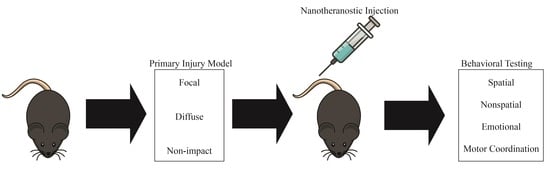
The Nanotheranostic Researcher’s Guide for Use of Animal Models of Traumatic Brain Injury
Abstract
1. Introduction
2. Classification of TBI Injury Severity in Humans
2.1. Glasgow Coma Scale
2.2. Mayo Classification of TBI
2.3. Collaborative European NeuroTrauma Effectiveness for Research for TBI (CENTER-TBI)
3. Categories of TBI
4. TBI Animal Models
5. Focal TBI
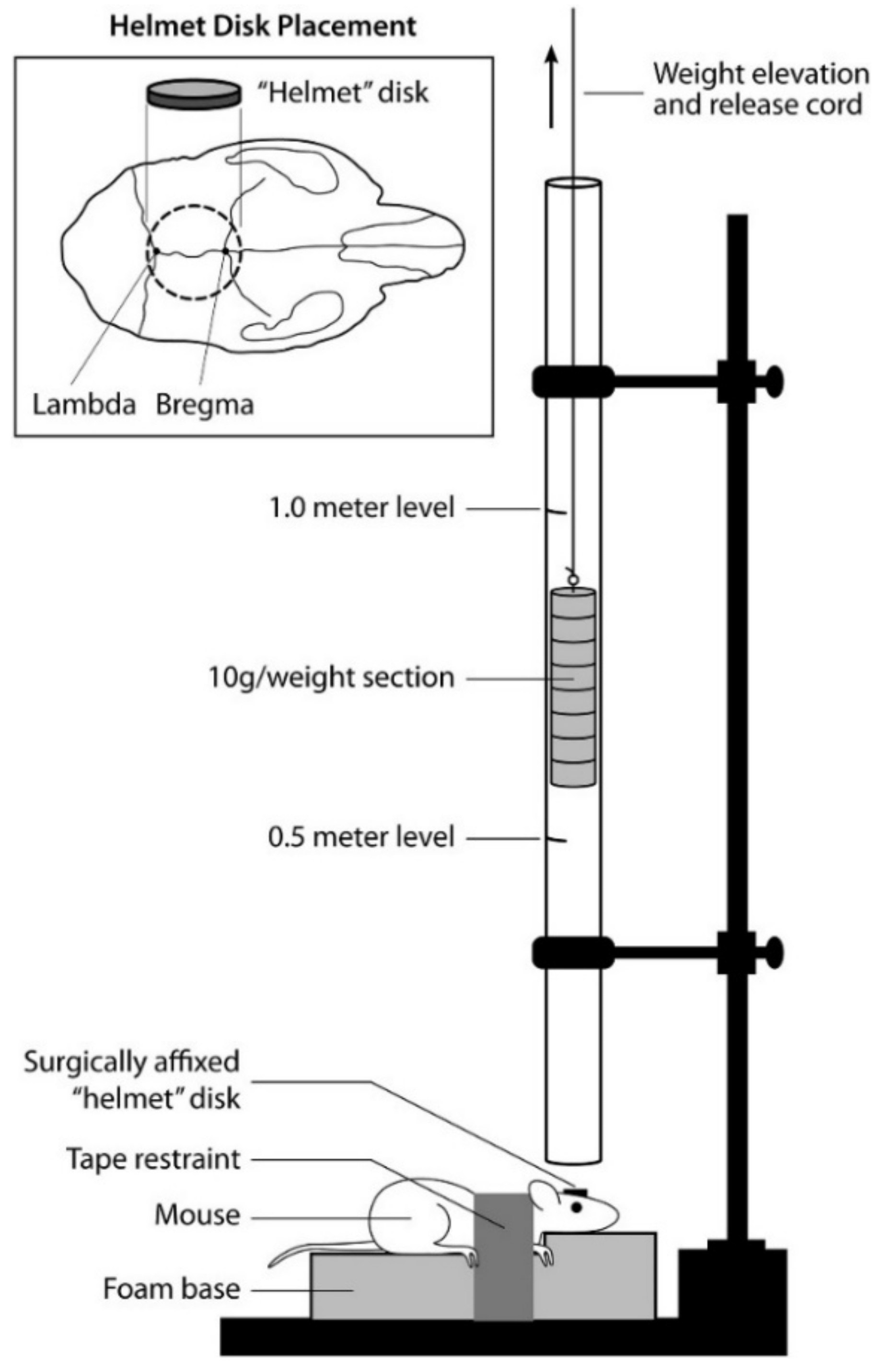
5.1. Weight Drop
5.2. Feeney’s Weight Drop Model
5.3. Shohami’s Weight Drop Model
5.4. Fluid Percussion Injury
5.5. Lateral Fluid Percussion Injury
5.6. Penetrating Ballistic-Like Brain Injury
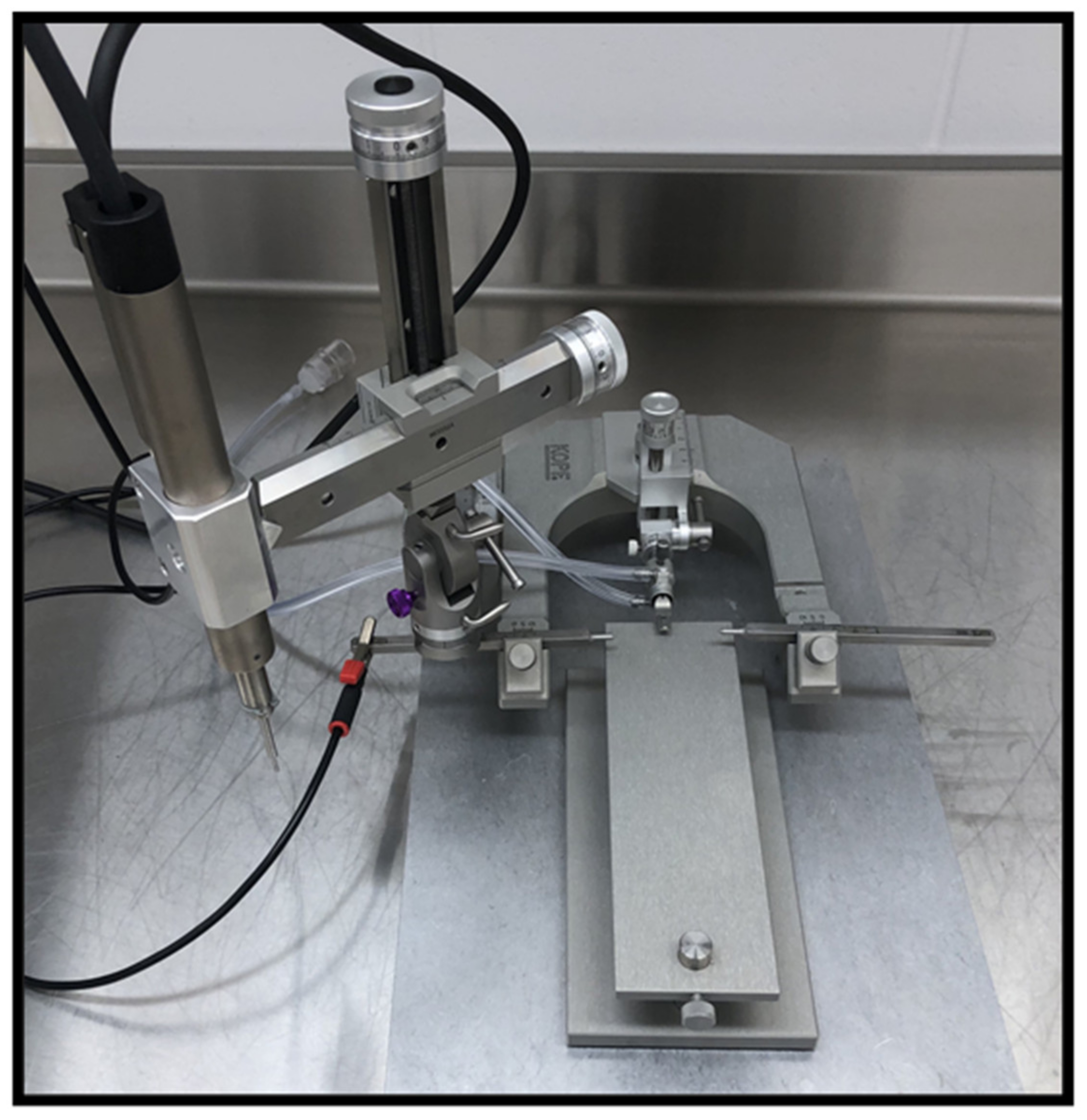
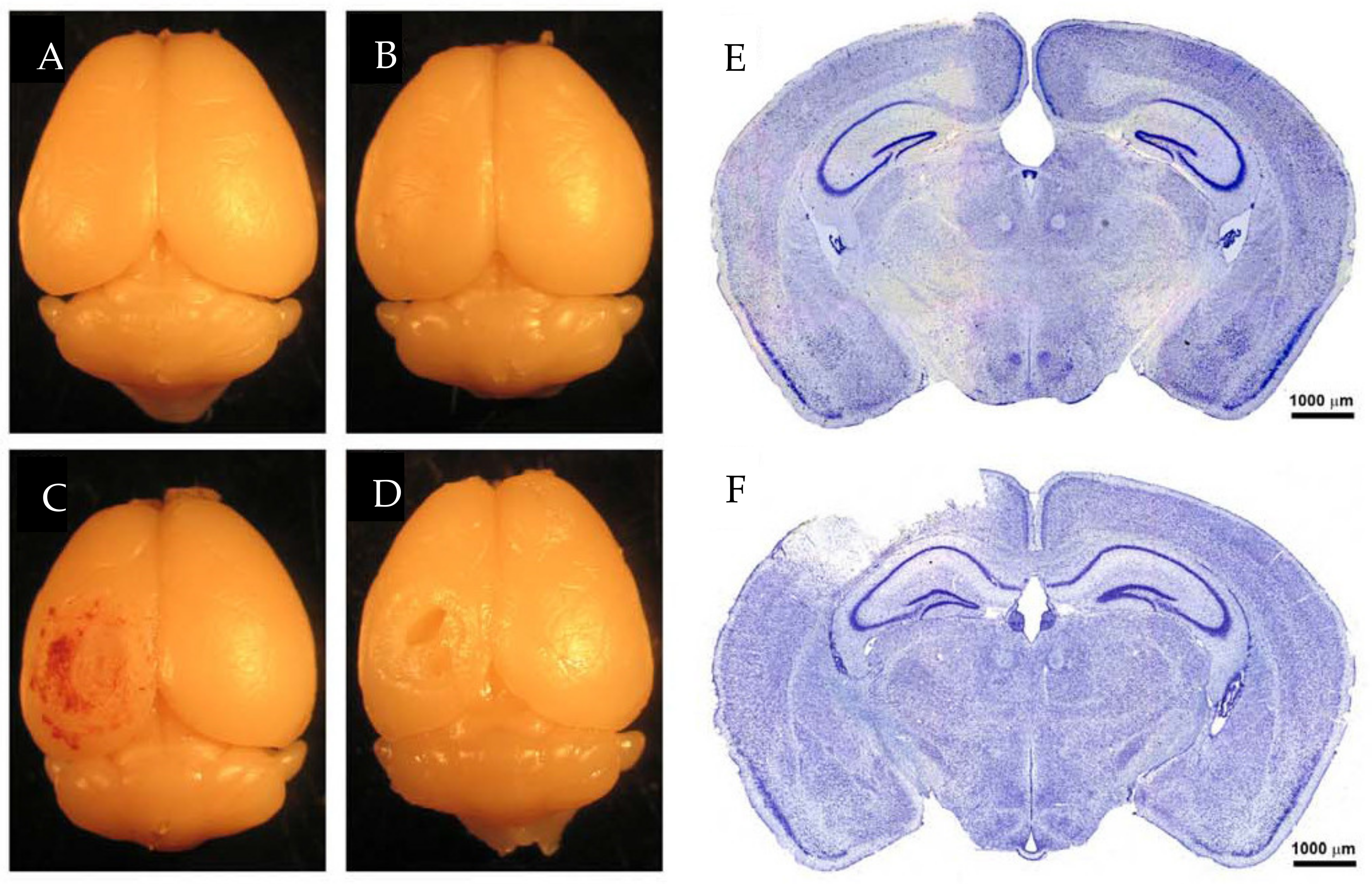
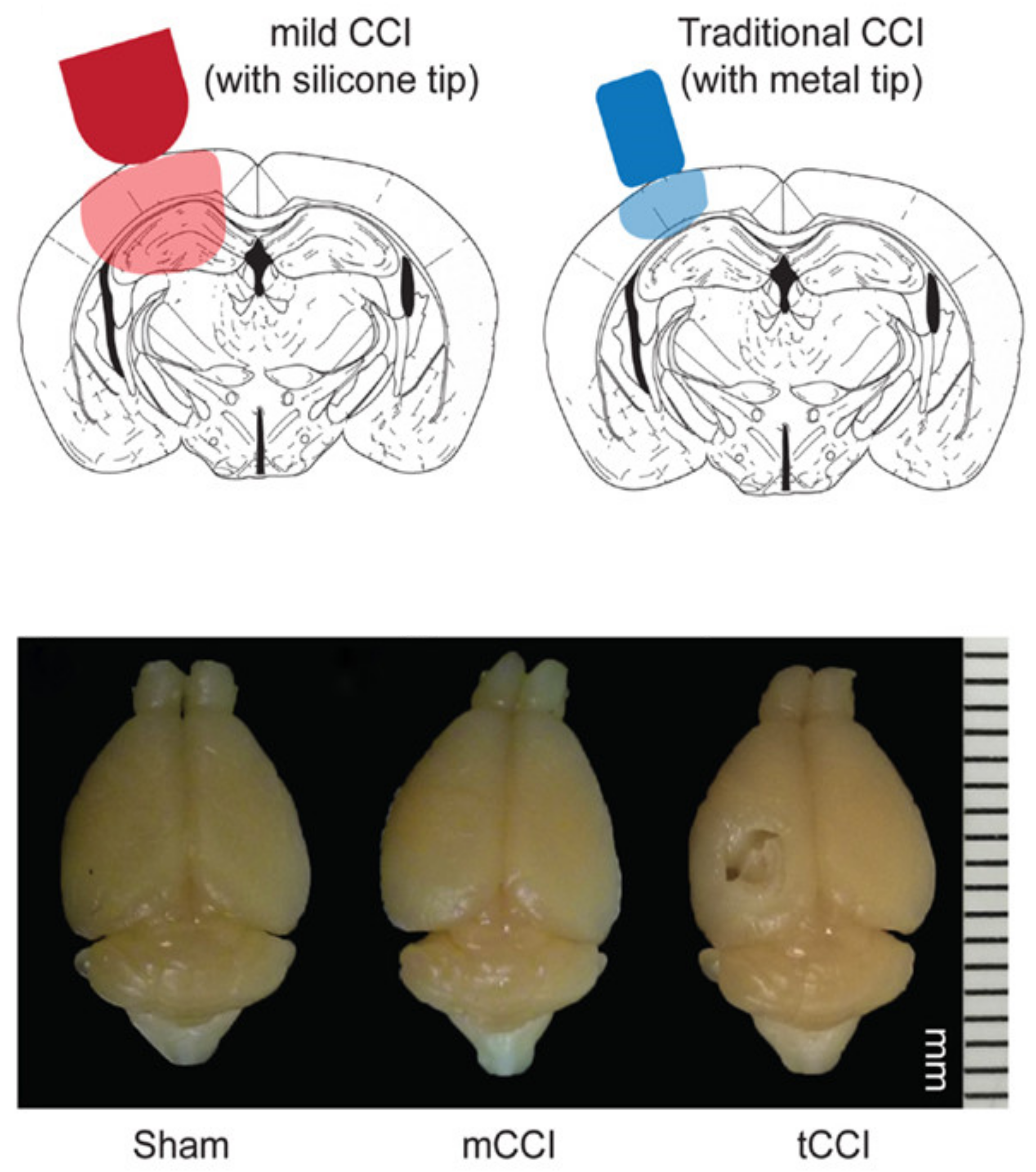
5.7. Controlled Cortical Impact
6. Diffuse TBI
6.1. Marmarou Weight Drop Model
6.2. Modified Marmarou Weight Drop Model
6.3. Modified Controlled Cortical Impact
6.4. Repeated Mild TBI
7. Non-Impact TBI
7.1. Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA)
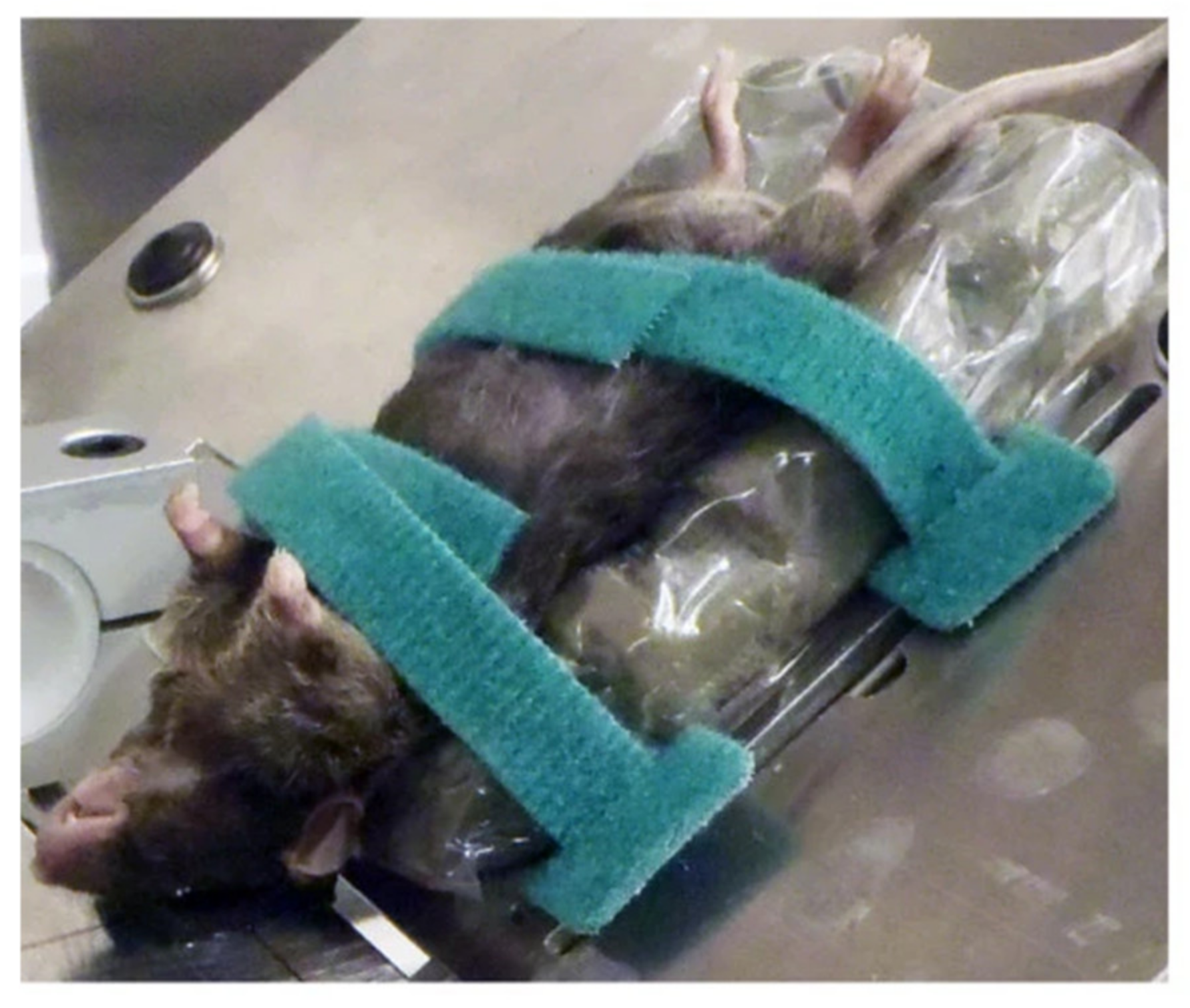
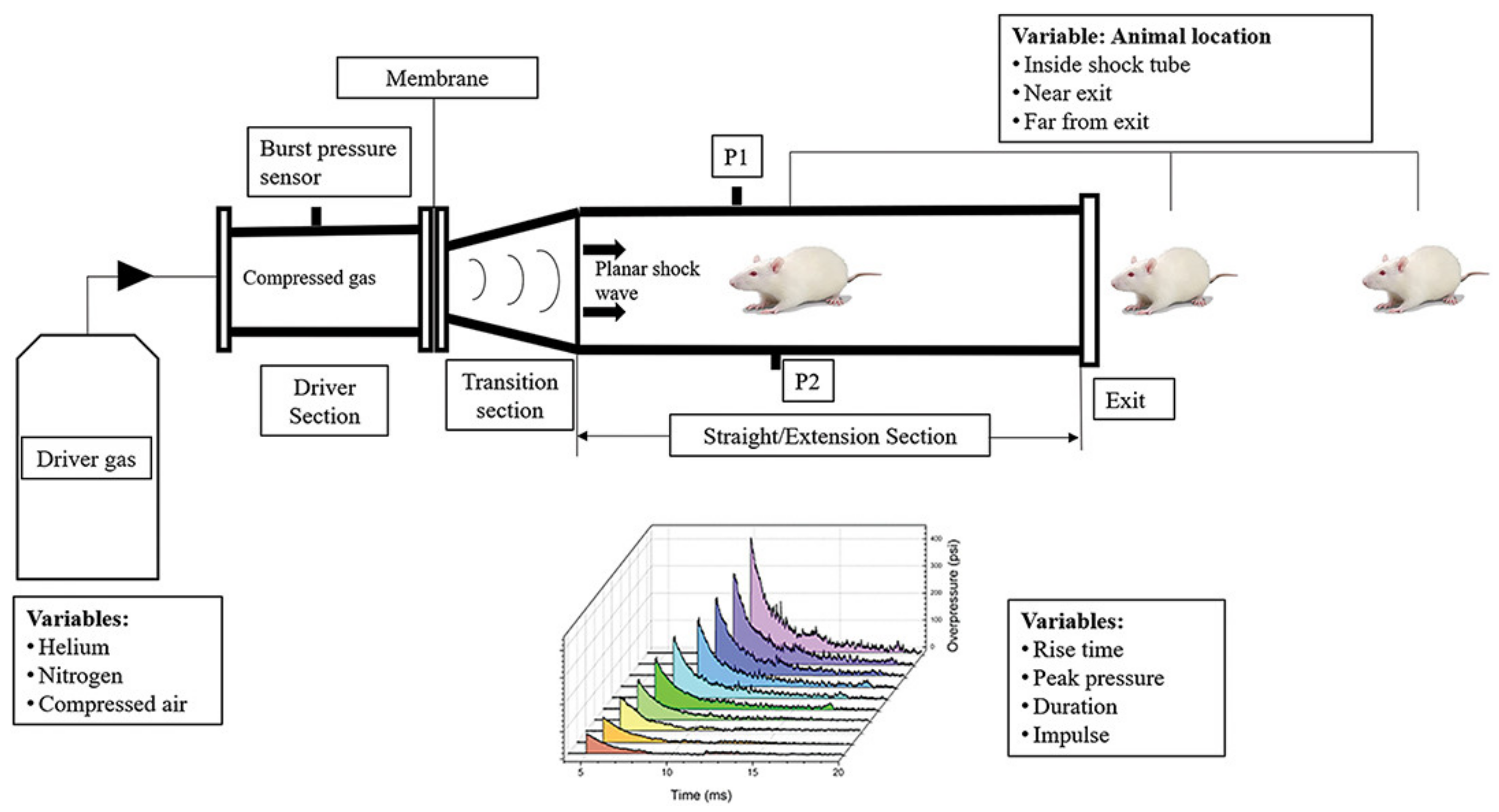
7.2. Blast Injury Model
8. Behavioral Analysis
9. Spatial Learning and Memory Tasks
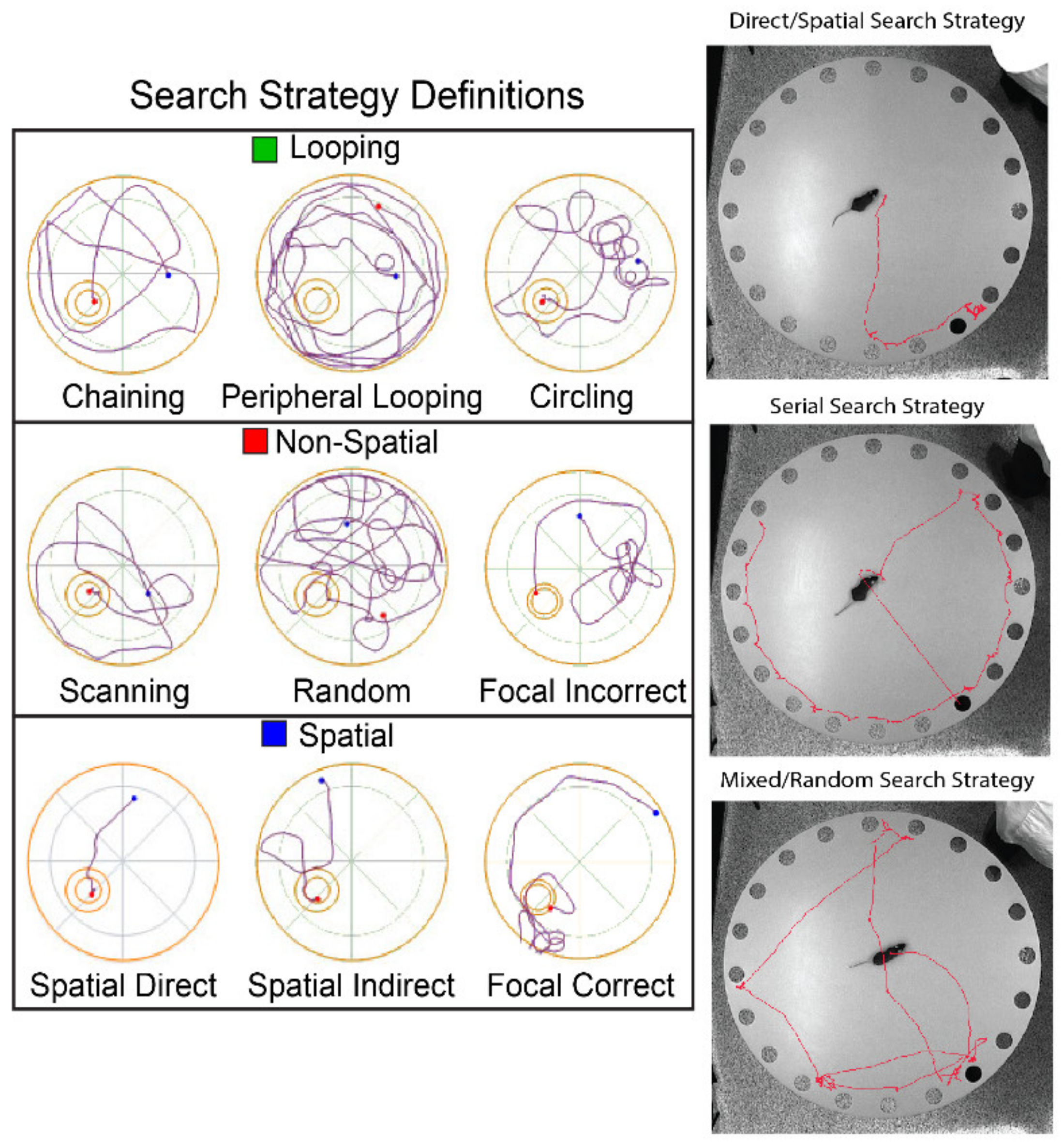
9.1. Morris Water Maze and Barnes Maze
9.2. Radial Arm Maze
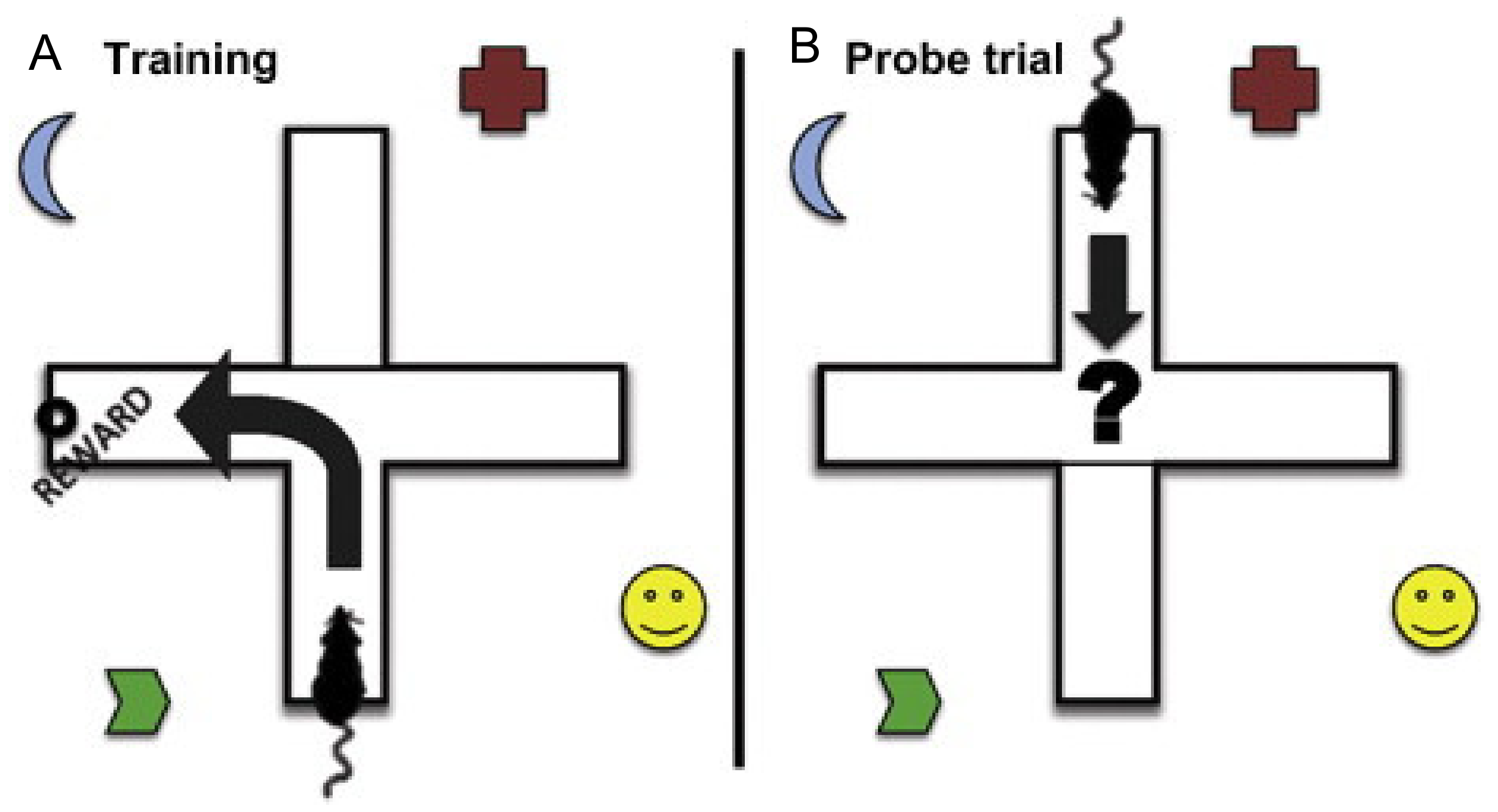
9.3. T and Y Maze
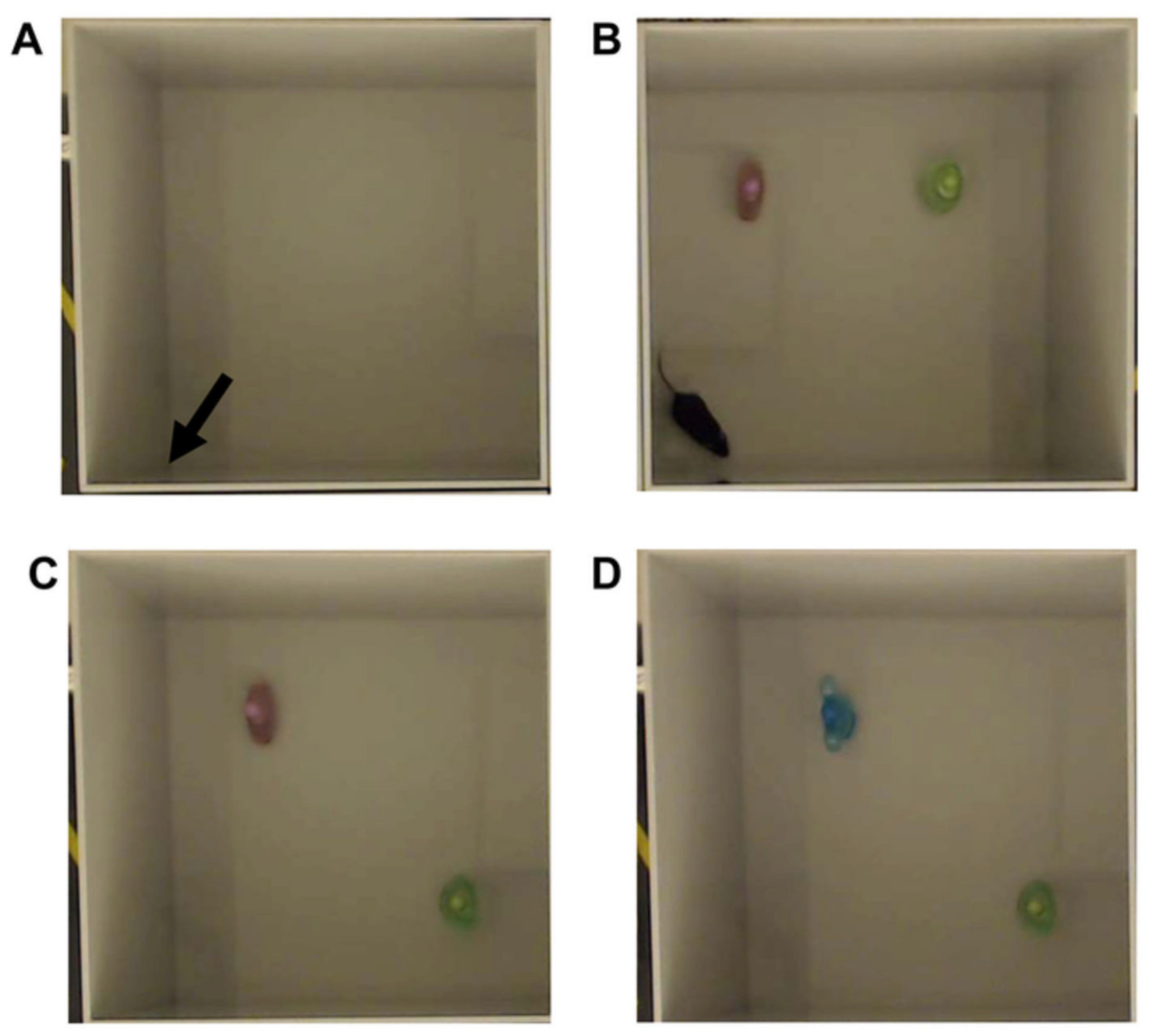
9.4. Novel Object Location Test
10. Nonspatial Learning and Memory
Spatial Learning Task Variations for Nonspatial Learning
11. Emotional Tests
11.1. Forced Swim Test
11.2. Dark/Light Avoidance Test
11.3. Open Field Test
11.4. Resident Intruder Test
12. Motor Coordination
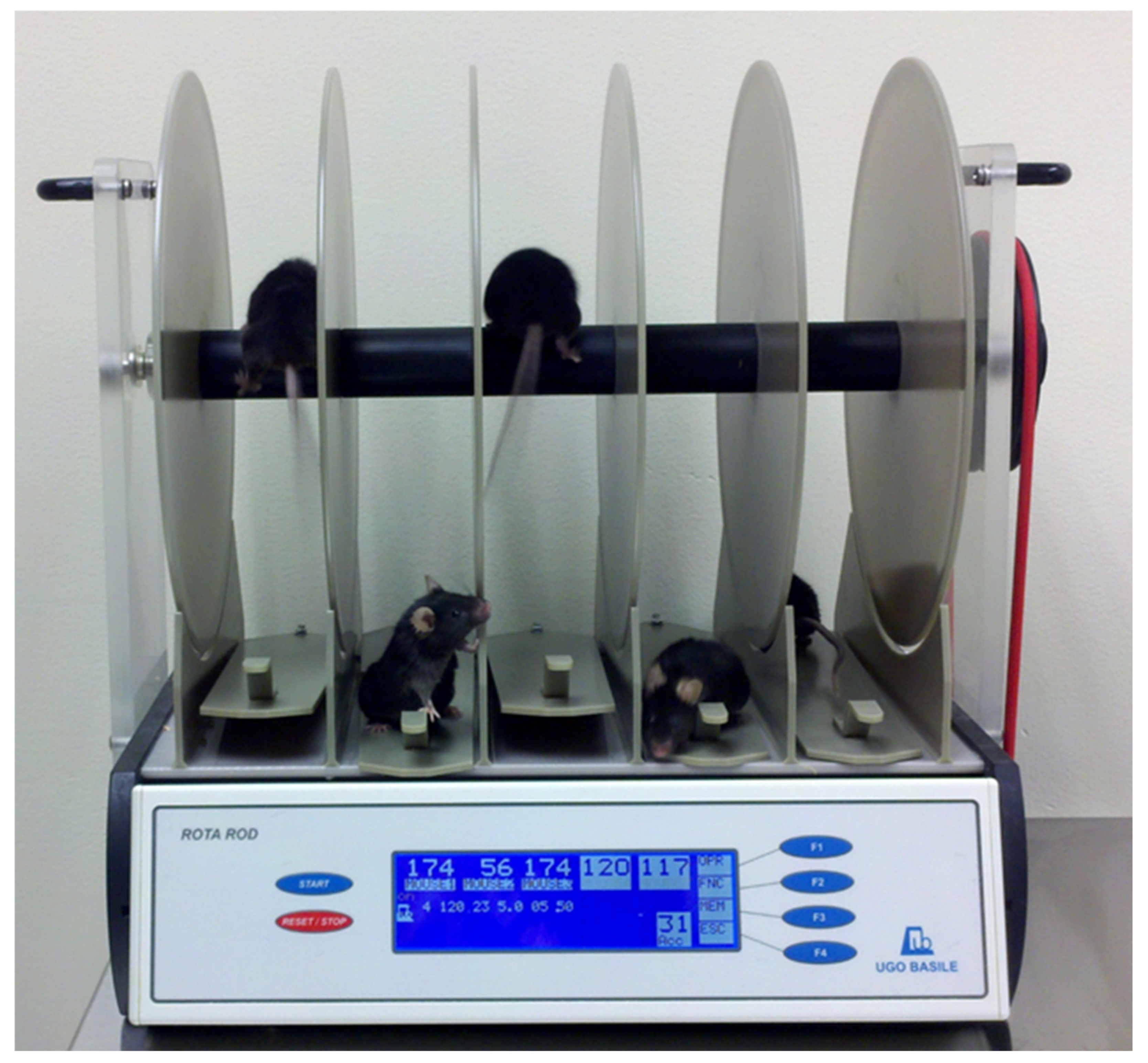
12.1. Rotarod
12.2. Open Field Test
12.3. Footprint Pattern Assay
13. Comparison of TBI Animal Models to Human Injury
14. Perspective and Recommendations for the Nanotheranostics Researcher
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Buki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Najem, D.; Rennie, K.; Ribecco-Lutkiewicz, M.; Ly, D.; Haukenfrers, J.; Liu, Q.; Nzau, M.; Fraser, D.D.; Bani-Yaghoub, M. Traumatic brain injury: Classification, models, and markers. Biochem. Cell Biol. 2018, 96, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Long, J.B.; Bentley, T.L.; Wessner, K.A.; Cerone, C.; Sweeney, S.; Bauman, R.A. Blast overpressure in rats: Recreating a battlefield injury in the laboratory. J. Neurotrauma 2009, 26, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, D.R.; Cheng, W.H.; McInnes, K.A.; Martens, K.M.; Carr, M.; Wilkinson, A.; Fan, J.; Robert, J.; Hayat, A.; Cripton, P.A.; et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): A novel, surgery-free model of traumatic brain injury. Mol. Neurodegener. 2014, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef] [PubMed]
- When Will a Clinical Trial for Traumatic Brain Injury Succeed? 2016. Available online: https://aansneurosurgeon.org/will-clinical-trial-traumatic-brain-injury-succeed/ (accessed on 15 June 2021).
- Howard, R.B.; Sayeed, I.; Stein, D.G. Suboptimal Dosing Parameters as Possible Factors in the Negative Phase III Clinical Trials of Progesterone for Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Dixon, C.E.; Mondello, S.; Wang, K.K.K.; Lafrenaye, A.; Bramlett, H.M.; Dietrich, W.D.; Hayes, R.L.; Shear, D.A.; Gilsdorf, J.S.; et al. Multi-Center Pre-clinical Consortia to Enhance Translation of Therapies and Biomarkers for Traumatic Brain Injury: Operation Brain Trauma Therapy and Beyond. Front. Neurol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Malec, J.F.; Brown, A.W.; Leibson, C.L.; Flaada, J.T.; Mandrekar, J.N.; Diehl, N.N.; Perkins, P.K. The mayo classification system for traumatic brain injury severity. J. Neurotrauma 2007, 24, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, B.Y.; Sewalt, C.A.; Ercole, A.; Akerlund, C.; Nelson, D.; Maas, A.I.R.; Menon, D.; Lingsma, H.F.; Steyerberg, E.W.; Collaborative European NeuroTrauma Effectiveness Research for Traumatic Brain Injury Collaborators. Toward a New Multi-Dimensional Classification of Traumatic Brain Injury: A Collaborative European NeuroTrauma Effectiveness Research for Traumatic Brain Injury Study. J. Neurotrauma 2020, 37, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, C.N.; Roberts, K.N.; Higgins, E.K.; Bachstetter, A.D. A Systematic Review of Closed Head Injury Models of Mild Traumatic Brain Injury in Mice and Rats. J. Neurotrauma 2019, 36, 1683–1706. [Google Scholar] [CrossRef] [PubMed]
- Griffen, J.; Hanks, R. Cognitive and Behavioral Outcomes from Traumatic Brain Injury. In Handbook on the Neuropsychology of Traumatic Brain Injury; Springer: New York, NY, USA, 2014; pp. 25–45. [Google Scholar]
- Gupte, R.; Brooks, W.; Vukas, R.; Pierce, J.; Harris, J. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J. Neurotrauma 2019, 36, 3063–3091. [Google Scholar] [CrossRef] [PubMed]
- Alam Bony, B.; Kievit, F.M. A Role for Nanoparticles in Treating Traumatic Brain Injury. Pharmaceutics 2019, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Nguyen, D.T.; Kodibagkar, V.D.; Stabenfeldt, S.E. Nanoparticle-Based Therapeutics for Brain Injury. Adv. Healthc. Mater. 2018, 7, 1700668. [Google Scholar] [CrossRef] [PubMed]
- Onyeje, C.; Lavik, E. Highlighting the usage of polymeric nanoparticles for the treatment of traumatic brain injury: A review study. Neurochem. Int. 2021, 147, 105048. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Teasdale, G.; Maas, A.I.R.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow Coma Scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Sternbach, G.L. The Glasgow coma scale. J. Emerg. Med. 2000, 19, 67–71. [Google Scholar] [CrossRef]
- Prins, M.; Greco, T.; Alexander, D.; Giza, C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Model. Mech. 2013, 6, 1307–1315. [Google Scholar] [CrossRef]
- Nissen, J.J.; Jones, P.A.; Signorini, D.F.; Murray, L.S.; Teasdale, G.M.; Miller, J.D. Glasgow head injury outcome prediction program: An independent assessment. J. Neurol. Neurosurg. Psychiatry 1999, 67, 796–799. [Google Scholar] [CrossRef]
- Dong, P.V.; Cremer, O.L. Limitations of the use of the Glasgow Coma Scale in intensive care patients with non-neurological primary disease: A search for alternatives. Crit. Care 2011, 15, P506. [Google Scholar] [CrossRef][Green Version]
- Andriessen, T.M.; Jacobs, B.; Vos, P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell Mol. Med. 2010, 14, 2381–2392. [Google Scholar] [CrossRef]
- Doppenberg, E.M.; Choi, S.C.; Bullock, R. Clinical trials in traumatic brain injury: Lessons for the future. J. Neurosurg. Anesthesiol. 2004, 16, 87–94. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Duchen, M.R. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim. Biophys. Acta 2008, 1777, 953–964. [Google Scholar] [CrossRef]
- Rosenfeld, J.V.; McFarlane, A.C.; Bragge, P.; Armonda, R.A.; Grimes, J.B.; Ling, G.S. Blast-related traumatic brain injury. Lancet Neurol. 2013, 12, 882–893. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, J.; Ma, Y.; Yang, T.; Kuang, Y.; Li, B.; Kang, J. Development of a rat model for studying blast-induced traumatic brain injury. J. Neurol. Sci. 2010, 294, 23–28. [Google Scholar] [CrossRef]
- Chen, Y.; Constantini, S.; Trembovler, V.; Weinstock, M.; Shohami, E. An experimental model of closed head injury in mice: Pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 1996, 13, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Feeney, D.M.; Boyeson, M.G.; Linn, R.T.; Murray, H.M.; Dail, W.G. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981, 211, 67–77. [Google Scholar] [CrossRef]
- Marmarou, A.; Foda, M.A.; van den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef]
- Cooper, G.M.; Mooney, M.P.; Gosain, A.K.; Campbell, P.G.; Losee, J.E.; Huard, J. Testing the critical size in calvarial bone defects: Revisiting the concept of a critical-size defect. Plast. Reconstr. Surg. 2010, 125, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Zhao, Y.; Buza, J.A., III; Li, W.; Wang, W.; Jia, T. Surgicallyinduced mouse models in the study of bone regeneration: Current models and future directions (Review). Mol. Med. Rep. 2017, 15, 1017–1023. [Google Scholar] [CrossRef]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, C.; Arfai, N.; Smid, N.; Feeney, D.M. Decrease and recovery of N-acetylaspartate/creatine in rat brain remote from focal injury. J. Neurotrauma 2001, 18, 241–246. [Google Scholar] [CrossRef]
- Kalish, B.T.; Whalen, M.J. Weight Drop Models in Traumatic Brain Injury. Methods Mol. Biol. 2016, 1462, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Shohami, E.; Sidi, A.; Soffer, D.; Freeman, S.; Cotev, S. Experimental closed head injury in rats: Mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 1988, 16, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Albert-Weissenberger, C.; Varrallyay, C.; Raslan, F.; Kleinschnitz, C.; Siren, A.L. An experimental protocol for mimicking pathomechanisms of traumatic brain injury in mice. Exp. Transl. Stroke Med. 2012, 4, 1. [Google Scholar] [CrossRef]
- Luh, C.; Gierth, K.; Timaru-Kast, R.; Engelhard, K.; Werner, C.; Thal, S.C. Influence of a brief episode of anesthesia during the induction of experimental brain trauma on secondary brain damage and inflammation. PLoS ONE 2011, 6, e19948. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.K.; Vink, R.; Noble, L.; Yamakami, I.; Fernyak, S.; Soares, H.; Faden, A.L. Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience 1989, 28, 233–244. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Noble, L.; Andrews, B.; Faden, A.I. Traumatic brain injury in the rat: Characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma. 1987, 4, 119–134. [Google Scholar] [CrossRef]
- Thompson, H.J.; Lifshitz, J.; Marklund, N.; Grady, M.S.; Graham, D.I.; Hovda, D.A.; McIntosh, T.K. Lateral fluid percussion brain injury: A 15-year review and evaluation. J. Neurotrauma 2005, 22, 42–75. [Google Scholar] [CrossRef]
- Rowe, R.K.; Harrison, J.L.; Ellis, T.W.; Adelson, P.D.; Lifshitz, J. Midline (central) fluid percussion model of traumatic brain injury in pediatric and adolescent rats. J. Neurosurg. Pediatr. 2018, 22, 22–30. [Google Scholar] [CrossRef]
- Dixon, C.E.; Lyeth, B.G.; Povlishock, J.T.; Findling, R.L.; Hamm, R.J.; Marmarou, A.; Young, H.F.; Hayes, R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987, 67, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kabadi, S.V.; Hilton, G.D.; Stoica, B.A.; Zapple, D.N.; Faden, A.I. Fluid-percussion-induced traumatic brain injury model in rats. Nat. Protoc. 2010, 5, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Arundine, M.; Sun, H.S.; Jones, M.; Tymianski, M. Inhibition of caspase-mediated apoptosis by peroxynitrite in traumatic brain injury. J. Neurosci. 2006, 26, 11540–11553. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.; Soares, H.; Smith, D.; McIntosh, T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Bramlett, H.M.; Dietrich, W.D. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002, 103, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Hartings, J.A.; Lu, X.C.; Rolli, M.L.; Dave, J.R.; Tortella, F.C. Characterization of a new rat model of penetrating ballistic brain injury. J. Neurotrauma 2005, 22, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Hartings, J.A.; Lu, X.C.; Rolli, M.L.; Tortella, F.C. Penetrating ballistic-like brain injury in the rat: Differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J. Neurotrauma 2006, 23, 1828–1846. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I.; Wing, I.D.; Davidsson, J.; Plantman, S. A novel mouse model of penetrating brain injury. Front. Neurol. 2014, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, B.; Wilfred, B.S.; Johnson, D.; Gilsdorf, J.S.; Shear, D.A.; Boutte, A.M. Penetrating Ballistic-Like Brain Injury Leads to MicroRNA Dysregulation, BACE1 Upregulation, and Amyloid Precursor Protein Loss in Lesioned Rat Brain Tissues. Front. Neurosci. 2020, 14, 915. [Google Scholar] [CrossRef] [PubMed]
- Risling, M.; Davidsson, J. Experimental animal models for studies on the mechanisms of blast-induced neurotrauma. Front. Neurol. 2012, 3, 30. [Google Scholar] [CrossRef]
- Osier, N.; Dixon, C.E. The Controlled Cortical Impact Model of Experimental Brain Trauma: Overview, Research Applications, and Protocol. Methods Mol. Biol. 2016, 1462, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Alluri, H.; Shaji, C.A.; Davis, M.L.; Tharakan, B. A Mouse Controlled Cortical Impact Model of Traumatic Brain Injury for Studying Blood-Brain Barrier Dysfunctions. Methods Mol. Biol. 2018, 1717, 37–52. [Google Scholar] [CrossRef]
- Dixon, C.E.; Clifton, G.L.; Lighthall, J.W.; Yaghmai, A.A.; Hayes, R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 1991, 39, 253–262. [Google Scholar] [CrossRef]
- Romine, J.; Gao, X.; Chen, J. Controlled cortical impact model for traumatic brain injury. J. Vis. Exp. 2014, e51781. [Google Scholar] [CrossRef]
- Marmarou, C.R.; Prieto, R.; Taya, K.; Young, H.F.; Marmarou, A. Marmarou Weight Drop Injury Model. In Animal Models of Acute Neurological Injuries; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; pp. 393–407. [Google Scholar]
- Xu, L.; Nguyen, J.V.; Lehar, M.; Menon, A.; Rha, E.; Arena, J.; Ryu, J.; Marsh-Armstrong, N.; Marmarou, C.R.; Koliatsos, V.E. Repetitive mild traumatic brain injury with impact acceleration in the mouse: Multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp. Neurol. 2016, 275 Pt 3, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I.; Vink, R.; Zapple, D.N.; Cruz, M.I.; Ahmed, F.; Chang, T.; Fricke, S.T.; Faden, A.I. The pathobiology of moderate diffuse traumatic brain injury as identified using a new experimental model of injury in rats. Neurobiol. Dis. 2004, 17, 29–43. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, H.; Yang, K.H.; Abel, T.; Meaney, D.F. A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice. Front. Neurol. 2014, 5, 100. [Google Scholar] [CrossRef]
- Omalu, B.I.; DeKosky, S.T.; Minster, R.L.; Kamboh, M.I.; Hamilton, R.L.; Wecht, C.H. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005, 57, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hiskens, M.I.; Angoa-Perez, M.; Schneiders, A.G.; Vella, R.K.; Fenning, A.S. Modeling sports-related mild traumatic brain injury in animals—A systematic review. J. Neurosci. Res. 2019, 97, 1194–1222. [Google Scholar] [CrossRef] [PubMed]
- Sauerbeck, A.D.; Fanizzi, C.; Kim, J.H.; Gangolli, M.; Bayly, P.V.; Wellington, C.L.; Brody, D.L.; Kummer, T.T. modCHIMERA: A novel murine closed-head model of moderate traumatic brain injury. Sci. Rep. 2018, 8, 7677. [Google Scholar] [CrossRef]
- Cheng, W.H.; Martens, K.M.; Bashir, A.; Cheung, H.; Stukas, S.; Gibbs, E.; Namjoshi, D.R.; Button, E.B.; Wilkinson, A.; Barron, C.J.; et al. CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice. Alzheimers Res. Ther. 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Aravind, A.; Ravula, A.R.; Chandra, N.; Pfister, B.J. Behavioral Deficits in Animal Models of Blast Traumatic Brain Injury. Front. Neurol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Garman, R.H.; Jenkins, L.W.; Switzer, R.C., 3rd; Bauman, R.A.; Tong, L.C.; Swauger, P.V.; Parks, S.A.; Ritzel, D.V.; Dixon, C.E.; Clark, R.S.; et al. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma 2011, 28, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kaneko, Y.; Bae, E.; Stahl, C.E.; Wang, Y.; van Loveren, H.; Sanberg, P.R.; Borlongan, C.V. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009, 1287, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; McDonald, S.J.; Corrigan, F.; Semple, B.D.; Salberg, S.; Zamani, A.; Jones, N.C.; Mychasiuk, R. Clinical Relevance of Behavior Testing in Animal Models of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2381–2400. [Google Scholar] [CrossRef]
- Shinohara, Y.; Hosoya, A.; Yamasaki, N.; Ahmed, H.; Hattori, S.; Eguchi, M.; Yamaguchi, S.; Miyakawa, T.; Hirase, H.; Shigemoto, R. Right-hemispheric dominance of spatial memory in split-brain mice. Hippocampus 2012, 22, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014, 55, 310–332. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents-methodological consideration. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.E.; Hosseini, A.H.; McDonald, M.P. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 2009, 198, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Morellini, F. Spatial memory tasks in rodents: What do they model? Cell Tissue Res. 2013, 354, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.L.; Marchette, S.A.; Furman, A.J. A Mechanistic Approach to Individual Differences in Spatial Learning, Memory, and Navigation. In Psychology of Learning and Motivation; Academic Press: Cambridge, MA, USA, 2013; pp. 223–259. [Google Scholar]
- Skelton, R.W.; Ross, S.P.; Nerad, L.; Livingstone, S.A. Human spatial navigation deficits after traumatic brain injury shown in the arena maze, a virtual Morris water maze. Brain Inj. 2006, 20, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Tarudji, A.W.; Gee, C.C.; Romereim, S.M.; Convertine, A.J.; Kievit, F.M. Antioxidant thioether core-crosslinked nanoparticles prevent the bilateral spread of secondary injury to protect spatial learning and memory in a controlled cortical impact mouse model of traumatic brain injury. Biomaterials 2021, 272, 120766. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Osugi, T.; Satoh, H.; McIntosh, T.K.; Nabeshima, T. Pre-Injury magnesium treatment prevents traumatic brain injury-induced hippocampal ERK activation, neuronal loss, and cognitive dysfunction in the radial-arm maze test. J. Neurotrauma 2005, 22, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Park, H.K.; Kim, T.W.; Ji, E.S.; Lee, J.M.; Choi, H.S.; Kim, M.Y.; Kim, Y.P. Neuroprotective Effects of Bone Marrow Stromal Cell Transplantation in Combination With Treadmill Exercise Following Traumatic Brain Injury. Int. Neurourol. J. 2016, 20, S49–S56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Floresco, S.B.; Seamans, J.K.; Phillips, A.G. Selective Roles for Hippocampal, Prefrontal Cortical, and Ventral Striatal Circuits in Radial-Arm Maze Tasks With or Without a Delay. J. Neurosci. 1997, 17, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Darwish, H.; Hasan, H. Y-Shaped Maze to Test Spontaneous Object Recognition and Temporal Order Memory After Traumatic Brain Injury. Methods Mol. Biol. 2019, 2011, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Pioli, E.Y.; Gaskill, B.N.; Gilmour, G.; Tricklebank, M.D.; Dix, S.L.; Bannerman, D.; Garner, J.P. An automated maze task for assessing hippocampus-sensitive memory in mice. Behav. Brain Res. 2014, 261, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kobayashi, Y.; Goto, H.; Itohara, S. An Automated T-maze Based Apparatus and Protocol for Analyzing Delay- and Effort-based Decision Making in Free Moving Rodents. J. Vis. Exp. 2018, e57895. [Google Scholar] [CrossRef]
- Davis, K.E.; Burnett, K.; Gigg, J. Water and T-maze protocols are equally efficient methods to assess spatial memory in 3xTg Alzheimer’s disease mice. Behav. Brain Res. 2017, 331, 54–66. [Google Scholar] [CrossRef]
- Farr, S.A.; Niehoff, M.L.; Kumar, V.B.; Roby, D.A.; Morley, J.E. Inhibition of Glycogen Synthase Kinase 3beta as a Treatment for the Prevention of Cognitive Deficits after a Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1869–1875. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. Spatial Memory: Assessment in Animals. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Wolf, A.; Bauer, B.; Abner, E.L.; Ashkenazy-Frolinger, T.; Hartz, A.M. A Comprehensive Behavioral Test Battery to Assess Learning and Memory in 129S6/Tg2576 Mice. PLoS ONE 2016, 11, e0147733. [Google Scholar] [CrossRef]
- Hattiangady, B.; Mishra, V.; Kodali, M.; Shuai, B.; Rao, X.; Shetty, A.K. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front. Behav. Neurosci. 2014, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Dolan, R.J. Differential Neural Responses during Performance of Matching and Nonmatching to Sample Tasks at Two Delay Intervals. J. Neurosci. 1999, 19, 5066–5073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Brody, D.L.; Holtzman, D.M. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 2006, 197, 330–340. [Google Scholar] [CrossRef]
- Iaria, G.; Petrides, M.; Dagher, A.; Pike, B.; Bohbot, V.D. Cognitive Strategies Dependent on the Hippocampus and Caudate Nucleus in Human Navigation: Variability and Change with Practice. J. Neurosci. 2003, 23, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Popovitz, J.; Mysore, S.P.; Adwanikar, H. Long-Term Effects of Traumatic Brain Injury on Anxiety-Like Behaviors in Mice: Behavioral and Neural Correlates. Front. Behav. Neurosci. 2019, 13, 6. [Google Scholar] [CrossRef]
- Juengst, S.B.; Terhorst, L.; Kew, C.L.; Wagner, A.K. Variability in daily self-reported emotional symptoms and fatigue measured over eight weeks in community dwelling individuals with traumatic brain injury. Brain Inj. 2019, 33, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, e3638. [Google Scholar] [CrossRef] [PubMed]
- Sunal, R.; Gümüşel, B.; Kayaalp, S.O. Effect of changes in swimming area on results of “behavioral despair test”. Pharmacol. Biochem. Behav. 1994, 49, 891–896. [Google Scholar] [CrossRef]
- Arrant, A.E.; Schramm-Sapyta, N.L.; Kuhn, C.M. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013, 256, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, e52434. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Coppens, C.M.; de Boer, S.F.; Buwalda, B.; Meerlo, P.; Timmermans, P.J. The resident-intruder paradigm: A standardized test for aggression, violence and social stress. J. Vis. Exp. 2013, e4367. [Google Scholar] [CrossRef] [PubMed]
- De Jong, T.R.; Beiderbeck, D.I.; Neumann, I.D. Measuring virgin female aggression in the female intruder test (FIT): Effects of oxytocin, estrous cycle, and anxiety. PLoS ONE 2014, 9, e91701. [Google Scholar] [CrossRef]
- Yang, S.H.; Gustafson, J.; Gangidine, M.; Stepien, D.; Schuster, R.; Pritts, T.A.; Goodman, M.D.; Remick, D.G.; Lentsch, A.B. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J. Surg. Res. 2013, 184, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.T.; Longhi, L.; Saatman, K.E.; Conte, V.; Stocchetti, N.; McIntosh, T.K. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004, 28, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Osmon, K.J.; Vyas, M.; Woodley, E.; Thompson, P.; Walia, J.S. Battery of Behavioral Tests Assessing General Locomotion, Muscular Strength, and Coordination in Mice. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.S.; Bartos, I.; Marka, Z.; Akay, T.; Marka, S.; Mann, R.S. Quantification of gait parameters in freely walking rodents. BMC Biol. 2015, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; Shieh, J. Chapter 2—Animal Behavior. In Guide to Research Techniques in Neuroscience; Academic Press: San Diego, CA, USA, 2015; pp. 39–71. [Google Scholar]
- Kappos, E.A.; Sieber, P.K.; Engels, P.E.; Mariolo, A.V.; D’Arpa, S.; Schaefer, D.J.; Kalbermatten, D.F. Validity and reliability of the CatWalk system as a static and dynamic gait analysis tool for the assessment of functional nerve recovery in small animal models. Brain Behav. 2017, 7, e00723. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/0470119055 (accessed on 15 June 2021).
- Buhlman, L.M.; Krishna, G.; Jones, T.B.; Thomas, T.C. Drosophila as a model to explore secondary injury cascades after traumatic brain injury. Biomed. Pharm. 2021, 142, 112079. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, A. A new zebrafish model to study the link between TBI and dementia. Lab. Animal. 2021, 50, 65. [Google Scholar] [CrossRef]

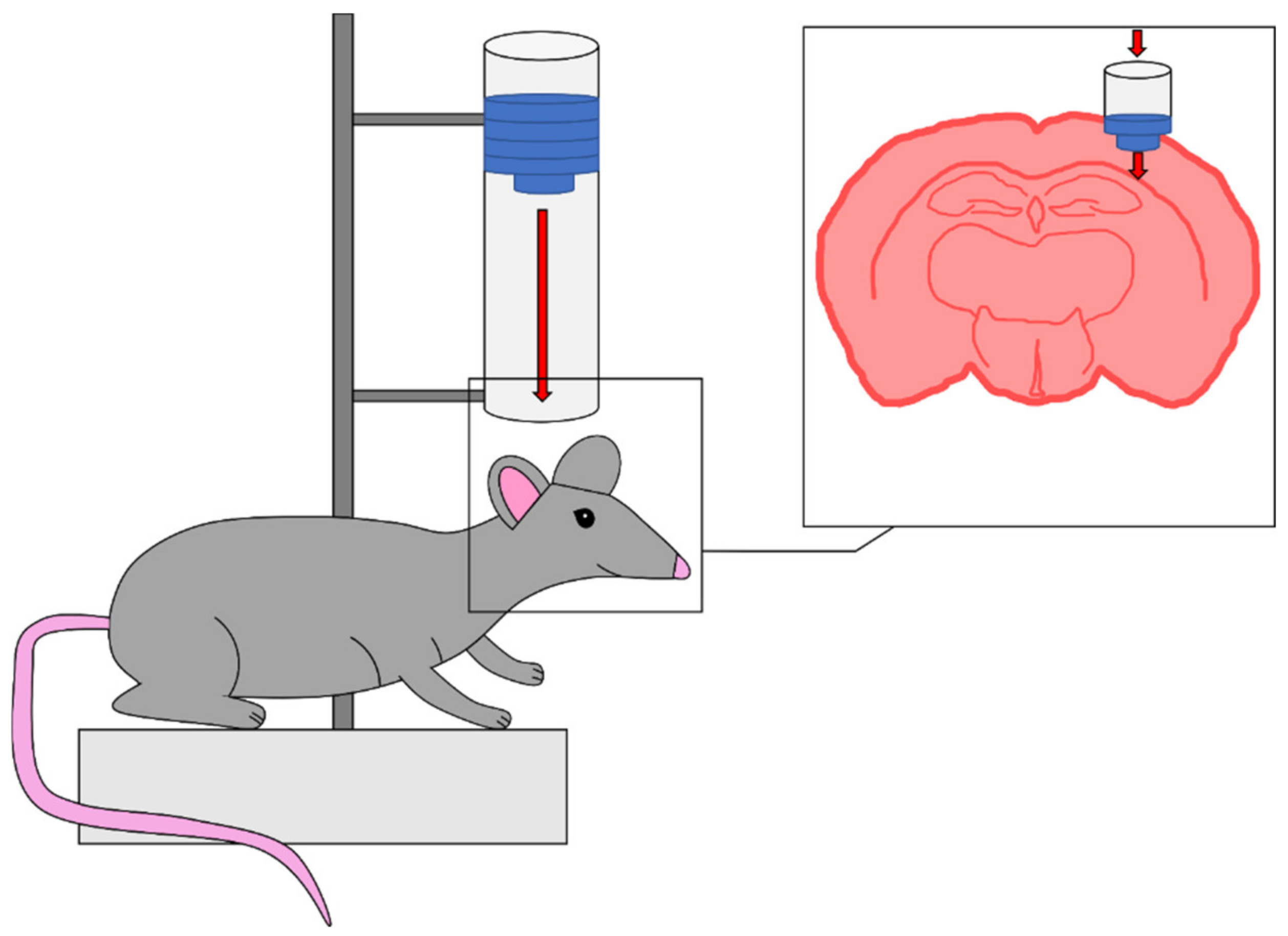

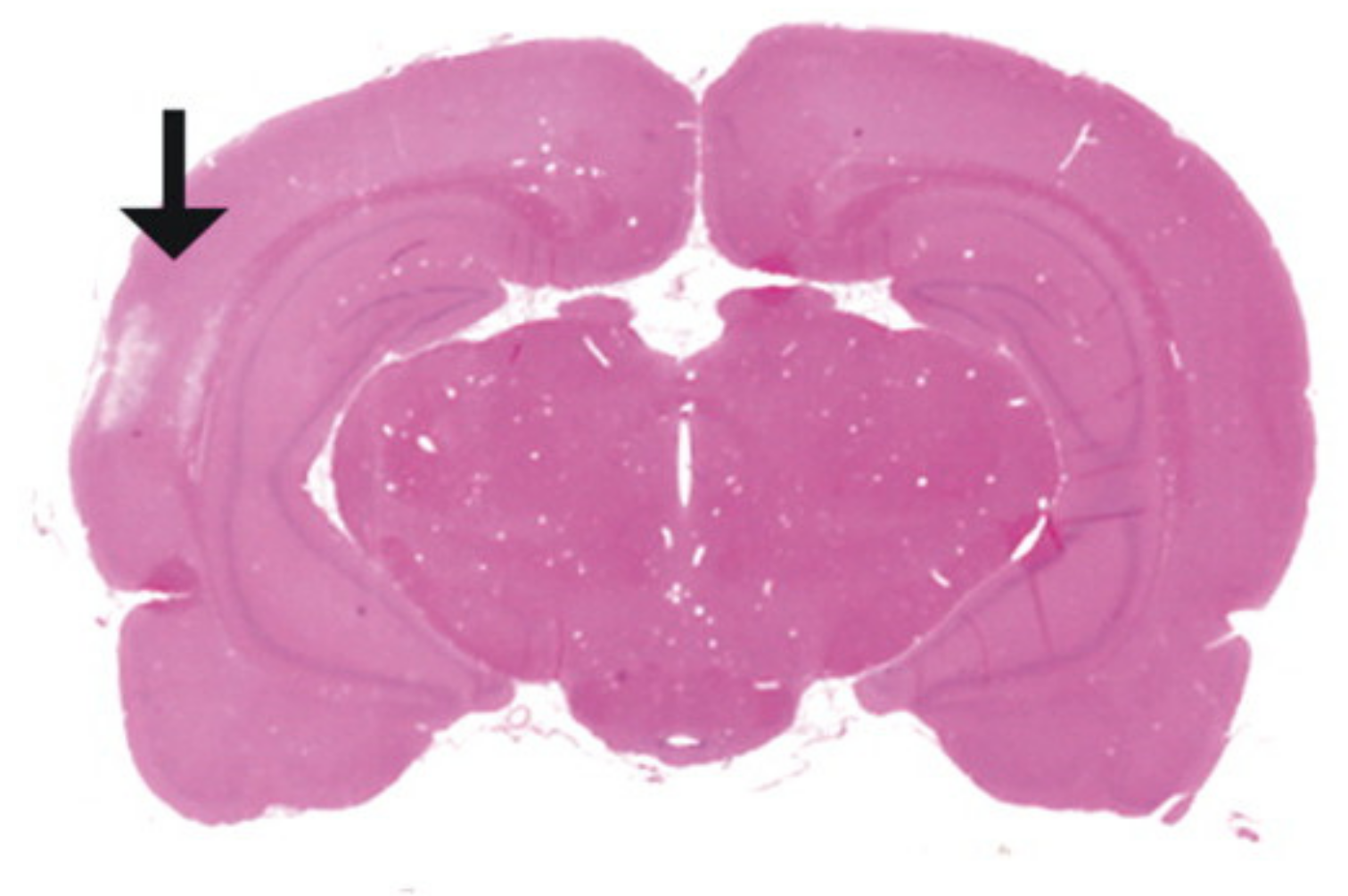
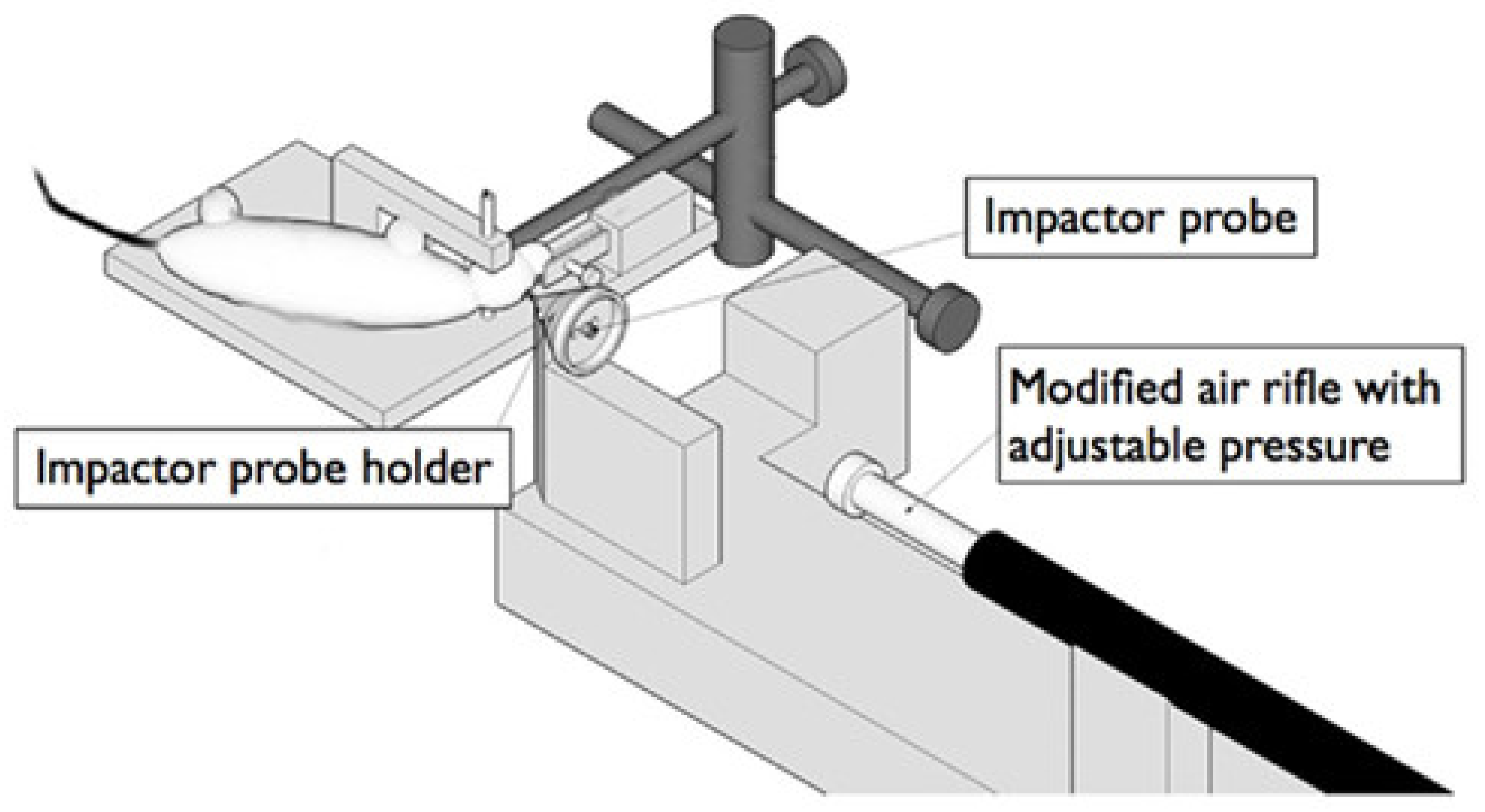






| Response | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Eye | None | To Pain | To Speech | Spontaneous | N/A | N/A |
| Verbal | None | Incomprehensible Sounds | Inappropriate Words | Confused Conversation | Oriented | N/A |
| Motor | None | Extension (Decerebrate) | Abnormal Flexion (Decorticate) | Withdrawal (Normal Flexion) | Localizes Pain | Obeys Commands |
| Behavioral Task | Data Type | Description | Expected Result (Compared to Control Group) | Meaning of Results |
|---|---|---|---|---|
| Spatial Learning and Memory | ||||
| MWM | Latency to Platform (s) | The amount of time it takes an animal to escape the maze. | TBI should take longer | Decreased latency shows a higher amount of spatial learning. |
| Percent in Quadrant (% or fraction) | The percentage of time spent in a specific quadrant over the total time in maze. | TBI should spend less time near the escape and more time in quadrants away from the escape | High percentages in the quadrant of the platform show higher learning; however, high percentages in the reversal week in the former escape quadrant show an inability to relearn. | |
| Percent of Time in the Outer Annulus (% or fraction) | The percentage of time spent in the outer annulus of the maze. | TBI should spend more time in the outer annulus | Higher percentages in the outer annulus show thigmotaxis, which shows no learning or confusion. | |
| Path Length (cm) | The length of the path made while moving through the maze. | TBI should have a large path length | Higher path length shows more movement and a lower understanding of how to escape the maze and thus, less ability to learn and memorize the maze. | |
| Cumulative Distance from the Platform (cm or m) | The distance, measured every few seconds or milliseconds, from the platform. | TBI should have a larger cumulative distance | Longer distances show a lack of spatial or non-spatial search strategies, which indicate worse learning or memory. | |
| First Bearing (Degrees or radians) | The angle between the first movement of the animal and a direct line to the platform. | TBI should have a larger degree of first bearing | Higher degree of first bearing shows a deficit in memory of where the platform lies spatially. | |
| Search Strategy | The strategy (i.e., spatial, nonspatial, or random) the animal uses to find the platform. | TBI should use more random or nonspatial strategies | Higher use of random search strategies indicates lower learning and memory while the inverse of higher spatial strategies shows an increase in learning and memory. | |
| Probe Trial Time in Target Quadrant (% or fraction) | The time spent in the quadrant where the platform should be as a percentage of total time. | TBI should spend less time in the target quadrant | Higher percentage of time in the target quadrant shows an increased ability in learning and memory of the maze. | |
| Probe Trial Platform Crossings (Frequency) | The number of times the area where the platform should be is passed over. | TBI should pass over less | Higher frequency of platform crossing shows better learning and memory. | |
| Swim Speed (m/s) | The velocity at which animals are travelling in the maze | TBI should be relatively similar in order to rule out motor deficits; however, this is specific to post-acute phase testing | Lower swim speed shows either a motor coordination deficit, or, potentially but unlikely, a lower ability to learn and remember the maze. These should, in most circumstances, be very similar. | |
| BM | Primary Latency (s) | The amount of time it takes an animal to find the escape and enter (head only). | TBI should take longer | Lower primary latencies show a better understanding of the escape and how to reach it via nonspatial navigation or spatial navigation, depending on search strategy. |
| Total Latency (s) | The amount of time it takes an animal to find and fully enter the escape hole. | TBI should take longer | Lower total latency shows learning and memory into which method will provide escape the quickest. | |
| Reference Errors (Frequency) | The number of times an animal enters a non-escape hole with its head. | TBI should have more errors | Higher reference errors show a decreased ability to learn and memorize the maze. | |
| Working Errors (Frequency) | The number of times an animal makes a reference error after having visited that hole before. | TBI should have more errors | Higher working errors show a decreased understanding of the maze along with potential confusion regarding visited areas, showing a lack of memory. | |
| Perseverative Errors (Frequency) | The number of times an animal repeats searching the same hole before moving on to another. | TBI should have more errors | Higher perseverative errors show a lack of learning and memory of places previously visited and may, in reversal trials, indicate an inability to relearn. | |
| Primary Errors (Frequency) | The number of times an animal enters a non-escape hole with its head before finding the escape hole. | TBI should have more errors | Higher primary errors indicate deficits in learning and memory of the maze. | |
| Total Errors (Frequency) | The number of times an animal enters a non-escape hole with its head before entering the escape hole with its whole body. | TBI should have more errors | Higher total errors indicate deficits in learning and memory of escape of the maze, or, when combined with low primary latency, more curiosity from the animals, indicating comfort in the maze. | |
| Hole Deviation Score | The number of non-escape hole visits between the first visited hole and the escape. | TBI should have a higher score | Higher hole deviation scores show a lack of learning and memory when related to finding the correct path in the maze. Spatial learning will show lower scores than nonspatial learning. | |
| Primary Path Length (cm) | The distance an animal has travelled before reaching the escape hole with only its head. | TBI should have a longer distance | Path length, in either context, shows a decreased ability to understand and memorize the maze. | |
| Total Path Length (cm) | The distance an animal has travelled before entering the escape hole with its whole body. | TBI should have a longer distance | Path length, in either context, shows a decreased ability to understand and memorize the maze. | |
| Search Strategy | The strategy (i.e., direct/spatial, serial, or mixed/random) the animal uses to find the escape hole. | TBI should use more mixed/random strategies and fewer direct/spatial strategies | Higher use of mixed/random search strategies show a decreased ability to learn the maze; however, an increase in serial strategies after a large number of spatial strategies show complacency within the maze | |
| Velocity (cm/s) | The change in distance over time at which animals are travelling in the maze. | TBI should be similar during chronic phase, acute phase measurements may be lower for TBI | Lower velocity can indicate motor coordination issues within the maze. These should stay relatively similar throughout both weeks of trials. | |
| RAM | Errors (Frequency) | For delayed test, the number of entries into non-baited arms. For the non-delayed, re-entries into the arms entered previously that trial. | TBI should have more errors | Higher frequency of errors shows a lack of memory, |
| Across-Phase Error (Frequency) | Entry to an arm previously entered during the training phase (delayed test only). | TBI should have more errors | Higher frequency of these errors shows a poor ability to learn from the training phase and thus a worse long-term memory, | |
| Within-Phase Error (Frequency) | Entry into an arm entered within the test phase (delayed test only). | TBI should have more errors | Higher frequency of these errors shows a poor ability to remember what has been visiting, showing a worse short-term memory, | |
| Baited Arm Re-entry (Frequency) | A second entry into an arm that had been baited at the beginning of the trial but was already discovered (non-delayed test only). | TBI should have more errors | Higher re-entries of this type show a lack of learning. | |
| Non-baited Arm Re-entry (Frequency) | A second entry into an arm that was not baited at the beginning of the trial but was already discovered (non-delayed test only). | TBI should have more errors | Higher re-entries of this type show a lack of memory. | |
| First Latency (s) | The time it takes for the animal to first visit a baited or non-baited food cup. | TBI should take longer | Higher first latency shows a hesitancy to explore the maze and potential deficits in memory or learning. This may also indicate a nonperformer. | |
| Total Latency (s) | The time it takes for the animal to retrieve all food pellets. | TBI should take longer | Higher total latency shows a lack of learning and memory. | |
| T and Y Maze | Time Spent in Novel Arm (% or fraction) | The amount of time the animal spends in the opened arm during the second trial (alternating T/Y maze only). | TBI should spend about equal time exploring both arms | A lower percentage of time spent in the novel arm shows memory deficits. |
| Forced Alternation (% or fraction) | The percentage or fraction of animals that enter the novel arm first during the second trial (alternating T/Y maze only). | TBI should enter the novel arm less | A lower percentage of forced alternation shows a lack of learning. | |
| Place Versus Response Learning | When the direction of the entrance arm is switched, the animal will either use spatial learning and turn toward goal or nonspatial learning and turn the direction turned during training. | TBI should more often use nonspatial learning and turn in the direction it did during training | This shows the difference between place learning (spatial learning) and response learning (nonspatial learning). | |
| Novel Object Location | Percent of Total Investigation Time (% or fraction) | The time spent exploring the novel location divided by the total time spent exploring either object. | TBI should spend about 50% of the time or less exploring the novel location | A lower percentage of novel investigation shows an inability to remember the familiar object. |
| Discrimination Index | The time spent exploring the novel location minus time spent exploring the familiar location divided by total time exploring either object. | TBI should be closer to zero; positive values show more time investigating the novel location | A higher discrimination index shows a preference to explore the novel object rather than the familiar object. | |
| Nonspatial Learning and Memory | ||||
| Novel Object Recognition | Percent of Total Investigation Time (% or fraction) | The time spent exploring the novel object divided by the total time spent in the exploring either object. | TBI should spend about 50% of the time or less exploring the novel object | A lower percentage of novel investigation shows an inability to remember the familiar object. |
| Discrimination Index | The time spent exploring the novel object minus time spent exploring the familiar object divided by total time exploring either object. | TBI should be closer to zero; positive values show more time investigating the novel object | A higher discrimination index shows a preference to explore the novel object rather than the familiar object. | |
| Nonspatial Variants of Spatial | Same data as described above | Nonspatial variants simply take away spatial cues for each task. | Refer to above corresponding expectation for spatial tasks. | |
| Emotional | ||||
| Forced Swim Test | Time Spent Immobile (s) | The time spent not attempting to climb, move, or leave the swimming column. | TBI should spend a longer time immobile; however, depression-like activity is still controversial | A longer time spent immobile shows a larger number of depressive-like symptoms. |
| Dark/Light Avoidance Test | Time Spent in Either Zone | The time spent in either the light or dark zones. These will amount to complimentary measurements. | TBI should spend more time in the dark zone | Longer time spent in the dark zone shows a higher level of anxiety-like behaviors, while a longer time in the light zone shows the inverse. |
| Distance Travelled in Each Zone (cm) | The distance travelled while in either the dark or light zone. This will also contain two separate data points for light and dark zones. | TBI should travel a greater distance in the dark zone | Higher distance travelled in the dark zone shows a higher level of anxiety-like behaviors, while a higher distance travelled in the light zone shows the inverse. | |
| Latency to Light Zone (s) | The amount of time it takes an animal to first explore the light zone. | TBI should take longer to explore the light zone | A greater latency to the light zone shows an increased amount of anxiety-like behavior. | |
| Number of Entries into the Light Zone (Frequency) | The number of times an animal enters and renters the light zone. | TBI should have fewer entries into the light zone | A lower number of entries into the light zone shows an increased amount of anxiety-like behavior. | |
| Open Field Test | Time Spent in the Outer Zone (s or %/fraction) | The amount of time the animal stays on the outside of the open field, measured either as seconds or as a percentage or fraction of total time spent in the open field. | TBI should spend more time in the outer zone | A longer time spent in the outer zone infers an increased anxiety-like response to the open field. |
| Time Spent in the Central Zone (s or %/fraction) | The amount of time the animal spends in the center of the open field, measured either as seconds or as a percentage or fraction of total time spent in the open field. | TBI should spend less time in the center zone | A higher amount of time spent in the central zone shows a decrease in anxiety-like responses. | |
| Total Distance Travelled (cm) | The distance the animal travels through the entire trial regardless of zone. | Differences could be from locomotor issues or a greater stress response from a change in general activity. It is important researchers take notice when using this measurement. | Total distance travelled should, normally, be relatively similar. However, a greater total distance travelled along with a significantly larger time spent in the outer zone may show increased anxiety-like behaviors. Additionally, decreased total distance travelled along with a significantly greater percentage of time spent in the center may show a decrease in anxiety-like behaviors. | |
| Resident Intruder Test | Attack Latency (s) | The amount of time between introduction and the first clinch attack for either animal. | TBI should attack earlier and usually first | Lower attack latencies show a higher aggression if the animal attacking is the resident animal. |
| Total Offense Score | The sum of lateral threat, upright standing, clinch attacking, keeping down the intruder, and chasing. | TBI animals should have higher total offense scores | A higher total offense score shows a higher level of aggression. | |
| Social Exploration Score | The sum of social exploration, genital sniffing, and social grooming. | TBI animals should have lower social exploration scores | A higher social exploration score shows a lower level of anxiety. | |
| Both above can be measured as a sum of frequencies; however, these data are usually seen as percentages of total observation time. | ||||
| Motor Coordination | ||||
| Rotarod | Latency to Fall (s) | The amount of time it takes an animal to fall off of the rotating rod. | TBI animals should perform worse during the acute phase | |
| Open Field Test | Total Distance Travelled (cm) | The distance the animal travels through the entire trial regardless of zone. | TBI animals should have less distance travelled. This is mainly true for the acute phase of injury. | Lower distance travelled can mean worse motor coordination. See above for the relation between total distance travelled and anxiety-like behaviors. Time after injury is an important parameter when interpreting these results. |
| Footprint Assay | Step Length (mm) | The distance between steps of the same paw. | Dependent on time; TBI animals should show differences during acute and subacute phases | A shorter step length in the acute and subacute phases shows poor motor coordination. |
| Step Duration (ms) | The length of time one step takes. | Dependent on time; TBI animals should show differences during acute and subacute phases | A shorter step duration in the acute and subacute phases shows poor motor coordination. | |
| Inter-Leg Coordination | The coordination to keep both legs on each respective side within a straight line. This datum is quantitative. | Dependent on time; TBI animals should show differences during acute and subacute phases | A worse outcome of inter-leg coordination in the acute and subacute phases shows poor motor coordination. | |
| Injury Severity | Injury Mechanism | Presence of MEI | Imaging Characteristics | Animal Models |
|---|---|---|---|---|
| Mild Upper Intermediate | Diffuse Blunt Force Trauma Fall Sports Injury Rotational Acceleration of Brain | No | Cerebral Edema Concussion Grade 1 DAI No Presence of Lesion or Cortical Tissue Loss | CHIMERA Modified Marmarou Modified CCI Weight Drop (Marmarou) Midline FPI |
| Lower Intermediate | Fall RTI Focal Blunt Force Trauma | Possible | Diffuse Cortical Contusion Intraventricular Hemorrhage Subarachnoid Hemorrhage | Weight Drop (Shohami and Marmarou) CCI Blast Injury Lateral FPI |
| Severe | Focal Penetration Laceration GSW | Probable | Skull Fracture Focal Cortical Contusion Cortical Tissue Loss Cavity Formation Subdural Hematoma Epidural Hematoma | Weight Drop (Feeney and Shohami) CCI Lateral FPI PBBI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonald, B.Z.; Gee, C.C.; Kievit, F.M. The Nanotheranostic Researcher’s Guide for Use of Animal Models of Traumatic Brain Injury. J. Nanotheranostics 2021, 2, 224-268. https://doi.org/10.3390/jnt2040014
McDonald BZ, Gee CC, Kievit FM. The Nanotheranostic Researcher’s Guide for Use of Animal Models of Traumatic Brain Injury. Journal of Nanotheranostics. 2021; 2(4):224-268. https://doi.org/10.3390/jnt2040014
Chicago/Turabian StyleMcDonald, Brandon Z., Connor C. Gee, and Forrest M. Kievit. 2021. "The Nanotheranostic Researcher’s Guide for Use of Animal Models of Traumatic Brain Injury" Journal of Nanotheranostics 2, no. 4: 224-268. https://doi.org/10.3390/jnt2040014
APA StyleMcDonald, B. Z., Gee, C. C., & Kievit, F. M. (2021). The Nanotheranostic Researcher’s Guide for Use of Animal Models of Traumatic Brain Injury. Journal of Nanotheranostics, 2(4), 224-268. https://doi.org/10.3390/jnt2040014