Revisiting Atmospheric Oxidation Kinetics of Nitrogen Oxides: The Use of Low-Cost Electrochemical Sensors to Measure Reaction Kinetics
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
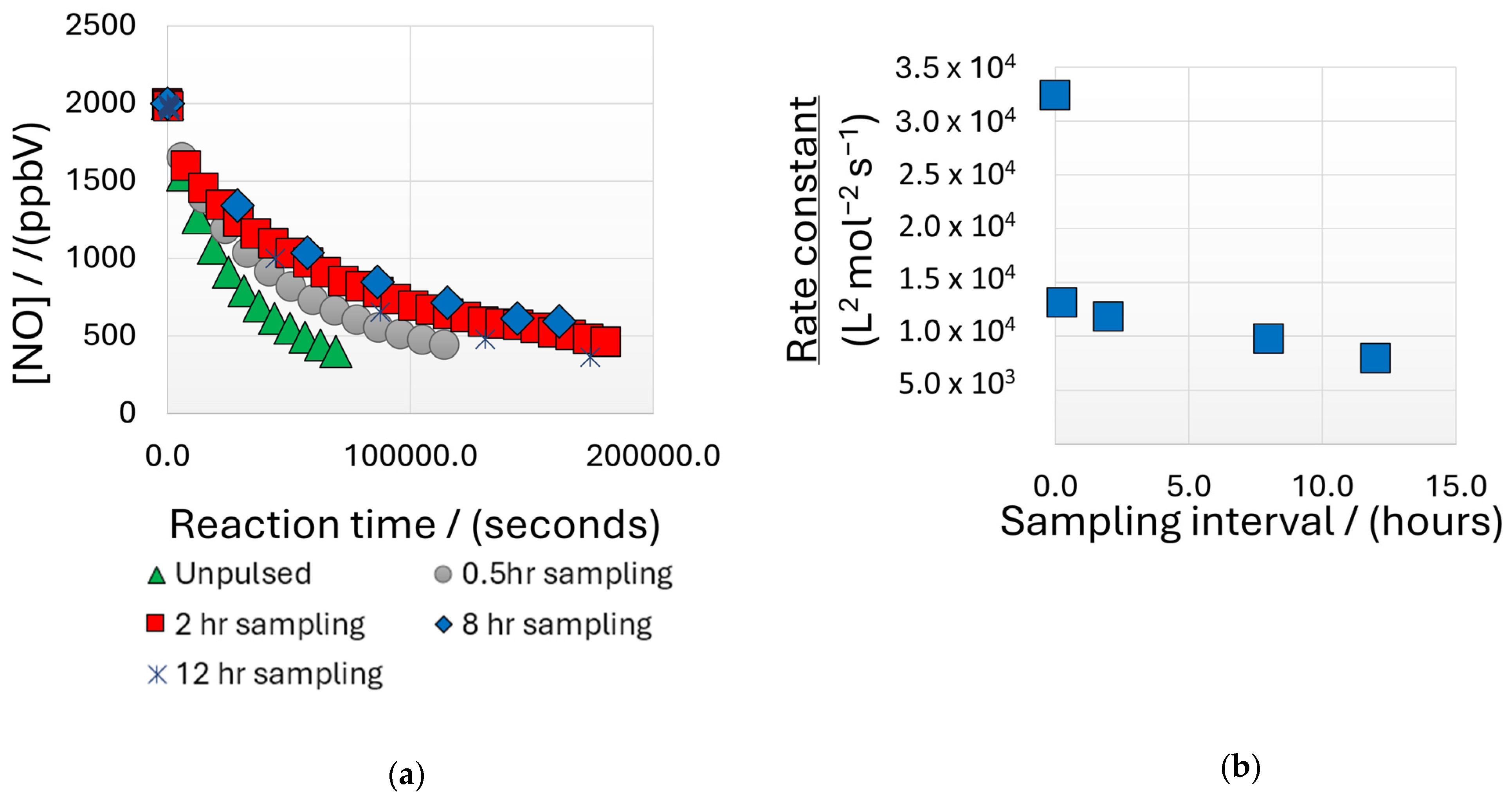
3.1. Determination of Appropriate Measurement Cycle Durations
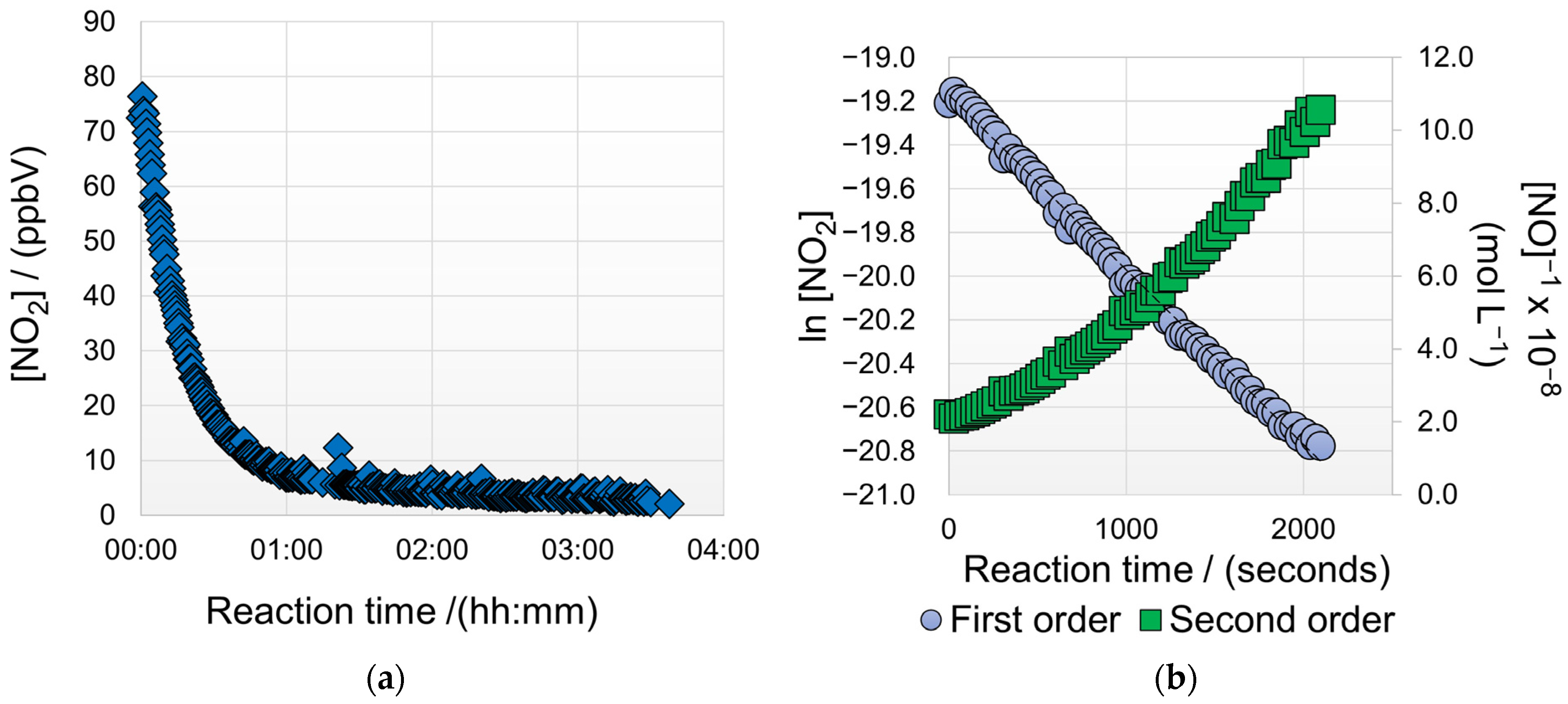
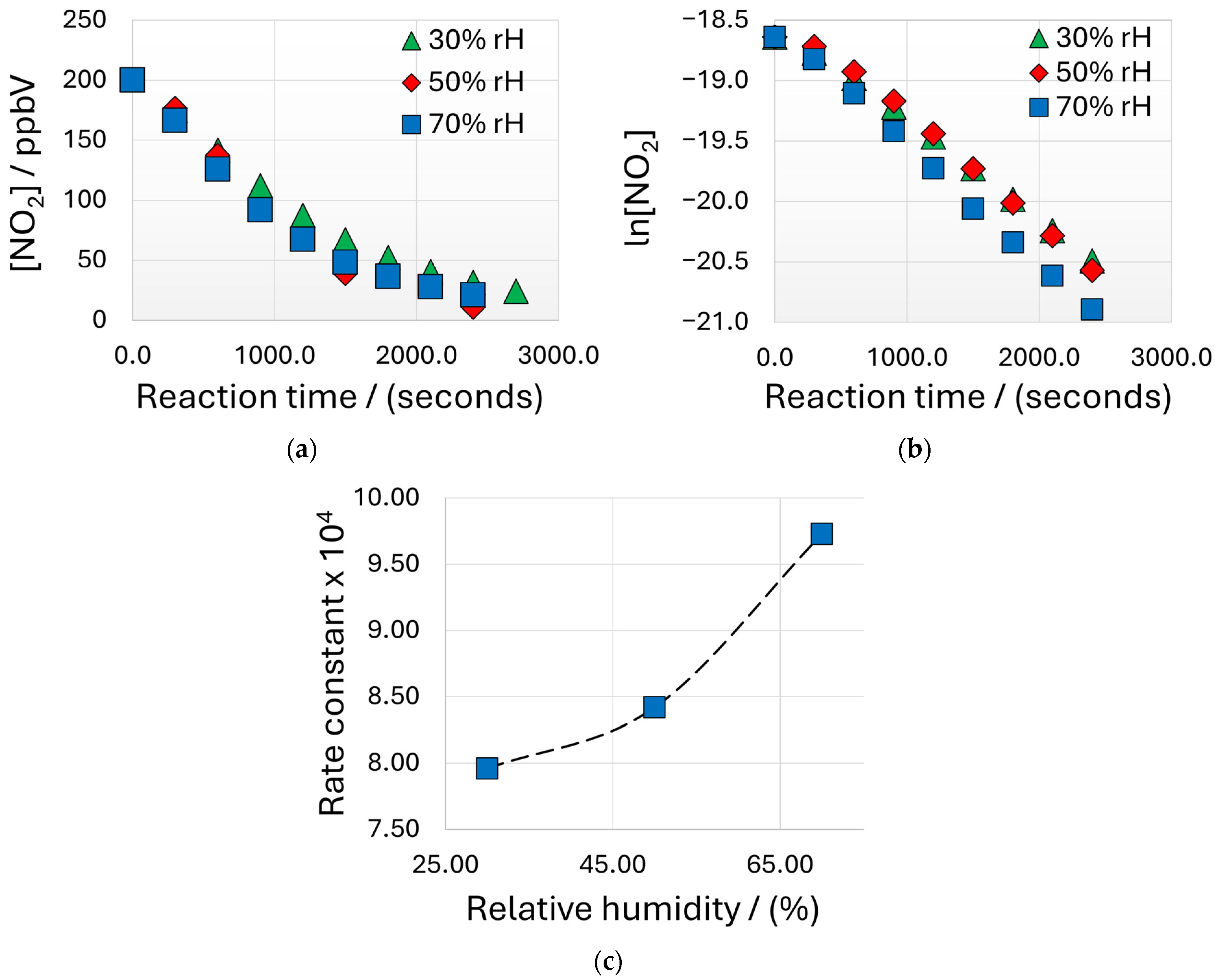
3.2. Measurement of Reaction Kinetics Using LCESs
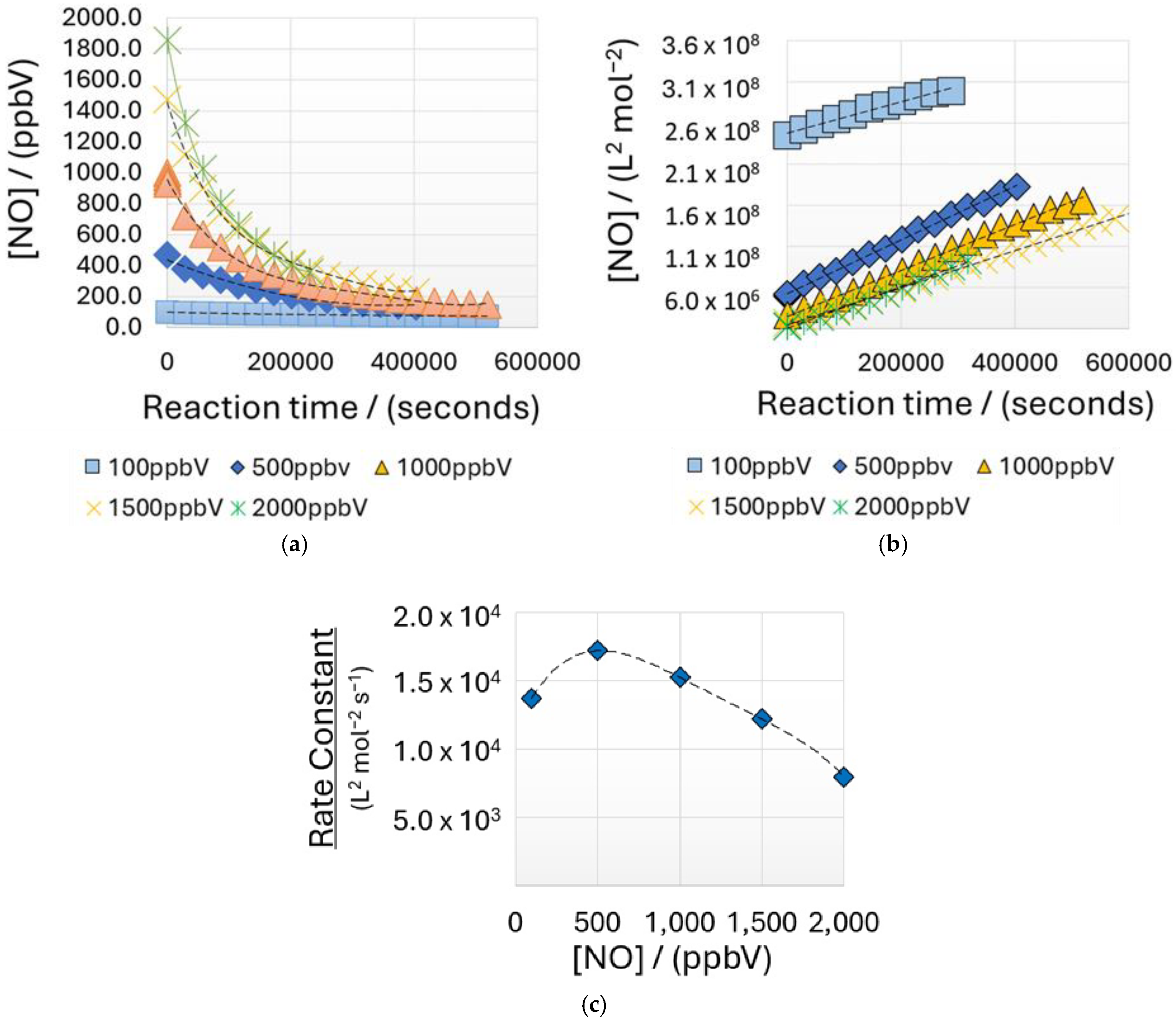
3.3. Nitric Oxide Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodenstein, M.; Wachenheim, L. Die Geschwindigkeit der Reaktion zwischen Stickoxyd und Sauerstoff. Z. Elektrochem. 1918, 24, 183–201. [Google Scholar]
- Tsukahara, H.; Ishida, T.; Mayumi, M. Gas-Phase Oxidation of Nitric Oxide: Chemical Kinetics and Rate Constant. Nitric Oxide 1999, 3, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Aida, A.; Miyamoto, K.; Saito, S.; Nakano, T.; Nishimura, M.; Kawakami, Y.; Omori, Y.; Ando, S.; Ichida, T.; Ishibe, Y. Effects of temperature and humidity on the stability of nitric oxide, and efficacy of soda lime as a selective absorber of nitrogen dioxide. Jpn. J. Thorac. Dis. 1995, 33, 306–311. [Google Scholar]
- Greig, J.D.; Hall, P.G. Infra-red spectrophotometric study of the oxidation of nitric oxide. Trans. Faraday Soc. 1966, 62, 652–658. [Google Scholar] [CrossRef]
- Stedman, D.H.; Niki, H. Kinetics and mechanism for the photolysis of nitrogen dioxide in air. J. Phys. Chem. 1973, 77, 2604–2609. [Google Scholar] [CrossRef]
- Treacy, J.C.; Daniels, F. Kinetic study of the oxidation of nitric oxide with oxygen in the pressure range 1 to 20 mm. J. Am. Chem. Soc 1955, 77, 2033–2036. [Google Scholar] [CrossRef]
- England, C. The Rate and Mechanism of the Air Oxidation of Parts-per-Million Concentrations of Nitric Oxide in the Presence of Water Vapour. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 1970. [Google Scholar]
- Government, N. Air Quality Data Services: Data Download Facility. 2024. Available online: https://www.airquality.nsw.gov.au/air-quality-data-services/data-download-facility (accessed on 3 February 2024).
- AS/NZS 1580.101.1:1999; Paints and Related Materials—Methods of Test Conditions of Test—Temperature, Humidity and Airflow Control. Standards Australia: Sydney, Australia, 1999.
- AS/NZS 3580.5.1-2011; Methods for Sampling and Analysis of Ambient Air Determination of Oxides of Nitrogen—Direct-reading Instrumental Method. SAI Global: Sydney, Australia, 2011.
- BS EN 14211:2012; Ambient Air. Standard Method for the Measurement of the Concentration of Nitrogen Dioxide and Nitrogen Monoxide by Chemiluminescence. British Standards Institute: London, UK, 2012.
- Department of Climate Change, Energy, the Environment and Water. National Environmental Protection (Ambient Air Quality) Measure. Available online: https://www.nepc.gov.au/nepms/ambient-air-quality (accessed on 3 February 2022).
- Department of Planning Industry and Environment. NSW Compliance Report 2020. National Environment Protection (Ambient Air Quality) Measure 2021. Available online: https://www.environment.nsw.gov.au/research-and-publications/publications-search/new-south-wales-annual-compliance-report-2020 (accessed on 3 February 2024).
- Mead, M.I.; Popoola, O.A.M.; Stewart, G.B.; Landshoff, P.; Calleja, M.; Hayes, M.; Baldovi, J.J.; McLeod, M.W.; Hodgson, T.F.; Dicks, J.; et al. The use of electrochemical sensors for monitoring urban air quality in low-cost, high-density networks. Atmos. Environ. 2013, 70, 186–203. [Google Scholar] [CrossRef]
- Popoola, O.A.M.; Stewart, G.B.; Mead, M.I.; Jones, R.L. Development of a baseline-temperature correction methodology for electrochemical sensors and its implications for long-term stability. Atmos. Environ. 2016, 147, 330–343. [Google Scholar] [CrossRef]
- Lewis, A.C.; Lee, J.D.; Edwards, P.M.; Shaw, M.D.; Evans, M.J.; Moller, S.J.; Smith, K.R.; Buckley, J.W.; Ellis, M.; Gillot, S.R.; et al. Evaluating the performance of low cost chemical sensors for air pollution research. Faraday Discuss. 2016, 189, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Zajda, J.; Meyerhoff, M.E. Comparison of electrochemical nitric oxide detection methods with chemiluminescence for measuring nitrite concentration in food samples. Anal. Chim. Acta 2019, 1077, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.S.; Williams, L.R.; Lewis, D.K.; Magoon, G.R.; Onasch, T.B.; Kaminsky, M.L.; Worsnop, D.R.; Jayne, J.T. Use of electrochemical sensors for measurement of air pollution: Correcting interference response and validating measurements. Atmos. Meas. Tech. 2017, 10, 3575–3588. [Google Scholar] [CrossRef]
- Kamionka, M.; Breuil, P.; Pijolat, C. Calibration of a multivariate gas sensing device for atmospheric pollution measurement. Sens. Actuators B Chem. 2006, 118, 323–327. [Google Scholar] [CrossRef]
- Malings, C.; Tanzer, R.; Hauryliuk, A.; Kumar, S.P.N.; Zimmerman, N.; Kara, L.B.; Presto, A.A.; Subramanian, R. Development of a general calibration model and long-term performance evaluation of low-cost sensors for air pollutant gas monitoring. Atmos. Meas. Tech. 2019, 12, 903–920. [Google Scholar] [CrossRef]
- Zimmerman, N.; Presto, A.A.; Kumar, S.P.N.; Gu, J.; Hauryliuk, A.; Robinson, E.S.; Robinson, A.L.; Subramanian, R. A machine learning calibration model using random forests to improve sensor performance for lower-cost air quality monitoring. Atmos. Meas. Tech. 2018, 11, 291–313. [Google Scholar] [CrossRef]
- Wei, P.; Ning, Z.; Ye, S.; Sun, L.; Yang, F.; Wong, K.C.; Westerdahl, D.; Louie, P. Impact Analysis of Temperature and Humidity Conditions on Electrochemical Sensor Response in Ambient Air Quality Monitoring. Sensors 2018, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Rogulski, M.; Badyda, A.; Gayer, A.; Reis, J. Improving the Quality of Measurements Made by Alphasense NO2 Non-Reference Sensors Using the Mathematical Methods. Sensors 2022, 22, 3619. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.; Yee, L.H.; Maher, D.T. Low-Cost Nitric Oxide Sensors: Assessment of Temperature and Humidity Effects. Sensors 2022, 22, 9013. [Google Scholar] [CrossRef] [PubMed]
- Skalska, K.; Miller, J.; Ledakowicz, S. Kinetics of nitric oxide oxidation. Chem. Pap. 2010, 64, 269–272. [Google Scholar] [CrossRef]
- England, C.; Corcoran, W.H. The Rate and Mechanism of the Air Oxidation of Parts-per-Million Concentrations of Nitric Oxide in the Presence of Water Vapor. Ind. Eng. Chem. Fundam. 1975, 14, 55–63. [Google Scholar] [CrossRef]






| Component | Composition (mol/mole) |
|---|---|
| Nitrogen | 78.1% |
| Oxygen | 20.9% |
| Argon | 0.9% |
| Water | <25 ppm |
| [NO]/ppbV | [NO]/(mol L−1) | Rate Constant/(L2 mol−2 s−1) |
|---|---|---|
| 100 500 1000 1500 2000 | 4.09 × 10−9 2.04 × 10−8 4.09 × 10−8 6.13 × 10−8 8.18 × 10−8 | 1.37 × 104 1.72 × 104 1.52 × 104 1.22 × 104 7.95 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, S.M.; Yee, L.H.; Maher, D.T. Revisiting Atmospheric Oxidation Kinetics of Nitrogen Oxides: The Use of Low-Cost Electrochemical Sensors to Measure Reaction Kinetics. Reactions 2024, 5, 789-799. https://doi.org/10.3390/reactions5040040
Owen SM, Yee LH, Maher DT. Revisiting Atmospheric Oxidation Kinetics of Nitrogen Oxides: The Use of Low-Cost Electrochemical Sensors to Measure Reaction Kinetics. Reactions. 2024; 5(4):789-799. https://doi.org/10.3390/reactions5040040
Chicago/Turabian StyleOwen, Steven M., Lachlan H. Yee, and Damien T. Maher. 2024. "Revisiting Atmospheric Oxidation Kinetics of Nitrogen Oxides: The Use of Low-Cost Electrochemical Sensors to Measure Reaction Kinetics" Reactions 5, no. 4: 789-799. https://doi.org/10.3390/reactions5040040
APA StyleOwen, S. M., Yee, L. H., & Maher, D. T. (2024). Revisiting Atmospheric Oxidation Kinetics of Nitrogen Oxides: The Use of Low-Cost Electrochemical Sensors to Measure Reaction Kinetics. Reactions, 5(4), 789-799. https://doi.org/10.3390/reactions5040040







