Efficient Synthesis of Eight-Membered Cyclic Diaryl Sulfides via an Aryne Reaction with 2-Methylenebenzothiophene-3-Ones
Abstract
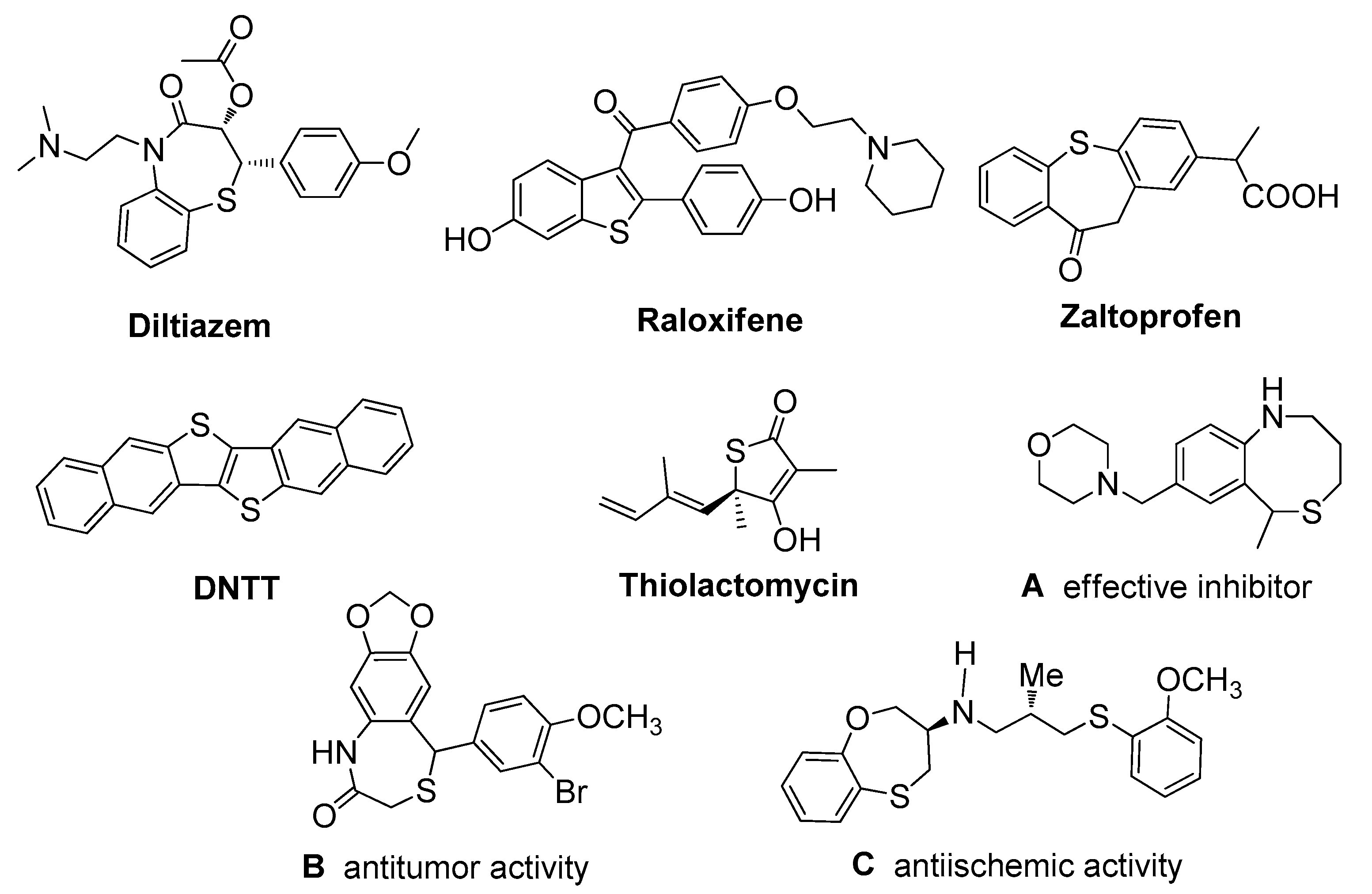
1. Introduction
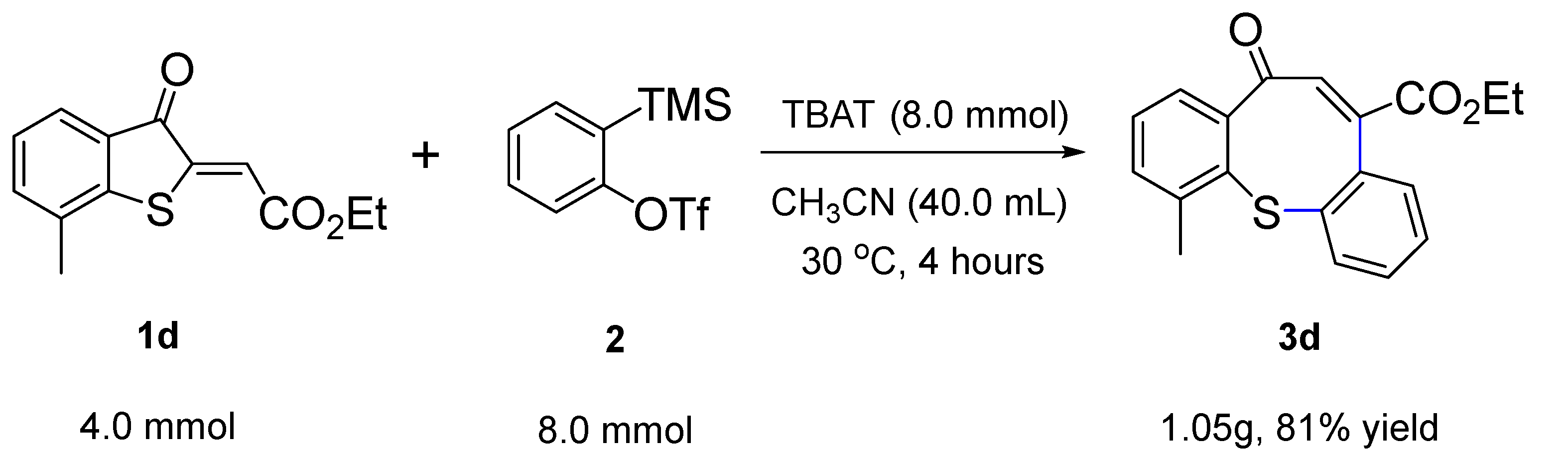
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suchý, M.; Kutschy, P.; Monde, K.; Goto, H.; Harada, N.; Takasugi, M.; Dzurilla, M.; Balentová, E. Synthesis, Absolute Configuration, and Enantiomeric Enrichment of a Cruciferous Oxindole Phytoalexin, (S)-(−)-Spirobrassinin, and Its Oxazoline Analog. J. Org. Chem. 2001, 66, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Bois-Choussy, M.; Zhu, J. Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc. 2006, 128, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Hale, C.R.H.; Nilewski, C.; Ioannidou, H.A. Constructing Molecular Complexity and Diversity: Total Synthesis of Natural Products of Biological and Medicinal Importance. Chem. Soc. Rev. 2012, 41, 5185–5238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Jiang, X. Sulfur-Center-Involved Photocatalyzed Reactions. Chem. Asian J. 2018, 13, 2208–2242. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Ma, D. A Scalable Total Synthesis of the Antitumor Agents Et-743 and Lurbinectedin. Angew. Chem. Int. Ed. 2019, 58, 3972–3975. [Google Scholar] [CrossRef]
- Yoneya, T.; Taniguchi, K.; Nakamura, R.; Tsunenari, T.; Ohizumi, I.; Kanbe, Y.; Morikawa, K.; Kaiho, S.-I.; Yamada-Okabe, H. Thiochroman Derivative CH4986399, A New Nonsteroidal Estrogen Receptor Down-regulator, Is Effective in Breast Cancer Models. Anticancer Res. 2010, 30, 873–878. [Google Scholar]
- Berrade, L.; Aisa, B.; Ramirez, M.J.; Galiano, S.; Guccione, S.; Moltzau, L.R.; Levy, F.O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; et al. Novel Benzo[b]thiophene Derivatives as New Potential Antidepressants with Rapid Onset of Action. J. Med. Chem. 2011, 54, 3086–3090. [Google Scholar] [CrossRef]
- Zaher, A.F.; Abuel-Maaty, S.M.; El-Nassan, H.B.; Amer, S.A.S.; Abdelghany, T.M. Synthesis, Antitumor Screening and Cell Cycle Analysis of Novel Benzothieno[3,2-b]pyran Derivatives. J. Enzyme Inhib. Med. Chem. 2016, 31, 145–153. [Google Scholar] [CrossRef]
- Boyd-Kimball, D.; Gonczy, K.; Lewis, B.; Mason, T.; Siliko, N.; Wolfe, J. Classics in Chemical Neuroscience: Chlorpromazine. ACS Chem. Neurosci. 2019, 10, 79–88. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; van den Oord, S.C.H.; Dimitriu-Leen, A.; Maas, A.H.E.M.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA: The EDIT-CMD Randomized Clinical Trial. JACC Cardiovasc. Imaging 2022, 15, 1473–1484. [Google Scholar] [CrossRef]
- Viscardi, S.; Topola, E.; Sobieraj, J.; Duda-Madej, A. Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia. Antibiotics 2024, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Shinamura, S.; Osaka, I.; Miyazaki, E. Thienoacene-Based Organic Semiconductors. Adv. Mater. 2011, 23, 4347–4370. [Google Scholar] [CrossRef] [PubMed]
- Lehnherr, D.; Hallani, R.; McDonald, R.; Anthony, J.E.; Tykwinski, R.R. Synthesis and Properties of Isomerically Pure Anthrabisbenzothiophenes. Org. Lett. 2012, 14, 62–65. [Google Scholar] [CrossRef]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic Semiconductors Based on [1]Benzothieno[3,2-b][1]benzothiophene Substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, Z.; Lin, Y.; Tang, S.; Yang, J.; Hu, J.; Peng, Q.; Ma, D.; Li, Q.; Li, Z. New AIEgens Containing Dibenzothiophene-S,S-dioxide and Tetraphenylethene Moieties: Similar Structures but Much Different Hole/Electron Transport Properties. J. Mater. Chem. C 2015, 3, 5903–5909. [Google Scholar] [CrossRef]
- Banerjee, T.; Sharma, S.K.; Kapoor, N.; Dwivedi, V.; Surolia, N.; Surolia, A. Benzothiophene Carboxamide Derivatives as Inhibitors of Plasmodium Falciparum Enoyl-ACP Reductase. IUBMB Life 2011, 63, 1101–1110. [Google Scholar] [CrossRef]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent Developments and Biological Activities of Thiazolidinone Derivatives: A Review. Bioorg. Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Lopez-Cara, C.; Preti, D.; Tabrizi, M.A.; Balzarini, J.; Bassetto, M.; Brancale, A.; Fu, X.-H.; Gao, Y.; et al. Concise Synthesis and Biological Evaluation of 2-Aroyl-5-Amino Benzo[b]thiophene Derivatives As a Novel Class of Potent Antimitotic Agents. J. Med. Chem. 2013, 56, 9296–9309. [Google Scholar] [CrossRef]
- Scott, S.A.; Spencer, C.T.; O’Reilly, M.C.; Brown, K.A.; Lavieri, R.R.; Cho, C.-H.; Jung, D.-I.; Larock, R.C.; Alex Brown, H.; Lindsley, C.W. Discovery of Desketoraloxifene Analogues as Inhibitors of Mammalian, Pseudomonas aeruginosa, and NAPE Phospholipase D Enzymes. ACS Chem. Biol. 2015, 10, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Dao, P.; Ye, F.; Liu, Y.; Du, Z.Y.; Zhang, K.; Dong, C.Z.; Meunier, B.; Chen, H. Development of Phenothiazine-Based Theranostic Compounds That Act Both as Inhibitors of β-Amyloid Aggregation and as Imaging Probes for Amyloid Plaques in Alzheimer’s Disease. ACS Chem. Neurosci. 2017, 8, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Kugita, H.; Inoue, H.; Ikezaki, M.; Konda, M.; Takeo, S. Synthesis of 1,5-Benzothiazepine Derivatives. II. Chem. Pharm. Bull. 1970, 18, 2284–2299. [Google Scholar] [CrossRef]
- Asano, K.; Matsubara, S. Catalytic Approaches to Optically Active 1,5-Benzothiazepines. ACS Catal. 2018, 8, 6273–6282. [Google Scholar] [CrossRef]
- Jones, C.D.; Jevnikar, M.G.; Pike, A.J.; Peters, M.K.; Black, L.J.; Thompson, A.R.; Falcone, J.F.; Clemens, J.A. Antiestrogens. 2. Structure-Activity Studies in a Series of 3-Aroyl-2-arylbenzo[b]thiophene Derivatives Leading to [6-Hydroxy-2-(4-hydroxyphenyl) benzo[b]thien-3-yl] [4-[2-(1-piperidinyl) ethoxy]-phenyl] methanone Hydrochloride (LY156758), a Remarkably Effective Estrogen Antagonist with Only Minimal Intrinsic Estrogenicity. J. Med. Chem. 1984, 27, 1057–1066. [Google Scholar]
- Yasuo, F.; Shigeru, Y. DE2941869. 1980. Available online: https://patents.google.com/patent/DE2941869C2/d (accessed on 26 May 2025).
- Shoji, M.; Mikio, W. Production of 2-(10,11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl) Propionic Acid. JP61251682A, 8 November 1986. [Google Scholar]
- Mitsuo, M.; Hiromitsu, T.; Naoya, M. Verfahren zur Herstellung von Dibenzothiepin-Derivaten. EP0309626 A1, 5 April 1989. [Google Scholar]
- Yamamoto, T.; Takimiya, K. Facile Synthesis of Highly π-Extended Heteroarenes, Dinaphtho [2,3-b: 2′, 3′-f] chalcogenopheno [3,2-b] chalcogenophenes, and Their Application to Field-Effect Transistors. J. Am. Chem. Soc. 2007, 129, 2224–2225. [Google Scholar] [CrossRef]
- Kang, M.J.; Doi, I.; Mori, H.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H. Alkylated Dinaphtho [2,3-b:2′,3′-f] Thieno [3,2-b] Thiophenes (Cn-DNTTs): Organic Semiconductors for High-Performance Thin-Film Transistors. Adv. Mater. 2011, 23, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Nishida, I.; Kawaguchi, A.; Yamada, M. Effect of Thiolactomycin on the Individual Enzymes of the Fatty Acid Synthase System in Escherichia coli1. J. Biochem. 1986, 99, 1447–1454. [Google Scholar] [CrossRef]
- Slayden, R.A.; Lee, R.E.; Armour, J.W.; Cooper, A.M.; Orme, I.M.; Brennan, P.J.; Besra, G.S. Antimycobacterial Action of Thiolactomycin: An Inhibitor of Fatty Acid and Mycolic Acid Synthesis. Antimicrob. Agents Chemother. 1996, 40, 2813–2819. [Google Scholar] [CrossRef]
- Heath, R.J.; White, S.W.; Rock, C.O. Lipid Biosynthesis as a Target for Antibacterial Agents. Prog. Lipid Res. 2001, 40, 467–497. [Google Scholar] [CrossRef]
- Tomita, K.; Sato, S.; Kobayashi, S. Condensed Heterocyclic Compound. JP 60132972 A, 12 December 1983. [Google Scholar]
- Wu, L.-Q.; Yang, X.-J.; Peng, Q.-J.; Sun, G.-F. Synthesis and Anti-proliferative Activity Evaluation of Novel Benzo[d][1,3] dioxoles-fused 1,4-Thiazepines. Eur. J. Med. Chem. 2017, 127, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Grand, B.L.; Pignier, C.; Létienne, R.; Cuisiat, F.; Rolland, F.; Mas, A.; Vacher, B. Sodium Late Current Blockers in Ischemia Reperfusion: Is the Bullet Magic? J. Med. Chem. 2008, 51, 3856–3866. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Chen, D.-L. Eight-Membered Thiocycloether via Indium-Mediated Ring Enlargement. Synlett 1999, 1999, 735–736. [Google Scholar] [CrossRef]
- Qiao, Z.; Liu, H.; Xiao, X.; Fu, Y.; Wei, J.; Li, Y.; Jiang, X. Efficient Access to 1,4-Benzothiazine: Palladium-Catalyzed Double C–S Bond Formation Using Na2S2O3 as Sulfurating Reagent. Org. Lett. 2013, 15, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Sivaguru, M.; Suresh, E. Indium(III) Chloride Catalyzed Highly Diastereoselective Domino Synthesis of Indenodithiepines and Indenodithiocines. Chem. Commun. 2015, 51, 707–710. [Google Scholar] [CrossRef]
- Acharya, A.; Vijay Kumar, S.; Ila, H. Diversity-Oriented Synthesis of Substituted Benzo[b]thiophenes and Their Hetero-Fused Analogues through Palladium-Catalyzed Oxidative C-H Functionalization/Intramolecular Arylthiolation. Chem. Eur. J. 2015, 21, 17116–17125. [Google Scholar] [CrossRef]
- Masuya, Y.; Tobisu, M.; Chatani, N. Palladium-Catalyzed Synthesis of 2,3-Disubstituted Benzothiophenes via the Annulation of Aryl Sulfides with Alkynes. Org. Lett. 2016, 18, 4312–4315. [Google Scholar] [CrossRef]
- Meng, L.; Fujikawa, T.; Kuwayama, M.; Segawa, Y.; Itami, K. Thiophene-Fused π-Systems from Diarylacetylenes and Elemental Sulfur. J. Am. Chem. Soc. 2016, 138, 10351–10355. [Google Scholar] [CrossRef]
- Simlandy, A.K.; Mukherjee, S. Catalytic Enantioselective Synthesis of 3,4-Unsubstituted Thiochromenes through Sulfa-Michael/Julia–Kocienski Olefination Cascade Reaction. J. Org. Chem. 2017, 82, 4851–4858. [Google Scholar] [CrossRef]
- Yugandar, S.; Konda, S.; Ila, H. Synthesis of Substituted Benzo[b]thiophenes via Sequential One-Pot, Copper-Catalyzed Intermolecular C–S Bond Formation and Palladium-Catalyzed Intramolecular Arene–Alkene Coupling of Bis(het)aryl/alkyl-1,3-monothiodiketones and o-Bromoiodoarenes. Org. Lett. 2017, 19, 1512–1515. [Google Scholar] [CrossRef]
- Song, J.; Wu, H.; Sun, W.; Wang, S.; Sun, H.; Xiao, K.; Qian, Y.; Liu, C. A Pd-catalyzed Optional Approach for the Synthesis of Dibenzothiophenes. Org. Biomol. Chem. 2018, 16, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, Y.; Hou, X.; Zeng, W.; Yu, A.; Zhao, X.; Meng, X. Darzens Reaction of Thioisatins and Sulfonium Salts: Approach to the Synthesis of Thiochromenone Derivatives with Anticancer Potency. Org. Biomol. Chem. 2018, 16, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, L.; Qian, C.; Zhu, X.; Yang, Y.; Liu, J.; Yang, Y.; Liang, Y. Copper-Catalyzed Synthesis of 2-Acylbenzo[b]thiophenes from 3-(2-Iodophenyl)-1-arylpropan-1-ones and Potassium Sulfide under Aerobic Conditions. Org. Biomol. Chem. 2018, 16, 8020–8024. [Google Scholar] [CrossRef]
- Garg, P.; Singh, A. Unmasking Dipole Character of Acyl Ketene Dithioacetals via a Cascade Reaction with Arynes: Synthesis of Benzo[b]thiophenes. Org. Lett. 2018, 20, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kinoshita, H.; Miura, K. Diisobutylaluminum Hydride Promoted Selectivity-Switchable Synthesis of Benzothiophene Oxides and Benzothiophenes via an Al–Li-Dimetalated Intermediate. Org. Lett. 2020, 22, 3123–3127. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Q.; Zhang, J.; Deng, G. Regioselective Reversal Cyclization to Access either Eight-Membered Sulfur-Containing Heterocycle-Fused γ-Pyrones or 2-(1,4-Dithianyl)-4-pyrones from the Same Precursors. J. Org. Chem. 2020, 85, 2575–2584. [Google Scholar] [CrossRef]
- An, Y.; Zhang, F.; Du, G.; Cai, Z.; He, L. Construction of 6H-benzo[c]thiochromenes via a Tandem Reaction of Arynes with Thionoesters. Org. Chem. Front. 2021, 8, 6979–6984. [Google Scholar] [CrossRef]
- Sundaravelu, N.; Nandy, A.; Sekar, G. Visible Light Mediated Photocatalyst Free C–S Cross Coupling: Domino Synthesis of Thiochromane Derivatives via Photoinduced Electron Transfer. Org. Lett. 2021, 23, 3115–3119. [Google Scholar] [CrossRef]
- Molander, G.A. Diverse Methods for Medium Ring Synthesis. Acc. Chem. Res. 1998, 31, 603–609. [Google Scholar] [CrossRef]
- Maier, M.E. Synthesis of Medium-Sized Rings by the Ring-Closing Metathesis Reaction. Angew. Chem. Int. Ed. 2000, 39, 2073–2077. [Google Scholar] [CrossRef]
- Mukherjee, C.; Biehl, E. An Efficient Synthesis of Benzene Fused Six-, Seven- and Eight-membered Rings Containing Nitrogen and Sulfur by Benzyne Ring Closure Reaction. Heterocycles 2004, 63, 2309–2318. [Google Scholar] [CrossRef]
- Lu, S.-M.; Alper, H. Intramolecular Carbonylation Reactions with Recyclable Palladium-Complexed Dendrimers on Silica: Synthesis of Oxygen, Nitrogen, or Sulfur-Containing Medium Ring Fused Heterocycles. J. Am. Chem. Soc. 2005, 127, 14776–14784. [Google Scholar] [CrossRef] [PubMed]
- Foubelo, F.; Moreno, B.; Soler, T.; Yus, M. Reductive Ring Opening of Dihydrodibenzothiepine and Dihydrodinaphtho-Oxepine and -Thiepine. Tetrahedron 2005, 61, 9082–9096. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Q.; Jiang, X. Transition-Metal-Free Diarylannulated Sulfide and Selenide Construction via Radical/Anion-Mediated Sulfur−Iodine and Selenium−Iodine Exchange. Org. Lett. 2016, 18, 5756–5759. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, J.; Fan, Q.; Jiang, X. Cu(II)-catalyzed Sulfide Construction: Both Aryl Groups Utilization of Intermolecular and Intramolecular Diaryliodonium Salt. Chem. Commun. 2017, 53, 2918–2921. [Google Scholar] [CrossRef]
- Ogo, N.; Ishikawa, Y.; Sawada, J.; Matsuno, K.; Hashimoto, A.; Asai, A. Structure-Guided Design of Novel L-Cysteine Derivatives as Potent KSP Inhibitors. ACS Med. Chem. Lett. 2015, 6, 1004–1009. [Google Scholar] [CrossRef]
- Xiao, P.; Su, S.; Wang, W.; Cao, W.; Chen, J.; Li, J.; Chen, Y. Sequential Cycloaddition and Ring Expansion Reaction of Arynes and Methylenebenzothiopheneones: Synthesis of a Benzo-Fused Eight-Membered Ring via Sulfonium Ylides. RSC Adv. 2019, 9, 39119–39123. [Google Scholar] [CrossRef]
- Ding, W.; Yu, A.; Zhang, L.; Meng, X. Construction of Eight-Membered Cyclic Diaryl Sulfides via Domino Reaction of Arynes with Thioaurone Analogues and DFT Study on the Reaction Mechanism. Org. Lett. 2019, 21, 9014–9018. [Google Scholar] [CrossRef]
- Reddy, R.J.; Waheed, M.; Kumari, A.H.; Krishna, G.R. Interrupted CuAAC-Thiolation for the Construction of 1,2,3-Triazole-Fused Eight-Membered Heterocycles from O-/N-Propargyl derived Benzyl Thiosulfonates with Organic Azides. Adv. Synth. Catal. 2022, 364, 319–325. [Google Scholar] [CrossRef]

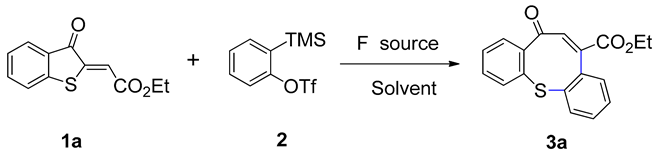 | ||||
|---|---|---|---|---|
| Entry | Solvent | F Source | T (°C) | Yield (%) b |
| 1 | EtOAc | CsF | 30 | Trace |
| 2 | THF | CsF | 30 | Trace |
| 3 | CH2Cl2 | CsF | 30 | Trace |
| 4 | EtOH | CsF | 30 | Trace |
| 5 | Toluene | CsF | 30 | N.R. c |
| 6 | Acetone | CsF | 30 | 33 |
| 7 | CH3CN | CsF | 30 | 52 |
| 8 | CH3CN | KF | 30 | 16 |
| 9 | CH3CN | NaF | 30 | Trace |
| 10 | CH3CN | TBAF | 30 | Trace |
| 11 | CH3CN | TBAT | 30 | 92 |
| 12 d | CH3CN | TBAT | 30 | 73 |
| 13 | CH3CN | TBAT | 40 | 77 |
| 14 | CH3CN | TBAT | 50 | 84 |
| 15 | CH3CN | TBAT | 60 | 91 |
| 16 e | CH3CN | TBAT | 30 | 92 |
| 17 f | CH3CN | TBAT | 30 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Zou, W.; Zhang, H.; Huang, Q.; Huang, A.; Liu, K.; Yue, G. Efficient Synthesis of Eight-Membered Cyclic Diaryl Sulfides via an Aryne Reaction with 2-Methylenebenzothiophene-3-Ones. Reactions 2025, 6, 35. https://doi.org/10.3390/reactions6020035
Feng J, Zou W, Zhang H, Huang Q, Huang A, Liu K, Yue G. Efficient Synthesis of Eight-Membered Cyclic Diaryl Sulfides via an Aryne Reaction with 2-Methylenebenzothiophene-3-Ones. Reactions. 2025; 6(2):35. https://doi.org/10.3390/reactions6020035
Chicago/Turabian StyleFeng, Juhua, Wenjie Zou, Haokun Zhang, Qilin Huang, Ailin Huang, Kuan Liu, and Guizhou Yue. 2025. "Efficient Synthesis of Eight-Membered Cyclic Diaryl Sulfides via an Aryne Reaction with 2-Methylenebenzothiophene-3-Ones" Reactions 6, no. 2: 35. https://doi.org/10.3390/reactions6020035
APA StyleFeng, J., Zou, W., Zhang, H., Huang, Q., Huang, A., Liu, K., & Yue, G. (2025). Efficient Synthesis of Eight-Membered Cyclic Diaryl Sulfides via an Aryne Reaction with 2-Methylenebenzothiophene-3-Ones. Reactions, 6(2), 35. https://doi.org/10.3390/reactions6020035






