Increased Yields of the Guanine Oxidative Damage Product Imidazolone Following Exposure to LED Light
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Reanalysis of Iz Generation Using Transilluminator
3.2. Analysis of Iz Yields Using LEDs at Various Light Intensities
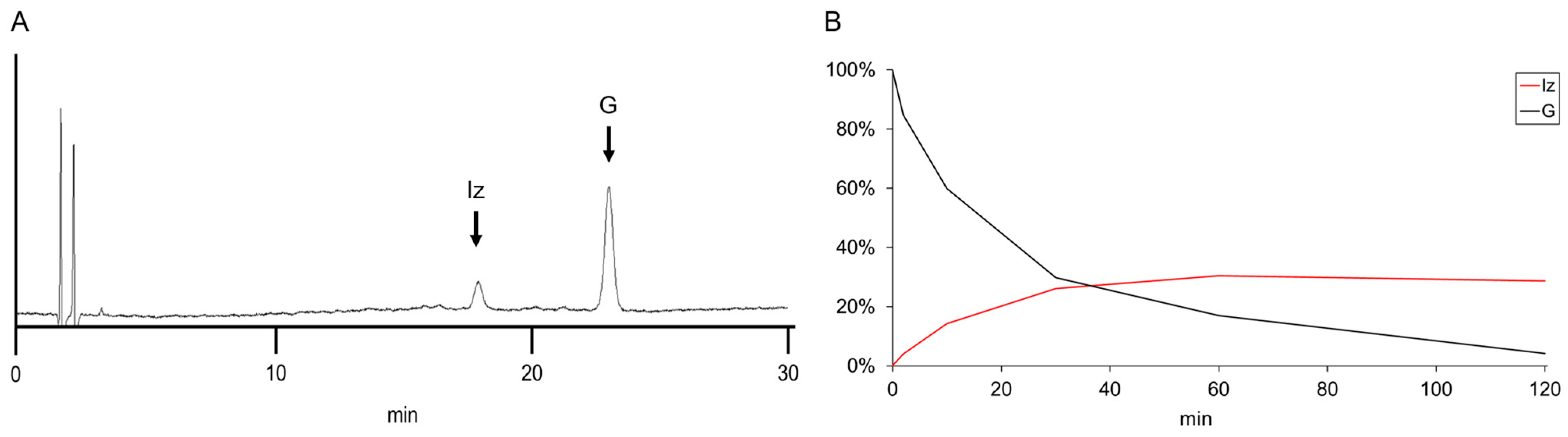
3.3. Analysis of the Efficiency of Iz Generation at Various LED Irradiation Times
3.4. Exploring Why the Yield of Iz Decreases at Higher Light Intensities or Longer Irradiation Times
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negishi, K.; Hao, W. Spectrum of mutations in single-stranded DNA phage M13mp2 exposed to sunlight: Predominance of G-to-C transversion. Carcinogenesis 1992, 13, 1615–1618. [Google Scholar] [CrossRef]
- Takimoto, K.; Tano, K.; Hashimoto, M.; Hori, M.; Akasaka, S.; Utsumi, H. Delayed transfection of DNA after riboflavin mediated photosensitization increases G:C to C:G transversions of supF gene in Escherichia coli mutY strain. Mutat. Res. 1999, 445, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Schulz, I.; Mahler, H.-C.; Boiteux, S.; Epe, B. Oxidative DNA base damage induced by singlet oxygen and photosensitization: Recognition by repair endonucleases and mutagenicity. Mutat. Res. 2000, 461, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Tano, K.; Iwamatsu, Y.; Yasuhira, S.; Utsumi, H.; Takimoto, K. Increased base change mutations at G:C pairs in Escherichia coli deficient in endonuclease III and VIII. J. Radiat. Res. 2001, 42, 409–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kino, K.; Miyazawa, H.; Sugiyama, H. User-friendly synthesis and photoirradiation of a flavin-linked oligomer. Genes Environ. 2007, 29, 23–28. [Google Scholar] [CrossRef]
- Mcbride, T.J.; Schneider, J.E.; Floyd, R.A.; Loeb, L.A. Mutations induced by methylene blue plus light in single-stranded M13mp2. Proc. Natl. Acad. Sci. USA 1992, 89, 6866–6870. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Negishi, K.; Hayatsu, H. Spectra of superoxide-induced mutations in the lacI gene of a wild-type and a mutM strain of Escherichia coli K-12. Mutat. Res. 1995, 326, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Macbride, T.J.; Preston, B.D.; Loeb, L.A. Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry 1991, 30, 207–213. [Google Scholar] [CrossRef]
- Akasaka, S.; Takimoto, K. Hydrogen peroxide induces G:C to T:A and G:C to C:G transversions in the supF gene of Escherichia coli. Mol. Gen. Genet. 1994, 243, 500–505. [Google Scholar] [CrossRef]
- Valentine, M.R.; Rodriguez, H.; Termini, J. Mutagenesis by peroxyradical is dominated by transversions at deoxyguanosine: Evidence for the lack of involvement of 8-oxo-dG and/or abasic site formation. Biochemistry 1998, 37, 7030–7038. [Google Scholar] [CrossRef]
- Emmert, S.; Epe, B.; Saha-Möller, C.R.; Adam, W.; Rünger, T.M. Assessment of genotoxicity and mutagenicity of 1,2-dioxetanes in human cells using a plasmid shuttle vector. Photochem. Photobiol. 1995, 61, 136–141. [Google Scholar] [CrossRef]
- Sargentini, N.J.; Smith, K.C. DNA sequence analysis of γ-radiation (anionic)-induced and spontaneous lacId mutations in Escherichia coli K-12. Mutat. Res. 1994, 309, 147–163. [Google Scholar] [CrossRef]
- Shin, C.Y.; Ponomareva, O.N.; Connolly, L.; Turker, M.S. A mouse kidney cell line with a G:C->C:G transversion mutator phenotype. Mutat. Res. 2002, 503, 69–76. [Google Scholar] [CrossRef]
- Cadet, J.; Mouret, S.; Ravanat, J.-L.; Douki, T. Photoinduced damage to cellular DNA: Direct and photosensitized reactions. Photochem. Photobiol. 2012, 88, 1048–1065. [Google Scholar] [CrossRef]
- Epe, B. DNA damage spectra induced by photosensitization. Photochem. Photobiol. Sci. 2012, 11, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, T.; Kaneko, S.; Hamamoto, K.; Yuasa, H. Mapping the diffusion pattern of 1O2 along DNA duplex by guanine photooxidation with an appended biphenyl photosensitizer. Sci. Rep. 2023, 13, 288. [Google Scholar] [CrossRef]
- Maisuls, I.; Cabrerizo, F.M.; David-Gara, P.M.; Epe, B.; Ruiz, G.T. DNA oxidation photoinduced by norharmane rhenium(I) polypyridyl complexes: Effect of the bidentate N,N′-ligands on the damage profile. Chem. Eur. J. 2018, 24, 12902–12911. [Google Scholar] [CrossRef]
- Hirakawa, K.; Okazaki, S.; Murakami, H.; Kanayama, N. Development of cancer-selective and effective photosensitizers through electron transfer mechanism. J. Jpn. Soc. Laser Surg. Med. 2021, 41, 349–355. [Google Scholar] [CrossRef]
- Kawai, K.; Osakada, Y.; Fujitsuka, M.; Majima, T. Consecutive adenine sequences are potential targets in photosensitized DNA damage. Chem. Biol. 2005, 12, 1049–1054. [Google Scholar] [CrossRef]
- Yun, B.H.; Dedon, P.C.; Geacintov, N.E.; Shafirovich, V. One-electron oxidation of a pyrenyl photosensitizer covalently attached to DNA and competition between its further oxidation and DNA hole injection. Photochem. Photobiol. 2010, 86, 563–570. [Google Scholar] [CrossRef]
- Dumont, E.; Monari, A. Understanding DNA under oxidative stress and sensitization: The role of molecular modeling. Front. Chem. 2015, 3, 43. [Google Scholar] [CrossRef]
- Aerssens, D.; Cadoni, E.; Tack, L.; Madder, A. A photosensitized singlet oxygen (1O2) toolbox for bio-organic applications: Tailoring 1O2 generation for DNA and protein labelling, targeting and biosensing. Molecules 2022, 27, 778. [Google Scholar] [CrossRef]
- Roberts, L.W.; Schuster, G.B. Synthesis and study of naphthacenedione (TQ) as a photosensitizer for one-electron oxidation of DNA. Org. Lett. 2004, 6, 3813–3816. [Google Scholar] [CrossRef]
- Kino, K.; Morikawa, M.; Kobayashi, T.; Kobayashi, T.; Komori, R.; Sei, Y.; Miyazawa, H. The oxidation of 8-oxo-7,8-dihydroguanine by iodine. Bioorg. Med. Chem. Lett. 2010, 20, 3818–3820. [Google Scholar] [CrossRef]
- Steenken, S.; Jovanovic, S.V. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 397–407. [Google Scholar] [CrossRef]
- Sugiyama, H.; Saito, I. Theoretical studies of GG-specific photocleavage of DNA via Electron Transfer: Significant lowering of ionization potential and 5′-localization of HOMO of stacked GG bases in B-form DNA. J. Am. Chem. Soc. 1996, 118, 7063–7068. [Google Scholar] [CrossRef]
- Saito, I.; Nakamura, T.; Nakatani, K.; Yoshioka, Y.; Yamaguchi, K.; Sugiyama, H. Mapping of the hot spots for DNA damage by one-electron oxidation: Efficacy of GG doublets and GGG triplets as a trap in long-range hole migration. J. Am. Chem. Soc. 1998, 120, 12686–12687. [Google Scholar] [CrossRef]
- Morikawa, M.; Kino, K.; Oyoshi, T.; Suzuki, M.; Kobayashi, T.; Miyazawa, H. Product analysis of photooxidation in isolated quadruplex DNA; 8-oxo-7,8-dihydroguanine and its oxidation product at 3′-G are formed instead of 2,5-diamino-4H-imidazol-4-one. RSC Adv. 2013, 3, 25694–25697. [Google Scholar] [CrossRef]
- Morikawa, M.; Kino, K.; Oyoshi, T.; Suzuki, M.; Kobayashi, T.; Miyazawa, H. Calculation of the HOMO localization of Tetrahymena and Oxytricha telomeric quadruplex DNA. Bioorg. Med. Chem. Lett. 2015, 25, 3359–3362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, R.; Nash, H.M.; Verdine, G.L. A mammalian DNA repair enzyme that excises oxidatively damaged guanines maps to a locus frequently lost in lung cancer. Curr. Biol. 1997, 7, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Maki, H.; Sekiguchi, M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 1992, 355, 273–275. [Google Scholar] [CrossRef]
- Brunner, S.D.; Norman, D.P.G.; Verdine, G.L. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 2000, 403, 859–866. [Google Scholar] [CrossRef]
- Grollman, A.P.; Moriya, M. Mutagenesis by 8-oxoguanine: An enemy within. Trends Genet. 1993, 9, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, L.A.; Peek, M.E.; Morningstar, M.L.; Verghis, S.M.; Miller, E.M.; Rich, A.; Essigmann, J.M.; Williams, L.D. X-ray structure of a DNA decamer containing 7,8-dihydro-8-oxoguanine. Proc. Natl. Acad. Sci. USA 1995, 92, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Moriya, M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-Oxoguanine in DNA induces targeted G:C->T:A transversions in simian kidney cells. Proc. Natl. Acad. Sci. USA 1993, 90, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Berger, M.; Buchko, G.W.; Joshi, P.C.; Raoul, S.; Ravanat, J.-L. 2,2-Diamino-4-[(3,5-di-O-acetyl-2-deoxy-L-D-erythro-pentofuranosyl)amino]-5-(2H)-oxazolone: A novel and predominant radical oxidation product of 3,5-di-O-acetyl-2-deoxyguanosine. J. Am. Chem. Soc. 1994, 116, 7403–7404. [Google Scholar] [CrossRef]
- Raoul, S.; Berger, M.; Buchko, G.W.; Joshi, P.C.; Morin, B.; Weinfeld, M.; Cadet, J. 1H, 13C and 15N Nuclear magnetic resonance analysis and chemical features of the two main radical oxidation products of 2-deoxyguanosine: Oxazolone and imidazolone nucleosides. J. Chem. Soc. Perkin Trans. 1996, 2, 371–381. [Google Scholar] [CrossRef]
- Duarte, V.; Muller, J.G.; Burrows, C.J. Insertion of dGMP and dAMP during in vitro DNA synthesis opposite an oxidized form of 7,8-dihydro-8-oxoguanine. Nucleic Acids Res. 1999, 27, 496–502. [Google Scholar] [CrossRef]
- Leipold, M.D.; Muller, J.G.; Burrows, C.J.; David, S.S. Removal of hydantoin products of 8-oxoguanine oxidation by the Escherichia coli. Biochemistry 2000, 39, 14984–14992. [Google Scholar] [CrossRef]
- Luo, W.; Muller, J.G.; Rachlin, E.M.; Burrows, C.J. Characterization of spiroiminodihydantoin as a product of one-electron oxidation of 8-oxo-7,8-dihydroguanosine. Org. Lett. 2000, 2, 613–616. [Google Scholar] [CrossRef]
- Kino, K.; Sugiyama, H. Possible cause of G-C-->C-G transversion mutation by guanine oxidation product, imidazolone. Chem. Biol. 2001, 8, 369–378. [Google Scholar] [CrossRef]
- Neeley, W.L.; Delaney, J.C.; Henderson, P.T.; Essigmann, J.M. In vivo bypass efficiencies and mutational signatures of the guanine oxidation products 2-aminoimidazolone and 5-guanidino-4-nitroimidazole. J. Biol. Chem. 2004, 279, 43568–43573. [Google Scholar] [CrossRef] [PubMed]
- Kino, K.; Sugasawa, K.; Mizuno, T.; Bando, T.; Sugiyama, H.; Akita, M.; Miyazawa, H.; Hanaoka, F. Eukaryotic DNA polymerases α, β and ε incorporate guanine opposite 2,2,4-triamino-5(2H)-oxazolone. ChemBioChem 2009, 10, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kino, K.; Kawada, T.; Morikawa, M.; Kobayashi, T.; Miyazawa, H. Analysis of nucleotide insertion opposite 2,2,4-triamino-5(2H)-oxazolone by eukaryotic B- and Y-family DNA polymerases. Chem. Res. Toxicol. 2015, 28, 1307–1316. [Google Scholar] [CrossRef]
- Kornyushyna, O.; Berges, A.M.; Muller, J.G.; Burrows, C.J. In vitro nucleotide misinsertion opposite the oxidized guanosine lesions spiroiminodihydantoin and guanidinohydantoin and DNA synthesis past the lesions using Escherichia coli DNA polymerase I (Klenow Fragment). Biochemistry 2002, 41, 15304–15314. [Google Scholar] [CrossRef]
- Henderson, P.T.; Delaney, J.C.; Muller, J.G.; Neeley, W.L.; Tannenbaum, S.R.; Burrows, C.J.; Essigmann, J.M. The hydantoin lesions formed from oxidation of 7,8-dihydro-8-oxoguanine are potent sources of replication errors in vivo. Biochemistry 2003, 42, 9257–9262. [Google Scholar] [CrossRef]
- Neeley, W.L.; Delaney, S.; Alekseyev, Y.O.; Jarosz, D.F.; Delaney, J.C.; Walker, G.C.; Essigmann, J.M. DNA polymerase V allows bypass of toxic guanine oxidation products in vivo. J. Biol. Chem. 2007, 282, 12741–12748. [Google Scholar] [CrossRef]
- Delaney, S.; Neeley, W.L.; Delaney, J.C.; Essigmann, J.M. The substrate specificity of MutY for hyperoxidized guanine lesions in vivo. Biochemistry 2007, 46, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Kino, K.; Kawada, T.; Hirao-Suzuki, M.; Morikawa, M.; Miyazawa, H. Products of oxidative guanine damage form base pairs with guanine. Int. J. Mol. Sci. 2020, 21, 7645. [Google Scholar] [CrossRef]
- McNally, A.; Prier, C.K.; MacMillan, D.W.C. Discovery of an α-amino C-H arylation reaction using the strategy of accelerated serendipity. Science 2011, 334, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Dong, V.M. An enlightening reactor. ACS Cent. Sci. 2017, 3, 526. [Google Scholar] [CrossRef]
- Lin, S.; Ischay, M.A.; Fry, C.G.; Yoon, T.P. Radical cation Diels-Alder cycloadditions by visible light photocatalysis. J. Am. Chem. Soc. 2011, 133, 19350–19353. [Google Scholar] [CrossRef]
- DiRocco, D.A.; Dykstra, K.; Krska, S.; Vachal, P.; Conway, D.V.; Tudge, M. Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew. Chem. Int. Ed. 2014, 53, 4802–4806. [Google Scholar] [CrossRef]
- Ito, E.; Fukushima, T.; Kawakami, T.; Murakami, K.; Itami, K. Catalytic dehydrogenative C-H imitation of arenas enabled by photo-generated hole donation to sulfonimide. Chem. 2017, 2, 383–392. [Google Scholar] [CrossRef]
- Zeitler, K. Photoredox catalysis with visible light. Angew. Chem. Int. Ed. 2009, 48, 9757–9987. [Google Scholar] [CrossRef]
- Patel, S.C.; Burns, N.Z. Conversion of aryl azides to aminopyridines. J. Am. Chem. Soc. 2022, 144, 17797–17802. [Google Scholar] [CrossRef]
- Woo, J.; Stein, C.; Christian, A.H.; Levin, M.D. Carbon-to-nitrogen single-atom transmutation of azaarenes. Nature 2023, 623, 77–82. [Google Scholar] [CrossRef]
- Kino, K.; Kobayashi, T.; Arima, E.; Komori, R.; Kobayashi, T.; Miyazawa, H. Photoirradiation products of flavin derivatives, and the effects of photooxidation on guanine. Bioorg. Med. Chem. Lett. 2009, 19, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Kino, K.; Nakatsuma, A.; Nochi, H.; Kiriyama, Y.; Kurita, T.; Kobayashi, T.; Miyazawa, H. Commentary on the phototoxicity and absorption of vitamin B2 and its degradation product, lumichrome. Pharm. Anal. Acta 2015, 6, 403. [Google Scholar]
- Ahmad, I.; Vaid, F.H.M. Flavins: Photochemistry and Photobilogy; Silva, E., Edwards, A.M., Eds.; RSC Publishing: Cambridge, UK, 2006; pp. 13–40. [Google Scholar]
- Posthuma, J.; Berends, W. Energy transfer in aqueous solution. Biochim. Biophys. Acta 1966, 112, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Penzer, G.R.; Radda, G.K. The chemistry and biological function of isoalloxazines (flavines). Q. Rev. Chem. Soc. 1967, 21, 43–65. [Google Scholar] [CrossRef]
- Gore, D.M.; Margineanu, A.; French, P.; O’Brart, D.; Dunsby, C.; Allan, B.D. Two-photon fluorescence microscopy of corneal riboflavin absorption. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2476–2481. [Google Scholar] [CrossRef]
- Zhang, Y.; Sukthankar, P.; Tomich, J.M.; Conrad, G.W. Effect of the synthetic NC-1059 peptide on diffusion of riboflavin across an intact corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Thakuri, P.S.; Joshi, R.; Basnet, S.; Pandey, S.; Taujale, S.D.; Mishra, N. Antibacterial photodynamic therapy on staphylococcus aureus and pseudomonas aeruginosa in-vitro. Nepal. Med. Coll. J. 2011, 13, 281–284. [Google Scholar] [PubMed]
- de Jesus, M.B.; Fraceto, L.F.; Martini, M.F.; Pickholz, M.; Ferreira, C.V.; de Paula, E. Non-inclusion complexes between riboflavin and cyclodextrins. J. Pharm. Pharmacol. 2012, 64, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. A DFT study on the degradation mechanism of vitamin B2. Food Chem. Mol. Sci. 2022, 4, 100080. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Abbas, S.H.; Anwar, Z.; Sheraz, M.A.; Ahmed, S.; Arsalan, A.; Bano, R. Stability-indicating photochemical method for the assay of riboflavin: Lumichrome Method. J. Chem. 2015, 2015, 256087. [Google Scholar] [CrossRef][Green Version]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef]
- Sheraz, M.; Kazi, S.; Ahmed, S.; Qadeer, K.; Khan, M.; Ahmad, I. Multicomponent spectrometric analysis of riboflavin and photoproducts and their kinetic applications. Open Chem. 2014, 12, 635–642. [Google Scholar] [CrossRef]
- McCormick, D.B. Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; International Life Sciences Institute: Washington, DC, USA, 2012; pp. 280–292. [Google Scholar]
- Chaudhuri, S.; Batabyal, S.; Polley, N.; Pal, S.K. Vitamin B2 in nanoscopic environments under visible light: Photosensitized antioxidant or phototoxic drug? J. Phys. Chem. A 2014, 118, 3934–3943. [Google Scholar] [CrossRef] [PubMed]
- Remucal, C.K.; McNeill, K. Photosensitized amino acid degradation in the presence of riboflavin and its derivatives. Environ. Sci. Technol. 2011, 45, 5230–5237. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawada, T.; Maehara, M.; Kino, K. Increased Yields of the Guanine Oxidative Damage Product Imidazolone Following Exposure to LED Light. Reactions 2023, 4, 801-810. https://doi.org/10.3390/reactions4040046
Kawada T, Maehara M, Kino K. Increased Yields of the Guanine Oxidative Damage Product Imidazolone Following Exposure to LED Light. Reactions. 2023; 4(4):801-810. https://doi.org/10.3390/reactions4040046
Chicago/Turabian StyleKawada, Taishu, Moka Maehara, and Katsuhito Kino. 2023. "Increased Yields of the Guanine Oxidative Damage Product Imidazolone Following Exposure to LED Light" Reactions 4, no. 4: 801-810. https://doi.org/10.3390/reactions4040046
APA StyleKawada, T., Maehara, M., & Kino, K. (2023). Increased Yields of the Guanine Oxidative Damage Product Imidazolone Following Exposure to LED Light. Reactions, 4(4), 801-810. https://doi.org/10.3390/reactions4040046





