Aromatics Alkylated with Olefins Utilizing Zeolites as Heterogeneous Catalysts: A Review
Abstract
1. Introduction
2. Previous Studies
3. Mechanism of Alkylation Reaction
4. Catalysts for Alkylation Reaction
5. Zeolites
5.1. Zeolite Classification
5.1.1. Zeolite Y
5.1.2. Zeolite A
5.1.3. Zeolite Mordenite
5.1.4. Zeolite MFI
5.1.5. Zeolite Beta
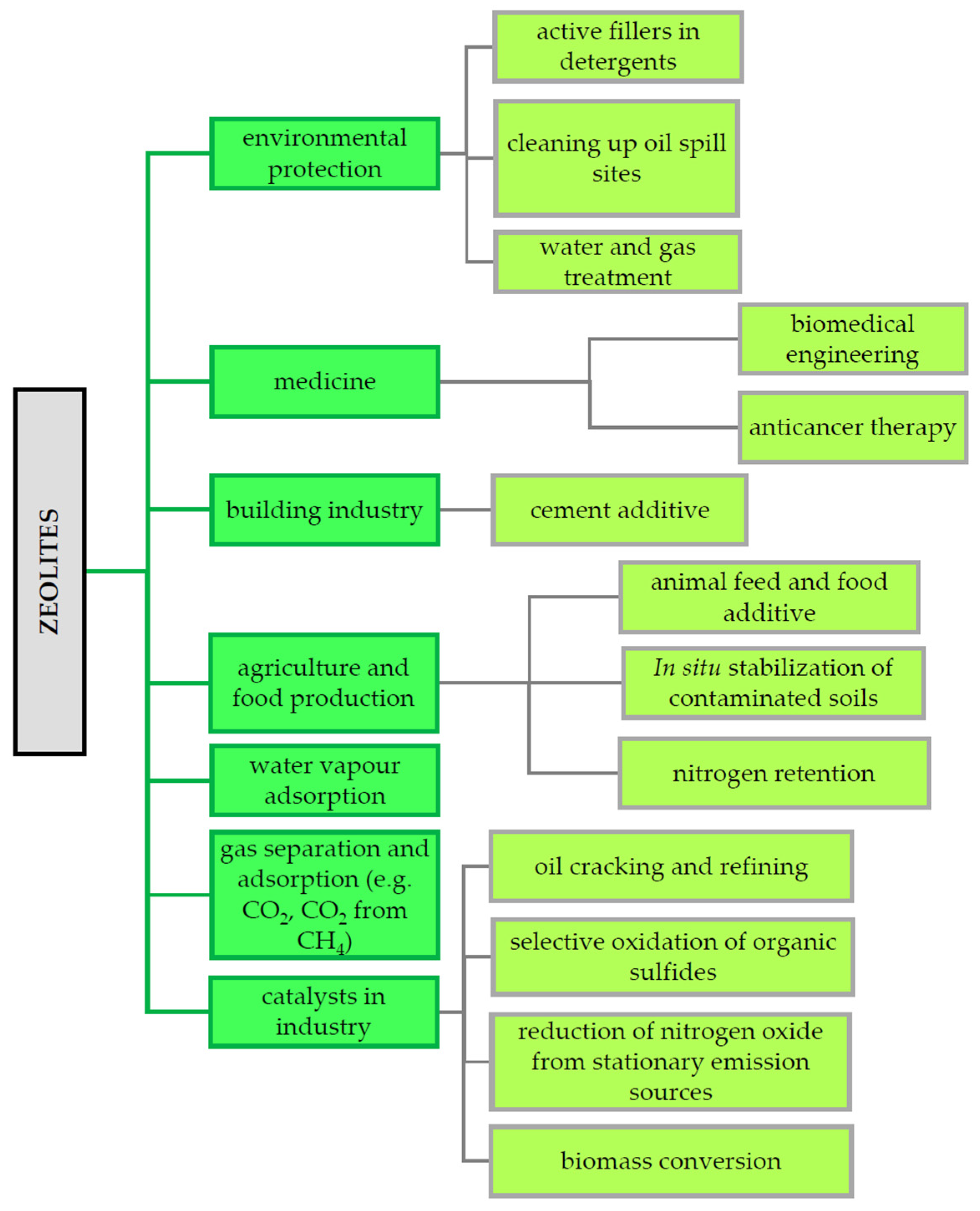
6. The Utilization and Applications of Zeolites as a Catalyst
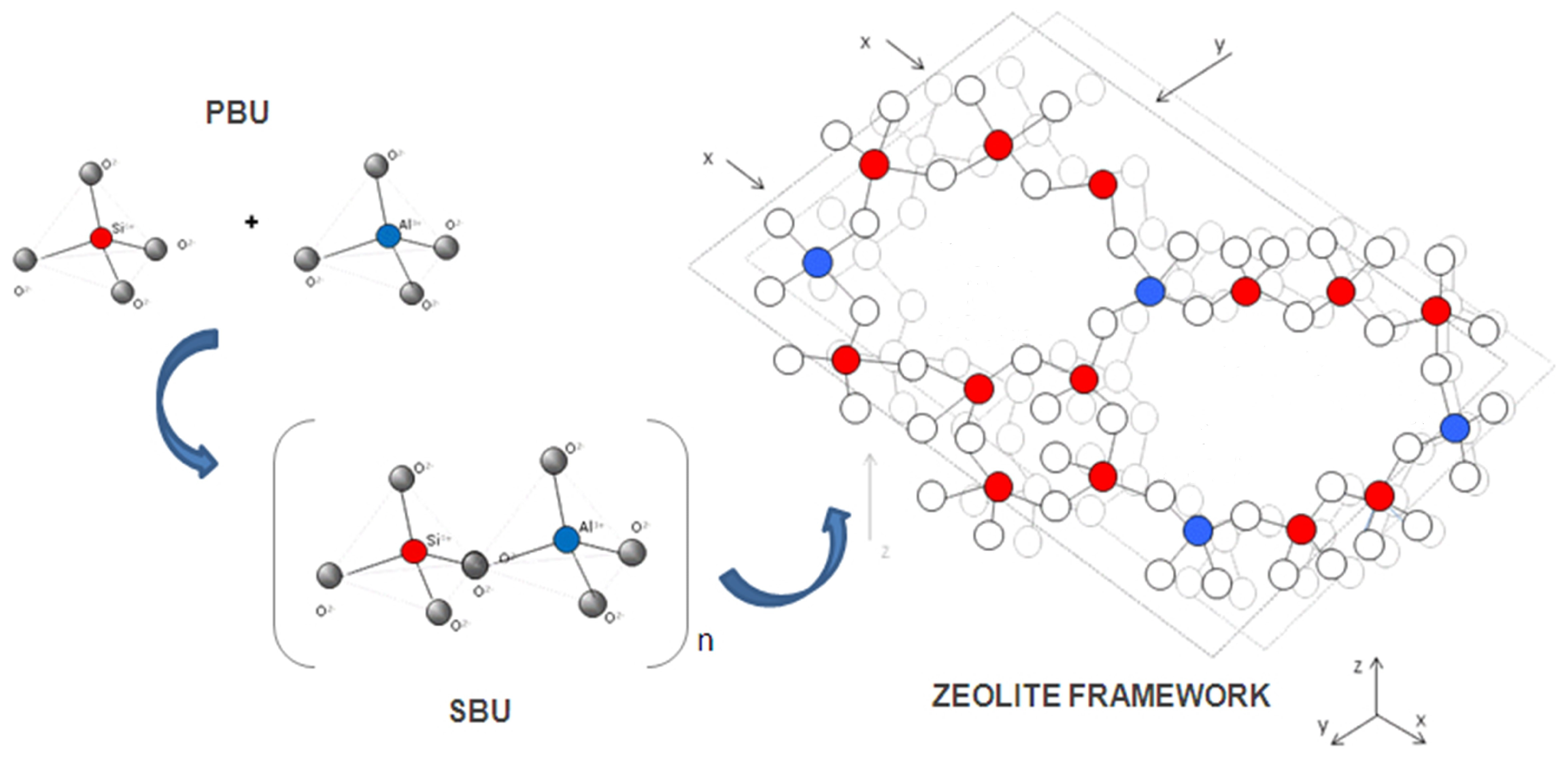
7. Structures of Zeolite
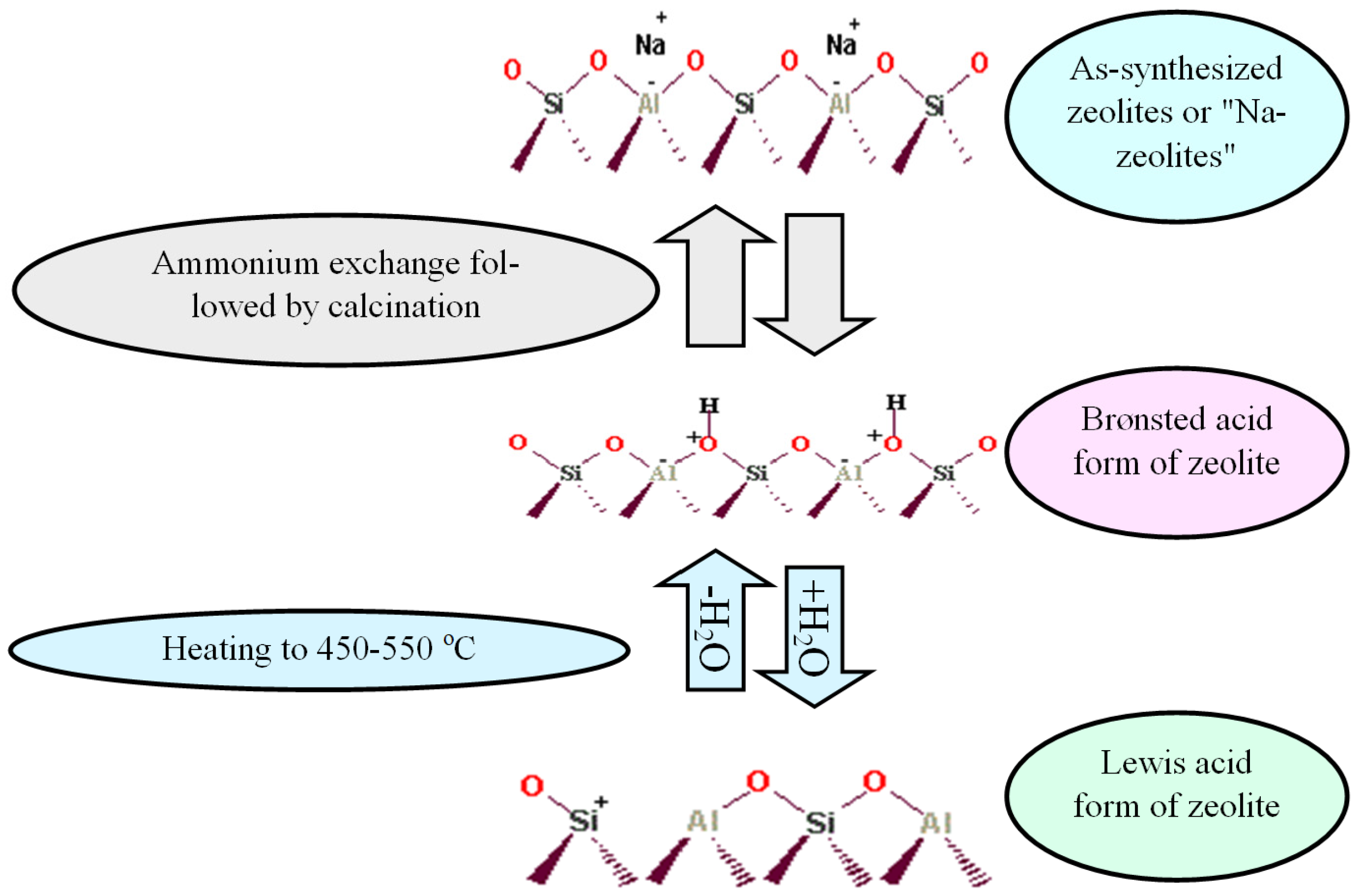
- The stability of the crystal structure remains intact upon dehydration (i.e., the elimination of water from the zeolite crystals), a trait commonly observed in numerous zeolite types, with dehydration occurring at temperatures below 400 °C.
- The adsorption of gases, vapors, and various molecules within the microporous channels is facilitated by their adequate size to accommodate guest species. Alongside a substantial void volume, the majority of zeolite materials are characterized by low density and uniformly sized molecular channels.
- Additionally, a range of physical properties such as electrical conductivity, cation exchange, and catalytic capabilities are observed in zeolite materials.
8. The Zeolite Features
- (a)
- Catalytic activity:
- (b)
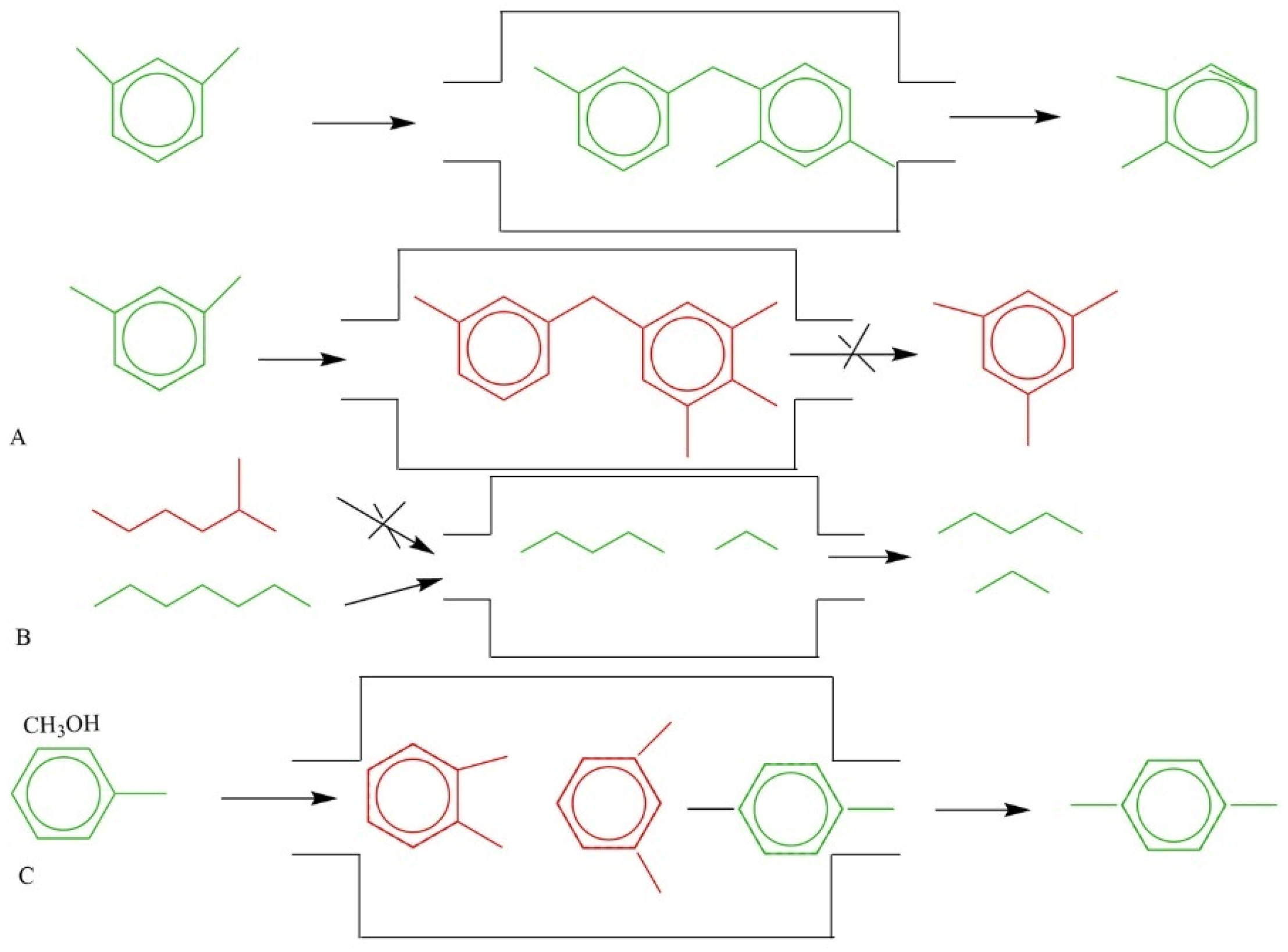
- Shape selectivity:
- (c)
- Thermal stability:
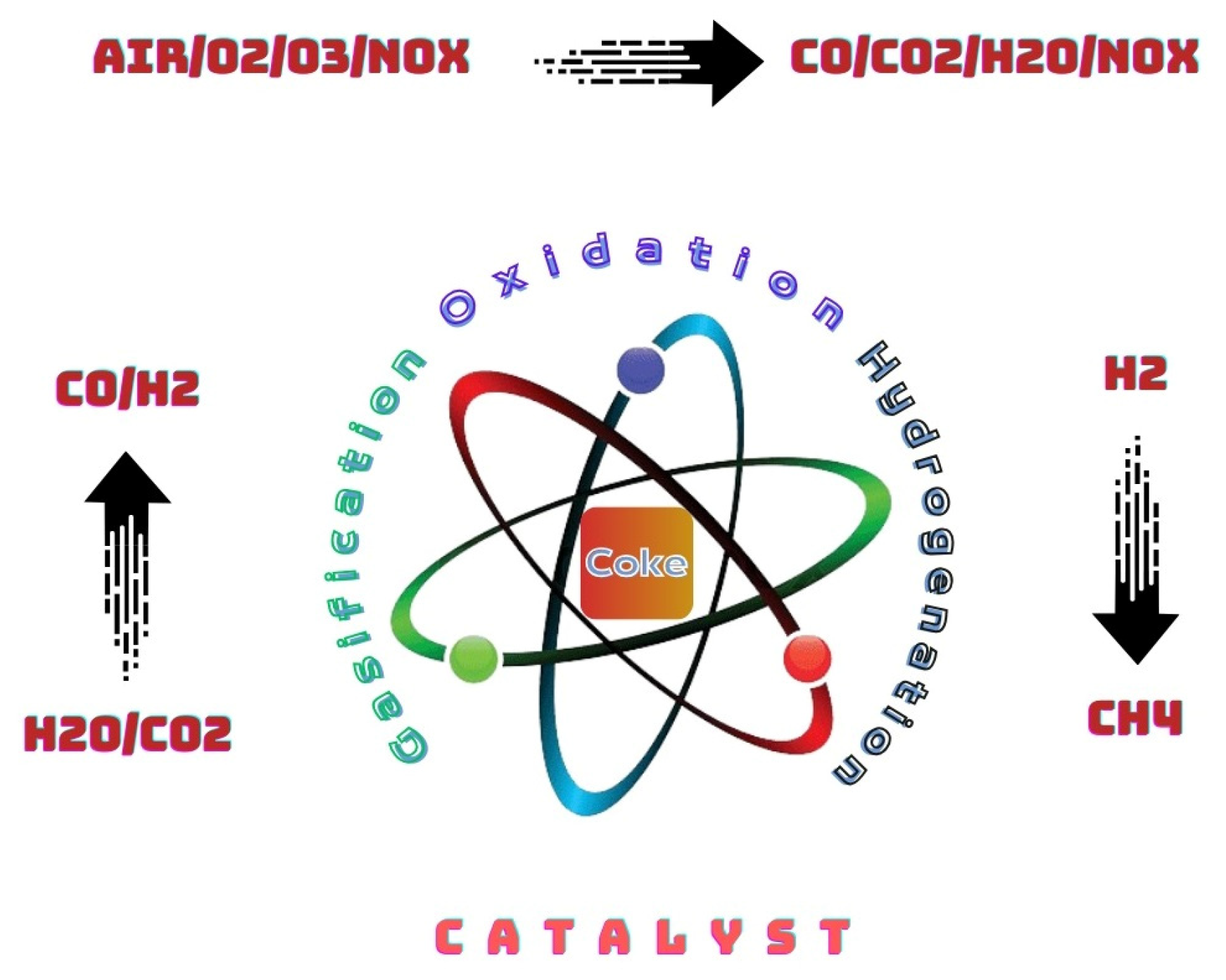
9. Role of Coke and Catalyst Deactivation
10. Catalysts Regeneration (Deactivated by Coke Deposition)
11. Benefits of Coke
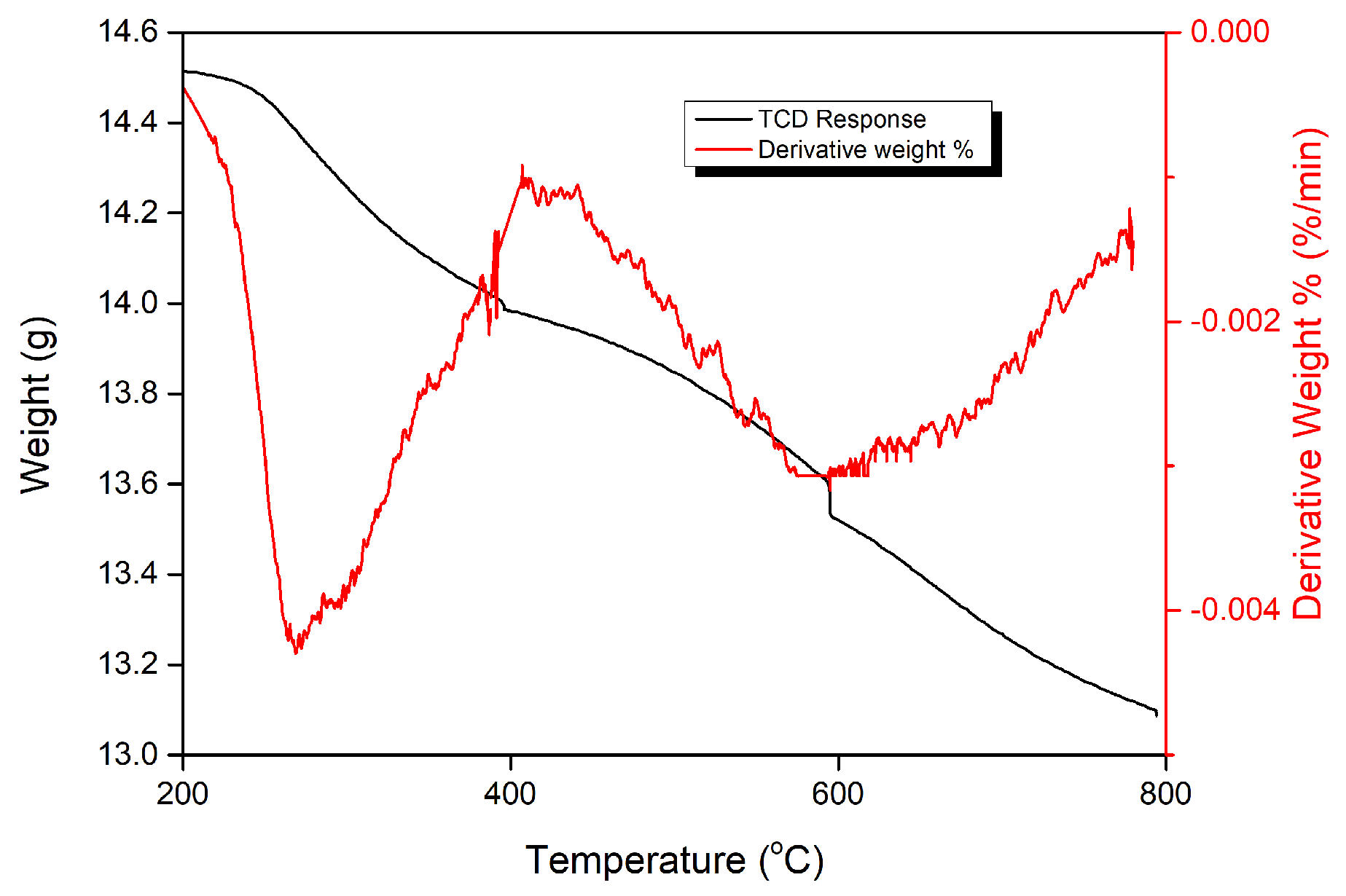
12. Coke Characterization
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galadima, A.; Muraza, O. Role of zeolite catalysts for benzene removal from gasoline via alkylation: A review. Microporous Mesoporous Mater. 2015, 213, 169–180. [Google Scholar] [CrossRef]
- Guisnet, M. “Coke” molecules trapped in the micropores of zeolites as active species in hydrocarbon transformations. J. Mol. Catal. A Chem. 2002, 182–183, 367–382. [Google Scholar] [CrossRef]
- Perego, C.; Amarilli, S.; Carati, A.; Flego, C.; Pazzuconi, G.; Rizzo, C.; Bellussi, G. Mesoporous silica-aluminas as catalysts for the alkylation of aromatic hydrocarbons with olefins. Microporous Mesoporous Mater. 1999, 27, 345–354. [Google Scholar] [CrossRef]
- Horňáček, M.; Hudec, P.; Smiešková, A.; Jakubík, T. Alkylation of Benzene with 1-Alkenes over Zeolite Y and Mordenite. Acta Chim. Slovaca 2009, 2, 31–45. [Google Scholar]
- Gancedo, J.; Faba, L.; Ordóñez, S. Role of Reactant Alkylation Grade in the Selectivity and Stability of Furan–Alkene Diels–Alder Reactions. ACS Sustain. Chem. Eng. 2022, 10, 3057–3065. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Abduljawad, M.M.; Alassmy, Y.A.; Alnafisah, M.S.; El Hariri El Nokab, M.; Van Steenberge, P.H.M.; Sebakhy, K.O. Synergistic Catalytic Effects of Alloys of Noble Metal Nanoparticles Supported on Two Different Supports: Crystalline Zeolite Sn-Beta and Carbon Nanotubes for Glycerol Conversion to Methyl Lactate. Catalysts 2023, 13, 1486. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Koelewijn, R.; El Hariri El Nokab, M.; van der Wel, P.C.A.; Sebakhy, K.O.; Pescarmona, P.P. Binder-free Zeolite Beta Beads with Hierarchical Porosity: Synthesis and Application as Heterogeneous Catalysts for Anisole Acylation. ChemCatChem 2022, 14, e202200518. [Google Scholar] [CrossRef]
- Shokri, A.; Karimi, S. A Review in Linear Alkylbenzene (LAB) Production Processes in the Petrochemical Industry. Russ. J. Appl. Chem. 2021, 94, 1546–1559. [Google Scholar] [CrossRef]
- Cao, Y.; Kessas, R.; Naccache, C.; Ben Taarit, Y. Alkylation of benzene with dodecene. The activity and selectivity of zeolite type catalysts as a function of the porous structure. Appl. Catal. A Gen. 1999, 184, 231–238. [Google Scholar] [CrossRef]
- de Almeida, J.G.; Dufaux, M.; Ben Taarit, Y.; Naccache, C. Effect of pore size and aluminium content on the production of linear alkylbenzenes over HY, H-ZSM-5 and H-ZSM-12 zeolites: Alkylation of benzene with 1-dodecene. Appl. Catal. A Gen. 1994, 114, 141–159. [Google Scholar] [CrossRef]
- Imarc. Linear Alkylbenzene (LAB) Market Report by Application (Linear Alkylbenzene Sulfonates (LAS), Non-Surfactant), End User (Laundry Detergents, Light-Duty Dishwashing Liquids, Industrial Cleaners, Household Cleaners, and Others), and Region 2024–2032. 2024. Available online: https://www.imarcgroup.com/linear-alkylbenzene-market#:~:text=Market%20Overview%3A,4.4%20Million%20Tons%20in%202023 (accessed on 1 August 2024).
- Lin, J.-S.; Wang, J.-J.; Wang, J.; Wang, I.; Balasamy, R.J.; Aitani, A.; Al-Khattaf, S.; Tsai, T.-C. Catalysis of alkaline-modified mordenite for benzene alkylation of diolefin-containing dodecene for linear alkylbenzene synthesis. J. Catal. 2013, 300, 81–90. [Google Scholar] [CrossRef]
- Ren, S.; Yang, F.; Tian, C.; Yue, Y.; Zou, W.; Hua, W.; Gao, Z. Selective Alkylation of Benzene with Methanol to Toluene and Xylene over H-ZSM-5 Zeolites: Impact of Framework Al Spatial Distribution. Catalysts 2023, 13, 1295. [Google Scholar] [CrossRef]
- Ting, K.W.; Kamakura, H.; Poly, S.S.; Takao, M.; Siddiki, S.M.A.H.; Maeno, Z.; Matsushita, K.; Shimizu, K.-i.; Toyao, T. Catalytic Methylation of m-Xylene, Toluene, and Benzene Using CO2 and H2 over TiO2-Supported Re and Zeolite Catalysts: Machine-Learning-Assisted Catalyst Optimization. ACS Catal. 2021, 11, 5829–5838. [Google Scholar] [CrossRef]
- Larson, R.J.; Rothgeb, T.M.; Shimp, R.J.; Ward, T.E.; Ventullo, R.M. Kinetics and practical significance of biodegradation of linear alkylbenzene sulfonate in the environment. J. Am. Oil Chem. Soc. 1993, 70, 645–657. [Google Scholar] [CrossRef]
- Ravishankar, R.; Sen, T.; Ramaswamy, V.; Soni, H.S.; Ganapathy, S.; Sivasanker, S. Synthesis, Characterization and Catalytic properties of Zeolite PSH-3/MCM-22. In Studies in Surface Science and Catalysis; Weitkamp, J., Karge, H.G., Pfeifer, H., Hölderich, W., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 331–338. [Google Scholar]
- Yadav, G.D.; Doshi, N.S. Synthesis of Linear Phenyldodecanes by the Alkylation of Benzene with 1-Dodecene over Non-Zeolitic Catalysts. Org. Process Res. Dev. 2002, 6, 263–272. [Google Scholar] [CrossRef]
- Awate, S.V.; Waghmode, S.B.; Agashe, M.S. Synthesis, characterization and catalytic evaluation of zirconia-pillared montmorillonite for linear alkylation of benzene. Catal. Commun. 2004, 5, 407–411. [Google Scholar] [CrossRef]
- Clark, J.H.; Monks, G.L.; Nightingale, D.J.; Price, P.M.; White, J.F. A New Solid Acid-Based Route to Linear Alkylbenzenes. J. Catal. 2000, 193, 348–350. [Google Scholar] [CrossRef]
- Yadav, G.D.; Siddiqui, M.I.N.I. UDCaT-5: A Novel Mesoporous Superacid Catalyst in the Selective Synthesis of Linear Phenyldodecanes by the Alkylation of Benzene with 1-Dodecene. Ind. Eng. Chem. Res. 2009, 48, 10803–10809. [Google Scholar] [CrossRef]
- Hu, X.; Foo, M.L.; Chuah, G.K.; Jaenicke, S. Pore Size Engineering on MCM-41: Selectivity Tuning of Heterogenized AlCl3 for the Synthesis of Linear Alkyl Benzenes. J. Catal. 2000, 195, 412–415. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.-O. Alkylation of 1-Dodecene with Benzene over H3PW12O40 Supported on Mesoporous Silica SBA-15. Catal. Lett. 2004, 93, 209–212. [Google Scholar] [CrossRef]
- Huseynova, G.; Muxtarova, G.; Aliyeva, N.; Gasimova, G.; Rashidova, S. Zeolite-Containing Catalysts in Alkylation Processes. Catal. Res. 2022, 2, 19. [Google Scholar] [CrossRef]
- Deka, R.C.; Pal, S.; Goursot, A.; Vetrivel, R. Influence of zeolite composition on the selectivity of alkylation reaction for the synthesis of p-isobutylethylbenzene: A computational study. Catal. Today 1999, 49, 221–227. [Google Scholar] [CrossRef]
- Guo, Y.; Du, X.; Liu, L.; Dong, Y.; Lei, Z. Reaction mechanism of benzene alkylation with propylene catalyzed by HZSM-5 zeolite and H-Beta zeolite. Mater. Today Commun. 2021, 26, 101757. [Google Scholar] [CrossRef]
- Da, Z.; Magnoux, P.; Guisnet, M. Alkylation of toluene with 1-dodecene over HFAU zeolite. Deactivation and regeneration. Catal. Lett. 1999, 61, 203–206. [Google Scholar] [CrossRef]
- Da, Z.; Magnoux, P.; Guisnet, M. Liquid phase alkylation of toluene with 1-heptene over a HFAU zeolite: Evidence for transalkylation between toluene and non-desorbed products. Appl. Catal. A Gen. 1999, 182, 407–411. [Google Scholar] [CrossRef]
- Da, Z.; Han, Z.; Magnoux, P.; Guisnet, M. Liquid-phase alkylation of toluene with long-chain alkenes over HFAU and HBEA zeolites. Appl. Catal. A Gen. 2001, 219, 45–52. [Google Scholar] [CrossRef]
- Horňáček, M.; Hudec, P.; Nociar, A.; Smiešková, A.; Jakubík, T. Activity and regenerability of dealuminated zeolite Y in liquid phase alkylation of benzene with 1-alkene. Chem. Pap. 2010, 64, 469–474. [Google Scholar] [CrossRef]
- Ithisuphalap, K.; Nolen, M.A.; Monroe, H.; Kwon, S. Kinetic, Spectroscopic, and Theoretical Study of Toluene Alkylation with Ethylene on Acidic Mordenite Zeolite. ACS Catal. 2023, 13, 16012–16031. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamizhdurai, P.; Mangesh, V.L.; Ragupathi, C.; Santhana krishnan, P.; Ramesh, A. Recent advances in the synthesis and applications of mordenite zeolite—Review. RSC Adv. 2021, 11, 250–267. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Al-Zaidi, B.Y.; Shehab, A.K.; Shakoor, Z.M.; Aal-Kaeb, S.; Gomez, L.Q.; Majdi, H.S.; Al-Shafei, E.N.; AbdulRazak, A.A.; McGregor, J. Experimental and kinetic studies of the advantages of coke accumulation over Beta and Mordenite catalysts according to the pore mouth catalysis hypothesis. Catal. Commun. 2023, 181, 106718. [Google Scholar] [CrossRef]
- Horňáček, M.; Hudec, P.; Smiešková, A.; Jakubík, T. Alkylation of benzene with 1-alkenes over beta zeolite in liquid phase. React. Kinet. Mech. Catal. 2010, 99, 431–437. [Google Scholar] [CrossRef]
- Borutskii, P.N.; Kozlova, E.G.; Podkletnova, N.M.; Gil’chenok, N.D.; Sokolov, B.G.; Zuev, V.A.; Shatovkin, A.A. Alkylation of benzene with higher olefins on heterogeneous catalysts. Pet. Chem. 2007, 47, 250–261. [Google Scholar] [CrossRef]
- Moljord, K.; Magnoux, P.; Guisnet, M. Coking, aging and regeneration of zeolites XV. Influence of the composition of HY zeolites on the mode of formation of coke from propene at 450 °C. Appl. Catal. A Gen. 1995, 122, 21–32. [Google Scholar] [CrossRef]
- Shakor, Z.; Shafei, E. The mathematical catalyst deactivation models: A mini review. RSC Adv. 2023, 13, 22579–22592. [Google Scholar] [CrossRef]
- Banerjee, S.; Aurangzeb, M.; Kumar, A. A kinetic model and parameters estimate for the synthesis of 2-phenyloctane: A starting material of bio-degradable surfactant. Indian Chem. Eng. 2023, 65, 1–13. [Google Scholar] [CrossRef]
- Lachter, E.R.; da Silva San Gil, R.A.; Tabak, D.; Costa, V.G.; Chaves, C.P.S.; dos Santos, J.A. Alkylation of toluene with aliphatic alcohols and 1-octene catalyzed by cation-exchange resins. React. Funct. Polym. 2000, 44, 1–7. [Google Scholar] [CrossRef]
- Craciun, I.; Reyniers, M.-F.; Marin, G.B. Effects of acid properties of Y zeolites on the liquid-phase alkylation of benzene with 1-octene: A reaction path analysis. J. Mol. Catal. A Chem. 2007, 277, 1–14. [Google Scholar] [CrossRef]
- Viswanathan, B.; Jacob, B. Alkylation, Hydrogenation and Oxidation Catalyzed by Mesoporous Materials. Catal. Rev. 2005, 47, 1–82. [Google Scholar] [CrossRef]
- Han, M.; Cui, Z.; Xu, C.; Chen, W.; Jin, Y. Synthesis of linear alkylbenzene catalyzed by Hβ-zeolite. Appl. Catal. A Gen. 2003, 238, 99–107. [Google Scholar] [CrossRef]
- Cadenas, M.; Bringué, R.; Fité, C.; Iborra, M.; Ramírez, E.; Cunill, F. Alkylation of toluene with 1-hexene over macroreticular ion-exchange resins. Appl. Catal. A Gen. 2014, 485, 143–148. [Google Scholar] [CrossRef][Green Version]
- Craciun, I.; Reyniers, M.-F.; Marin, G.B. Liquid-phase alkylation of benzene with octenes over Y zeolites: Kinetic modeling including acidity descriptors. J. Catal. 2012, 294, 136–150. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Lachter, E.R. Evaluation of sulfonic resins for liquid phase alkylation of toluene. Catal. Commun. 2005, 6, 550–554. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Deactivation by coking of zeolite catalysts. Prevention of deactivation. Optimal conditions for regeneration. Catal. Today 1997, 36, 477–483. [Google Scholar] [CrossRef]
- Liang, W.; Jin, Y.; Yu, Z.; Wang, Z.; Han, B.; He, M.; Min, E. Alkylation of benzene with dodecene over HY zeolite: Deactivation, regeneration, and product distribution. Zeolites 1996, 17, 297–303. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Fundamental description of deactivation and regeneration of acid zeolites. In Studies in Surface Science and Catalysis; Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 53–68. [Google Scholar]
- Liu, Y.; Xu, L.; Xu, B.; Li, Z.; Jia, L.; Guo, W. Toluene alkylation with 1-octene over supported heteropoly acids on MCM-41 catalysts. J. Mol. Catal. A Chem. 2009, 297, 86–92. [Google Scholar] [CrossRef]
- Mayerová, J.; Pawlesa, J.; ilková, N.; Košová, G.; Čejka, J. Microporous and mesoporous molecular sieves for alkylation of toluene with olefins. In Studies in Surface Science and Catalysis; Čejka, J., Žilková, N., Nachtigall, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1945–1952. [Google Scholar]
- Cowley, M.; de Klerk, A.; Nel, R.J.J. Amylation of Toluene by Solid Acid Catalysis. Ind. Eng. Chem. Res. 2005, 44, 5535–5541. [Google Scholar] [CrossRef]
- Magnoux, P.; Mourran, A.; Bernard, S.; Guisnet, M. Influence of the acidity and of the pore structure of zeolites on the alkylation of toluene by 1-heptene. In Studies in Surface Science and Catalysis; Blaser, H.U., Baiker, A., Prins, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 107–114. [Google Scholar]
- Yuan, X.-D.; Park, J.-N.; Wang, J.; Lee, C.; Park, S.-E. Alkylation of benzene with 1- dodecene over usy zeolite catalyst: Effect of pretreatment and reaction conditions. Korean J. Chem. Eng. 2002, 19, 607–610. [Google Scholar] [CrossRef]
- Wang, B.; Lee, C.W.; Cai, T.X.; Park, S.E. Benzene alkylation with 1-dodecene over Y zeolite. Bull. Korean Chem. Soc. 2001, 22, 1056–1058. [Google Scholar]
- Horňáček, M.; Hudec, P.; Smiešková, A.; Jakubík, T. Alkylation of benzene with linear 1-alkenes in liquid phase. influence of zeolite type and chain length of 1-alkenes on the activity and selectivity. Int. Pet. Conf. 2009, 44, 1–12. [Google Scholar]
- Peregoa, C.; Amarillia, S.; Bellussia, G.; Cappellazzob, O.; Girotti, G.; Schenato, M. The Development of a New Zeolite Catalyst for the Production of Cumene: A Case History. Available online: http://www.researchgate.net/publication/236874011 (accessed on 11 August 2024).
- Bhore, N.A.; Klein, M.T.; Bischoff, K.B. The delplot technique: A new method for reaction pathway analysis. Ind. Eng. Chem. Res. 1990, 29, 313–316. [Google Scholar] [CrossRef]
- Gerzeliev, I.M.; Zhmylev, V.P.; Khusaimova, D.O.; Shkuropatov, A.V.; Knyazeva, E.E.; Ponomareva, O.A.; Ivanova, I.I.; Maksimov, A.L. Effect of Binder on the Properties of MWW Zeolite Catalysts in Benzene Alkylation with Propylene. Pet. Chem. 2019, 59, 695–700. [Google Scholar] [CrossRef]
- Namuangruk, S.; Pantu, P.; Limtrakul, J. Alkylation of benzene with ethylene over faujasite zeolite investigated by the ONIOM method. J. Catal. 2004, 225, 523–530. [Google Scholar] [CrossRef]
- Armor, J.N. A history of industrial catalysis. Catal. Today 2011, 163, 3–9. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.M.; Faria, J. Catalysis from Theory to Application; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2008. [Google Scholar]
- Ali, N.S.; Salih, I.K.; Harharah, H.N.; Majdi, H.S.; Salih, H.G.; Kalash, K.R.; Al-Shathr, A.; Al-Sudani, F.T.; Abdulrahman, M.A.; Alrubaye, J.M.; et al. Utilization of Loaded Cobalt onto MCM-48 Mesoporous Catalyst as a Heterogeneous Reaction in a Fixed Bed Membrane Reactor to Produce Isomerization Product from n-Heptane. Catalysts 2023, 13, 1138. [Google Scholar] [CrossRef]
- Guisnet, M.; Pinard, L. Characterization of acid-base catalysts through model reactions. Catal. Rev. 2018, 60, 337–436. [Google Scholar] [CrossRef]
- Al-Zaidi, B.Y.; Holmes, R.J.; Garforth, A.A. Study of the Relationship between Framework Cation Levels of Y Zeolites and Behavior during Calcination, Steaming, and n-Heptane Cracking Processes. Ind. Eng. Chem. Res. 2012, 51, 6648–6657. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Shakor, Z.M.; Majdi, H.S.; AbdulRazak, A.A.; Albayati, T.M. Comparison between Artificial Neural Network and Rigorous Mathematical Model in Simulation of Industrial Heavy Naphtha Reforming Process. Catalysts 2021, 11, 1034. [Google Scholar] [CrossRef]
- Khalil, M.; Al-Zaidi, B.Y.; Shakor, Z.M.; Hussein, S.J.; Al-Shathr, A. Experimental and Kinetic Study of the Catalytic Behavior of Sulfate-Treated Nanostructured Bifunctional Zirconium Oxide Catalysts in n-Heptane Hydroisomerization Reactions. ChemEngineering 2023, 7, 115. [Google Scholar] [CrossRef]
- Hagen, J. Industrial Catalysis: A Practical Approach; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Quintana-Gómez, L.; Connolly, M.; Shehab, A.K.; Al-Shathr, A.; McGregor, J. “Reverse combustion” of carbon dioxide in water: The influence of reaction conditions. Front. Energy Res. 2022, 10, 917943. [Google Scholar] [CrossRef]
- Wang, B.; Lee, C.W.; Cai, T.-X.; Park, S.-E. Benzene Alkylation with 1-Dodecene over H-Mordenite Zeolite. Catal. Lett. 2001, 76, 99–103. [Google Scholar] [CrossRef]
- Perego, C.; Ingallina, P. Recent advances in the industrial alkylation of aromatics: New catalysts and new processes. Catal. Today 2002, 73, 3–22. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.Q.; Shehab, A.K.; Al-Shathr, A.; Ingram, W.; Konstantinova, M.; Cumming, D.; McGregor, J. H2-free Synthesis of Aromatic, Cyclic and Linear Oxygenates from CO2. ChemSusChem 2020, 13, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Kulprathipanja, S. Zeolites in Industrial Separation and Catalysis; WILEY-VCH: Weinheim, Germany, 2010. [Google Scholar]
- van Bekkum, H.; Flanigen, E.M.; Jacobs, P.A.; Jansen, J.C. Introduction to Zeolite Science and Practice; Elsevier: Amsterdam, The Netherlands, 2001; Volume 58. [Google Scholar]
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Shakor, Z.M.; Al-Zaidi, B.Y.; Majdi, H.S.; AbdulRazak, A.A.; Aal-Kaeb, S.; Shohib, A.A.; McGregor, J. Reaction Kinetics of Cinnamaldehyde Hydrogenation over Pt/SiO2: Comparison between Bulk and Intraparticle Diffusion Models. Int. J. Chem. Eng. 2022, 2022, 8303874. [Google Scholar] [CrossRef]
- Al-Zaidi, B.Y.; Al-Shathr, A.; Shehab, A.K.; Shakor, Z.M.; Majdi, H.S.; AbdulRazak, A.A.; McGregor, J. Hydroisomerisation and Hydrocracking of n-Heptane: Modelling and Optimisation Using a Hybrid Artificial Neural Network–Genetic Algorithm (ANN–GA). Catalysts 2023, 13, 1125. [Google Scholar] [CrossRef]
- Zaera, F. New Challenges in Heterogeneous Catalysis for the 21st Century. Catal. Lett. 2012, 142, 501–516. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Appl. Catal. A Gen. 2001, 212, 83–96. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, X.; Song, H.; Xia, G.; Shen, Z.-Y.; Yu, R.; Moskovits, M. Microwave synthesis of zeolites and their related applications. Microporous Mesoporous Mater. 2021, 323, 111262. [Google Scholar] [CrossRef]
- Verdoliva, V.; Saviano, M.; De Luca, S. Zeolites as Acid/Basic Solid Catalysts: Recent Synthetic Developments. Catalysts 2019, 9, 248. [Google Scholar] [CrossRef]
- Liu, M.; Miao, C.; Wu, Z. Recent advances in the synthesis, characterization, and catalytic consequence of metal species confined within zeolite for hydrogen-related reactions. Ind. Chem. Mater. 2024, 2, 57–84. [Google Scholar] [CrossRef]
- Sandoval-Díaz, L.-E.; González-Amaya, J.-A.; Trujillo, C.-A. General aspects of zeolite acidity characterization. Microporous Mesoporous Mater. 2015, 215, 229–243. [Google Scholar] [CrossRef]
- Vaughan, O. Porous by design. Nature 2014, 511, 19. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and Clay Minerals as Sorbents and Molecular Sieves; Academic Press: London, UK; New York, NY, USA, 1978. [Google Scholar]
- Mon, M.; Leyva-Pérez, A. Chapter Two—Zeolites catalyze selective reactions of large organic molecules. In Advances in Catalysis; Diéguez, M., Pàmies, O., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 59–102. [Google Scholar]
- Verma, R.; Nair, N.N. Proton-Exchange Reaction in Acidic Zeolites: Mechanism and Free Energetics. J. Phys. Chem. C 2022, 126, 19169–19177. [Google Scholar] [CrossRef]
- Ferreira Young, A.; Nothaft Romano, P.; Monnerat Araújo Ribeiro de Almeida, J.; Gomes Aranda, D.A. Isomerization of Oleic Acid over Acid Zeolites: Design of Experiments and Proposal of a Novel Kinetic Mechanism. Ind. Eng. Chem. Res. 2021, 60, 14051–14059. [Google Scholar] [CrossRef]
- Li, B.; Huang, P.; Cao, P.; Gao, W.; Zheng, W.; Lian, C.; Sun, W.; Zhao, L. Understanding the structural properties of zeolites for isobutane alkylation based on adsorption/diffusion behaviors. Microporous Mesoporous Mater. 2022, 341, 112040. [Google Scholar] [CrossRef]
- Chaihad, N.; Karnjanakom, S.; Abudula, A.; Guan, G. Zeolite-based cracking catalysts for bio-oil upgrading: A critical review. Resour. Chem. Mater. 2022, 1, 167–183. [Google Scholar] [CrossRef]
- Golubev, K.B.; Zhang, K.; Su, X.; Kolesnichenko, N.V.; Wu, W. Dimethyl ether aromatization over nanosized zeolites: Effect of preparation method and zinc modification on catalyst performance. Catal. Commun. 2021, 149, 106176. [Google Scholar] [CrossRef]
- Barrer, R.M. Hydrothermal Chemistry of Zeolites; Academic Press: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Vinaches, P.; Bernardo-Gusmão, K.; Pergher, S.B.C. An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents. Molecules 2017, 22, 1307. [Google Scholar] [CrossRef]
- Sharma, V.; Javed, B.; Byrne, H.; Curtin, J.; Tian, F. Zeolites as Carriers of Nano-Fertilizers: From Structures and Principles to Prospects and Challenges. Appl. Nano 2022, 3, 163–186. [Google Scholar] [CrossRef]
- Raman, G. Identifying extra-large pore structures in zeolites with a machine learning approach and its deployment into production. Microporous Mesoporous Mater. 2023, 348, 112362. [Google Scholar] [CrossRef]
- Lin, Q.F.; Gao, Z.R.; Lin, C.; Zhang, S.; Chen, J.; Li, Z.; Liu, X.; Fan, W.; Li, J.; Chen, X.; et al. A stable aluminosilicate zeolite with intersecting three-dimensional extra-large pores. Science 2021, 374, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Len, T.; de Oliveira, A.d.N.; Costa, A.A.F.d.; Souza, A.R.d.S.; Costa, C.E.F.d.; Luque, R.; Rocha Filho, G.N.d.; Noronha, R.C.R.; Nascimento, L.A.S.d. Zeolites: A Theoretical and Practical Approach with Uses in (Bio)Chemical Processes. Appl. Sci. 2023, 13, 1897. [Google Scholar] [CrossRef]
- Catuzo, G.L.; Santilli, C.V.; Martins, L. Hydrophobic-hydrophilic balance of ZSM-5 zeolites on the two-phase ketalization of glycerol with acetone. Catal. Today 2021, 381, 215–223. [Google Scholar] [CrossRef]
- Cruciani, G.; Gualtieri, A. Dehydration dynamics of analcime by in situ synchrotron powder diffraction. Am. Mineral. 1999, 84, 112–119. [Google Scholar] [CrossRef]
- Zang, J.; Yu, H.; Liu, G.; Hong, M.; Liu, J.; Chen, T. Research Progress on Modifications of Zeolite Y for Improved Catalytic Properties. Inorganics 2023, 11, 22. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9, 013007. [Google Scholar] [CrossRef]
- Simoncic, P.; Armbruster, T. Peculiarity and defect structure of the natural and synthetic zeolite mordenite: A single-crystal X-ray study. Am. Mineral. 2004, 89, 421–431. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Pan, J.; Chow, T.P.; Aboalsaud, A.M.; Lai, Z.; Sheng, P. Peierls-type metal-insulator transition in carbon nanostructures. Carbon 2021, 172, 106–111. [Google Scholar] [CrossRef]
- Busca, G. Heterogeneous Catalytic Materials; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Čejka, J.; Bekkum, H.V.; Corma, A.; Schüth, F. Introduction to Zeolite Science and Practice; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Younus, H.K.; Bashir, Y.S.A.-Z.; Zaidoon, M.S. Experimental and Kinetic Study of the Effect of using Zr- and Pt-loaded Metals on Y-zeolite-based Catalyst to Improve the Products of n-heptane Hydroisomerization Reactions. Orbital Electron. J. Chem. 2022, 14, 153–167. [Google Scholar] [CrossRef]
- Saleh, N.J.; Al-Zaidi, B.Y.S.; Sabbar, Z.M. A Comparative Study of Y Zeolite Catalysts Derived from Natural and Commercial Silica: Synthesis, Characterization, and Catalytic Performance. Arab. J. Sci. Eng. 2018, 43, 5819–5836. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Lutz, W. Zeolite Y: Synthesis, Modification, and Properties—A Case Revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. [Google Scholar] [CrossRef]
- Li, J.; Gao, M.; Yan, W.; Yu, J. Regulation of the Si/Al ratios and Al distributions of zeolites and their impact on properties. Chem. Sci. 2023, 14, 1935–1959. [Google Scholar] [CrossRef] [PubMed]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; Noyes Publications: Park Ridge, NJ, USA, 2001. [Google Scholar]
- Kocal, J.A.; Vora, B.V.; Imai, T. Production of linear alkylbenzenes. Appl. Catal. A Gen. 2001, 221, 295–301. [Google Scholar] [CrossRef]
- Aslam, W.; Siddiqui, M.A.B.; Rabindran Jermy, B.; Aitani, A.; Čejka, J.; Al-Khattaf, S. Selective synthesis of linear alkylbenzene by alkylation of benzene with 1-dodecene over desilicated zeolites. Catal. Today 2014, 227, 187–197. [Google Scholar] [CrossRef]
- Julbe, A.; Drobek, M. Zeolite A Type. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 2055–2056. [Google Scholar] [CrossRef]
- McCusker, L.B.; Baerlocher, C. Chapter 3 Zeolite structures. In Studies in Surface Science and Catalysis; van Bekkum, H., Flanigen, E.M., Jacobs, P.A., Jansen, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 37–67. [Google Scholar]
- Ozekmekci, M.; Salkic, G.; Fellah, M.F. Use of zeolites for the removal of H2S: A mini-review. Fuel Process. Technol. 2015, 139, 49–60. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Leonard, R.J. The hydrothermal alteration of certain silicate minerals. Econ. Geol. 1927, 22, 18–43. [Google Scholar] [CrossRef]
- Li, X.; Prins, R.; van Bokhoven, J.A. Synthesis and characterization of mesoporous mordenite. J. Catal. 2009, 262, 257–265. [Google Scholar] [CrossRef]
- Xu, C.; Guo, Y.; Xiao, Q.; Zhong, Y.; Zhu, W. Synthesis and characterization of large, pure mordenite crystals. J. Porous Mater. 2012, 19, 847–852. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Yang, G.; Zhou, J.; Wang, C.; Teng, J.; Wang, Y.; Xie, Z. A diffusion anisotropy descriptor links morphology effects of H-ZSM-5 zeolites to their catalytic cracking performance. Commun. Chem. 2021, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sutar, P.P.; Xiao, H.; Zhang, Q. The untapped potential of zeolites in techno-augmentation of the biomaterials and food industrial processing operations: A review. J. Future Foods 2023, 3, 127–141. [Google Scholar] [CrossRef]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meier, W.M. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981, 85, 2238–2243. [Google Scholar] [CrossRef]
- Sharbini Kamaluddin, H.; Gong, X.; Ma, P.; Narasimharao, K.; Dutta Chowdhury, A.; Mokhtar, M. Influence of zeolite ZSM-5 synthesis protocols and physicochemical properties in the methanol-to-olefin process. Mater. Today Chem. 2022, 26, 101061. [Google Scholar] [CrossRef]
- Niu, X.; Bai, Y.; Du, Y.-e.; Qi, H.; Chen, Y. Size controllable synthesis of ZSM-5 zeolite and its catalytic performance in the reaction of methanol conversion to aromatics. R. Soc. Open Sci. 2022, 9, 211284. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, N.; Dai, C.; Xu, R.; Yu, G.; Chen, B.; Wang, N. Recent advances in shape selectivity of MFI zeolite and its effect on the catalytic performance. Chem. Synth. 2023, 3, 2. [Google Scholar] [CrossRef]
- Borade, R.B.; Clearfield, A. Preparation of aluminum-rich Beta zeolite. Microporous Mater. 1996, 5, 289–297. [Google Scholar] [CrossRef]
- Liu, S.-b.; Wu, J.-F.; Ma, L.-J.; Tsai, T.-C.; Wang, I. On the thermal stability of zeolite beta. J. Catal. 1991, 132, 432–439. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, H.; Lv, G.; Liao, W.; Lü, H.; Zhu, Z.; Yang, K. Hydrothermal synthesis of nanosized Sn-beta zeolites by interzeolite transformation for glucose isomerization. CrystEngComm 2023, 25, 5588–5596. [Google Scholar] [CrossRef]
- Weitkamp, J.; Traa, Y. Isobutane/butene alkylation on solid catalysts. Where do we stand? Catal. Today 1999, 49, 193–199. [Google Scholar] [CrossRef]
- Yoo, K.; Burckle, E.C.; Smirniotis, P.G. Comparison of protonated zeolites with various dimensionalities for the liquid phase alkylation of i-butane with 2-butene. Catal. Lett. 2001, 74, 85–90. [Google Scholar] [CrossRef]
- Han, S.; Linares, N.; Terlier, T.; Hoke, J.B.; García Martínez, J.; Li, Y.; Rimer, J.D. Cooperative Surface Passivation and Hierarchical Structuring of Zeolite Beta Catalysts. Angew. Chem. Int. Ed. 2022, 61, e202210434. [Google Scholar] [CrossRef] [PubMed]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of Synthesis, and Selected Applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef]
- Klerk, A. Zeolites as Catalysts for Fuels Refining after Indirect Liquefaction Processes. Molecules 2018, 23, 115. [Google Scholar] [CrossRef]
- Hambali, H.U.; Jalil, A.A.; Abdulrasheed, A.A.; Siang, T.J.; Gambo, Y.; Umar, A.A. Zeolite and clay based catalysts for CO2 reforming of methane to syngas: A review. Int. J. Hydrogen Energy 2022, 47, 30759–30787. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Dann, S.E.; Mead, P.J.; Weller, M.T. Löwenstein’s Rule Extended to an Aluminum Rich Framework. The Structure of Bicchulite, Ca8(Al2SiO6)4(OH)8, by MASNMR and Neutron Diffraction. Inorg. Chem. 1996, 35, 1427–1428. [Google Scholar] [CrossRef]
- Margeta, K.; Logar, N.Z.; Šiljeg, M.; Farkaš, A. Natural Zeolites in Water Treatment—How Effective is Their Use. In Water Treatment; Elshorbagy, W., Chowdhury, R.K., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Crum, J.T.; Crum, J.R.; Taylor, C.; Schneider, W.F. Characterization and analysis of ring topology of zeolite frameworks. Microporous Mesoporous Mater. 2023, 351, 112466. [Google Scholar] [CrossRef]
- Van Speybroeck, V.; Hemelsoet, K.; Joos, L.; Waroquier, M.; Bell, R.G.; Catlow, C.R.A. Advances in theory and their application within the field of zeolite chemistry. Chem. Soc. Rev. 2015, 44, 7044–7111. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Yang, X.; Dib, E.; Lang, Q.; Guo, H.; Fu, G.; Wang, J.; Yi, Q.; Zhao, H.; Valtchev, V. Silicalite-1 formation in acidic medium: Synthesis conditions and physicochemical properties. Microporous Mesoporous Mater. 2022, 329, 111537. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Yue, B.; Wang, C.; Qin, B.; Chai, Y.; Wu, G.; Li, J.; Han, X.; da-Silva, I.; et al. Regulating Extra-Framework Cations in Faujasite Zeolites for Capture of Trace Carbon Dioxide. Chem. A Eur. J. 2022, 28, e202201659. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.; Puppe, L. Catalysis and Zeolites; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically Structured Zeolites: From Design to Application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating Hierarchical Pores in Zeolite Catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Derbe, T.; Temesgen, S.; Bitew, M. A Short Review on Synthesis, Characterization, and Applications of Zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898. [Google Scholar] [CrossRef]
- Barbosa, A.d.S.; Rodrigues, M.G.F. Synthesis of NaA Zeolite: Conventional Route and Green Route. Catal. Res. 2024, 4, 2. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Deng, F. Recent advances in solid-state NMR of zeolite catalysts. Natl. Sci. Rev. 2022, 9, nwac155. [Google Scholar] [CrossRef]
- Rocha, L.C.C.; Zuquette, L.V. Evaluation of Zeolite as a Potential Reactive Medium in a Permeable Reactive Barrier (PRB): Batch and Column Studies. Geosciences 2020, 10, 59. [Google Scholar] [CrossRef]
- Han, Y.; Larmier, K.; Rivallan, M.; Pirngruber, G.D. Generation of mesoporosity in H–Y zeolites by basic or acid/basic treatments: Towards a guideline of optimal Si/Al ratio and basic reagent. Microporous Mesoporous Mater. 2024, 365, 112906. [Google Scholar] [CrossRef]
- Guisnet, M.; Gilson, J.-P. Zeolites for Cleaner Technologies; Imperial College Press: London, UK, 2002; Volume 3. [Google Scholar]
- Prodinger, S.; Derewinski, M.A. Recent Progress to Understand and Improve Zeolite Stability in the Aqueous Medium. Pet. Chem. 2020, 60, 420–436. [Google Scholar] [CrossRef]
- Trachta, M.; Bulánek, R.; Bludský, O.; Rubeš, M. Brønsted acidity in zeolites measured by deprotonation energy. Sci. Rep. 2022, 12, 7301. [Google Scholar] [CrossRef] [PubMed]
- Haw, J.F. Zeolite acid strength and reaction mechanisms in catalysis. Phys. Chem. Chem. Phys. 2002, 4, 5431–5441. [Google Scholar] [CrossRef]
- Trachta, M.; Bludský, O.; Vaculík, J.; Bulánek, R.; Rubeš, M. Investigation of Brønsted acidity in zeolites through adsorbates with diverse proton affinities. Sci. Rep. 2023, 13, 12380. [Google Scholar] [CrossRef]
- Ravi, M.; Sushkevich, V.L.; van Bokhoven, J.A. On the location of Lewis acidic aluminum in zeolite mordenite and the role of framework-associated aluminum in mediating the switch between Brønsted and Lewis acidity. Chem. Sci. 2021, 12, 4094–4103. [Google Scholar] [CrossRef]
- Palčić, A.; Valtchev, V. Analysis and control of acid sites in zeolites. Appl. Catal. A Gen. 2020, 606, 117795. [Google Scholar] [CrossRef]
- Li, G.; Pidko, E.A. The Nature and Catalytic Function of Cation Sites in Zeolites: A Computational Perspective. ChemCatChem 2019, 11, 134–156. [Google Scholar] [CrossRef]
- Van Speybroeck, V.; Bocus, M.; Cnudde, P.; Vanduyfhuys, L. Operando Modeling of Zeolite-Catalyzed Reactions Using First-Principles Molecular Dynamics Simulations. ACS Catal. 2023, 13, 11455–11493. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A. Factors Controlling the Acidity of Zeolites. Catal. Lett. 2015, 145, 162–172. [Google Scholar] [CrossRef]
- Grifoni, E.; Piccini, G.; Lercher, J.A.; Glezakou, V.-A.; Rousseau, R.; Parrinello, M. Confinement effects and acid strength in zeolites. Nat. Commun. 2021, 12, 2630. [Google Scholar] [CrossRef] [PubMed]
- van Vreeswijk, S.H.; Weckhuysen, B.M. Emerging analytical methods to characterize zeolite-based materials. Natl. Sci. Rev. 2022, 9, nwac047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, X.; Yi, H.; Yu, Q.; Zhang, Y.; Wei, J.; Yuan, Y. Synthesis, characterization and application of Fe-zeolite: A review. Appl. Catal. A Gen. 2022, 630, 118467. [Google Scholar] [CrossRef]
- Serrano, D.P.; Centi, G.; Diddams, P.A.; Čejka, J. Outlooks for zeolite catalysts in a low-carbon scenario. Catal. Today 2024, 426, 114365. [Google Scholar] [CrossRef]
- Zhang, H.; Samsudin, I.b.; Jaenicke, S.; Chuah, G.-K. Zeolites in catalysis: Sustainable synthesis and its impact on properties and applications. Catal. Sci. Technol. 2022, 12, 6024–6039. [Google Scholar] [CrossRef]
- Csicsery, S.M. Shape-selective catalysis in zeolites. Zeolites 1984, 4, 202–213. [Google Scholar] [CrossRef]
- Csicsery, S.M. Catalysis by shape selective zeolites-science and technology. Pure Appl. Chem. 1986, 58, 841–856. [Google Scholar] [CrossRef]
- Nyankson, E.; Efavi, J.K.; Yaya, A.; Manu, G.; Asare, K.; Daafuor, J.; Abrokwah, R.Y. Synthesis and characterisation of zeolite-A and Zn-exchanged zeolite-A based on natural aluminosilicates and their potential applications. Cogent Eng. 2018, 5, 1440480. [Google Scholar] [CrossRef]
- Bellussi, G.; Millini, R. Zeoliti. Enciclopedia. Available online: http://www.treccani.it/enciclopedia/zeoliti_(Enciclopedia_della_Scienza_e_della_Tecnica)/ (accessed on 7 July 2015).
- Rzepka, P.; Sheptyakov, D.; Wang, C.; van Bokhoven, J.A.; Paunović, V. How Micropore Topology Influences the Structure and Location of Coke in Zeolite Catalysts. ACS Catal. 2024, 14, 5593–5604. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity; Academic Press: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Rouquerol, J.; Rouquerol, F.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: Cambridge, MA, USA, 1999; p. 467. [Google Scholar]
- Nishi, Y.; Inagaki, M. Chapter 11—Gas Adsorption/Desorption Isotherm for Pore Structure Characterization. In Materials Science and Engineering of Carbon; Inagaki, M., Kang, F., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 227–247. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent advances of pore system construction in zeolite-catalyzed chemical industry processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar] [CrossRef]
- Cruciani, G. Zeolites upon heating: Factors governing their thermal stability and structural changes. J. Phys. Chem. Solids 2006, 67, 1973–1994. [Google Scholar] [CrossRef]
- Hoff, T.C.; Thilakaratne, R.; Gardner, D.W.; Brown, R.C.; Tessonnier, J.-P. Thermal Stability of Aluminum-Rich ZSM-5 Zeolites and Consequences on Aromatization Reactions. J. Phys. Chem. C 2016, 120, 20103–20113. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Hums, E.; Kuhnt, A.; Schwieger, W. Thermal stability studies of zeolites A and X synthesized from South African coal fly ash. Res. Chem. Intermed. 2015, 41, 575–582. [Google Scholar] [CrossRef]
- Padamurthy, A.; Nandanavanam, J.; Rajagopalan, P. Thermal stability evaluation of selected zeolites for sustainable thermochemical energy storage. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–14. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Zhang, J.; Zhang, T.; Ye, M.; Liu, Z. Regeneration of catalysts deactivated by coke deposition: A review. Chin. J. Catal. 2020, 41, 1048–1061. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Chetty, M.; Khotseng, L.; Kiambi, S.L.; Maharaj, L.; Oboirien, B.; Isa, Y.M. Stability, deactivation and regeneration study of a newly developed HZSM-5 and Ni-doped HZSM-5 zeolite catalysts for ethanol-to-hydrocarbon conversion. Catal. Commun. 2024, 186, 106802. [Google Scholar] [CrossRef]
- Kadja, G.T.M.; Azhari, N.J.; Mardiana, S.; Culsum, N.T.U.; Maghfirah, A. Recent advances in the development of nanosheet zeolites as heterogeneous catalysts. Results Eng. 2023, 17, 100910. [Google Scholar] [CrossRef]
- Lundegaard, L.F.; Berdiell, I.C.; König, N.; Junge, N.H.; Beato, P.; Chernyshov, D.; Svelle, S.; Wragg, D.S. Tracking deactivation of zeolite beta with and without a detailed structure model: XRD analysis and in situ studies. Microporous Mesoporous Mater. 2024, 366, 112911. [Google Scholar] [CrossRef]
- Xing, S.; Liu, X.; Cui, Y.; Zhao, Y.; Chen, Z.; Xiang, S.; Han, M. Elucidating the deactivation mechanism of beta zeolite catalyzed linear alkylbenzene production with oxygenated organic compound contaminated feedstocks. RSC Adv. 2024, 14, 9243–9253. [Google Scholar] [CrossRef]
- Guisnet, M.; Ribeiro, F.R. Deactivation and Regeneration of Zeolite Catalysts; Imperial College Press: London, UK, 2011. [Google Scholar]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, J.; Li, M.; Liu, W.; Wu, X.; Liu, S. Model Ag/CeO2 catalysts for soot combustion: Roles of silver species and catalyst stability. Chem. Eng. J. 2022, 430, 132802. [Google Scholar] [CrossRef]
- Wan, Z.; Li, G.K.; Wang, C.; Yang, H.; Zhang, D. Relating coke formation and characteristics to deactivation of ZSM-5 zeolite in methanol to gasoline conversion. Appl. Catal. A Gen. 2018, 549, 141–151. [Google Scholar] [CrossRef]
- Díaz, M.; Epelde, E.; Valecillos, J.; Izaddoust, S.; Aguayo, A.T.; Bilbao, J. Coke deactivation and regeneration of HZSM-5 zeolite catalysts in the oligomerization of 1-butene. Appl. Catal. B Environ. 2021, 291, 120076. [Google Scholar] [CrossRef]
- Guisnet, M.; Costa, L.; Ribeiro, F.R. Prevention of zeolite deactivation by coking. J. Mol. Catal. A Chem. 2009, 305, 69–83. [Google Scholar] [CrossRef]
- Morales, M.V.; Góra-Marek, K.; Musch, H.; Pineda, A.; Murray, B.; Stefanidis, S.; Falco, L.; Tarach, K.; Ponomareva, E.; Marsman, J.H.; et al. Advanced oxidation process for coke removal: A systematic study of hydrogen peroxide and OH-derived-Fenton radicals of a fouled zeolite. Appl. Catal. A Gen. 2018, 562, 215–222. [Google Scholar] [CrossRef]
- Karpe, S.; Veser, G. Coke Formation and Regeneration during Fe-ZSM-5-Catalyzed Methane Dehydro-Aromatization. Catalysts 2024, 14, 292. [Google Scholar] [CrossRef]
- Jong, S.-J.; Pradhan, A.R.; Wu, J.-F.; Tsai, T.-C.; Liu, S.-B. On the Regeneration of Coked H-ZSM-5 Catalysts. J. Catal. 1998, 174, 210–218. [Google Scholar] [CrossRef]
- Glasson, C.; Geantet, C.; Lacroix, M.; Labruyere, F.; Dufresne, P. Beneficial Effect of Carbon on Hydrotreating Catalysts. J. Catal. 2002, 212, 76–85. [Google Scholar] [CrossRef]
- Collett, C.H.; McGregor, J. Things go better with coke: The beneficial role of carbonaceous deposits in heterogeneous catalysis. Catal. Sci. Technol. 2015, 6, 363–378. [Google Scholar] [CrossRef]
- Kaeding, W.W.; Chu, C.; Young, L.B.; Weinstein, B.; Butter, S.A. Selective alkylation of toluene with methanol to produce para-Xylene. J. Catal. 1981, 67, 159–174. [Google Scholar] [CrossRef]
- Menon, P.G. Coke on catalysts-harmful, harmless, invisible and beneficial types. J. Mol. Catal. 1990, 59, 207–220. [Google Scholar] [CrossRef]
- Querini, C.A. Coke characterization. In Catalysis: Volume 17; Spivey, J.J., Roberts, G.W., Eds.; The Royal Society of Chemistry: London, UK, 2004; pp. 166–209. [Google Scholar] [CrossRef]
- Bauer, F.; Karge, H.G. Characterization of Coke on Zeolites. In Characterization II; Karge, H.G., Weitkamp, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 249–364. [Google Scholar] [CrossRef]
- Suwardiyanto; Howe, R.F.; Gibson, E.K.; Catlow, C.R.A.; Hameed, A.; McGregor, J.; Collier, P.; Parker, S.F.; Lennon, D. An assessment of hydrocarbon species in the methanol-to-hydrocarbon reaction over a ZSM-5 catalyst. Faraday Discuss. 2017, 197, 447–471. [Google Scholar] [CrossRef] [PubMed]
- Gomez Sanz, S.; McMillan, L.; McGregor, J.; Zeitler, J.A.; Al-Yassir, N.; Al-Khattaf, S.; Gladden, L.F. The enhancement of the catalytic performance of CrOx/Al2O3 catalysts for ethylbenzene dehydrogenation through tailored coke deposition. Catal. Sci. Technol. 2016, 6, 1120–1133. [Google Scholar] [CrossRef]
- Verdeş, O.; Popa, A.; Borcănescu, S.; Suba, M.; Sasca, V. Thermogravimetry Applied for Investigation of Coke Formation in Ethanol Conversion over Heteropoly Tungstate Catalysts. Catalysts 2022, 12, 1059. [Google Scholar] [CrossRef]
- Mekki-Berrada, A.; Auroux, A. Characterization of Solid Materials and Heterogeneous Catalysts; Wiley-VCH: Weinheim, Germany, 2012; Volume 1. [Google Scholar]
- Martín, N.; Viniegra, M.; Lima, E.; Espinosa, G. Coke Characterization on Pt/Al2O3−β-Zeolite Reforming Catalysts. Ind. Eng. Chem. Res. 2004, 43, 1206–1210. [Google Scholar] [CrossRef]
- Husham, K.; Khdier, H.; Saadoon, N.; Ahmed, A. Preparation of CuO/PVA Nanocomposite Thin Films for Gamma Ray Attenuation via PLA Method. J. Nano Struct. 2024, 14, 712–722. [Google Scholar]
- Ibáñez, M.; Gamero, M.; Ruiz-Martínez, J.; Weckhuysen, B.M.; Aguayo, A.T.; Bilbao, J.; Castaño, P. Simultaneous coking and dealumination of zeolite H-ZSM-5 during the transformation of chloromethane into olefins. Catal. Sci. Technol. 2016, 6, 296–306. [Google Scholar] [CrossRef]
- Devaraj, A.; Vijayakumar, M.; Bao, J.; Guo, M.F.; Derewinski, M.A.; Xu, Z.; Gray, M.J.; Prodinger, S.; Ramasamy, K.K. Discerning the Location and Nature of Coke Deposition from Surface to Bulk of Spent Zeolite Catalysts. Sci. Rep. 2016, 6, 37586. [Google Scholar] [CrossRef]
- Tsiao, C.; Dybowski, C.; Gaffney, A.M.; Sofranko, J.A. Coke formation on ZSM-5 zeolites: Evidence from NMR spectrometry of sorbed xenon gas. J. Catal. 1991, 128, 520–525. [Google Scholar] [CrossRef]
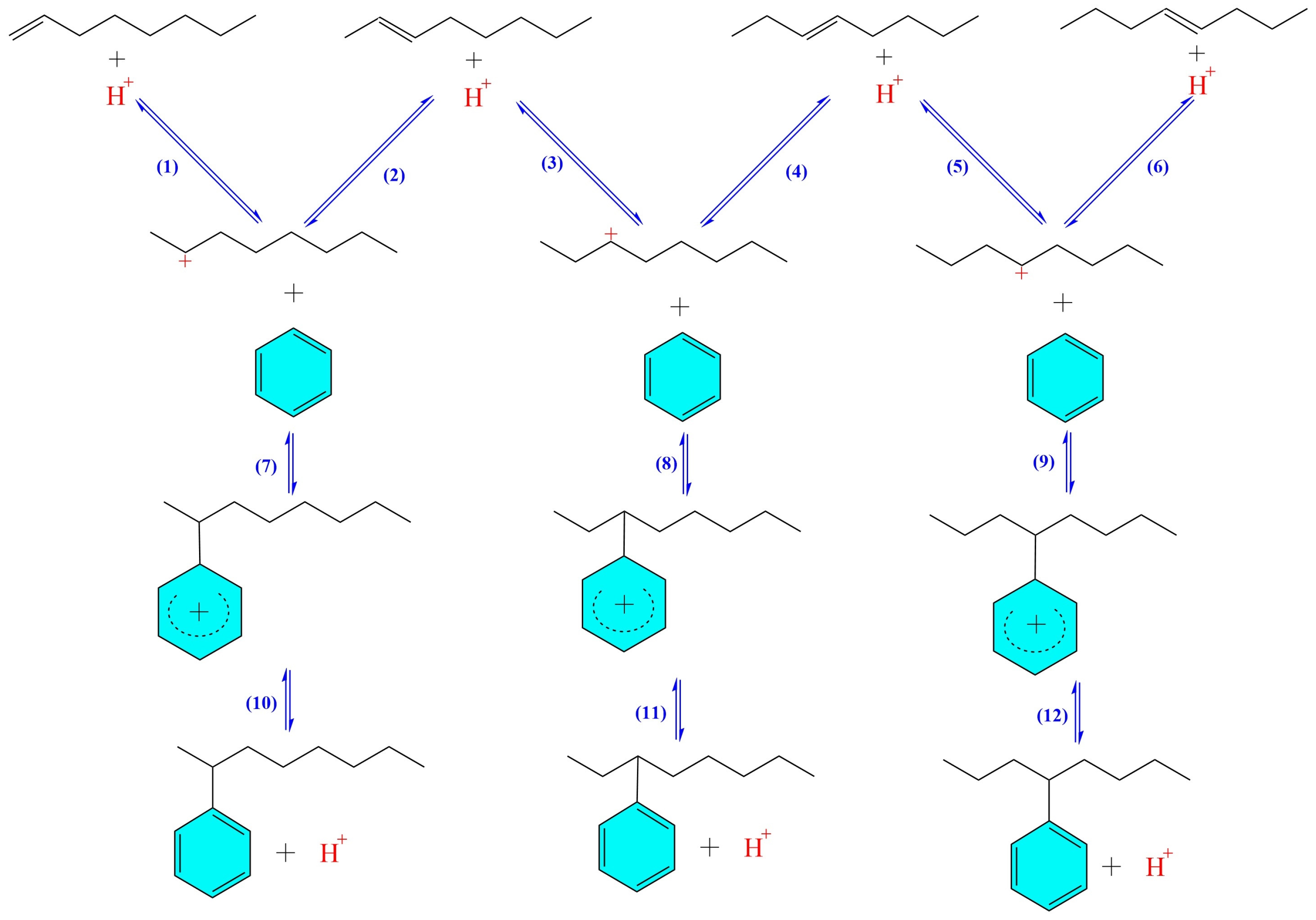
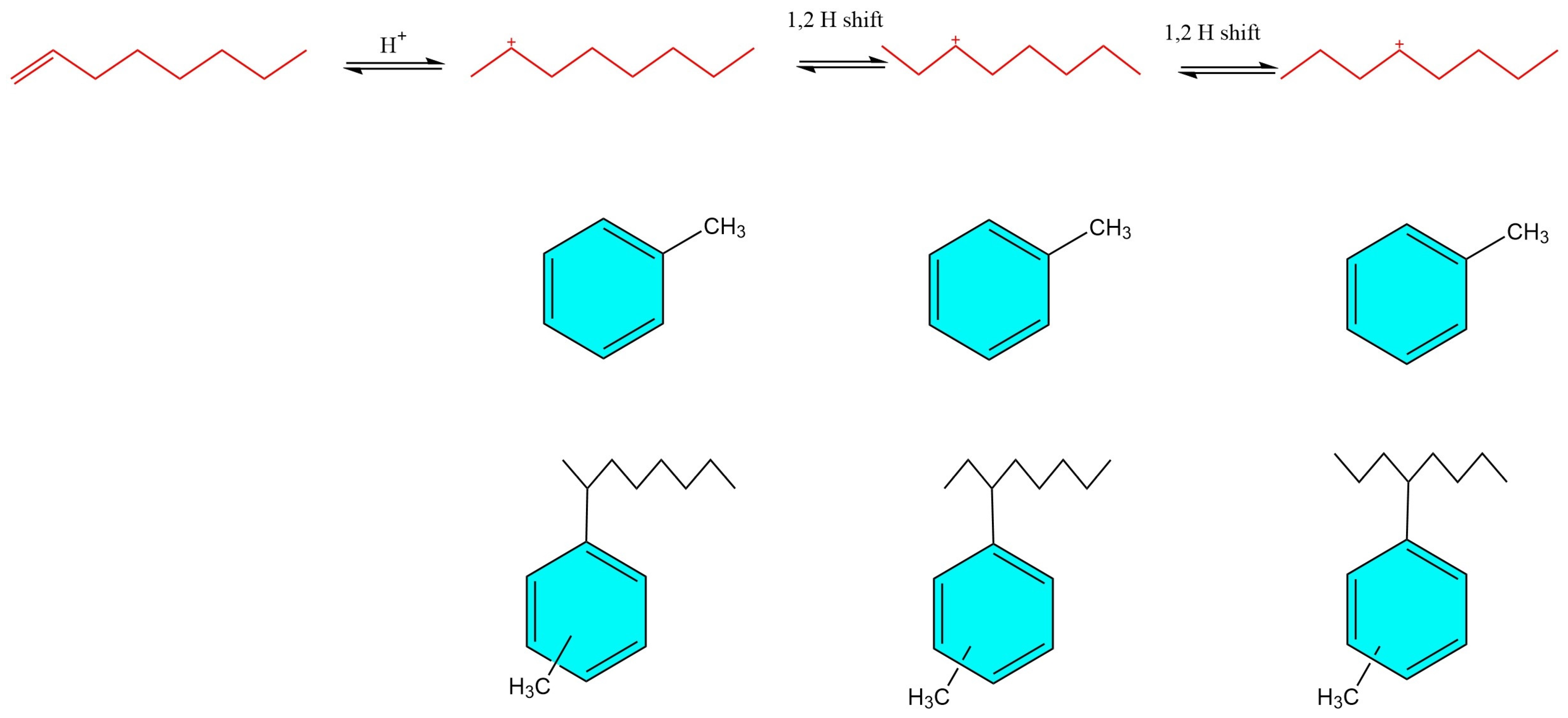

| Mechanism | Type | Brief Definition/Description |
|---|---|---|
| Poisoning | Chemical | Strong chemisorption of species on catalytic sites which block sites for catalytic reaction |
| Fouling | Mechanical | Physical deposition of species from fluid phase onto the catalytic surface and in catalyst pores |
| Thermal degradation and sintering | Thermal Thermal/chemical | Thermally induced loss of catalytic surface area, support area, and active phase-support reactions |
| Vapor formation | Chemical | Reaction of gas with catalyst phase to produce volatile compound |
| Vapor–solid and solid–solid reactions | Chemical | Reaction of vapor, support, or promoter with catalytic phase to produce inactive phase |
| Attrition/crushing | Mechanical | Loss of catalytic material due to abrasion; loss of internal surface area due to mechanical-induced crushing of the catalyst particle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sultani, S.H.; Al-Shathr, A.; Al-Zaidi, B.Y. Aromatics Alkylated with Olefins Utilizing Zeolites as Heterogeneous Catalysts: A Review. Reactions 2024, 5, 900-927. https://doi.org/10.3390/reactions5040048
Al-Sultani SH, Al-Shathr A, Al-Zaidi BY. Aromatics Alkylated with Olefins Utilizing Zeolites as Heterogeneous Catalysts: A Review. Reactions. 2024; 5(4):900-927. https://doi.org/10.3390/reactions5040048
Chicago/Turabian StyleAl-Sultani, Samaa H., Ali Al-Shathr, and Bashir Y. Al-Zaidi. 2024. "Aromatics Alkylated with Olefins Utilizing Zeolites as Heterogeneous Catalysts: A Review" Reactions 5, no. 4: 900-927. https://doi.org/10.3390/reactions5040048
APA StyleAl-Sultani, S. H., Al-Shathr, A., & Al-Zaidi, B. Y. (2024). Aromatics Alkylated with Olefins Utilizing Zeolites as Heterogeneous Catalysts: A Review. Reactions, 5(4), 900-927. https://doi.org/10.3390/reactions5040048







