Efficient Copolymerization of CO2 and Propylene Oxide via ZnGA/Zn-Co DMC Composite Catalysts: Synergistic Catalysis for High-Performance Polypropylene Carbonate
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst Preparation
2.2.1. ZnGA Synthesis
2.2.2. Preparation of Zn-Co DMC
2.2.3. Preparation of the ZnGA/DMC Catalyst
2.3. Copolymerization
2.4. Characterizations
3. Result and Discussion
3.1. ZnGA Catalysts
3.2. Zn-Co DMC Catalyst
3.3. Analysis of ZnGA/DMC Catalysts and Its Effect on PPC Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- North, M.; Styring, P. Perspectives and visions on CO2 capture and utilisation. Faraday Discuss. 2015, 183, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Tricase, C.; Cholewa-Wójcik, A.; Kawecka, A.; Rana, R.; Siracusa, V. Polylactic acid trays for fresh-food packaging A Carbon Footprint assessment. Sci. Total Environ. 2015, 537, 85–398. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Li, K.; Yu, H.; Li, F. Materials and system design for direct electrochemical CO2 conversion in capture media. J. Mater. Chem. A 2021, 9, 18785–18792. [Google Scholar] [CrossRef]
- Stegmann, P.; Daioglou, V.; Londo, M.; van Vuuren, D.P.; Junginger, M. Plastic futures and their CO2 emissions. Nature 2022, 612, 272–276. [Google Scholar] [CrossRef]
- Yan, X.; Duan, C.; Yu, S.; Dai, B.; Sun, C.; Chu, H. Recent advances on CO2 reduction reactions using single-atom catalysts. Renew. Sustain. Energy Rev. 2024, 190, 114086. [Google Scholar] [CrossRef]
- Decortes, A.; Castilla, A.M.; Kleij, A.W. Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew. Chem. Int. Ed. 2010, 49, 9822–9837. [Google Scholar] [CrossRef] [PubMed]
- Shaarani, F.W.; Bou, J.J. Synthesis of vegetable-oil based polymer by terpolymerization of epoxidized soybean oil, propylene oxide and carbon dioxide. Sci. Total Environ. 2017, 598, 931–936. [Google Scholar] [CrossRef]
- Scharfenberg, M.; Hilf, J.; Frey, H. Functional polycarbonates from carbon dioxide and tailored epoxide monomers: Degradable materials and their application potential. Adv. Funct. Mater. 2018, 28, 1704302. [Google Scholar] [CrossRef]
- Luinstra, G.A. Poly (propylene carbonate) old copolymers of propylene oxide and carbon dioxide with new interests: Catalysis and material properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wilson, S.J. What’s new with CO2? Recent advances in its copolymerization with oxiranes. Green Chem. 2012, 14, 2665–2671. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxid. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 287–292. [Google Scholar] [CrossRef]
- Inoue, S. Polymerization of Epidermis Influenced by Skin Tension Using Tritiated Thymidine Auto Radiography. Jpn. J. Plast. Reconstr. Surg. 1975, 18, 380–384. [Google Scholar]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide with organometallic compounds. Die Makromol. Chem. Macromol. Chem. Phys. 1969, 130, 210–220. [Google Scholar] [CrossRef]
- Yoo, J.; Na, S.J.; Park, H.C.; Cyriac, A.; Lee, B.Y. Anion variation on a cobalt (iii) complex of salen-type ligand tethered by four quaternary ammonium salts for CO2/epoxide copolymerization. Dalton Trans. 2010, 39, 2622–2630. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Yarbrough, J.C.; Ortiz, C.; Fang, C.C. Comparative kinetic studies of the copolymerization of cyclohexene oxide and propylene oxide with carbon dioxide in the presence of chromium salen derivatives. In situ FTIR measurements of copolymer vs cyclic carbonate production. J. Am. Chem. Soc. 2003, 125, 7586–7591. [Google Scholar] [CrossRef]
- Pang, M.Z.; Qiao, J.J.; Jiao, J.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Miscibility and properties of completely biodegradable blends of poly (propylene carbonate) and poly (butylene succinate). J. Appl. Polym. Sci. 2008, 107, 2854–2860. [Google Scholar] [CrossRef]
- Qin, Y.S.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Copolymerization of carbon dioxide and cyclohexene oxide catalyzed by aluminum porphyrin-quaternary ammonium salt in the presence of bulky lewis aci. Chin. J. Polym. Sci. 2008, 26, 241–247. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, X.; Zhang, Y. New catalytic systems for the fixation of carbon dioxide, 2. Synthesis of high molecular weight epichlorohydrin/carbon dioxide copolymer with rare earth phosphonates/triisobutyl-aluminium systems. Macromol. Chem. Phys. 1994, 195, 2003–2011. [Google Scholar] [CrossRef]
- Cheng, M.; Moore, D.R.; Reczek, J.J.; Chamberlain, B.M.; Lobkovsky, E.B.; Coates, G.W. Single-site β-diiminate zinc catalysts for the alternating copolymerization of CO2 and epoxides: Catalyst synthesis and unprecedented polymerization activity. J. Am. Chem. Soc. 2001, 123, 8738–8749. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Herdtweck, E.; Deglmann, P.; Ott, A.K.; Rieger, B. Mechanistic insights into heterogeneous zinc dicarboxylates and theoretical considerations for CO2–epoxide copolymerization. J. Am. Chem. Soc. 2011, 133, 13151–13161. [Google Scholar] [CrossRef]
- Buzdugan, E.; Beckman, E.J. Propylene oxide copolymerization with carbon dioxide using sterically hindered aluminium catalysts. Mater. Plast. 2007, 44, 278–283. [Google Scholar]
- Boualleg, M.; Norsic, S.; Baudouin, D.; Sayah, R.; Quadrelli, E.A.; Basset, J.M.; Candy, J.P.; Délichère, P.; Pelzer, K.; Veyre, L.; et al. Selective and regular localization of accessible Pt nanoparticles inside the walls of an ordered silica: Application as a highly active and well-defined heterogeneous catalyst for propene and styrene hydrogenation reactions. J. Catal. 2011, 284, 184–193. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Yuan, G. Cycloaddition of CO2 and epoxide catalyzed by amino-and hydroxyl-rich graphitic carbon nitride. Catal. Sci. Technol. 2016, 6, 2942–2948. [Google Scholar] [CrossRef]
- Yang, G.W.; Zhang, Y.Y.; Xu, C.K.; Wu, G.P. Evolution of copolymers of epoxides and CO2: Catalysts, monomers, architectures, and applications. Chem. Rev. 2024, 124, 12305–12380. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and application of polypropylene carbonate composite materials: A review. Polymers 2022, 14, 2159. [Google Scholar] [CrossRef]
- Ang, R.R.; Sin, L.T.; Bee, S.T. Determination of zinc glutarate complexes synthesis factors affecting production of propylene carbonate from carbon dioxide and propylene oxide. Chem. Eng. J. 2017, 327, 120–127. [Google Scholar] [CrossRef]
- Gao, L.; Luo, Y.; Lin, Y.; Su, T.; Su, R.; Feng, J. Silica-supported zinc glutarate catalyst synthesized by rheological phase reaction used in the copolymerization of carbon dioxide and propylene oxide. J. Polym. Res. 2015, 22, 220. [Google Scholar] [CrossRef]
- Ree, M.; Hwang, Y.; Kim, J.S.; Kim, H.; Kim, G.; Kim, H. New findings in the catalytic activity of zinc glutarate and its application in the chemical fixation of CO2 into polycarbonates and their derivatives. Catal. Today 2006, 115, 134–145. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Adams, M.J.; Yarbrough, J.C.; Phelps, A.L. Synthesis and structural characterization of double metal cyanides of iron and zinc: Catalyst precursors for the copolymerization of carbon dioxide and epoxides. Inorg. Chem. 2003, 42, 7809–7818. [Google Scholar] [CrossRef]
- Kim, I.; Byun, S.H.; Ha, C.S. Ring-opening polymerizations of propylene oxide by double metal cyanide catalysts prepared with ZnX2 (X = F, Cl, Br, or I). J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4393–4404. [Google Scholar] [CrossRef]
- Varghese, J.K.; Park, D.S.; Jeon, J.Y.; Lee, B.Y. Double metal cyanide catalyst prepared using H3Co(CN)6 for high carbonate fraction and molecular weight control in carbon dioxide/propylene oxide copolymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4811–4818. [Google Scholar] [CrossRef]
- Meng, Q.; Cheng, R.; Li, J.; Wang, T.; Liu, B. Copolymerization of CO2 and propylene oxide using ZnGA/DMC composite catalyst for high molecular weight poly (propylene carbonate). J. CO2 Util. 2016, 16, 86–96. [Google Scholar] [CrossRef]
- Wang, W.Z.; Zhang, K.Y.; Jia, X.G.; Wang, L.; Li, L.L.; Fan, W.; Xia, L. A new dinuclear cobalt complex for copolymerization of CO2 and propylene oxide: High activity and selectivity. Molecules 2020, 25, 4095. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W.; Xu, C.K.; Xie, R.; Zhang, Y.Y.; Lu, C.; Qi, H.; Yang, L.; Wang, Y.; Wu, G.P. Precision copolymerization of CO2 and epoxides enabled by organoboron catalysts. Nat. Synth. 2022, 1, 892–901. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Lavernia, E.J. On the analysis of grain size in bulk nanocrystalline materials via X-ray diffraction. Metall. Mater. Trans. A 2003, 34, 1349–1355. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, I.K.; Ha, J.Y.; Jo, J.K.; Park, I.; Ha, C.S.; Suh, H.; Kim, I. Tuning of the activity and induction period of the polymerization of propylene oxide catalyzed by double metal cyanide complexes bearing β-alkoxy alcohols as complexing agents. Ind. Eng. Chem. Res. 2010, 49, 4107–4116. [Google Scholar] [CrossRef]
- An, N.; Li, Q.; Yin, N.; Kang, M.; Wang, J. Facile preparation and synergy study of DMC/ZnGA composite catalyst for the synthesis of oligo (propylene-carbonate) diols. Appl. Organomet. Chem. 2019, 33, 4999. [Google Scholar] [CrossRef]
- Yang, G.W.; Zhang, Y.Y.; Xie, R.; Wu, G.P. Scalable bifunctional organoboron catalysts for copolymerization of CO2 and epoxides with unprecedented efficiency. J. Am. Chem. Soc. 2020, 142, 12245–12255. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Rodgers, J.L.; Mackiewicz, R.M.; Phelps, A.L. Probing the mechanistic aspects of the chromium salen catalyzed carbon dioxide/epoxide copolymerization process using in situ ATR/FTIR. Catal. Today 2004, 98, 485–492. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Feng, L.; Wang, L.; Gu, X. Pressure dependence of the CO2/propylene oxide copolymerization catalyzed by zinc glutarate. J. Appl. Polym. Sci. 2010, 118, 366–371. [Google Scholar]

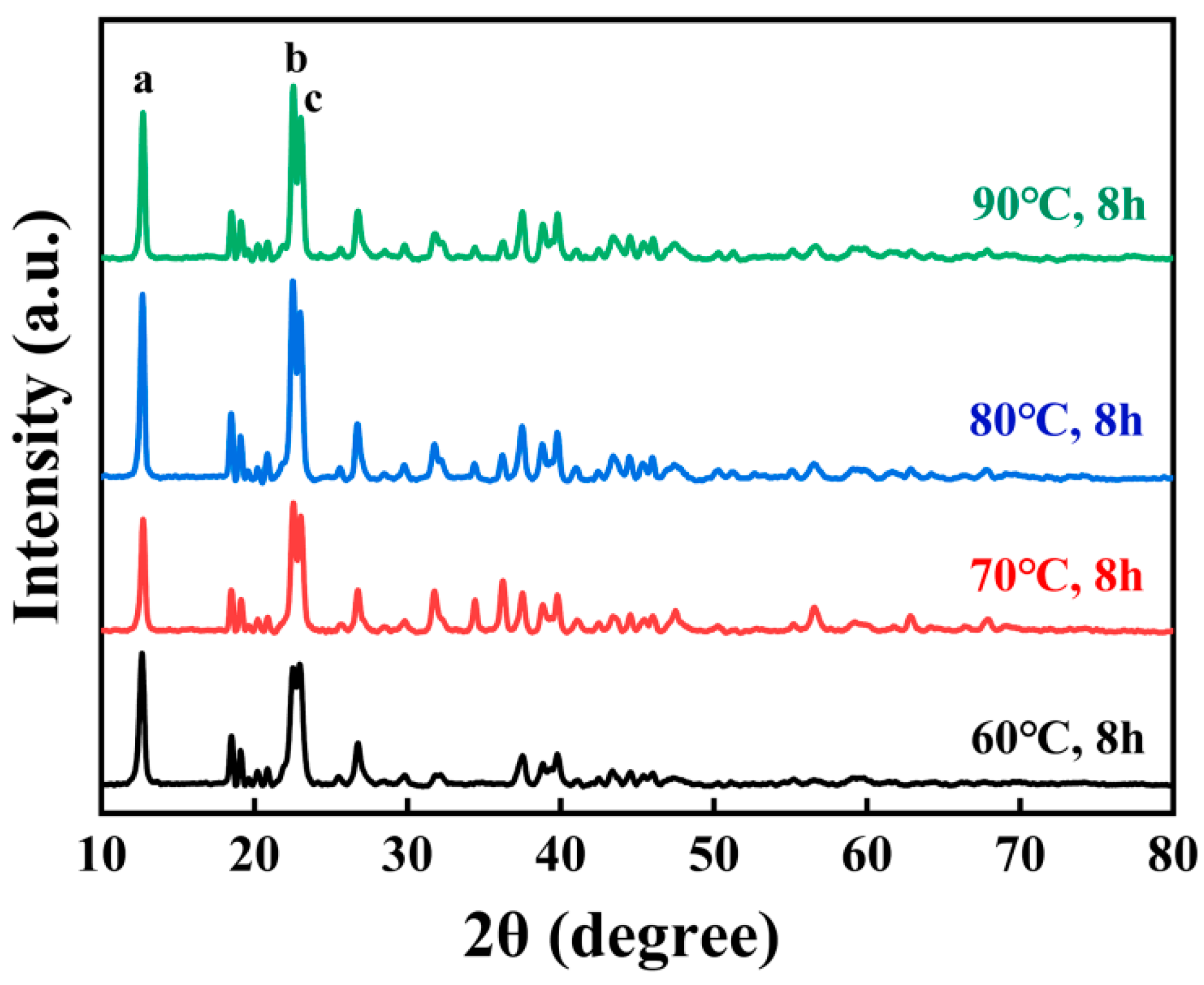
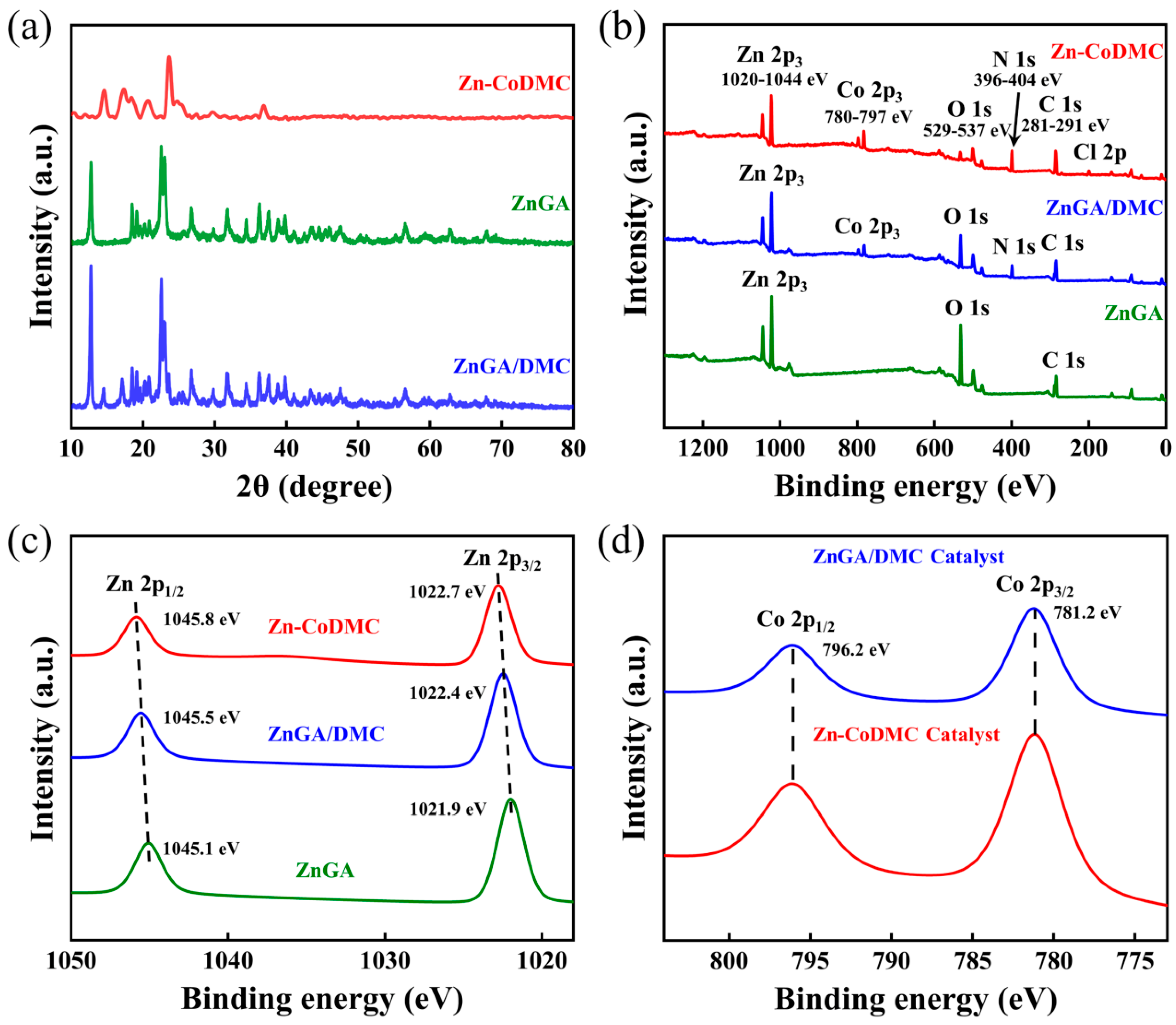

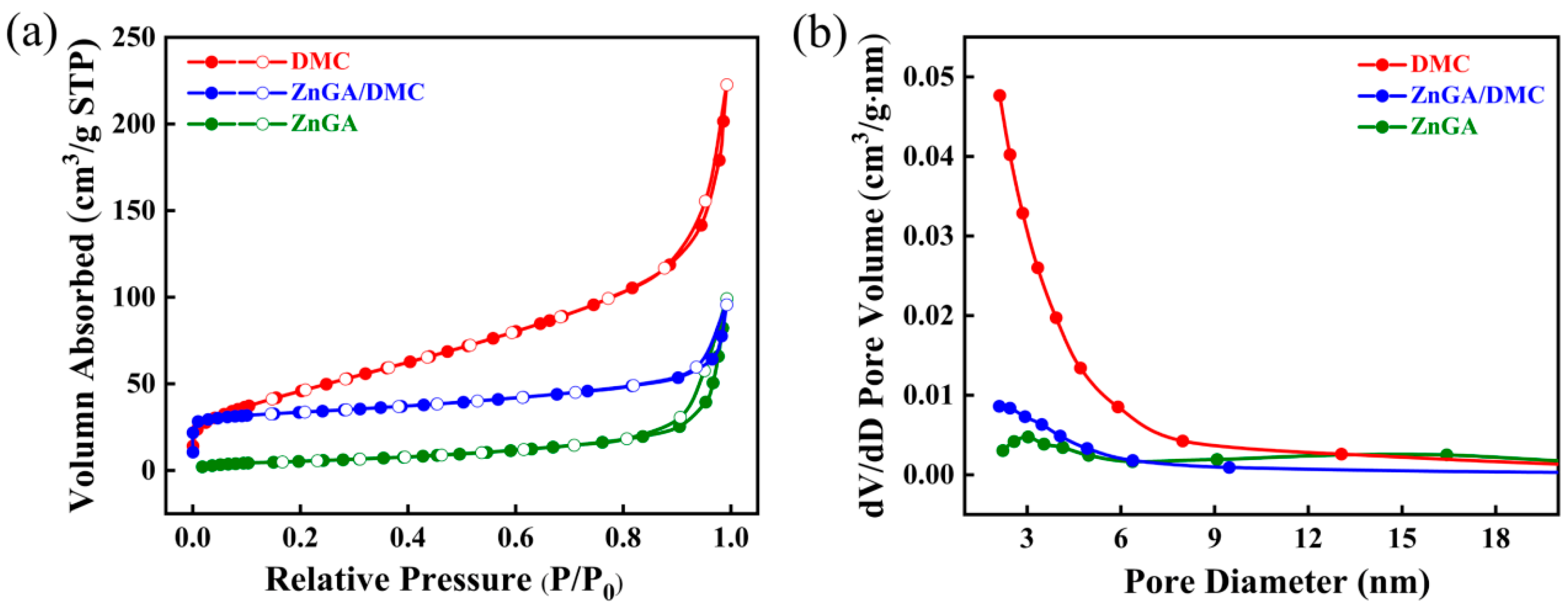
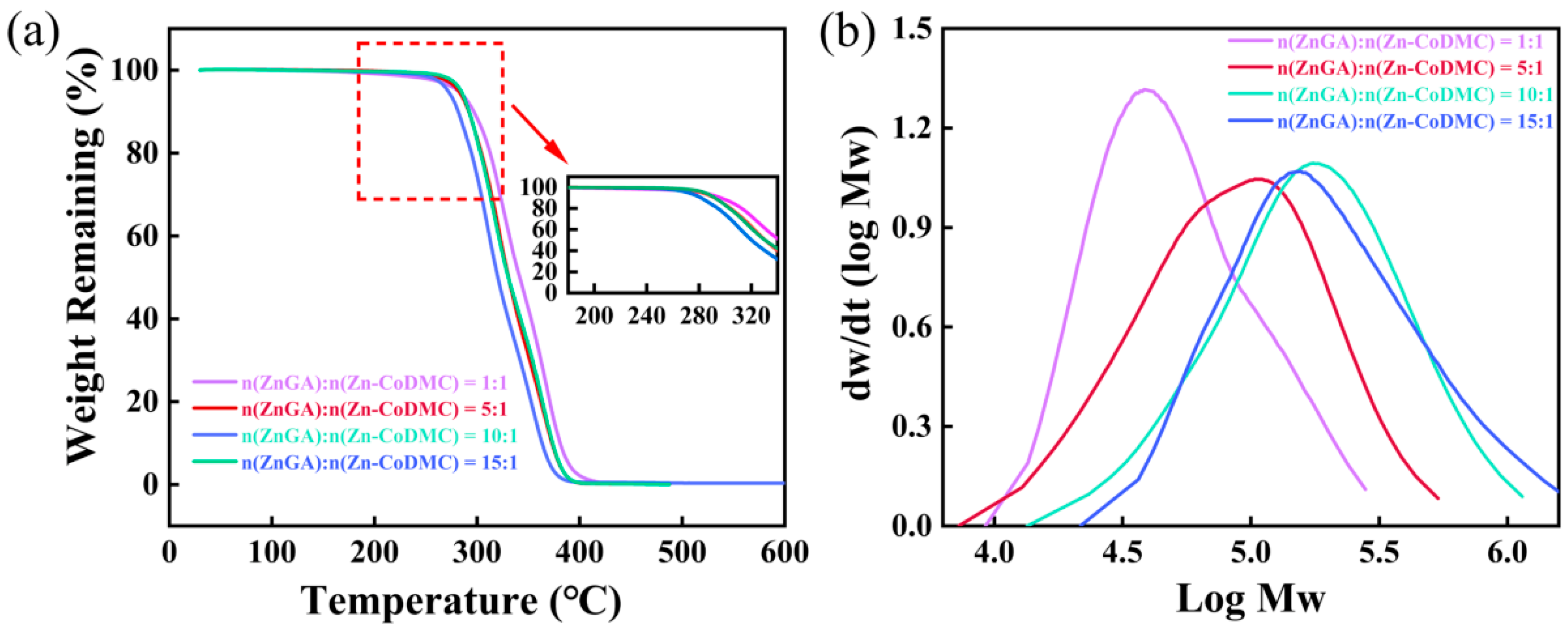
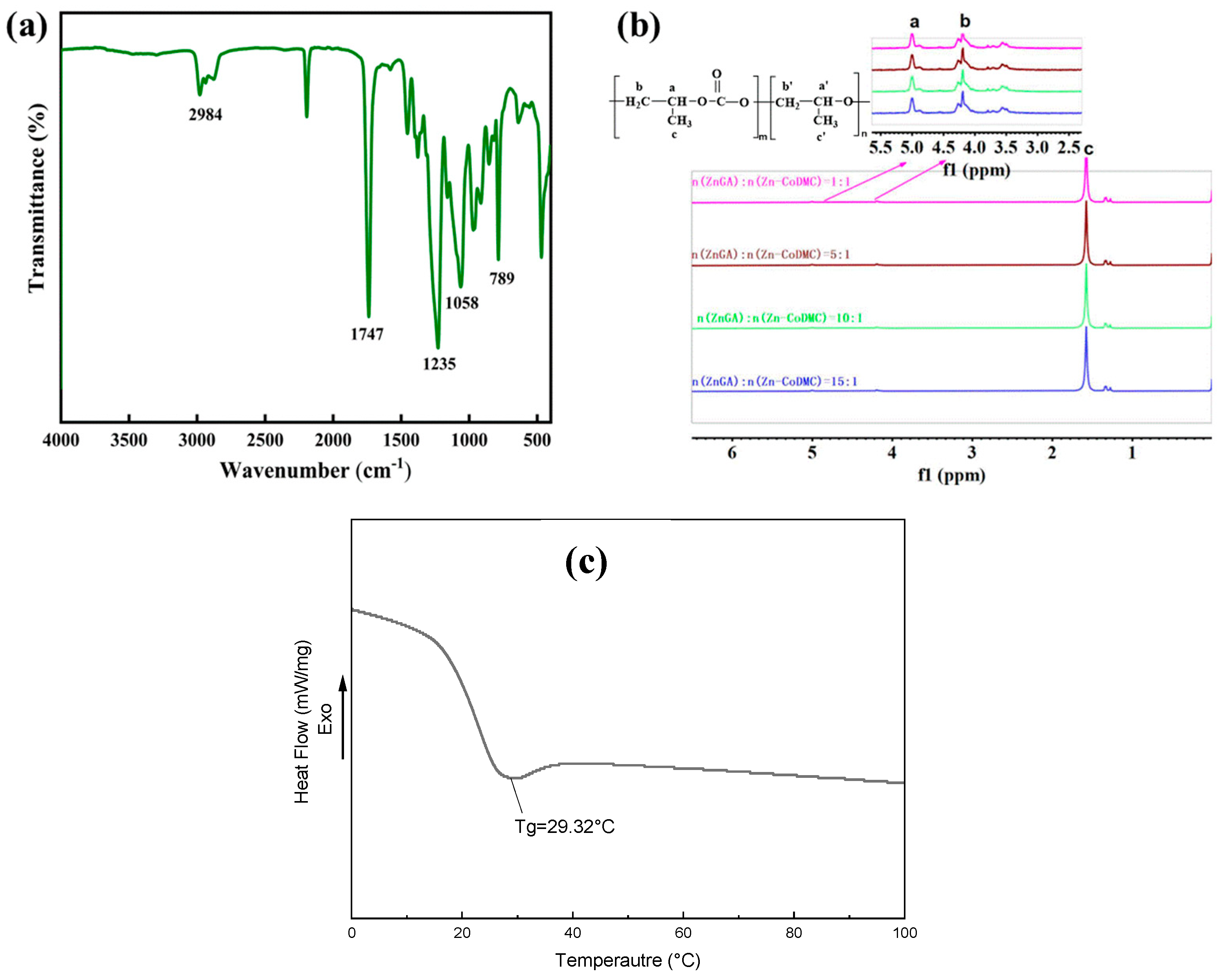
| Catalyst | Reaction a Time/h | Selectity b /% | Yield c /(gpolymer/gcatalyst) | Mn d /(kg/mol) | Mw d /(kg/mol) | PDI d | Td–5% e/(°C) |
|---|---|---|---|---|---|---|---|
| ZnGA f | 40 | 99 | 50 | 16.94 | 22.90 | 1.35 | 210 |
| DMC f | 30 | 99 | 8.48 | 13.79 | 16.07 | 1.16 | 170 |
| ZnGA/DMC (1:10) g | 24 | 99 | 55.87 | 136.12 | 518.66 | 3.81 | 256 |
| ZnGA/DMC (1:1) g | 24 | 99 | 160 | 40.23 | 65.83 | 1.63 | 279 |
| ZnGA/DMC (5:1) g | 24 | 99 | 680 | 56.44 | 105.86 | 1.87 | 281 |
| ZnGA/DMC (10:1) g | 24 | 99 | 951 | 210.07 | 512.79 | 2.44 | 284 |
| ZnGA/DMC (15:1) g | 24 | 99 | 750 | 139.49 | 296.58 | 2.12 | 280 |
| ZnGA/DMC /(g) | PO /(g) | m(PO)/m(ZnGA/DMC) | Selectity b /% | Yield c /(gpolymer/gcatalyst) | Mn d /(kg/mol) | Mw d /(kg/mol) | PDI d | Td–5% e /(°C) |
|---|---|---|---|---|---|---|---|---|
| 0.20 | 65 | 325 | 98 | 398.5 | 98.46 | 229.86 | 2.33 | 278 |
| 0.18 | 65 | 361 | 99 | 433.3 | 200.98 | 263.00 | 1.31 | 281 |
| 0.15 | 65 | 433 | 98 | 570 | 121.10 | 288.69 | 2.38 | 280 |
| 0.10 | 65 | 650 | 99 | 885.0 | 210.07 | 512.79 | 2.44 | 284 |
| 0.05 | 65 | 1300 | 98 | 1510 | 137.25 | 472.97 | 3.44 | 280 |
| Temperture/°C | FCO2 /% b | Yield b /(gpolymer/gcatalyst) | Mn c /(Kg/mol) | Mw c /(Kg/mol) | PDI c | Td–5% d /(°C) |
|---|---|---|---|---|---|---|
| 60 | 70.55 | 450 | 46.72 | 93.70 | 2.01 | 278 |
| 70 | 72.53 | 885 | 210.07 | 512.79 | 2.44 | 284 |
| 80 | 67.64 | 680 | 75.41 | 185.55 | 2.46 | 280 |
| 90 | 64.48 | 340 | 45.93 | 106.84 | 2.32 | 276 |
| Pressure /MPa | FCO2 b /% | Yield b /(gpolymer/gcat) | Mn c /(kg/mol) | Mw c /(Kg/mol) | PDI c | Td–5% d /(°C) |
|---|---|---|---|---|---|---|
| 4.5 | 66.81 | 885 | 118.75 | 243.35 | 2.05 | 279 |
| 4.0 | 72.53 | 951 | 210.07 | 512.79 | 2.44 | 284 |
| 3.5 | 65.88 | 730 | 70.60 | 218.02 | 3.08 | 278 |
| 3.0 | 63.63 | 480 | 55.05 | 133.58 | 2.42 | 278 |
| 2.5 | 60.46 | 434 | 55.06 | 137.87 | 2.50 | 279 |
| 2.0 | 61.94 | 340 | 46.70 | 101.83 | 2.18 | 278 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, S.; Wu, X.; Ding, D.; Ge, C.; Shen, W.; Yang, Y.; Fang, Y. Efficient Copolymerization of CO2 and Propylene Oxide via ZnGA/Zn-Co DMC Composite Catalysts: Synergistic Catalysis for High-Performance Polypropylene Carbonate. Reactions 2025, 6, 30. https://doi.org/10.3390/reactions6020030
Miao S, Wu X, Ding D, Ge C, Shen W, Yang Y, Fang Y. Efficient Copolymerization of CO2 and Propylene Oxide via ZnGA/Zn-Co DMC Composite Catalysts: Synergistic Catalysis for High-Performance Polypropylene Carbonate. Reactions. 2025; 6(2):30. https://doi.org/10.3390/reactions6020030
Chicago/Turabian StyleMiao, Shuqin, Xiaojiong Wu, Delong Ding, Chunliang Ge, Weihua Shen, Yi Yang, and Yunjin Fang. 2025. "Efficient Copolymerization of CO2 and Propylene Oxide via ZnGA/Zn-Co DMC Composite Catalysts: Synergistic Catalysis for High-Performance Polypropylene Carbonate" Reactions 6, no. 2: 30. https://doi.org/10.3390/reactions6020030
APA StyleMiao, S., Wu, X., Ding, D., Ge, C., Shen, W., Yang, Y., & Fang, Y. (2025). Efficient Copolymerization of CO2 and Propylene Oxide via ZnGA/Zn-Co DMC Composite Catalysts: Synergistic Catalysis for High-Performance Polypropylene Carbonate. Reactions, 6(2), 30. https://doi.org/10.3390/reactions6020030






