-AuNP/kG body/day batch (black arrow).
Nanoparticle-associated spectral signals were prominently detected in the spleen (Figure 8), indicating substantial nanoparticle accumulation. This accumulation is consistent with prior in vitro internalization patterns [11].
Moderate presence in the liver (Figure 9) and myocardium suggests the partial uptake of functionalized NPs. The presence of CEA-AuNPs was minimal to undetectable in the kidneys, lungs, and cortical brain tissue, implying limited or no nanoparticle retention in these regions. Qualitative analysis suggests a dose-dependent relationship between signal intensity and nanoparticle accumulation, reinforcing the biodistribution profile inferred from organ-specific reflectance patterns.
3.4. Cytokine Profile Analysis
Cytokine measurements in spleen and liver homogenates showed variable expression patterns following the oral administration of CEA-AuNPs at increasing doses. The results are derived from single ELISA measurements performed on pooled tissue lysates from each group (n = 6 animals/group). As such, the values are presented descriptively to illustrate the general trends in cytokine modulation. No statistical comparisons or standard deviations were reported due to the lack of replicated data. These findings serve as a preliminary assessment of immunomodulatory activity and inform future studies involving full statistical validation.
In the spleen, analysis revealed a predominantly anti-inflammatory response characterized by the elevation of IL-10 levels relative to IL-12. Notable trends included an increase in IL-10 levels at higher doses, suggesting anti-inflammatory and immunomodulatory effects. IL-4 levels decreased at intermediate doses but rose sharply at 50 mg/kg. IL-12 peaked at 5 mg/kg but declined at higher doses, indicating a shift in immune activation balance. The pro-inflammatory markers IL-1β and TNF-α showed variable responses, while IFN-γ increased progressively with the dose. These profiles support a dynamic immunological response modulated by nanoparticle dosage. This cytokine shift suggests macrophage activation and cell-mediated immunological polarization, which is consistent with an immunostimulatory effect and potentially supportive of a vaccine-like antitumoral response (Figure 10).
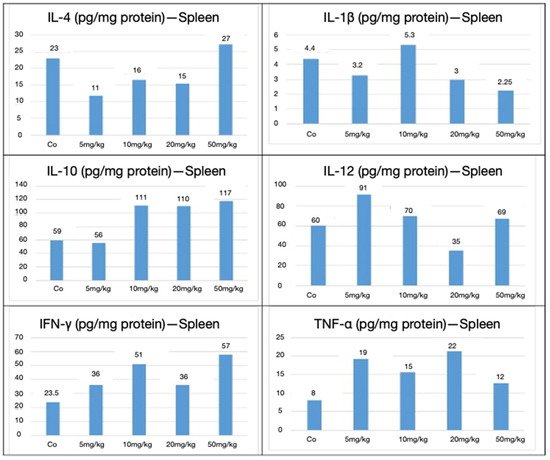
Figure 10.
Splenic cytokines expressed in pg/mg of protein for IL-4, IL-1β, IL-10, IL-12, IFN-γ, and TNF-α, following CEA-AuNP administration at increasing doses. Note: Cytokine concentrations (pg/mg protein) are shown in the spleen from pooled organ lysates (n = 6 animals/group). Results reflect single measurements per group and are presented for descriptive purposes only. No statistical comparisons were conducted.
In contrast, the hepatic cytokine profile exhibited a more pro-inflammatory tendency, particularly at higher nanoparticle doses. Although both IL-10 and IL-12 levels were elevated, the absence of IL-10’s predominance, as observed in the spleen, indicated a more balanced pro- and anti-inflammatory environment. Additionally, sporadic increases in TNF-α and IL-1β levels were noted, suggesting localized immune activation and early signs of hepatic functional stress. IL-10 levels remained elevated across all doses, peaking at 10 mg/kg, indicating sustained anti-inflammatory signaling. IL-4 expression decreased at intermediate doses but rebounded by 50 mg/kg. IL-12 and IL-1β levels declined to 20 and 50 mg/kg, suggesting the suppression of some pro-inflammatory pathways. In contrast, TNF-α exhibited an increase at higher doses, with the highest expression at 50 mg/kg. IFN-γ responses were variable, with a transient spike at 5 mg/kg. These hepatic cytokine dynamics suggest a more balanced or mildly pro-inflammatory immune profile, particularly at higher doses, potentially reflecting the localized immune activation associated with hepatic nanoparticle processing (Figure 11).
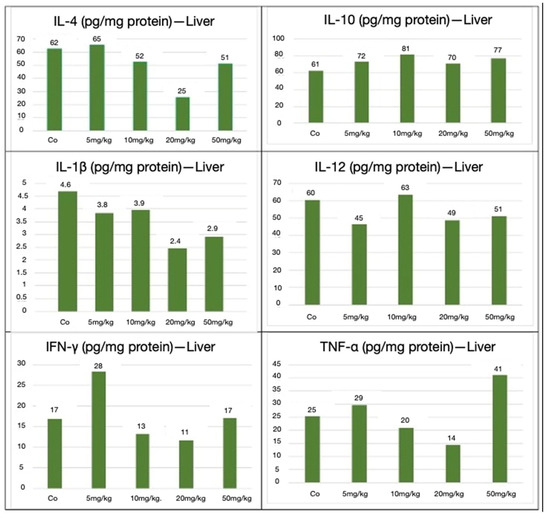
Figure 11.
Hepatic cytokines expressed in pg/mg of protein for IL-4, IL-1β, IL-10, IL-12, IFN-γ, and TNF-α, following CEA-AuNP administration at increasing doses. Note: Cytokine concentrations (pg/mg protein) are shown in the liver from pooled organ lysates (n = 6 animals/group). Results reflect single measurements per group and are presented for descriptive purposes only. No statistical comparisons were made.
Collectively, these findings indicate that CEA-AuNP exposure leads to targeted immune activation within the spleen, which is conducive to an antitumoral nanovaccine effect while maintaining controlled inflammatory responses within the liver at higher concentrations without evidence of systemic toxicity. Moreover, the results suggest a dose-dependent biodistribution profile with associated histological changes, particularly within reticuloendothelial tissues. These findings provide preliminary insights into cytokine dynamics following repeated nanoparticle exposure, warranting further quantitative and statistically powered studies. A summary of cytokine modulation is presented in Table 2.

Table 2.
Summary of organ-specific cytokine profile changes. Note: The interpretations are exploratory in nature.
4. Discussion
The present in vivo study evaluated the safety, biodistribution, and immunological impact of CEA-AuNPs administered subacutely over 30 days in a murine model. The key findings include dose-dependent histological changes, splenic immune activation, and limited organ toxicity, suggesting a favorable immunostimulatory profile for potential vaccine development. Building upon these in vivo observations, the Discussion section considers how prior preclinical validations alongside the present findings collectively support the immunological potential and biocompatibility of the CEA-AuNP nanovaccine platform.
Prior to in vivo testing, the CEA-AuNP nanoconstruct underwent extensive physicochemical and biological validation to ensure its suitability as a nanovaccine candidate [11]. The successful synthesis and functionalization of gold nanoparticles (AuNPs) with CEA-derived peptides, confirmed through UV-Vis spectroscopy and dynamic light scattering (DLS) analyses, established a stable, monodisperse nanoplatform with favorable physicochemical properties. These biophysical characteristics—specifically the plasmonic peak at 522 nm, post-functionalization hydrodynamic size of approximately 80 nm, and retention of colloidal stability—are consistent with the literature describing effective peptide conjugation to metallic nanoparticles [14,15,16].
In vitro validation further confirmed the biocompatibility and functional capacity of the CEA-AuNP construct. Cell viability remained above 85% even at the highest tested dose (50 µg/mL), with a modest, dose-dependent decline likely reflecting adaptive cellular responses rather than toxicity [17,18]. The observed upregulation in caspase-3 activity suggested a controlled apoptotic response, which is a beneficial phenomenon in enhancing antigen presentation by macrophages [19]. Additionally, the effective cellular internalization and perinuclear localization of the nanoconjugates were consistent with antigen trafficking toward proteasomal pathways essential for MHC-mediated immune activation [20].
Following this preclinical validation, the in vivo administration of CEA-AuNPs revealed consistent and promising immunological effects, particularly in splenic architecture, cytokine modulation, and systemic safety. The logical progression from in vitro validation to in vivo efficacy strengthens the translational relevance of the CEA-AuNP nanoconstruct. These in vivo findings provide important confirmation that the physicochemical integrity and immunological function observed in vitro are retained and effectively translated into a living system, supporting the further development of this platform for cancer immunoprophylaxis.
Histological evaluation was performed for all experimental groups, including controls; representative micrographs from the control group were not retained for publication. However, low-dose groups, which exhibited no significant alterations, served as a valid visual comparator and are included in the revised manuscript. Moreover, histological figures have been organized to present side-by-side sections for the organ and dose level, thereby improving comparative interpretations across experimental conditions.
The most pronounced response was observed in the spleen, both morphologically and functionally. Significant splenomegaly and histological alterations—such as lymphoid hyperplasia, active hematopoiesis, and the presence of megakaryocytes—were particularly evident in animals treated with intermediate and high doses of CEA-AuNPs. These changes indicate the stimulation of both innate and adaptive immune compartments, consistent with an active immunological response to the nanoconstructs and CEA exposure [21].
One of the notable histological findings was the development of splenic amyloidosis in the group exposed to the highest dose (50 mg/kg). Amyloidosis is characterized by the extracellular deposition of amorphous proteinaceous material in multiple tissues, particularly within the red pulp and marginal zones of the spleen, as observed here. In murine models, inflammation-induced amyloidosis is predominantly mediated by serum amyloid A (SAA), an acute-phase reactant produced during systemic inflammatory responses [22]. Given the relatively young age of the experimental mice, the observed amyloid deposits are likely triggered secondary to sustained immune activation by prolonged nanoparticle exposure rather than senile amyloidosis [23].
The detection of extramedullary hematopoiesis in both the spleen and liver at intermediate and high doses further supports systemic hematologic adaptation, possibly reflecting increased demands for immune cell production [24]. According to the literature, such reactive changes are non-neoplastic and occur in response to persistent immune stimulation [25,26].
Despite these histological changes, no overt tissue damage was detected in renal, pulmonary, or cerebral tissues across all the groups. Minor findings, such as brown granular deposits in the renal cortex at intermediate doses, were isolated and did not indicate significant organ impairment.
Hyperspectral imaging confirmed that gold nanoparticle accumulation was primarily localized to the spleen and liver, with lower presence in the heart and negligible accumulation in the kidneys and brain, consistent with the known biodistribution of nanoparticles favoring the reticuloendothelial system [27,28]. Reflectance intensity comparisons were made visually between dose groups and were not subjected to automated image quantification. This semi-quantitative approach is noted as a limitation, and future studies will include numerical image analysis.
This biodistribution pattern, coupled with the absence of major tissue damage, prompted a deeper evaluation of the immunological microenvironment through cytokine profiling. Although cytokine measurements were limited to mean values without replicate-level statistical analysis, the observed trends offer preliminary insights into the immune response triggered by CEA-AuNP exposure. In the spleen, increased levels of IL-4 and IL-10 at higher nanoparticle doses may suggest a shift toward an anti-inflammatory or regulatory immune phenotype, which is consistent with macrophage activation and immune modulation [29]. Although IL-10 is often associated with immune tolerance, in the context of vaccine platforms, controlled IL-10 elevation may enhance antigen presentation and limit excessive inflammation, possibly leading to effective T-cell activation [9,30]. Concurrent elevations in IFN-γ and TNF-α—although moderate—hint at a balanced activation of pro-inflammatory responses, possibly reflecting early immune engagement.
Similar trends were observed in hepatic tissue, where a slight increase in pro-inflammatory cytokines (TNF-α and IL-1β) was noted at higher doses. However, these effects were mild and sporadic, and the balanced IL-10/IL-12 ratio in the liver suggests organ-specific immunomodulation rather than overt toxicity [31].
The efficient localization of nanoparticles within macrophage-rich organs and the associated cytokine profiles align with prior studies on nanoparticle-based vaccine platforms, where gold nanoparticles facilitated enhanced antigen uptake and promoted robust cellular immune responses [10,32,33].
While these observations cannot be confirmed statistically due to the lack of raw replicated data, they highlight potential organ-specific cytokine effects that merit further investigation using fully quantitative methods. Future studies incorporating replicate-level ELISA measurements, multiplex cytokine panels, and appropriate controls will be essential to confirm and expand on these preliminary findings.
Taken together, our findings support the relative biocompatibility and immunogenic potential of CEA-AuNPs when administered at low and intermediate doses. The observed splenic immune activation, without evidence of systemic toxicity at these doses, highlights the promise of these nanoconstructs to become carriers for tumor-associated antigens. Such platforms could improve antigen delivery, processing, and presentation, ultimately stimulating cytotoxic T-cell responses against CEA-expressing malignancies, including colorectal, pancreatic, and lung carcinomas [6,34,35].
The development of amyloidosis at high doses emphasizes the need for careful dose optimization in future applications. Further studies on tumor-bearing models are necessary to validate these findings, assess long-term efficacy and immunological memory, and explore potential synergy with immune checkpoint inhibitors [36]. Additionally, mechanistic investigations into antigen cross-presentation pathways (e.g., via MHC class I) will be critical for advancing translational development.
5. Conclusions
This study demonstrates that CEA-functionalized gold nanoparticles exhibit favorable biodistribution, immunogenic activation, and a strong safety profile at low-to-intermediate doses in vivo. These findings, supported by prior in vitro validation, highlight the potential of CEA-AuNPs as a promising nanovaccine platform for colorectal cancer immunoprophylaxis. Further studies focusing on therapeutic efficacy, dose optimization, and combination immunotherapy strategies are warranted to advance this platform toward clinical translation.
Author Contributions
All authors have contributed equally to this work. Their involvement includes the conception and design of the study, data acquisition, analysis, and interpretation, as well as drafting the manuscript and critically revising it for important intellectual content. All authors have reviewed and approved the final version for publication. Each author accepts full responsibility for every aspect of the work, ensuring the accuracy and integrity of the entire study. Should any questions arise regarding the reliability or validity of any component, all authors are committed to promptly investigating and resolving such issues. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors used artificial intelligence (AI)-based tools, including ChatGPT (OpenAI), DeepL Translator, and Perplexity AI, to assist with language editing, phrasing suggestions, and improving manuscript clarity. No AI tool was used for data analysis, scientific interpretation, or decision-making. All intellectual content, analysis, and conclusions are the original work of the authors, who assume full responsibility for the integrity and accuracy of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AuNPs | Gold nanoparticles |
| CEA | Carcinoembrionic antigen |
| CEA-AuNP | CEA-functionalized gold nanoparticles |
| HE | Hematoxilin & eozine |
References
- Granados-Romero, J.J.; Valderrama-Treviño, A.I.; Contreras-Flores, E.H.; Barrera-Mera, B.; Herrera Enríquez, M.; Uriarte-Ruíz, K.; Ceballos-Villalba, J.C.; Estrada-Mata, A.G.; Alvarado Rodríguez, C.; Arauz-Peña, G. Colorectal cancer: A review. Int. J. Res. Med. Sci. 2017, 5, 4667. [Google Scholar] [CrossRef]
- Stintzing, S. Management of colorectal cancer. F1000Prime Rep. 2014, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Bhagat, A.; Lyerly, H.K.; Morse, M.A.; Hartman, Z.C. CEA vaccines. Hum. Vaccin. Immunother. 2023, 19, 2291857. [Google Scholar] [CrossRef]
- Hammarström, S. (Ed.) The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness-A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Almeida, J.P.; Figueroa, E.R.; Drezek, R.A. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine 2014, 10, 503–514. [Google Scholar] [CrossRef]
- Ferrando, R.M.; Lay, L.; Polito, L. Gold nanoparticle-based platforms for vaccine development. Drug Discov. Today Technol. 2020, 38, 57–67. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Zdrehus, R.S.; Mocan, T.; Sabau, L.I.; Matea, C.T.; Tăbăran, F.; Pop, T.; Delcea, C.; Mosteanu, O.; Mocan, L. CEA-Functionalized Gold Nanoparticles as a Nanovaccine Platform: In Vitro Evaluation of Cytocompatibility, Cellular Uptake, and Antigen Processing. Vaccines 2025, 13, 668. [Google Scholar] [CrossRef]
- OECD. Repeated Dose 28-Day Oral Toxicity Study in Rodents. OECD Guidelines for the Testing of Chemicals, Section 4, Test No. 407; Organisation for Economic Co-operation and Development: Paris, France, 2008. [Google Scholar]
- Broadhurst, D.I. Kell DB. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2006, 2, 171–196. [Google Scholar] [CrossRef]
- Zaccariotto, G.D.C.; Bistaffa, M.J.; Zapata, A.M.M.; Rodero, C.; Coelho, F.; Quitiba, J.V.B.; Lima, L.; Sterman, R.; Cardoso, V.M.D.O.; Zucolotto, V. Cancer Nanovaccines: Mechanisms, Design Principles, and Clinical Translation. ACS Nano 2025, 19, 16204–16223. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Zhu, X.; Wang, L.; Wu, C. Synthesis, properties, and optical applications of noble metal nanoparticle-biomolecule conjugates. Chin. Sci. Bull. 2012, 57, 238–246. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef]
- Zhang, Q.; Pi, J.; Woods, C.G.; Jarabek, A.M.; Clewell, H.J., III; Andersen, M.E. Hormesis and adaptive cellular control systems. Dose-Response 2008, 6, 07–028. [Google Scholar] [CrossRef]
- Zhang, Q.; Bhattacharya, S.; Pi, J.; Clewell, R.A.; Carmichael, P.L.; Andersen, M.E. Adaptive posttranslational control in cellular stress response pathways and its relationship to toxicity testing and safety assessment. Toxicol. Sci. 2015, 147, 302–316. [Google Scholar] [CrossRef]
- Byrne, H.J.; Maher, M.A. Numerically modelling time and dose dependent cytotoxicity. Comput. Toxicol. 2019, 12, 100090. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Roy, A.A.; Pokale, R.; Mukharya, A.; Jadhav, S.R.; Naik, G.A.R.R.; Rachana, S.P.; Colaco, V.; Hedayat, P.; Kudarha, R.; Mutalik, S.; et al. Innovative Organic Core–Nonmagnetic Shell Nanoconstructs: Pioneering Precision in Cancer Theranostics. In Core-Shell Nano Constructs for Cancer Theragnostic: Current Scenario, Challenges and Regulatory Aspects; Springer: Berlin/Heidelberg, Germany, 2025; pp. 415–449. [Google Scholar]
- Ge, F.; Yao, J.; Fu, X.; Guo, Z.; Yan, J.; Zhang, B.; Zhang, H.; Tomozawa, H.; Miyazaki, J.; Sawashita, J.; et al. Amyloidosis in transgenic mice expressing murine amyloidogenic apolipoprotein A-II (Apoa2c). Lab. Investig. 2007, 87, 633–643. [Google Scholar] [CrossRef]
- Kakinen, A.; Javed, I.; Davis, T.P.; Ke, P.C. In vitro and in vivo models for anti-amyloidosis nanomedicines. Nanoscale Horizons. 2021, 6, 95–119. [Google Scholar] [CrossRef]
- Cenariu, D.; Iluta, S.; Zimta, A.A.; Petrushev, B.; Qian, L.; Dirzu, N.; Tomuleasa, C.; Bumbea, H.; Zaharie, F. Extramedullary Hematopoiesis of the Liver and Spleen. J. Clin. Med. 2021, 10, 5831. [Google Scholar] [CrossRef]
- Kim, C.H. Homeostatic and pathogenic extramedullary hematopoiesis. J. Blood Med. 2010, 1, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Miwa, Y.; Abe-Suzuki, S.; Abe, S.; Kirimura, S.; Onishi, I.; Kitagawa, M.; Kurata, M. Extramedullary hematopoiesis: Elucidating the function of the hematopoietic stem cell niche. Mol. Med. Rep. 2016, 13, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Ma, X. TNF-α and IL-12: A balancing act in macrophage functioning. Microbes Infect. 2001, 3, 121–129. [Google Scholar] [CrossRef]
- He, J.S.; Liu, S.J.; Zhang, Y.R.; Chu, X.D.; Lin, Z.B.; Zhao, Z.; Qui, S.H.; Guo, Y.G.; Ding, H.; Pan, Y.L. The application of and strategy for gold nanoparticles in cancer immunotherapy. Front. Pharmacol. 2021, 12, 687399. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Finn, O.J. Cancer immunology. N. Engl. J. Med. 2008, 358, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.M.; Wright, J.A.; Blake, S.J.; Page, A.J.; Worthley, D.L.; Woods, S.L. Advancing translational research for colorectal immuno-oncology. Br. J. Cancer 2023, 129, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).