One-Pot Reactions of Triethyl Orthoformate with Amines
Abstract
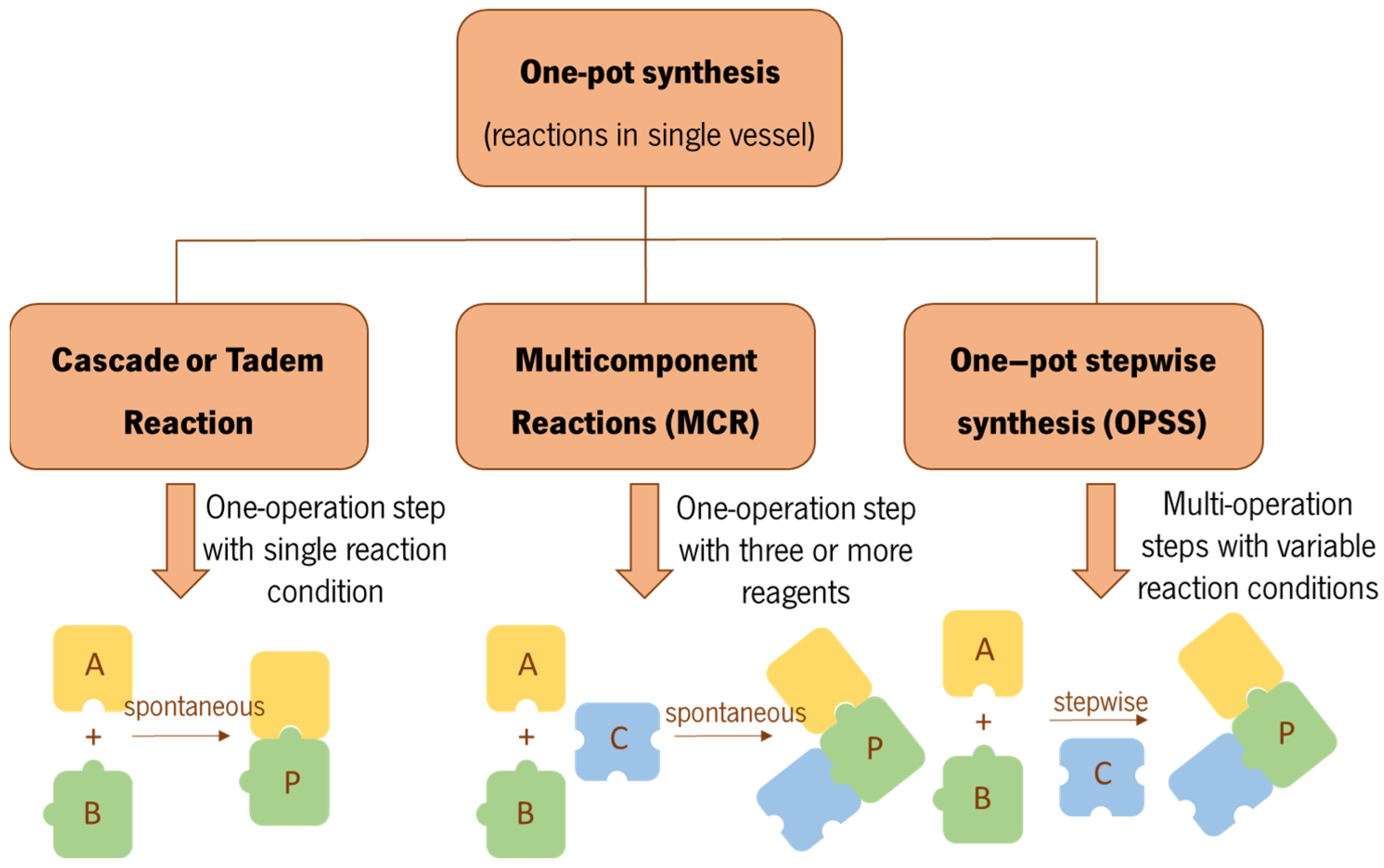
:1. Introduction
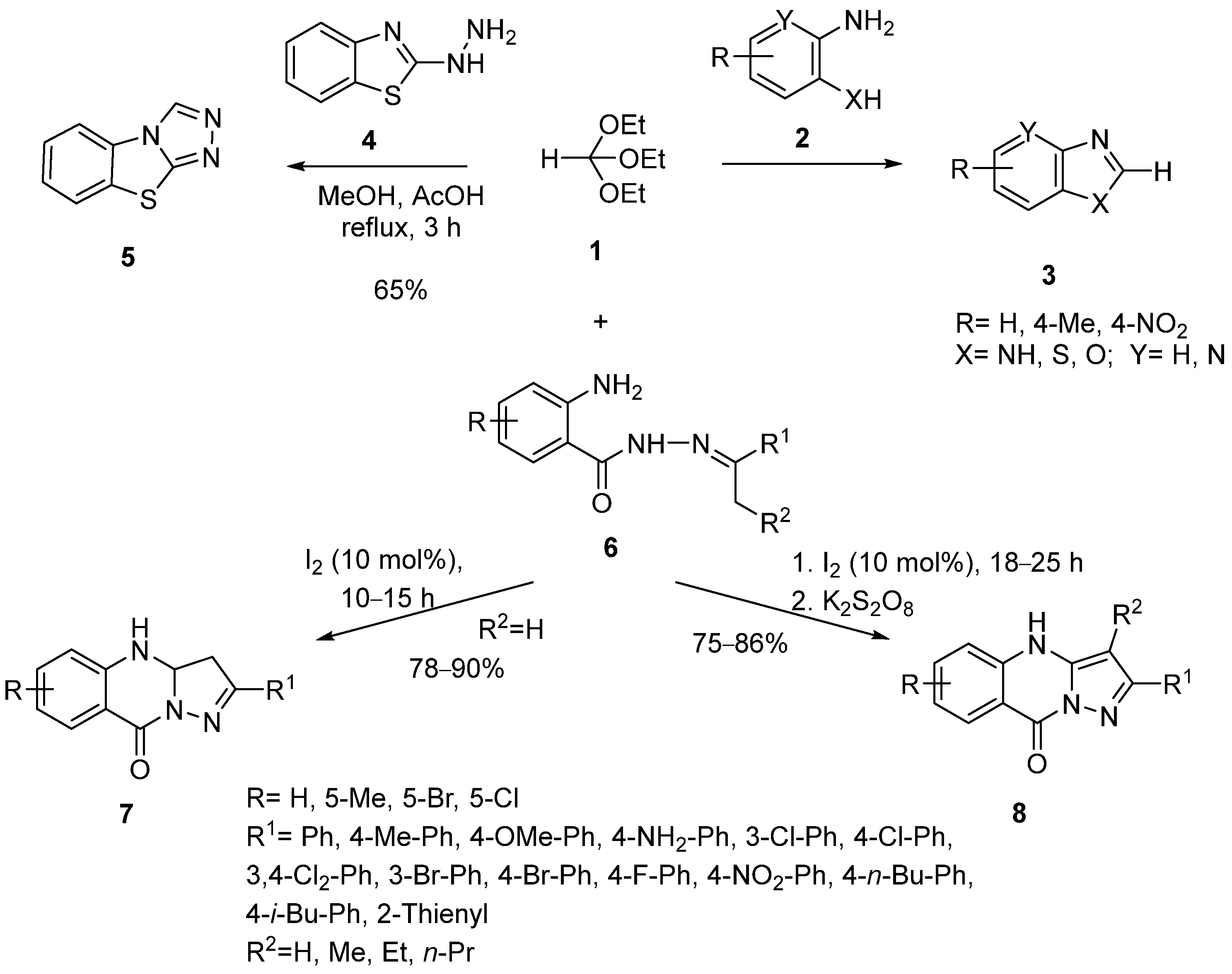
2. Synthesis by Two-Component Reaction
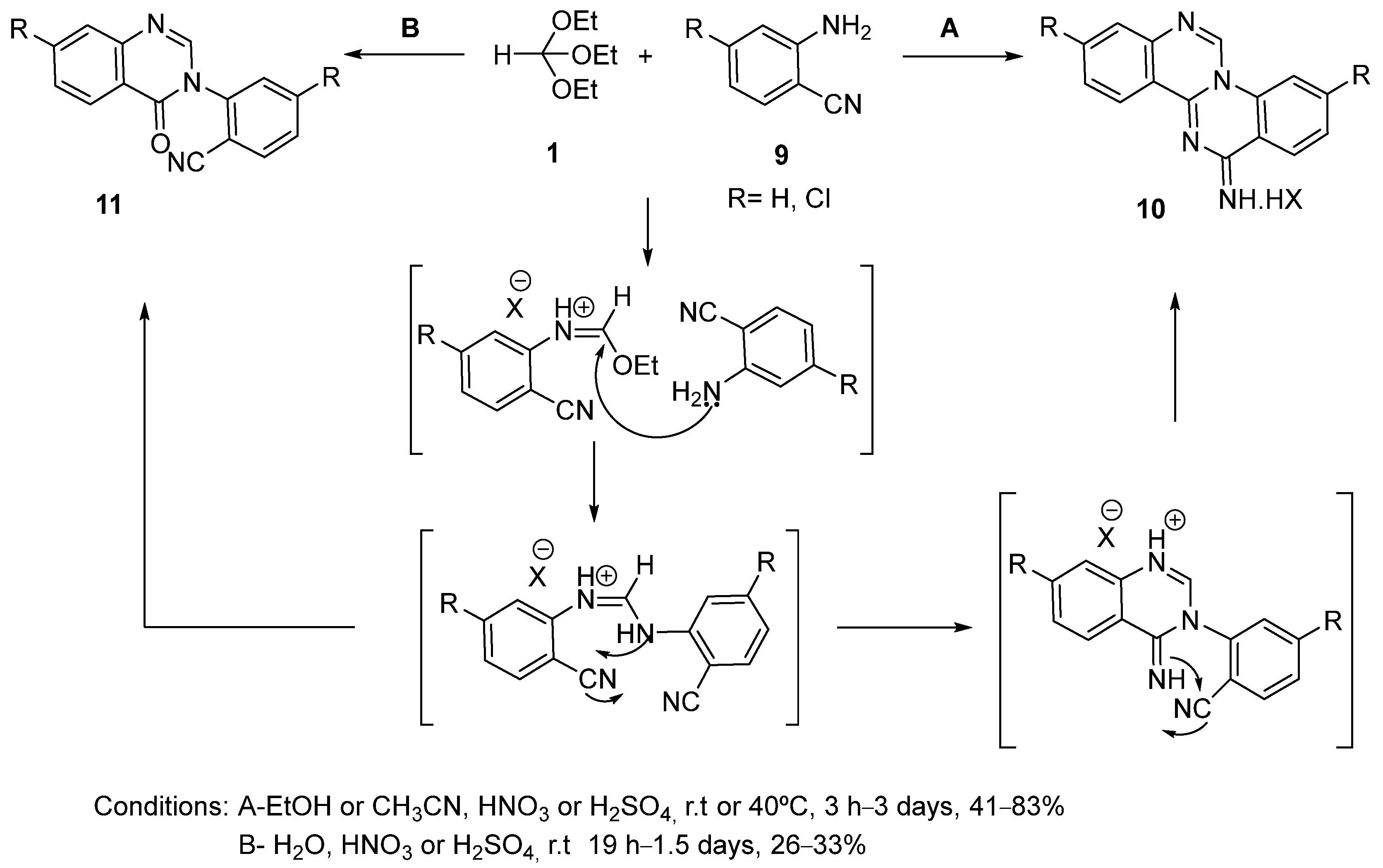
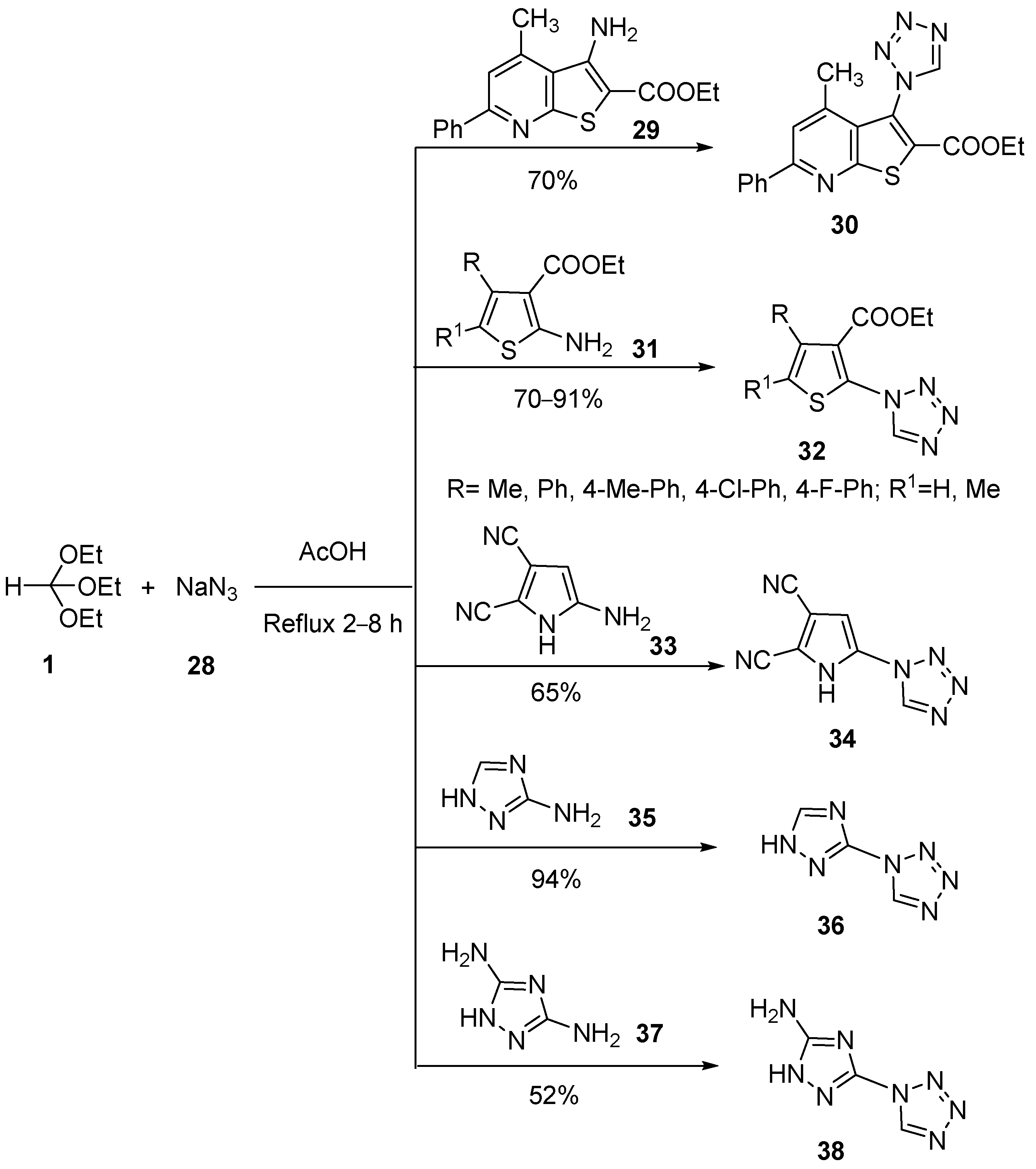
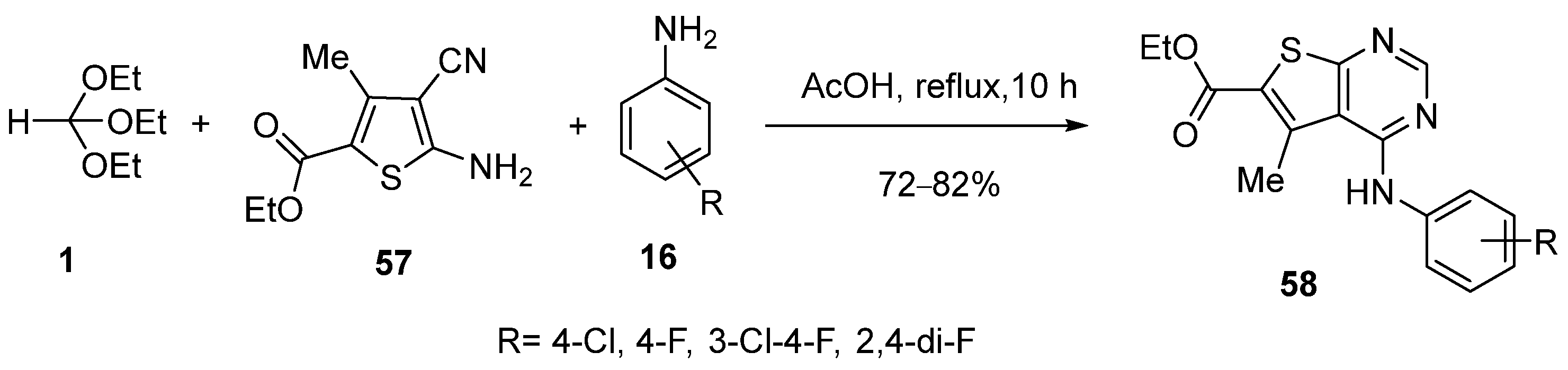
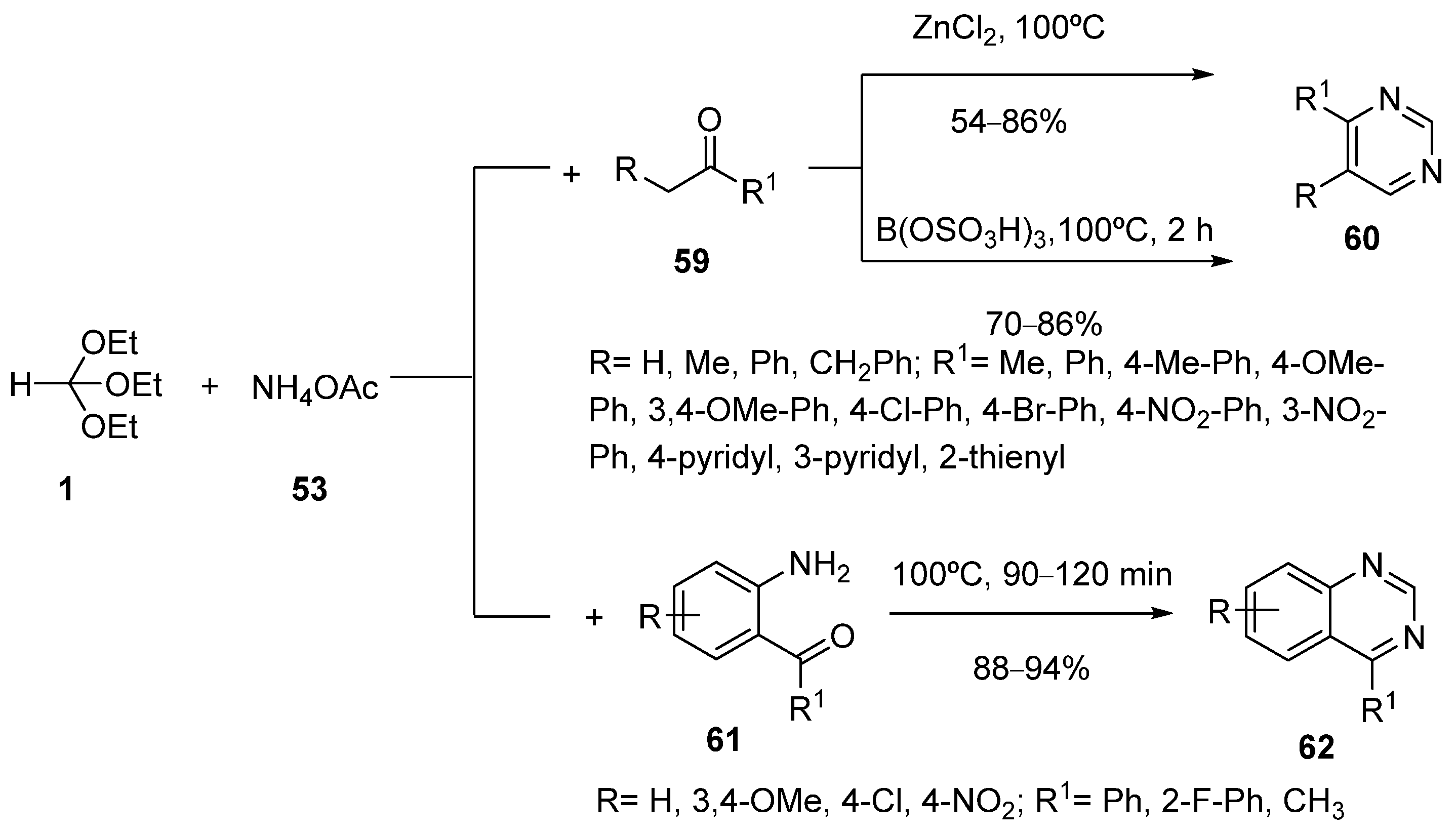
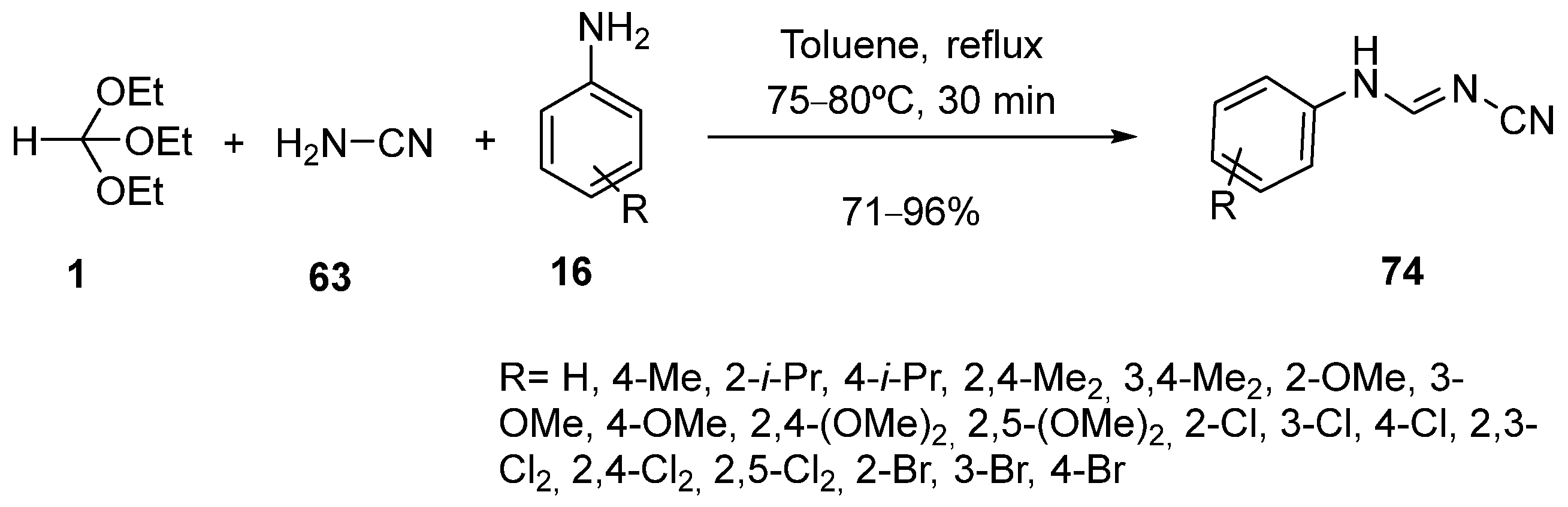
3. Synthesis by Three-Component Reaction
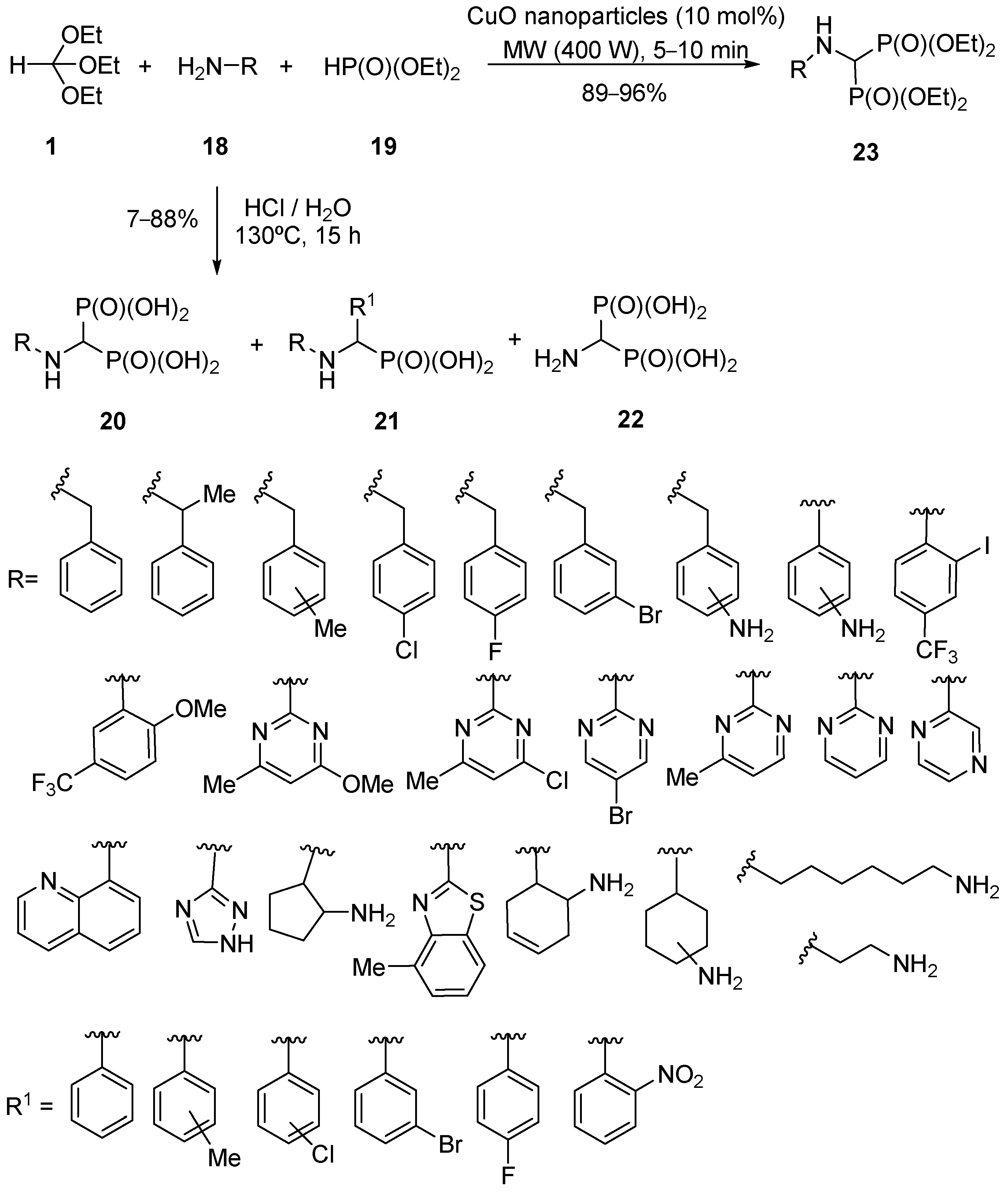
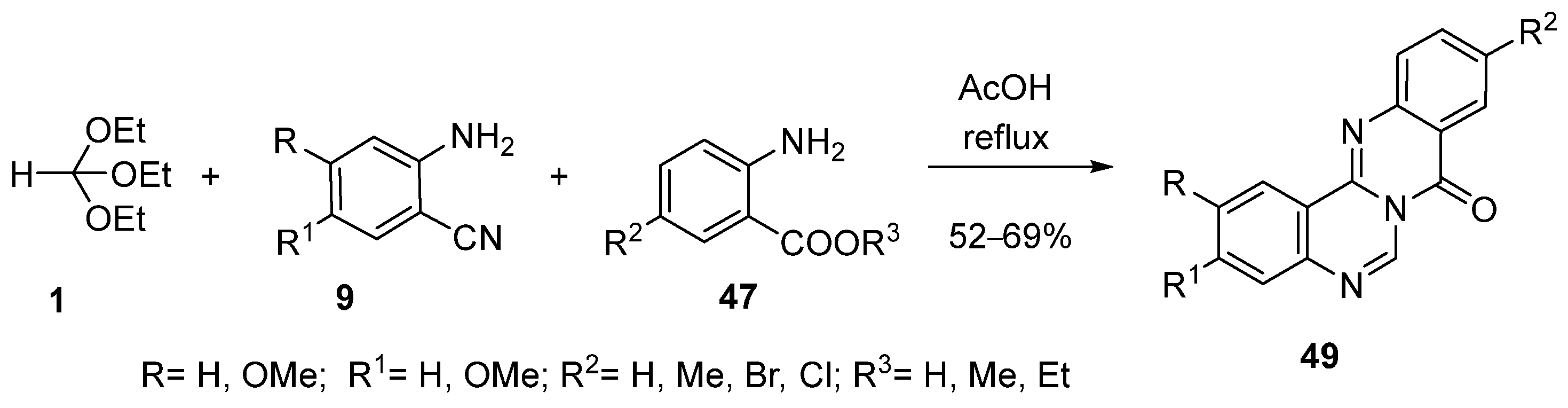
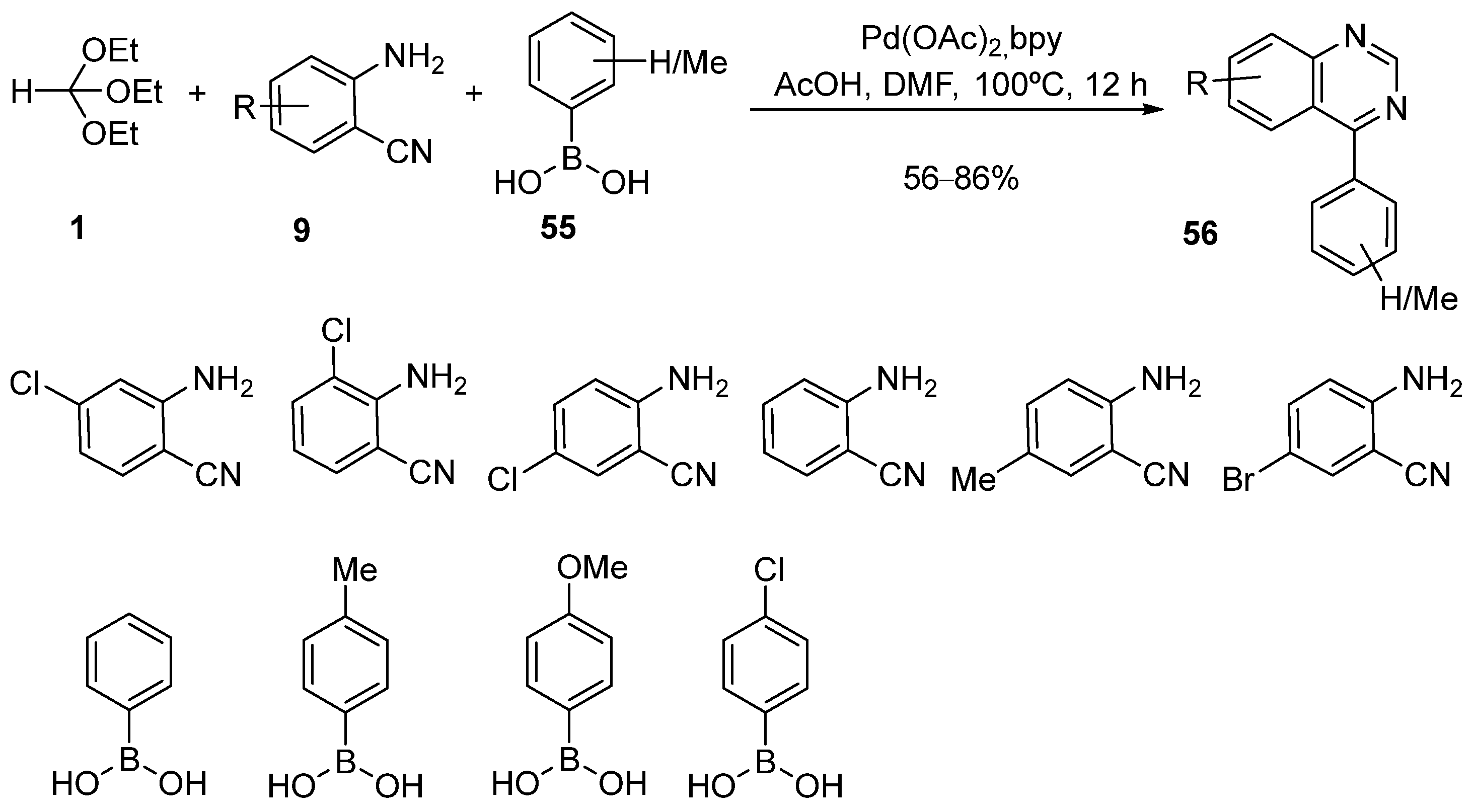
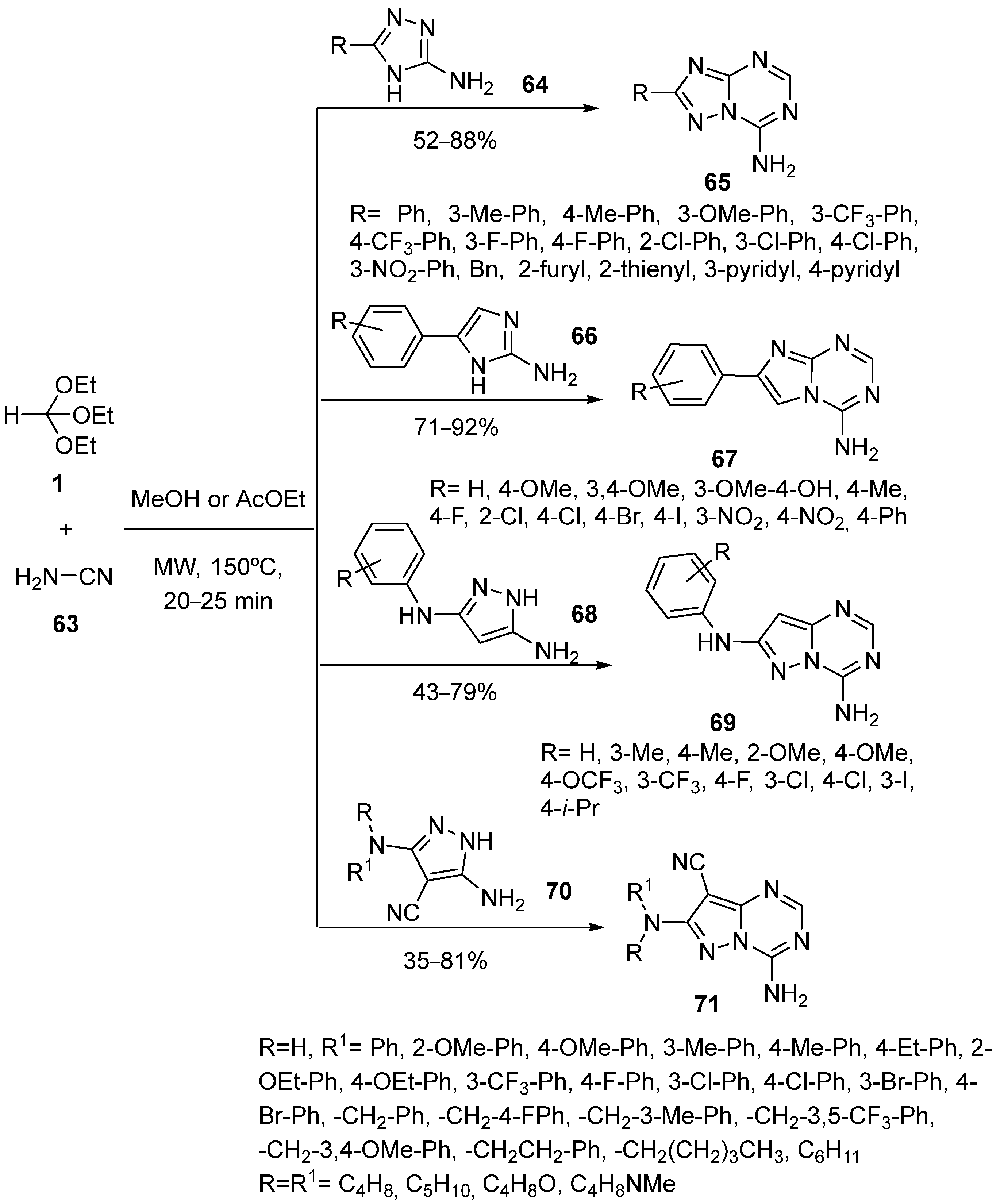
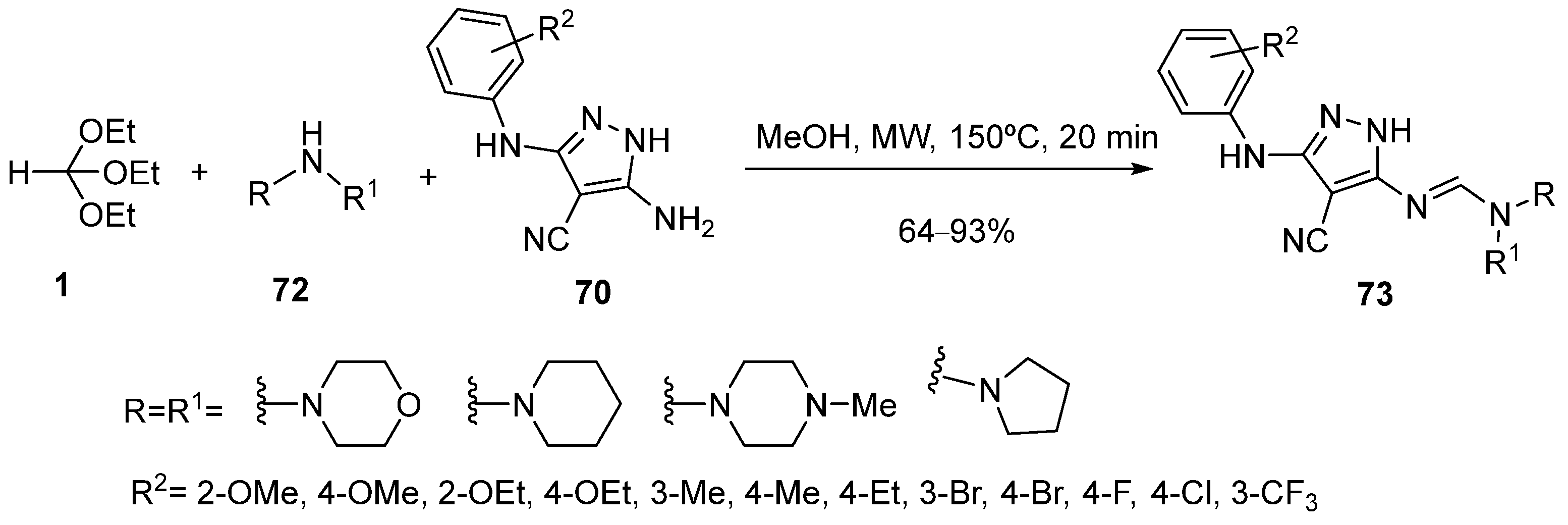
4. Synthesis by Four-Component Reaction
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Damera, T.; Pagadala, R.; Rana, S.; Jonnalagadda, S.B. A Concise Review of Multicomponent Reactions Using Novel Heterogeneous Catalysts under Microwave Irradiation. Catalysts 2023, 13, 1034. [Google Scholar] [CrossRef]
- Bai, H.; Sun, R.; Liu, S.; Yang, L.; Chen, X.; Huang, C. Construction of Fully Substituted 2-Pyridone Derivatives via Four-Component Branched Domino Reaction Utilizing Microwave Irradiation. J. Org. Chem. 2018, 83, 12535–12548. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef]
- Biesen, L.; Müller, T.J.J. Multicomponent and One-pot Syntheses of Quinoxalines. Adv. Synth. Catal. 2021, 363, 980–1006. [Google Scholar] [CrossRef]
- Shivam Tiwari, G.; Kumar, M.; Chauhan, A.N.S.; Erande, R.D. Recent advances in cascade reactions and their mechanistic insights: A concise strategy to synthesize complex natural products and organic scaffolds. Org. Biomol. Chem. 2022, 20, 3653–3674. [Google Scholar] [CrossRef]
- Brusa, A.; Iapadre, D.; Casacchia, M.E.; Carioscia, A.; Giorgianni, G.; Magagnano, G.; Pesciaioli, F.; Carlone, A. Acetaldehyde in the Enders triple cascade reaction via acetaldehyde dimethyl acetal. Beilstein J. Org. Chem. 2023, 19, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.G.; Zimmermann, S.; Kumar, K. Cascade reaction based synthetic strategies targeting biologically intriguing indole polycycles. Org. Biomol. Chem. 2019, 17, 413–431. [Google Scholar] [CrossRef]
- Bunce, R.A. Orthoesters in heterocycle synthesis. Arkivoc 2020, i, 400–436. [Google Scholar] [CrossRef]
- Marinho, E.; Araújo, R.; Proença, F. The reaction of anthranilonitrile and triethylorthoformate revisited: Formation of dimeric and trimeric species. Tetrahedron 2010, 66, 8681–8689. [Google Scholar] [CrossRef]
- Hamed, M.M.; Abou El Ella, D.A.; Keeton, A.B.; Piazza, G.A.; Engel, M.; Hartmann, R.W.; Abadi, A.H. Quinazoline and tetrahydropyridothieno[2,3-d]pyrimidine derivatives as irreversible EGFR tyrosine kinase inhibitors: Influence of the position 4 substituent. Med.Chem.Commun. 2013, 4, 1202–1207. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, X.-T.T.; Nguyen, T.-L.H.; Tran, P.H. Synthesis of Benzoxazoles, Benzimidazoles, and Benzothiazoles Using a Brønsted Acidic Ionic Liquid Gel as an Efficient Heterogeneous Catalyst under a Solvent-Free Condition. ACS Omega 2019, 4, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Bastug, G.; Eviolitte, C.; Markó, I.E. Functionalized orthoesters as powerful building blocks for the efficient preparation of heteroaromatic bicycles. Org. Lett. 2012, 14, 3502–3505. [Google Scholar] [CrossRef] [PubMed]
- Grieco, G.; Blacque, O.; Berke, H. A facile synthetic route to benzimidazolium salts bearing bulky aromatic N-substituents. Beilstein J. Org. Chem. 2015, 11, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Mazloumi, M.; Shirini, F.; Goli-Jolodar, O.; Seddighi, M. Nanoporous TiO2 containing an ionic liquid bridge as an efficient and reusable catalyst for the synthesis of N,N’-diarylformamidines, benzoxazoles, benzothiazoles and benzimidazoles. New J. Chem. 2018, 42, 5742–5752. [Google Scholar] [CrossRef]
- Al-Majidi, S.M.H. Synthesis of some new 4-oxo-thiazolidines, tetrazole and triazole derived from 2-SH-benzothiazole and antimicrobial screening of some Synthesized. J. Saudi Chem. Soc. 2014, 18, 893–901. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Zhang, M.-M.; Li, Y.-L.; Liu, Y.; Wang, X.-S. Iodine-catalyzed synthesis of 2-arylpyrazolo[5,1-b]quinazolin-9(3H)-one derivatives in ionic liquids via domino reaction. Tetrahedron 2014, 70, 3440–3446. [Google Scholar] [CrossRef]
- Szczepankiewicz, W.; Kuźnik, N. Synthesis of 3-arylquinazolin-4(3H)-imines from 2-amino-N′-arylbenzamidines and triethyl orthoformate. Tetrahedron Lett. 2015, 56, 1198–1199. [Google Scholar] [CrossRef]
- Gavin, J.T.; Annor-Gyamfi, J.K.; Bunce, R.A. Quinazolin-4(3H)-ones and 5,6-Dihydropyrimidin-4(3H)-ones from β-Aminoamides and Orthoesters. Molecules 2018, 23, 2925. [Google Scholar] [CrossRef]
- Haji, M. Multicomponent reactions: A simple and efficient route to heterocyclic phosphonates. Beilstein J. Org. Chem. 2016, 12, 1269–1301. [Google Scholar] [CrossRef]
- Miszczyk, P.; Turowska-Tyrk, I.; Kafarski, P.; Chmielewska, E. Three-Component Reaction of Benzylamines, Diethyl Phosphite and Triethyl Orthoformate: Dependence of the reaction Course on the Structural Features of the Substrates and Reaction Conditions. Molecules 2017, 22, 450. [Google Scholar] [CrossRef]
- Miszczyk, P.; Wieczorek, D.; Gałęzowska, J.; Dziuk, B.; Wietrzyk, J.; Chmielewska, E. Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities of the Products. Molecules 2017, 22, 254. [Google Scholar] [CrossRef] [PubMed]
- Petruczynik, P.; Kafarski, P.; Psurski, M.; Wietrzyk, J.; Kiełbowicz, Z.; Kuryszko, J.; Chmielewska, E. Three-Component Reaction of Diamines with Triethyl Orthoformate and Diethyl Phosphite and Anti-Proliferative and Antiosteoporotic Activities of the Products. Molecules 2020, 25, 1424. [Google Scholar] [CrossRef]
- Tellamekala, S.; Gundluru, M.; Sudileti, M.; Sarva, S.; Putta, C.R.K.; Cirandur, S.R. Green one-pot synthesis of N-bisphosphonates as antimicrobial and antioxidant agents. Monatshefte Chem. Chem. Mon. 2020, 151, 251–260. [Google Scholar] [CrossRef]
- Amira, A.; K’tir, H.; Aouf, Z.; Khaldi, T.; Bentoumi, H.; Khattabi, L.; Zerrouki, R.; Ibrahim-Ouali, M.; Aouf, N.-E. One-Pot Microwave-Assisted Synthesis, in Vitro Antiinflammatory Evaluation and Computer-Aided Molecular Design of Novel Sulfamide-Containing Bisphosphonates Derivatives. ChemistrySelect 2022, 7, e202201889. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef]
- Dhiman, N.; Kaur, K.; Jaitak, V. Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Bioorganic Med. Chem. 2020, 28, 115599. [Google Scholar] [CrossRef] [PubMed]
- Darvish, F.; Khazraee, S. FeCl3 Catalyzed One Pot Synthesis of 1-Substituted 1H-1,2,3,4-Tetrazoles under Solvent-Free Conditions. Int. J. Org. Chem. 2015, 5, 75–80. [Google Scholar] [CrossRef]
- Naeimi, H.; Mohamadabadi, S. Sulfonic acid-functionalized silica-coated magnetic nanoparticles as an efficient reusable catalyst for the synthesis of 1-substituted 1H-tetrazoles under solvent-free conditions. Dalton Trans. 2014, 43, 12967–12973. [Google Scholar] [CrossRef]
- Naeimi, H.; Kiani, F. Ultrasound-promoted one-pot three component synthesis of tetrazoles catalyzed by zinc sulfide nanoparticles as a recyclable heterogeneous catalyst. Ultrason. Sonochemistry 2015, 27, 408–415. [Google Scholar] [CrossRef]
- Naeimi, H.; Kiani, F.; Moradian, M. Rapid microwave promoted heterocyclization of primary amines with triethyl orthoformate and sodium azide using zinc sulfide nanoparticles as recyclable catalyst. Green Chem. Lett. Rev. 2018, 11, 361–369. [Google Scholar] [CrossRef]
- Khan, F.A.K.; Zaheer, Z.; Sangshetti, J.N.; Ahmed, R.Z. Facile one-pot synthesis, antibacterial activity and in silico ADME prediction of 1-substituted-1H-1,2,3,4-tetrazoles. Chem. Data Collect. 2018, 15–16, 107–114. [Google Scholar] [CrossRef]
- Salimi, M.; Zamanpour, A. Green synthesis of the 1-substituted 1H-1,2,3,4-tetrazoles over bifunctional catalyst based on copper intercalated into Mg/Al hydrotalcite modified magnetite nanoparticles. Appl. Organomet. Chem. 2020, 34, e5682. [Google Scholar] [CrossRef]
- Mashhoori, M.-S.; Sandaroos, R. New ecofriendly heterogeneous nano-catalyst for the synthesis of 1-substituted and 5-substituted 1H-tetrazole derivatives. Sci. Rep. 2022, 12, 15364. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.Y.; Sarg, M.T.; Said, M.M.; El-Sebaey, S.A. Utility of thieno[2,3-b] pyridine derivatives in the synthesis of some condensed heterocyclic compounds with expected biological activity. Univers. Org. Chem. 2013, 1, 2. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, M.D. New convenient synthesis of 2,3-diaminothieno[2,3-d] pyrimidin-4(3H)-one derivatives from substituted alkyl 2-(1H-tetrazol-1-yl)thiophene-3-carboxylates. Tetrahedron 2008, 64, 1430–1434. [Google Scholar] [CrossRef]
- Srinivas, D.; Ghule, V.D.; Muralidharan, K. Synthesis of nitrogen-rich imidazole, 1,2,4-triazole and tetrazole-based compounds. RSC Adv. 2014, 4, 7041–7051. [Google Scholar] [CrossRef]
- Nagaraju, K.; Lalitha, G.; Singh, P.; Rao, C.V. One-pot synthesis of 1-substituted 1H-1,2,3,4-tetrazoles from 2-aminothiazoles using tributylmethylammonium chloride as a catalyst. Heterocycl. Commun. 2017, 23, 365–368. [Google Scholar] [CrossRef]
- Marinho, E.; Proença, M.F. The Reaction of 2-(Acylamino)Benzonitriles with Primary Aromatic Amines: A Convenient Synthesis of 2-Substituted 4-(Arylamino)Quinazolines. Synthesis 2015, 47, 1623–1632. [Google Scholar] [CrossRef]
- Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf, Z.; Iqbal, J.; Saeed, A. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: An update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 2016, 24, 2361–2381. [Google Scholar] [CrossRef]
- Ismail, R.S.M.; Ismail, N.S.M.; Abuserii, S.; El Ella, D.A.A. Recent advances in 4-aminoquinazoline based scaffold derivatives targeting EGFR kinases as anticancer agents. Future J. Pharm. Sci. 2016, 2, 9–19. [Google Scholar] [CrossRef]
- Marinho, E.; Proença, M.F. Reactivity and regioselectivity in the acylation of 2,4-diaminoquinazolines. Tetrahedron 2016, 72, 4383–4389. [Google Scholar] [CrossRef]
- Khabnadideh, S.; Sadeghian, S. A Review on Current Synthetic Methods of 4-Aminoquinazoline Derivatives. J. Chem. 2022, 2022, 8424838. [Google Scholar] [CrossRef]
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases. Sci. Pharm. 2023, 91, 18. [Google Scholar] [CrossRef]
- Devi, P.; Srivastava, A.; Srivastava, K.; Bishnoi, A. Green approaches towards the synthesis of substituted quinazolines. Curr. Green Chem. 2017, 4, 25–37. [Google Scholar] [CrossRef]
- Das, D.; Hong, J. Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur. J. Med. Chem. 2019, 170, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Tamatam, R.; Kim, S.-H.; Shin, D. Transition-metal-catalyzed synthesis of quinazolines: A review. Front. Chem. 2023, 11, 1140562. [Google Scholar] [CrossRef] [PubMed]
- Gore, R.P.; Rajput, A.P. A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invent. Today 2013, 5, 148–152. [Google Scholar] [CrossRef]
- Habib, N.S.; Soliman, R.; El-Tombary, A.A.; El-Hawash, S.A.; Shaaban, O.G. Synthesis and biological evaluation of novel series of thieno[2,3-d]pyrimidine derivatives as anticancer and antimicrobial agents. Med. Chem. Res. 2013, 22, 3289–3308. [Google Scholar] [CrossRef]
- Jing, X.-B.; Li, Z.; Pan, X.; Shi, Y.-C. A Novel Method for the Synthesis of 4(3H)-Quinazolinones. J. Chin. Chem. Soc. 2008, 55, 1145–1149. [Google Scholar] [CrossRef]
- Wu, L.; Ma, W.; Yang, L.; Yan, F. Silica-Supported Boron Trifluoride (BF3-SiO2): An Efficient, Environment Frendly and Recyclable Catalyst for The One-Pot Synthesis of 4(3H)-quinazolinones. Asian J. Chem. 2010, 22, 6053–6058. [Google Scholar]
- Nasreen, A.; Borik, R.M. Cobalt(II) Chloride Catalyzed one Pot Synthesis of 2-substituted and 3-substituted-4(3H)-Quinazolinones. Orient. J. Chem. 2014, 30, 761–768. [Google Scholar] [CrossRef]
- Kawade, D.S.; Chaudhari, M.A.; Gujar, J.B.; Shingare, M.S. Thiamine hydrochloride (vitamin B1) as an efficient catalyst for the synthesis of 4-(3H)-Quinazolinone derivatives using grinding method. Iran. J. Catal. 2016, 6, 313–318. [Google Scholar]
- Fan, Y.; Luo, F.; Su, M.; Li, Q.; Zhong, T.; Xiong, L.; Li, M.; Yuan, M.; Wang, D. Structure optimization, synthesis, and biological evaluation of 6-(2-amino-1H-benzo[d]imidazole-6-yl)-quinazolin-4(3H)-one derivatives as potential multi-targeted anticancer agents via Aurora A/PI3K/BRD4 inhibition. Bioorganic Chem. 2023, 132, 106352. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Satyanarayana, M.; Ravikiran, P.; Siddaiah, V. Reaction of Imidoformates with Anthranilates: Facile, One-Pot, Three-Component Synthesis of 8H-Quinazolino[4,3-b]quinazolin-8-ones. J. Heterocycl. Chem. 2013, 50, 1089–1093. [Google Scholar] [CrossRef]
- Zaman, A.U.; Khan, M.A.; Munawar, M.A.; Athar, M.M.; Pervaiz, M.; Pervaiz, A.; Mahmood, A. Microwave Assisted Gould-Jacobs Reaction for Synthesis of 3-Acetyl-4-hydroxyquinoline Derivatives. Asian J. Chem. 2015, 27, 2823–2826. [Google Scholar] [CrossRef]
- Bai, H.; Liu, F.; Wang, X.; Wang, P.; Huang, C. Three-Component One-Pot Approach to Highly Efficient and Sustainable Synthesis of the Functionalized Quinolones via Linear/Branched Domino Protocols, Key Synthetic Methods for the Floxacin of Quinolone Drugs. ACS Omega 2018, 3, 11233–11251. [Google Scholar] [CrossRef] [PubMed]
- Rad-Moghadam, K.; Samavi, L. One-pot Three-component Synthesis of 2-Substituted 4-Aminoquinazolines. J. Heterocycl. Chem. 2006, 43, 913–916. [Google Scholar] [CrossRef]
- Hussen, A.S.; Bagchi, S.; Sharma, A. Ammonium Chloride Assisted Microwave Mediated Domino Multicomponent Reaction: An Efficient and Sustainable Synthesis of Quinazolin-4(3H)-imines under Solvent Free Condition. ChemistrySelect 2019, 4, 10169–10173. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; He, C.; Zhang, G.; Yu, Y. Palladium(II)-Catalyzed Three-Component Tandem Cyclization Reaction for the One-Pot Assembly of 4-Arylquinazolines. Synthesis 2021, 53, 1356–1364. [Google Scholar] [CrossRef]
- Kotaiah, Y.; Harikrishna, N.; Nagaraju, K.; Rao, C.V. Synthesis and antioxidant activity of 1,3,4-oxadiazole tagged thieno[2,3-d]pyrimidine derivatives. Eur. J. Med. Chem. 2012, 58, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Sasada, T.; Kobayashi, F.; Sakai, N.; Konakahara, T. An Unprecedented Approach to 4,5-Disubstituted Pyrimidine Derivatives by a ZnCl2-Catalyzed Three-Component Coupling Reaction. Org. Lett. 2009, 11, 2161–2164. [Google Scholar] [CrossRef] [PubMed]
- Soheilizad, M.; Adib, M.; Sajjadifar, S. One-pot and three-component synthesis of substituted pyrimidines catalysed by boron sulfuric acid under solvent-free conditions. J. Chem. Res. 2014, 38, 524–527. [Google Scholar] [CrossRef]
- Bhat, S.I.; Das, U.K.; Trivedi, D.R. An Efficient Three-component, One-pot Synthesis of Quinazolines under Solvent-free and Catalyst-free Condition. J. Heterocycl. Chem. 2015, 52, 1253–1259. [Google Scholar] [CrossRef]
- Kalinina, S.A.; Kalinin, D.V.; Dolzhenko, A.V. A one-pot, three-component, microwave-promoted synthesis of 2-amino-substituted 7-amino-1,2,4-triazolo[1,5-a]-[1,3,5]triazines. Tetrahedron Lett. 2013, 54, 5537–5540. [Google Scholar] [CrossRef]
- Dolzhenko, A.V.; Kalinina, S.A.; Kalinin, D.V. A novel multicomponent microwave-assisted synthesis of 5-aza-adenines. RSC Adv. 2013, 3, 15850–15855. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Low, S.T.; Ho, E.L.K.; Halcovitch, N.R.; Tiekink, E.R.T.; Dolzhenko, A.V. A multicomponent reaction of 2-aminoimidazoles: Microwave-assisted synthesis of novel 5-aza-7-deaza-adenines. RSC Adv. 2017, 7, 51062–51068. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A new, one-pot, multicomponent synthesis of 5-aza-9-deaza-adenines under microwave irradiation. Tetrahedron Lett. 2014, 55, 5159–5163. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Tan, K.C.; Tiekink, E.R.T.; Dolzhenko, A.V. A synthesis of new 7-amino-substituted 4-aminopyrazolo[1,5-a][1,3,5]triazines via a selective three-component triazine ring annulation. Tetrahedron 2019, 75, 2322–2329. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A one-pot, three-component, microwave-assisted synthesis of novel 7-amino-substituted 4-aminopyrazolo[1,5-a][1,3,5]triazine-8-carbonitriles. Tetrahedron Lett. 2015, 56, 7016–7019. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A one-pot, three-component aminotriazine annulation onto 5-aminopyrazole-4-carbonitriles under microwave irradiation. Tetrahedron Lett. 2015, 56, 521–524. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A New, One-Pot, Multicomponent Synthesis of Bioactive N-Pyrazolylformamidines under Microwave Irradiation. Synthesis 2015, 48, 2423–2428. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Nardi, M.; Pasceri, R.; Procopio, A.; Russo, B. Development of one-pot three component reaction for the synthesis of N’-aryl-N-cyanoformamidines, essential precursors of formamidine pesticides family. Arab. J. Chem. 2016, 9, 32–37. [Google Scholar] [CrossRef]
- Siutkina, A.I.; Kalinina, S.; Liu, R.; Heitman, L.H.; Junker, A.; Daniliuc, C.G.; Kalinin, D.V. Microwave-Assisted Synthesis, Structure, and Preliminary Biological Evaluation of Novel 6-Methoxy-5,6-dihydro-5-azapurines. ACS Omega 2023, 8, 14097–14112. [Google Scholar] [CrossRef] [PubMed]
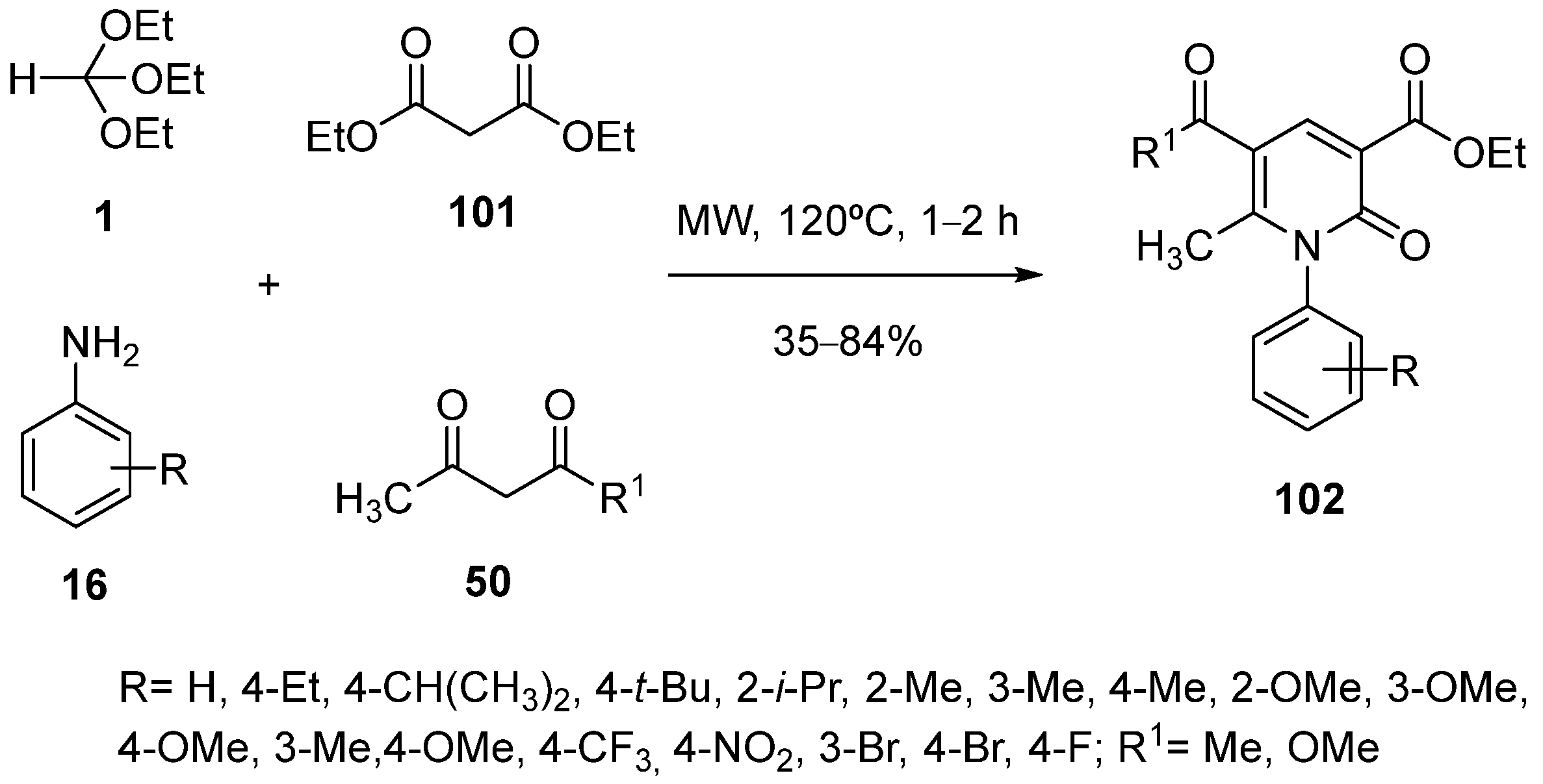
- Prior, A.M.; Gunaratna, M.J.; Kikuchi, D.; Desper, J.; Kim, Y.; Chang, K.-O.; Maezawa, I.; Jin, L.-W.; Hua, D.H. Syntheses of 3-[(Alkylamino)methylene]-6-methyl-1H-pyridine-2,4-diones, Fluorescence Probes 3-Substituted 7-Methyl-6H-pyrano[3,2-c]pyridine-2,5-diones, and Tetrahydro-6H-2,10-dioxa-9-azaanthracen-1-ones. Synthesis 2014, 46, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Olyaei, A.; Mohamadi, A.; Rahmani, N. Green synthesis of new lawsone enaminones and their Z/E(C=C)-isomerization induced by organic solvente. RSC Adv. 2021, 11, 12990–12994. [Google Scholar] [CrossRef]
- Hameed, A.M.A.; Nour-Eldin, A.M.; Ibrahim, M.M.; Sadek, K.U. Regioselectivity in the Multicomponent Reaction of 5-aminopyrazoles, Meldrum’s Acid and Triethyl Orthoformate. Am. Chem. Sci. J. 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Adnan, A.I.; Pungot, N.H.; Ash’ari, N.A.N. Convenient Synthesis of 5-arylidene Meldrum’s Acid Derivatives via Knoevenagel Condensation. J. Acad. 2021, 9, 80–84. [Google Scholar]
- Hussien, H.A.K.; Al-Messri, Z.A.K. Synthesis, Characterization and Evaluation of Some Meldrum’s Acid Derivatives as Lubricant Additives. Iraqi J. Sci. 2023, 64, 1041–1048. [Google Scholar] [CrossRef]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Potapov, A.Y.; Krysin, M.Y. Cascade two- and three-component cyclization reactions using 1,2-diamino-4-phenylimidazole and cyclohexane-1,3-diones. Chem. Heterocycl. Compd. 2014, 50, 1428–1433. [Google Scholar] [CrossRef]
- Kruzhilin, A.A.; Kosheleva, E.A.; Shikhaliev, K.S.; Denisov, G.L.; Vandyshev, D.Y. Regioselective Synthesis of Imidazo[1,5-b]pyridazines by Cascade Cyclizations of 1,2-Diamino-4H-phenylimidazole with 1,3-Diketones, Acetoacetic Ester and Their Derivatives. ChemistrySelect 2021, 6, 5801–5806. [Google Scholar] [CrossRef]
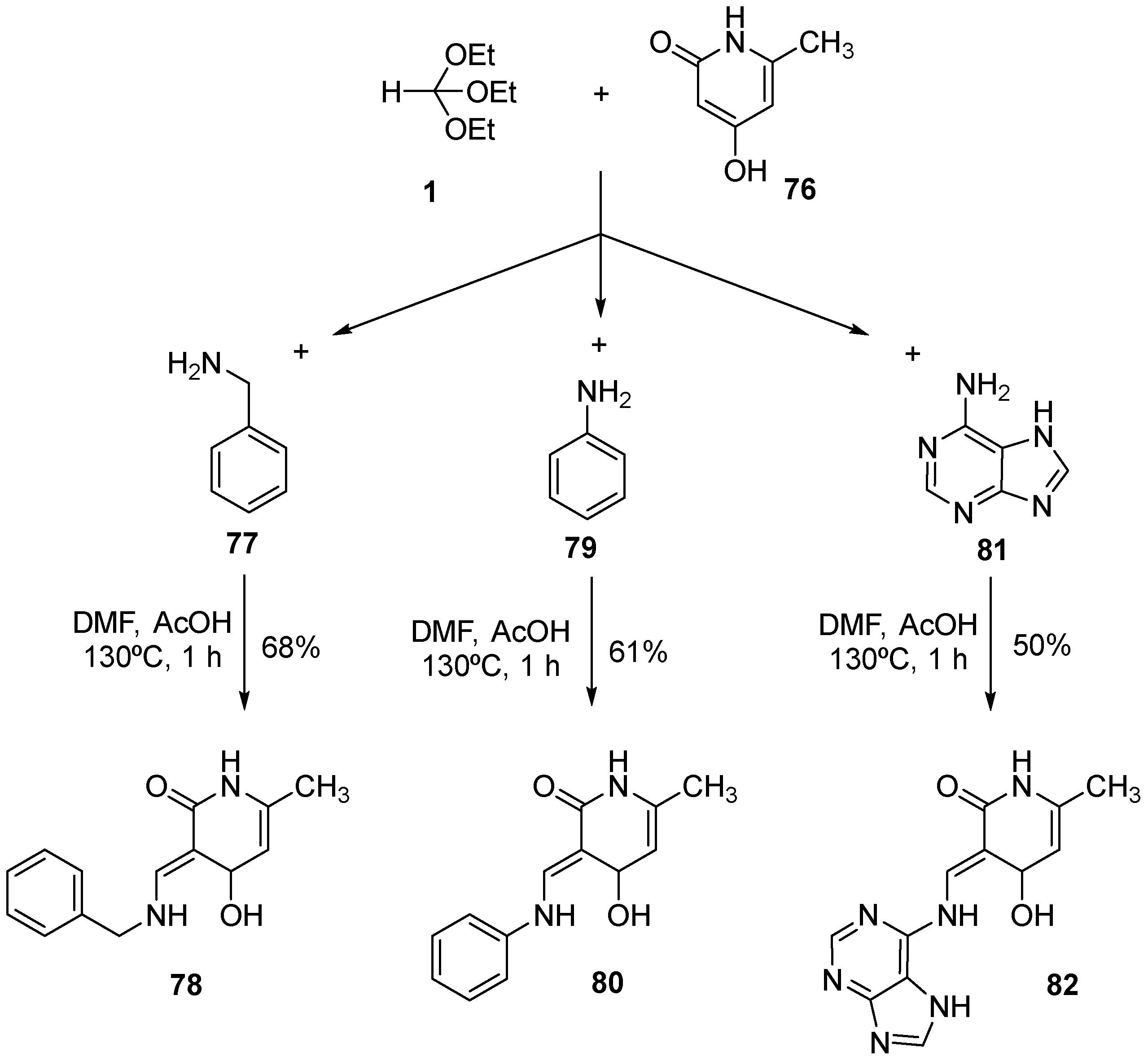
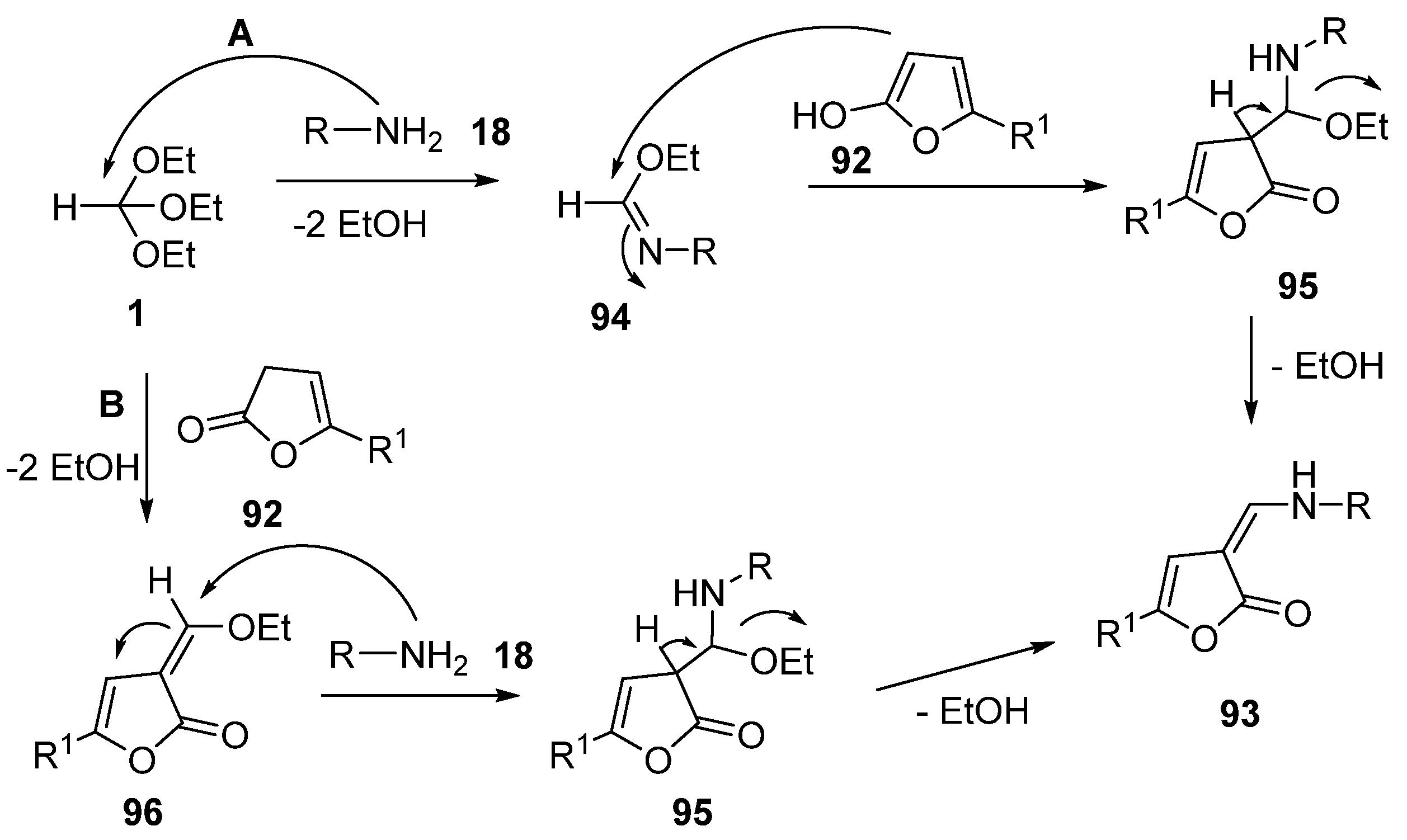
- Tikhomolova, A.S.; Grinev, V.S.; Yegorova, A.Y. One-Pot Synthesis, E-/Z-Equilibrium in Solution of 3-Hetarylaminomethylidenefuran-2(3H)-ones and the Way to Selective Synthesis of the E-Enamines. Molecules 2023, 28, 963. [Google Scholar] [CrossRef] [PubMed]
- Mehiaoui, N.; Hassaine, R.; Berrichi, A.; Kibou, Z.; Choukchou-Braham, N. Synthesis of Highly Heterocyclic Fluorescent Molecules: 2-imino-2H-pyrano[3,2-c] Pyridin-5(6H)-ones Derivatives. J. Fluoresc. 2023, 33, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
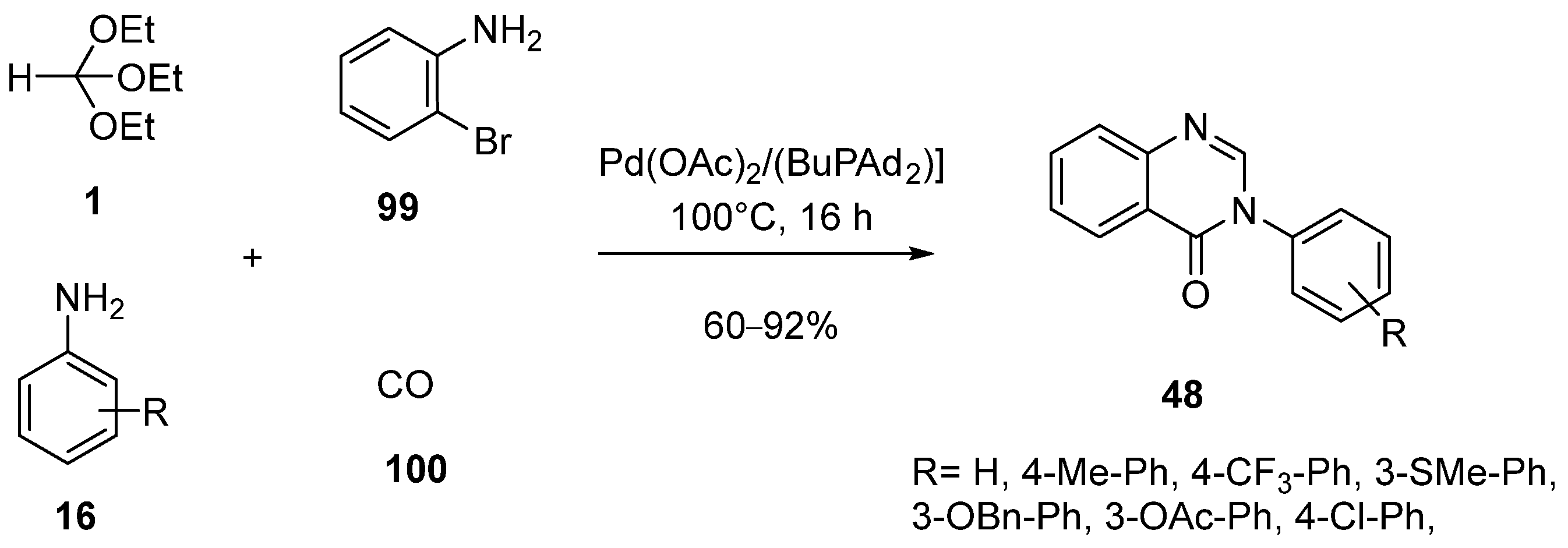
- He, L.; Li, H.; Neumann, H.; Beller, M.; Wu, X.-F. Highly efficient four-component synthesis of 4(3H)-Quinazolinones: Palladium-catalyzed carbonylative coupling reactions. Angew. Chem. Int. Ed. 2014, 53, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, S.; Yadav, N.; Kumar, R.; Chauhan, S.; Dhanda, V.; Walia, P.; Duhan, A. A score years’ update in the synthesis and biological evaluation of medicinally important 2-pyridones. Eur. J. Med. Chem. 2022, 232, 114199. [Google Scholar] [CrossRef]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinho, E. One-Pot Reactions of Triethyl Orthoformate with Amines. Reactions 2023, 4, 779-800. https://doi.org/10.3390/reactions4040045
Marinho E. One-Pot Reactions of Triethyl Orthoformate with Amines. Reactions. 2023; 4(4):779-800. https://doi.org/10.3390/reactions4040045
Chicago/Turabian StyleMarinho, Elina. 2023. "One-Pot Reactions of Triethyl Orthoformate with Amines" Reactions 4, no. 4: 779-800. https://doi.org/10.3390/reactions4040045
APA StyleMarinho, E. (2023). One-Pot Reactions of Triethyl Orthoformate with Amines. Reactions, 4(4), 779-800. https://doi.org/10.3390/reactions4040045





