Hydroxyl Radical-Initiated Reaction of Nerol: A Pathway to Secondary Pollutants in an Indoor Environment
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Abstraction and Addition Pathways of Nerol with OH Radical
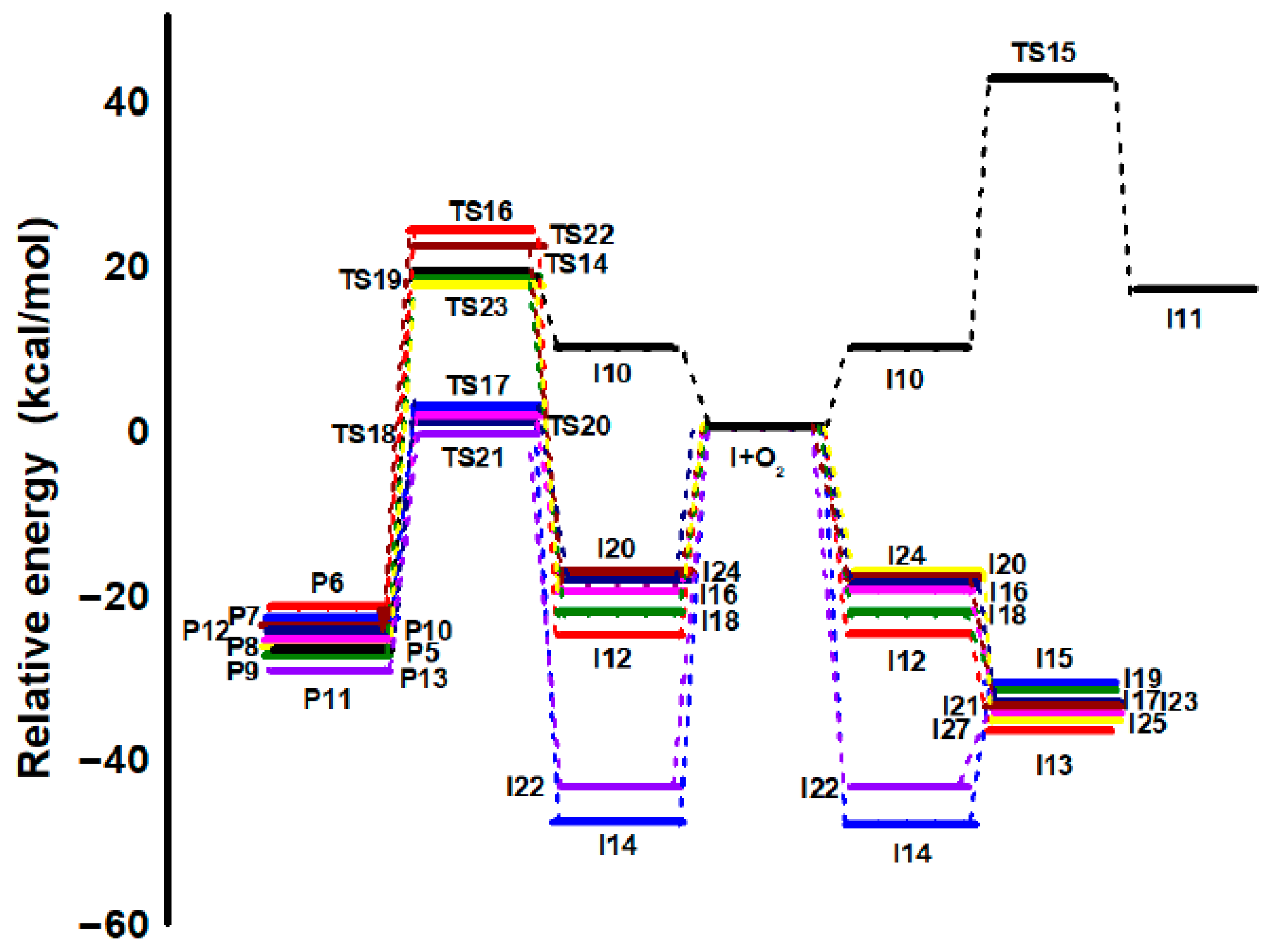
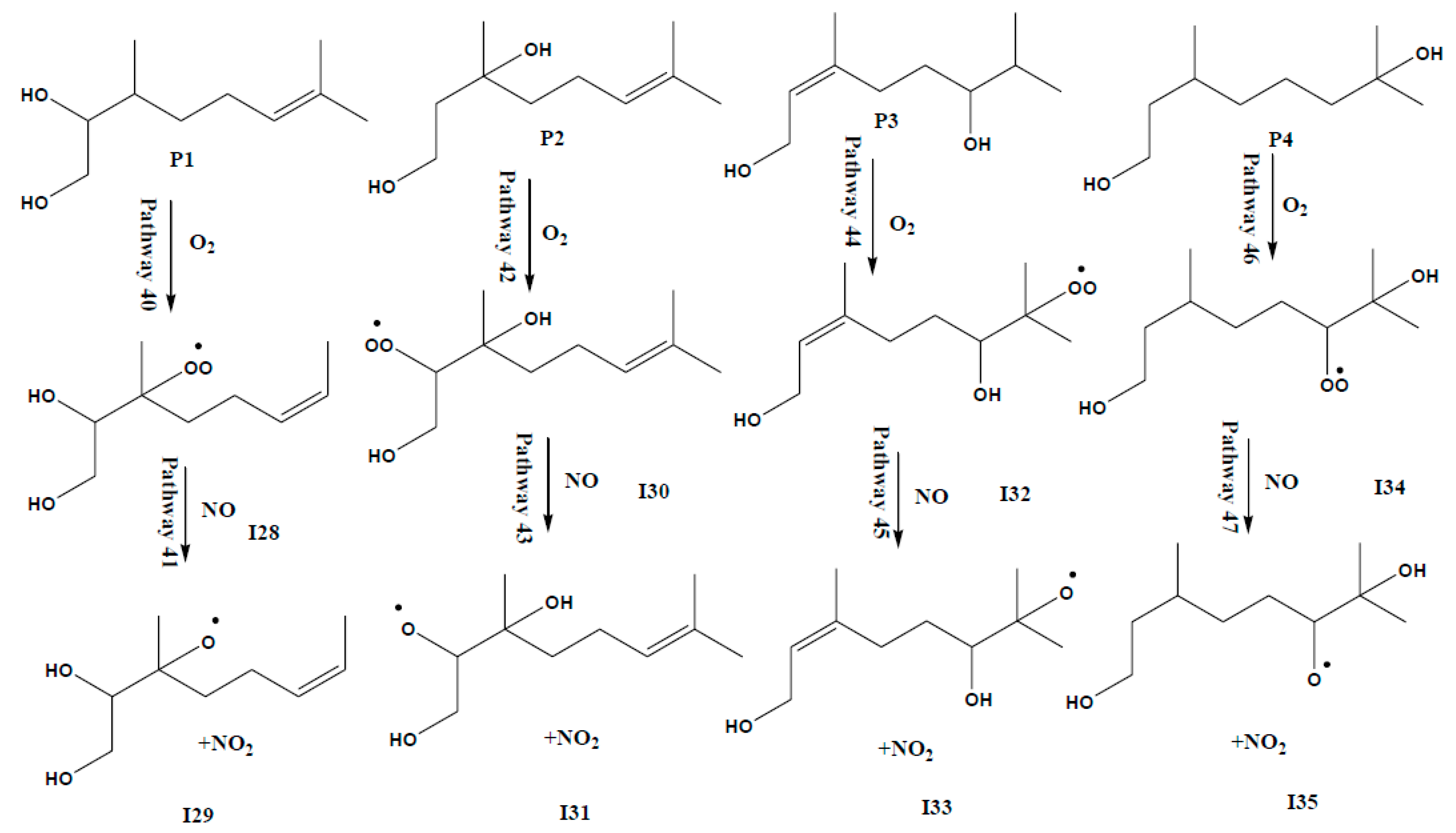
3.2. Competing Pathways of RO2 and Their Reactions with NO and HO2
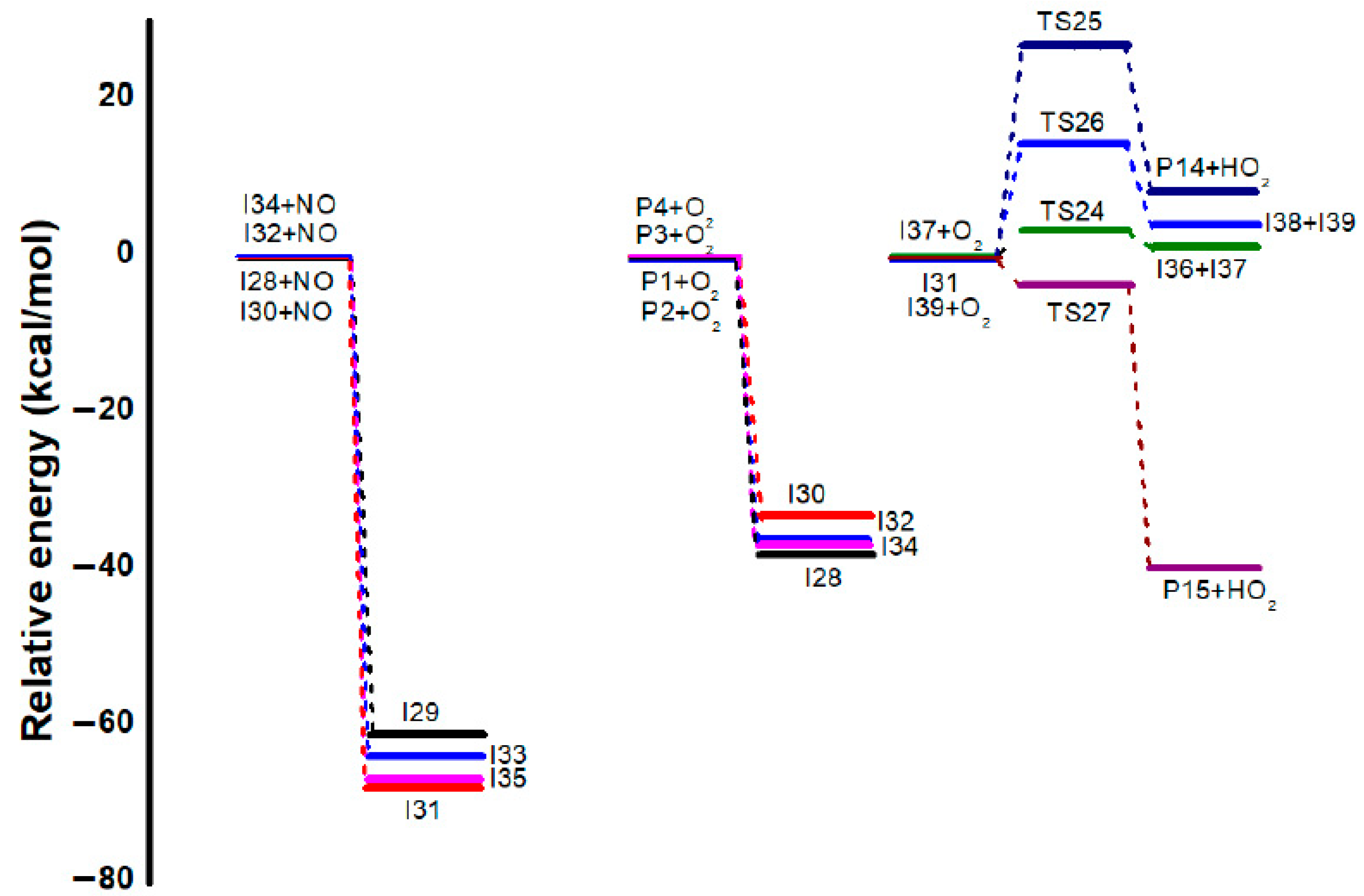
3.3. Mechanistic Study: Formation of Formaldehyde, Glycoaldehyde and 6-Methyl-hept-5-en-2-ol from Intermediate I31
3.4. Rate Coefficient, Branching Ratio of Nerol with OH Radical
4. Evaluating the Role of Nerol in Atmospheric Chemistry and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirose, S.; Satake, A. Theoretical analyses for the evolution of biogenic volatile organic compounds (BVOC) emission strategy. Ecol. Evol. 2024, 14, e11548. [Google Scholar] [CrossRef]
- Gu, S.; Guenther, A.; Faiola, C. Effects of Anthropogenic and Biogenic Volatile Organic Compounds on Los Angeles Air Quality. Environ. Sci. Technol. 2021, 55, 12191–12201. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Carslaw, N.; Fletcher, L.; Heard, D.; Ingham, T.; Walker, H. Significant OH production under surface cleaning and air cleaning conditions: Impact on indoor air quality. Indoor Air 2017, 27, 1091–1100. [Google Scholar] [CrossRef]
- Wang, N.; Zannoni, N.; Ernle, L.; Bekö, G.; Wargocki, P.; Li, M.; Weschler, C.J.; Williams, J. Total OH Reactivity of Emissions from Humans: In Situ Measurement and Budget Analysis. Environ. Sci. Technol. 2021, 55, 149–159. [Google Scholar] [CrossRef]
- Weschler, C.J.; Shields, H.C. Production of the Hydroxyl Radical in Indoor Air. Environ. Sci. Technol. 1996, 30, 3250–3258. [Google Scholar] [CrossRef]
- Weschler, C.J.; Shields, H.C. Measurements of the Hydroxyl Radical in a Manipulated but Realistic Indoor Environment. Environ. Sci. Technol. 1997, 31, 3719–3722. [Google Scholar] [CrossRef]
- Lelieveld, J.; Butler, T.M.; Crowley, J.N.; Dillon, T.J.; Fischer, H.; Ganzeveld, L.; Harder, H.; Lawrence, M.G.; Martinez, M.; Taraborrelli, D.; et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature 2008, 452, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Whalley, L.K.; Edwards, P.M.; Furneaux, K.L.; Goddard, A.; Ingham, T.; Evans, M.J.; Stone, D.; Hopkins, J.R.; Jones, C.E.; Karunaharan, A.; et al. Quantifying the magnitude of a missing hydroxyl radical source in a tropical rainforest. Atmos. Chem. Phys. 2011, 11, 7223–7233. [Google Scholar] [CrossRef]
- Calogirou, A.; Larsen, B.R.; Kotzias, D. Gas-phase terpene oxidation products: A review. Atmos. Environ. 1999, 33, 1423–1439. [Google Scholar] [CrossRef]
- Mano Priya, A.; El Dib, G. Mechanistic and kinetic study of limona ketone oxidation initiated by hydroxyl radical: Impact of indoor air pollution. New J. Chem. 2024, 48, 3036–3044. [Google Scholar] [CrossRef]
- Wolkoff, P.; Larsen, S.T.; Hammer, M.; Kofoed-Sørensen, V.; Clausen, P.A.; Nielsen, G.D. Human reference values for acute airway effects of five common ozone-initiated terpene reaction products in indoor air. Toxicol. Lett. 2013, 216, 54–64. [Google Scholar] [CrossRef]
- Braure, T.; Bedjanian, Y.; Romanias, M.N.; Morin, J.; Riffault, V.; Tomas, A.; Coddeville, P. Experimental Study of the Reactions of Limonene with OH and OD Radicals: Kinetics and Products. J. Phys. Chem. A 2014, 118, 9482–9490. [Google Scholar] [CrossRef]
- Chuong, B.; Davis, M.; Edwards, M.; Stevens, P.S. Measurements of the kinetics of the OH + α-pinene and OH + β-pinene reactions at low pressure. Int. J. Chem. Kinet. 2002, 34, 300–308. [Google Scholar] [CrossRef]
- Tan, Z.; Hantschke, L.; Kaminski, M.; Acir, I.H.; Bohn, B.; Cho, C.; Dorn, H.P.; Li, X.; Novelli, A.; Nehr, S.; et al. Atmospheric photo-oxidation of myrcene: OH reaction rate constant, gas-phase oxidation products and radical budgets. Atmos. Chem. Phys. 2021, 21, 16067–16091. [Google Scholar] [CrossRef]
- Durello, R.S.; Silva, L.M.; Bogusz, S. Química do Lúpulo. Química Nova 2019, 42, 900–919. [Google Scholar] [CrossRef]
- Escobar, P.; Milena Leal, S.; Herrera, L.V.; Martinez, J.R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp. essential oils and their major components. Memórias Do Inst. Oswaldo Cruz 2010, 105, 184–190. [Google Scholar] [CrossRef]
- Mahecha, G.L.; Echeverria, G.C.; Barrera, J.A.; Garavagno, M.d.l.A.; Pino, G.A. Tropospheric degradation of nerol: Kinetics with OH radicals, product distribution in the presence of NOx and atmospheric implications. Atmos. Environ. 2025, 346, 121055. [Google Scholar] [CrossRef]
- Coêlho, M.L.; Islam, M.T.; Oliveira, G.L.d.S.; de Alencar, M.V.O.B.; Santos, J.V.d.O.; dos Reis, A.C.; da Mata, A.M.O.F.; Correia Jardim Paz, M.F.; Docea, A.O.; Calina, D.; et al. Cytotoxic and Antioxidant Properties of Natural Bioactive Monoterpenes Nerol, Estragole, and 3,7-Dimethyl-1-Octanol. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 8002766. [Google Scholar] [CrossRef] [PubMed]
- Lapczynski, A.; Foxenberg, R.J.; Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on nerol. Food Chem. Toxicol. 2008, 46, S241–S244. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.d.S.e.; Marques, J.N.d.J.; Linhares, E.P.M.; Bonora, C.M.; Costa, É.T.; Saraiva, M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem.-Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, K.; Chen, L.; Yan, R.; Qu, S.; Li, Y.; Liu, M.; Zeng, H.; Tian, J. Activities of Nerol, a natural plant active ingredient, against Candida albicans in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 5039–5052. [Google Scholar] [CrossRef]
- Alossaily, M.G.; Shamas, M.; Chakir, A.; Roth, E. Temperature-Dependent Kinetic Study of the Gas Phase Ozonolysis of Linalool, Nerol, and Citronellol. Int. J. Chem. Kinet. 2025, 57, 342–350. [Google Scholar] [CrossRef]
- Aazaad, B.; Mano Priya, A.; Subramani, R. Environmental implications of oxalic and malonic acids with tropospheric oxidants. J. Phys. Org. Chem. 2024, 37, e4639. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Noga, J.; Bartlett, R.J. The full CCSDT model for molecular electronic structure. J. Chem. Phys. 1987, 86, 7041–7050. [Google Scholar] [CrossRef]
- Ramabhadran, R.O.; Raghavachari, K. Extrapolation to the Gold-Standard in Quantum Chemistry: Computationally Efficient and Accurate CCSD(T) Energies for Large Molecules Using an Automated Thermochemical Hierarchy. J. Chem. Theory Comput. 2013, 9, 3986–3994. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.A., Jr.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J. Chem. Phys. 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Werner, H.-J.; Knowles, P.J.; Celani, P.; Györffy, W.; Hesselmann, A.; Kats, D.; Knizia, G.; Köhn, A.; Korona, T.; Kreplin, D.; et al. MOLPRO, a Package of Ab Initio Programs, version 2015; University of Cardiff: Cardiff, UK, 2015. [Google Scholar]
- Garrett, B.C.; Truhlar, D.G. Generalized transition state theory. Bond energy-bond order method for canonical variational calculations with application to hydrogen atom transfer reactions. J. Am. Chem. Soc. 1979, 101, 4534–4548. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Criterion of minimum state density in the transition state theory of bimolecular reactions. J. Chem. Phys. 1979, 70, 1593–1598. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G.; Grev, R.S.; Magnuson, A.W. Improved treatment of threshold contributions in variational transition-state theory. J. Phys. Chem. 1980, 84, 1730–1748. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, S.; Corchado, J.; Chuang, Y.; Coitino, E.; Ellingson, B.; Truhlar, D. Gaussrate, Version 2017-B; University of Minnesota: Minneapolis, MN, USA, 2010. [Google Scholar]
- Zheng, J.; Zhang, S.; Lynch, B.; Corchado, J.; Chuang, Y.; Fast, P.; Hu, W.; Liu, Y.; Lynch, G.; Nguyen, K. Polyrate, version 2017; University of Minnesota: Minneapolis, MN, USA, 2010. [Google Scholar]
- Ingold, K.U. Peroxy radicals. Acc. Chem. Res. 1969, 2, 1–9. [Google Scholar] [CrossRef]
- Lightfoot, P.D.; Cox, R.A.; Crowley, J.N.; Destriau, M.; Hayman, G.D.; Jenkin, M.E.; Moortgat, G.K.; Zabel, F. Organic peroxy radicals: Kinetics, spectroscopy and tropospheric chemistry. Atmos. Environ. 1992, 26, 1805–1961. [Google Scholar] [CrossRef]
- Zhang, J.; Dransfield, T.; Donahue, N.M. On the Mechanism for Nitrate Formation via the Peroxy Radical + NO Reaction. J. Phys. Chem. A 2004, 108, 9082–9095. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Monks, P.S. Gas-phase radical chemistry in the troposphere. Chem. Soc. Rev. 2005, 34, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Idaboy, J.R.; Mora-Diez, N.; Boyd, R.J.; Vivier-Bunge, A. On the Importance of Prereactive Complexes in Molecule-Radical Reactions: Hydrogen Abstraction from Aldehydes by OH. J. Am. Chem. Soc. 2001, 123, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Atkinson, R. Atmospheric lifetimes and fates of a series of sesquiterpenes. J. Geophys. Res. Atmos. 1995, 100, 7275–7281. [Google Scholar] [CrossRef]

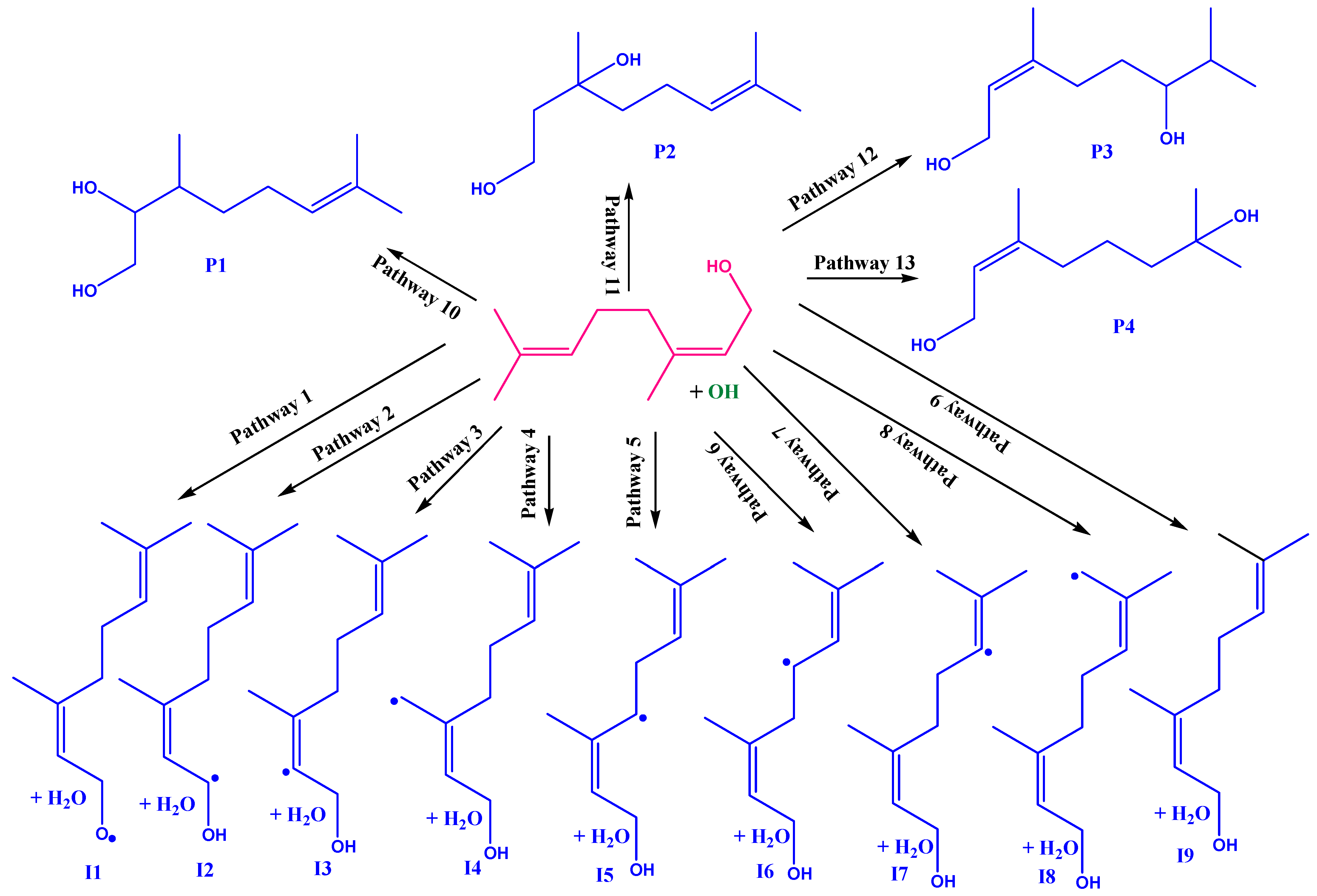

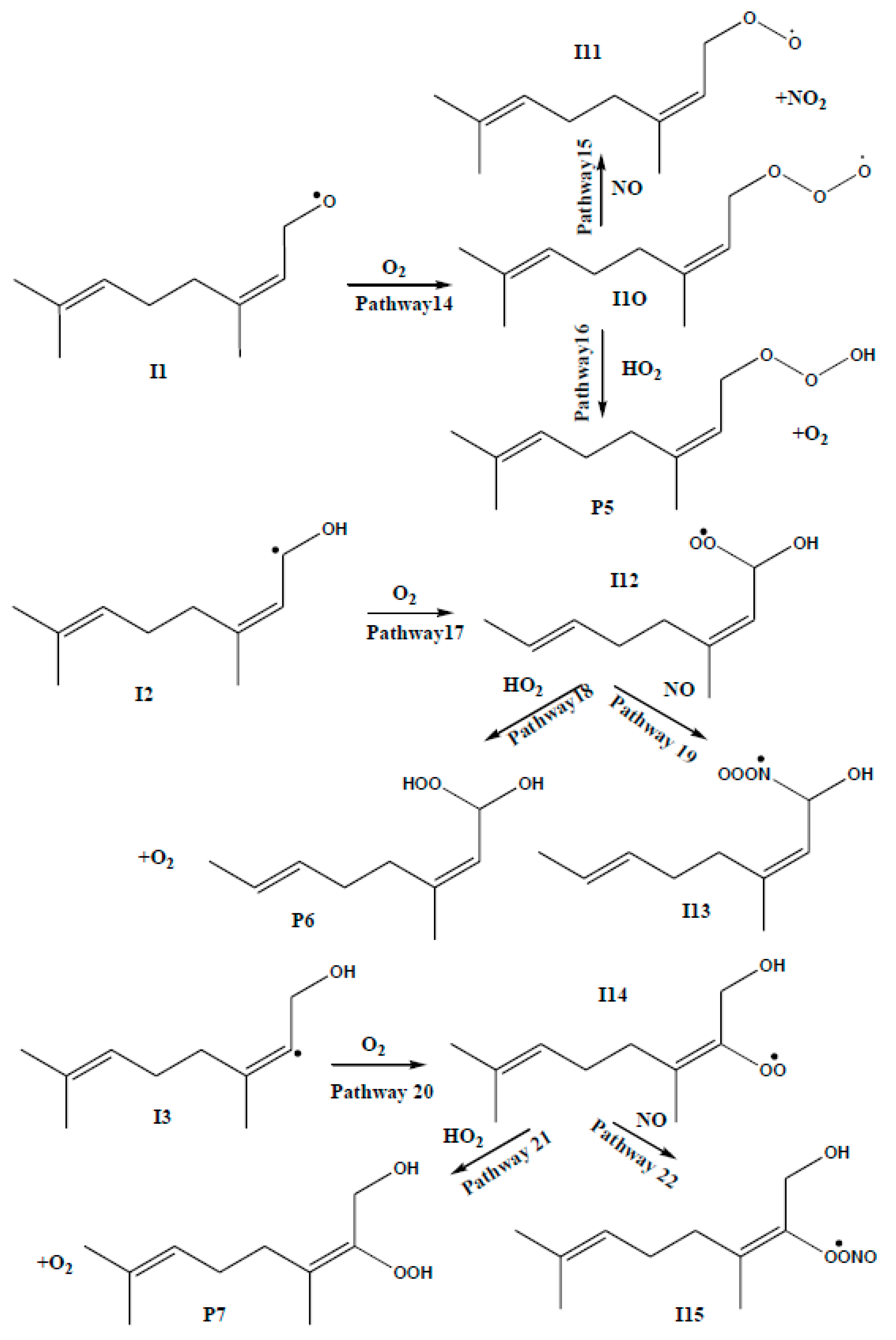
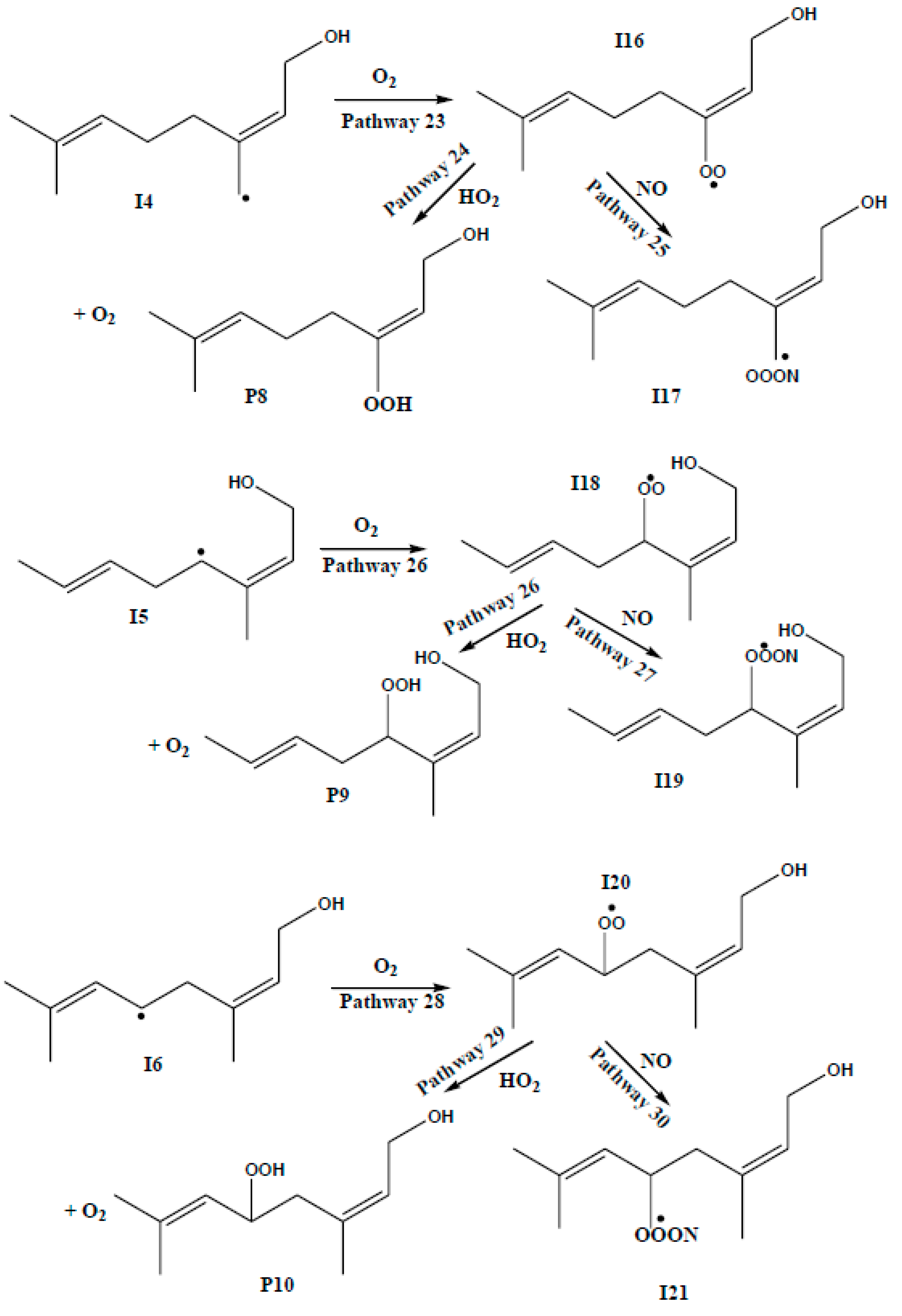
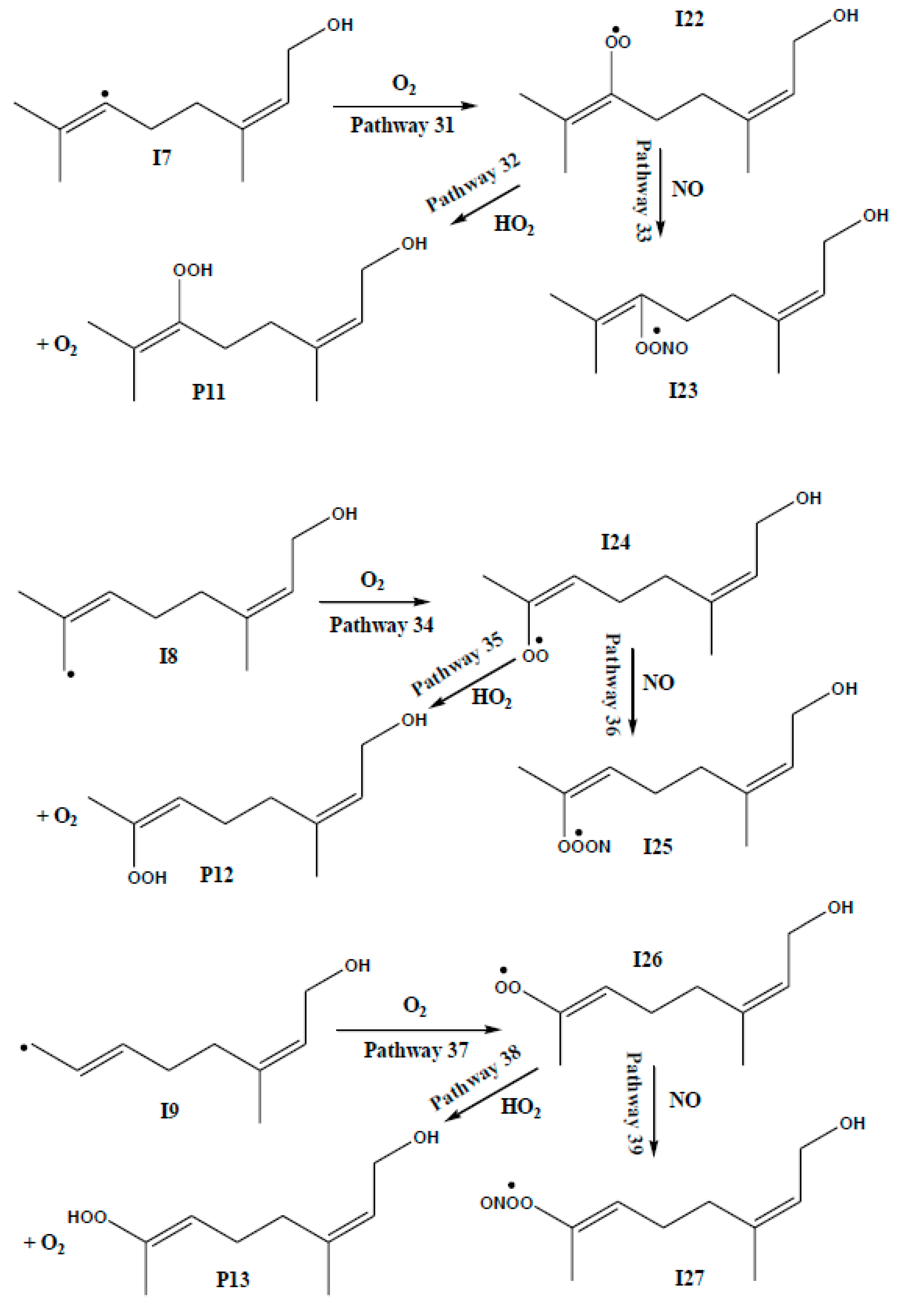

| REACTION PATHWAYS | M06-2X | CCSD(T) | CBS-QB3 | |||
|---|---|---|---|---|---|---|
| ΔE | ΔH | ΔG | ΔE | ΔE | ||
| PATHWAY 1 | R | 0 | 0 | 0 | 0 | 0 |
| RC1 | −10.81 | −8.84 | −0.34 | −8.31 | −6.32 | |
| TS1 | 1.13 | 0.11 | 8.54 | 3.83 | 2.22 | |
| IC1 | −19.71 | −18.96 | −12.38 | −17.22 | −16.95 | |
| I1+H2O | −13.40 | −14.27 | −15.03 | −11.79 | −14.13 | |
| PATHWAY 2 | TS2 | −2.09 | −2.73 | 5.93 | 0.08 | −2.59 |
| IC2 | −45.52 | −44.43 | −38.00 | −41.75 | −44.01 | |
| I2+H2O | −37.14 | −37.87 | −38.59 | −33.78 | −39.57 | |
| PATHWAY 3 | TS3 | 1.21 | −0.36 | 8.59 | 3.43 | −0.93 |
| IC3 | −16.19 | −14.51 | −8.32 | −12.83 | −12.92 | |
| I3+H2O | −8.17 | −8.44 | −10.24 | −6.49 | −9.42 | |
| PATHWAY 4 | TS4 | 0.90 | −0.15 | 8.05 | 2.35 | −0.90 |
| IC4 | −37.16 | −35.68 | −27.51 | −34.88 | −36.25 | |
| I4+H2O | −28.66 | −29.27 | −29.88 | −27.16 | −31.98 | |
| PATHWAY 5 | TS5 | −0.66 | −1.56 | 8.42 | 1.15 | −2.38 |
| IC5 | −31.72 | −32.09 | −28.16 | −29.02 | −35.98 | |
| I5+H2O | −30.47 | −31.60 | −32.65 | −25.22 | −33.70 | |
| PATHWAY 6 | TS6 | −1.24 | −2.29 | 6.71 | 0.61 | −1.95 |
| IC6 | −38.94 | −38.38 | −33.21 | −36.30 | −38.58 | |
| I6+H2O | −32.97 | −33.77 | −35.35 | −30.82 | −36.06 | |
| PATHWAY 7 | TS7 | 1.95 | 0.53 | 8.84 | 3.69 | −0.28 |
| IC7 | −16.54 | −15.46 | −9.79 | −13.48 | −13.55 | |
| I7+H2O | −9.85 | −10.08 | −11.81 | −8.07 | −10.89 | |
| PATHWAY 8 | TS8 | 1.96 | 0.96 | 9.09 | 3.17 | −0.25 |
| IC8 | −34.57 | −33.77 | −26.79 | −31.61 | −33.08 | |
| I8+H2O | −28.63 | −29.14 | −30.33 | −27.30 | −31.73 | |
| PATHWAY 9 | TS9 | 3.62 | 2.18 | 10.42 | 5.52 | 2.10 |
| IC9 | −33.65 | −32.89 | −26.59 | −31.39 | −33.26 | |
| I9+H2O | −28.76 | −29.16 | −29.68 | −27.08 | −31.54 | |
| PATHWAY 10 | TS10 | −6.61 | −4.91 | 4.83 | −4.45 | −5.93 |
| P1 | −39.04 | −35.28 | −25.56 | −33.90 | −33.99 | |
| PATHWAY 11 | TS11 | −8.04 | −5.83 | 4.76 | −6.86 | −6.92 |
| P2 | −39.00 | −35.28 | −24.22 | −34.26 | −33.61 | |
| PATHWAY 12 | TS12 | −5.17 | −3.54 | 6.13 | −2.81 | −3.37 |
| P3 | −35.64 | −32.08 | −22.85 | −31.11 | −30.46 | |
| PATHWAY 13 | TS13 | −5.99 | −4.41 | 5.12 | −4.35 | −4.91 |
| P4 | −34.80 | −31.74 | −21.54 | −30.80 | −31.96 | |
| Reaction Pathways | ΔE | ΔH | ΔG |
|---|---|---|---|
| P1+O2 | 0 | 0 | 0 |
| I28 | −37.62 | −34.17 | −28.31 |
| I28+NO | 0 | 0 | 0 |
| I29 | −60.79 | −58.86 | −59.46 |
| P2+O2 | 0 | 0 | 0 |
| I30 | −33.13 | −29.17 | −24.39 |
| I30+NO | 0 | 0 | 0 |
| I31 | −67.74 | −65.68 | −64.56 |
| P3+O2 | 0 | 0 | 0 |
| I32 | −36.49 | −32.86 | −26.28 |
| I32+NO | 0 | 0 | 0 |
| I33 | −63.83 | −62.00 | −61.25 |
| P4+O2 | 0 | 0 | 0 |
| I34 | −36.74 | −32.90 | −28.54 |
| I34+NO | 0 | 0 | 0 |
| I35 | −66.72 | −64.97 | −64.92 |
| I31 | 0 | 0 | 0 |
| TS24 | 3.47 | 2.74 | 2.69 |
| P14+I36 | 1.52 | −0.03 | −2.78 |
| I36+O2 | 0 | 0 | 0 |
| TS25 | 26.92 | 25.68 | 29.45 |
| P15+HO2 | 8.57 | 6.78 | −1.68 |
| I31 | 0 | 0 | 0 |
| TS26 | 14.60 | 13.13 | 12.54 |
| I37+I38 | 4.34 | 1.96 | −1.41 |
| I38+O2 | 0 | 0 | 0 |
| TS27 | −3.57 | −5.75 | −2.18 |
| P16+HO2 | −39.62 | −38.05 | −34.47 |
| T (K) | kI1 (10−13) | kI2 | kI3 (10−13) | kI4 | kI5 (10−12) | kI6 (10−12) | kI7 (10−13) | kI8 (10−14) | kI9 (10−14) | kII10 | kI11 | kI12 (10−11) | kI13 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 278 | 4.49 | 5.19 × 10−11 | 2.08 | 1.03 × 10−12 | 1.53 | 7.25 | 1.94 | 5.98 | 1.35 | 2.11 × 10−10 | 1.11 × 10−9 | 9.01 | 2.00 × 10−10 | 1.67 × 10−9 |
| 288 | 4.64 | 4.46 × 10−11 | 2.15 | 1.07 × 10−12 | 1.42 | 6.73 | 2.09 | 6.36 | 1.51 | 1.47 × 10−10 | 8.31 × 10−10 | 7.37 | 1.54 × 10−10 | 1.26 × 10−9 |
| 298 | 4.80 | 3.88 × 10−11 | 2.23 | 1.12 × 10−12 | 1.32 | 6.29 | 2.24 | 7.16 | 1.69 | 1.05 × 10−10 | 6.34 × 10−10 | 6.12 | 1.20 × 10−10 | 9.68 × 10−10 |
| 308 | 4.9 | 3.41 × 10−11 | 2.30 | 1.16 × 10−12 | 1.24 | 5.92 | 1.46 | 7.54 | 1.88 | 7.63 × 10−11 | 4.93 × 10−10 | 2.52 | 9.53 × 10−11 | 7.33 × 10−10 |
| 318 | 5.14 | 3.02 × 10−11 | 2.38 | 4.75 × 10−13 | 1.17 | 5.61 | 1.02 | 7.93 | 2.09 | 5.69 × 10−11 | 3.90 × 10−10 | 2.13 | 7.70 × 10−11 | 5.84 × 10−10 |
| 328 | 5.31 | 2.70 × 10−11 | 2.46 | 4.80 × 10−13 | 1.11 | 5.33 | 1.09 | 8.32 | 2.31 | 4.32 × 10−11 | 3.14 × 10−10 | 1.83 | 6.31 × 10−11 | 4.74 × 10−10 |
| 338 | 5.49 | 2.44 × 10−11 | 2.55 | 4.86 × 10−13 | 1.06 | 5.10 | 1.17 | 8.73 | 2.55 | 3.34 × 10−11 | 2.56 × 10−10 | 1.58 | 5.24 × 10−11 | 3.90 × 10−10 |
| 348 | 3.48 | 9.75 × 10−12 | 2.64 | 4.92 × 10−13 | 1.02 | 4.90 | 1.25 | 9.14 | 2.81 | 2.63 × 10−11 | 2.12 × 10−10 | 1.38 | 4.41 × 10−11 | 3.13 × 10−10 |
| 350 | 3.50 | 9.57 × 10−12 | 2.66 | 4.94 × 10−13 | 1.01 | 4.86 | 1.26 | 9.23 | 2.86 | 2.51 × 10−11 | 2.04 × 10−10 | 1.35 | 4.27 × 10−11 | 3.02 × 10−10 |
| T (K) | kI1 | kI2 | kI3 | kI4 | kI5 | kI6 | kI7 | kI8 | kI9 | kI10 | kI11 | kI12 | kI13 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 278 | 0.03 | 3.10 | 0.01 | 0.06 | 0.09 | 0.43 | 0.01 | 0.00 | 0.00 | 12.61 | 66.30 | 5.38 | 11.97 | 100.00 |
| 288 | 0.04 | 3.54 | 0.02 | 0.09 | 0.11 | 0.53 | 0.02 | 0.01 | 0.00 | 11.64 | 65.97 | 5.85 | 12.19 | 100.00 |
| 298 | 0.05 | 4.00 | 0.02 | 0.12 | 0.14 | 0.65 | 0.02 | 0.01 | 0.00 | 10.79 | 65.48 | 6.33 | 12.39 | 100.00 |
| 308 | 0.07 | 4.65 | 0.03 | 0.16 | 0.17 | 0.81 | 0.02 | 0.01 | 0.00 | 10.40 | 67.25 | 3.43 | 13.00 | 100.00 |
| 318 | 0.09 | 5.18 | 0.04 | 0.08 | 0.20 | 0.96 | 0.02 | 0.01 | 0.00 | 9.74 | 66.83 | 3.65 | 13.20 | 100.00 |
| 328 | 0.11 | 5.71 | 0.05 | 0.10 | 0.23 | 1.13 | 0.02 | 0.02 | 0.00 | 9.13 | 66.31 | 3.86 | 13.33 | 100.00 |
| 338 | 0.14 | 6.25 | 0.07 | 0.12 | 0.27 | 1.31 | 0.03 | 0.02 | 0.01 | 8.58 | 65.68 | 4.06 | 13.46 | 100.00 |
| 348 | 0.11 | 3.11 | 0.08 | 0.16 | 0.32 | 1.56 | 0.04 | 0.03 | 0.01 | 8.39 | 67.68 | 4.42 | 14.08 | 100.00 |
| 350 | 0.12 | 3.17 | 0.09 | 0.16 | 0.33 | 1.61 | 0.04 | 0.03 | 0.01 | 8.31 | 67.54 | 4.46 | 14.12 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mano Priya, A.; El Dib, G. Hydroxyl Radical-Initiated Reaction of Nerol: A Pathway to Secondary Pollutants in an Indoor Environment. Reactions 2025, 6, 49. https://doi.org/10.3390/reactions6030049
Mano Priya A, El Dib G. Hydroxyl Radical-Initiated Reaction of Nerol: A Pathway to Secondary Pollutants in an Indoor Environment. Reactions. 2025; 6(3):49. https://doi.org/10.3390/reactions6030049
Chicago/Turabian StyleMano Priya, Angappan, and Gisèle El Dib. 2025. "Hydroxyl Radical-Initiated Reaction of Nerol: A Pathway to Secondary Pollutants in an Indoor Environment" Reactions 6, no. 3: 49. https://doi.org/10.3390/reactions6030049
APA StyleMano Priya, A., & El Dib, G. (2025). Hydroxyl Radical-Initiated Reaction of Nerol: A Pathway to Secondary Pollutants in an Indoor Environment. Reactions, 6(3), 49. https://doi.org/10.3390/reactions6030049







