Optimization and Kinetic Study of Palmitic Acid Esterification with Subcritical Methanol via Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. The Esterification Reaction of Palmitic Acid and Methanol
2.3. Product Analysis
2.4. Experimental Design of Surface Response Method
3. Results and Discussion
3.1. Effects of Different Reaction Parameters on the Esterification of Methanol and Palmitic Acid
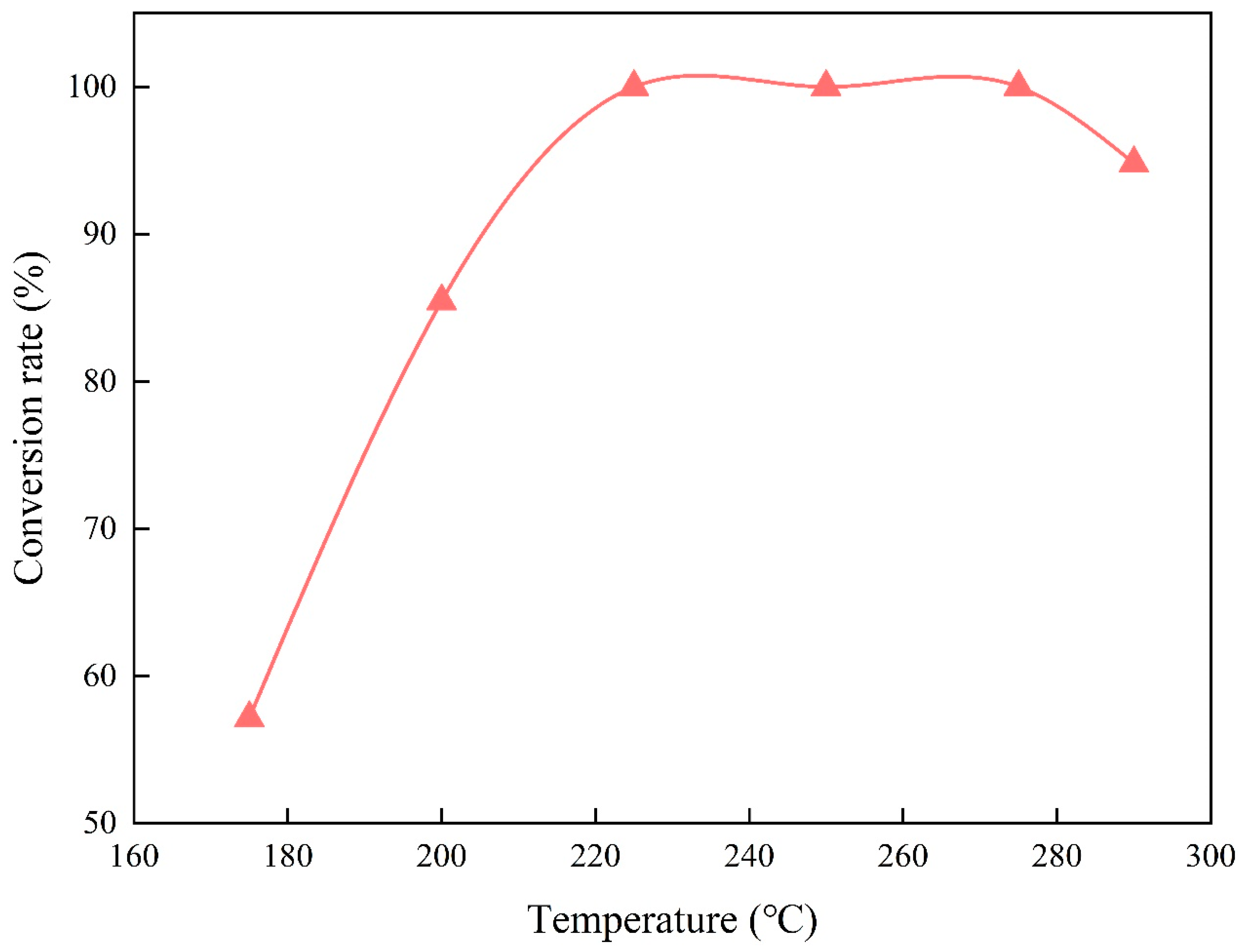
3.1.1. Effects of Reaction Temperature on the Esterification of Methanol and Palmitic Acid
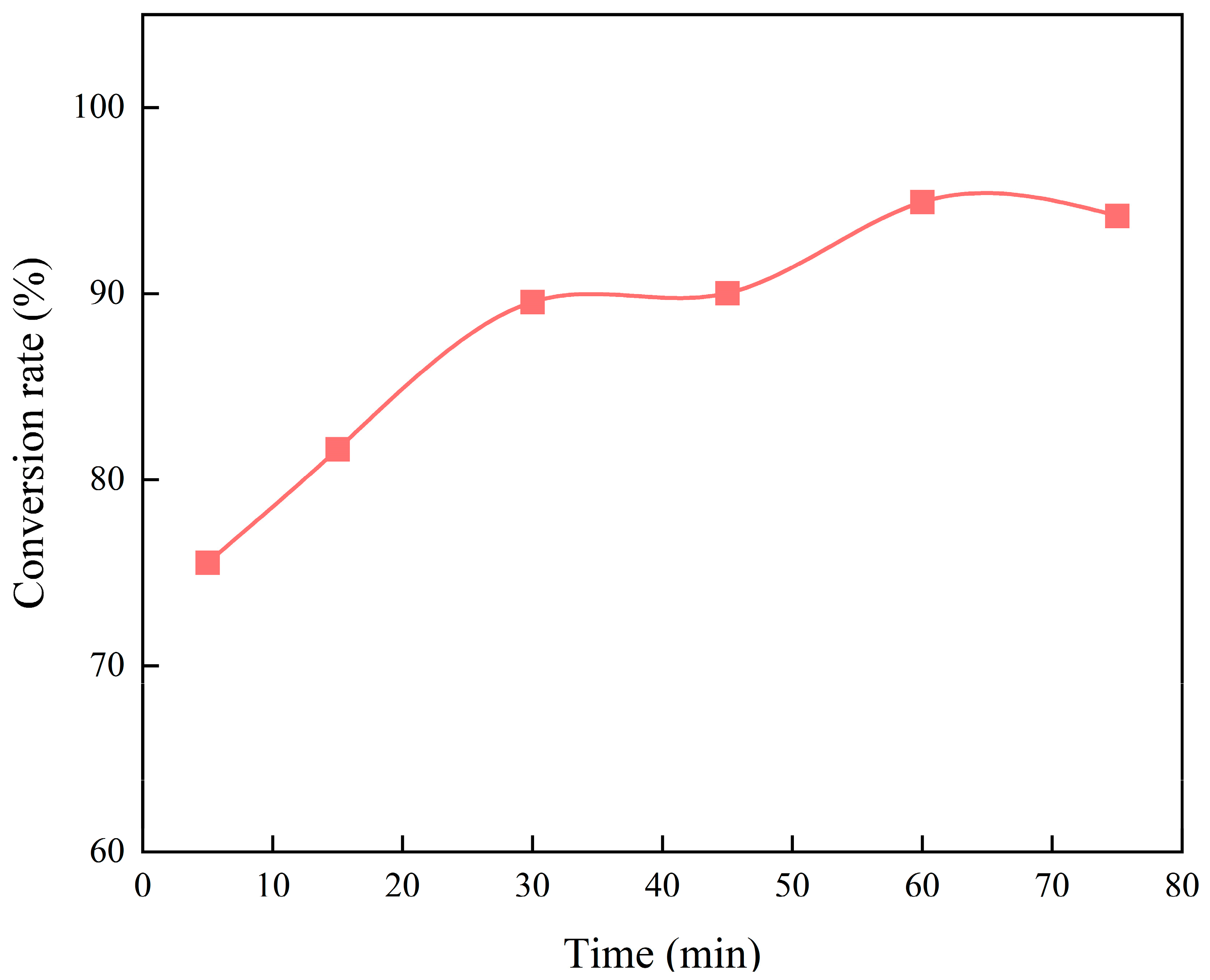
3.1.2. Effects of Residence Time on the Esterification of Methanol and Palmitic Acid
3.1.3. Effects of the Molar Ratio of Methanol and Palmitic Acid on Their Esterification
3.2. Response Surface Method Modeling and Variance Analysis
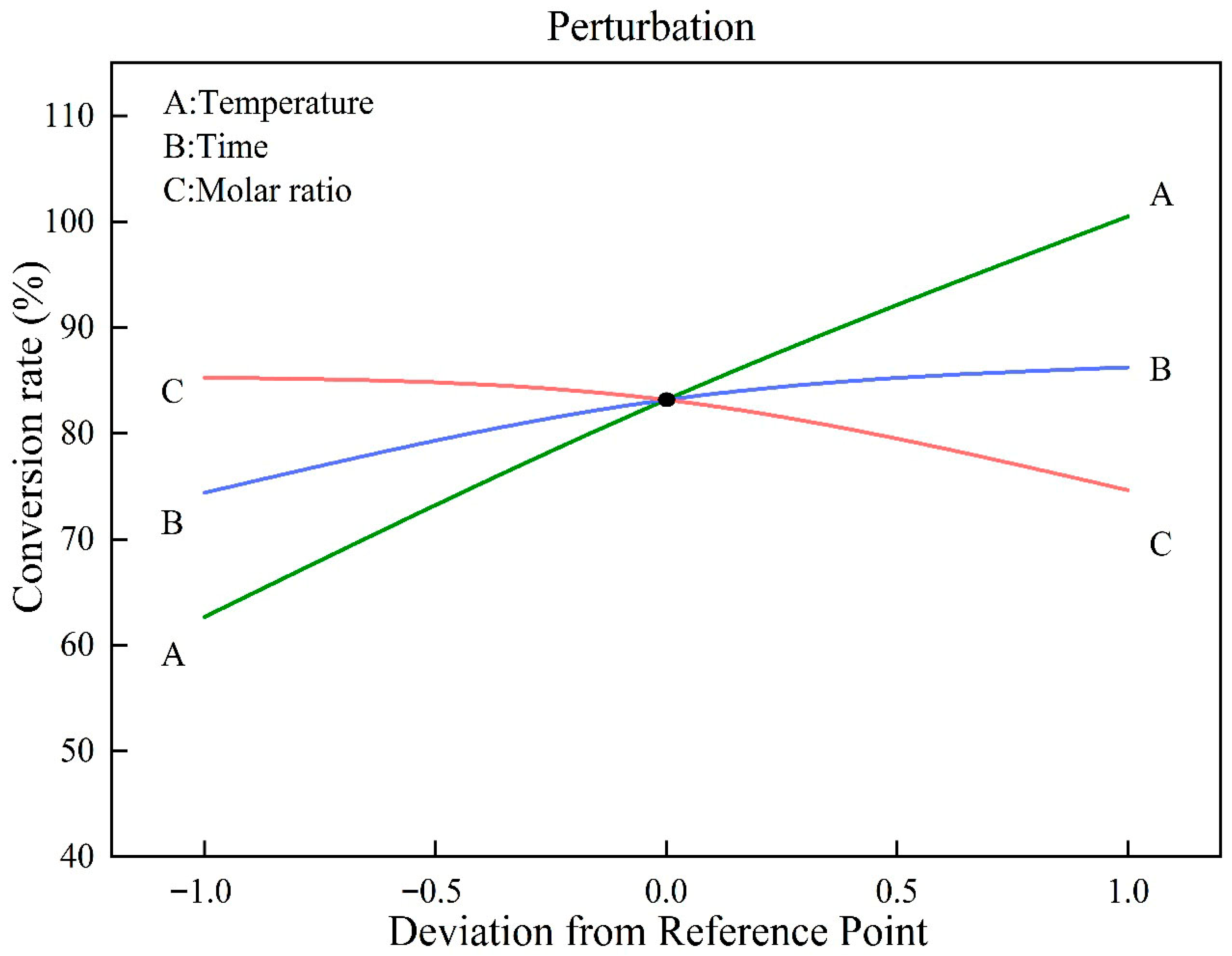
3.2.1. The Impact of a Single Factor on the Esterification of Methanol and Palmitic Acid
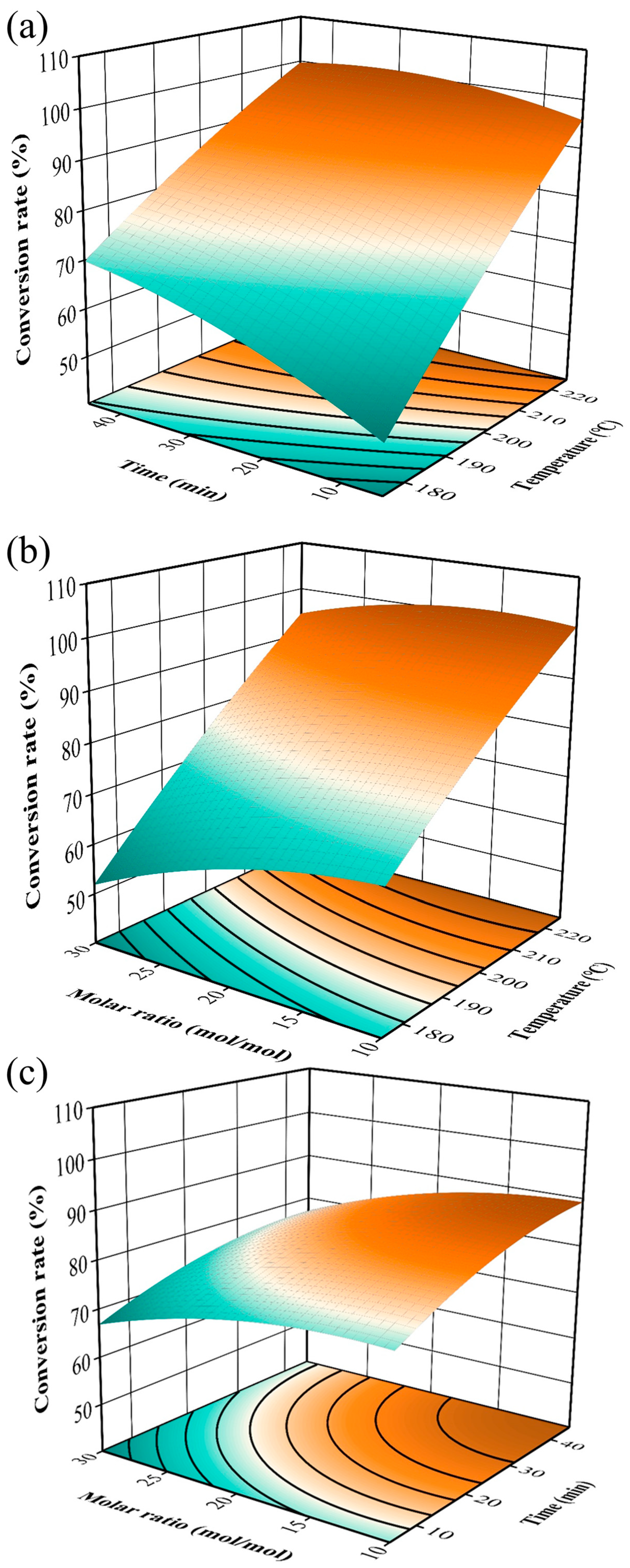
3.2.2. The Surface Response Analysis of Two Factors on the Esterification of Methanol and Palmitic Acid
3.3. Establishment of the Kinetic Model
4. Conclusions
- (1)
- In the esterification reaction of methanol and palmitic acid, the conversion rate of palmitic acid first increased and then remained unchanged with the increasing reaction temperature, which reached complete conversion at 225 °C but decreased above 275 °C. It first increased and then changed slightly with increasing residence time, obtaining a maximum value of 94.92% at 60 min. It first increased and then decreased with the increasing molar ratio of methanol to palmitic acid, achieving a maximum value of 85.46% at 15:1.
- (2)
- Both reaction temperature and residence time exhibited a positive correlation with the conversion rate of palmitic acid, while the methanol-to- palmitic acid molar ratio displayed a negative correlation. The relative influence of these factors was determined to follow the order: reaction temperature > residence time > molar ratio. The conversion rate of palmitic acid reached 99.30% under the optimal reaction parameters, which were a reaction temperature of 224 °C, a residence time of 26 min, and a molar ratio of methanol to palmitic acid of 16:1.
- (3)
- Within a temperature range of 175–225 °C, the reaction of palmitic acid with subcritical methanol follows a first-order reaction kinetic model. The activation energy and pre-exponential factor for their esterification reaction are 57.20 kJ/mol and 4.986 × 104, respectively.
- (4)
- The catalyst-free esterification in subcritical methanol presents a promising route for scalable biodiesel production. The absence of catalysts enables a simpler continuous process, such as in tubular reactors, without the need for separation units. Combined with its moderate temperature and lower methanol consumption, this method shows significant potential for reducing energy use and operational costs. These advantages align well with lifecycle carbon reduction and net-zero goals, supporting sustainable biodiesel production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akram, F.; ul Haq, I.; Raja, S.I.; Mir, A.S.; Qureshi, S.S.; Aqeel, A.; Shah, F.I. Current Trends in Biodiesel Production Technologies and Future Progressions: A Possible Displacement of the Petro-Diesel. J. Clean. Prod. 2022, 370, 133479. [Google Scholar] [CrossRef]
- Go, A.W.; Tran Nguyen, P.L.; Huynh, L.H.; Liu, Y.T.; Sutanto, S.; Ju, Y.H. Catalyst Free Esterification of Fatty Acids with Methanol under Subcritical Condition. Energy 2014, 70, 393–400. [Google Scholar] [CrossRef]
- Lüneburger, S.; Lazarin Gallina, A.; Cabreira Soares, L.; Moter Benvegnú, D. Biodiesel Production from Hevea Brasiliensis Seed Oil. Fuel 2022, 324, 124639. [Google Scholar] [CrossRef]
- Ganesan, S.; Nadarajah, S.; Khairuddean, M.; Teh, G.B. Studies on Lauric Acid Conversion to Methyl Ester via Catalytic Esterification Using Ammonium Ferric Sulphate. Renew. Energy 2019, 140, 9–16. [Google Scholar] [CrossRef]
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel Production from Waste Cooking Oil: An Efficient Technique to Convert Waste into Biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Kuo, J.Y. Optimized Conversion of Waste Cooking Oil to Biodiesel Using Modified Calcium Oxide as Catalyst via a Microwave Heating System. Fuel 2020, 266, 117114. [Google Scholar] [CrossRef]
- Abu-Ghazala, A.H.; Abdelhady, H.H.; Mazhar, A.A.; El-Deab, M.S. Exceptional Room Temperature Catalytic Transesterification of Waste Cooking Oil to Biodiesel Using Environmentally-Benign K2CO3/γ-Al2O3 Nano-Catalyst. Chem. Eng. J. 2023, 474, 145784. [Google Scholar] [CrossRef]
- Argaw Shiferaw, K.; Mathews, J.M.; Yu, E.; Choi, E.Y.; Tarte, N.H. Sodium Methoxide/Zeolite-Supported Catalyst for Transesterification of Soybean Waste Cooking Oil for Biodiesel Production. Inorganics 2023, 11, 163. [Google Scholar] [CrossRef]
- Falizi, N.J.; Madenoğlu, T.G.; Yüksel, M.; Kabay, N. Biodiesel Production Using Gel-type Cation Exchange Resin at Different Ionic Forms. Int. J. Energy Res. 2019, 43, 2188–2199. [Google Scholar] [CrossRef]
- Shin, H.Y.; Lee, S.H.; Ryu, J.H.; Bae, S.Y. Biodiesel Production from Waste Lard Using Supercritical Methanol. J. Supercrit. Fluids 2012, 61, 134–138. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Olivares-Carrillo, P.; Paneque, M.; Palacios-Nereo, F.J.; Quesada-Medina, J. High-Yield Production of Biodiesel by Non-Catalytic Supercritical Methanol Transesterification of Crude Castor Oil (Ricinus communis). Energy 2016, 107, 165–171. [Google Scholar] [CrossRef]
- Mohod, A.V.; Subudhi, A.S.; Gogate, P.R. Intensification of Esterification of Non Edible Oil as Sustainable Feedstock Using Cavitational Reactors. Ultrason. Sonochem. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- Jin, T.; Wang, B.; Zeng, J.; Yang, C.; Wang, Y.; Fang, T. Esterification of Free Fatty Acids with Supercritical Methanol for Biodiesel Production and Related Kinetic Study. RSC Adv. 2015, 5, 52072–52078. [Google Scholar] [CrossRef]
- Ang, G.T.; Tan, K.T.; Lee, K.T.; Mohamed, A.R. Biodiesel Production via Injection of Superheated Methanol Technology at Atmospheric Pressure. Energy Convers. Manag. 2014, 87, 1231–1238. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Biodiesel Production from Waste Cooking Oil via Supercritical Methanol: Optimisation and Reactor Simulation. Renew. Energy 2018, 124, 144–154. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Moein, P. Biodiesel Synthesis from Waste Vegetable Oil via Transesterification Reaction in Supercritical Methanol. J. Supercrit. Fluids 2013, 76, 24–31. [Google Scholar] [CrossRef]
- dos Santos, P.R.S.; Voll, F.A.P.; Ramos, L.P.; Corazza, M.L. Esterification of Fatty Acids with Supercritical Ethanol in a Continuous Tubular Reactor. J. Supercrit. Fluids 2017, 126, 25–36. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Madhu, D.; Chandra Sharma, Y. A Clean Approach of Biodiesel Production from Waste Cooking Oil by Using Single Phase BaSnO3 as Solid Base Catalyst: Mechanism, Kinetics & E-Study. J. Clean. Prod. 2020, 265, 121440. [Google Scholar] [CrossRef]
- Minami, E.; Kawamoto, H. Methyl Esterification of Oleic Acid in Supercritical Methanol with Methyl Formate. J. Jpn. Pet. Inst. 2021, 64, 188–196. [Google Scholar] [CrossRef]
- Kamjam, M.; Wongsawaeng, D.; Sawangkeaw, R.; Supang, W.; Hosemann, P.; Sola, P.; Ngamprasertsith, S. Valorization of Rambutan Seed Waste into Biodiesel via Non-Catalytic Supercritical Ethanol and Ethyl Acetate. Energies 2025, 18, 6004. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Comprehensive Optimisation of Biodiesel Production Conditions via Supercritical Methanolysis of Waste Cooking Oil. Energies 2022, 15, 3766. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Methyl Esterification of Free Fatty Acids of Rapeseed Oil as Treated in Supercritical Methanol. J. Chem. Eng. Jpn. 2001, 34, 383–387. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 6th ed.; Pearson: Boston, MA, USA, 2020; pp. 825–841. [Google Scholar]
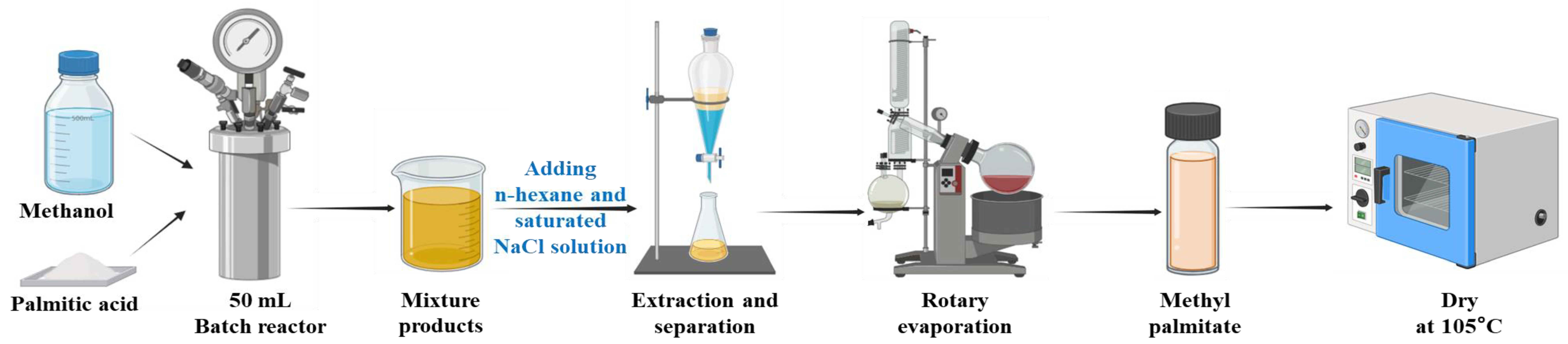





| Symbols | Variables | Low Level | Central Level | High Level |
|---|---|---|---|---|
| X1 | Reaction temperature (°C) | 175 | 200 | 225 |
| X2 | Residence time (min) | 5 | 25 | 45 |
| X3 | Molar ratio (mol/mol) | 10 | 20 | 30 |
| No. | Reaction Temperature (°C) | Residence Time (min) | Methanol to Palmitic Acid Molar Ratio (mol/mol) | Conversion Rate (%) |
|---|---|---|---|---|
| 1 | 175 | 25 | 30 | 54.13 |
| 2 | 175 | 25 | 10 | 65.81 |
| 3 | 175 | 45 | 20 | 69.36 |
| 4 | 175 | 5 | 20 | 49.78 |
| 5 | 200 | 45 | 30 | 75.17 |
| 6 | 200 | 45 | 10 | 91.17 |
| 7 | 200 | 5 | 30 | 64.87 |
| 8 | 200 | 5 | 10 | 77.62 |
| 9 | 200 | 25 | 20 | 83.17 |
| 10 | 200 | 25 | 20 | 84.16 |
| 11 | 200 | 25 | 20 | 81.83 |
| 12 | 225 | 5 | 20 | 96.23 |
| 13 | 225 | 25 | 10 | 98.01 |
| 14 | 225 | 25 | 30 | 96.06 |
| 15 | 225 | 45 | 20 | 100 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F Value | Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 3513.08 | 9 | 390.34 | 60.17 | 0.0001 | Significant |
| X1 | 2858.44 | 1 | 2858.44 | 440.6 | <0.0001 | |
| X2 | 278.48 | 1 | 278.48 | 42.92 | 0.0012 | |
| X3 | 224.51 | 1 | 224.51 | 34.61 | 0.002 | |
| X1X2 | 62.49 | 1 | 62.49 | 9.63 | 0.0267 | |
| X1X3 | 23.67 | 1 | 23.67 | 3.65 | 0.1144 | |
| X2X3 | 2.64 | 1 | 2.64 | 0.41 | 0.5516 | |
| X12 | 7.85 | 1 | 7.85 | 1.21 | 0.3215 | |
| X22 | 27.98 | 1 | 27.98 | 4.31 | 0.0924 | |
| X32 | 35.32 | 1 | 35.32 | 5.44 | 0.0669 | |
| Residual | 32.44 | 5 | 6.49 | |||
| Lack of Fit | 29.7 | 3 | 9.9 | 7.24 | 0.1238 | Not Significant |
| Pure Error | 2.73 | 2 | 1.37 | |||
| Cor Total | 3545.52 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Que, Z.; Zhang, K.; Fu, Y.; Cheng, X.; Huang, R.; Shi, J.; Jiang, H.; Ai, X.; Deng, T.; et al. Optimization and Kinetic Study of Palmitic Acid Esterification with Subcritical Methanol via Response Surface Methodology. Reactions 2025, 6, 69. https://doi.org/10.3390/reactions6040069
Luo J, Que Z, Zhang K, Fu Y, Cheng X, Huang R, Shi J, Jiang H, Ai X, Deng T, et al. Optimization and Kinetic Study of Palmitic Acid Esterification with Subcritical Methanol via Response Surface Methodology. Reactions. 2025; 6(4):69. https://doi.org/10.3390/reactions6040069
Chicago/Turabian StyleLuo, Jie, Zhigang Que, Ke Zhang, Yinxuan Fu, Xiaodi Cheng, Rong Huang, Jinming Shi, Haiwei Jiang, Xianbin Ai, Tonghui Deng, and et al. 2025. "Optimization and Kinetic Study of Palmitic Acid Esterification with Subcritical Methanol via Response Surface Methodology" Reactions 6, no. 4: 69. https://doi.org/10.3390/reactions6040069
APA StyleLuo, J., Que, Z., Zhang, K., Fu, Y., Cheng, X., Huang, R., Shi, J., Jiang, H., Ai, X., Deng, T., Qiu, X., & Xu, C. (2025). Optimization and Kinetic Study of Palmitic Acid Esterification with Subcritical Methanol via Response Surface Methodology. Reactions, 6(4), 69. https://doi.org/10.3390/reactions6040069




_Xu.png)


