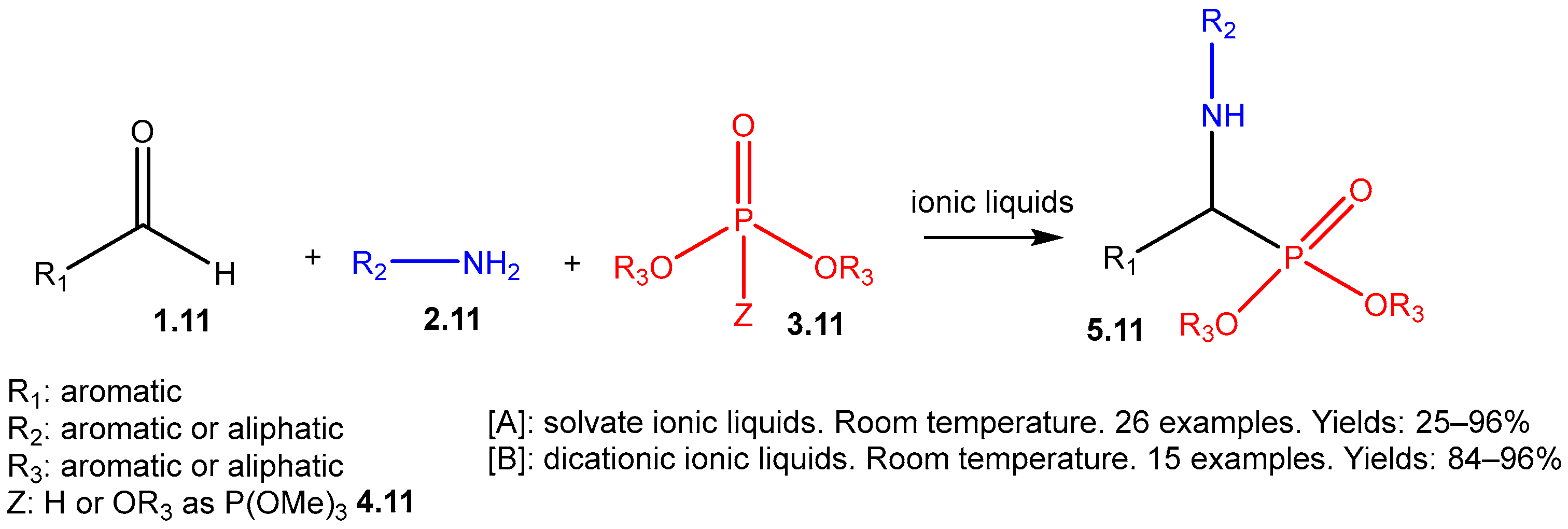
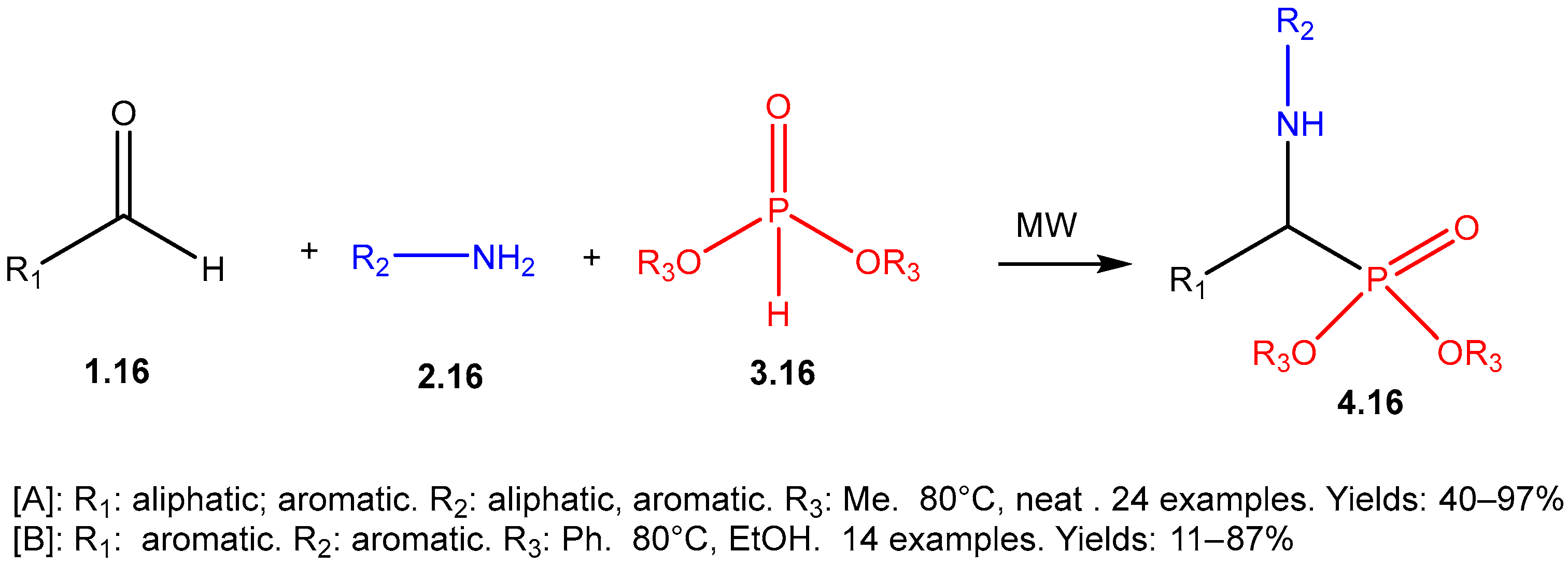
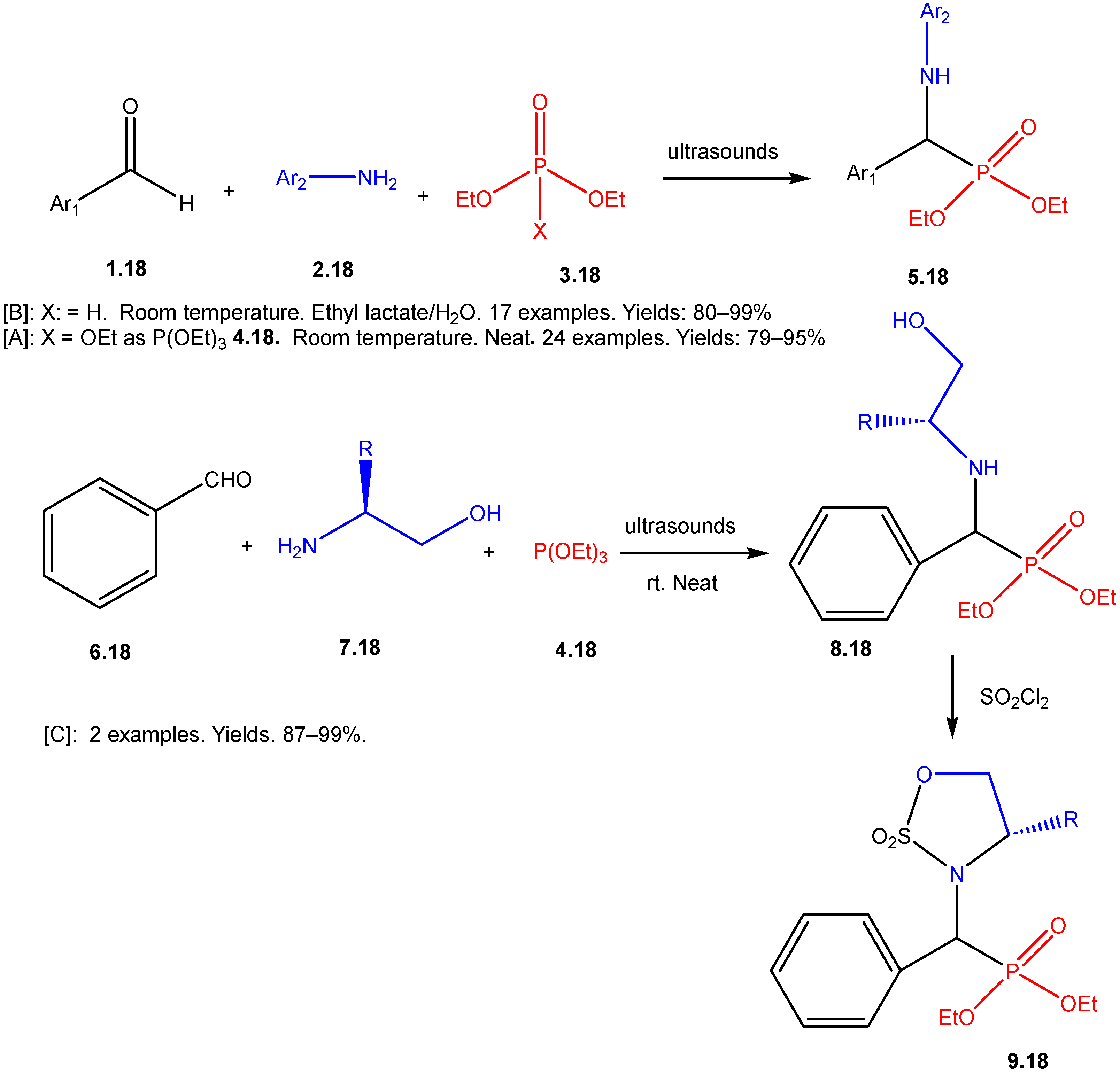
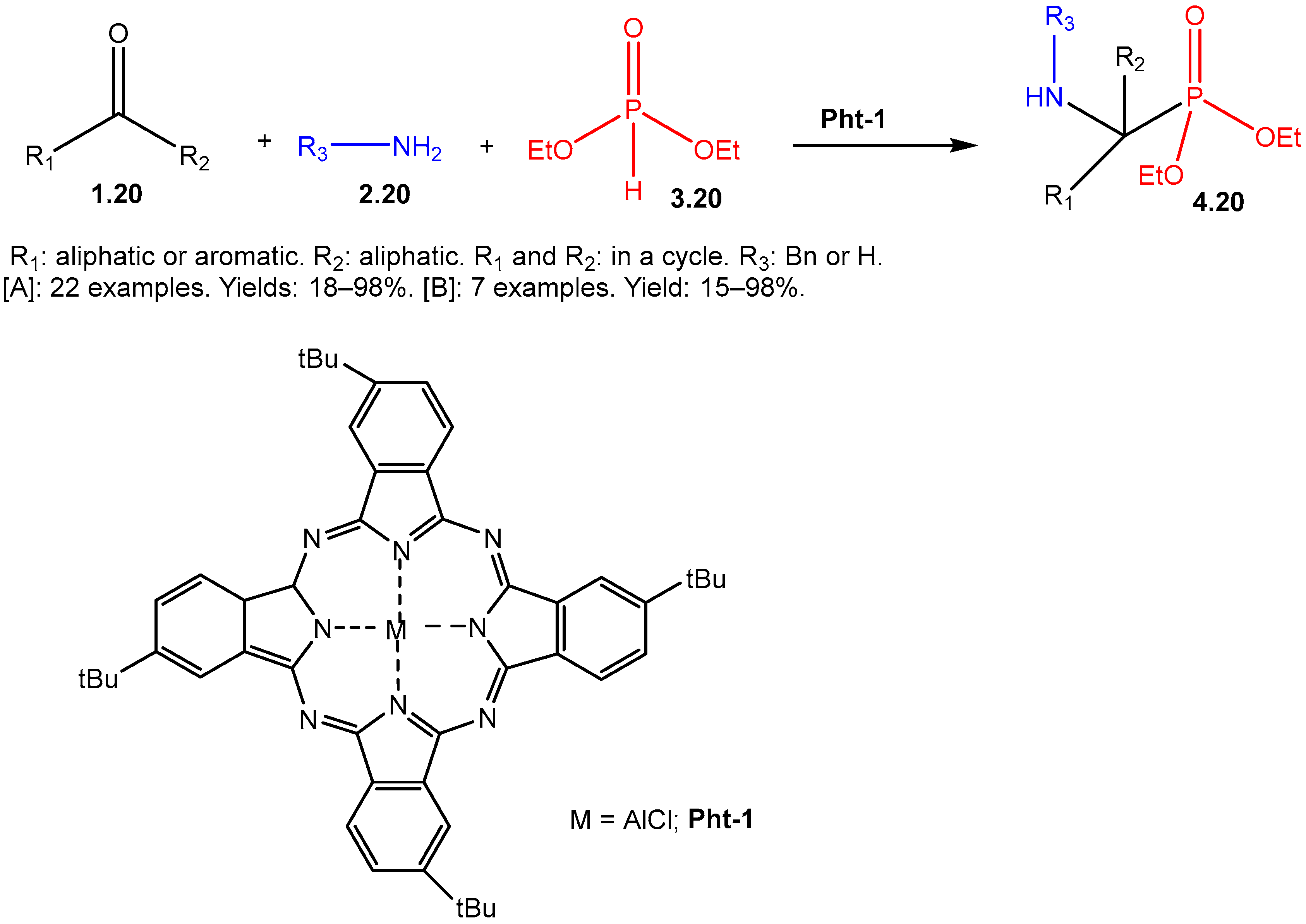
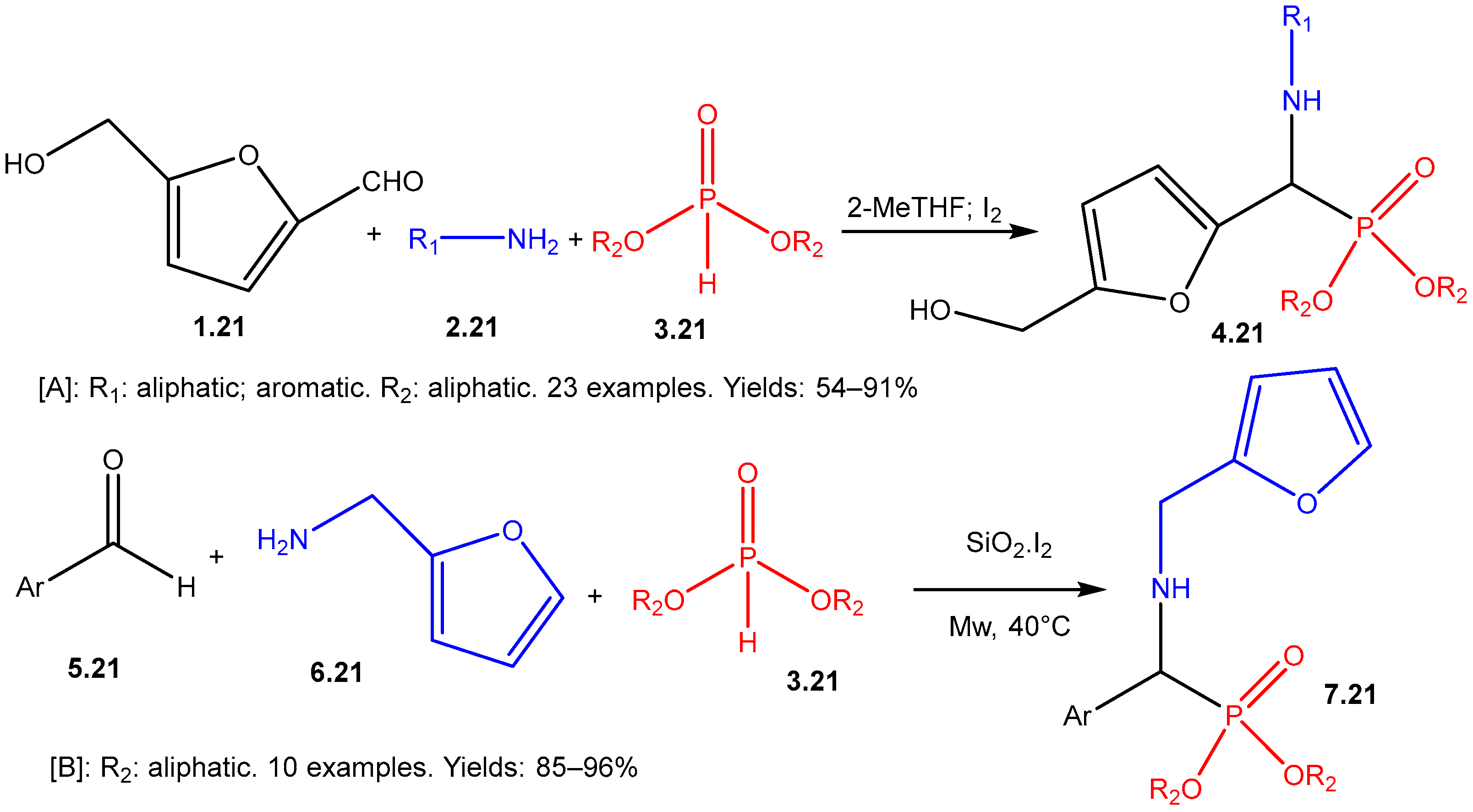
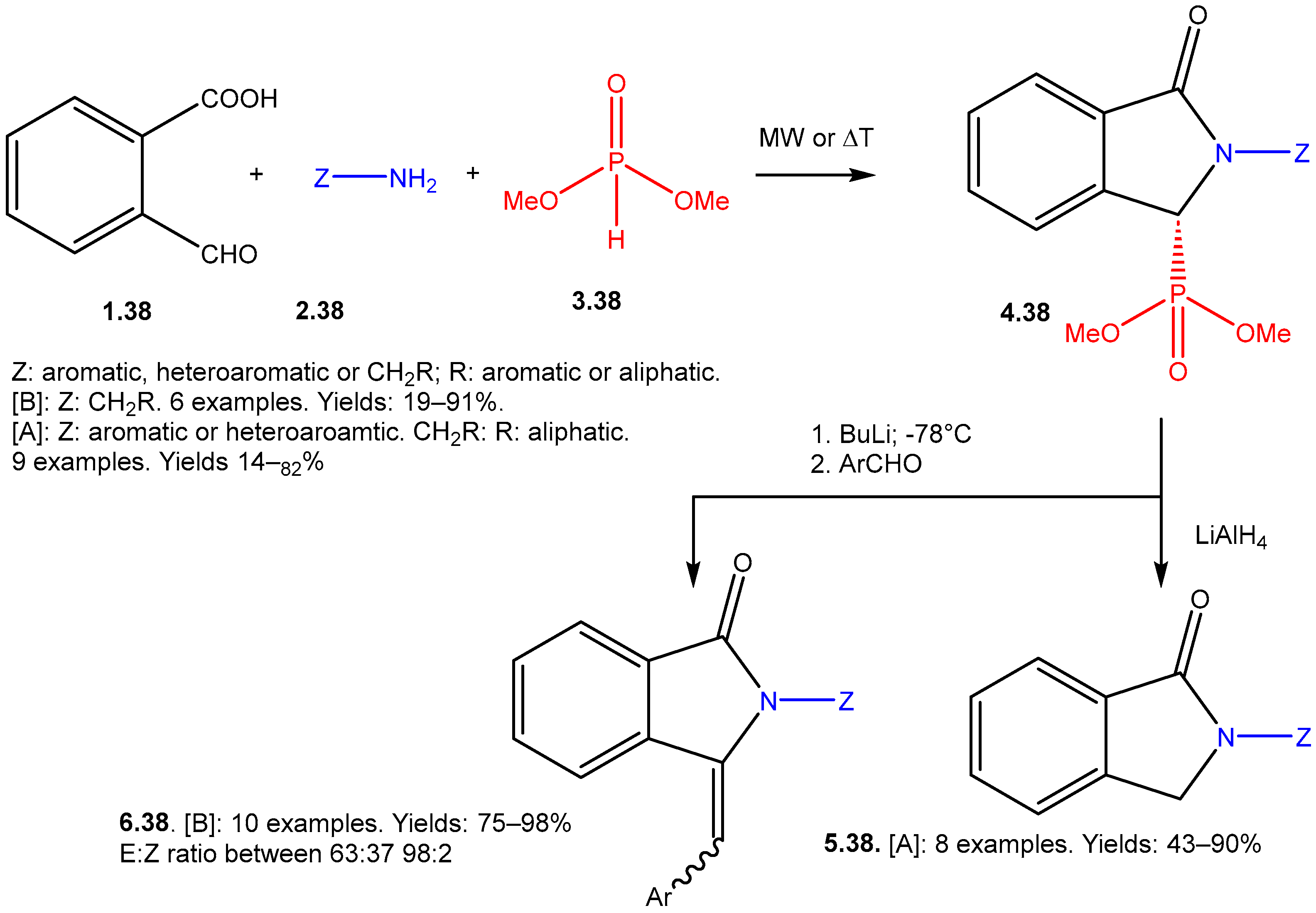
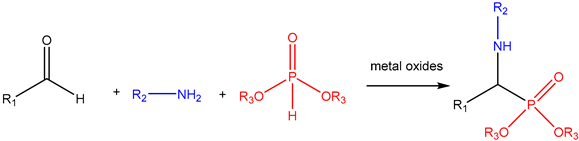
The Kabachnik–Fields Reaction: A Key Transformation in Organophosphorus Chemistry
Abstract
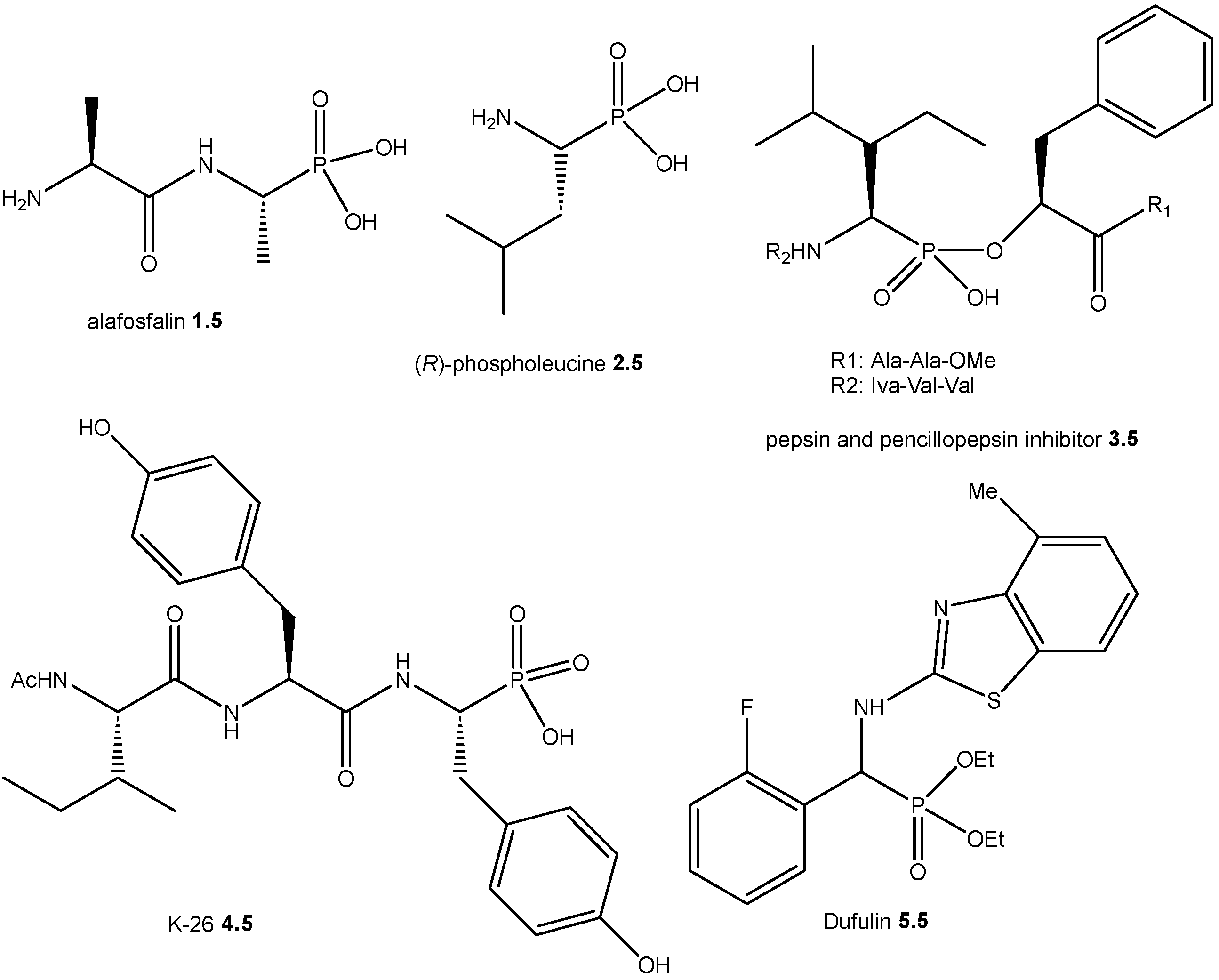
1. Introduction
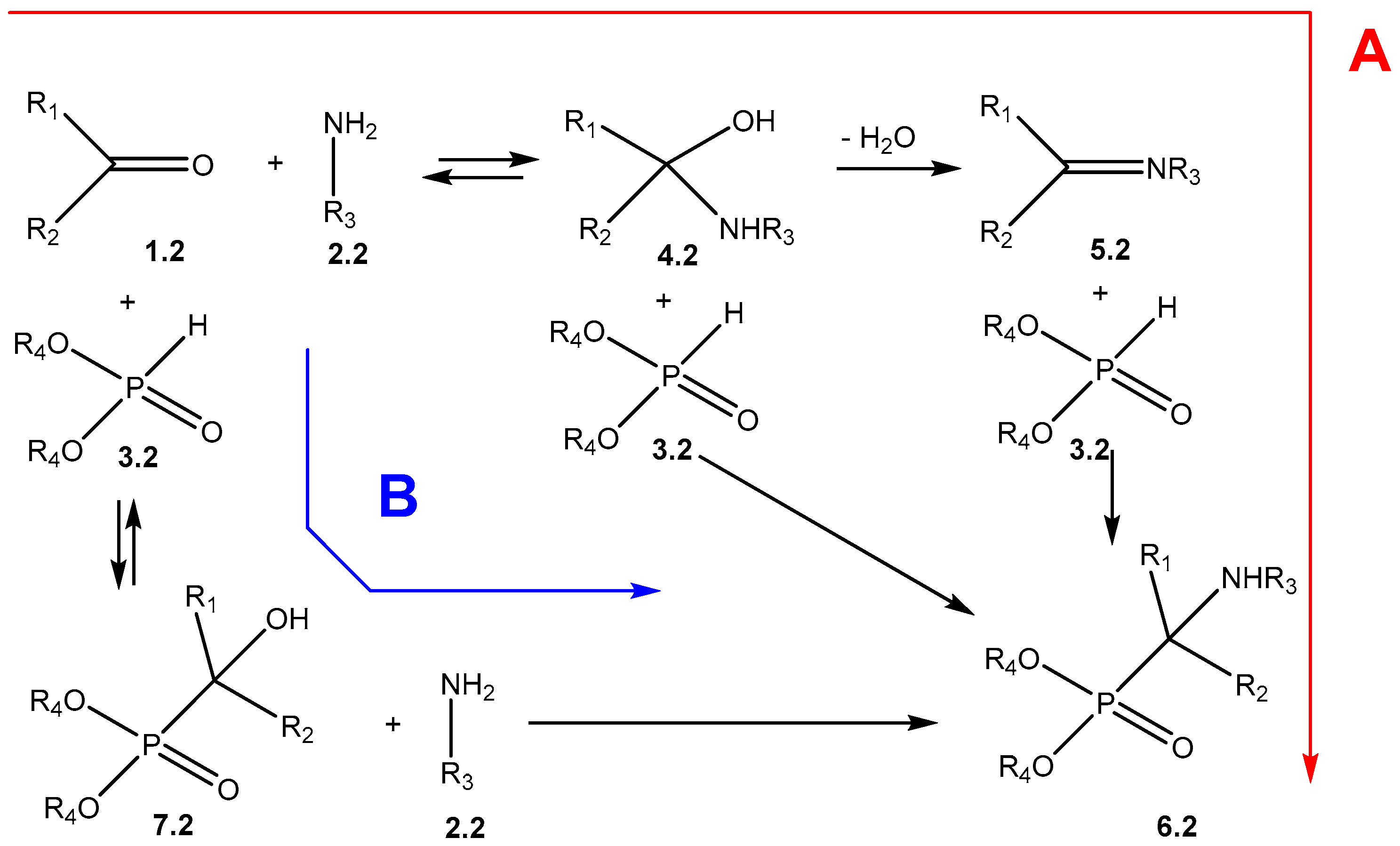
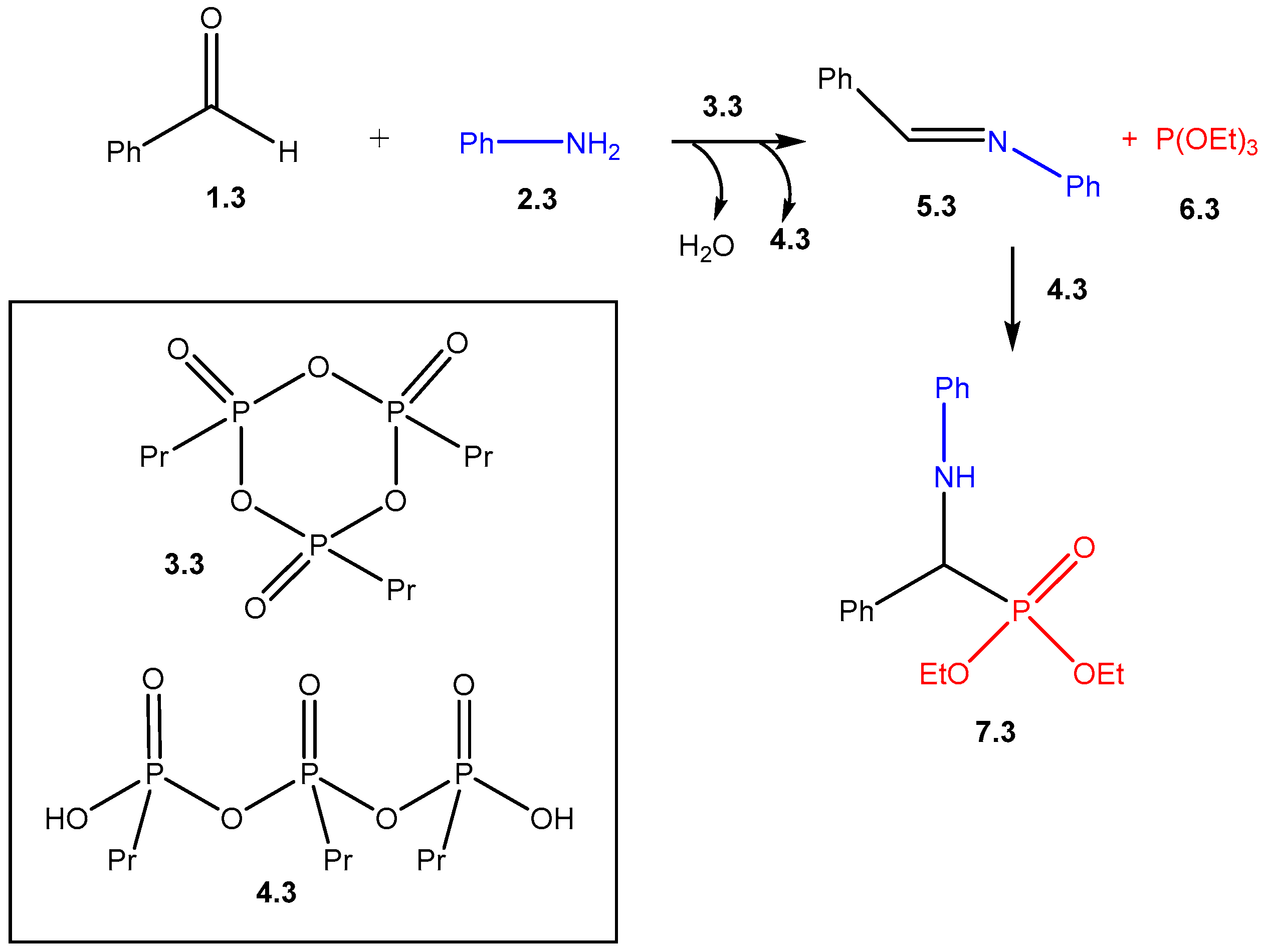
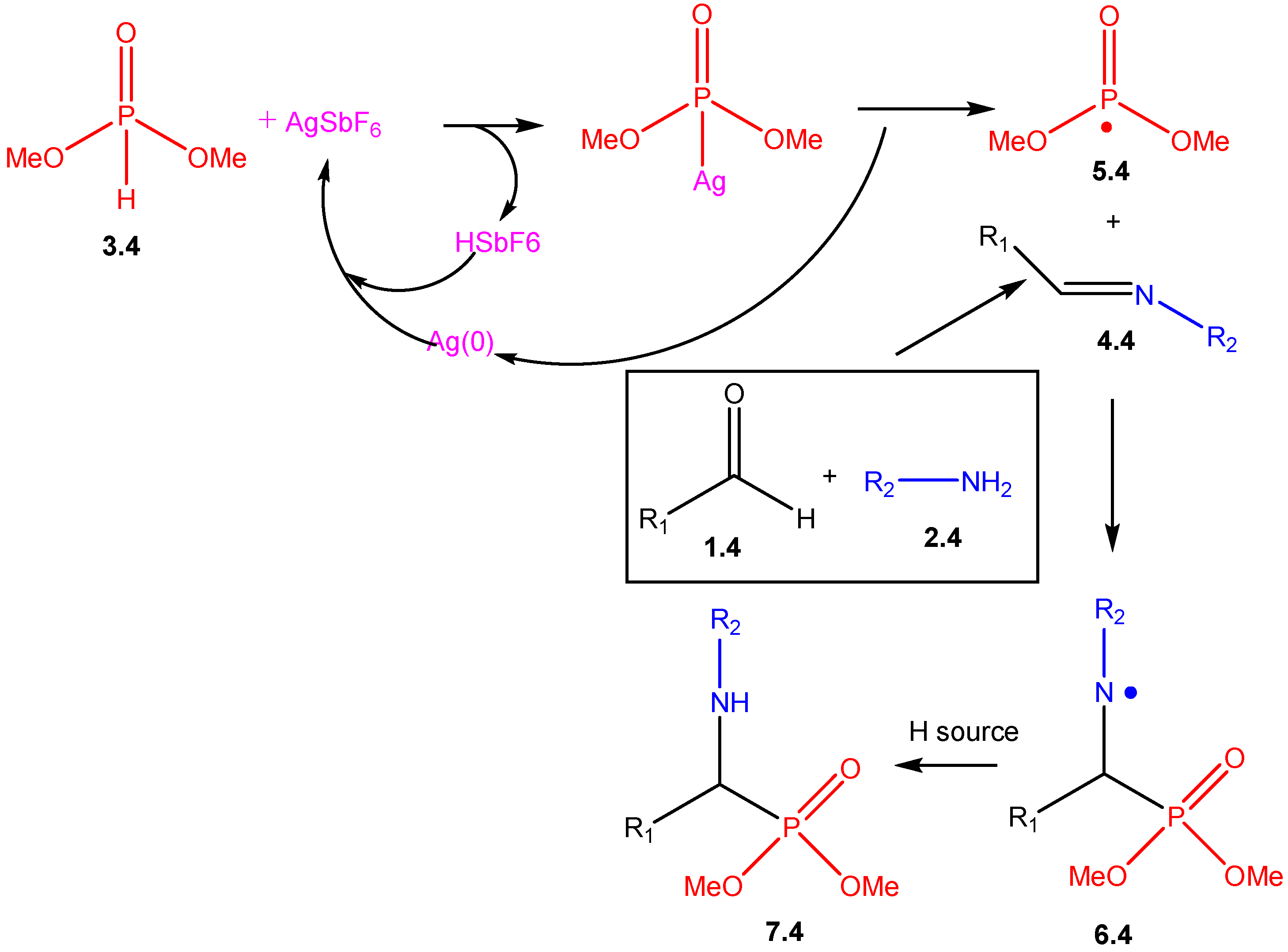
2. Mechanistic Studies
3. Catalyst-Free Kabachnik–Fields Reactions
4. Lewis Acid-Catalyzed Kabachnik–Fields Reactions
5. Brønsted Acid-Catalyzed Kabachnik–Fields Reactions
6. Other Catalysts for Kabachnik–Fields Reactions
7. Enantioselective Kabachnik–Fields Reactions
7.1. Lewis Acid-Catalyzed Reactions
7.2. Brønsted Acid-Catalyzed Reactions
7.3. Enantioselective Synthesis Without Chiral Catalysts
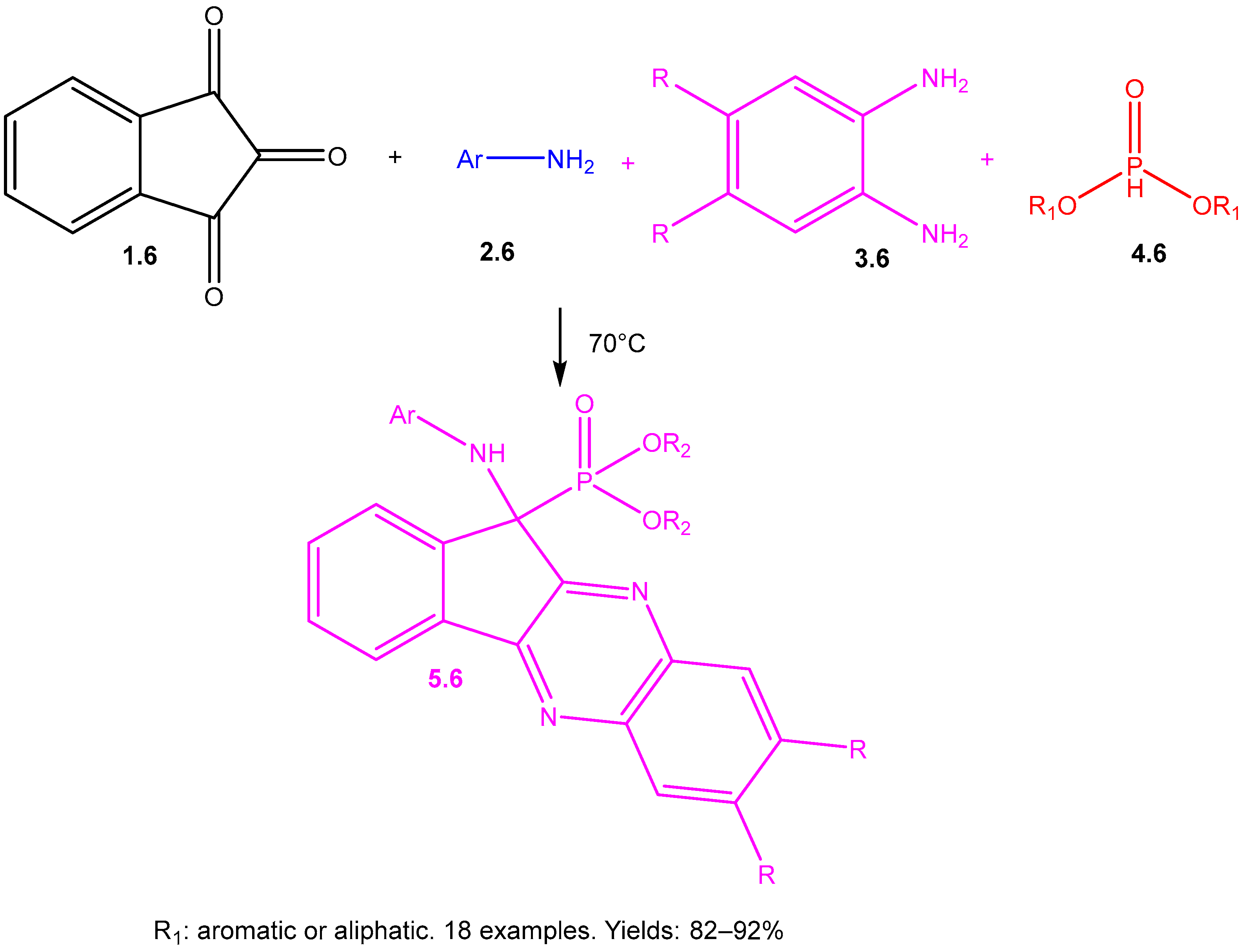
8. Derivatives of α-Aminophosphonates
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabachnik, M.I.; Medved, T.Y. New synthesis of aminophosphonic acids. Dokl. Akad. Nauk SSSR 1952, 83, 689–692. [Google Scholar]
- Fields, E.K. The synthesis of esters of substituted amino phosphonic acids. J. Am. Chem. Soc. 1952, 74, 1528–1531. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Matveeva, E.D. Catalytic Kabachnik-Fields reaction: New horizons for old reaction. Arkivoc 2008, 40, 1–17. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821–12835. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.; Keglevich, G. Synthesis of α-Aminophosphonates and Related Derivatives; The Last Decade of the Kabachnik–Fields Reaction. Molecules 2021, 26, 2511. [Google Scholar] [CrossRef]
- Yuan, R.; He, X.; Zhu, C.; Tao, L. Recent Developments in Functional Polymers via the Kabachnik–Fields Reaction: The State of the Art. Molecules 2024, 29, 727. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Galkin, V.I. The Kabachnik-Fields reaction: Synthetic potential and the problem of the mechanism. Russ. Chem. Rev. 1998, 67, 857–882. [Google Scholar] [CrossRef]
- Shilpa, T.; Harry, N.A.; Ujwaldev, S.M.; Anilkumar, G. An Overview of Microwave-Assisted Kabachnik-Fields Reactions. ChemistrySelect 2020, 5, 4422–4436. [Google Scholar] [CrossRef]
- Sravya, G.; Balakrishna, A.; Zyryanov, G.V.; Mohan, G.; Reddy, C.S.; Reddy, N.B. Synthesis of a-aminophosphonates by the Kabachnik-Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 353–381. [Google Scholar] [CrossRef]
- Gábor, D.; Pollák, P.; Volk, B.; Dancsó, A.; Simig, G.; Milen, M. Catalyst- and Solvent-Free Room Temperature Synthesis of α-Aminophosphonates: Green Accomplishment of the Kabachnik–Fields Reaction. ChemistrySelect 2023, 8, e202301460. [Google Scholar] [CrossRef]
- Wu, M.; Liu, R.; Wan, D. Convenient One-Pot Synthesis of α-Amino Phosphonates in Water Using p-Toluenesulfonic Acid as Catalyst for the Kabachnik–Fields Reaction. Heteroat. Chem. 2013, 24, 110–115. [Google Scholar] [CrossRef]
- Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. Synthesis and use of α-aminophosphine oxides and N,N-bis(phosphinoylmethyl)amines—A study on the related ring platinum complexes. J. Organomet. Chem. 2016, 801, 111–121. [Google Scholar] [CrossRef]
- Pudovik, A.N. Addition of dialkyl phosphites to imines. New method of synthesis of esters of amino phosphonic acids. Dokl. Akad. Nauk SSSR 1952, 83, 865–868. [Google Scholar]
- Galkin, V.I.; Zvereva, E.R.; Sobanov, A.A.; Galkina, I.V.; Cherkasov, R.A. A study on the Kabachnik-Fields reaction of benzaldehyde, cyclohexylamine and dialkyl phosphites. Zh. Obshch. Khim. 1993, 63, 2225–2227. [Google Scholar]
- Dimukhametov, M.N.; Bayandina, E.V.; Davydova, E.Y.; Gubaidullin, A.T.; Litvinov, I.A.; Alfonsov, V.A. A stereochemical approach to the Kabachnik–Fields reaction mechanism. Mendel. Comm. 2003, 3, 150–151. [Google Scholar] [CrossRef]
- Matveeva, E.D.; Zefirov, N.S. On the Mechanism of the Kabachnik–Fields Reaction: Does a Mechanism of Nucleophilic Amination of α-Hydroxyphosphonates Exist? Dokl. Chem. 2008, 420, 137–140. [Google Scholar] [CrossRef]
- Keglevich, G.; Kiss, N.Z.; Menyhard, D.K.; Fehervari, A.; Csontos, I. A Study on the Kabachnik–Fields Reaction of Benzaldehyde, Cyclohexylamine, and Dialkyl Phosphites. Heteroat. Chem. 2012, 23, 171–178. [Google Scholar] [CrossRef]
- Keglevich, G.; Fehervari, A.; Csontos, I. A Study on the Kabachnik–Fields Reaction of Benzaldehyde, Propylamine, and Diethyl Phosphite by In Situ Fourier Transform IR Spectroscopy. Heteroat. Chem. 2011, 22, 599–604. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Z.; Xu, Y.; Lv, K.; Zhang, I.; Liu, T. Molecular synthesis mechanism of α-aminophosphonate using DES: Ring-tension-controlled reactivity. Inorg. Chim. Acta 2025, 578, 122549. [Google Scholar] [CrossRef]
- Engel, R. Phosphorus Addition at sp2 Carbon. In Organic Reactions; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 175–248. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Medved, T.Y. New method for the synthesis of 1-aminoalkylphosphonic acids Communication 1. In Bulletin of the Academy of Sciences of the USSR, Division of chemical Science; Springer: Berlin/Heidelberg, Germany, 1953; Volume 2, pp. 769–777. [Google Scholar] [CrossRef]
- Galkina, I.V.; Sobanov, A.A.; Galkin, V.I.; Cherkasov, R.A. Kinetics and mechanism of the Kabachnik–Fields reaction: IV. Salicyaldehyde in the Kabachnik–Fields reaction. Russ. J. Gen. Chem. 1998, 68, 1398–1401. [Google Scholar]
- Gancarz, R.; Gancarz, I. Failure of Aminophosphonate Synthesis Due to Facile Hydroxyphosphonate–Phosphate Rearrangement. Tetrahedron Lett. 1993, 34, 145–148. [Google Scholar] [CrossRef]
- Gancarz, R.; Gancarz, I.; Walkowjak, U. On the reversibility of hydroxyphosphonate formation in the in the Kabachnik-Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 1995, 104, 45–52. [Google Scholar] [CrossRef]
- Gancarz, R. Unexpeced products in the Kabachnik-Fields synthesis of aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 1993, 83, 59–64. [Google Scholar] [CrossRef]
- Gancarz, R. Nucleophilic Addition to Carbonyl Compounds. Competition between Hard (Amine) and Soft (Phosphite) Nucleophile. Tetrahedron 1995, 51, 10627–10632. [Google Scholar] [CrossRef]
- Bhagat, S.; Chakraborti, A.K. An Extremely Efficient Three-Component Reaction of Aldehydes/Ketones, Amines, and Phosphites (Kabachnik-Fields Reaction) for the Synthesis of α-Aminophosphonates Catalyzed by Magnesium Perchlorate. J. Org. Chem. 2007, 72, 1263–1270. [Google Scholar] [CrossRef]
- Fiore, C.; Sovic, I.; Lukin, S.; Halasz, I.; Martina, K.; Delogu, F.; Ricci, P.C.; Porcheddu, A.; Shemchuk, O.; Braga, D.; et al. Kabachnik−Fields Reaction by Mechanochemistry: New Horizons from Old Methods. ACS Sustain. Chem. Eng. 2020, 8, 18889–18902. [Google Scholar] [CrossRef]
- Milen, M.; Ábrányi-Balogh, P.; Dancsó, A.; Frigyes, D.; Pongó, L.; Keglevich, G. T3P®-promoted Kabachnik–Fields reaction: An efficient synthesis of α-aminophosphonates. Tetrahedron Lett. 2013, 54, 5430–5433. [Google Scholar] [CrossRef]
- Das, S.; Rawal, P.; Bhattacharjee, J.; Devadkar, A.; Pal, K.; Gupta, P.; Panda, T.K. Indium promoted C(sp3)–P bond formation by the Domino A3-coupling method—A combined experimental and computational study. Inorg. Chem. Front. 2021, 8, 1142–1153. [Google Scholar] [CrossRef]
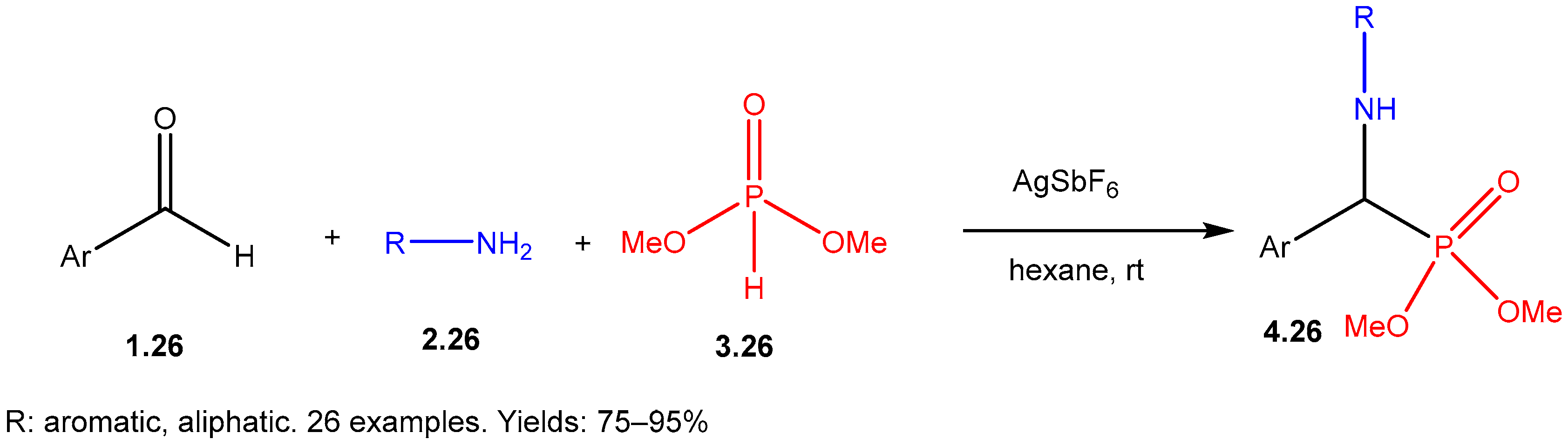
- Pandey, V.K.; Tiwari, C.S.; Rit, A. Silver-Catalyzed One-Pot Three-Component Synthesis of α- Aminonitriles and Biologically Relevant α-Amino-phosphonates. Chem. Asian J. 2022, 17, e202200703. [Google Scholar] [CrossRef] [PubMed]
- Kaboudin, B.; Faghih, S.; Alavi, S.; Naimi-Jamal, M.R.; Fattahi, A. An Efficient One-Pot Synthesis of 1-Aminophosphonates. Synthesis 2023, 55, 121–130. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A. A simple and green procedure for the synthesis of a-aminophosphonate by a one-pot three-component condensation of carbonyl compound, amine and diethyl phosphite without solvent and catalyst. Green Chem. 2002, 4, 551–554. [Google Scholar] [CrossRef]
- Rashid, Z.; Naeimi, H.; Ghahremanzadeh, R. Highly efficient one-pot four-component Kabachnik–Fields synthesis of novel α-amino phosphonates under solvent-free and catalyst-free conditions. RSC Adv. 2015, 5, 99148–99152. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Wang, Y.; Yang, J.; Lu, H. A novel route for the synthesis of phosphonate-containing siloxanes by the catalyst-free Kabachnik—Fields reaction. Russ. Chem. Bull. 2024, 73, 2725–2729. [Google Scholar] [CrossRef]
- Soliya, S.; Kuperkar, K.; Ashalu, K.C.; Naveen, T. Catalyst-Free Three-Component Synthesis of α-Amino Phosphonates. Asian J. Org. Chem. 2024, 13, e202300572. [Google Scholar] [CrossRef]
- Ke, S.; Zhang, D.; Li, Y.; Gong, Z.; Tang, P.; Tang, W. One-pot synthesis and fluorescent property of novel syringaldehyde α-aminophosphonate derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 607–614. [Google Scholar] [CrossRef]
- Sampath, C.; Harika, P.; Revaprasadu, N. Design, green synthesis, anti-microbial, and anti-oxidant activities of novel α-aminophosphonates via Kabachnik-Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1081–1085. [Google Scholar] [CrossRef]
- Kumar, M.A.; Lee, K.D. A Simple and Catalyst-Free One-Pot Synthesis of α-Aminophosphonates in Polyethylene Glycol. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 899–905. [Google Scholar] [CrossRef]
- Azizi, K.; Karimi, M.; Heydari, A. A catalyst-free synthesis of α-aminophosphonates in glycerol. Tetrahedron Lett. 2014, 55, 7236–7239. [Google Scholar] [CrossRef]
- Mandal, S.; Narvariya, R.; Sunar, S.L.; Paul, I.; Jain, A.; Panda, T.K. Synthesis of α-aminophosphorous derivatives using a deep eutectic solvent (DES) in a dual role. Green Chem. 2023, 25, 8266–8272. [Google Scholar] [CrossRef]
- Shaibuna, S.; Sreekumar, K. Experimental Investigation on the Correlation between the Physicochemical Properties and Catalytic Activity of Six DESs in the Kabachnik-Fields Reaction. ChemistrySelect 2020, 5, 13454–13460. [Google Scholar] [CrossRef]
- Haghayegh, M.S.; Azizi, N.; Shahabi, S.S.; Gu, Y. Pvp-based deep eutectic solvent polymer: Sustainable Brønsted-Lewis acidic catalyst in the synthesis of α-aminophosphonate and bisindole. J. Mol. Liq. 2023, 387, 122677. [Google Scholar] [CrossRef]
- Benzaim, A.Y.; Cheraiet, Z.; Guezane-Lakoud, S.; Zadem, A.; Boukhari, A. Natural deep eutectic solvents [BetaineCl][Lactic acid] as efficient and sustainable catalyst for one-pot synthesis of α-aminophosphonates. J. Indian Chem. Soc. 2025, 102, 101593. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Henderson, L.C. Synthesis of α-aminophosphonates using solvate ionic liquids. RSC Adv. 2017, 7, 27900–27904. [Google Scholar] [CrossRef]
- Fang, N.; Yang, J.; Ni, C. Dicationicionic liquids as recyclable catalysts for one-pot solvent-free synthesis of α-aminophosphonates. Heteroat. Chem. 2010, 22, 5–10. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrenyi, A. Eco-Friendly Accomplishment of the Extended Kabachnik–Fields Reaction; a Solvent- and Catalyst-Free Microwave-Assisted Synthesis of α-Aminophosphonates and α-Aminophosphine Oxides. Lett. Org. Chem. 2008, 5, 616–622. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A.; Kovács, R.; Grün, A. Microwave Irradiation as a Useful Tool in Organophosphorus Syntheses. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1648–1652. [Google Scholar] [CrossRef]
- Bálint, E.; Takács, J.; Drahos, L.; Juranovič, A.; Kočevar, M.; Keglevich, G. α-Aminophosphonates and α-Aminophosphine Oxides by the Microwave-Assisted Kabachnik-Fields Reactions of 3-Amino-6-methyl-2H-pyran-2-ones. Heteroat. Chem. 2013, 24, 221–225. [Google Scholar] [CrossRef]
- Bálint, E.; Fazekas, E.; Tripolszky, A.; Kangyal, R.; Milen, M.; Keglevich, G. Synthesis of α-Aminophosphonate Derivatives by Microwave-Assisted Kabachnik–Fields Reaction. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 655–659. [Google Scholar] [CrossRef]
- Tajti, Á.; Balint, E.; Keglevich, G. Synthesis of Ethyl Octyl α-Aminophosphonate Derivatives. Curr. Org. Synth. 2016, 13, 638–645. [Google Scholar] [CrossRef]
- Bálint, E.; Tóth, R.E.; Keglevich, G. Synthesis of alkyl α-aminomethyl-phenylphosphinates and N,N-bis(alkoxyphenylphosphinylmethyl)amines by the microwave-assisted Kabachnik-Fields reaction. Heteroat. Chem. 2016, 27, 323–335. [Google Scholar] [CrossRef]
- Tajti, Á.; Szatmári, E.; Perdih, F.; Keglevich, G.; Bálint, E. Microwave-Assisted Kabachnik-Fields Reaction with Amino Alcohols as the Amine Component. Molecules 2019, 24, 1640. [Google Scholar] [CrossRef]
- Bálint, E.; Tripolszky, A.; Hegedűs, L.; Keglevich, G. Microwave-assisted synthesis of N,N-bis(phosphinoylmethyl)amines and N,N,N-tris(phosphinoylmethyl)amines bearing different substituents on the phosphorus atoms. Beilstein J. Org. Chem. 2019, 15, 469–473. [Google Scholar] [CrossRef]
- Hudson, H.R.; Tajti, Á.; Bálint, E.; Czugler, M.; Karaghiosoff, K.; Keglevich, G. Microwave-assisted synthesis of α- aminophosphonates with sterically demanding α-aryl substituents. Synth. Commun. 2020, 50, 1446–1455. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, A.; Ladányi-Pára, L.; Tóth, N.; Mátravölgyi, B.; Keglevich, G. Continuous flow synthesis of α-aryl-α-aminophosphonates. Pure Appl. Chem. 2019, 91, 67–76. [Google Scholar] [CrossRef]
- Mu, X.; Lei, M.; Zou, J.; Zhang, W. Microwave-Assisted Solvent-Free and Catalyst-Free Kabachnik—Fields Reactions for α-Amino Phosphonates. Tetrahedron Lett. 2006, 47, 1125–1127. [Google Scholar] [CrossRef]
- Martínez-Campos, Z.; Elizondo-Zertuche, M.; Hernández-Núñez, E.; Hernández-Fernández, E.; Robledo-Leal, E.; López-Cortina, S.T. Microwave-Assisted Synthesis of Aminophosphonic Derivatives and Their Antifungal Evaluation against Lomentospora prolificans. Molecules 2023, 28, 3995. [Google Scholar] [CrossRef]
- Tajti, Á.; Tóth, N.; Rávai, B.; Csontos, I.; Szabó, P.T.; Bálint, E. Study on the Microwave-Assisted Batch and Continuous Flow Synthesis of N-Alkyl-Isoindolin-1-One-3-Phosphonates by a Special Kabachnik–Fields Condensation. Molecules 2020, 25, 3307. [Google Scholar] [CrossRef]
- Gao, G.; Chen, M.N.; Mo, L.-P.; Zhang, Z.-H. Catalyst free one-pot synthesis of α-aminophosphonates in aqueous ethyl lactate. Phosphorus Sulfur Silicon Relat. Elem. 2018, 194, 528–532. [Google Scholar] [CrossRef]
- Dar, B.; Singh, A.; Sahu, A.; Patidar, P.; Chakraborty, A.; Sharma, M.; Singh, B. Catalyst and solvent-free, ultrasound promoted rapid protocol for the one-pot synthesis of α-aminophosphonates at room temperature. Tetrahedron Lett. 2012, 53, 5497–5502. [Google Scholar] [CrossRef]
- K’tir, H.; Amira, A.; Benzaid, C.; Aouf, Z.; Benharoun, S.; Chemam, Y.; Zerrouki, R.; Aouf, N.E. Synthesis, bioinformatics and biological evaluation of novel α-aminophosphonates as antibacterial agents: DFT, molecular docking and ADME/T studies. J. Mol. Struct. 2022, 1250, 131635. [Google Scholar] [CrossRef]
- Matveeva, E.D.; Podrugina, T.A.; Tishkovskaya, E.V.; Tomilova, L.G.; Zefirov, N.S. A Novel Catalytic Three-Component Synthesis (Kabachnick-Fields Reaction) of α-Aminophosphonates from Ketones. Synlett 2003, 2003, 2321–2322. [Google Scholar] [CrossRef]
- Shuvalov, M.V.; Maklakova, S.Y.; Rudakova, E.V.; Kovaleva, N.V.; Makhaeva, G.F.; Podrugina, T.A. New Possibilities of the Kabachnik–Fields and Pudovik Reactions in the Phthalocyanine-Catalyzed Syntheses of α-Aminophosphonic and α-Aminophosphinic Acid Derivatives. Russ. J. Gen. Chem. 2018, 88, 1761–1775. [Google Scholar] [CrossRef]
- Fan, W.; Queneau, Y.; Popowycz, F. The synthesis of HMF-based α-amino phosphonates via one-pot Kabachnik–Fields reaction. RSC Adv. 2018, 8, 31496–31501. [Google Scholar] [CrossRef] [PubMed]
- Nadiveedhi, M.R.; Nuthalapati, P.; Gundluru, M.; Yanamula, M.R.; Kallimakula, S.V.; Pasupuleti, V.R.; Avula, V.K.R.; Vallela, S.; Zyryanov, G.V.; Balam, S.K.; et al. Green Synthesis, Antioxidant, and Plant Growth Regulatory Activities of Novel α-Furfuryl-2-alkylaminophosphonates. ACS Omega 2021, 6, 2934–2948. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.A.; Nazarpour, M.; Abdollahi-Alibeik, M. CeCl3·7H2O-Catalyzed One-Pot Kabachnik–Fields Reaction: A Green Protocol for Three-Component Synthesis of α-Aminophosphonates. Heteroat. Chem. 2010, 21, 397–404. [Google Scholar] [CrossRef]
- Rezaei, Z.; Firouzabadi, H.; Iranpoor, N.; Ghaderi, A.; Jafari, M.R.; Jafari, A.A.; Zare, H.R. Design and one-pot synthesis of α-aminophosphonates and bis(α-aminophosphonates) by iron(III) chloride and cytotoxic activity. Eur. J. Med. Chem. 2009, 44, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Manjula, A.; Rao, B.; Neelakantan, P. One-pot synthesis of α-aminophosphonates: An inexpensive approach. Synth. Comm. 2003, 33, 2963–2969. [Google Scholar] [CrossRef]
- Ambica, S.; Kumar, S.C.; Taneja, M.S.; Hundal, K.; Kapoor, K. One-pot synthesis of a-aminophosphonates catalyzed by antimony trichloride adsorbed on alumina. Tetrahedron Lett. 2008, 49, 2208–2212. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Prakash, S.J.; Jagadeshwar, V.; Narsihmulu, C. Three component coupling catalyzed by TaCl5–SiO2: Synthesis of α-amino phosphonates. Tetrahedron Lett. 2001, 42, 5561–5563. [Google Scholar] [CrossRef]
- Zhan, Z.P.; Li, J.P. Bismuth(III) Chloride–Catalyzed Three-Component Coupling: Synthesis of α-Amino Phosphonates. Synth. Commun. 2005, 35, 2501–2508. [Google Scholar] [CrossRef]
- Bhagat, S.; Chakraborti, A.K. Zirconium(IV) compounds as efficient catalysts for synthesis of α-aminophosphonates. J. Org. Chem. 2008, 73, 6029–6032. [Google Scholar] [CrossRef]
- Azizi, N.; Rajabi, F.; Saidi, M.R. A mild and highly efficient protocol for the one-pot synthesis of primary α-amino phosphonates under solvent-free conditions. Tetrahedron Lett. 2004, 45, 9233–9236. [Google Scholar] [CrossRef]
- Hou, J.-T.; Gao, J.-W.; Zhang, Z.-H. NbCl5: An efficient catalyst for one-pot synthesis of α-aminophosphonates under solvent-free conditions. Appl. Organomet. Chem. 2010, 25, 47–53. [Google Scholar] [CrossRef]
- Li, X.-C.; Gong, S.-S.; Zeng, D.-Y.; You, Y.-H.; Sun, Q. Highly efficient synthesis of α-aminophosphonates catalyzed by hafnium(IV) chloride. Tetrahedron Lett. 2016, 57, 1782–1785. [Google Scholar] [CrossRef]
- Anand, A.S.V.; Sivaramakrishna, A. Facile InCl3-catalyzed room temperature synthesis and electrochemical behavior of ferrocene appended (R,S)-α-aminophosphonates. J. Mol. Struct. 2024, 1311, 138361. [Google Scholar] [CrossRef]
- Mirzaei, M.; Eshghi, H.; Sabbaghzadeh, R. LaCl3·7H2O as an Effective Catalyst for the Synthesis of α-Aminophosphonates under Solvent-Free Conditions and Docking Simulation of Ligand Bond Complexes of Cyclin-Dependent Kinase 2. Polycycl. Aromat. Comp. 2022, 42, 5882–5892. [Google Scholar] [CrossRef]
- Basha, S.K.T.; Kalla, R.M.N.; Varalakshmi, M.; Sudhamani, H.; Appa, R.M.; Hong, S.C.; Raju, C.N. Heterogeneous catalyst SiO2–LaCl3·7H2O: Characterization and microwave-assisted green synthesis of α-aminophosphonates and their antimicrobial activity. Mol. Divers. 2022, 26, 2703–2715. [Google Scholar] [CrossRef]
- Lee, S.-G.; Park, J.H.; Kang, J.; Lee, J.K. Lanthanide triflate-catalyzed three component synthesis of α-amino phosphonates in ionic liquids. A catalyst reactivity and reusability study. Chem. Commun. 2001, 47, 1698–1699. [Google Scholar] [CrossRef]
- Paraskar, A.S.; Sudalai, A. A novel Cu(OTf)2 mediated three component high yield synthesis of α-aminophosphonates. ARKIVOC 2006, 2006, 183–189. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S.; Chakraborty, A.; Maiti, D.K. In(OTf)3 catalysed simple one-pot synthesis of α -amino phosphonates. J. Mol. Catal. A: Chem. 2004, 210, 53–57. [Google Scholar] [CrossRef]
- Essid, I.; Touil, S. Efficient and Green One-Pot Multi-Component Synthesis of α-Aminophosphonates Catalyzed by Zinc Triflate. Curr. Org. Synth. 2017, 14, 272–278. [Google Scholar] [CrossRef]
- Ewies, E.F.; El-Hussieny, M.; El-Sayed, N.F.; Fouad, M.A. Design, synthesis and biological evaluation of novel α-aminophosphonate oxadiazoles via optimized iron triflate catalyzed reaction as apoptotic inducers. Eur. J. Med. Chem. 2019, 180, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.K.; Kaur, T. An Efficient One-Pot Synthesis of α-Amino Phosphonates Catalyzed by Bismuth Nitrate Pentahydrate. Synlett 2007, 2007, 745–748. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Madan, C. Montmorillonite Clay-Catalyzed One-Pot Synthesis of a-Amino Phosphonates. Synlett 2001, 2001, 1131–1133. [Google Scholar] [CrossRef]
- Cabrita, I.R.; Sousa, S.C.; Florindo, P.R.; Fernandes, A.C. Direct Aminophosphonylation of aldehydes catalyzed by cyclopentadienyl ruthenium(II) complexes. Tetrahedron 2018, 74, 1817–1825. [Google Scholar] [CrossRef]
- Keri, R.; Patil, M.; Brahmkhatri, V.P.; Budagumpi, S.; Adimule, V. Copper (II)-β-Cyclodextrin Promoted Kabachnik-Fields Reaction: An Efficient, One-Pot Synthesis of α-Aminophosphonates. Top. Catal. 2025, 68, 1243–1256. [Google Scholar] [CrossRef]
- Kaboudin, B.; Moradi, K. A simple and convenient procedure for the synthesis of 1-aminophosphonates from aromatic aldehydes. Tetrahedron Lett. 2005, 46, 2989–2991. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Garifzyanov, A.R.; Koshkin, S.A. Synthesis of α-Aminophosphine Oxides with Chiral Phosphorus and Carbon Atoms. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 782–784. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Garifzyanov, A.R.; Koshkin, S.A. Synthesis of optically active α-aminophosphine oxides and enantioselective membrane transport of acids with their participation. Russ. J. Gen. Chem. 2011, 81, 773–774. [Google Scholar] [CrossRef]
- Vagapova, L.I.; Burilov, A.R.; Voronina, J.K.; Syakaev, V.V.; Sharafutdinova, D.R.; Amirova, L.R.; Pudovik, M.A.; Garifzyanov, A.R.; Sinyashin, O.G. Phosphorylated Aminoacetal in the Synthesis of New Acyclic, Cyclic, and Heterocyclic Polyphenol Structures. Heteroat. Chem. 2014, 25, 178–185. [Google Scholar] [CrossRef]
- Vagapova, L.I.; Burilov, A.R.; Amirova, L.R.; Voronina, J.K.; Garifzyanov, A.R.; Abdrachmanova, N.F.; Pudovik, M.A. New aminophosphonates (aminophosphine oxides) containing acetal groups in reactions with polyatomic phenols. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1527–1529. [Google Scholar] [CrossRef]
- Ravi, N.; Venkatanarayana, N.; Sharathbabu, H.; Ravendra Babu, K. Synthesis of novel α-aminophosphonates by methanesulfonic acid catalyzed Kabachnik–Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 1018–1024. [Google Scholar] [CrossRef]
- Mitragotri, S.D.; Pore, S.D.; Desai, U.V.; Wadgaonkar, P.P. Sulfamic acid: An efficient and cost-effective solid acid catalyst for the synthesis of a-aminophosphonates at ambient temperature. Catal. Commun. 2008, 9, 1822–1826. [Google Scholar] [CrossRef]
- Akiyama, T.; Sanada, M.; Fuchibe, K. Brønsted Acid-Mediated Synthesis of α-Amino Phosphonates under Solvent-Free Conditions. Synlett 2003, 2003, 1463–1464. [Google Scholar] [CrossRef]
- Kudrimoti, S.; Bommena, V.R. (Bromodimethyl)sulfonium bromide: An inexpensive reagent for the solvent-free, one-pot synthesis of a-aminophosphonates. Tetrahedron Lett. 2005, 46, 1209–1210. [Google Scholar] [CrossRef]
- Gangwar, N.; Kasana, V.K. Tartaric Acid–Catalyzed Synthesis of α-Aminophosphonates Under Solvent-Free Conditions. Synth. Commun. 2011, 41, 2800–2804. [Google Scholar] [CrossRef]
- Ordóñez, M.; Tibhe, G.; Bedolla-Medrano, M.; Cativiela, C. Phenylboronic Acid as Efficient and Eco-Friendly Catalyst for the One-Pot, Three-Component Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Synlett 2012, 23, 1931–1936. [Google Scholar] [CrossRef]
- Ordóñez, M.; Bedolla-Medrano, M.; Hernández-Fernández, E. Phenylphosphonic Acid as Efficient and Recyclable Catalyst in the Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Synlett 2014, 25, 1145–1149. [Google Scholar] [CrossRef]
- Hellal, A.; Chafaa, S.; Touafri, L. An eco-friendly procedure for the efficient synthesis of diethyl α-aminophosphonates in aqueous media using natural acids as a catalyst. Korean J. Chem. Eng. 2016, 33, 2366–2373. [Google Scholar] [CrossRef]
- Reddy, P.S.; Reddy, P.V.G.; Reddy, S.M. Phosphomolybdic acid promoted Kabachnik–Fields reaction: An efficient one-pot synthesis of α-aminophosphonates from 2-cyclopropylpyrimidine-4-carbaldehyde. Tetrahedron Lett. 2014, 55, 3336–3339. [Google Scholar] [CrossRef]
- Jaydeokara, S.; Yadava, V.G.; Bandivadekarb, P.V.; Chaturbhujb, G.; Chavan, V.L. N-(1-hydroxybutan-2-yl)-4-nitrobenzene sulfonamide: A novel organocatalyst for an effective one-pot synthesis of α-amino phosphonates. Results Chem. 2025, 16, 102295. [Google Scholar] [CrossRef]
- Khatri, C.K.; Satalkar, V.B.; Chaturbhuj, G.U. Sulfated polyborate catalyzed Kabachnik-Fields reaction: An efficient and eco- friendly protocol for synthesis of α-amino phosphonates. Tetrahedron Lett. 2017, 58, 694–698. [Google Scholar] [CrossRef]
- Maghsoodlou, M.T.; Khorassani, S.M.H.; Hazeri, N.; Rostamizadeh, M.; Sajadikhah, S.S.; Shahkarami, Z.; Maleki, N. An Efficient Synthesis of α-Amino Phosphonates Using Silica Sulfuric Acid As a Heterogeneous Catalyst. Heteroat. Chem. 2009, 20, 316–318. [Google Scholar] [CrossRef]
- Sun, G.; Hou, J.; Dou, J.; Lu, J.; Hou, Y.; Xue, T.; Zhang, Z. Xanthan sulfuric acid as an efficient biodegradable and recyclable catalyst for the one-pot synthesis of α-amino phosphonates. J. Chin. Chem. Soc. 2010, 57, 1315–1320. [Google Scholar] [CrossRef]
- Sobhani, S.; Falatoonia, Z.M.; Honarmand, M. Synthesis of phosphoric acid supported on magnetic core–shell nanoparticles: A novel recyclable heterogeneous catalyst for Kabachnik– Fields reaction in water. RSC Adv. 2014, 4, 15797–15805. [Google Scholar] [CrossRef]
- Saberi, D.; Cheraghi, S.; Mahdudi, S.; Akbari, J.; Heydari, A. Dehydroascorbic acid (DHAA) capped magnetite nanoparticles as an efficient magnetic organocatalyst for the one-pot synthesis of α-aminonitriles and α-aminophosphonates. Tetrahedron Lett. 2013, 54, 6403–6406. [Google Scholar] [CrossRef]
- Gangireddy, C.S.R.; Chinthaparthi, R.R.; Mudumala, V.R.; Mamilla, M.; Arigala, U.R.S. An Efficient Green Synthesis of a New Class of α-Aminophosphonates under Microwave Irradiation Conditions in the Presence of PS/PTSA. Heteroat. Chem. 2014, 25, 147–156. [Google Scholar] [CrossRef]
- Gundluru, M.; Badavath, V.N.; Shaik, H.Y.; Sudileti, M.; Nemallapudi, B.R.; Gundala, S.; Zyryanov, G.V.; Cirandur, S.R. Design, synthesis, cytotoxic evaluation and molecular docking studies of novel thiazolyl α-aminophosphonates. Res. Chem. Intermed. 2021, 47, 1139–1160. [Google Scholar] [CrossRef]
- Reddy, C.B.; Kumar, K.S.; Kumar, M.A.; Reddy, M.V.N.; Krishna, B.S.; Naveen, M.; Arunasree, M.; Raju, C.N. PEG-SO3H catalyzed synthesis and cytotoxicity of α-aminophosphonates. Eur. J. Med. Chem. 2012, 47, 553–559. [Google Scholar] [CrossRef]
- Tillu, V.; Dumbre, D.; Wakharkar, R.; Choudhary, V. One-pot three-component Kabachnik–Fields synthesis of α-aminophosphonates using H-beta zeolite catalyst. Tetrahedron Lett. 2011, 52, 863–866. [Google Scholar] [CrossRef]
- Datta, K.; Mitra, B.; Ghosh, P. Humic Acid: A Green-Catalyst-Mediated Solvent-Free Synthesis of Functionalized Diethyl(Phenyl(phenylamino)methyl)phosphonate. ChemistrySelect 2023, 8, e202301255. [Google Scholar] [CrossRef]
- Kunde, S.P.; Kanade, K.G.; Karale, B.K.; Akolkar, H.N.; Arbuj, S.S.; Randhavane, P.V.; Shinde, S.T.; Shaikha, M.H.; Kulkarni, A.K. Nanostructured N doped TiO2 efficient stable catalyst for Kabachnik–Fields reaction under microwave irradiation. RSC Adv. 2020, 10, 26997–27005. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, M.H. TiO2 as a new and reusable catalyst for one-pot three-component syntheses of α-aminophosphonates in solvent-free conditions. Tetrahedron 2008, 64, 5459–5466. [Google Scholar] [CrossRef]
- Agawane, S.M.; Nagarkar, J.M. Nanoceria catalyzed synthesis of α-aminophosphonates under ultrasonication. Tetrahedron Lett. 2011, 52, 3499–3504. [Google Scholar] [CrossRef]
- Reddy, K.M.K.; Santhisudha, S.; Mohan, G.; Peddanna, K.; Rao, C.A.; Reddy, C.S. Nano Gd2O3 catalyzed synthesis and anti-oxidant activity of new α-aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 933–938. [Google Scholar] [CrossRef]
- Sreelakshmi, P.; Santhisudha, S.; Reddy, G.R.; Subbarao, Y.; Peddanna, K.; Apparao, C.; Reddy, C.S. Nano-Cuo–Au-catalyzed solvent-free synthesis of α-aminophosphonates and evaluation of their antioxidant and α-glucosidase enzyme inhibition activities. Synth. Commun. 2018, 48, 1148–1163. [Google Scholar] [CrossRef]
- Ummadi, R.R.; Ratnakaram, V.R.; Devineni, S.R.; Subramanyam, C.; Pavuluri, C.M. Eco-friendly TiO2–ZnO nano-catalysts: Synthesis, catalytic performance in synthesis of α-amino/hydroxyphosphonates and their ADME, docking and antimicrobial studies. Res. Chem. Intermed. 2025, 51, 3767–3795. [Google Scholar] [CrossRef]
- Sahani, A.; Rao, R.S.; Vadakkayl, A.; Santhosh, M.; Mummoorthi, M.; Karthick, M.; Ramanathan, C.R. Niobium pentoxide, a recyclable heterogeneous solid surface catalyst for the synthesis of α-amino phosphonates. J. Chem. Sci. 2021, 133, 4. [Google Scholar] [CrossRef]
- Popovics-Tóth, N.; Szabó, K.E.; Bálint, E. Study of the Three-Component Reactions of 2-Alkynylbenzaldehydes, Aniline, and Dialkyl Phosphites—The Significance of the Catalyst System. Materials 2021, 14, 6015. [Google Scholar] [CrossRef]
- Ando, K.; Egami, T. Facile synthesis of α-amino phosphonates in water by Kabachnik-Fields reaction using magnesium dodecylsulfate. Heteroat. Chem. 2011, 22, 358–362. [Google Scholar] [CrossRef]
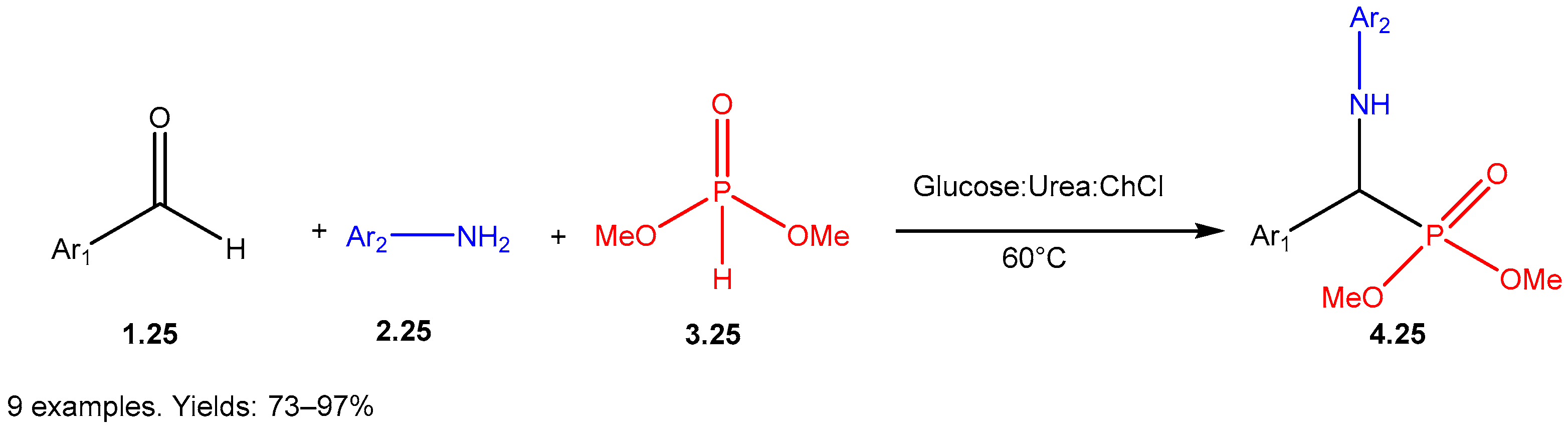
- Anupama, B.; Andria, A.A.; Jisha, M.T.; Cherian, A.L.; Letcy, V.T. Glucose-Urea-Choline chloride: A versatile catalyst and solvent for the Kabachnik-Fields’ reaction. J. Mol. Liq. 2025, 434, 128079. [Google Scholar] [CrossRef]
- Varga, P.R.; Keglevich, G. The Last Decade of Optically Active α-Aminophosphonates. Molecules 2023, 28, 6150. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable Potential of the r-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef] [PubMed]
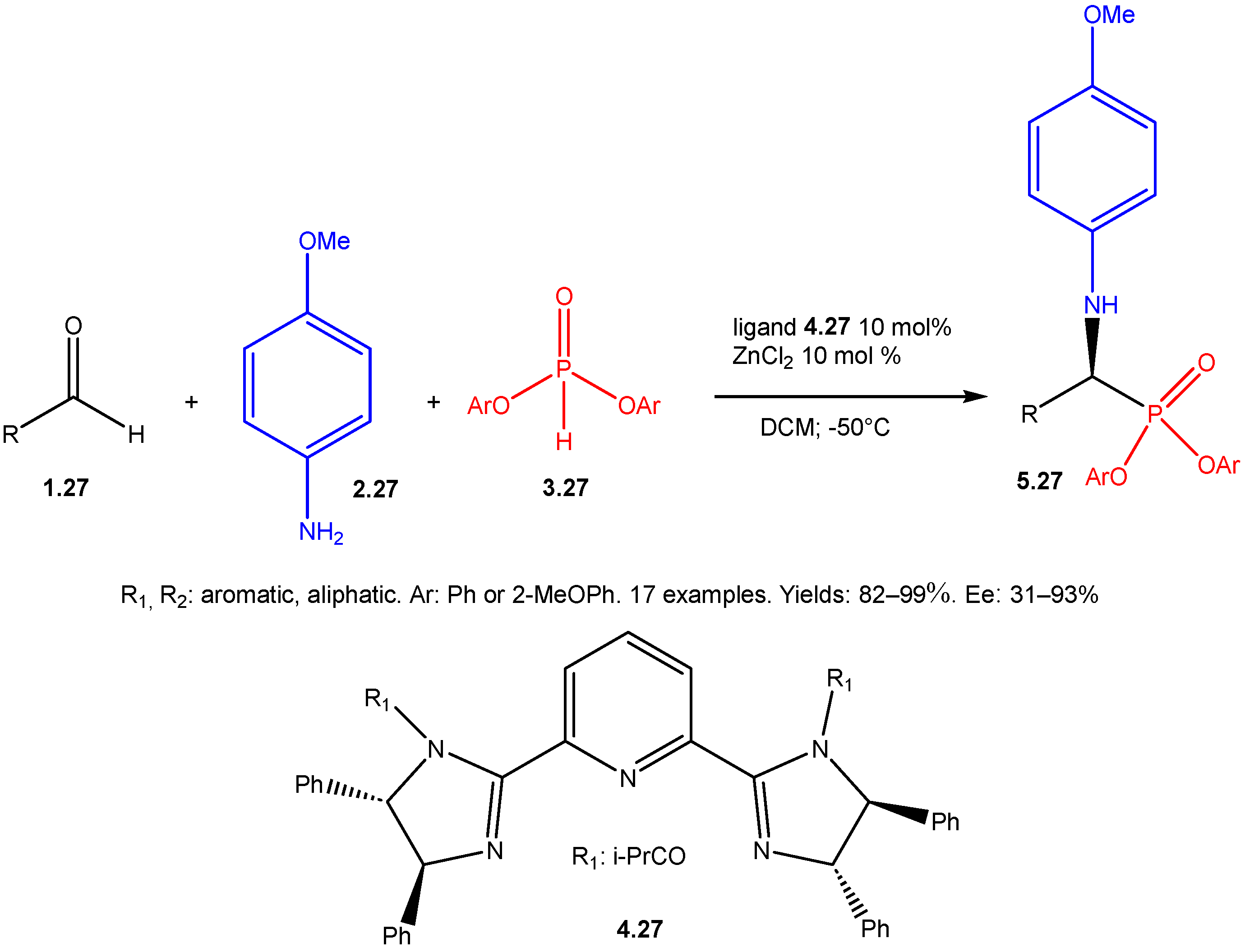
- Ohara, M.; Nakamura, S.; Shibata, N. Direct Enantioselective Three-Component Kabachnik–Fields Reaction Catalyzed by Chiral Bis(Imidazoline)-Zinc(II) Catalysts. Adv. Synth. Catal. 2011, 353, 3285–3289. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, L.; Chakraborty, D.; Borhan, B.; Wulff, W.D. Zirconium-Catalyzed Asymmetric Kabachnik–Fields Reactions of Aromatic and Aliphatic Aldehydes. Chem. Sci. 2021, 12, 12333–12345. [Google Scholar] [CrossRef]
- Reddy, P.S.; Reddy, M.V.K.; Reddy, P.V.G. Camphor-Derived Thioureas: Synthesis and Application in Asymmetric Kabachnik–Fields Reaction. Chin. Chem. Lett. 2016, 27, 943–947. [Google Scholar] [CrossRef]
- Saito, B.; Egami, H.; Katsuki, T. Synthesis of an Optically Active Al(Salalen) Complex and Its Application to Catalytic Hydrophosphonylation of Aldehydes and Aldimines. J. Am. Chem. Soc. 2007, 129, 1978–1986. [Google Scholar] [CrossRef]
- Zhou, X.; Shang, D.; Zhang, Q.; Lin, L.; Liu, X.; Feng, X. Enantioselective Three-Component Kabachnik-Fields Reaction Catalyzed by Chiral Scandium(III)-N,N′-Dioxide Complexes. Org. Lett. 2009, 11, 1401–1404. [Google Scholar] [CrossRef]
- Cheng, X.; Goddard, R.; Buth, G.; List, B. Direct Catalytic Asymmetric Three-Component Kabachnik–Fields Reaction. Angew. Chem. Int. Ed. 2008, 47, 5079–5081. [Google Scholar] [CrossRef]
- Wang, L.; Cui, S.; Meng, W.; Zhang, G.; Nie, J.; Ma, J. Asymmetric Synthesis of α-Aminophosphonates by Means of Direct Organocatalytic Three-Component Hydrophosphonylation. Chin. Sci. Bull. 2010, 55, 1729–1731. [Google Scholar] [CrossRef]
- Zou, L.; Huang, J.; Liao, N.; Liu, Y.; Guo, Q.; Peng, Y. Catalytic Asymmetric Three-Component Reaction of 2-Alkynylbenzaldehydes, Amines, and Dimethylphosphonate. Org. Lett. 2020, 22, 6932–6937. [Google Scholar] [CrossRef] [PubMed]
- Thorat, P.B.; Goswami, S.V.; Magar, R.L.; Patil, B.R.; Bhusare, S.R. An Efficient Organocatalysis: A One-Pot Highly Enantioselective Synthesis of α-Aminophosphonates. Eur. J. Org. Chem. 2013, 24, 5509–5516. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, Á.; Kalocsai, D.; Mátravölgyi, B.; Karaghiosoff, K.; Czugler, M.; Keglevich, G. Synthesis and utilization of optically active α-aminophosphonate derivatives by Kabachnik-Fields reaction. Tetrahedron 2017, 73, 5659–5667. [Google Scholar] [CrossRef]
- Viveros-Ceballos, J.L.; Cativiela, C.; Ordóñez, M. One-pot three-component highly diastereoselective synthesis of isoindolin-1-one-3-phosphonates under solvent and catalyst free-conditions. Tetrahedron Asymmetry 2011, 22, 1479–1484. [Google Scholar] [CrossRef]
- Ordóñez, M.; Tibhe, G.; Zamudio-Medina, A.; Viveros-Ceballos, J. An Easy Approach for the Synthesis of N-Substituted Isoindolin-1-ones. Synthesis 2012, 2012, 569–574. [Google Scholar] [CrossRef]
- Reyes-Gonzalez, M.A.; Zamudio-Medina, A.; Ordóñez, M. Practical and highstereoselective synthesis of 3-(arylmethylene)isoindolin-1-ones from 2-formylbenzoic acid. Tetrahedron Lett. 2012, 53, 5756–5758. [Google Scholar] [CrossRef]
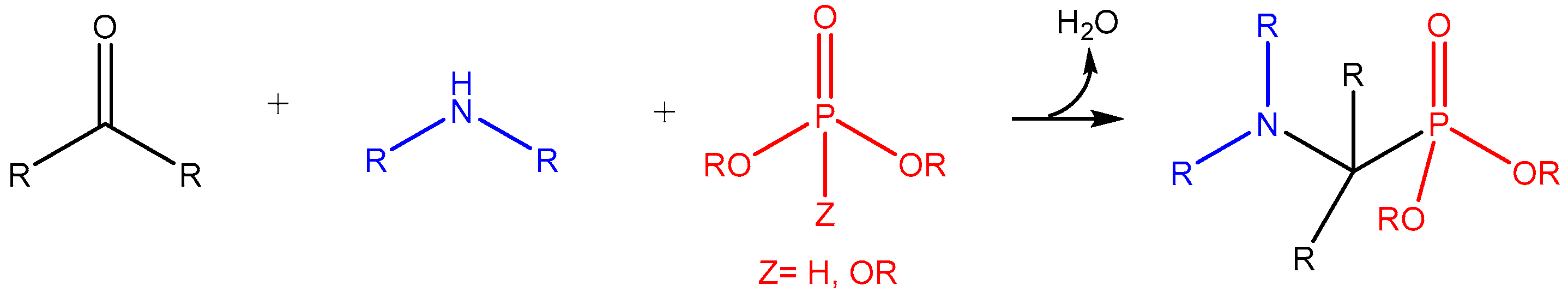
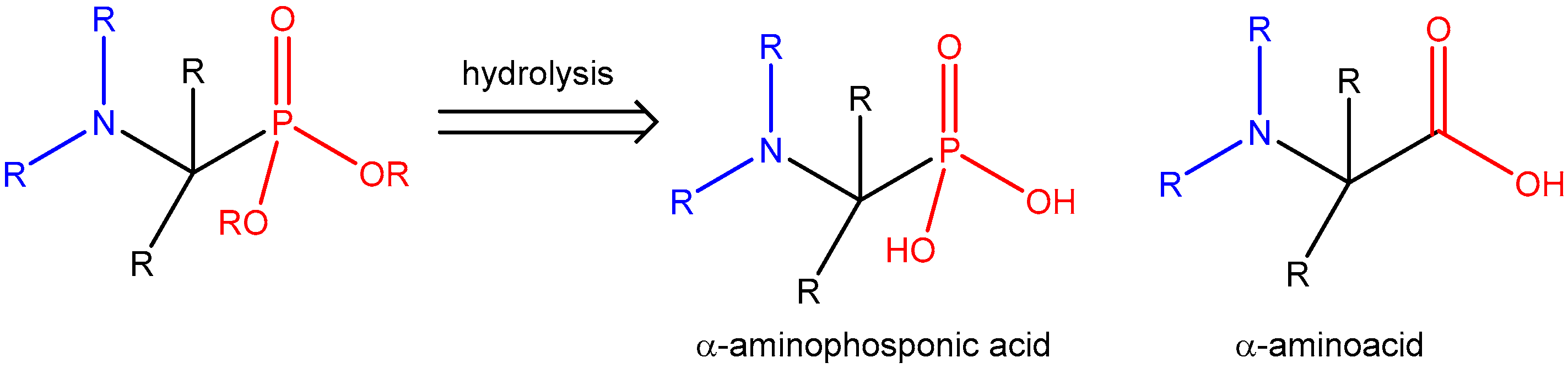
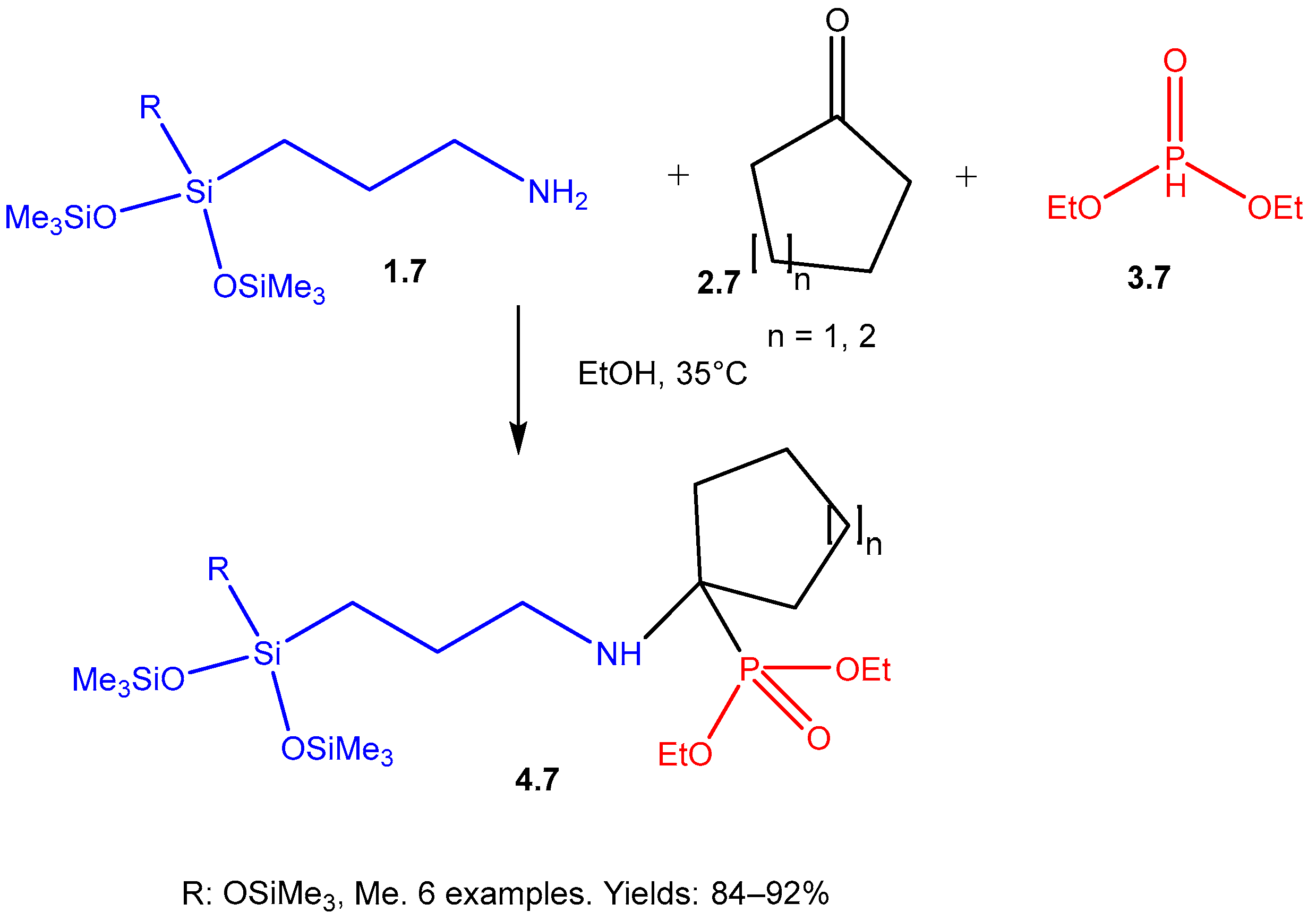
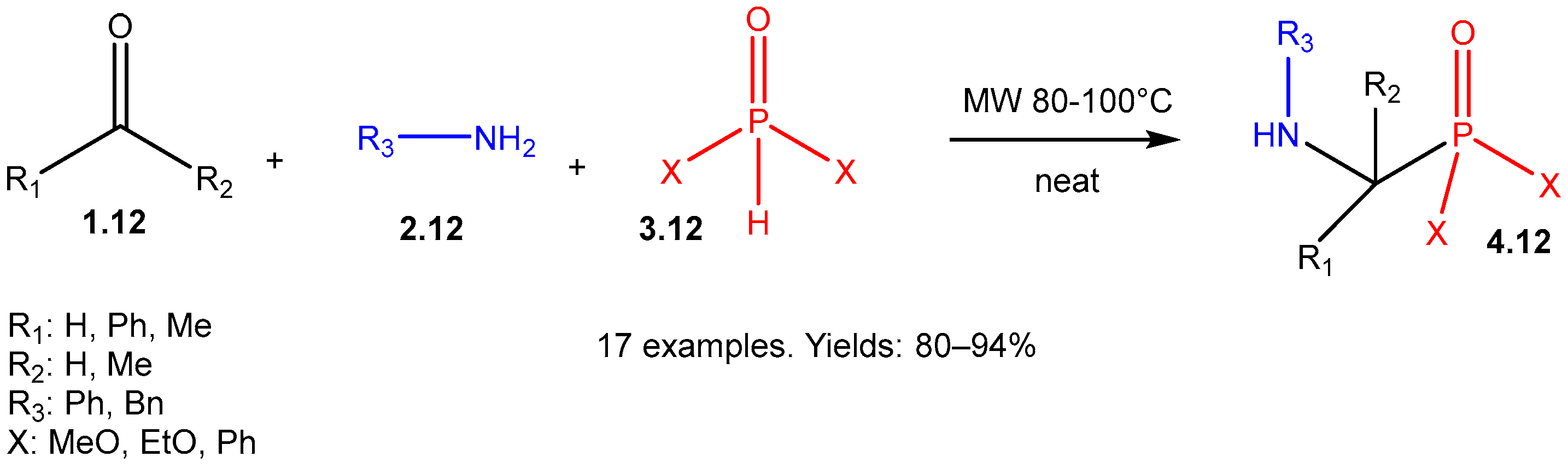
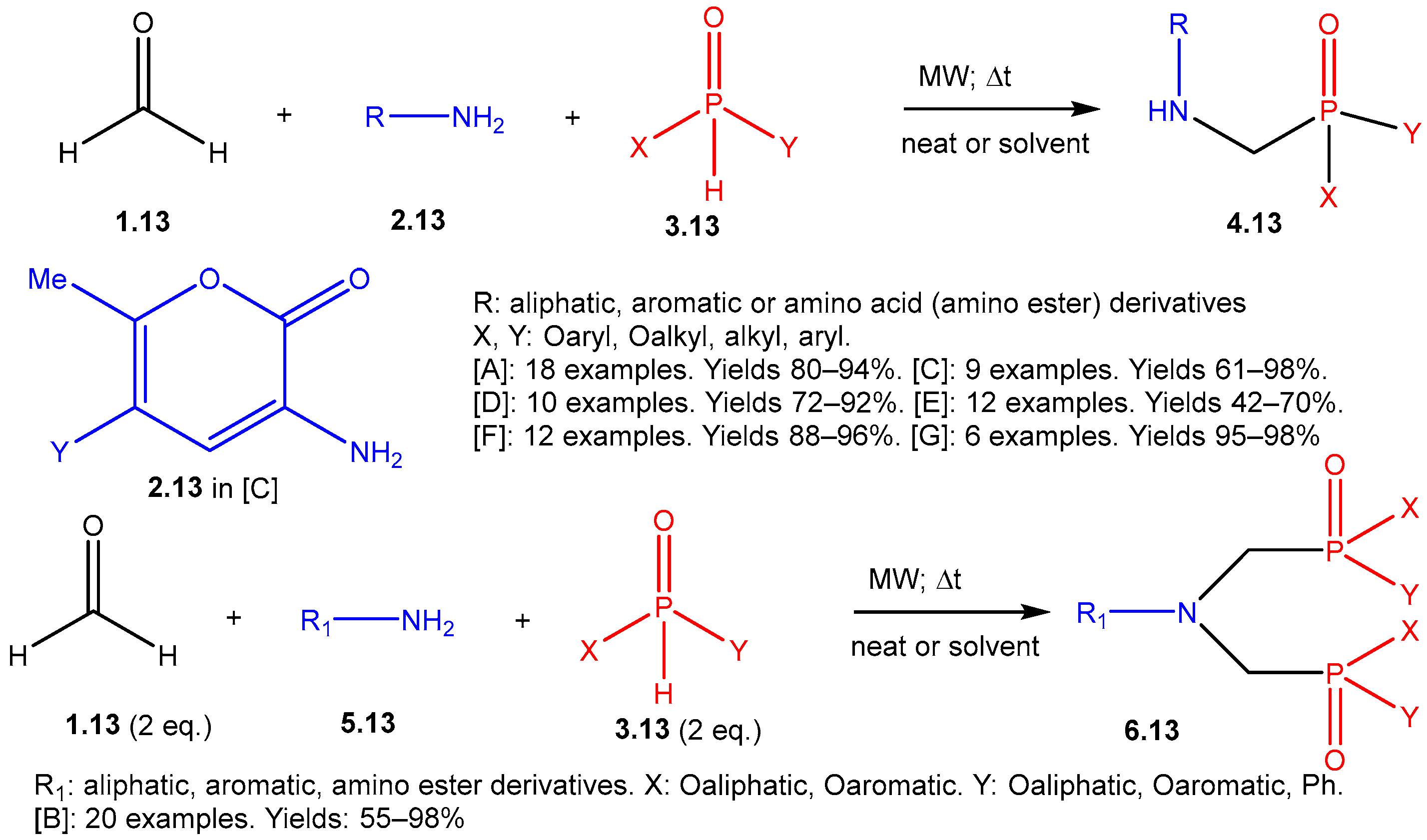
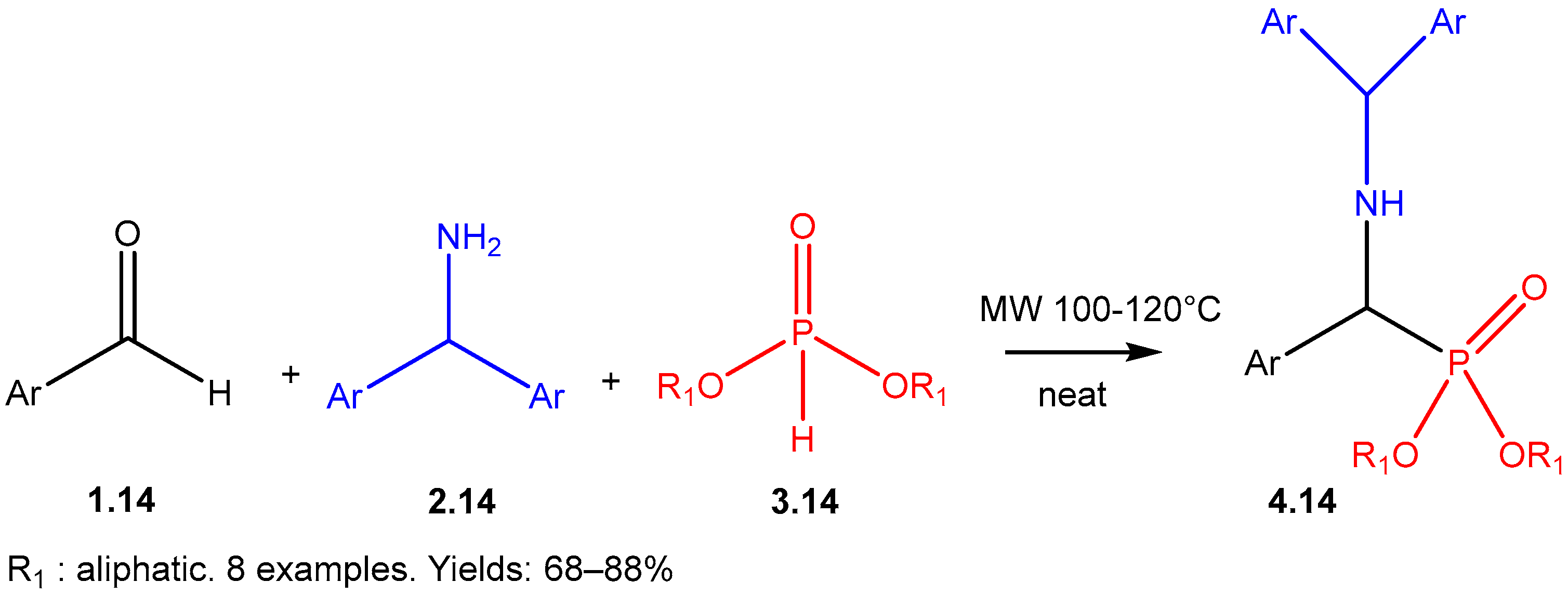
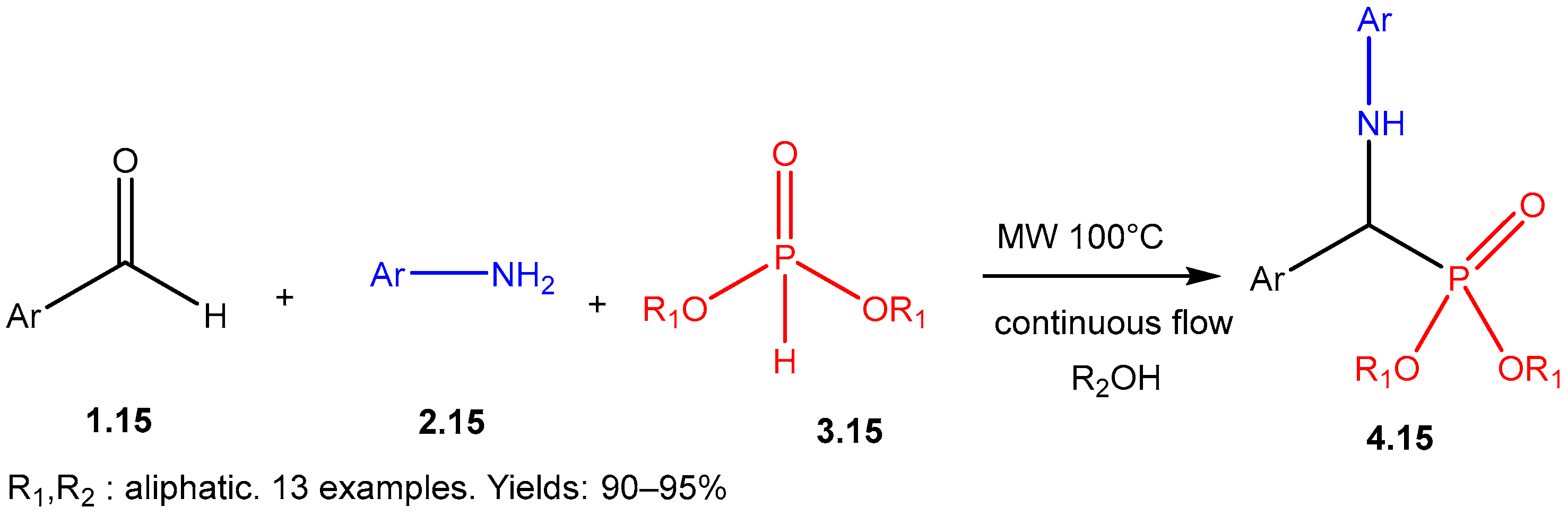
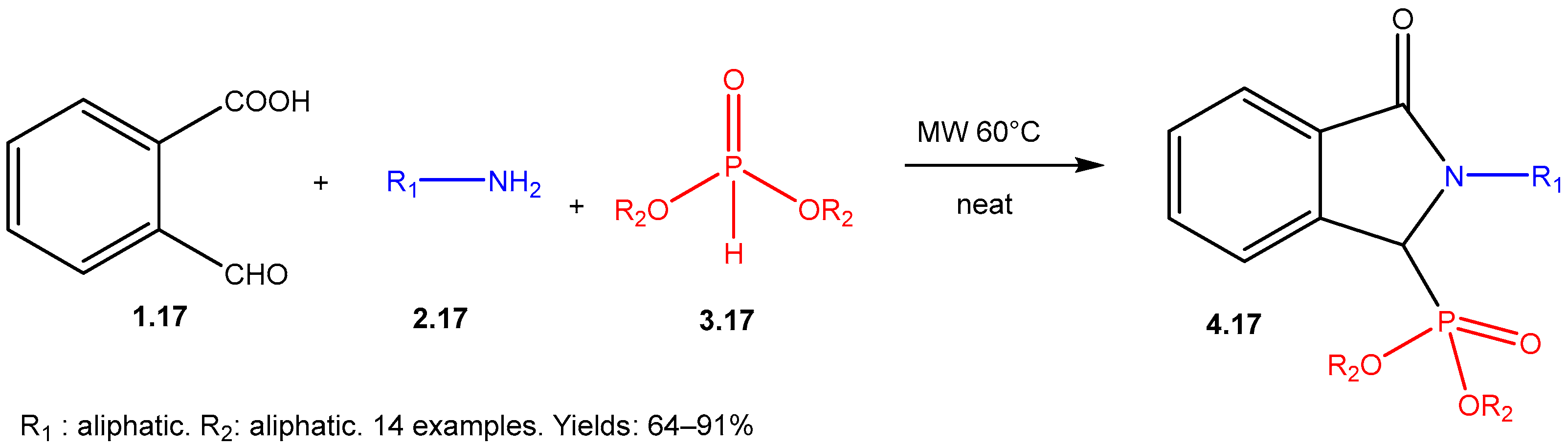
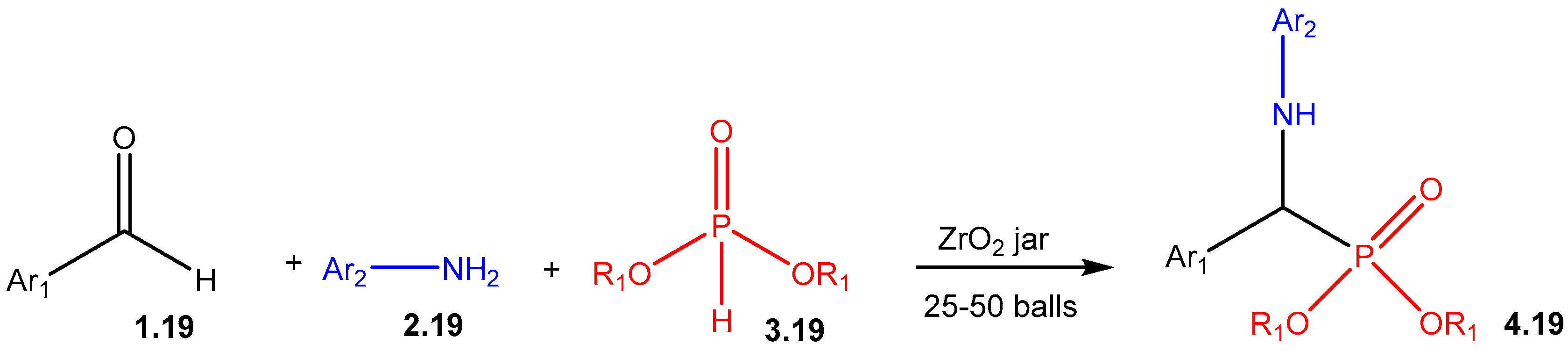
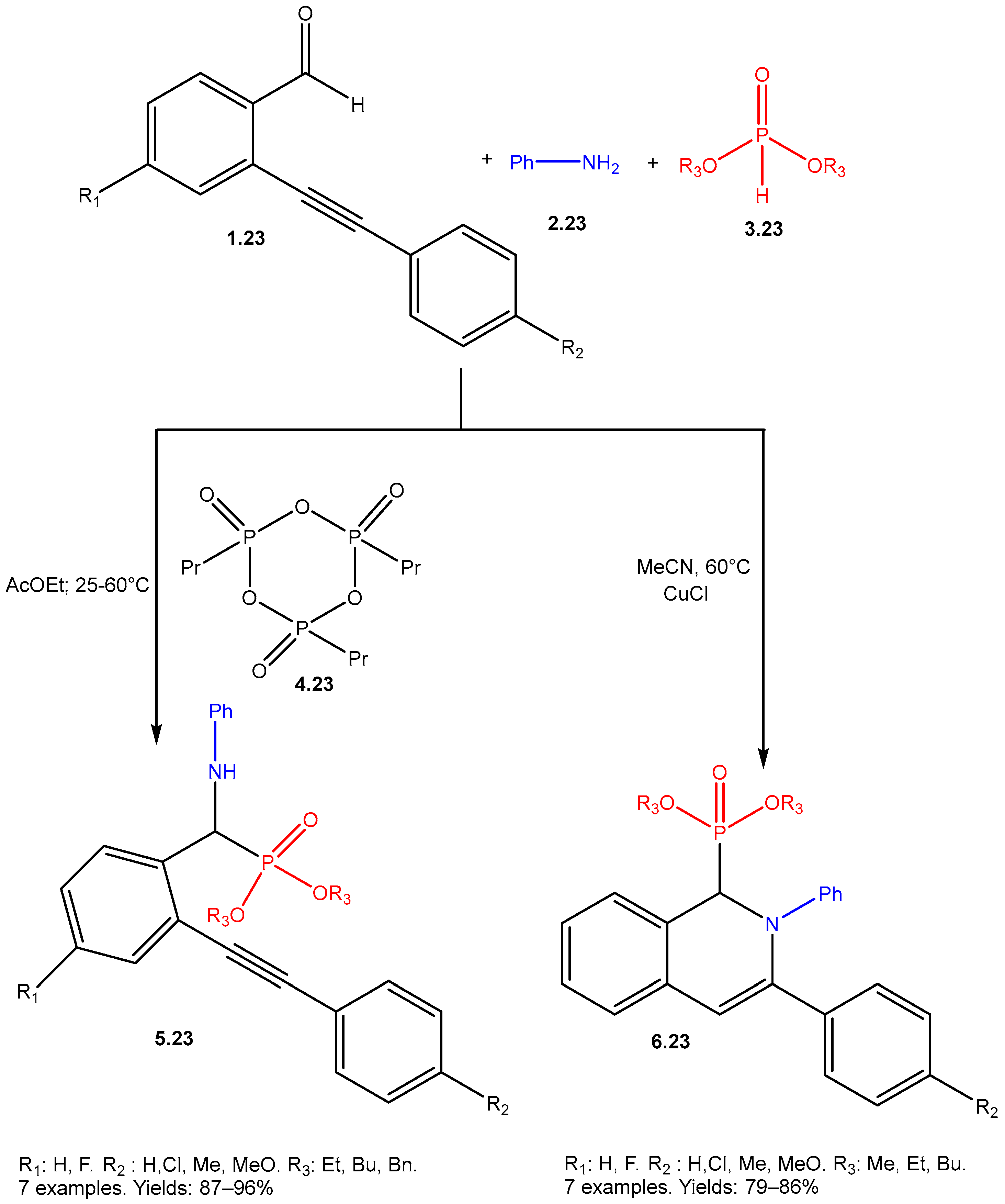

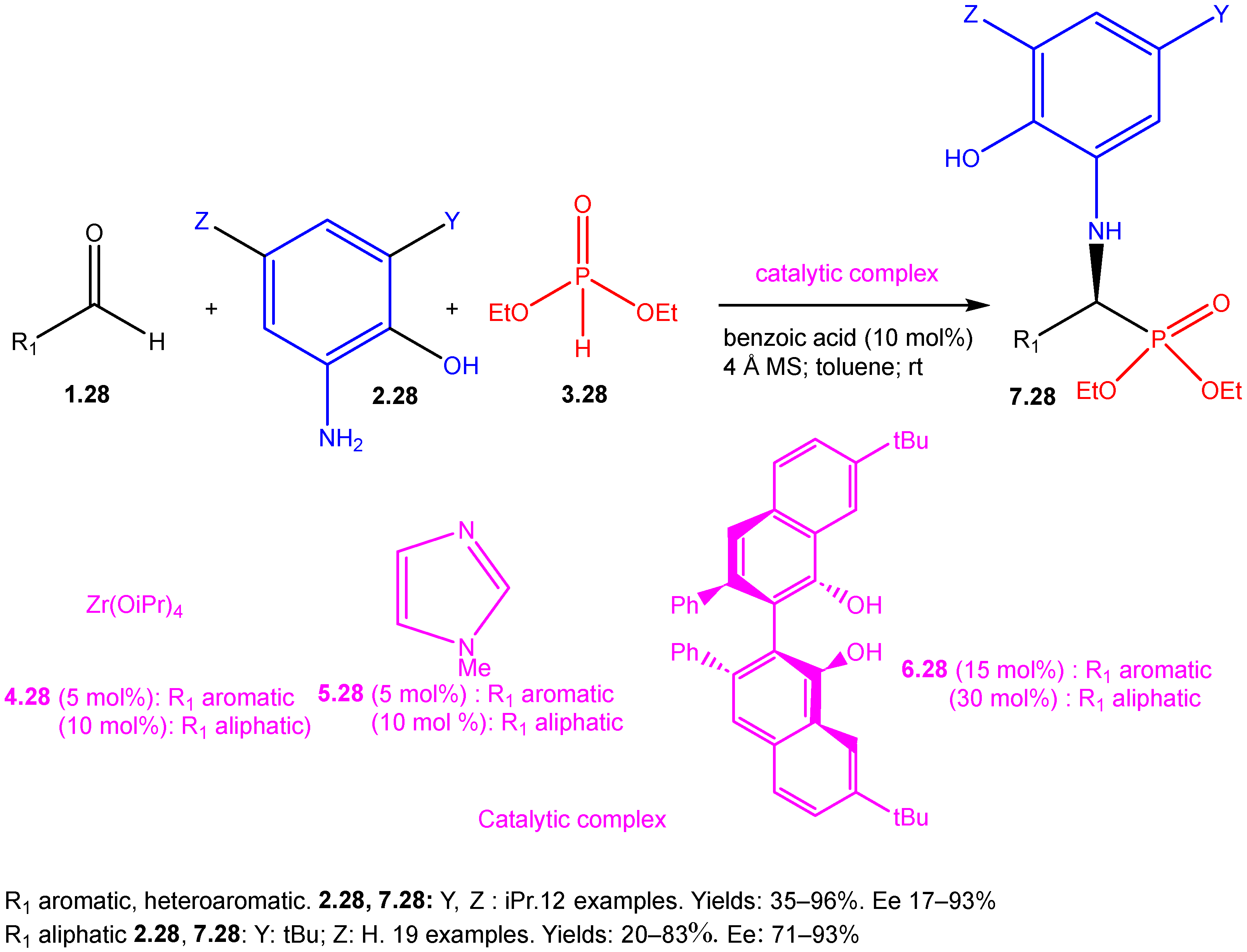
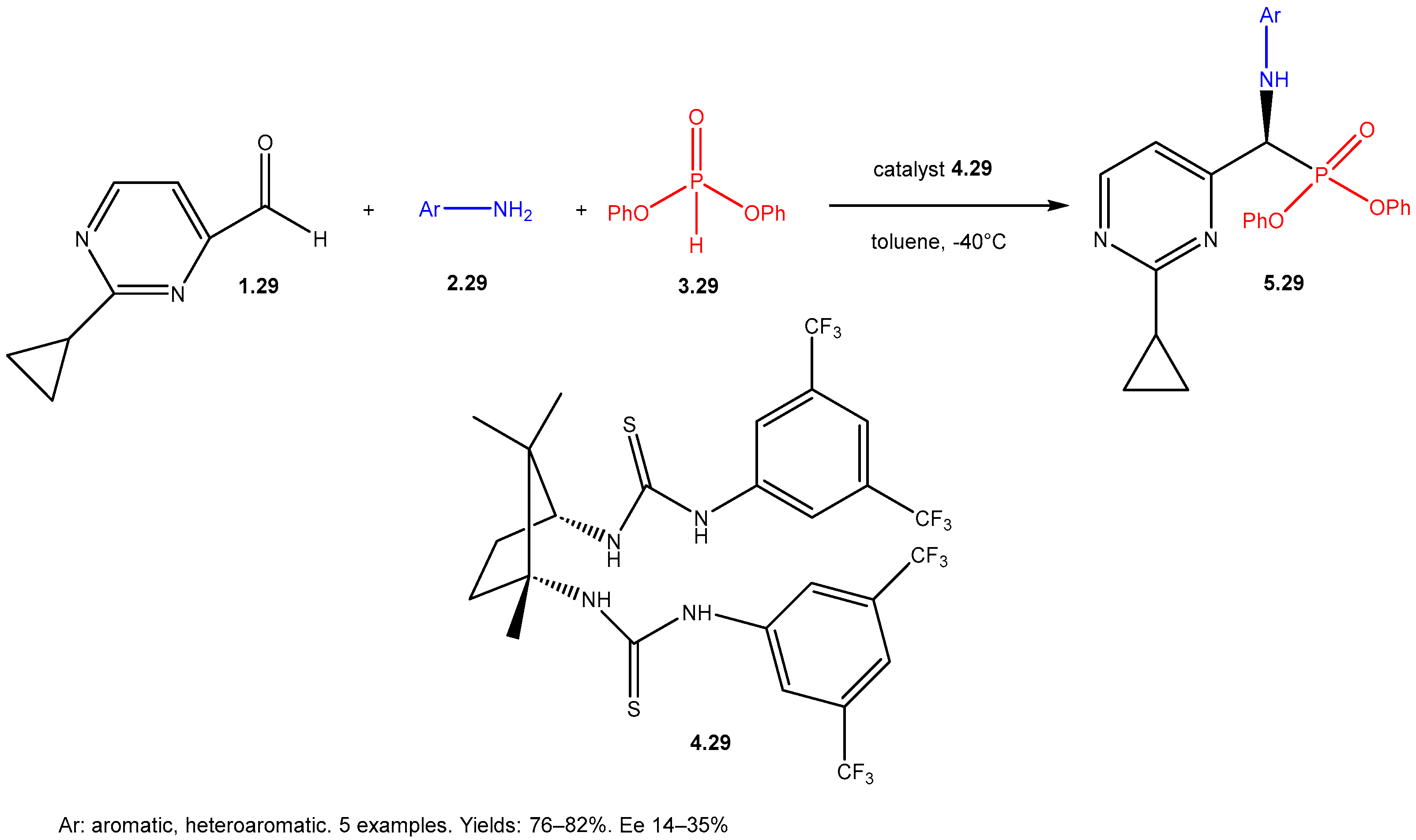
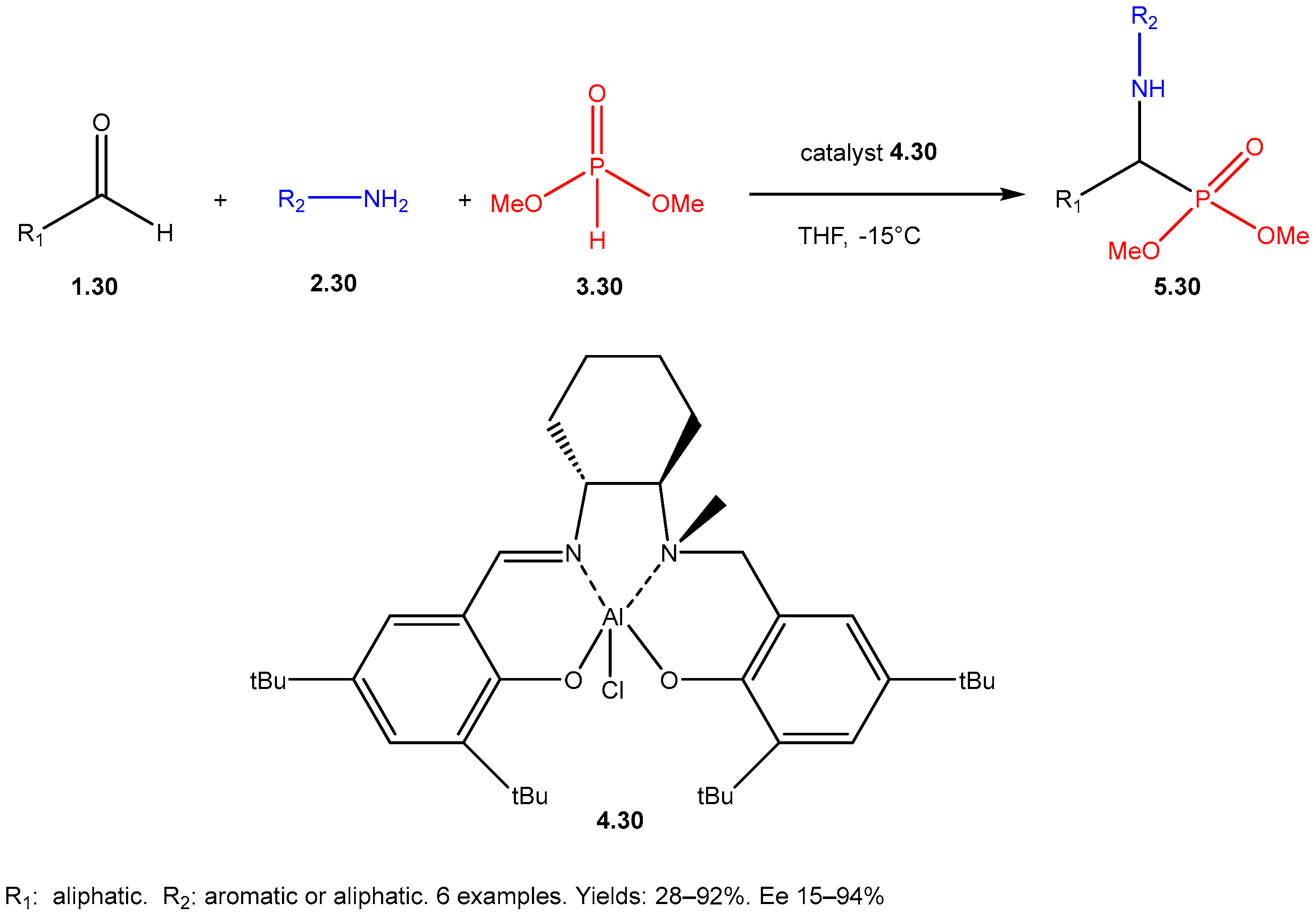
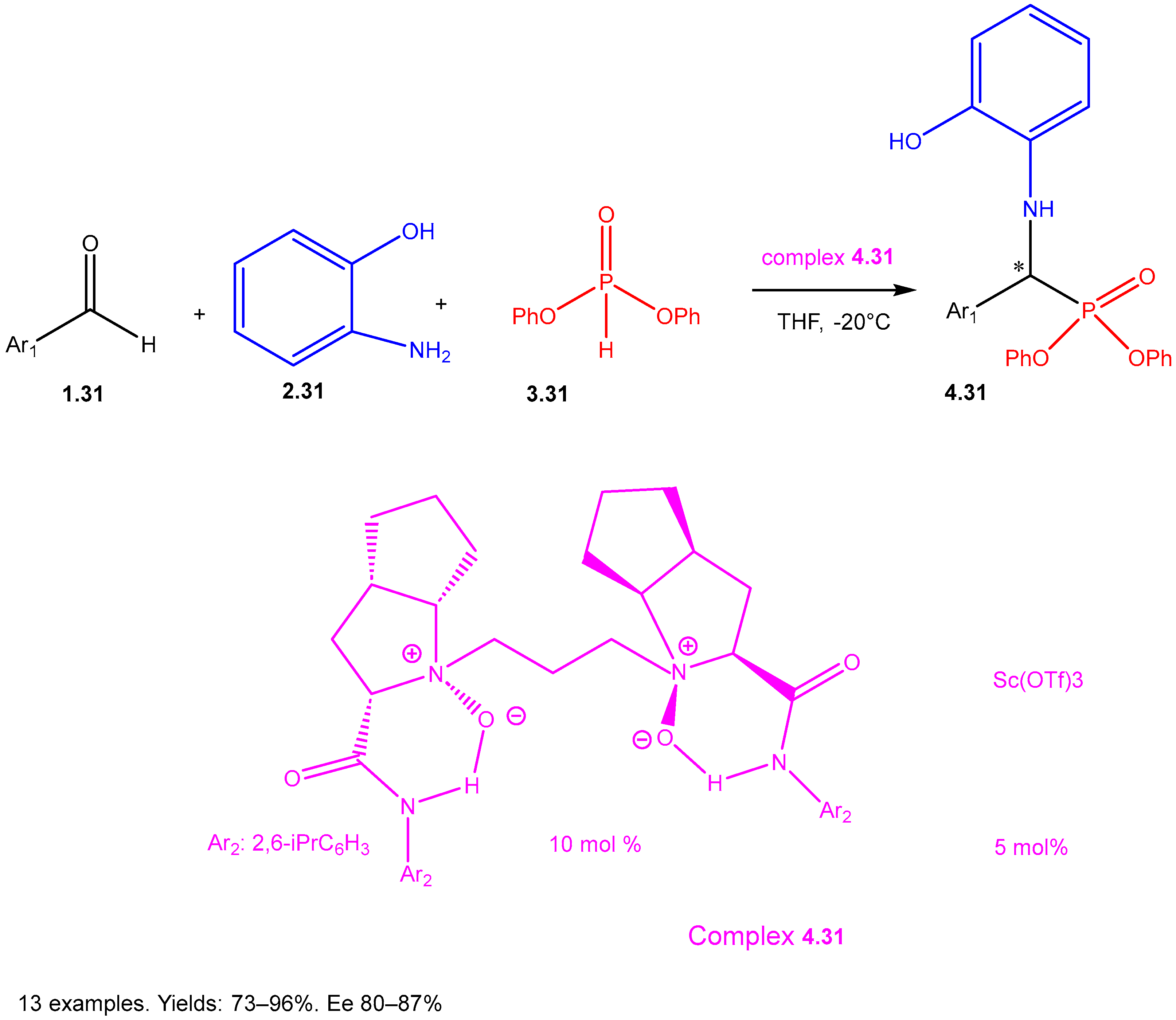
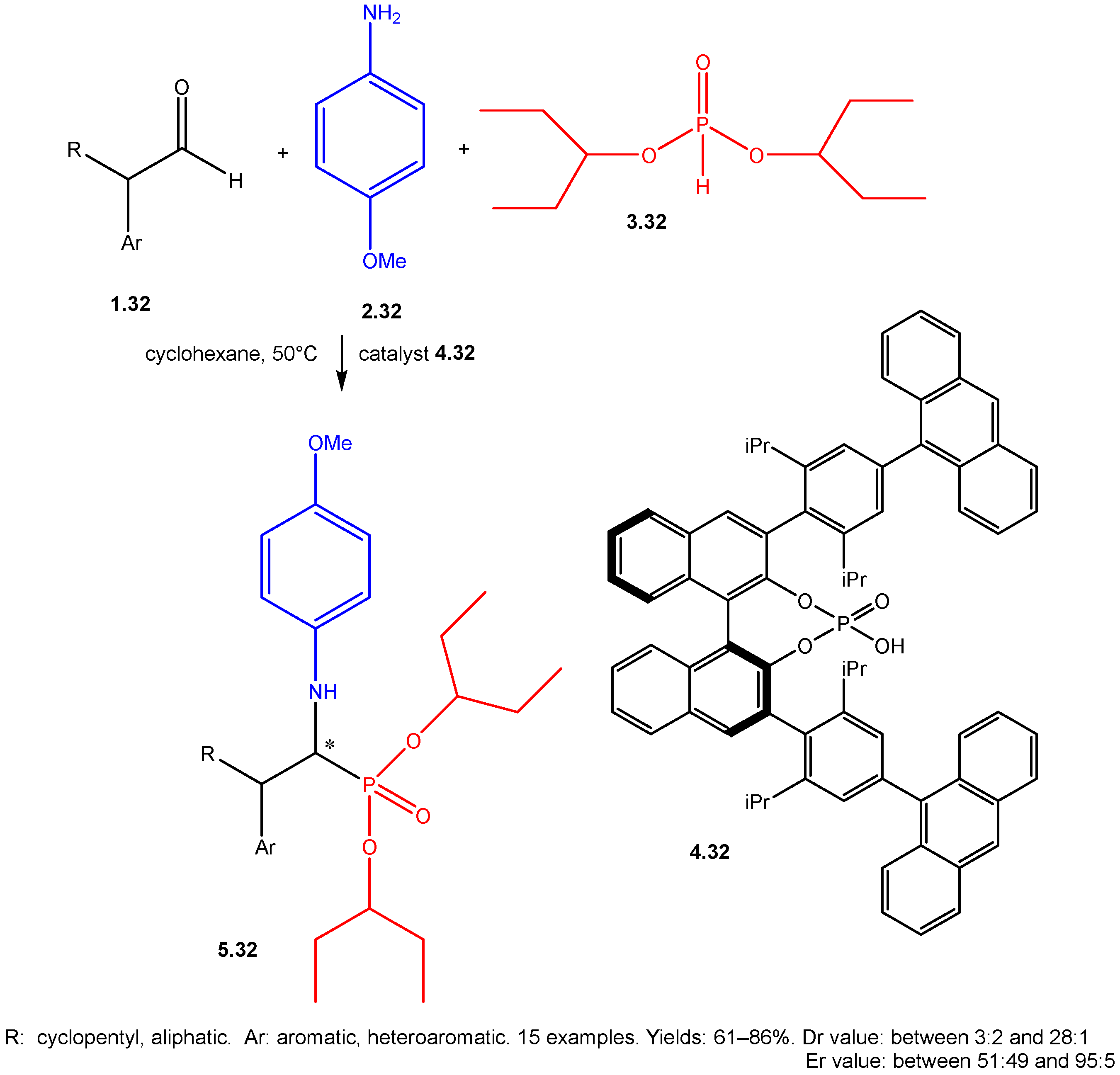
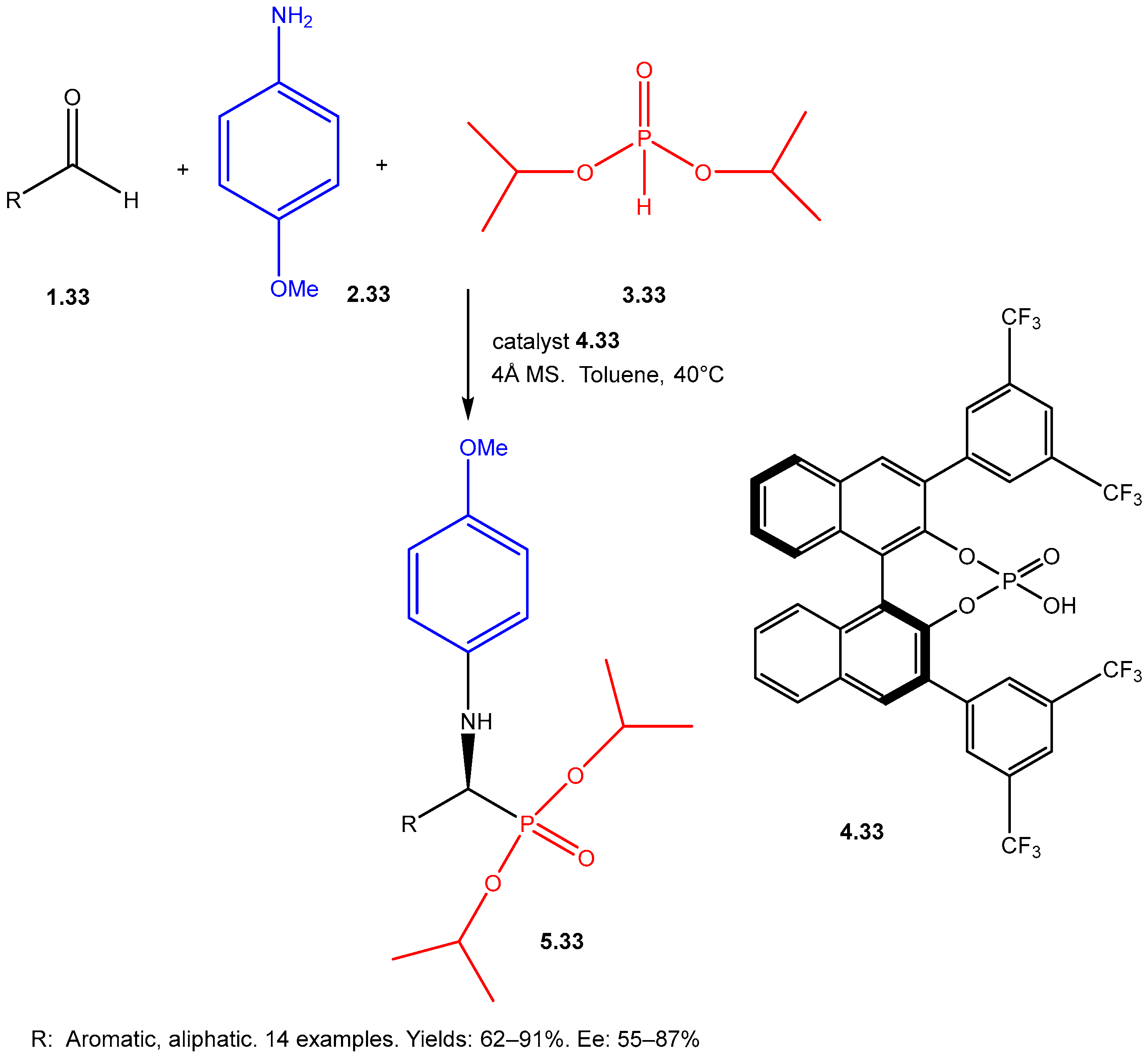
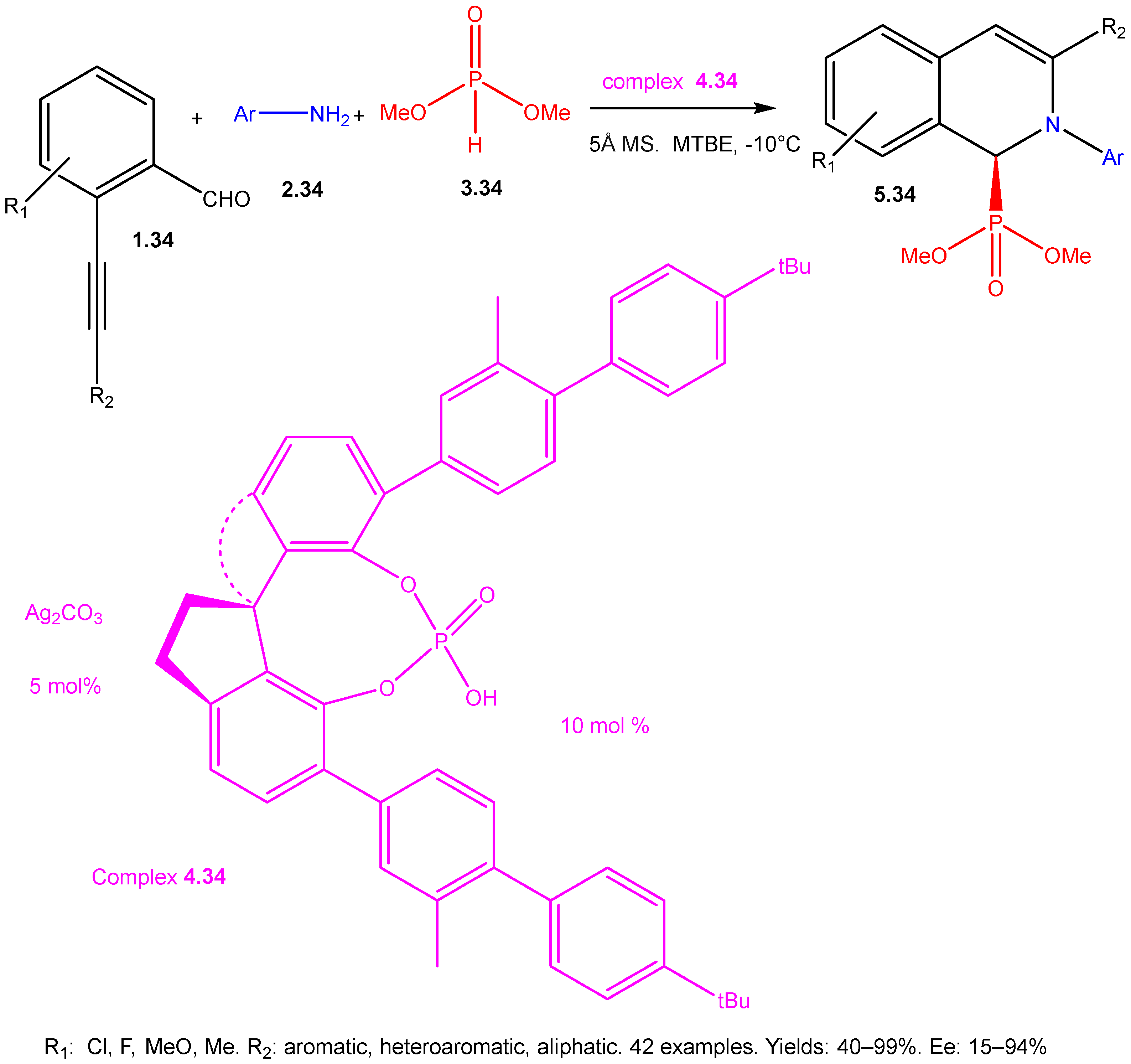
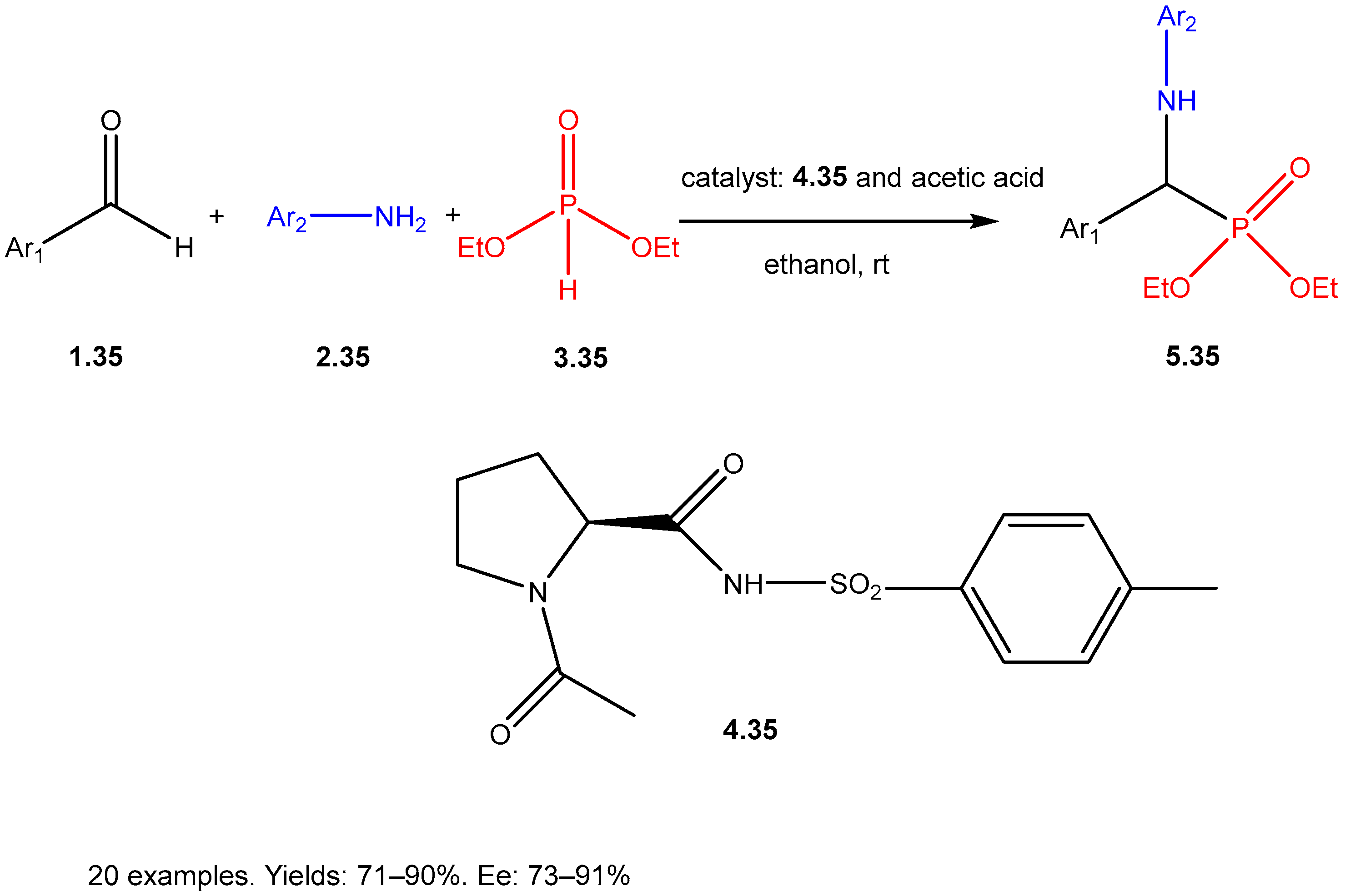
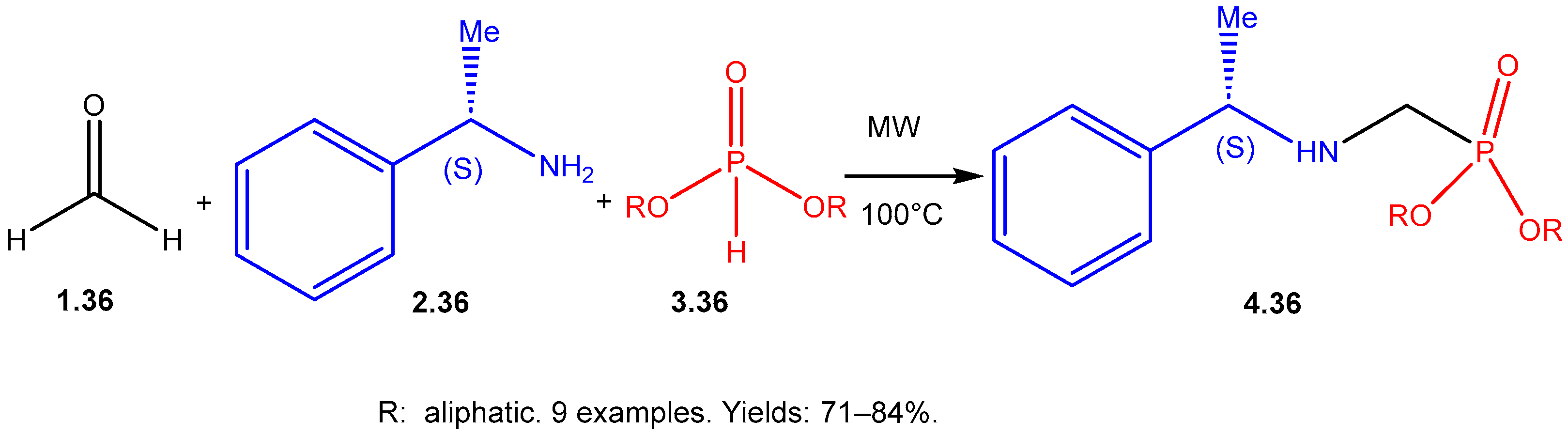
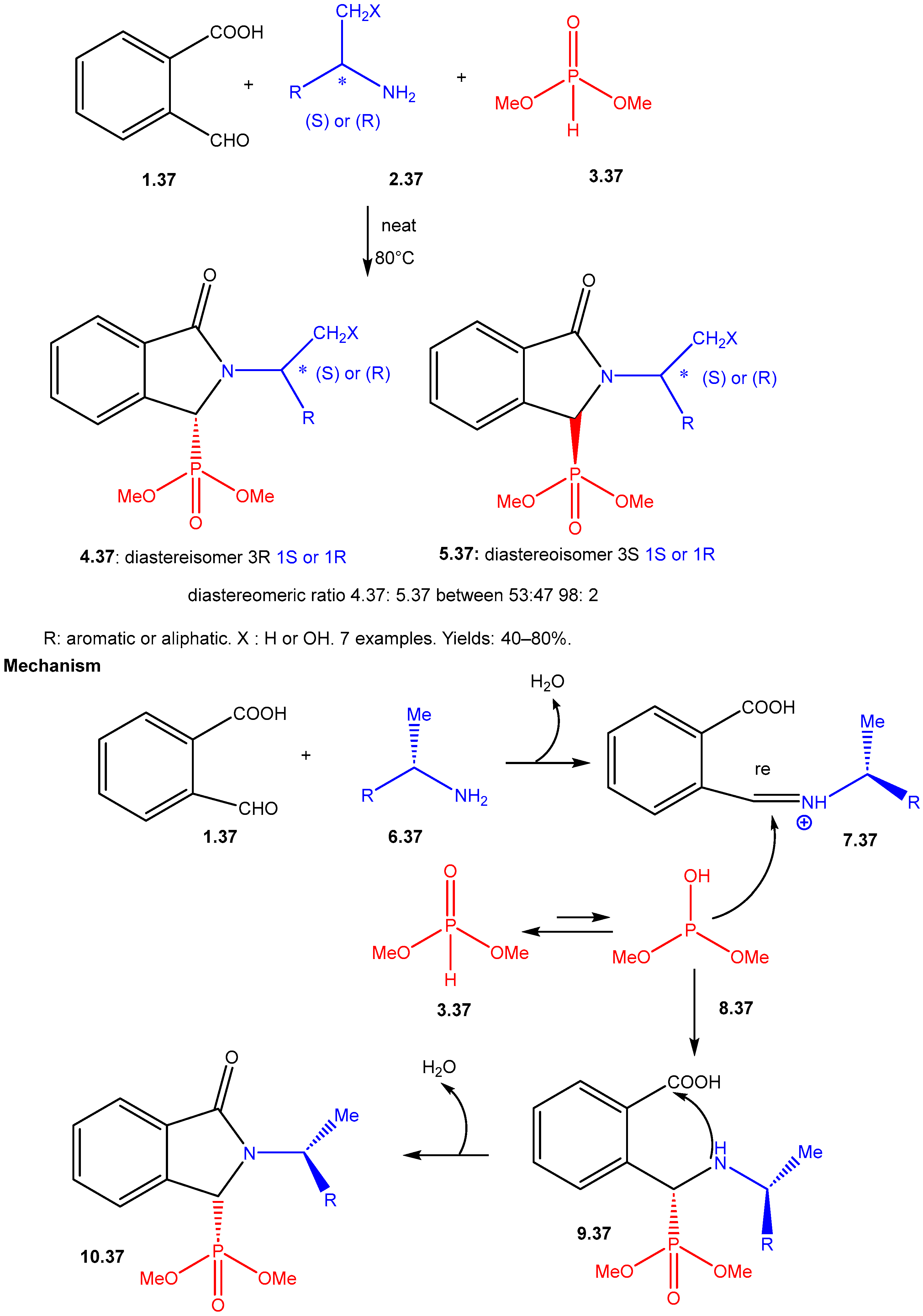
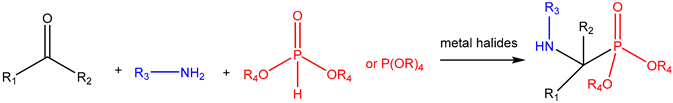 | ||||||
| Entry | Aldehydes or Ketones | Amines | Phosphites | Catalyst | Yields | Ref. |
| 1 | Aromatic Cyclohexanone | Aromatic | Diethyl | CeCl3·7H2O | 21 examples: 87–95% | [67] |
| 2 | Aromatic | Aromatic | Diethyl | FeCl3 | 9 examples: 87–95% | [68] |
| 3 | Aromatic Heteroaromatic Aliphatic | Aromatic Heteroaromatic Aliphatic | Trimethyl | AlCl3 or ZrCl4 | 11 examples: 66.87% | [69] |
| 4 | Aromatic | Aromatic, Heteroaromatic, Aliphatic | Dimethyl Diethyl | SbCl3 on SiO2 | 26 examples: 49–92% | [70] |
| 5 | Aromatic Heteroaromatic Aliphatic Acetophenone | Aromatic | Diethyl | TaCl5 on SiO2 | 18 examples: 81–94% | [71] |
| 6 | Aromatic Aliphatic Cyclic ketones | Aromatic Aliphatic | Dimethyl Diethyl | BiCl3 | 18 examples: 70–95% | [72] |
| 7 | Aromatic Heteroaromatic Aliphatic Cyclohexanone | Aromatic Heteroaromatic Aliphatic | Dimethyl Diethyl | ZrOCl2·8H2O | 56 examples: 70–96% | [73] |
| 8 | Aromatic | (Me3Si)2NH | Trimethyl Triethyl | LiClO4 | 9 examples: 82–92% | [74] |
| 9 | Aromatic | Aromatic | Diethyl | NbCl5 | 19 examples: 87–95% | [75] |
| 10 | Aromatic Aliphatic | Aromatic Aliphatic | Dimethyl Diethyl Trimethyl Triethyl | HfCl4 | 23 examples: 82–98% | [76] |
| 11 | Ferrocene 2-carboxaldehyde | Aromatic | Diethyl Diphenyl | InCl3 | 8 examples: 88–95% | [77] |
| 12 | Aromatic | Aromatic | Dimethyl | LaCl3·7H2O | 10 examples: 60–96% | [78] |
| 13 | Aromatic | Derivatives of benzothiazole or thiadiazole | Diethyl | LaCl3·7H2O on SiO2 | 32 examples: 87–97% | [79] |
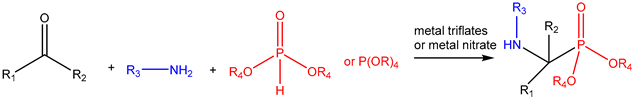 | ||||||
| Entry | Aldehydes or Ketones | Amines | Phosphites | Catalyst | Yields | Ref. |
| 1 | Aromatic | Aniline | Diethyl Triethyl | Lanthanides triflates | 23 examples: 18–99% | [80] |
| 2 | Aromatic Aliphatic | Aromatic Aliphatic | Trimethyl | Cu(OTf)2 | 11 examples: 57–97% | [81] |
| 3 | Aromatic Aliphatic Cyclohexanone | Aromatic Aliphatic | Diethyl | In(OTf)3 | 21 examples: 16–99% | [82] |
| 4 | Aromatic Aliphatic | Aromatic, Aliphatic | Dimethyl Diethyl | Zn(OTf)2 | 20 examples: 72–93% | [83] |
| 5 | Aromatic Heteroaromatic | 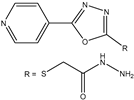 | Diethyl | Fe(OTf)3 | 13 examples: 65–73% | [84] |
| 6 | Aromatic | Heteroaromatic | Diethyl Diphenyl | 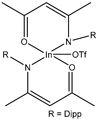 | 18 examples: 86–97% | [30] |
| 7 | Aromatic Aliphatic | Aromatic | Diethyl | Bi(NO3)3.5H2O | 18 examples: 80–95% | [85] |
 | ||||||
| Entry | Aldehydes or Ketones | Amines | Phosphites | Catalyst | Yields | Ref. |
| 1 | Aromatic | NH4OH | Diethyl | 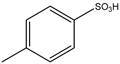 | 9 examples: 53–81% | [89] |
| 2 | HCHO | Aromatic Aliphatic | Aromatic Aliphatic | 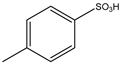 | 8 examples: 51–95% | [90] |
| 3 | HCHO | 2-Aminopyridine or 2-Phenylethan-1-amine | Didecyl or Decyl phenyl | 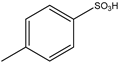 | 2 examples: 94% | [91] |
| 4 | HCHO | aminoacetaldehyde dimethylacetal | Dihexyl | 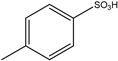 | 1 example: 91% | [92] |
| 5 | HCHO | aminoaaldehyde dimethylacetals | Aliphatic | 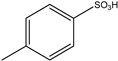 | 8 examples: Yields n.a. | [93] |
| 6 | Salicylaldehydes | Aromatic | Triphenyl | 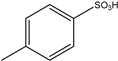 | 12 examples: 82–94% | [11] |
| 7 | Aromatic | 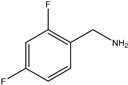 | Diethyl | MeSO3H | 7 examples: 75–92% | [94] |
| 8 | Aromatic | Aromatic Aliphatic | Diethyl | Sulfamic acid | 17 examples: 81–100% | [95] |
| 9 | Aromatic Heteroaromatic | p-Anisidine | Diethyl | CF3COOH | 9 examples: 87–95% | [96] |
| 10 | Aromatic Aliphatic | Aromatic | Trimethyl | Me2S+Br Br− | 14 examples: 87–95% | [97] |
| 11 | Aromatic | Aromatic | Triethyl | Tartaric acid | 12 examples: 65–89% | [98] |
| 12 | Aromatic Aliphatic Cyclic Ketones Aliphatic Ketones | Benzylamine | Dimethyl | Phenyl boronic acid | 22 examples: 28–93% | [99] |
| 13 | Aromatic Aliphatic Aliphatic Ketones | Benzylamine | Dimethyl | Phenyl phosphonic acid | 20 examples: 47–98% | [100] |
| 14 | Aromatic | Aromatic | Diethyl | Citric acid Malic acid Tartaric acid Oxalic acid | 84 examples: 54–95% | [101] |
| 15 | 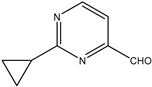 | Aromatic Heteroaromatic | Aliphatic Diphenyl | H3PMo12O40 | 14 examples: 89–96% | [102] |
| 16 | Aromatic Heteroaromatic Aliphatic | Aromatic | Diethyl | 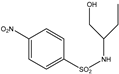 | 26 examples: 87–96% | [103] |
 | ||||||
| Entry | Aldehydes or Ketones | Amines | Phosphites | Catalyst | Yields | Ref. |
| 1 | Aromatic | Aromatic | Diethyl | Sulfated polyborate | 20 examples: 90–98% | [104] |
| 2 | Aromatic | Aniline | Dimethyl Diethyl | Silica sulfuric acid | 11 examples: 80–95% | [105] |
| 3 | Aromatic Heteroaromatic | Aromatic | Diethyl | Xanthan sulfuric acid | 32 examples: 88–95% | [106] |
| 4 | Aromatic Aliphatic Cyclohexanone | Aromatic Aliphatic | Triethyl | Phosphoric acid on γ-Fe2O3@SiO2 | 16 example: 82–95% | [107] |
| 5 | Aromatic Heteroaromatic Aliphatic Cyclohexanone | Aromatic Aliphatic | Methyl | DHAA-Fe3O4 | 10 examples: 75–95% | [108] |
| 6 | Aromatic Aliphatic | 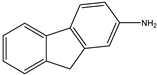 | Diethyl | polystyrene-supported  | 18 examples: Yields: n.a | [109] |
| 7 | 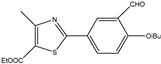 | Aromatic | Diethyl | β-cyclodextrin-supported sulfonic acid | 10 examples: 91–96% | [110] |
| 8 | 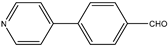 | Aromatic Heteroaromatic | Triethyl | polyethylene glycol sulfonic acid | 10 examples: 82–96% | [111] |
| 9 | Aromatic Heteroaromatic Aliphatic Acetophenone | Aromatic Aliphatic | Dialkyl | H-beta zeolite | 15 examples: 76–93% | [112] |
| 10 | Aromatic Heteroaromatic | Aromatic Aliphatic | Diethyl | Humic acid | 25 examples: 78–93% | [113] |
| 11 | 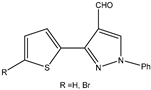 | Aromatic | Triethyl | N-TiO2 | 11 examples: 71–95% | [114] |
| 12 | Aromatic Heteroaromatic Aliphatic Cyclohexanone Acetophenone | Aromatic Aliphatic | Diethyl | TiO2 | 36 examples: 50–98% | [115] |
 | ||||||
| Entry | Aldehydes | Amines | Phosphites | Catalyst | Yields | Ref. |
| 1 | Aromatic Heteroaromatic | Aromatic Heteroaromatic Aliphatic | Diethyl | Nano CeO2 | 16 examples: 67–99% | [116] |
| 2 | 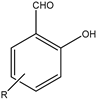 | 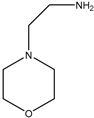 | Dimethyl | Nano Gd2O3 | 10 examples: Yields: n.a | [117] |
| 3 | Aromatic | 2-aminophenol | Dimethyl | Nano CuO-Au | 10 examples: 87–96% | [118] |
| 4 | Aromatic | 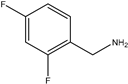 | Diethyl | TiO2-ZnO | 12 examples: 91–95% | [119] |
| 5 | Aromatic Aliphatic | Aromatic Heteroaromatic | Diethyl | Nb2O5 | 43 examples: 40–97% | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ghigo, G.; Nicoletti, S.; Dughera, S. The Kabachnik–Fields Reaction: A Key Transformation in Organophosphorus Chemistry. Reactions 2026, 7, 3. https://doi.org/10.3390/reactions7010003
Ghigo G, Nicoletti S, Dughera S. The Kabachnik–Fields Reaction: A Key Transformation in Organophosphorus Chemistry. Reactions. 2026; 7(1):3. https://doi.org/10.3390/reactions7010003
Chicago/Turabian StyleGhigo, Giovanni, Sara Nicoletti, and Stefano Dughera. 2026. "The Kabachnik–Fields Reaction: A Key Transformation in Organophosphorus Chemistry" Reactions 7, no. 1: 3. https://doi.org/10.3390/reactions7010003
APA StyleGhigo, G., Nicoletti, S., & Dughera, S. (2026). The Kabachnik–Fields Reaction: A Key Transformation in Organophosphorus Chemistry. Reactions, 7(1), 3. https://doi.org/10.3390/reactions7010003