Abstract
Lung cancer remains one of the main causes of cancer-related death globally and a significant global health concern. There is an urgent need for safer and more effective therapeutic alternatives despite notable progress in therapy; issues such as drug resistance, side effects, metastasis, and recurrence still affect patient outcome and quality of life. The aim of this review is to examine recent developments in the application of herbal-drug-loaded nanoparticles as a new strategy for treating lung cancer. A thorough examination of different drug delivery systems based on nanoparticles is provided, highlighting their function in improving the solubility, bioavailability, and targeted delivery of herbal compounds. In addition, the review evaluates the biomarkers used for targeted therapy and examines how new personalised treatment approaches like wearable electronic patches, robotics-assisted interventions, smartphone-enabled therapies, AI-driven diagnostics, and lung-on-a-chip technologies can be integrated to improve the accuracy and effectiveness of lung cancer treatment. In conclusion, the combination of personalised medicine and nanotechnology may lead to revolutionary changes in lung cancer treatment in the future.
1. Introduction
Lung cancer is the primary cause of cancer-related death globally and one of the most diagnosed cancers, with around 2.48 million new cases (12.4%) and 1.82 million deaths (18.7%) from the disease in 2022, according to estimates from the GLOBOCAN report. Due to a number of successful tobacco control initiatives, the incidence of lung cancer in men has been decreasing in many nations, but rates in women are still increasing [1]. It is, at present, the second most prominent malignant tumour in women and the first in men in terms of both incidence and fatality [2]. There are primarily two major histological subtypes of lung cancer, i.e., small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) [3]. SCLCs are highly aggressive tumours accounting for 15–20% of all primary lung malignancies and are generally instigated by smoking [4]. NSCLC is a prevalent subtype that accounts for over 85% of all lung cancer occurrences and thus is a major threat to human life, health, and social and economic advancement [5]. There are four subtypes of NSCLC: lung adenocarcinoma (LAUD), lung squamous cell carcinoma (LUSC), large-cell carcinoma, and bronchial carcinoid tumour. Out of these, LUAD is the most frequently occurring in female non-smokers. Conventional therapies for NSCLC that have been used for the last two decades include immunotherapy, radiochemotherapy, and surgical resection [6]. Surgery is the primary treatment method for NSCLC in the early stages, but over 81% of medically confirmed cases are ineligible for surgical methods. The platinum-based chemotherapeutic technique is considered to be the primary medication for advanced NSCLC, yet the prognosis remains poor. Radio-chemotherapy frequently leads to adverse effects, and drug resistance can develop, potentially worsening the condition [7]. Therefore, there is an immediate requirement to develop novel therapeutic strategies for the management of lung cancer. Over the last twenty years, a global surge has been observed in research and development of anticancer medication, with many novel treatments making their way into clinical trials and use. The idea and approach to cancer, from diagnosis to therapy, are undergoing novel developments in the age of precision medicine. According to a WHO report, around 80% of individuals living in rural areas rely mainly on herbal medicines as their primary healthcare system. Recently, herbal medicines have gained wide popularity as an alternative to traditional medicines. This is because herbal medicines have several advantages over conventional therapy. Conventional medicines often involve chemicals and radiation that can adversely affect normal cells in the body, while herbal medicines are much cheaper and are believed to have fewer side effects. Herbal medicines derived from plants have been utilised for their therapeutic effects since ancient times. Many plant formulations have been developed and are popularly used for treating various diseases, mainly cancer, diabetes, cardiovascular disease, obesity, microbial contamination, chronic inflammatory disease, and liver disease. Herbal medicines have abundant active ingredients that provide several medicinal properties, such as antitumour, anti-inflammatory, antioxidant, antibacterial, and antiproliferative activities [8]. They have been demonstrated to have remarkable anticancer effects. They can activate DNA repair mechanisms, trigger apoptosis, modulate miRNAs, elevate levels of protective enzymes, enhance the immune system, and display antioxidant activity. There has thus been a keen interest in finding novel herbal drugs in the pharmaceutical industry. Currently, 50% of the medications used in the healthcare system are of natural origin [9], and almost 77% of lung cancer patients use herbal remedies as adjuvants in addition to conventional (like chemotherapy) treatment [10]. It has been demonstrated that the traditional Chinese herb Selaginella tamariscina inhibits the formation of malignant lung cells and stops them from spreading. The flowering plant Crocus sativus L. is most known for its saffron. Its aqueous extract is frequently used in complementary and alternative applications. Research on its impact on cancer cells revealed that apoptosis was associated with the capacity to impede the advancement of lung cancer. In experimental models for both prevention and treatment, a bioactive component discovered in Toona sinensis leaves has been shown to slow the growth of H441 xenograft tumours. In human lung cancer cell lines, it was demonstrated that the methanolic fraction of Sesbania grandiflora exhibited potent antiproliferative properties. Reactive oxygen species (ROS) of intermediate leaves were found to increase the chance of apoptosis in cells [11]. Natural products have consistently demonstrated their potential as efficient agents in treating lung cancer. Still, unfortunately, the low solubility and low absorption of herbal medications decrease their bioavailability and limit their practical applications. Over the last decade, significant research has been conducted to advance novel drug delivery systems to enhance bioavailability, lessen the side effects, and prevent drug degradation [12]. Using herbal medicines in the configuration of nanoparticles offers a potential strategy that overcomes this limitation and enhances scientific investigations, offering a promising avenue for effective and precise cancer treatment. This involves altering absorption processes, increasing specific surface area, and decreasing particle size—all of which improve solubility and bioavailability. Such nanoformulations can significantly enhance drug effectiveness by diminishing toxicity and increasing bioavailability. This approach minimises the need for repeated administration, promoting greater compliance with therapeutic regimens.
This review summarises the advantages of employing nanosystems for delivering herbal drugs as a novel treatment approach. This review has also examined various intelligent detection methods, innovative therapies, and robotic surgery techniques for lung cancer management, leading to the term “therasurgynostic.” This concept integrates diagnostic and therapeutic research with advanced surgical procedures, offering a unified approach that has the potential to significantly improve lung cancer care and support global efforts to control and eventually eliminate this devastating disease. To enable this, a comprehensive and systematic review of the existing literature was conducted in an orderly manner (Figure 1).
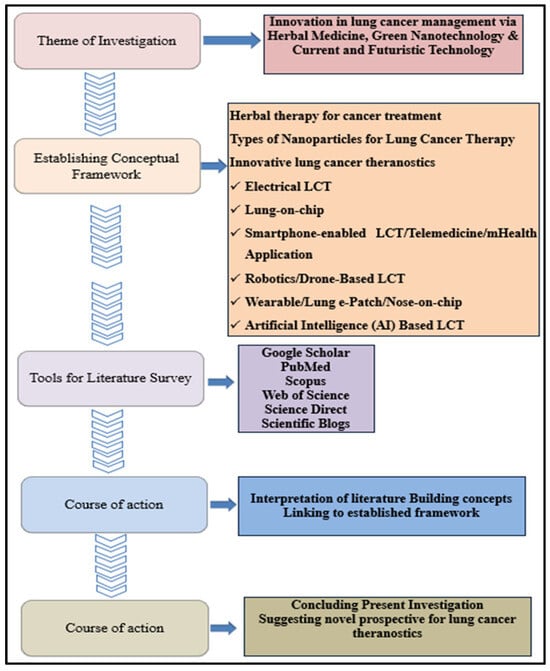
Figure 1.
Systematic literature Review analysis: under the direction of a conceptual framework, the review of the literature concentrated on the current and future management of lung cancer. Key insights were obtained from scholarly sources, culminating in novel prospects on theranostics of lung cancer, including their incorporation with AI.
2. Biomarkers Targeted for Lung Cancer Treatment
Immune checkpoints are small proteins on tumour cells and T-cells that help check the immune cell responses and keep them from being too strong. When these surface proteins of both cancer cells and T-cells interact, it sends an “off” signal to T-cells, inhibiting them from destroying the tumour cells [13]. Immune checkpoint inhibitors, or ICIs, are immunotherapy drugs that can block such immune checkpoints, enhancing T-cell efficiency to kill cancer cells [14]. ICIs, unlike specially targeted medications that bind to target gene mutation sites and dampen proliferative impulses or cytocidal anticancer agents that disrupt the cell cycle, employ host autoimmune processes to exert their antitumour effects. PD-1/PD-L1 and CTLA-4 are the most common targets for ICI therapy for lung cancer treatment [15]. Some examples of ICI drugs are atezolizumab (Tecentriq), ipilimumab (Yervoy), pembrolizumab (Keytruda), and nivolumab (Opdivo) [16,17].
2.1. Signalling Pathways Targeting Tumour Immunity
2.1.1. CTLA-4
CTLA-4 is a T-lymphocyte surface protein receptor regarded as a primary immune checkpoint inhibitor (ICI). It hinders the activity of autoreactive T-cells during the initial activation stage of naive T-cells by binding CD80 or B7.1 ligand, CD86 or B7.2 ligand, and CD28 receptor [17,18]. The activity of this particular receptor is essential for regulating self-tolerance and preventing autoimmune disorders [18,19]. The B7, CD80, and CD86 found on APCs are primary CTLA-4 and CD28 T-cell receptor ligands. CD28 is a co-stimulatory molecule that efficiently activates T-cells through IL-2 gene transcription and T-cell proliferation [20]. CTLA-4 is homologous to CD28 but has a greater affinity for the B7 ligand and acts as a negative regulator for T-cell activation. B7 ligands bind CD28 and induce immune responses through T-cell multiplication, whereas binding B7 ligands to CTLA-4 inhibits their activity. The extent of CTLA:B7 interactions compared to CD28:B7 interactions determines if the T-cell becomes activated or remains inactive [21] (Figure 2).
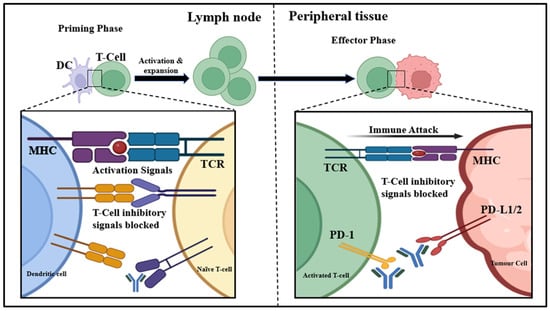
Figure 2.
Importance of CTLA-4/PD-1/PD-L1 in tumour suppression: CTLA-4 is an inhibitory receptor on T-cells that binds to B7 on APCs, suppressing T-cell activation. Tumours exploit this to evade immune response. Blocking CTLA-4 with specific drugs restores T-cell activation, promoting tumour cell destruction. PD-L1, expressed on tumour cells and APCs, binds to PD-1 on T-cells, leading to T-cell inactivation. Anti-PD-1/PD-L1 therapies block this interaction, reactivating T-cells to target and kill tumour cells.
Targeting the CTLA-4 pathway shows excellent potential in cancer treatment [22,23]. The first ever evidence of antitumour effects of inhibiting CTLA-4 activity was suggested by Leach and Krummel in 1996 [24]. Various anti-CTLA-4 antibodies are now being used to suppress the binding of B7 and CTLA-4, leading to increased binding of B7 to CD28 receptors, stimulating T-cell activation, and enhanced antitumour effects. The anti-CTLA-4 antibodies work through the initial phase of antigen presentation, typically when dendritic cells expose T-cells to antigens and stimulate their activation [25]. Many monoclonal antibodies like ipilimumab (Yervoy) and tremelimumab (Imjuno) are CTLA-4 inhibitors used as immunotherapy drugs for cancer treatment [26]. Ipilimumab is a recombinant human IgG1 monoclonal antibody that works by lowering CTLA4-induced T-cell inhibitory roles by binding to its MYPPPY motif, which is essential for its interaction with B7, and by targeting effector cells expressing an activatory Fc receptor against CTLA-4-expressing Tregs. Tremelimumab is a human monoclonal IgG2 antibody that operates through a similar mechanism [27,28]. These drugs are generally paired with a PD-1 or PD-L1 inhibitor and used to treat several types of cancer [29].
2.1.2. PD-L1
Programmed death-1 (PD-1) is an immune checkpoint like CTLA-4, expressed on activated T- and B-cell surfaces. It interacts with PD-L1 (present on several cells and tumour cells) and PD-L2 (found on haematopoietic cells) ligands and acts as an inhibitor of immunity by suppressing T-cell activity, comprising interferon-γ production, multiplication, and promoting apoptosis of T-cells [30]. Programmed death ligand-1 (PD-L1), a receptor within the immunoglobulin superfamily, negatively regulates T-cell antigen receptor signalling by binding to PD-1, contributing significantly to self-tolerance maintenance. Tumour cells often take advantage of the PD-1/PD-L1 pathway by expressing multiple PD-L1 proteins on their surface, suppressing T-cell activity, and thus can evade immune responses. Blocking the interaction between PD-1 and PD-L1 presents an appealing immunotherapeutic approach for cancer cells expressing PD-L1. The presence of PD-L1 on cancer cells and immune cells serves as a crucial biomarker for estimating the treatment outcome of anti-PD-1/PD-L1 in patients with SCLC.
Using monoclonal antibodies as PD-1 and PD-L1 inhibitors has exhibited remarkable outcomes [31]. In recent years, various anti-PD-1 antibodies like nivolumab (Opdivo), pembrolizumab (Keytruda), cemiplimab (Libtayo), and their ligands, anti-PD-L1 antibodies like atezolizumab (Tecentriq), avelumab (Bavencio), and durvalumab (Imfinzi), have demonstrated advantageous effects in treating several cancer types. The year 2014 marked the development of the first monoclonal antibody, nivolumab, which works as an ICI and was approved for treating melanoma [32,33]. Atezolizumab was, however, the first PD-L1 inhibitor approved by the FDA. In 2021, the FDA also approved atezolizumab as adjuvant therapy for patients with stage II NSCLC whose tumours exhibited PD-L1 expression on less than one per cent of tumour cells [34]. Despite the success of mAbs, certain limitations, including poor oral bioavailability, poor tissue and tumour penetration, and higher production cost, forced researchers to find potential alternatives like peptide, non-peptide, and small-molecule inhibitors [35]. Another approach is to incorporate antibody-based inhibition of PD-1/PD-L1 and CTLA-4. Response strength and the proportion of patients reporting response were both significantly improved by combination therapy as compared to monotherapies [36].
2.2. Signalling Pathways Targeting Angiogenesis
Angiogenesis is how existing blood vessels give rise to new ones. Tumour growth relies on the development of a blood supply facilitated by angiogenesis [37]. This process is primarily driven by three angiokinase pathways involving vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). Cancer cells often upregulate these angiokinase expressions to ensure synthesis of new blood vessels, integrity, and maintenance, thereby enabling them to evade inhibition of VEGF by increasing the production of PDGF and FGF. To address this challenge, multitargeted tyrosine kinase inhibitors have been created to hinder multiple vital receptors simultaneously. Nintedanib, a potent oral inhibitor, effectively targets VEGFR, PDGFR, and FGFR, earning approval as a second-line treatment for NSCLC and idiopathic lung fibrosis [38]. Developing novel angiogenic agents inhibiting VEGF and PDGF holds promise in enhancing antiangiogenic therapies by expanding the range of targeted receptors and improving pharmacokinetic properties [39].
2.2.1. PDGF
Platelet-derived growth factor (PDGF) and its receptors are the major factors responsible for cancer progression and drug resistance, making them appropriate targets for cancer treatment. PDGF exists in heterodimer and homodimer forms formed by combining polypeptide chains like A, B, C, and D. The PDGF-AA, AB, and BB dimers are secreted as proteolytically processed molecules in secretory vesicles, while PDGF-CC and DD are secreted in an inactive state. PDGF isoforms transmit intracellular signals by binding to receptor tyrosine kinases, namely PDGFα and PDGFβ [40,41]. PDGFRs/PDGFs are present in several cancer cells, and their levels correlate with invasiveness, tumour size, chemoresistance, and poor clinical outcomes. Commonly in lung epithelial cells, PDGFRs are not expressed, but high PDGFα is displayed on lung cancer cell lines and tumour tissue, while PDGFβ is mainly expressed in stromal cells. The presence of these receptors appeared to be linked with poor diagnosis.
The PDGF pathway is crucial for recruiting tumour stromal cells, including cancer-associated fibroblasts, that maintain the tumour microenvironment. Initially found in platelets, PDGF is created in an autocrine manner following the activation of platelets. This proangiogenic factor regulates healthy and pathological blood vessel development. PDGFs are expressed in numerous cell types under normal conditions and exert their functions through autocrine or paracrine mechanisms. The heightened activity of PDGF in both malignant and non-malignant tumours led to the development of various inhibitors targeting PDGF signalling pathways, which are currently undergoing preclinical and clinical assessment. These inhibitors are antibodies, DNA aptamers, or soluble portions of receptors binding with PDGF isoforms and hindering their interaction with signalling receptors. Various low-molecular-weight receptor kinase inhibitors have been developed, such as imatinib, sorafenib, sunitinib, pazopanib, and nilotinib, all demonstrating potent inhibition of PDGF receptor kinases [42]. Crenolanib is an efficient inhibitor against PDGFR, which triggers apoptosis in the NSCLC A549 cell line [43].
2.2.2. VEGF
Vascular endothelial growth factor (VEGF) is a formidable growth factor with proangiogenic properties, influencing endothelial cells by promoting mitosis, preventing apoptosis, increasing vascular permeability, and facilitating cell migration. These effects have a significant role in regulating both normal and pathological angiogenesis processes [44]. VEGF aids in sustaining lung cancer progression and could be an attractive target for targeted therapy [45]. The VEGF family, which includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor (PIGF or PGF), regulates blood vessel growth and lymph angiogenesis. VEGF-A, often called VEGF, primarily drives angiogenesis and disease progression by binding to VEGF receptor 1 (VEGFR1) and VEGF receptor 2 (VEGFR2), with VEGFR2 being the principal receptor. During tumorigenesis, VEGF overexpression is associated with higher vascular density, invasiveness, metastasis, cancer recurrence, and poor prognosis. In response, therapeutic strategies targeting the VEGF-VEGFR signalling pathway have been developed.
Neutralising monoclonal antibodies together with VEGF, exemplified by bevacizumab, were the first antiangiogenic drugs approved for metastatic colorectal cancer treatment in 2004 [46,47]. Regulatory agencies like the US FDA and the European Medicines Agency (EMEA) have authorised their use in numerous cancers, including NSCLC. Endostatin/Endostar is another drug used for NSCLC treatment [48].
3. Nanoparticles as Drug Delivery Vehicles
Nanoparticles comprise an array of materials, including organic and inorganic materials, with a size ranging between 1 and 100 nanometres. By discovering that the size of various materials can influence their physicochemical properties, the importance of nanomaterials came to light [49]. Some common characteristics include a higher surface-to-volume ratio, more excellent electrical conduction, supermagnetic behaviour, spectral shifts in optical absorption, and distinctive fluorescence. In medicine, nanomaterials have applications in drug delivery and controlled release systems. Significant progress has been made in advancing nanoparticles (NPs) for drug delivery to target tumour cells selectively. Nanostructures benefit this purpose because these substances exhibit exceptional attributes, including minimal non-specific toxicity, biodegradability, compatibility with biological systems, and prolonged circulation duration, thus minimising drug adverse effects while enhancing treatment efficacy. It may also help to improve the transport of drugs across the membrane [50] (Figure 3).
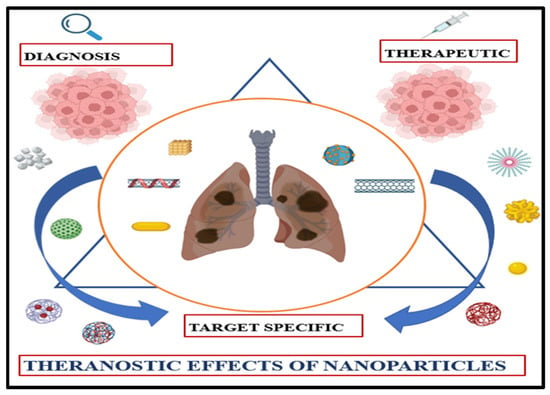
Figure 3.
Theranostic nanoparticles in oncology: A convergent platform for tumour-targeted drug delivery and real-time molecular imaging.
3.1. Barriers to Pulmonary Drug Targeting
Despite the potential of the inhaled nanoparticles system for targeted lung cancer therapy, a number of barriers impede pulmonary drugs from efficiently reaching the targeted lung sites. These challenges include technological, physiological, and biological factors, and each calls for specialised approaches to ensure efficient medication delivery. The mucociliary escalator, a defense system made of cilia and mucus lining the respiratory tract, is one of the primary barriers. Drug accumulations in the deep lung reduce when inhaled particles, including therapeutic medication, become stuck in the mucus and are propelled upward by coordinated ciliary movement toward the throat, where they are swallowed or released [51]. The mononuclear phagocyte system (MPS), in particular alveolar macrophages, which strongly phagocytose foreign particles, including nanoparticles, while patrolling the lung alveoli, is another important barrier. As a result, the drug is cleared up before it reaches the tumour cells. Furthermore, by changing surface tension at the air–liquid interface, pulmonary surfactant—a lipoprotein-rich layer released by alveolar epithelial cells—can destabilise nanoparticle formulations or have an impact on drug absorption [52]. Other barriers include rapid systemic absorption or clearance through the rich pulmonary vasculature, tight junctions of the alveolar epithelium that impede paracellular transport, and enzymatic degradation inside the lung tissue that breaks down specific drug molecules. For maximum therapeutic efficacy, these obstacles must be combined to create sophisticated drug delivery systems that can withstand mucociliary clearance, avoid immune detection, and efficiently enter lung tissues [53].
3.2. Passive and Active Targeting
Drug-conjugated nanoparticles offer two main targeting strategies for tumour tissue: passive and active. The goal of passive targeting is to take advantage of the differences between normal and cancer tissue. Drugs are effectively transported to the target site in passive targeting so they can perform their therapeutic function. Neovascularisation is brought on by high cancer cell proliferation, and the permeability of tumour artery walls relative to normal vessels deteriorates as a result of increased vascular wall openings. Nanoparticles and other macromolecules can enter tumours through permeable blood vessels and build up inside the tumour tissue due to rapid and aberrant angiogenesis. At the same time, tumours that compromise lymphatic outflow lessen the removal of these NPs, which results in their long-term retention. These elements work together to produce the enhanced permeability and retention (EPR) effect, a crucial mechanism underlying passive targeting in cancer treatment. The size of NPs affects the EPR effect because, as many studies have shown, smaller NPs are more penetrable yet do not leak into healthy vessels. However, the immune system is more likely to eliminate bigger particles [54]. Active targeting is the process of delivering nanoparticles (NPs) to cancer cells selectively by use of particular interactions between ligands and cell surface receptors that are overexpressed. To distinguish between malignant and healthy tissues, ligands are designed on the surface of NPs to recognise and bind to molecules that are predominantly or exclusively expressed on cancer cells. This binding promotes the internalisation of NPs and the effective release of therapeutic substances within the targeted cells by inducing receptor-mediated endocytosis upon engagement. When it comes to administering macromolecular medications like protein and small interfering RNAs (siRNAs), this method works particularly well. Monoclonal antibodies, peptides, amino acids, vitamins, and carbohydrates are examples of common targeting ligands that bind preferentially to a particular receptor [55].
3.3. Lymphatic Drug Delivery: An Alternative Route for Improved Pulmonary Targeting
A potential method to enhance pulmonary drug deposition is lymphatic drug delivery, in addition to passive and active targeting techniques. The bronchi, pleura, and alveolar regions are the focal points of the lungs’ vast network of lymphatic capillaries. In conditions like lung cancer, where lymphatic metastasis is frequent, targeting the pulmonary lymphatic system improves local medication retention, lowers systemic circulation, and improves therapeutic results. After inhalation or interstitial delivery, nanoparticles with the right physicochemical characteristics, such as size (usually 10–100 nm), surface charge, and hydrophilicity, can preferentially enter lymphatic capillaries. These nanocarriers can transport drugs to peribranchial regions and draining lymph nodes once they are inside the lymphatic system, enabling longer exposure at the disease site and sustained drug release. Major pulmonary delivery obstacles, such as enzymatic degradation, quick mucociliary clearance, and alveolar macrophage phagocytosis, can also be overcome with this tactic. Thus, by lowering off-target toxicity, the lymphatic-targeted delivery method provides a useful supplement to nanoparticle-based lung cancer therapy [56].
Drugs reaching tumours induce cell rupture, triggering immune responses. Antigens associated with tumour cells are expelled from ruptured tissue and stimulate T-cells via antigen-presenting cells (APCs) for immune responses. Original tumour tissue signals APCs for an immune response. Nanoparticles are effective carriers for safe drug delivery to cancerous cells, facilitating targeted immune therapy. In the past few years, nanoparticles have surfaced as potent agents for lung cancer treatment, with clinical trials and preclinical studies demonstrating their efficacy in this context. These nanoparticle-based drugs directly or indirectly affect lung cancer treatment [57]. Synthesis of nanoparticles (NPs) should be performed under a highly controlled process. The particular shape and size of NPs need to be adequately maintained as their properties rely mainly on their dimensions [58]. There are two significant ways of nanoparticle synthesis: (a) top-down approach, which requires breaking down more extensive materials into nanoscale structures, and (b) bottom-up approach, which involves creating nanomaterials by aggregating or grouping smaller atoms and molecules [59,60,61]. The top-down method utilises physical methods such as mechanical machining, thermal evaporation, pyrolysis, physical vapour deposition (PVD), and lithography. In contrast, the bottom-up approach involves biological and chemical techniques. Chemical methods include sol–gel, chemical co-precipitation, chemical vapour deposition (CVD), microemulsions, microwave-assisted, hydrothermal processes, and sono-chemical synthesis [62].
3.4. Types of Nanoparticles Used for Lung Cancer Therapy
Broadly, nanomaterials are classified into two types: (1) organic nanomaterials and (2) inorganic nanomaterials. (Figure 4)
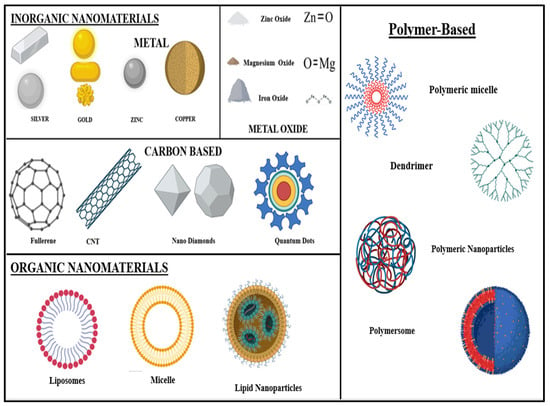
Figure 4.
Nanomaterials are broadly classified into inorganic and organic categories based on their chemical composition: inorganic nanomaterials typically include metals, metal oxides, quantum dots, and ceramic-based structures, known for their unique optical, electrical, and magnetic properties. Organic NPs, on the other hand, comprise carbon-based structure such as liposomes, dendrimers, polymeric NPs, and micelles, offering enhanced biocompatibility and versatility for drug delivery and biomedical applications.
- (A)
- Inorganic Nanomaterials: Various metals like gold (Au), silver (Ag), aluminium (Al), zinc (Zn), copper (Cu), iron (Fe), cadmium (Cd), and lead (Pb), as well as metal oxides like zinc oxide (ZnO), iron oxide (Fe2O3 or Fe3O4), copper oxide (CuO), titanium dioxide (TiO2), magnesium aluminium oxide (MgAl2O4), cerium oxide (CeO2), silica (SiO2), etc., are used to synthesise nanoparticles [63].
- (B)
- Organic Nanomaterials: Nanomaterials are made of organic matter—liposomes, dendrimers, micelles, cyclodextrin, nanogels, and compact polymers. Some organic nanoparticles, like liposomes and micelles, contain a hollow sphere and are biodegradable and non-toxic. This is beneficial for drug delivery purposes [64].
Also, nanoparticles can be subdivided into four groups based on their external form and structure: zero-dimensional NPs are small, spherically shaped particles having nano-sized radii, 1D NPs are nanowires and nanorods, 2D NPs are nanosheets or nanoplates, and 3D nanomaterials include nanocubes and nanocages [65,66]. Several materials can be used for NP production for lung cancer treatment. More precisely, noble metal NPs, including silver, gold, zinc, etc., have shown promise in biomedicine due to their exceptional potential in treating cancer. Their small size allows them to efficiently engage with biomolecules inside and outside cells. NPs can also shield biomolecules like DNA and RNA from enzymatic breakdown [67].
3.5. Gold-Based Lung Cancer Therapy
Gold nanoparticles (AuNPs) stand out from other noble metal nanoparticles for their exceptional optical characteristics, superior biocompatibility, and minimal toxicity. Moreover, AuNPs are small, ranging from 3 to 200 nm in diameter [68], and can enter every cancer cell with relative consistency. AuNPs produced by the bottom-up method have more consistent sizes and shapes and are considered more appropriate for biomedical applications. AuNPs are known to be some of the most stable and thus have immense possibilities for use in cancer therapy as contrast agents, drug transporters, photothermal agents, radiosensitisers, and in other applications [69]. Production of metal or metal oxide NPs is relatively simple through various physical, chemical, and biological methods. AuNPs can be formed in different shapes according to their applications, such as gold nanorods, nanospheres, nanocages, nanoshells, and nanostars [70]. The management and detection of several cancer types, including lung, breast, cervical, liver, rectal, and more, are greatly aided by gold nanoparticles [71]. They have been extensively studied to be used as tumour radiosensitisers for their characteristics like high Z number giving strong photoelectric absorption coefficients; superior biocompatibility and lower biological toxicity compared to conventional agents; high surface-area-to-volume ratio that facilitates attachment of therapeutic agents for targeted tumour treatment; EPR effect, especially accumulating at tumour sites while sparing normal tissues; effective imaging contrast for disease diagnosis and well-controlled size distribution along with distinct electrical, chemical, and optical properties within a size range of 1–150 nm [72].
Giulio F. Paciotti et al. were the first to describe the application of colloidal AuNPs for targeted drug delivery at the cancer site [73]. Jacob D. Gibson et al. detailed the production of drug-loaded NPs in their article, marking the initial description of attaching paclitaxel, a chemotherapeutic drug, onto a 2 nm AuNP surface through covalent bonding [74]. In Romy Mueller et al.’s experiment, A549 cell lines (human lung cancer cell lines) were exposed to radiation with AuNPs and it was observed that the carcinoembryonic antigen was expressed on the cell surface, escalating with higher radiation doses and longer exposure times. This study demonstrates that gold nanoparticles effectively enhance the expression of CEA [75]. The survey conducted by Crus and Abrahamse aimed to improve photodynamic therapy for lung cancer using AuNPs, along with targeted antibody selection of cancer stem cells using a nanobioconjugate. Photodynamic therapy effects were notably enhanced with the nanobioconjugate, leading to substantial lung cancer stem cell destruction and potential eradication [76]. Peng and Wang et al. developed novel gold nanorods coated with RGD peptide and polyethyleneimine to target cancer-specific nanoparticles and deliver miRNA [77].
3.6. Silver-Based Lung Cancer Therapy
Silver nanoparticles (AgNPs) are widely recognised for their diverse pharmacological properties, including antibacterial, antifungal, anticancer, and antioxidant activities, and, thus, AgNPs with controlled morphology find extensive use in various bio-related applications. According to their application, AgNPs can be made in multiple sizes and shapes with specific properties. The surface properties, size distribution, particle morphology and composition, dissolution rate, and the type of capping and reducing agents used are essential to consider while synthesising AgNPs. Their distinct physicochemical characteristics, like high conductive, optical, and biological properties, provide significant promise in cancer therapy. They can trigger apoptosis in tumour cells [78], restrain tumour growth, and amplify the effectiveness of traditional cancer therapy methods like chemotherapy and radiotherapy. Despite their therapeutic potential, AgNPs present some challenges, including the toxicity associated with them, and the primary cause is the production of ROS. This may cause issues and extend to normal or non-cancerous cells when used in treatment [79]. Therefore, several passive and active targeting methods are employed for silver-based nanosystems to ensure that nanoparticles accumulate primarily in cancerous tissues while sparing non-target organs [80].
Recently, the green synthesis method of NPs has been a topic of study. The formation of nanoparticles with the help of plant extracts, natural drugs, biomolecules, and even microorganisms is beneficial for producing non-toxic and ecologically friendly NPs with a definite shape and size. The careful selection of capping agents ensures the production of highly pure and stable nanoparticles. Organic compounds found in plants, such as flavonoids, phenols, alkaloids, and co-enzymes, serve as both reducing and stabilising agents, particularly in the case of AgNPs. Alkaloids, a class of organic compounds containing nitrogen, are found in various organisms, including bacteria, fungi, plants, and animals. Camptothecin (CMT), a specific alkaloid isolated from Camptotheca acuminata, demonstrates anticancer and anti-HIV activities, particularly against lung, ovarian, breast, and colon cancers [81]. A challenge faced during AgNP synthesis is the aggregation of AgNPs due to their small size, which alters their properties. Various methods have been established to address this, like the synthesis of highly dispersed AgNPs utilising solution-based methods. These days, the focus has been on introducing AgNPs on solid surfaces like metal oxides or polymers using various nanostructures like spheres and mesoporous silica to generate composite catalysts [82].
3.7. Zinc-Based Lung Cancer Therapy
Zinc oxide nanoparticles (ZnONPs) are extensively utilised across various domains, including medicinal applications, drug delivery, anticancer effects, anti-inflammatory effects, antibacterial effects, wound healing, diabetic therapy, and bioimaging, because of their remarkable physical and chemical properties. ZnONPs with a hydrodynamic diameter of <100 nm are acknowledged as the most effectively administered in vivo. Generally, bulk ZnONPs are considered safe and have received approval from the US Food and Drug Administration (FDA), thus recognising their potential in drug delivery solutions. ZnO nanoparticles have been observed to impact several cancer cells in vitro, as Zn2+ stimulates ROS generation. UV light can also encourage ZnO electrons to move from the valence band to the conduction band to create photocatalytic ROS.
A study by Tanino et al. stated that ZnONPs administered intravenously accumulate in lung tissues and cause ROS-related death in healthy mice. Also, a report suggests that ZnO shields macrophages from the adverse effects of anticancer drugs. These observations indicate that ZnO particles might target SCLC cells through a pathway different from the present chemotherapies [83]. A study by Prakriti et al. revealed the novel conjugation of biogenically synthesised ZnONPs using Aspergillus niger (fungus) with DOX. It evaluated its medicinal effectiveness against A549 lung cancer cells for the first time [84]. Mishra et al. demonstrated the efficiency of methotrexate-conjugated-ZnONPs against lung cancer. It was observed that ZnONP-conjugated MTX showed higher drug-loading efficiency and enhanced cytotoxic effects than MTX alone [85]. Zhou et al. developed tumour-microenvironment-responsive nanoparticles (MONPs) that induce cancer cell death and activate the STING pathway, enhancing immune responses. These nanoparticles also reduce immunosuppression and, when combined with anti-PD-1 therapy, successfully hinder the growth and spread of tumours [86].
3.8. Diamond-Based Lung Cancer Therapy
NDs, due to their biocompatibility, are used as non-toxic drug carriers for targeted drug delivery. They are great candidates for binding active pharmaceutical components due to their high surface-area-to-volume ratio and functional surface groups [87]. Huang et al. explored the leading and releasing efficiency of the chemotherapy drug doxorubicin hydrochloride (DOX) using NDs. They formed loose ND-DOX clusters and observed that DOX was both adsorbed on the ND surface. Cytotoxicity studies showed that, with sustained release, DOX-NDs had lower toxicity than free DOX [88].
Along with minimising the drug side effects, NDs also guard them from being expelled by resistance pumps. However, one disadvantage of NDs as colloids is their size, since they tend to aggregate. One singular ND ranges from 2–5 nm, but their aggregates are usually ~70–80 nm in size. So, developing dry forms of NDs, like dry powder inhalers (DPIs), can help overcome this challenge. Nebulised chemotherapy presents some issues that can be addressed using DPIs, including limited solubility, aerosol air pollution, and the requirement for extra measures to be taken before delivery. To attain deep lung deposition, DPI powder formulations must have an aerodynamic diameter from 1–3 µm. Because of their small size, nanoparticles are mostly expelled after inhalation, even if they have a large surface area, can evade alveolar macrophages, and can transport or release medications in the deep lungs. This problem was solved by encasing nanodiamonds in microspheres that quickly release the trapped nanoparticles when they go deep within [89]. Local photothermal therapy is another promising approach for treating malignant neoplasms, which involves selectively destroying tumour cells through heating. This is achieved by introducing nanoparticles with a high light absorption coefficient into target tissues, followed by local heating using focused laser radiation. Diamond nanoparticles, mainly those heavily doped with boron and exhibiting increased absorption within the near-infrared range, show great potential for photothermal therapy. Their advantages include exceptional thermal conductivity, biocompatibility, and easy bioconjugation due to remarkable surface functionalisation. These diamond nanoparticle characteristics make them appealing for biomedical applications, such as cell labelling and photothermal therapy. Laser-synthesised NDs coated with polydopamine demonstrate robust photothermal characteristics and fluorescent imaging capabilities [90].
3.9. Two-Dimensional-Based Lung Cancer Therapy
Two-dimensional (2D) nanomaterials are a newly emerging class of nanomaterials. The hallmark property of these nanomaterials is their sheet-like structure with substantially lateral dimensions up to a few microns, but their thickness is minimal (<5 nm). These advanced materials have garnered considerable attention, particularly in biomedical applications, including their potential in lung cancer treatment [91]. There are abundant resources for 2D nanomaterials, like graphitic carbon nitride, transition metal carbides, transition metal dichalcogenides, hexagonal boron nitride, and group-VA semiconductors [92].
Graphene, a prototypical 2D material characterised by its sheet-like porous assembly, has gained attention in research domains because of its remarkable traits, such as large surface area, ultra-high electron mobility, and exceptional thermal conductivity. This has facilitated its use in various applications, such as hybrid materials, optical devices, biological and chemical sensors, lithium-ion batteries, and supercapacitors. The family of 2DMs has been significantly expanded over the past decade. Over a hundred graphene-like nanomaterials have been made, including graphitic carbon nitride, transition metals, hexagonal boron nitride, black phosphorus, and specific organic polymers. When compared to a single graphene element, graphene-like 2D materials are made up of several components that are more useful, versatile, and flexible [93].
The unique properties of 2D-NPs of hexagonal boron nitride, also known as white graphite, render them highly suitable for delivering chemotherapeutic agents in cancer therapy. Still, less research has been carried out on them than on 2D graphene oxide, which is more popular. The various physical and chemical characteristics of hexagonal boron nitride nanosheets as potential anticancer drug carriers are discussed by Sharker et al. in their review [94]. An article by Bhatt et al. highlights the possible use of 2D nanomaterials as photothermal agents to enhance their effect as phytomedicine for cancer treatment [95]. A novel sensor utilising 2D nanosheets, specifically Ti3C2 MXene, has been developed and applied to detect the response of 8-hydroxyoctanoic acid (8-HOA) and prostaglandin E2 (PGE2) in lung cancer cells. Initial findings suggest a significant outcome: this innovative Ti3C2-based sensor offers a straightforward approach for guiding anticancer treatments. Moreover, the heightened sensitivity of this sensor suggests a potential avenue for cancer detection at early stages by observing fluctuations in PGE2 and 8-HOA levels within cells. In contrast to the heavy, costly, and time-consuming GC–MS method typically used to aid in anticancer treatments, this sensor-based method presents a rapid, uncomplicated, cost-effective, highly efficient, and minimally invasive tool for cancer detection and treatment [96].
3.10. Three-Dimensional-Based Lung Cancer Therapy
Self-assembly is a phenomenon where individual nanoparticles spontaneously assemble through weak forces like electrostatic interactions, hydrogen bonds, non-covalent interactions, etc. to form a complex nanostructure without external assistance [97]. This results in the production of mechanically stable 2D and 3D materials with efficient encapsulation and controlled release of drugs. Several approaches can be employed for their synthesis, including fluid interfaces. A review by Sujit and Alexander briefly explains how the liquid–liquid interface is used to produce colloidal 2D and 3D superlattices [98].
Three-dimensional scaffolds have become popular in cancer immunotherapy because of their distinctive features, including sizeable surface-to-volume ratio, high porosity, and adjustable mechanical traits, which help their interaction with cytotoxic T-lymphocytes. Several FDA-approved biocompatible polymers are being used to create 3D scaffolds for cancer therapy. The choice of scaffolds and chemotherapeutic agents is based on the specific type of cancer under treatment. Some examples include intelligent scaffolds, expandable scaffolds, microneedle patches, and microspheres [99]. Due to their remarkable structural and functional properties, self-assembled peptide hydrogels are popularly used as nanocarriers for delivering antitumour drugs. The first efficient drug delivery tool using self-assembled peptide hydrogel was developed by Mao et al. [100]. Graphite oxide, carbon nanotubes, and nanodiamonds are also used for cancer therapy.
4. Herbal Nanomedicine in Lung Cancer Therapy
In order to combat cancer, it is essential to find new medications that target neoplasms. Numerous findings suggest that herbal medicine may have anticancer benefits through a variety of mechanisms, such as apoptosis and autophagy, cell proliferation suppression, and modulation of cancer-related signalling pathways. Several cancers can be treated with herbal medicine. Thevetin peruviana (pers.) K. Schum. (TPKS) contains a monomer called thevebioside (THB), which may have pharmacological effects. THB has demonstrated anti-inflammatory properties for skin diseases [101]. In P53 wild-type A549 and H460 cell lines, researchers found that THB exhibits the most cytotoxic activity in a dose- and time-dependent manner. THB has been demonstrated to prevent NSCLC from proliferating by inducing apoptosis, with a decrease in BCL-XL expression and an increase in Bad, cleaved caspase-3, and cleaved PARP expression [102]. A variety of plants, such as Satureja thymbra L. and Ocimum gratissimum L., contain thymol. Thymol is a monoterpene phenol that occurs naturally. The antiproliferative effect of thymol on A549 cells was shown by Balan et al. to be dosage- and time-dependent, with IC50 values of 745 µm after 24 h. Through cell cycle arrest, thymol caused cell death. The number of cells grew significantly during the G0/G1 phase. Additionally, it was found that apoptotic DNA fragmentation, Bcl-2 downregulation, and Bax overexpression increased cell death. Thymol alters the mitochondrial membrane potential and raises reactive oxygen species (ROS) levels [103]. Direct administration of herbal therapeutic agents, however, comes with multiple drawbacks, including poor permeability, poor solubility, and poor bioavailability for some herbal components; additionally, it has fast metabolism and an off-target effect, meaning that the therapeutic agents may bind to molecules in the human body other than the target site to which the therapy was intended to bind. Unexpected negative repercussions could arise from this. Additionally, biologically active, water-soluble herbal components like tannins, flavonoids, and so on also showed low absorption across cell lipid membranes when combined with terpenoids in chemotherapy, hormone therapy, and gene therapy. This resulted in decreased efficacy and bioavailability, which would promote a synergistic effect for the eradication of cancer. The optimum nanoparticle design was examined in order to produce this representation [104]. A biodegradable carrier containing a strong herbal medication and a sensitiser that works in many ways to increase the susceptibility of cancer to death by the targeted therapy has become popular in cancer treatment. In this case, the effective dose lessens toxicity to healthy cells, achieves effective targeting, improves component solubility, improves drug action, achieves sustained controlled drug release, and, using both passive and active targeting, improves therapeutic accumulation in tumour tissue [105] (Table 1 and Table 2).
The herbal drug ellagic acid (EA) was found to have antifibrotic and antioxidant properties in a study. Additionally, the chemotherapeutic drug DOX was used in conjunction with EA’s promising antitumour effect to induce cell apoptosis by activating various molecular signals from AMP-activated protein kinase to affect the Bcl-2/Bax apoptosis pathway, where EA can further inhibit NF-κB activation in addition to its direct action on mitochondria [106].
In order to facilitate deep lung deposition and advance drug delivery through the lung by combining the advantages of both nanoparticles and microparticles, Elwakil et al. developed a spray-dried inhalable nanocarrier incorporating DOX and EA in combination based on a lactoferrin chondroitin sulfate (LF-CS) electrostatic nanocomplex. When evaluated in mice with lung cancer, the LF chondroitin nanocomplex demonstrated improved entrapment into A549 lung carcinoma cells and subsequent superior cytotoxicity via Tf and CD44 receptors overexpressed by lung cancer cells [107]. A different form of drug administration, known as nano-in-nano, was created to administer etoposide (ETP) and berberine (BER), a natural medication, via albumin nanoparticles. In order to limit ETP’s toxicity and solve the issue of resistance development, co-encapsulation of BER was proposed as a way to lower the therapeutic dose of ETP and improve its antitumour activity. Before the poorly soluble chemotherapeutic drug ETP was encapsulated into hydrophilic albumin nanoparticles, it was first preformulated into a phospholipid complex (SPC) or water-soluble nanocrystals. On the contrary, different albumin NP crosslinkers (GA, genipin, and Zn2+) were used for sustaining the release of BER as a hydrophilic herbal medication over a number of days. The mannose/phenylboronic acid (PBA) dual-targeted BER and ETP NPs demonstrated a possible decrease in vascular endothelial growth factor (VEGF) expression level when administered to mice with lung cancer. They also triggered caspase activation with tumour cell apoptosis and an enhanced antiangiogenic effect, which may be related to synergistic topoisomerase 2 inhibition and a decrease in the multidrug resistance (MDR0 effect in A549 cells) [108]. In a follow-up study, PBA-targeted albumin NPs were added to a spray-dried inhalable nanocomposite (NC) to improve deep lung deposition of ETP and BER. In terms of reducing tumour angiogenesis, the inhalation approach outperformed the intravenous route [109]. The herbal-multidrug-loaded nanosystem offers a viable platform for the delivery of anticancer drugs. The ability to synthesise nanoparticle systems with flexibility has allowed for unexpected success in providing a vast array of anticancer treatments.

Table 1.
Various herbal-drug-loaded Nanostructures used for lung cancer treatment.
Table 1.
Various herbal-drug-loaded Nanostructures used for lung cancer treatment.
| S. No. | Nanostructure | Herbs | Bioactive Compound | Biomarkers | Ref. |
|---|---|---|---|---|---|
| 1. | Gold and silver NPs | Platycodi Radix (Platycodon grandiflorum) | Platycodin D (PLD) | PD-L1 | [110] |
| 2. | Silver NPs | Morus alba (white mulberry) | Prenylated flavonoids like morusin | STAT3, NF-κB, caspases, EGFR, PDGFR, ROS, etc. | [111,112,113,114] |
| 3. | Gold nanoclusters | Most fruits and vegetables | Kaempferol (flavonoid) | TNF-α, IL, NF-κB, Akt, VEGF, AP-1, PIK3R1, AKT1, EGFR, and IGF1R | [115,116] |
| 4. | Lipid NPs | Found in many essential oils of plants | Geraniol (GOH) | Inhibit HMGCR | [117,118] |
| 5. | Biocompatible lipid NPs | Lippia alba and Clinopodium nepeta essential oils | Monoterpenes (limonene in L. alba and pulegone in C. nepeta) | Increase ROS generation | [119] |
| 6. | Solid lipid NPs | Myrica rubra tree bark | Myricetin (MYC) | RIPK3 and MLKL upregulation | [120] |
| 7. | Core–shell lipid–polymer hybrid NPs (LPNs) | Skin of grapes, raspberries, mulberries | Resveratrol (RSV) | VEGF, caspase-3 | [121,122] |
| 8. | Liquid crystalline NPs (LCNs) | Rhizoma coptidis | Berberine | VEGF, TF AP-1, NF-κB | [123] |
| 9. | Poly (lactic-co-glycolic acid)-based AE NPs (nanoAE) | Aloe vera and Rheum officinale | Aloe-emodin (AE) | Caspase-3, poly (ADP-ribose) polymerase (PARP), caspase-8, and caspase-9 | [124] |
| 10. | Poly (lactic acid)–poly (ethylene glycol) (PLA-PEG) NPs | Green vegetables like broccoli, cabbage, celery, spinach | Luteolin (flavonoid) | PD-L1 | [125,126] |
| 11. | Oil nanoemulsions | Piper nigrum (black pepper) | Piperine | Wnt, NF-κB, cAMP response element-BP, TF-2, PPAR- γ, human G-quadruplex DNA, | [127,128] |
| 12. | Nanoemulsions | Green tea | Epigallocatechin-3-gallate (EGCG) | ROS, NF-κB, Akt, VEGF, PPAR, Bcl-2, MAP kinases | [129] |

Table 2.
Combinations of herbal therapy nanomedicines with various antitumour treatments for lung cancer.
Table 2.
Combinations of herbal therapy nanomedicines with various antitumour treatments for lung cancer.
| Nanocarrier/Delivery System | Herbal Compound | Cancer Model/Type | Key Outcomes | Ref. |
|---|---|---|---|---|
| Baicalein/Paclitaxel | A549/PTX lung cancer | Highest tumour regression with dual targeting ligands | [130] |
| Berberine/Etoposide | A549/Lung-cancer-bearing mice | Improved cellular internalisation Elevated caspase-3 and downregulated VEGF | [131] |
| Resveratrol/Pemetrexed | A549/Lung-cancer-bearing mice | Inhibition of angiogenesis Induction of apoptosis | [132] |
| Doxorubicin/Ellagic acid | A549/lung-cancer-bearing mice | Improved cellular internalisation Improved the anticancer efficacy of DOX | |
| Curcumin/Berberine | A549 cell line | Inhibited MDR1 Activity Improved cellular internalisation | [133] |
| Berberine/Rapamycin | A549/Lung-cancer-bearing mice | Improved internalisation |
5. Innovative Lung Cancer Theranostics
Lung cancer treatment methods have evolved over the years. The earliest preferred treatment for lung cancer was lobectomy, supported by findings from the pivotal Lung Cancer Study Group trial in 1995. The study demonstrated an increase in death and reappearance rates linked to sub-lobar resection as opposed to lobectomy. Many patients now have small lung nodules found on computer tomography (CT) scans, which might be very early-stage lung cancers and could be treated with less invasive methods instead of surgery. With more people getting CT scans, lung cancer screening has become common, showing a decrease in lung cancer deaths. Some patients develop new suspicious nodules in their lungs after previous cancer treatment, which may need different treatments. In Asia, some patients have a genetic predisposition to multiple lung cancers. This highlights the need for less invasive therapies. Since more older adults are diagnosed with lung cancer, many with other health problems, patients are ineligible for surgery due to high clinical frailty scores, inadequate lung function, and multiple co-morbidities (Figure 5).
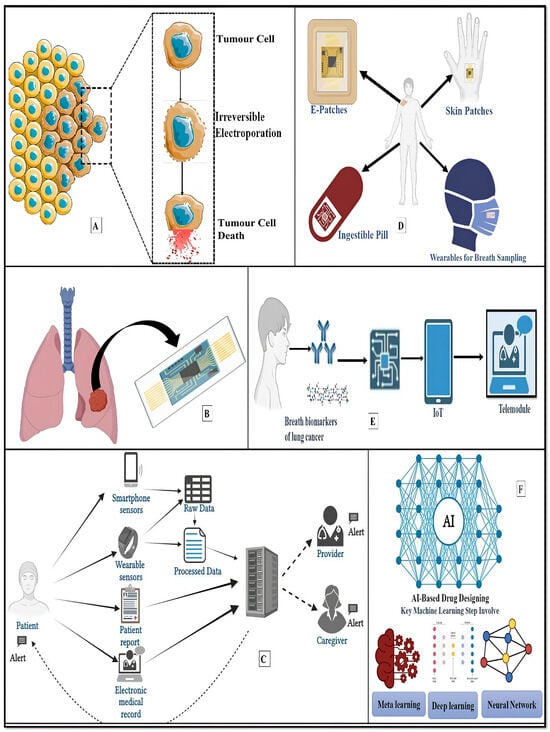
Figure 5.
Recapitulation of futuristic lung cancer therapy. (A) Electrical therapy for lung cancer employs short yet powerful electrical pulses to irreversibly disrupt cell membranes, leading to tumour cell death. (B) The development of microfluidic devices for replicating the lung microenvironment and physiology. (C) A mobile sensing framework used to monitor patients with lung cancer. Patients employ wearables and smartphone sensors to offer passive sensor data that can be used to create clinical risk prediction models in conjunction with patient-reported outcomes or data from electronic health records. These models can set off individualised intervention alerts for patients, caregivers, or providers. (D,E) Wearable Lung e-Patch/breathomic biosensors/Nose-on-a-chip for Continuous Monitoring of Lung Health. (F) AI-based drug design: artificial intelligence makes the drug designing process faster and more efficient. The vast amount of biological data from various sources presents opportunities and challenges. AI and machine learning techniques are crucial for efficient mining and extracting insights from this data.
5.1. Electrical LCT
Electricity-assisted cancer therapy is a recent development that aims to improve cancer treatment results by applying electrical stimulation. This technique targets cancer cells directly or supports other therapeutic approaches by utilising various electrical energy sources, such as low-frequency electric fields, electroporation, and electrical impulses (Figure 5A). The use of electrical stimulations in disease treatment was developed because electrical fields exist within the body and are necessary for development. Various exogenous physical stimuli have been developed for cancer therapy, such as radiation therapy, heat, light, magnetic field, electric field, etc. Later, the electroporation method was developed when Neumann and Rosenheck discovered that electrical signals can increase the permeability of cell membranes and even restore them after removing the electric field [134]. Reversible electroporation, which involves using electric pulses to temporarily increase cell membrane permeability and enhance cellular uptake of systemic drugs, has shown promise in improving the transport of medicinal medicines. Electrochemotherapy is based on reversible electroporation. Conversely, irreversible electroporation allows for higher intracellular concentrations of cytotoxic agents inducing apoptosis. Electroporation is presently being studied for treating melanoma and its cutaneous metastases, head and neck tumours, pancreatic cancer, and hepatobiliary tumours [135]. The study by Kodoma and his colleagues revealed that catheter-based endobronchial electroporation provides a dependable method for treating peribronchial tumours in conjunction with cisplatin [136]. Electric field lines exert force on charged molecules, particularly interacting with polar molecules such as tubulin, causing mitotic spindles to collapse and possibly halting cell proliferation. Tumour-treating fields (TTFields) is a new field that demonstrates antimitotic effects across various tumour types. Combining TTFields with paclitaxel enhances apoptosis-mediated inhibition of tumour progression [137]. Using a voltage pulse generator is considered the most effective way of electroporation [138]. A pilot study on the effectiveness of electrochemical treatment (ECT) as an NSCLC treatment method was conducted, and the results showed that ECT is simple, effective, safe, and least traumatic [139]. Electrocautery is a technique used to treat NSCLC where heat from an electric current destroys small tumours or early-stage cancer cells that have not yet metastasised.
5.2. Lung-on-a-Chip
Evaluation of lung cancer treatment methods and testing anticancer drug efficiency can be performed through in vitro and in vivo models. Two-dimensional cell cultures are commonly employed as models for investigating tissue pathophysiology and in vitro drug reactions. Still, the biggest downside of 2D cultures is the absence of a human-like microenvironment. Animal models have also been employed for preclinical drug synthesis and screening methods. Still, due to the differences in the physiological structure of animal models and humans, they also cannot predict exact drug interaction. This led to the development of 3D culture systems to overcome such issues since 3D systems replicate human organs using organoids and organ-on-a-chip structures. One disadvantage of this is the difficulty in constructing a lung or lung tumour 3D model that exactly reproduces the complex working of our system [140]. An organ-on-a-chip is a biomimetic device that mimics the physiological environment of an organ along with its microscale fluid or gas channels to replicate tissue and blood circulation systems. It enables the creation of tissue–tissue and organ–organ junction, providing a complicated structure, delicate microenvironment, and biophysical factors similar to real human organs. This model offers greater accuracy and realism than two-dimensional models and addresses limitations associated with animal experiments, including species variations, prolonged cycle times, high expenses, and ethical concerns related to animal testing [141] (Figure 5B).
A lung-on-a-chip is a microfluidic cell culture device engineered to closely replicate the three-dimensional structure, mechanical forces, cellular architecture, and microenvironment of the human lung. This advanced in vitro model enables the simulation of essential physiological processes, such as breathing movement and air–blood barrier functions. Lung-on-a-chip technology offers substantial advantages for lung physiological studies, disease modeling, toxicological studies, and drug screening. This technology represents a breakthrough in organ-on-a-chip systems, helping bridge the gap between traditional in vitro studies and clinical outcomes [142]. Hassell et al. developed a model lung cancer chip for human NSCLC to analyse cancer behaviour, propagation, and invasion in various microenvironments. They also studied the tumour’s response to tyrosine kinase inhibitors. The research revealed that, in human alveolus chips, NSCLC cancer cells multiplied more quickly than in airway chips, consistent with in vivo results observed in patients with lung adenocarcinoma [143]. Yang et al. built a lung-on-a-chip prototype using poly (lactic-co-glycolic acid) and co-cultured A549 cells with HFL1 (human fetal lung fibroblasts). They utilised this model to assess gefitinib, an EGFR-targeted antitumour drug. Their investigation revealed that insulin-like growth factor (IGF-1), secreted by HFL1 cells, hindered the EGFR-related pathway targeted by the drug, activating the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signal pathway to sustain tumour cells, potentially explaining reduced tumour cell sensitivity to the drug. Additionally, when A549, HFL1, and HUVECs were co-cultured, A549 cells induced tumour cell invasion by triggering endothelial cell death [144].
A lung alveolus chip coated with A549 cells (human lung alveolar epithelial cells) was employed to evaluate cytotoxic reactions to ZnONPs. These nanoparticles are utilised in drug delivery, biopharmaceutical production, and biomedical imaging. The investigation indicated that, under dynamic flow conditions in the lung chip, the toxicity of these nanoparticles was reduced compared to static conditions [145]. The lung-on-a-chip approach shows promise in increasing the understanding of complex lung diseases and accelerating drug development. As lung diseases and medication burdens increase, lung-on-a-chip platforms could revolutionise therapeutic identification, reducing reliance on costly and sometimes unreliable traditional models. By improving fabrication methods, lung-on-a-chip models can enhance studies of pulmonary diseases, personalised medicine, and more. Combining separate organ-on-a-chip models into a “body-on-a-chip” with standardised procedures and standard media could revolutionise drug development and clinical trials.
5.3. Smartphone-Enabled LCT/Telemedicine/mHealth Applications
Smartphones have ingrained themselves into our daily lives with numerous applications outside of communication. The use of smartphones in healthcare has created new opportunities for patient education, monitoring, diagnosis, and potentially therapeutic interventions, especially in lung cancer treatment (Figure 5C). The expected global cancer cases are predicted to reach up to 28.4 million by 2040, marking a 47% increase compared to the numbers recorded in 2020 [146]. Low- to middle-income countries are highly burdened by cancer, calling for urgent development of innovative and cost-effective approaches for early detection [147].
The COVID-19 pandemic has accelerated the adoption of telemedicine, including in the oncology domain. Due to the extraordinary hospital patient overload caused by the pandemic, national health systems have found it difficult to handle the increasing number of COVID-19 cases. This has had a severe negative impact on the treatment of various pathologic conditions, most notably the management of cancer patients. Elective surgical procedures and primary health services have been postponed [148]. A recent report by Dhawale et al. highlighted the potential of telemedicine-based serious illness conversations (SICs) to improve end-of-life (EOL) cancer care. Future research should focus on reducing barriers, improving patient access, and better understanding the impact of conversations on cancer care delivery [149]. Margarinos et al. addressed COVID-19 by implementing a single-encounter telemedicine (SET) lung cancer screening paradigm, demonstrating successful identification and treatment on paradigm with the single-visit, in-person (SIP) lung cancer screening methods used before COVID-19 [150].
The increasing use of mobile technology has set the stage for mobile health (mHealth) technologies that have the potential to revolutionise both clinical research and healthcare worldwide. These technologies include wearable sensors, diagnostic tools, and imaging of medical quality, all integrated with real-time data streams and bolstered by automated clinical decision-support systems. With the ability to transcend geographical limitations, it can facilitate improved delivery and deepen our understanding of physiological variability [151]. Allowing cancer patients to manage their symptoms makes them more informed and self-reliant, increasing their quality of life and reducing the strain on healthcare services [152].
In recent years, various mHealth (mobile device support for medical and public health practices) platforms like mobile phones, personal digital assistants, patient monitoring devices, and other wireless devices have been used to target lung cancer patients. Ciani et al. developed a mobile application, LuCApp or lung cancer app, to manage patient symptoms in real time and it was trialled in northern Italy. This app was released on iTunes and Play Store in April 2018 [153]. The study by Ji et al. intended to examine the result of using a personalised pulmonary rehabilitation program that used real-time data on the health of patients diagnosed with NSCLC and found that it significantly improved their exercise capacity, dyspnea symptoms, and quality of life [154]. Henshall et al. also developed iEXHALE, an app specifically aimed at increasing physical activity among lung cancer survivors to improve symptoms [155]. A report by Ni et al. showed that lung cancer patients, after their discharge, lacked self-management skills and a support platform. Thus, there is a need to develop a tool to meet their diverse support care needs. A public account on WeChat (a Chinese social-media platform) called “symptoms self-management” was created, which initially gave positive feedback [156].
5.4. Robotics/Drone-Based Lung Cancer Treatment
5.4.1. Robotics in Lung Cancer Diagnosis and Treatment
Robotic-assisted video thoracoscopic surgery (R-VATS) is growing in popularity as the least invasive technique for early detection and treatment of lung cancer. It offers improved visualisation and more precise manipulation of the surgical site than video-assisted thoracic surgery (VATS). Robotic methods have been employed in various mediastinal and lung resections. The long-term survival rates of lung cancer patients undergoing robotic surgery are similar to those of patients treated with VATS or open surgery. However, the drawbacks of such surgery are higher initial and ongoing costs compared to VATS and limited availability of instruments [157,158]. Robotic-assisted bronchoscopy (RAB) was developed to enhance lung nodule biopsy accuracy and yield. Compared to conventional and other guided bronchoscopies like virtual bronchoscopy (VB), radial endobronchial ultrasound (r-EBUS), and electromagnetic navigation bronchoscopy (ENB), RAB enables navigation up to the ninth airway generation and direct airway visualisation [159]. Chen and colleagues conducted a study to compare the reach of a robotic endoscopic system with conventional thin bronchoscopes in human cadaveric lungs. The study concluded that the robotic endoscopic system could reach farther into the periphery of human cadaveric lungs than conventional thin bronchoscopes with the same outer diameter. This enhanced reach into the lung periphery may help overcome some limitations associated with current bronchoscopic techniques for biopsying peripheral lesions [160].
5.4.2. Nanorobots in Targeted Drug Delivery
Nanorobotics, the study of nanoscale robots, offers a promising future in cancer treatment. Many anticancerous drugs have a narrow therapeutic window, i.e., the dosage range between minimum effective therapeutic concentration and minimum toxic concentration, which could have various side effects. Using nanotechnology as a drug delivery system (DDS) can reduce the side effects of traditional treatments as DDSs can deliver drugs to precise locations while maintaining dosage and release frequency. Also, using nanorobots can reduce the ill effects of conventional chemotherapy [161]. Nanorobots can be organic or inorganic. Organic nanorobots or bionanorobots are the least harmful since they are produced by fusing DNA cells from bacteria and viruses. Inorganic nanorobots include synthetic proteins, diamond structures, or other materials that are more toxic to the body [162]. Performing bronchoscopy for peripheral pulmonary lesions remains difficult for clinicians, primarily due to the inability to manoeuvre traditional bronchoscopes close enough to these lesions before taking a biopsy.
5.4.3. Drone Technology in Healthcare Delivery
Drones have been referred to as unmanned aerial vehicles, or UAVs, and unmanned aircraft systems, or UASs. Distribution is the first area that would gain the most from drone integration in the healthcare sector. Nations that have reaped some of the benefits from this unmanned aircraft application are starting to conduct in-depth studies in this area [163]. Abeygunawaradana et al. developed an e-medic platform for employing an autonomous drone to distribute medication. The platform comprises an autonomous drone that delivers medication to patients and a healthcare portal that links patients and clinicians. This platform primarily offers a number of features for managing drone deliveries and e-prescribing; the e-medic system uses a mobile application with face recognition-based authentication for patient administration. Additionally, this platform was created with a distinct web application to manage orders, deliveries, and prescriptions. This platform includes features to control the delivery drone via a web application, as the system uses an autonomous drone for deliveries. Regardless of the drone’s distance from the ground station, the system operates it via REST APIs. With the aid of computer-vision-based obstacle avoidance technology, the delivery drone can choose the shortest route to its destination and fly there independently [164].
5.5. Wearable/Lung e-Patch/Nose-on-a-Chip
The healthcare industry has changed dramatically in the last few years, from outdated equipment to advanced, cutting-edge technologies and devices based on biosensors, which power many of these developments. Wearable biosensors and other intelligent devices are non-invasive and offer patients continuous real-time monitoring (Figure 5D). Wearable sensors are becoming important instruments for lung cancer monitoring and management, employing technology to improve patient outcomes. A wearable chest patch that incorporates a bioimpedance (BioZ) sensor to detect alterations in chest impedance during breathing was developed by Qui et al. Additionally, it integrates a high-quality infrared temperature sensor designed for medical use for measuring body temperature. This patch employs a computing algorithm to compute the rate of breathing and temperature of the chest on each breath in real time with an accuracy of 97.8 to 98.5%. It features two wireless communication protocols, Bluetooth and long range (LoRa), facilitating long- and short-range data transfer. It helps in continuous monitoring, which plays a significant role in early detection [165]. Physio patch, a novel wearable sensor patch, assists in monitoring respiratory diseases by continuously capturing respiratory parameters through bioimpedance. The findings indicate that the Physio patch can derive respiratory parameters, demonstrating its potential for ongoing respiratory monitoring [166]. Moon et al. developed a distinctive wearable lung health monitoring system by integrating a piezoelectric sound sensor and an ECG sensor with electrodes. This system is designed to monitor respiratory and cardiovascular activities, providing a rapid digital assessment of lung health status [167]. Chromatography was used for the first time to analyse breath in the 1970s, revealing the presence of organic compounds in exhaled breath. Since then, over 3000 volatile organic compounds (VOCs) have been found in breath; these may have potential uses in medicine [168] yet only a few sensing devices aside from breath analysers that measure alcohol content have attained high technology readiness levels, and chromatographic laboratory tests have been the only avenue for diagnostic application. Breath analysis has been more popular recently due to developments in material science, synthetic biology and engineering, and a rise in interest in using exhaled breath to diagnose infectious diseases [169]. Breath analysis, also called breathomics, is the process of locating and quantifying several biomarkers in exhaled breath, including gases, volatile organic compounds, and biological components [170]. In addition to lung cancer, other widely recognised diseases like asthma, pneumonia, irritable bowel syndrome, diabetes, liver disorders, neurodegenerative diseases, renal disorder, breast cancer, gastric disorders, Helicobacter pylori infection, and even coronavirus disease (COVID-19) are reflected by the volatile organic compounds (VOCs), which act as prominent biomarkers [171]. Numerous biomarkers found in human breath have been established through significant investigation as potential disease indicators in the context of BBD of lung cancer. Based on their chemical makeup and source, these biomarkers can be divided into many groups, providing insight into the complex molecular environment related to lung cancer [172]. Researchers can create more precise and accurate diagnostic techniques by learning more about the intricate molecular mechanism underlying the synthesis of breath biomarkers in lung cancer. These techniques can potentially improve patient outcomes and raise survival rates by supporting early detection, surveillance, and customised lung cancer therapy.
Moreover, the development of nano-enabled VOC sensor arrays and the validation of the technology through clinical studies in a range of populations, coupled with integrating nanomaterials (NMs) in sensor technology, revolutionised the breathomic detection of LC [173]. In addition, the results’ accuracy, dependability, and reproducibility have recently increased with the integration of artificial intelligence (AI) with breathomic nanobiosensors [174]. By using sampling techniques and diagnostic algorithms, AI makes it possible to standardise and validate data, increasing BBD’s clinical applicability. Prospective research areas include integrating huge amounts of data, 5G/6G connectivity, the Internet of Things (IOT), the Internet of Medical Things, data clouding, and breathomic sensors to create point-of-care (POC) devices [175]. Smart nanobiosensors have been integrated with contemporary technologies to create a variety of POC modules, such as nanobiosensors for lab-on-a-chip (LOC) and hospital-on-a-chip (HOC) applications [176]. In the COVID-19 pandemic era, where remote testing, limited interaction, and isolation are essential for effectively managing the spread of illness, these 5th generation biosensors provide prospects for remote and tailored detection of numerous diseases [177]. The detection of biomarkers of many diseases from human breath is made possible by enabling POC modules, such as LOCs, with breathomics. This leads to the development of nose-on-a-chip (NOC) module-based nanobiosensors. Through non-invasive and non-contact techniques, these NOC nanobiosensors have the ability to screen for diseases such as LC, COVID-19, diabetes, renal problems, irritable bowel syndrome, antimicrobial resistance (AMR), and neurodegenerative diseases [178]. It may lessen the load on today’s medical diagnostic and treatment facilities and offer healthcare even in isolated locations. These nanobiosensors have the potential to improve healthcare fairness and accessibility regardless of human resource shortages or social and economic or geographic limitations [179].
5.6. Artificial Intelligence (AI)-Based LCT
AI has become an innovative tool in biomedical research in recent times, especially in the field of lung cancer. Through high-precision imaging and pattern recognition, AI improves the possibility of early diagnosis and opens up new possibilities for monitoring and predicting the course of disease. In addition, AI-driven strategies are essential for understanding the intricate molecular and genetic landscapes of lung cancer [180]. Lung cancer research is being revolutionised by AI, which is changing our understanding, diagnosis, and treatment of the disease. Deeper study of complex biological structures is now made possible by the widespread integration of AI technologies across a variety of omics disciplines, including pathology, microbiomics, radiomics, immunogenomics, proteomics, metabolomics, transcriptomics, and genomics [181]. The implementation of AI in lung cancer genomics includes the investigation of epigenetics, the evaluation of genomic instability, the analysis of gene mutations and variants, and the development of non-invasive liquid biopsy methods. These developments aid in early identification, targeted therapy, and a thorough comprehension of the genetic makeup of lung cancer, which may enhance patient outcomes and guide treatment plans [182]. AI has totally transformed the detection and analysis of mutation in lung cancer genomics, accelerating advances in precision medicine. Key mutations like EGFR, KRAS, and ALK can be accurately identified and classified as a result of deep learning (DL) models like convolutional neural networks (CNNs) and recurrent neural networks (RNNs), which enable high-resolution analysis of large-scale genomic information. This area of study is further enhanced by natural language processing (NLP), which improves the genomic knowledge base by automatically retrieving mutation-related information from genetic databases and scientific literature. Additionally, by forecasting the incidence and distribution of particular gene mutations across diverse populations, machine learning (ML) algorithms aid in the creation of tailored treatment plans for each patient [183]. Due to advancements in pharmacological data accumulation and machine learning, AI technology has emerged as an efficient data mining tool. In various aspects of drug design, including virtual screening, activity scoring, de novo drug design, in silico assessment of absorption, distribution, metabolism, excretion, and toxicity (ADME/T) properties, and quantitative structure–activity relationship (QSAR) analysis, machine learning and AI tools play an increasingly significant role. This has helped overcome many problems in new drug innovation and design, such as efficiency, accuracy, cost, and risk related to clinical and preclinical trials [184,185]. Lung cancer is believed to have the highest death rate due to patients showing no to minimal symptoms in the early stages, leading to 75 per cent of patients receiving a diagnosis in the later stages of illness [186]. AI is now being utilised, particularly in radiology and pathology, to enhance diagnostic accuracy through advanced picture processing. Deep learning algorithms reduce false positives and enable early intervention by detecting early-stage lung nodules in low-dose CT (LDCT) scans with higher sensitivity and specificity than human radiologists. Furthermore, tissue biopsies are analysed at the pixel level by AI-powered digital pathology tools, which uncover tumour microenvironment characteristics and histological subtypes that affect therapy choices. By combining multiomics data, AI helps with therapy selection in addition to diagnosis. This allows it to predict which molecular target medication or immunotherapies work for a particular patient [187]. The University of Texas Southwestern Medical Center’s treatment plans were developed more quickly because of the integration of AI and conventional pathology [188]. In order to help with the prognostication and severity evaluation of lung cancer, researchers at New York University Langone Health showed that AI tools with the ability to learn on their own could examine lung tissues and forecast cancer recurrence [189]. Pathchat is a multimodal generative AI helper designed especially for human pathologists developed by Lu et al. that efficiently processes and combines several data modalities through the use of visual language models. This cutting-edge method greatly improves diagnostic efficiency and accuracy while providing pathologists with strong support for their expert evaluations. AI has a wide range of potential uses in lung cancer pathological histology, with significant gains in diagnostic accuracy and efficiency as well as advancements in pathological research and customised therapy [190]. AI also aids in risk assessment and personalised screening. Now, computer-aided detection (CAD) systems allow the detection and characterisation (benign or malignant) of lung nodules with high sensitivity and shorter imaging time (Figure 5E).
6. Conclusions
Lung cancer is one of the deadliest diseases in the world as a result of delayed diagnosis and the poor efficacy of traditional treatment. Recent progress in cancer research has explored the potential of herbal medicines as a treatment method. Plant-derived herbal remedies have proved to be a promising alternative to conventional treatment methods by offering fewer side effects. These drugs can be used alone or in combination with other drugs to enhance their impact. Plant-derived substances with lower systemic toxicity, such resveratrol, paclitaxel, and curcumin, have shown strong anticancer potential. One significant problem observed with herbal drugs was their low solubility and poor absorption. Now, with the advancement of technology, specifically in nanotechnology, we can improve the effectiveness of these herbal drugs. Herbal extracts or their active compounds can be encapsulated within nano-sized particles to enhance herbal drugs’ solubility, stability, and bioavailability. This review explores the potential of several nanoparticles that can be used for lung cancer treatment, specifically focusing on gold, silver, zinc oxide, diamond, 2D, and 3D nanomaterials. Gold NPs are shown to have remarkable potential in targeted drug delivery due to their variable surface properties and biocompatibility. Silver nanoparticles also exhibit potent anticancer properties, making them valuable candidates for synergistic therapies with herbal medicines. Zinc nanoparticles possess antioxidant properties and anticancerous effects that could mitigate oxidative stress associated with cancer progression. Diamond NPs show biocompatibility, meaning they are the least toxic for the human body, thereby being suitable for various biomedical applications. A novel approach involves using 2D and 3D nanomaterials for precise drug delivery and controlled release, optimising therapeutic outcomes. These nanomaterials serve as customisable carriers for herbal compounds, ensuring their selective accumulation within only tumour cells and not harming normal cells. In order to assess nanotherapeutics in a physiologically realistic setting, new methods such as lung-on-a-chip models are currently being incorporated to replicate the lung microenvironment. Furthermore, smartphone-integrated biosensors and AI-enabled diagnostics are transforming tailored treatment and early detection. AI models, for example, may personalise nanomedicine-based treatment by analysing patient imaging and omics data. Precision medicine’s objectives are met by these technological synergies, which provide real-time monitoring early intervention and flexible treatment strategies. All things considered, the combination of digital health technology, sophisticated nanomaterials, and herbal nanomedicine offers a next-generation therapy paradigm for lung cancer.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ji, Y.; Zhang, Y.; Liu, S.; Li, J.; Jin, Q.; Wu, J.; Duan, H.; Liu, X.; Yang, L.; Huang, Y. The epidemiological landscape of lung cancer: Current status, temporal trend and future projections based on the latest estimates from GLOBOCAN 2022. J. Natl. Cancer Cent. 2025, 5, 278–286. [Google Scholar] [CrossRef]
- Jiang, P.; Liang, B.; Zhang, Z.; Fan, B.; Zeng, L.; Zhou, Z.; Mao, Z.; Xu, Q.; Yao, W.; Shen, Q. New insights into nanosystems for non-small-cell lung cancer: Diagnosis and treatment. RSC Adv. 2023, 13, 19540–19564. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and future development in lung cancer diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, J.-X.; Ma, L.-R.; Xu, D.-H.; Wang, P.; Li, L.-Q.; Yu, L.-L.; Li, Y.; Li, R.-Z.; Zhang, H.; et al. Traditional herbal medicine: A potential therapeutic approach for adjuvant treatment of non-small cell lung cancer in the future. Integr. Cancer Ther. 2022, 21, 15347354221144312. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-small cell lung cancer, Version 2.2021 featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Abdulridha, M.K.; Al-Marzoqi, A.H.; Al-Awsi, G.R.L.; Mubarak, S.M.; Heidarifard, M.; Ghasemian, A. Anticancer effects of herbal medicine compounds and novel formulations: A literature review. J. Gastrointest. Cancer 2020, 51, 765–773. [Google Scholar] [CrossRef]
- Guo, M.; Qin, S.; Wang, S.; Sun, M.; Yang, H.; Wang, X.; Fan, P.; Jin, Z. Herbal medicine nanocrystals: A potential novel therapeutic strategy. Molecules 2023, 28, 6370. [Google Scholar] [CrossRef]
- Barkat, M.A.; Goyal, A.; Barkat, H.A.; Salauddin, M.; Pottoo, F.H.; Anwer, E.T. Herbal medicine: Clinical perspective and regulatory status. Comb. Chem. High Throughput Screen. 2021, 24, 1573–1582. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Mason, M.D.; Sydes, M.R.; Glaholm, J.; Langley, R.E.; Huddart, R.A.; Sokal, M.; Stott, M.; Robinson, A.C.; James, N.D.; Parmar, M.K.; et al. Oral sodium clodronate for nonmetastatic prostate cancer—Results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J. Natl. Cancer Inst. 2007, 99, 765–776. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.X.; Chen, Z.S.; Hou, K. Nano-drug delivery systems entrapping natural bioactive compounds for cancer: Recent progress and future challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Taioli, E.; Flores, R.M.; Abdelhamid, A.; Untalan, M.; Ivic-Pavlicic, T.; Tuminello, S. Antibiotic use and survival in late-stage non-small cell lung cancer patients treated with chemo-immunotherapy. JTO Clin. Res. Rep. 2024, 5, 100710. [Google Scholar]
- Tinetti, A. Novel CAR design dually targets HER2+ breast cancer and MDSCs to improve efficacy in solid tumors. Preprint 2023. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune checkpoint inhibitors for lung cancer treatment: A review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- King, H.A.; Lewin, S.R. Immune checkpoint inhibitors in infectious disease. Immunol. Rev. 2024, 328, 350–371. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, L.; Zhou, Y.; He, W.; Li, W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: Current understanding in characteristics, diagnosis, and management. Front. Immunol. 2021, 12, 663986. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Babaloo, Z.; Baradaran, B. CTLA-4: From mechanism to autoimmune therapy. Int. Immunopharmacol. 2020, 80, 106221. [Google Scholar] [CrossRef]
- De Silva, P.; Aiello, M.; Gu-Trantien, C.; Migliori, E.; Willard-Gallo, K.; Solinas, C. Targeting CTLA-4 in cancer: Is it the ideal companion for PD-1 blockade immunotherapy combinations? Int. J. Cancer 2021, 149, 31–41. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, Y.; Arkenau, H.T.; Lao, T.; Chu, L.; Xu, Q. Signal pathways and precision therapy of small-cell lung cancer. Signal Transduct. Target. Ther. 2022, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood J. Am. Soc. Hematol. 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Sansom, D.M. CD28, CTLA-4 and their ligands: Who does what and to whom? Immunology 2000, 101, 169. [Google Scholar] [CrossRef]
- McLachlan, J. Immune checkpoint inhibitors and their side effects. Pathology 2019, 51, S17. [Google Scholar] [CrossRef]
- Ramagopal, U.A.; Liu, W.; Garrett-Thomson, S.C.; Bonanno, J.B.; Yan, Q.; Srinivasan, M.; Wong, S.C.; Bell, A.; Mankikar, S.; Rangan, V.S.; et al. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc. Natl. Acad. Sci. USA 2017, 114, E4223–E4232. [Google Scholar] [CrossRef]
- Malas, S.; Harrasser, M.; Lacy, K.E.; Karagiannis, S.N. Antibody therapies for melanoma: New and emerging opportunities to activate immunity. Oncol. Rep. 2014, 32, 875–886. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Parvez, A.; Choudhary, F.; Mudgal, P.; Khan, R.; Qureshi, K.A.; Farooqi, H.; Aspatwar, A. PD-1 and PD-L1: Architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front. Immunol. 2023, 14, 1296341. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.C.; He, Q.J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coyne, G.O.S.; Madan, R.A.; Gulley, J.L. Nivolumab: Promising survival signal coupled with limited toxicity raises expectations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 986. [Google Scholar] [CrossRef]
- Wilkinson, E. Nivolumab success in untreated metastatic melanoma. Lancet Oncol. 2015, 16, e9. [Google Scholar] [CrossRef]
- Mathieu, L.N.; Larkins, E.; Sinha, A.K.; Mishra-Kalyani, P.S.; Jafri, S.; Kalavar, S.; Ghosh, S.; Goldberg, K.B.; Pazdur, R.; Beaver, J.A.; et al. FDA approval summary: Atezolizumab as adjuvant treatment following surgical resection and platinum-based chemotherapy for stage II to IIIA NSCLC. Clin. Cancer Res. 2023, 29, 2973–2978. [Google Scholar] [CrossRef]
- Lin, X.; Lu, X.; Luo, G.; Xiang, H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020, 186, 111876. [Google Scholar] [CrossRef]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Combinational therapeutic strategies to overcome resistance to immune checkpoint inhibitors. Front. Immunol. 2025, 16, 1546717. [Google Scholar] [CrossRef]
- Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.Y.; Elserwy, W.S.; El-Mansy, M.F.; Serry, A.M.; Salem, A.M.; Abdou, A.M.; Abdelrahman, B.A.; Elsayed, K.H.; Elaziz, M.R.A. Angiokinase inhibition of VEGFR-2, PDGFR and FGFR and cell growth inhibition in lung cancer: Design, synthesis, biological evaluation and molecular docking of novel azaheterocyclic coumarin derivatives. Bioorganic Med. Chem. Lett. 2021, 48, 128258. [Google Scholar] [CrossRef] [PubMed]
- Homsi, J.; Daud, A.I. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control 2007, 14, 285–294. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Upadhyay, T.K.; Seungjoon, M.; Park, M.N.; Kim, B. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed. Pharmacother. 2023, 161, 114491. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Siddik, Z.H. Platelet-derived growth factor (PDGF) signalling in cancer: Rapidly emerging signalling landscape. Cell Biochem. Funct. 2015, 33, 257–265. [Google Scholar] [CrossRef]
- Heldin, C.H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013, 11, 1–18. [Google Scholar] [CrossRef]
- Wang, P.; Song, L.; Ge, H.; Jin, P.; Jiang, Y.; Hu, W.; Geng, N. Crenolanib, a PDGFR inhibitor, suppresses lung cancer cell proliferation and inhibits tumor growth in vivo. OncoTargets Ther. 2014, 7, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z. Non-small cell lung cancer targeted therapy: Drugs and mechanisms of drug resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef] [PubMed]
- Frezzetti, D.; Gallo, M.; Maiello, M.R.; D’Alessio, A.; Esposito, C.; Chicchinelli, N.; Normanno, N.; De Luca, A. VEGF as a potential target in lung cancer. Expert Opin. Ther. Targets 2017, 21, 959–966. [Google Scholar] [CrossRef]
- Niu, Z.; Jin, R.; Zhang, Y.; Li, H. Signaling pathways and targeted therapies in lung squamous cell carcinoma: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 353. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; Li, X.; et al. VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: Targeting the tumor microenvironment. Int. J. Biol. Sci. 2022, 18, 3845. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mathy, N.W.; Lu, H. The role of VEGF in the diagnosis and treatment of malignant pleural effusion in patients with non-small cell lung cancer. Mol. Med. Rep. 2018, 17, 8019–8030. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [Google Scholar] [CrossRef]
- Dev, M.; Chandel, P. Nanostructured Lipid Carriers in Pulmonary Drug Delivery: Progress and Prospects. Curr. Pharm. Res. 2025, 1, 131–143. [Google Scholar]
- Ge, D.; Ma, S.; Sun, T.; Li, Y.; Wei, J.; Wang, C.; Chen, X.; Liao, Y. Pulmonary delivery of dual-targeted nanoparticles improves tumor accumulation and cancer cell targeting by restricting macrophage interception in orthotopic lung tumors. Biomaterials 2025, 315, 122955. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhou, Q.; Pan, F.; Wang, R. Utilizing nanoparticles to overcome anti-PD-1/PD-L1 immunotherapy resistance in non-small cell lung cancer: A potential strategy. Int. J. Nanomed. 2025, 20, 2371–2394. [Google Scholar] [CrossRef] [PubMed]
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent advances and perspectives in liposomes for cutaneous drug delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci 2020, 7, 193. [Google Scholar] [CrossRef]
- McCright, J.; Naiknavare, R.; Yarmovsky, J.; Maisel, K. Targeting lymphatics for nanoparticle drug delivery. Front. Pharmacol. 2022, 13, 887402. [Google Scholar] [CrossRef]
- Huynh, K.H.; Lee, K.Y.; Chang, H.; Lee, S.H.; Kim, J.; Pham, X.H.; Lee, Y.S.; Rho, W.Y.; Jun, B.H. Bioapplications of Nanomaterials. In Nanotechnology for Bioapplications; Springer: Singapore, 2021; pp. 235–255. [Google Scholar]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Baimyrza, A. Bismuth Telluride Nanoparticles for Thermoelectrical Application, Synthesis and Incorporation into Polymer Hybrid Composites. Doctoral Dissertation, Normandie Université, Caen, France, 2022. [Google Scholar]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Gautam, B. Tuning the Thermal Conductivity of Lignin@ Fe3O4 Colloidal Suspension Through External Magnetic Field. Master’s Thesis, University of Dayton, Dayton, OH, USA, 2022. [Google Scholar]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and applications in theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, L.; Huang, H.; He, W.; Ming, W. Progress in laser ablation and biological synthesis processes:“Top-Down” and “Bottom-Up” approaches for the green synthesis of Au/Ag nanoparticles. Int. J. Mol. Sci. 2022, 23, 14658. [Google Scholar] [CrossRef] [PubMed]
- Giraldez-Perez, R.M.; Grueso, E.; Dominguez, I.; Pastor, N.; Kuliszewska, E.; Prado-Gotor, R.; Requena-Domenech, F. Biocompatible DNA/5-fluorouracil-gemini surfactant-functionalized gold nanoparticles as promising vectors in lung cancer therapy. Pharmaceutics 2021, 13, 423. [Google Scholar] [CrossRef]
- Woodman, C.; Vundu, G.; George, A.; Wilson, C.M. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 69, pp. 349–364. [Google Scholar]
- Jindal, M.; Nagpal, M.; Singh, M.; Aggarwal, G.; Dhingra, G.A. Gold nanoparticles-boon in cancer theranostics. Curr. Pharm. Des. 2020, 26, 5134–5151. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Xin, H.; Liu, R.; Wang, Q.; Cai, W.; Peng, X.; Yang, F.; Xin, H. Nanoparticles advanced from preclinical studies to clinical trials for lung cancer therapy. Cancer Nanotechnol. 2023, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, J.Q.; Ashby Jr, C.R.; Zeng, L.; Fan, Y.F.; Chen, Z.S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef]
- Mueller, R.; Yasmin-Karim, S.; DeCosmo, K.; Vazquez-Pagan, A.; Sridhar, S.; Kozono, D.; Hesser, J.; Ngwa, W. Increased carcinoembryonic antigen expression on the surface of lung cancer cells using gold nanoparticles during radiotherapy. Phys. Medica 2020, 76, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Crous, A.; Abrahamse, H. Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int. J. Mol. Sci. 2020, 21, 3742. [Google Scholar] [CrossRef]
- Peng, J.; Wang, R.; Sun, W.; Huang, M.; Wang, R.; Li, Y.; Wang, P.; Sun, G.; Xie, S. Delivery of miR-320a-3p by gold nanoparticles combined with photothermal therapy for directly targeting Sp1 in lung cancer. Biomater. Sci. 2021, 9, 6528–6541. [Google Scholar] [CrossRef]
- Kanipandian, N.; Li, D.; Kannan, S. Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol. Rep. 2019, 23, e00339. [Google Scholar] [CrossRef] [PubMed]
- Jakic, K.; Selc, M.; Macova, R.; Kurillova, A.; Kvitek, L.; Panacek, A.; Babelova, A. Effects of different-sized silver nanoparticles on morphological and functional alterations in lung cancer and non-cancer lung cells. Neoplasma 2023, 70, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer therapy by silver nanoparticles: Fiction or reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Raj, A.; Thomas, R.K.; Vidya, L.; Aparna, V.M.; Neelima, S.; Sudarsanakumar, C. Exploring the cytotoxicity on human lung cancer cells and DNA binding stratagem of camptothecin functionalised silver nanoparticles through multi-spectroscopic, and calorimetric approach. Sci. Rep. 2023, 13, 9045. [Google Scholar] [CrossRef]
- Singh, S.; Goel, T.; Singh, A.; Chugh, H.; Chakraborty, N.; Roy, I.; Tiwari, M.; Chandra, R. Synthesis and characterization of Fe3O4@ SiO2@ PDA@ Ag core–shell nanoparticles and biological application on human lung cancer cell line and antibacterial strains. Artif. Cells Nanomed. Biotechnol. 2024, 52, 46–58. [Google Scholar] [CrossRef]
- Tanino, R.; Amano, Y.; Tong, X.; Sun, R.; Tsubata, Y.; Harada, M.; Fujita, Y.; Isobe, T. Anticancer activity of ZnO nanoparticles against human small-cell lung cancer in an orthotopic mouse model. Mol. Cancer Ther. 2020, 19, 502–512. [Google Scholar] [CrossRef]
- Mishra, P.; Ahmad, A.; Al-Keridis, L.A.; Alshammari, N.; Alabdallah, N.M.; Muzammil, K.; Saeed, M.; Ansari, I.A. Doxorubicin-conjugated zinc oxide nanoparticles, biogenically synthesised using a fungus Aspergillus niger, exhibit high therapeutic efficacy against lung cancer cells. Molecules 2022, 27, 2590. [Google Scholar] [CrossRef]
- Mishra, P.; Ahmad, M.F.A.; Al-Keridis, L.A.; Saeed, M.; Alshammari, N.; Alabdallah, N.M.; Tiwari, R.K.; Ahmad, A.; Verma, M.; Fatima, S.; et al. Methotrexate-conjugated zinc oxide nanoparticles exert a substantially improved cytotoxic effect on lung cancer cells by inducing apoptosis. Front. Pharmacol. 2023, 14, 1194578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liang, S.; Liu, D.; Ma, K.; Yun, K.; Yao, J.; Peng, Y.; Hai, L.; Zhang, Q.; Wang, Z. Manganese-Enriched Zinc Peroxide Functional Nanoparticles for Potentiating Cancer Immunotherapy. Nano Lett. 2023, 23, 10350–10359. [Google Scholar] [CrossRef]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pierstorff, E.; Osawa, E.; Ho, D. Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 2007, 7, 3305–3314. [Google Scholar] [CrossRef]
- Ali, M.S.; Elhabak, M.; Osman, R.; Nasr, M. Towards more efficient inhalable chemotherapy: Fabrication of nanodiamonds-releasing microspheres. Int. J. Pharm. 2022, 626, 122169. [Google Scholar] [CrossRef]
- Grudinkin, S.A.; Bogdanov, K.V.; Tolmachev, V.A.; Baranov, M.A.; Kaliya, I.E.; Golubev, V.G.; Baranov, A.V. Multifunctional Core/Shell Diamond Nanoparticles Combining Unique Thermal and Light Properties for Future Biological Applications. Nanomaterials 2023, 13, 3124. [Google Scholar] [CrossRef] [PubMed]
- Padya, B.S.; Pandey, A.; NIkam, A.; Kulkarni, S.; Fernandes, G.; Mutalik, S. Two-dimensional materials-based nanoplatforms for lung cancer management: Synthesis, properties, and targeted therapy. In Advanced Drug Delivery Systems in the Management of Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 415–429. [Google Scholar]
- Zhu, W.; Li, H.; Luo, P. Emerging 2D nanomaterials for multimodel theranostics of cancer. Front. Bioeng. Biotechnol. 2021, 9, 769178. [Google Scholar] [CrossRef]
- Wen, W.; Song, Y.; Yan, X.; Zhu, C.; Du, D.; Wang, S.; Asiri, A.M.; Lin, Y. Recent advances in emerging 2D nanomaterials for biosensing and bioimaging applications. Mater. Today 2018, 21, 164–177. [Google Scholar] [CrossRef]
- Sharker, S.M. Hexagonal boron nitrides (white graphene): A promising method for cancer drug delivery. Int. J. Nanomed. 2019, 14, 9983–9993. [Google Scholar] [CrossRef]
- Bhatt, H.N.; Pena-Zacarias, J.; Beaven, E.; Zahid, M.I.; Ahmad, S.S.; Diwan, R.; Nurunnabi, M. Potential and progress of 2D materials in photomedicine for cancer treatment. ACS Appl. Bio Mater. 2023, 6, 365–383. [Google Scholar] [CrossRef]
- Sadiq, M.; Pang, L.; Johnson, M.; Sathish, V.; Zhang, Q.; Wang, D. 2D Nanomaterial, Ti3C2 MXene-based sensor to guide lung cancer therapy and management. Biosensors 2021, 11, 40. [Google Scholar] [CrossRef]
- Gholami, A.; Hashemi, S.A.; Yousefi, K.; Mousavi, S.M.; Chiang, W.H.; Ramakrishna, S.; Mazraedoost, S.; Alizadeh, A.; Omidifar, N.; Behbudi, G.; et al. 3D nanostructures for tissue engineering, cancer therapy, and gene delivery. J. Nanomater. 2020, 2020, 1852946. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Böker, A. Self-Assembly of Nanoparticles in 2D and 3D: Recent Advances and Future Trends. Macromol. Chem. Phys. 2019, 220, 1900196. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Andrabi, S.M.; Islam, F.; An, J.M.; Schindler, S.J.; Matis, M.P.; Lee, D.Y.; Lee, Y.-K. Next-generation 3D scaffolds for nano-based chemotherapeutics delivery and cancer treatment. Pharmaceutics 2022, 14, 2712. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, H.; Tan, M.; Ou, L.; Kong, D.; Yang, Z. Conjugation of two complementary anti-cancer drugs confers molecular hydrogels as a co-delivery system. Chem. Commun. 2012, 48, 395–397. [Google Scholar] [CrossRef]
- Rincón-Pérez, J.; Rodríguez-Hernández, L.; Ruíz-Valdiviezo, V.M.; Abud-Archila, M.; Luján-Hidalgo, M.C.; Ruiz-Lau, N.; González-Mendoza, D.; Gutiérrez-Miceli, F.A. Fatty Acids Profile, Phenolic Compounds and Antioxidant Capacity in Elicited Callus ofThevetia peruviana (Pers.) K. Schum. J. Oleo Sci. 2016, 65, 311–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, C.; Su, L.; Zhang, F.; Zhu, X.; Zhu, Y.; Wei, L.; Jiao, X.; Hou, Y.; Chen, X.; Wang, W.; et al. Thevebioside, the active ingredient of traditional Chinese medicine, promotes ubiquitin-mediated SRC-3 degradation to induce NSCLC cells apoptosis. Cancer Lett. 2020, 493, 167–177. [Google Scholar] [CrossRef]
- Balan, D.J.; Rajavel, T.; Das, M.; Sathya, S.; Jeyakumar, M.; Devi, K.P. Thymol induces mitochondrial pathway-mediated apoptosis via ROS generation, macromolecular damage and SOD diminution in A549 cells. Pharmacol. Rep. 2021, 73, 240–254. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; da Silva, P.B.; Ramos, M.A.D.S.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Ansari, S.H.; Islam, F.; Sameem, M. Influence of nanotechnology on herbal drugs: A Review. J. Adv. Pharm. Technol. Res. 2012, 3, 142–146. [Google Scholar] [CrossRef]
- Türk, G.; Ateşşahin, A.; Sönmez, M.; Çeribaşi, A.O.; Yüce, A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil. Steril. 2008, 89, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Abd Elwakil, M.M.; Mabrouk, M.T.; Helmy, M.W.; Abdelfattah, E.Z.A.; Khiste, S.K.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable lactoferrin–chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine 2018, 13, 2015–2035. [Google Scholar] [CrossRef]
- Mardani, R.; Hamblin, M.R.; Taghizadeh, M.; Banafshe, H.R.; Nejati, M.; Mokhtari, M.; Borran, S.; Davoodvandi, A.; Khan, H.; Jaafari, M.R.; et al. Nanomicellar-curcumin exerts its therapeutic effects via affecting angiogenesis, apoptosis, and T cells in a mouse model of melanoma lung metastasis. Pathol.-Res. Pract. 2020, 216, 153082. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, M.M.; Helmy, M.W.; Mortada, S.M.; Elzoghby, A.O. Dual-targeted nano-in-nano albumin carriers enhance the efficacy of combined chemo/herbal therapy of lung cancer. Nanomedicine 2018, 13, 2221–2224. [Google Scholar] [CrossRef]
- Kumkoon, T.; Srisaisap, M.; Boonserm, P. Biosynthesized silver nanoparticles using Morus alba (white mulberry) leaf extract as potential antibacterial and anticancer agents. Molecules 2023, 28, 1213. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Panek-Krzyśko, A.; Stompor-Gorący, M. The pro-health benefits of morusin administration—An update review. Nutrients 2021, 13, 3043. [Google Scholar] [CrossRef]
- Park, H.J.; Min, T.R.; Chi, G.Y.; Choi, Y.H.; Park, S.H. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018, 505, 194–200. [Google Scholar] [CrossRef]
- Govindaraju, S.; Roshini, A.; Lee, M.H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef]
- Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A.; Rahmani, A.H. Effects and mechanisms of kaempferol in the management of cancers through modulation of inflammation and signal transduction pathways. Int. J. Mol. Sci. 2023, 24, 8630. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhang, G.; Wu, J.; Liu, Z.; Liu, C.; Wang, H.; Miao, S.; Deng, L.; Cao, K.; et al. To explore the effect of kaempferol on non-small cell lung cancer based on network pharmacology and molecular docking. Front. Pharmacol. 2023, 14, 1148171. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Gambaro, R.; Cisneros, J.S.; Huck-Iriart, C.; Padula, G.; Castro, G.R.; Chain, C.Y.; Islan, G.A. Enhanced anticancer activity of encapsulated geraniol into biocompatible lipid nanoparticles against A549 human lung cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104159. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Castro, M.A.; Gambaro, R.C.; Girotti, J.; Cisneros, J.S.; Viña, S.; Padula, G.; Crespo, R.; Castro, G.R.; Gehring, S.; et al. Cytotoxic screening and enhanced anticancer activity of lippia alba and clinopodium nepeta essential oils-loaded biocompatible lipid nanoparticles against lung and colon cancer cells. Pharmaceutics 2023, 15, 2045. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Rocha-González, H.I.; Ambriz-Tututi, M.; Granados-Soto, V. Resveratrol: A natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci. Ther. 2008, 14, 234–247. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef]
- Paudel, K.R.; Mehta, M.; Yin, G.H.S.; Yen, L.L.; Malyla, V.; Patel, V.K.; Panneerselvam, J.; Madheswaran, T.; MacLoughlin, R.; Jha, N.K.; et al. Berberine-loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Environ. Sci. Pollut. Res. 2022, 29, 46830–46847. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhang, J.H.; Gao, J.H.; Li, Y.S. Aloe-emodin (AE) nanoparticles suppresses proliferation and induces apoptosis in human lung squamous carcinoma via ROS generation in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017, 490, 601–607. [Google Scholar] [CrossRef]
- Majumdar, D.; Jung, K.-H.; Zhang, H.; Nannapaneni, S.; Wang, X.; Amin, A.R.; Chen, Z.; Chen, Z.; Shin, D.M. Luteolin nanoparticle in chemoprevention: In vitro and in vivo anticancer activity. Cancer Prev. Res. 2014, 7, 65–73. [Google Scholar] [CrossRef]
- Rauf, A.; Wilairatana, P.; Joshi, P.B.; Ahmad, Z.; Olatunde, A.; Hafeez, N.; Hemeg, H.A.; Mubarak, M.S. Revisiting luteolin: An updated review on its anticancer potential. Heliyon 2024, 10, e26701. [Google Scholar] [CrossRef]
- Alshehri, S.; Bukhari, S.I.; Imam, S.S.; Hussain, A.; Alghaith, A.F.; Altamimi, M.A.; AlAbdulkarim, A.S.; Almurshedi, A. Formulation of Piperine-Loaded Nanoemulsion: In Vitro Characterization, Ex Vivo Evaluation, and Cell Viability Assessment. ACS Omega 2023, 8, 22406–22413. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef]
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy–Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef]
- Elgohary, M.M.; Helmy, M.W.; Abdelfattah, E.Z.A.; Ragab, D.M.; Mortada, S.M.; Fang, J.Y.; Elzoghby, A.O. Targeting sialic acid residues on lung cancer cells by inhalable boronic acid-decorated albumin nanocomposites for combined chemo/herbal therapy. J. Control Release 2018, 285, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.M.; Elzoghby, A.O.; Helmy, M.W.; Samaha, M.W.; Fang, J.Y.; Freag, M.S. Liquid crystalline assembly for potential combinatorial chemo–herbal drug delivery to lung cancer cells. Int. J. Nanomed. 2019, 14, 499–517. [Google Scholar] [CrossRef]
- Alemi, A.; Karamallah, M.H.; Sabaghan, M.; Hosseini, S.A.; Veisi, A.; Karamallah, S.H.; Farokhifar, M. Combination drug therapy by herbal nanomedicine prevent multidrug resistance protein 1: Promote apoptosis in Lung Carcinoma. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241235442. [Google Scholar] [CrossRef]
- Zhong, S.; Yao, S.; Zhao, Q.; Wang, Z.; Liu, Z.; Li, L.; Wang, Z.L. Electricity-Assisted Cancer Therapy: From Traditional Clinic Applications to Emerging Methods Integrated with Nanotechnologies. Adv. NanoBiomed Res. 2023, 3, 2200143. [Google Scholar] [CrossRef]
- Ahmad, U.; Machuzak, M. Electric shock therapy for lung cancer: Taking palliation to the next level. J. Thorac. Cardiovasc. Surg. 2018, 155, 2160–2161. [Google Scholar] [CrossRef]
- Kodama, H.; Vroomen, L.G.; Ueshima, E.; Reilly, J.; Brandt, W.; Paluch, L.R.; Monette, S.; Jones, D.; Solomon, S.B.; Srimathveeravalli, G. Catheter-based endobronchial electroporation is feasible for the focal treatment of peribronchial tumors. J. Thorac. Cardiovasc. Surg. 2018, 155, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Venugopal, A.; Chandrasekhar, M.; Suriyanarayanan, A.; Balasubramani, K.; Ilangovan, A.S.; Kamalakannan, S.; Gunaseelan, R.; Ayyadurai, N.; Gopalakrishnan, A.V.; et al. Electrical based cancer therapy for solid tumours-Theranostics approach. Biosens. Bioelectron. X 2022, 11, 100214. [Google Scholar] [CrossRef]
- Alshahat, M.A.; Elgenedy, M.A.; Aboushady, A.A.; Williams, M.T. Cancer Treatment: An Overview of Pulsed Electric Field Utilization and Generation. Appl. Sci. 2023, 13, 10029. [Google Scholar] [CrossRef]
- Xin, Y.L.; Xue, F.Z.; Ge, B.S.; Zhao, F.R.; Shi, B.; Zhang, W. Electrochemical treatment of lung cancer. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1997, 18, 8–13. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Guo, Q.; Kuang, J.; Li, D.; Wu, M.; Mo, Y.; Zhang, T.; Gao, X.; Tan, J. Advanced lung organoids and lung-on-a-chip for cancer research and drug evaluation: A review. Front. Bioeng. Biotechnol. 2023, 11, 1299033. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Ni, C.; Zhao, B.; Cheng, X. The application of patient-derived organoid in the research of lung cancer. Cell. Oncol. 2023, 46, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, J.; Razavi Bazaz, S.; Aboulkheyr Es, H.; Yaghobian Azari, D.; Thierry, B.; Ebrahimi Warkiani, M.; Ghadiri, M. Lung-on-a-chip: The future of respiratory disease models and pharmacological studies. Crit. Rev. Biotechnol. 2020, 40, 213–230. [Google Scholar] [CrossRef]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab A Chip 2018, 18, 486–495. [Google Scholar] [CrossRef]
- Arathi, A.; Joseph, X.; Akhil, V.; Mohanan, P.V. L-Cysteine capped zinc oxide nanoparticles induced cellular response on adenocarcinomic human alveolar basal epithelial cells using a conventional and organ-on-a-chip approach. Colloids Surf. B Biointerfaces 2022, 211, 112300. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, P.C. Promise of smartphone-enabled teleconsultation for global cancer prevention. JCO Glob. Oncol. 2020, 6, GO.20.00424. [Google Scholar] [CrossRef] [PubMed]
- Pardolesi, A.; Gherzi, L.; Pastorino, U. Telemedicine for management of patients with lung cancer during COVID-19 in an Italian cancer institute: SmartDoc Project. Tumori J. 2022, 108, 357–363. [Google Scholar] [CrossRef]
- Dhawale, T.M.; Bhat, R.S.; Johnson, P.C.; Srikonda, S.; Lau-Min, K.S.; Boateng, K.; Lee, H.; Amonoo, H.L.; Nipp, R.; Lindvall, C.; et al. Telemedicine-based serious illness conversations, healthcare utilization, and end of life care among patients with advanced lung cancer. The Oncologist 2024, 29, e1762–e1769. [Google Scholar] [CrossRef] [PubMed]
- Magarinos, J.; Lutzow, L.; Dass, C.; Ma, G.X.; Erkmen, C.P. Feasibility of single-encounter telemedicine lung cancer screening: A retrospective cohort study in an underserved population. Cancer Control 2023, 30, 10732748221121391. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Muse, E.D.; Topol, E.J. The emerging field of mobile health. Sci. Transl. Med. 2015, 7, rv3–rv283. [Google Scholar] [CrossRef]
- Vercell, A.; Gasteiger, N.; Yorke, J.; Dowding, D. Patient-facing cancer mobile apps that enable patient reported outcome data to be collected: A systematic review of content, functionality, quality, and ability to integrate with electronic health records. Int. J. Med. Inform. 2023, 170, 104931. [Google Scholar] [CrossRef]
- Ciani, O.; Cucciniello, M.; Petracca, F.; Apolone, G.; Merlini, G.; Novello, S.; Pedrazzoli, P.; Zilembo, N.; Broglia, C.; Capelletto, E.; et al. Lung Cancer App (LuCApp) study protocol: A randomised controlled trial to evaluate a mobile supportive care app for patients with metastatic lung cancer. BMJ Open 2019, 9, e025483. [Google Scholar] [CrossRef]
- Ji, W.; Kwon, H.; Lee, S.; Kim, S.; Hong, J.S.; Park, Y.R.; Kim, H.R.; Lee, J.C.; Jung, E.J.; Kim, D.; et al. Mobile health management platform–based pulmonary rehabilitation for patients with non–small cell lung cancer: Prospective clinical trial. JMIR Mhealth Uhealth 2019, 7, e12645. [Google Scholar] [CrossRef]
- Henshall, C.; Davey, Z. Development of an app for lung cancer survivors (iEXHALE) to increase exercise activity and improve symptoms of fatigue, breathlessness and depression. Psycho-Oncol. 2020, 29, 139–147. [Google Scholar] [CrossRef]
- Ni, X.; Lou, Y.; Hu, W.; Wang, H.; Xu, H.; Li, S.; Zhou, Y.; Ni, Y. Development of mobile health–based self-management support for patients with lung cancer: A stepwise approach. Nurs. Open 2022, 9, 1612–1624. [Google Scholar] [CrossRef]
- Berzenji, L.; Yogeswaran, K.; Van Schil, P.; Lauwers, P.; Hendriks, J.M. Use of robotics in surgical treatment of non-small cell lung cancer. Curr. Treat. Options Oncol. 2020, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G. Robotic surgery for the treatment of early-stage lung cancer. Curr. Opin. Oncol. 2013, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Siu, I.C.; Chang, A.T.; Li, M.S.; Lau, R.W.; Mok, T.S.; Ng, C.S. Transbronchial techniques for lung cancer treatment: Where are we now? Cancers 2023, 15, 1068. [Google Scholar] [CrossRef]
- Chen, A.C.; Gillespie, C.T. Robotic endoscopic airway challenge: REACH assessment. Ann. Thorac. Surg. 2018, 106, 293–297. [Google Scholar] [CrossRef]
- da Silva, L.G.V.; Barros, K.V.G.; de Araújo, F.V.C.; da Silva, G.B.; da Silva, P.A.F.; Condori, R.C.I.; Mattos, L. Nanorobotics in drug delivery systems for treatment of cancer: A review. J. Mat. Sci. Eng. A 2016, 6, 167–180. [Google Scholar]
- Muskan, A.; Kumar, S. The Use of Nanorobotics in the Treatment Therapy of Cancer and Its Future Aspects: A Review. Cureus 2022, 14, e29366. [Google Scholar]
- Oigbochie, A.E.; Odigie, E.B.; Adejumo, B.I.G. Importance of drones in healthcare delivery amid a pandemic: Current and generation next application. Open J. Med. Res. (ISSN: 2734-2093) 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Abeygunawaradana, P.; Gamage, N.; De Alwis, L.; Ashan, S.; Nilanka, C.; Godamune, P. E-Medic–Autonomous Drone for Healthcare System. In 2021 International Conference on Computing, Communication, and Intelligent Systems (ICCCIS); IEEE: Greater Noida, India, 2021; pp. 994–999. [Google Scholar]
- Qiu, C.; Wu, F.; Han, W.; Yuce, M.R. A wearable bioimpedance chest patch for real-time ambulatory respiratory monitoring. IEEE Trans. Biomed. Eng. 2022, 69, 2970–2981. [Google Scholar] [CrossRef]
- John, M.; Van Der Westen, R.G.; Yordanova, G.; Groenendaal, W.; Agell, C. Validation of a novel wearable monitoring patch for continuous respiratory monitoring. Eur. Respir. J. 2022, 60, 4391. [Google Scholar]
- Moon, K.S.; Lee, S.Q. A Wearable Multimodal Wireless Sensing System for Respiratory Monitoring and Analysis. Sensors 2023, 23, 6790. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 15, 1–23. [Google Scholar] [CrossRef]
- Guntner, A.T.; Abegg, S.; Konigstein, K.; Gerber, P.A.; Schmidt-Trucksass, A.; Pratsinis, S.E. Breath sensors for health monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef]
- Lin, L.P.; Tan, M.T.T. Biosensors for the detection of lung cancer biomarkers: A review on biomarkers, transducing techniques and recent graphene-based implementations. Biosens. Bioelectron. 2023, 237, 115492. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Hrinczenko, B.; Swain, G.M.; Peteu, S.F. Exhaled breath biomarker sensing. Biosens. Bioelectron. 2021, 182, 113193. [Google Scholar] [CrossRef]
- Schmidt, F.; Kohlbrenner, D.; Malesevic, S.; Huang, A.; Klein, S.D.; Puhan, M.A.; Kohler, M. Mapping the landscape of lung cancer breath analysis: A scoping review (ELCABA). Lung Cancer 2023, 175, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, Q. Ru-Doped MoS2 Monolayer for Exhaled Breath Detection on Early Lung Cancer Diagnosis: A First-Principles Investigation. ACS Omega 2024, 9, 13951–13959. [Google Scholar] [CrossRef]
- Lu, G.; Ji, T.; He, S.; Ai, F.; Yan, L.; Hu, J. Recent progress of exhaled gas-based diagnosis based on field effect transistor sensors. Adv. Funct. Mater. 2024, 35, 2309111. [Google Scholar] [CrossRef]
- Amalraj, J.J.; Banumathi, S.; John, J.J. IOT sensors and applications: A survey. Int. J. Sci. Technol. Res. 2019, 8, 998–1003. [Google Scholar]
- Chaudhary, V.; Khanna, V.; Awan, H.T.A.; Singh, K.; Khalid, M.; Mishra, Y.K.; Bhansali, S.; Li, C.-Z.; Kaushik, A. Towards hospital-on-chip supported by 2D MXenes-based 5th generation intelligent biosensors. Biosens. Bioelectron. 2023, 220, 114847. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaushik, A.; Furukawa, H.; Khosla, A. Towards 5th generation ai and iot driven sustainable intelligent sensors based on 2d mxenes and borophene. ECS Sens. Plus 2022, 1, 013601. [Google Scholar] [CrossRef]
- Taha, B.A.; Chaudhary, V.; Rustagi, S.; Singh, P. Fate of Sniff-the-Diseases Through Nanomaterials-Supported Optical Biochip Sensors. ECS J. Solid State Sci. Technol. 2024, 13, 047004. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gaur, P.; Rustagi, S. Sensors, society, and sustainability. Sustain. Mater. Technol. 2024, 40, e00952. [Google Scholar] [CrossRef]
- Rong, R.; Sheng, H.; Jin, K.W.; Wu, F.; Luo, D.; Wen, Z.; Tang, C.; Yang, D.M.; Jia, L.; Amgad, M.; et al. A deep learning approach for histology-based nucleus segmentation and tumor microenvironment characterization. Mod. Pathol. 2023, 36, 100196. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Jiang, T.; Yang, C.; Li, N. Artificial intelligence in lung cancer: Current applications, future perspectives, and challenges. Front. Oncol. 2024, 14, 1486310. [Google Scholar] [CrossRef] [PubMed]
- Kehl, K.L.; Xu, W.; Gusev, A.; Bakouny, Z.; Choueiri, T.K.; Bin Riaz, I.; Elmarakeby, H.; Van Allen, E.M.; Schrag, D. Artificial intelligence-aided clinical annotation of a large multi-cancer genomic dataset. Nat. Commun. 2021, 12, 7304. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Bonfante, M.; Zurek, E.; Cherezov, D.; Goldgof, D.; Hall, L.; Schabath, M. A radiogenomics ensemble to predict EGFR and KRAS mutations in NSCLC. Tomography 2021, 7, 154–168. [Google Scholar] [CrossRef]
- Duch, W.; Swaminathan, K.; Meller, J. Artificial intelligence approaches for rational drug design and discovery. Curr. Pharm. Des. 2007, 13, 1497–1508. [Google Scholar] [CrossRef]
- Zhong, F.; Xing, J.; Li, X.; Liu, X.; Fu, Z.; Xiong, Z.; Lu, D.; Wu, X.; Zhao, J.; Tan, X.; et al. Artificial intelligence in drug design. Sci. China Life Sci. 2018, 61, 1191–1204. [Google Scholar] [CrossRef]
- Murphy, A.; Skalski, M.; Gaillard, F. The utilisation of convolutional neural networks in detecting pulmonary nodules: A review. Br. J. Radiol. 2018, 91, 20180028. [Google Scholar] [CrossRef]
- Cellina, M.; Cacioppa, L.M.; Cè, M.; Chiarpenello, V.; Costa, M.; Vincenzo, Z.; Pais, D.; Bausano, M.V.; Rossini, N.; Bruno, A.; et al. Artificial intelligence in lung cancer screening: The future is now. Cancers 2023, 15, 4344. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Rong, R.; Zhou, Q.; Yang, D.M.; Zhang, X.; Zhan, X.; Bishop, J.; Chi, Z.; Wilhelm, C.J.; Zhang, S.; et al. Deep learning of cell spatial organizations identifies clinically relevant insights in tissue images. Nat. Commun. 2023, 14, 7872. [Google Scholar] [CrossRef] [PubMed]
- Quiros, A.C.; Coudray, N.; Yeaton, A.; Yang, X.; Liu, B.; Le, H.; Chiriboga, L.; Karimkhan, A.; Narula, N.; Moore, D.A.; et al. Mapping the landscape of histomorphological cancer phenotypes using self-supervised learning on unannotated pathology slides. Nat. Commun. 2024, 15, 4596. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Chen, B.; Williamson, D.F.K.; Chen, R.J.; Zhao, M.; Chow, A.K.; Ikemura, K.; Kim, A.; Pouli, D.; Patel, A.; et al. A multimodal generative AI copilot for human pathology. Nature 2024, 634, 466–473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).