Degradation and Nitrogen Transfer of 4-Aminophenol by Cavitation Induced by a Composite Hydrodynamic Cavitator
Abstract
1. Introduction
2. Structure Design and CFD Optimization of the Composite Hydrodynamic Cavitator
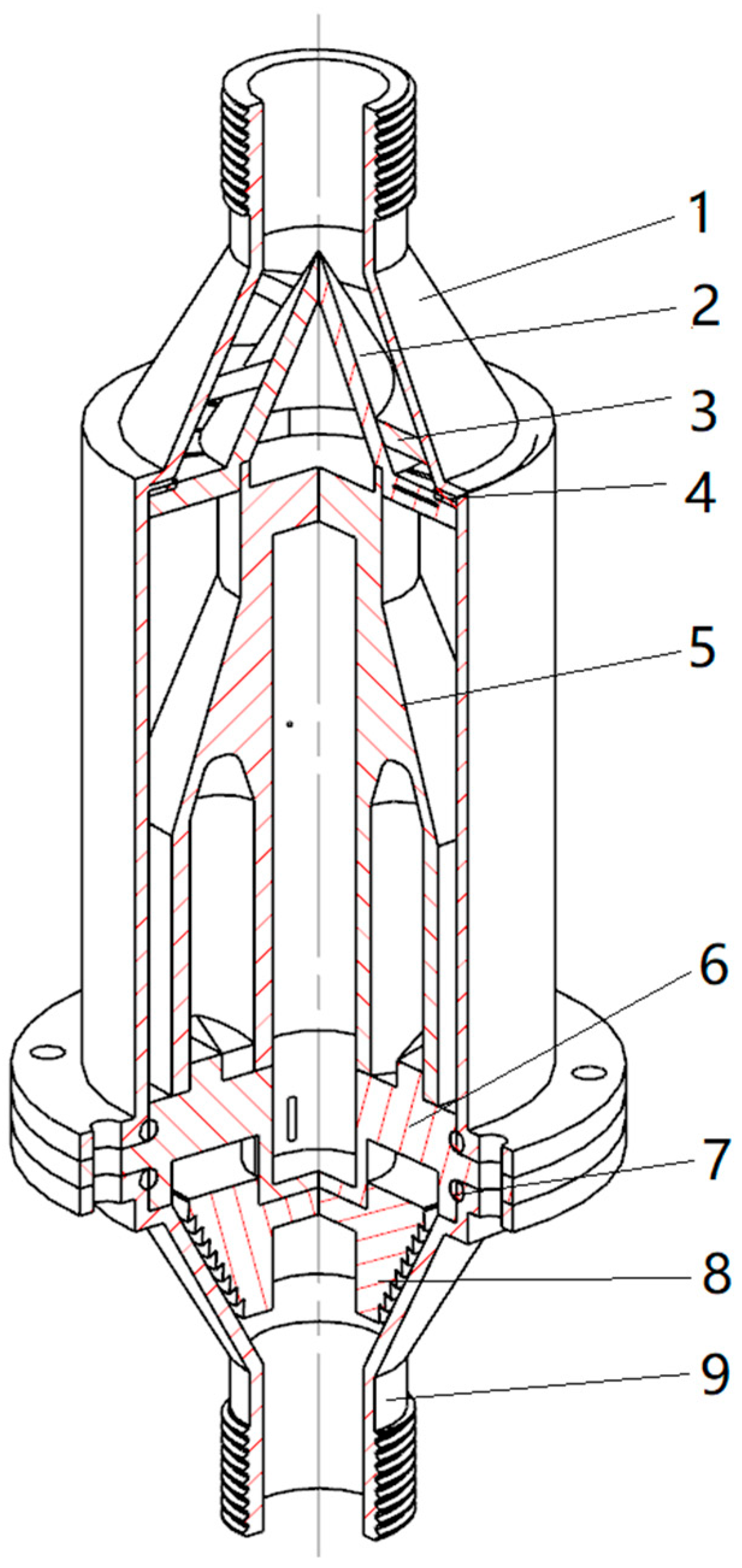
2.1. The Structure of the Composite Hydrodynamic Cavitator
2.2. Flow Field Simulation Method
2.2.1. Mesh Division
2.2.2. Simulation Conditions and Model Selection
3. Experimental Methods
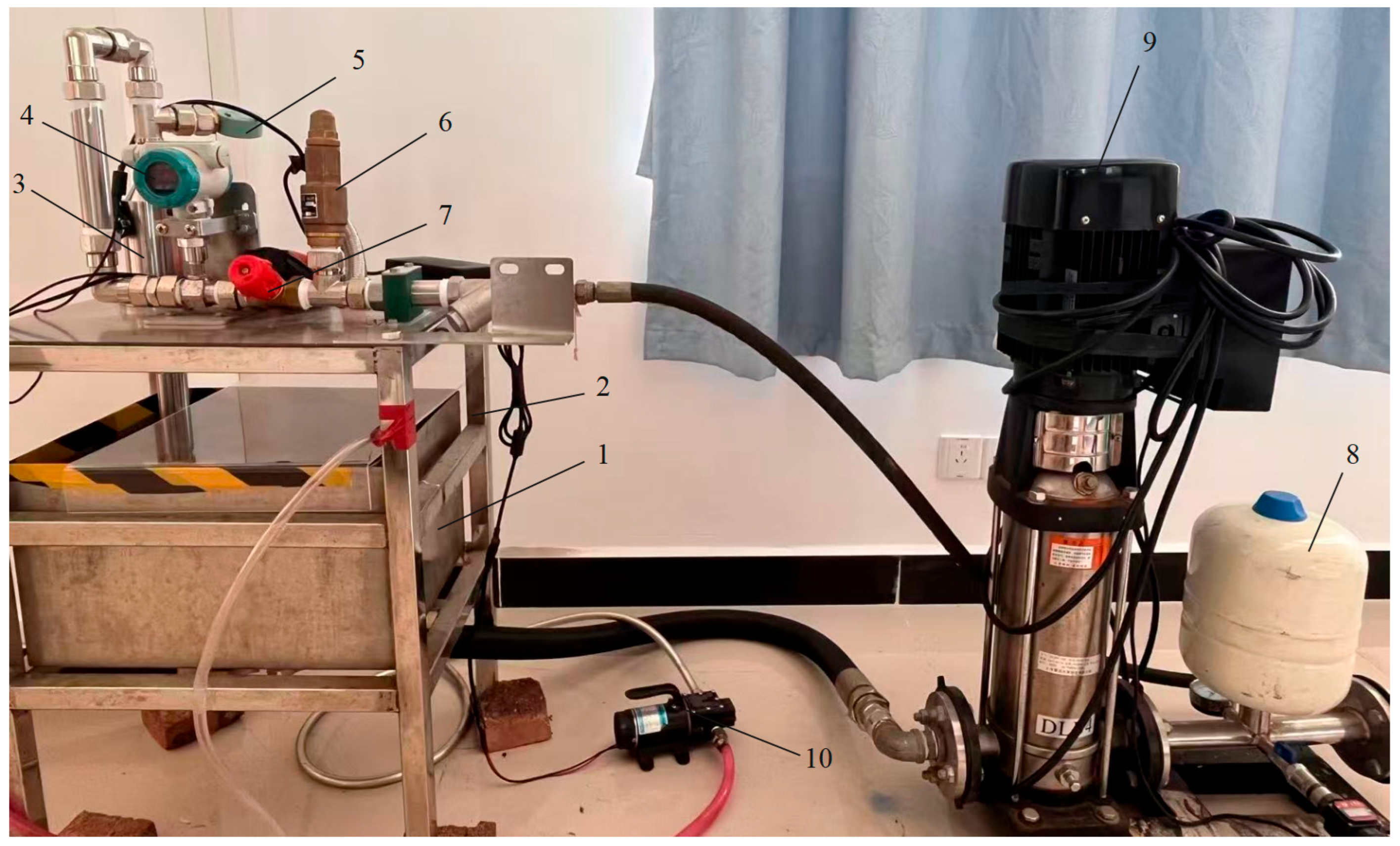
3.1. The Degradation Experimental Platform
3.2. The Hydroxyl Radical Generation
3.3. Degradation Experiment
4. Results and Discussions
4.1. Cavitation Characteristics Analysis of the Cavitator with Optimal Structural Parameters
4.1.1. The Absolute Pressure of the Fluid Inside the Cavitator
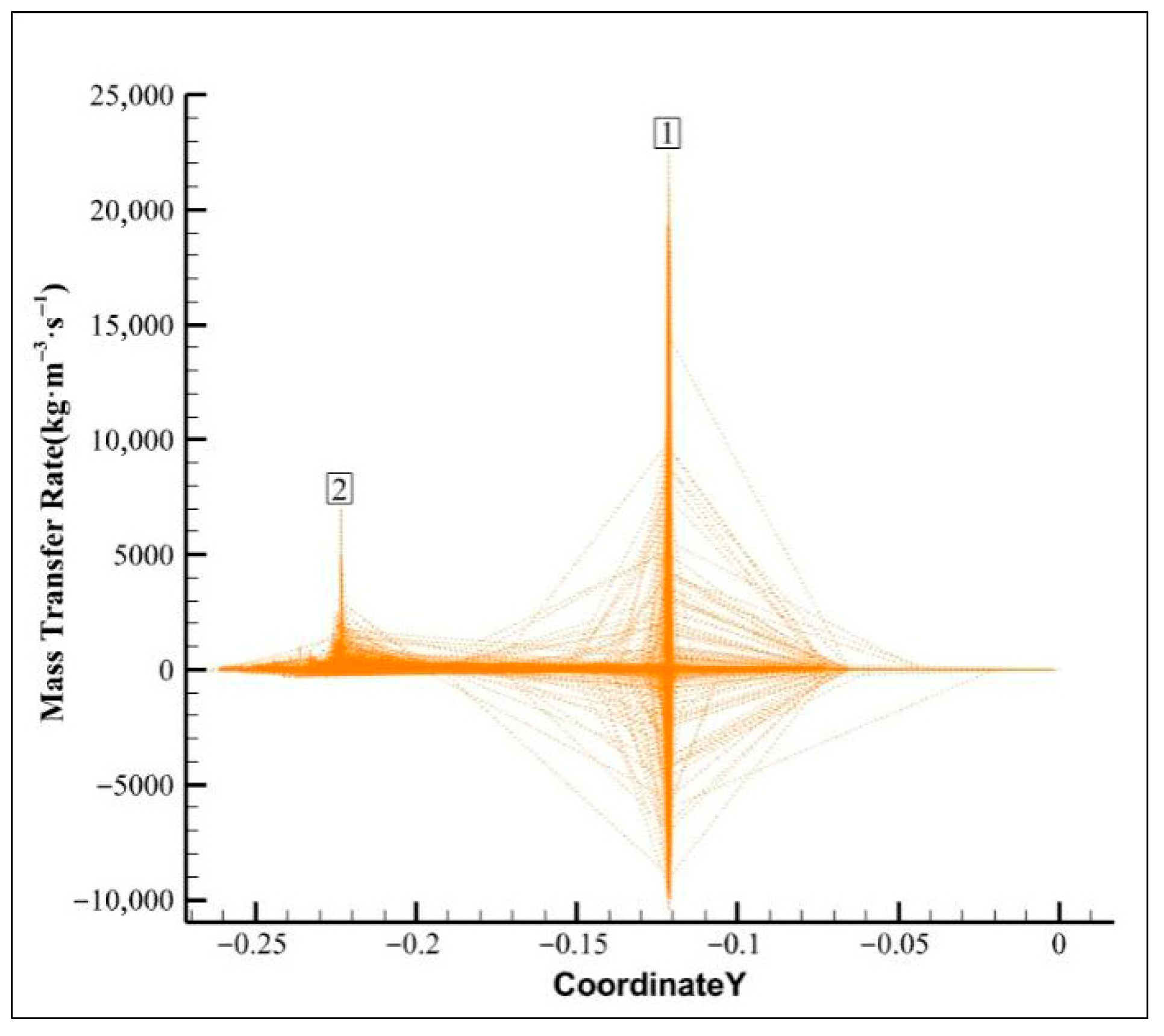
4.1.2. Mass Transfer Analysis
4.1.3. Gas Holdup Analysis
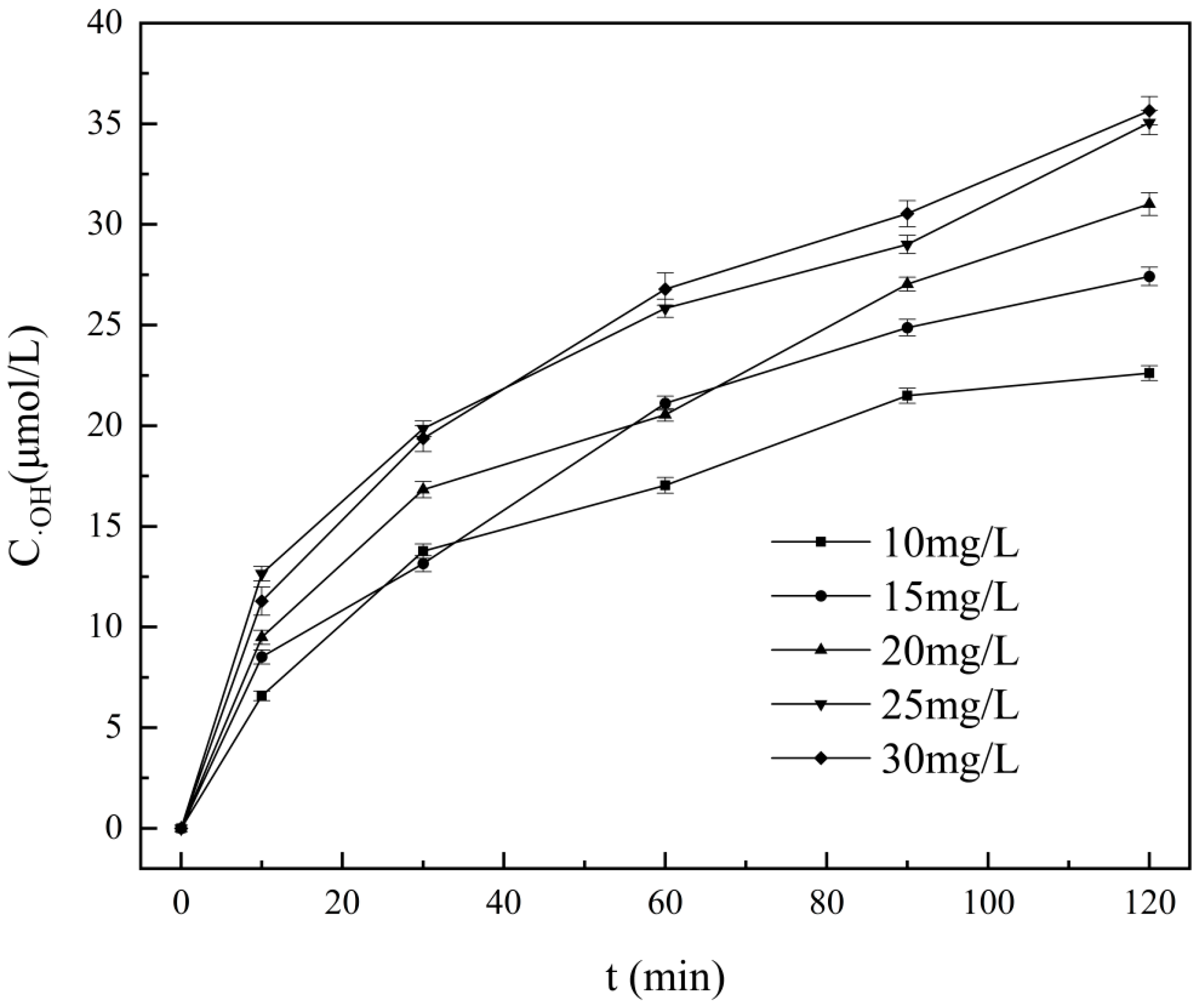
4.2. The Concentration of Hydroxyl Radical Produced During Cavitation
4.3. Degradation of 4-Aminophenol by the Cavitator and Nitrogen Removal Performance of the Solution
4.3.1. Influence of Cavitation Time
4.3.2. Influence of Inlet Pressure
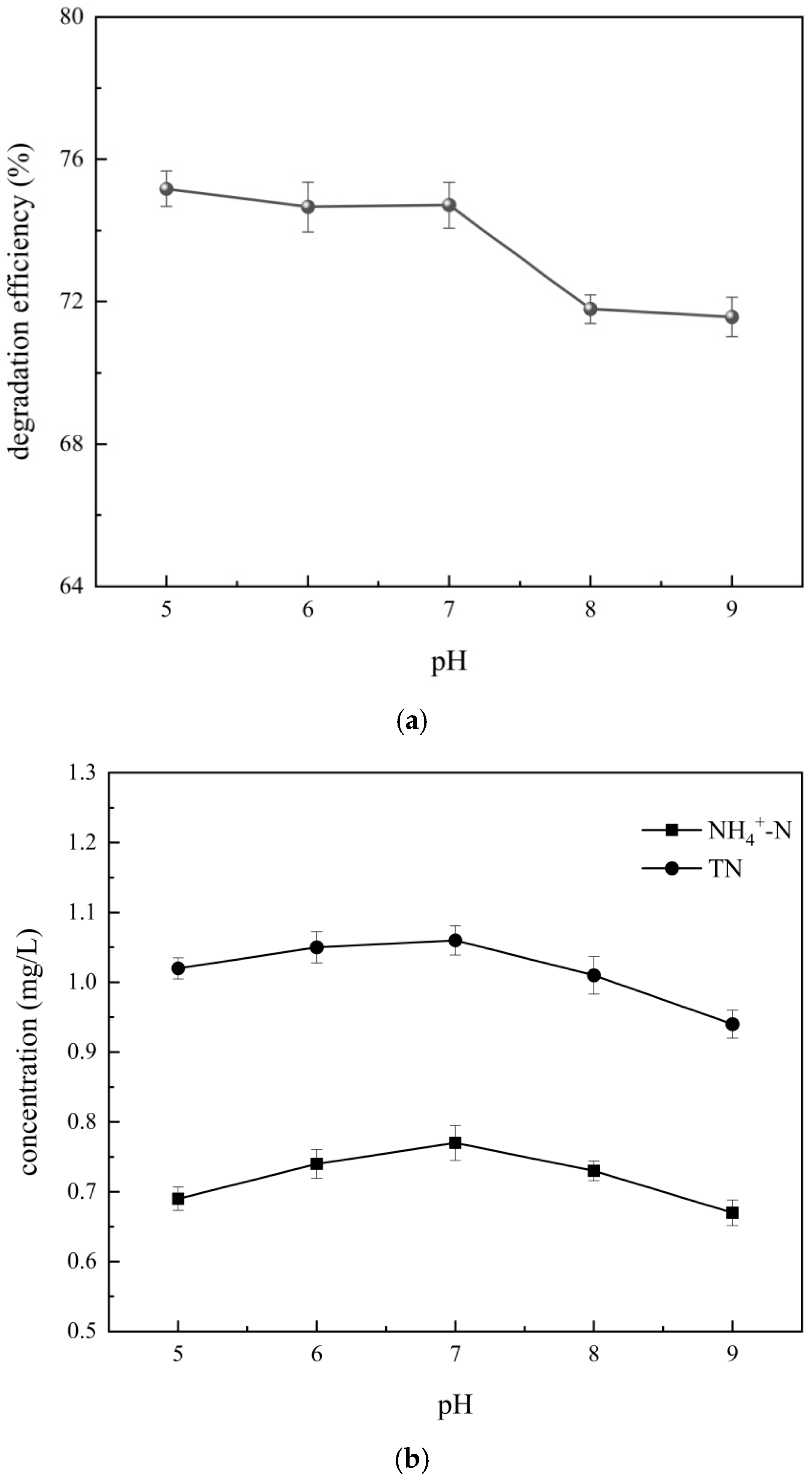
4.3.3. Influence of Initial pH
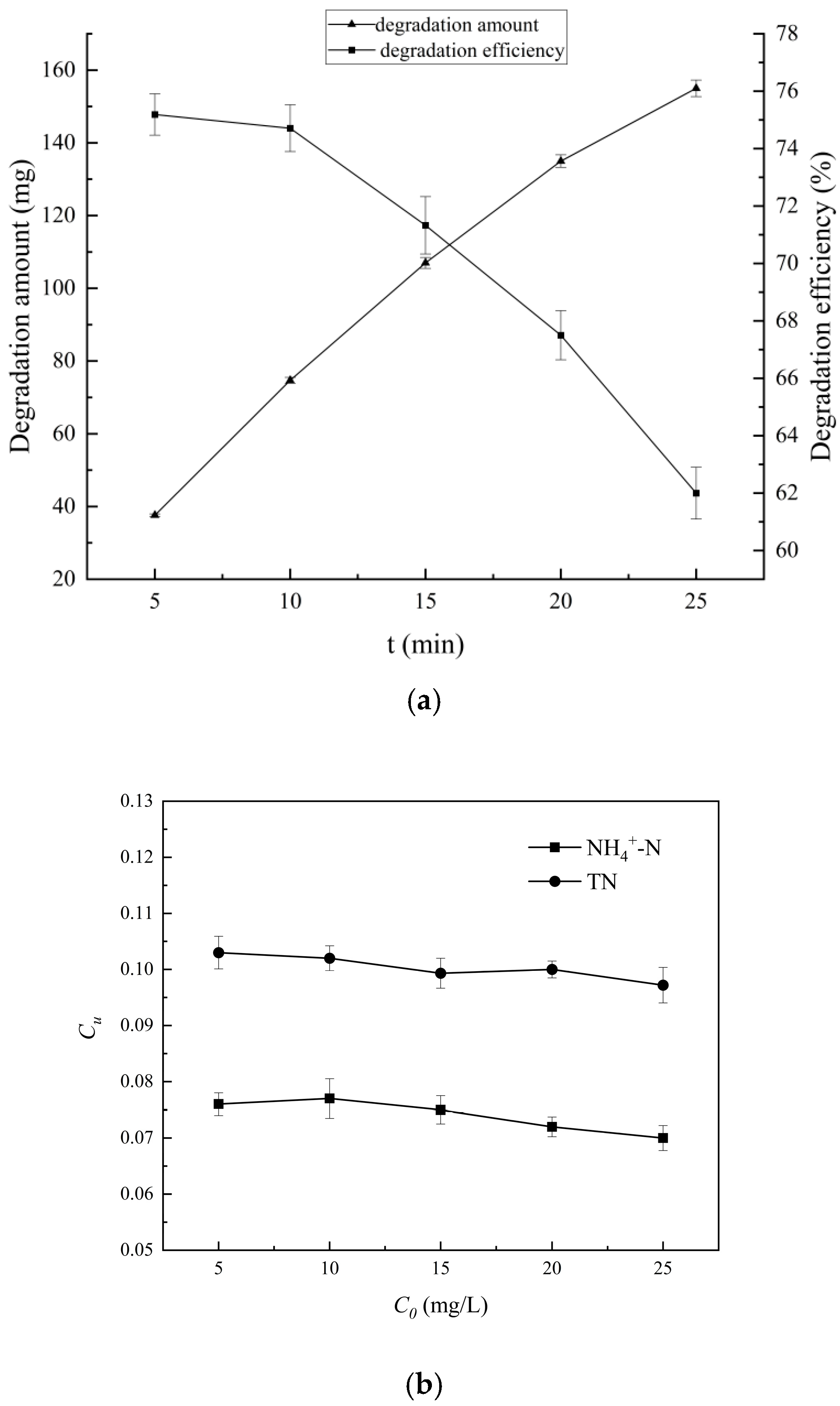
4.3.4. Influence on the Initial Concentration of 4-Aminophenol
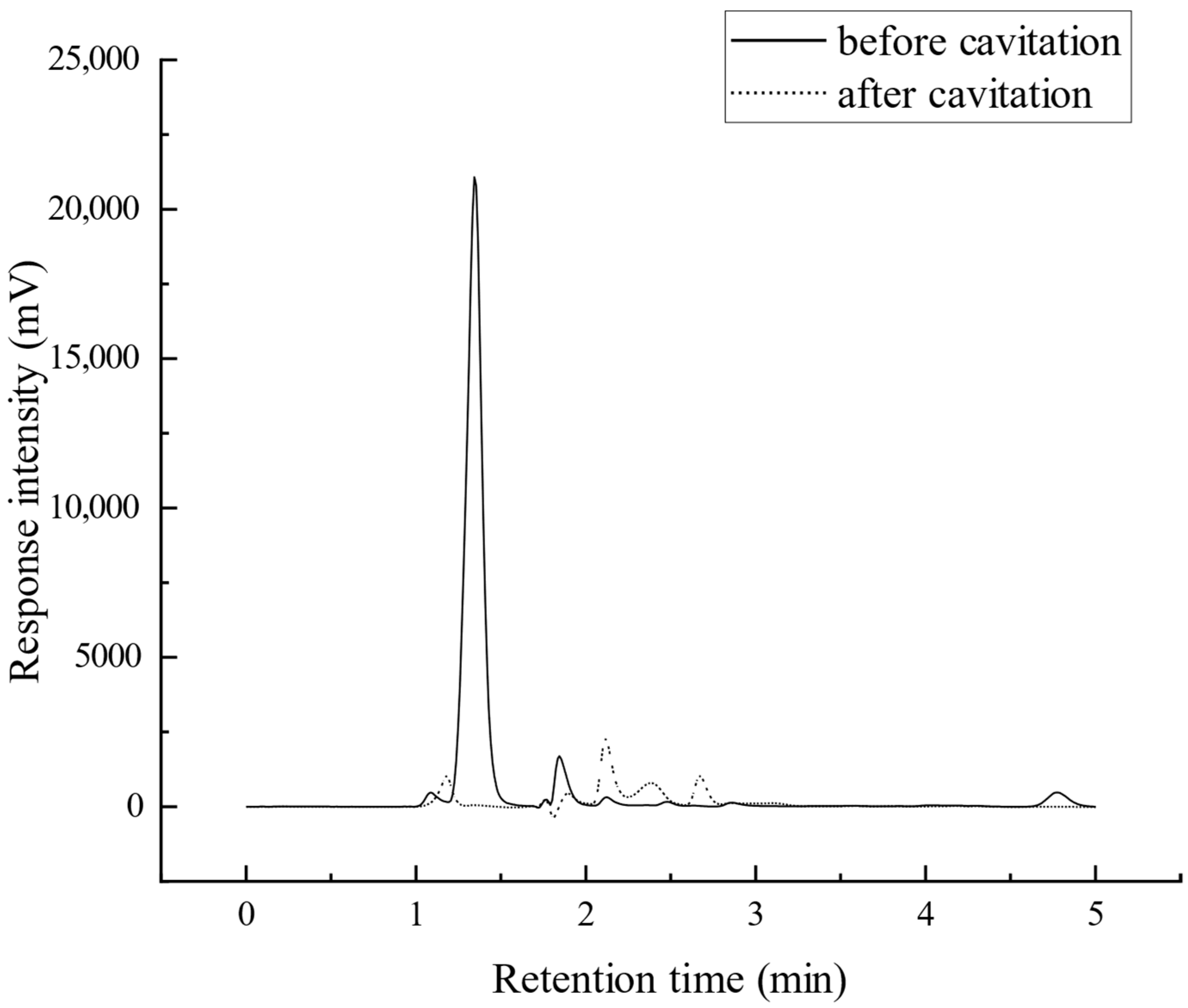
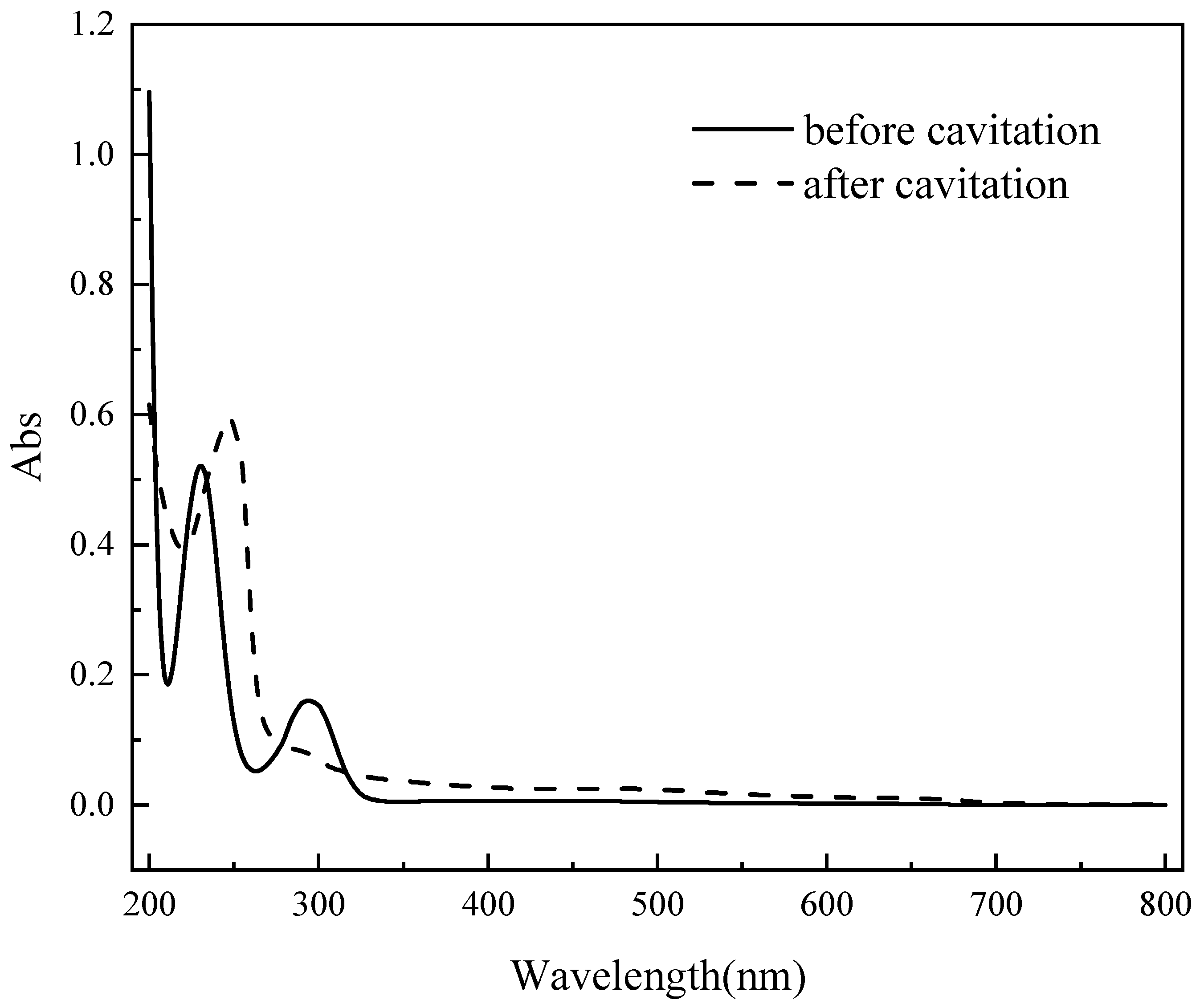
4.4. Degradation and Denitrification Chemical Reaction During Degradation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karimi-Maleh, H.; Darabi, R.; Karimi, F.; Karaman, C.; Shahidi, S.A.; Zare, N.; Baghayeri, M.; Fu, L.; Rostamnia, S.; Rouhi, J.; et al. State-of-art advances on removal, degradation and electrochemical monitoring of 4-aminophenol pollutants in real samples: A review. Environ. Res. 2023, 222, 115338. [Google Scholar] [CrossRef]
- Wu, C.W.; Li, Q.B. Characteristics of organic matter removed from highly saline mature landfill leachate by an emergency disk tube-reverse osmosis treatment system. Chemosphere 2021, 263, 128347. [Google Scholar] [CrossRef]
- Yuan, Y.; Qian, X.; Zhang, L.; Yin, W.; Chen, T.; Li, Z.; Ding, C.; Wang, B.; Liang, B.; Wang, A. Tailoring microbial redox with alternating current for efficient mineralization of refractory organic nitrogen compounds in wastewater. npj Clean Water 2025, 8, 4. [Google Scholar] [CrossRef]
- Huang, J.; Wang, C.; Zhang, S.; Han, X.; Feng, R.; Li, Y.; Huang, X.; Wang, J. Optimizing nitrogenous organic wastewater treatment through integration of organic capture, anaerobic digestion, and anammox technologies: Sustainability and challenges. Environ. Sci. Pollut. Res. 2023, 30, 76372–76386. [Google Scholar] [CrossRef] [PubMed]
- Tranchant, M.; Serrà Albert Gunderson, C.; Bertero, E.; García-Amorós, J.; Gómez, E.; Michler, J.; Philippe, L. Efficient and green electrochemical synthesis of 4-aminophenol using porous Au micropillars. Appl. Catal. A General. 2020, 602, 117698. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Amiri, M. CuO supported Clinoptilolite towards solar photocatalytic degradation of p-aminophenol. Powder. Technol. 2013, 235, 279–288. [Google Scholar] [CrossRef]
- Ratiu, C.; Manea, F.; Lazau, C.; Orha, C.; Burtica, G.; Grozescu, I.; Schoonman, J. Photocatalytically-assisted electrochemical degradation of p-aminophenol in aqueous solutions using zeolite-supported TiO2 catalyst. Chem. Pap. 2011, 65, 289–298. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, N.; Xing, M.; Xu, Z.; Shen, K.; Wu, J.; Feng, Y. Photoelectrocatalysis degradation of P-aminophenol using PbO2-TiO2 heterojunction electrode: Catalytic, theoretical calculating and mechanism. J. Environ. Chem. Eng. 2024, 12, 113304. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Fontecha-Cámara, M.Á.; Álvarez, M.A.; Mateus, L. Removal of phenolic compounds from water using copper ferrite nanosphere composites as fenton catalysts. Nanomaterials 2019, 9, 901. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, K.; Dixit, U.; Agarwal, A.; Bhat, R.A. Effective removal of 4-Aminophenol from aqueous environment by pea (Pisum sativum) shells activated with sulfuric acid: Characterization, isotherm, kinetics and thermodynamics. J. Ind. Chem. Soc. 2022, 99, 100528. [Google Scholar] [CrossRef]
- Afzal Khan, S.; Hamayun, M.; Ahmed, S. Degradation of 4-aminophenol by newly isolated Pseudomonas sp. strain ST-4. Enzym. Microb. Tech. 2006, 38, 10–13. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Jia, L.; Wang, G.; Zhao, S.; Yoon, J.Y.; Chen, S. A review on hydrodynamic cavitation disinfection: The current state of knowledge. Sci. Total Environ. 2020, 737, 139606. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Song, Y.; Liu, J.; Zhang, L.; Zhang, M.; Sun, X. Experimental and numerical investigation on the disinfection characteristics of a novel rotor-radial groove hydrodynamic cavitation reactor. Process Saf. Environ. 2023, 169, 260–269. [Google Scholar] [CrossRef]
- Gore, M.M.; Saharan, V.K.; Pinjari, D.V.; Chavan, P.V.; Pandit, A.B. Degradation of reactive orange 4 dye using hydrodynamic cavitation based hybrid techniques. Ultraso. Sonochem. 2014, 21, 1075–1082. [Google Scholar] [CrossRef]
- Innocenzi, V.; Prisciandaro, M.; Centofanti, M.; Vegliò, F. Comparison of performances of hydrodynamic cavitation in combined treatments based on hybrid induced advanced Fenton process for degradation of azo-dyes. J. Environ. Chem. Eng. 2019, 7, 103171. [Google Scholar] [CrossRef]
- Kore, V.S.; Manjare, S.D.; Patil, A.D.; Dhanke, P.B. A parametric study on intensified degradation of textile dye water using hydrodynamic cavitation based hybrid technique. Chem. Engin. Process.—Process Intensif. 2023, 193, 109550. [Google Scholar] [CrossRef]
- Thanekar, P.; Panda, M.; Gogate, P.R. Degradation of carbamazepine using hydrodynamic cavitation combined with advanced oxidation processes. Ultraso. Sonochem. 2018, 40, 567–576. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, R.; Li, Y.; Lian, X. CFD simulation of a swirling vortex cavitator and its degradation performance and pathway of tetracycline in aqueous solution. Int. J. Chem. React. Eng. 2022, 20, 955–963. [Google Scholar] [CrossRef]
- Roy, K.; Moholkar, V.S. Sulfadiazine degradation using hybrid AOP of heterogeneous Fenton/persulfate system coupled with hydrodynamic cavitation. Chem. Engin. J. 2020, 386, 121294. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Saini, D.; Sonawane, S.; Pandit, A. Effect of process intensifying parameters on the hydrodynamic cavitation based degradation of commercial pesticide (methomyl) in the aqueous solution. Ultraso. Sonochem. 2016, 28, 283–293. [Google Scholar] [CrossRef]
- Patil, P.N.; Bote, S.D.; Gogate, P. Degradation of imidacloprid using combined advanced oxidation processes based on hydrodynamic cavitation. Ultraso. Sonochem. 2014, 21, 1770–1777. [Google Scholar] [CrossRef]
- Abdelrahman, A.M.; Tebyani, S.; Talabazar, F.R.; Tabar, S.A.; Berenji, N.R.; Aghdam, A.S.; Koyuncu, I.; Kosar, A.; Guven, H.; Ersahin, M.E.; et al. The flow pattern effects of hydrodynamic cavitation on waste activated sludge digestibility. Chemosphere 2024, 357, 141949. [Google Scholar] [CrossRef]
- Patil, P.B.; Bhandari, V.M.; Ranade, V.V. Improving efficiency for removal of ammoniacal nitrogen from wastewaters using hydrodynamic cavitation. Ultraso. Sonochem. 2021, 70, 105306. [Google Scholar] [CrossRef]
- Patil, P.B.; Bhandari, V.M. Solvent-assisted cavitation for enhanced removal of organic pollutants—Degradation of 4-aminophenol. J. Environ. Manag. 2022, 311, 114857. [Google Scholar] [CrossRef]
- Hong, F.; Tian, H.; Yuan, X.; Liu, S.; Peng, Q.; Shi, Y.; Jin, L.; Ye, L.; Jia, J.; Ying, D.; et al. CFD-assisted modeling of the hydrodynamic cavitation reactors for wastewater treatment—A review. J. Environ. Manag. 2022, 321, 115982. [Google Scholar] [CrossRef]
- Dastane, G.G.; Thakkar, H.; Shah, R.; Perala, S.; Raut, J.; Pandit, A.B. Single and multiphase CFD simulations for designing cavitating venturi. Chem. Eng. Res. Des. 2019, 149, 1–12. [Google Scholar] [CrossRef]
- Iwamori, S.; Nishiyama, N.; Oya, K. A colorimetric indicator for detection of hydroxyl radicals in atmosphere using a methylene blue dye based on nafion film. Polym. Degrad. Stabil. 2016, 123, 131–136. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, M.; Deng, C.; Shi, X.; Deng, Y.; Ma, J.; Liu, H.; Chen, P. Analysis on reaction kinetics of methylene blue degraded by hydrodynamic cavitation. Ind. Wat. Treat. 2018, 38, 31–34. [Google Scholar]
- Saharan, V.K.; Badve, M.P.; Pandit, A.B. Degradation of reactive red 120 dye using hydrodynamic cavitation. Chem. Engin. J. 2011, 178, 100–107. [Google Scholar] [CrossRef]
- Maršálek, B.; Zezulka, Š.; Maršálková, E.; Pochylý, F.; Rudolf, P. Synergistic effects of trace concentrations of hydrogen peroxide used in a novel hydrodynamic cavitation device allows for selective removal of cyanobacteria. Chem. Engin. J. 2019, 382, 122383. [Google Scholar] [CrossRef]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Hydrodynamic cavitation of p-nitrophenol: A theoretical and experimental insight. Chem. Engin. J. 2014, 254, 1–8. [Google Scholar] [CrossRef]
- Kotronarou, A.; Mills, G.; Hoffmann, M.R. Ultrasonic irradiation of p-nitrophenol in aqueous solution. J. Phys. Chem. 1991, 95, 3630–3638. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Wen, P.; Li, Q. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 2019, 217, 411–422. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, R.; She, Z.; Li, Y. Degradation and Nitrogen Transfer of 4-Aminophenol by Cavitation Induced by a Composite Hydrodynamic Cavitator. Reactions 2025, 6, 68. https://doi.org/10.3390/reactions6040068
Wang B, Zhang R, She Z, Li Y. Degradation and Nitrogen Transfer of 4-Aminophenol by Cavitation Induced by a Composite Hydrodynamic Cavitator. Reactions. 2025; 6(4):68. https://doi.org/10.3390/reactions6040068
Chicago/Turabian StyleWang, Baoe, Rihong Zhang, Zipeng She, and Yiyong Li. 2025. "Degradation and Nitrogen Transfer of 4-Aminophenol by Cavitation Induced by a Composite Hydrodynamic Cavitator" Reactions 6, no. 4: 68. https://doi.org/10.3390/reactions6040068
APA StyleWang, B., Zhang, R., She, Z., & Li, Y. (2025). Degradation and Nitrogen Transfer of 4-Aminophenol by Cavitation Induced by a Composite Hydrodynamic Cavitator. Reactions, 6(4), 68. https://doi.org/10.3390/reactions6040068





