Multicomponent Reactions Promoted by Ecocatalyst from Metal Hyperaccumulating Plant Pluchea sagittalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Preparation of the Catalysts
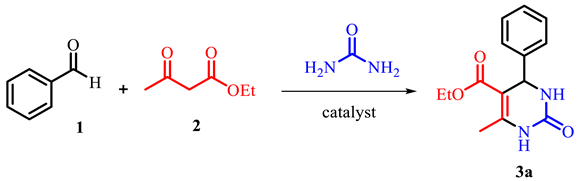
2.3. General Procedure for the Synthesis of Dihydropyrimidinones 3 (DHPMs)
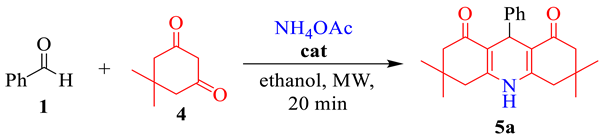
2.4. General Procedure for the Synthesis of 1,4-Dihydropyridines 5 (DHPs)
2.5. Recycling of the Cat B
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, Z.; Beach, E.S.; Anastas, P.T. Green chemistry in China. Pure Appl. Chem. 2011, 83, 1379–1390. [Google Scholar] [CrossRef]
- Sheldon, R.A. Engineering a more sustainable world through catalysis and green chemistry. J. R. Soc. Interface 2016, 13, 20160087. [Google Scholar] [CrossRef]
- Alponti, L.H.R.; Picinini, M.; Urquieta-Gonzales, E.A.; Corrêa, A.G. USY-zeolite catalyzed synthesis of 1,4-dihydropyridines under microwave irradiation: Structure and recycling of the catalyst. J. Mol. Struct. 2021, 1227, 129430. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Introduction: Green Chemistry and Catalysis. In Green Chemistry and Catalysis; Wiley-VCH Verlag: Weinheim, Germany, 2007; Chapter 1. [Google Scholar]
- Imamoto, T. Asymmetric Hydrogenation. In Hydrogenation; Karamé, I., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Devendar, P.; Qu, R.-Y.; Kang, W.-M.; He, B.; Yang, G.-F. Palladium-Catalyzed Cross-Coupling Reactions: A Powerful Tool for the Synthesis of Agrochemicals. J. Agric. Food Chem. 2018, 66, 8914–8934. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Resource Demand Scenarios for the Major Metals. Environ. Sci. Technol. 2018, 52, 2491–2497. [Google Scholar] [CrossRef]
- Patil, U.P.; Patil, S.S. Natural Feedstock in Catalysis: A Sustainable Route Towards Organic Transformations. Top. Curr. Chem. 2021, 379, 36. [Google Scholar] [CrossRef]
- Hechelski, M.; Ghinet, A.; Louvel, B.; Dufrénoy, P.; Rigo, B.; Daïch, A.; Waterlot, C. From Conventional Lewis Acids to Heterogeneous Montmorillonite K10: Eco-Friendly Plant-Based Catalysts Used as Green Lewis Acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef]
- Shikha, D.; Singh, P.K. In situ phytoremediation of heavy metal–contaminated soil and groundwater: A green inventive approach. Environ. Sci. Pollut. Res. 2021, 28, 4104–4124. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Ximba, B.J.; Fatoki, O.S.; Opeolu, B.O. Assessment of Phytoremediation Potential of Panicum maximum (Guinea Grass) for Selected Heavy Metal Removal from Contaminated Soils. Afr. J. Biotechnol. 2014, 13, 1979–1984. [Google Scholar] [CrossRef]
- Grison, C.; Escande, V.; Biton, J. Ecocatalysis: A New Integrated Approach to Scientific Ecology; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Grison, C.; Ki, Y.L.T. Ecocatalysis, a new vision of Green and Sustainable Chemistry. Curr. Opin. Green Sustain. Chem. 2021, 29, 100461. [Google Scholar] [CrossRef]
- Liu, H.; Ren, M.; Qu, J.; Feng, Y.; Song, X.; Zhang, Q.; Cong, Q.; Yuan, X. A cost-effective method for recycling carbon and metals in plants: Synthesizing nanomaterials. Environ. Sci. Nano 2017, 4, 461–469. [Google Scholar] [CrossRef]
- Harumain, Z.A.S.; Parker, H.L.; García, A.M.; Austin, M.J.; McElroy, C.R.; Hunt, A.J.; Clark, J.H.; Meech, J.A.; Anderson, C.W.N.; Ciacci, L.; et al. Toward Financially Viable Phytoextraction and Production of Plant-Based Palladium Catalysts. Environ. Sci. Technol. 2017, 51, 2992–3000. [Google Scholar] [CrossRef]
- Ruijter, E.; Orru, R.V. Multicomponent reactions—Opportunities for the pharmaceutical industry. Drug Discov. Today Technol. 2013, 10, e15. [Google Scholar] [CrossRef]
- Zarganes-Tzitzikas, T.; Domling, A. Modern multicomponent reactions for better drug syntheses. Org. Chem. Front. 2014, 1, 834–837. [Google Scholar] [CrossRef]
- Costanzo, P.; Nardi, M.; Oliverio, M. Similarity and Competition between Biginelli and Hantzsch Reactions: An Opportunity for Modern Medicinal Chemistry. Eur. J. Org. Chem. 2020, 2020, 3954–3964. [Google Scholar] [CrossRef]
- Patil, U.P.; Patil, R.C.; Patil, S.S. Biowaste-Derived Heterogeneous Catalyst for the One-Pot Multicomponent Synthesis of Diverse and Densely Functionalized 2-Amino-4H-Chromenes. Org. Prep. Proc. Int. 2021, 53, 190–199. [Google Scholar] [CrossRef]
- Jadhav, G.D.; Mujawar, T.A.P.; Tekale, S.U.; Pawar, R.P.; More, Y.W. Lemon Peel Powder: A Natural Catalyst for Multicomponent Synthesis of Coumarin Derivatives. Curr. Organocatal. 2020, 7, 140–148. [Google Scholar] [CrossRef]
- Patil, U.P.; Patil, R.C.; Patil, S.S. An Eco-friendly Catalytic System for One-pot Multicomponent Synthesis of Diverse and Densely Functionalized Pyranopyrazole and Benzochromene Derivatives. J. Heterocycl. Chem. 2019, 56, 1898–1913. [Google Scholar] [CrossRef]
- Grison, C.; Escande, V.; Petit, E.; Garoux, L.; Boulanger, C.; Grison, C. Psychotriadouarrei and Geissois pruinosa, novel resources for the plant-based catalytic chemistry. RSC Adv. 2013, 3, 22340–22345. [Google Scholar] [CrossRef]
- Escande, V.; Garoux, L.; Grison, C.M.; Thillier, Y.; Debart, F.; Vasseur, J.J.; Boulanger, C.; Grison, C. Ecological catalysis and phytoextraction: Symbiosis for future. Appl. Catal. B Environ. 2014, 146, 279–288. [Google Scholar] [CrossRef]
- Losfeld, G.; L’Huillier, L.; Fogliani, B.; Jaffré, T.; Grison, C. Mining in New Caledonia: Environmental stakes and restoration opportunities. Environ. Sci. Poll. Res. 2015, 22, 5592–5607. [Google Scholar] [CrossRef] [PubMed]
- Escande, V.; Renard, B.L.; Grison, C. Lewis acid catalysis and Green oxidations: Sequential tandem oxidation processes induced by Mn-hyperaccumulating plants. Environ. Sci. Poll. Res. 2015, 22, 5633–5652. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore Size Determination in Modified Micro- and Mesoporous Materials. Pitfalls and Limitations in Gas Adsorption Data Analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Chakraborti, A.; Roy, S.; Jadhavar, P.; Seth, K.; Sharma, K. Organocatalytic Application of Ionic Liquids: [bmim][MeSO4] as a Recyclable Organocatalyst in the Multicomponent Reaction for the Preparation of Dihydropyrimidinones and -thiones. Synthesis 2011, 24, 2261–2267. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Liu, F.; Zhao, X.; Xu, X.; An, Q.; Yun, K. Air-stable zirconium (IV)-salophen perfluorooctanesulfonate as a highly efficient and reusable catalyst for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under solvent-free conditions. Appl. Organomet. Chem. 2020, 34, e5454. [Google Scholar] [CrossRef]
- Shen, Y.-B.; Wang, G.-W. Solvent-free synthesis of xanthenediones and acridinediones. Arkivoc 2008, 16, 1–8. [Google Scholar] [CrossRef]
- Patil, M.; Karhale, S.; Kudale, A.; Kumbhar, A.; More, S.; Helavi, V. Green Protocol for the Synthesis of 1,8-Dioxo-Decahydroacridines by Hantzsch Condensation Using Citric Acid as Organocatalyst. Curr. Sci. 2019, 6, 936–942. [Google Scholar] [CrossRef]
- Navarro, C.A.; Sierra, C.A.; Ochoa-Puentes, C. Evaluation of sodium acetate trihydrate–urea DES as a benign reaction media for the Biginelli reaction. Unexpected synthesis of methylenebis(3-hydroxy-5,5-dimethylcyclohex-2-enones), hexahydroxanthene-1,8-diones and hexahydroacridine-1,8-diones. RSC Adv. 2016, 6, 65355–65365. [Google Scholar] [CrossRef]
- Chang, C.-C.; Cao, S.; Kang, S.; Kai, L.; Tian, X.; Pandey, P.; Silverman, R.B. Antagonism of 4-substituted 1,4-dihydropyridine-3,5-dicarboxylates toward voltage-dependent L-type Ca2+ channels CaV1.3 and CaV1.2. Bioorg. Med. Chem. 2010, 18, 3147–3158. [Google Scholar] [CrossRef]
- Teimouri, A.; Ghorbanian, L.; Moatari, A. A simple and efficient approach for synthesis of 1,4-dihydro-pyridines using nano-crystalline solid acid catalyst. Bull. Chem. Soc. Ethiop. 2013, 27, 427–437. [Google Scholar] [CrossRef]
- Carvalho, J.M.; Ramos, S.J.; Neto, A.E.; Gastauer, M.; Caldeira, C.F.; Siqueira, J.O.; Silva, M.L.S. Influence of nutrient management on growth and nutrient use efficiency of two plant species for mineland revegetation. Rest. Ecol. 2018, 26, 303–310. [Google Scholar] [CrossRef]
- Santana, B.V.N.; de Araújo, T.O.; Andrade, G.C.; de Freitas-Silva, L.; Kuki, K.N.; Pereira, E.G.; Azevedo, A.A.; da Silva, L.C. Leaf morphoanatomy of species tolerant to excess iron and evaluation of their phytoextraction potential. Environ. Sci. Pollut. Res. 2014, 21, 2550–2562. [Google Scholar] [CrossRef]
- Batista, A.A.; Santos, J.A.G.; Bomfim, M.R.; Moreira, F.M.; Leal, E.F.; da Conceicao, J.N. Induced changes in the growth of four plant species due to lead toxicity. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 327–332. [Google Scholar] [CrossRef]
- Rossato, L.V.; Nicoloso, F.T.; Farias, J.G.; Cargnelluti, D.; Tabaldi, L.A.; Antes, F.G.; Dressler, V.L.; Morsch, V.M.; Schetinger, M.R.C. Effects of lead on the growth, lead accumulation and physiological responses of Pluchea sagittalis. Ecotoxicology 2012, 21, 111–123. [Google Scholar]
- de Carvalho, C.F.M.; Viana, D.G.; Pires, F.R.; Egreja, F.B.; Bonomo, R.; Martins, L.F.; Cruz, L.B.S.; Nascimento, M.C.P.; Cargnelutti, A.; da Rocha, P.R. Phytoremediation of barium-affected flooded soils using single and intercropping cultivation of aquatic macrophytes. Chemosphere 2019, 214, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.L.; Hessevick, R.E.; Zoltai, T.; Finger, L.W. Refinement of the hematite structure. Am. Mineralog. 1966, 51, 123–129. [Google Scholar]
- Fiquet, G.; Richet, P.; Montagnac, G. High-temperature thermal expansion of lime, periclase, corundum and spinel. Phys. Chem. Miner. 1999, 27, 103–111. [Google Scholar] [CrossRef]
- Asbrink, S.; Norrby, L.J. A refinement of the crystal structure of copper (II) oxide with a discussion of some exceptional esd’s. Acta Cryst. 1970, B26, 8–15. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures, 2nd ed.; Interscience Publishers: New York, NY, USA, 1963; pp. 239–444. [Google Scholar]
- Hazen, R.M. Effects of temperature and pressure on the cell dimension and X-ray temperature factors of periclase. Am. Mineral. 1973, 61, 266–271. [Google Scholar]
- Fiameni, L.; Assi, A.; Fahimi, A.; Valentim, B.; Moreira, K.; Predeanu, G.; Slăvescu, V.; Vasile, B.Ş.; Nicoară, A.I.; Borgese, L.; et al. Simultaneous amorphous silica and phosphorus recovery from rice husk poultry litter ash. RSC Adv. 2021, 11, 8927–8939. [Google Scholar] [CrossRef]
- Stachel, D.; Svoboda, I.; Fuess, H. Phosphorus pentoxide at 233 K. Acta Cryst. 1995, C51, 1049–1050. [Google Scholar] [CrossRef]
- Levien, L.; Prewitt, C.T.; Weidner, D.J. Structure and elastic properties of quartz at pressure. Am. Mineral. 1980, 65, 920–930. [Google Scholar]
- Escande, V.; Petit, E.; Garoux, L.; Boulanger, C.; Grison, C. Switchable Alkene Epoxidation/Oxidative Cleavage with H2O2/NaHCO3: Efficient Heterogeneous Catalysis Derived from Biosourced Eco-Mn. ACS Sustain. Chem. Eng. 2015, 3, 2704–2715. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Dror, I.; Berkowitz, B. Atrazine degradation through PEI-copper nanoparticles deposited onto montmorillonite and sand. Sci. Rep. 2017, 7, 1415. [Google Scholar] [CrossRef]
- Marsh, A.; Heath, A.; Patureau, P.; Evernden, M.; Walker, P. Alkali activation behaviour of un-calcined montmorillonite and illite clay minerals. Appl. Clay Sci. 2018, 166, 250–261. [Google Scholar] [CrossRef]
- Escande, V.; Poullain, C.; Clavé, G.; Petit, E.; Masquelez, N.; Hesemann, P.; Grison, C. Bio-based and environmental input for transfer hydrogenation using EcoNi(0) catalyst in isopropanol. Appl. Catal. B Environ. 2017, 210, 495–503. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Mohajerani, B.; Heravi, M.M.; Ahmadi, A.N. Natural HEU type zeolite catalyzed Biginelli reaction for the synthesis of 3,4-dihydropyrimidin-2(1H) one derivatives. J. Mol. Catal. A 2005, 236, 216–219. [Google Scholar] [CrossRef]
- Lima, C.G.S.; Silva, S.I.R.M.; Gonçalves, R.H.; Leite, E.R.; Schwab, R.S.; Corrêa, A.G.; Paixão, M.W. Highly Efficient and Magnetically Recoverable Niobium Nanocatalyst for the Multicomponent Biginelli Reaction. ChemCatChem 2014, 6, 3455–3463. [Google Scholar] [CrossRef]
- Khasimbi, S.; Ali, F.; Manda, K.; Sharma, A.; Chauhan, G.; Wakode, S. Dihydropyrimidinones Scaffold as a Promising Nucleus for Synthetic Profile and Various Therapeutic Targets: A Review. Curr. Org. Synth. 2021, 18, 270–293. [Google Scholar] [CrossRef] [PubMed]
- Chopda, L.V.; Dave, N. Recent Advances in Homogeneous and Heterogeneous Catalyst in Biginelli Reaction from 2015-19: A Concise Review. ChemistrySelect 2020, 5, 5552–5572. [Google Scholar] [CrossRef]
- Kazemi, M. Magnetically reusable nanocatalysts in biginelli synthesis of dihydropyrimidinones (DHPMs). Synth. Commun. 2020, 50, 1409–1445. [Google Scholar] [CrossRef]
- Pawlowski, R.; Zaorska, E.; Staszko, S.; Szadkowska, A. Copper(I)-catalyzed multicomponent reactions in sustainable media: NHC copper complexes promote MCRs in sustainable solvents. Appl. Organomet. Chem. 2018, 32, e4256. [Google Scholar] [CrossRef]
- Anantha, I.S.S.; Kerru, N.; Maddila, S.; Jonnalagadda, S.B. Recent Progresses in the Multicomponent Synthesis of Dihydropyridines by Applying Sustainable Catalysts Under Green Conditions. Front. Chem. 2021, 9, 800236. [Google Scholar] [CrossRef]
- Sepehri, S.; Sanchez, H.P.; Fassihi, A. Hantzsch-Type Dihydropyridines and Biginelli-Type Tetra-hydropyrimidines: A Review of their Chemotherapeutic Activities. J. Pharm. Pharmaceut. Sci. 2015, 18, 1–52. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.M.; García, J.O.P.; Sánchez, L.R.B.; da Silva, M.G.C.; da Silva, V.L.; Arteaga-Pérez, L.E. Comprehensive Characterization of Sugarcane Bagasse Ash for its Use as an Adsorbent. Bioenergy Res. 2015, 8, 1885–1895. [Google Scholar] [CrossRef]
- Bentley, M.J.; Summers, R.S. Ash pretreatment of pine and biosolids produces biochars with enhanced capacity for organic micropollutant removal from surface water, wastewater, and stormwater. Environ. Sci. Water Res. Technol. 2020, 6, 635–644. [Google Scholar] [CrossRef]
- Khodamorady, M.; Sohrabnezhad, S.; Bahrami, K. Efficient One-Pot Synthetic Methods for the Preparation of 3,4-Dihydropyrimidinones and 1,4-Dihydropyridine Derivatives using BNPs@SiO2(CH2)3NHSO3H as a Ligand and Metal Free Acidic Heterogeneous Nano-catalyst. Polyhedron 2020, 178, 114340. [Google Scholar] [CrossRef]
- Mardani, F.; Khorshidi, A.; Gholampoor, S. Sulfonated Caspian Sea Sand: A Promising Heterogeneous Solid Acid Catalyst in Comparison with –SO3H Functionalized NiFe2O4@SiO2@KIT-6. ChemistrySelect 2019, 4, 8015–8020. [Google Scholar] [CrossRef]
- Wu, P.; Feng, L.; Liang, Y.; Zhang, X.; Mahmoudi, B.; Kazemnejadi, M. Magnetic Fe-C-O-Mo alloy nano-rods prepared from chemical decomposition of a screw (a top-down approach): An efficient and cheap catalyst for the preparation of dihydropyridine and dihydropyrimidone derivatives. Appl. Catal. Gen. A 2019, 590, 117301. [Google Scholar] [CrossRef]
- Chopda, L.V.; Dave, P.N. 12-Tungstosilicic Acid H4[W12SiO40] Over Natural Bentonite as a Heterogeneous Catalyst for the Synthesis of 3,4-dihydropyrimidin-2(1H)-ones. ChemistrySelect 2020, 5, 2395–2400. [Google Scholar] [CrossRef]
- Tayebee, R.; Ghadamgahi, M. Solvent free one-pot multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by mesoporous NH4H2PO4/MCM-41 as an environmentally friendly, cheap, and effective catalyst. Arab. J. Chem. 2017, 10, 757–764. [Google Scholar] [CrossRef]
- Saikia, M.; Bhuyan, D.; Saikia, L. Keggin type phosphotungstic acid encapsulated chromium (III) terephthalate metal organic framework as active catalyst for Biginelli condensation. Appl. Catal. Gen. 2015, 505, 501–506. [Google Scholar] [CrossRef]
- Kour, G.; Gupta, M.; Paul, S.; Rajnikant; Gupta, V.K. SiO2-CuCl2: An efficient and recyclable heterogeneous catalyst for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. J. Mol. Catal. A 2014, 392, 260–269. [Google Scholar] [CrossRef]
- Mustafa, A.; Siddiqui, Z.N. Silica-based ionic liquid supported on Xanthan [ImSi][PF6]@xanthan in the synthesis of acridine derivatives by multicomponent reaction. Sustain. Chem. Pharm. 2022, 29, 100775. [Google Scholar] [CrossRef]
- Pham, D.D.; Le, N.T.; Vo-Thanh, G. Fast and Efficient Hantzsch Synthesis Using Acid-Activated and Cation-Exchanged Montmorillonite Catalysts under Solvent-Free Microwave Irradiation Conditions. ChemistrySelect 2017, 2, 12041–12045. [Google Scholar] [CrossRef]
- Kumar, B.S.; Dhakshinamoorthy, A.; Pitchumani, K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396. [Google Scholar] [CrossRef]
- Bonacci, S.; Iriti, G.; Mancuso, S.; Novelli, P.; Paonessa, R.; Tallarico, S.; Nardi, M. Montmorillonite K10: An Efficient Organo- Heterogeneous Catalyst for Synthesis of Benzimidazole Derivatives. Catalysts 2020, 10, 845. [Google Scholar] [CrossRef]
- Safari, J.; Sadeghi, M. Montmorillonite K10: An effective catalyst for synthesis of 2-aminothiazoles. Res. Chem. Intermed. 2016, 42, 8175–8183. [Google Scholar] [CrossRef]
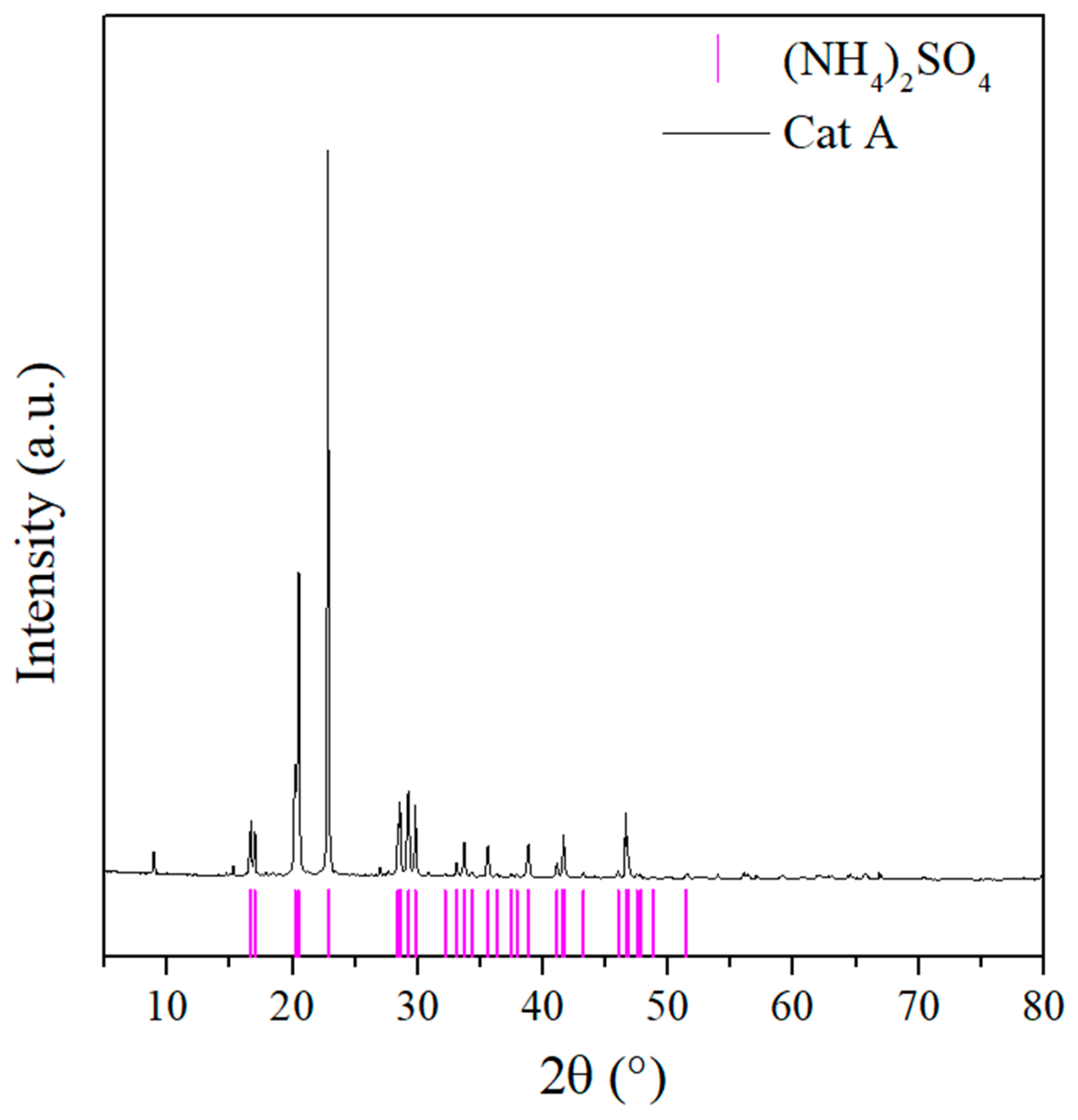
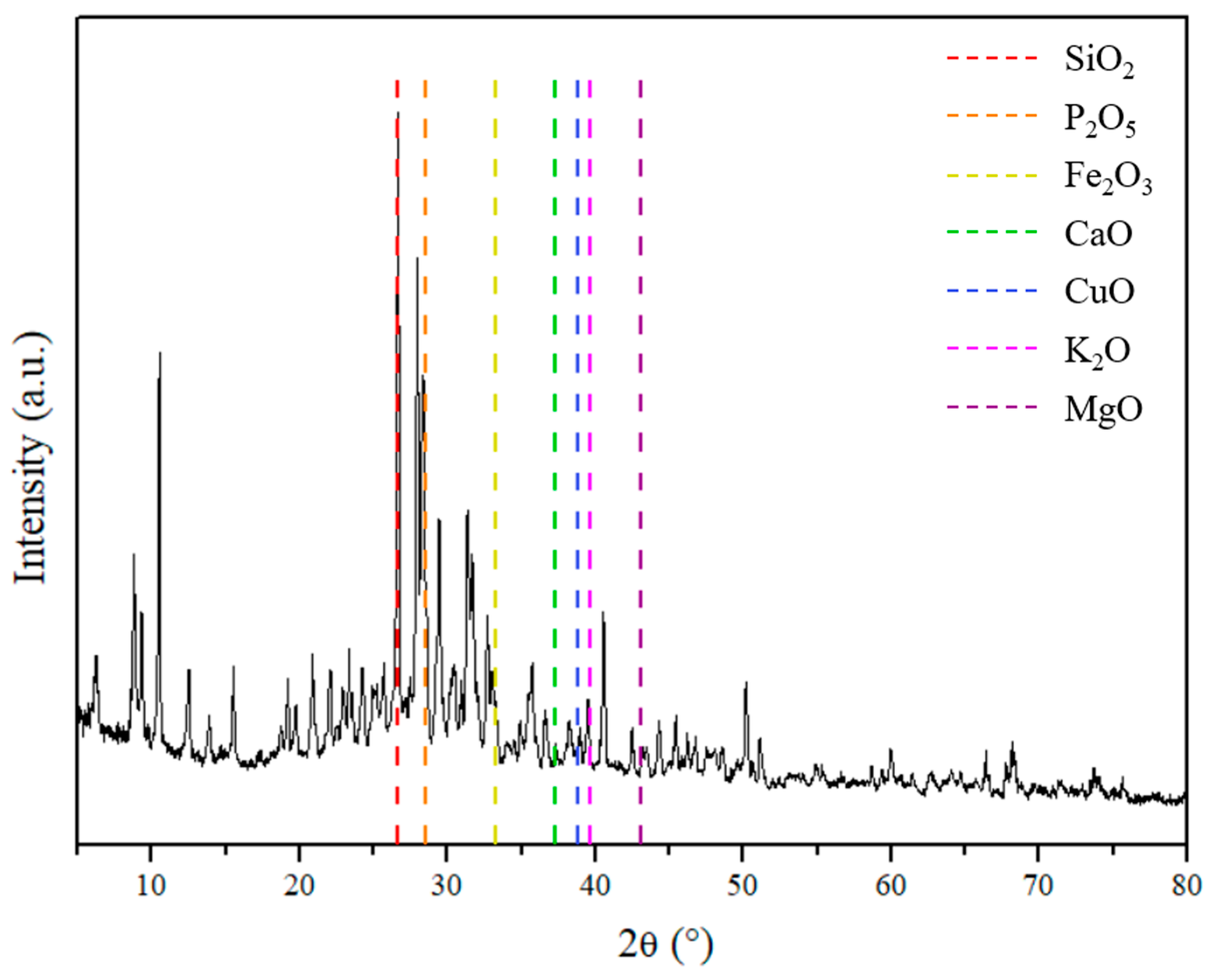

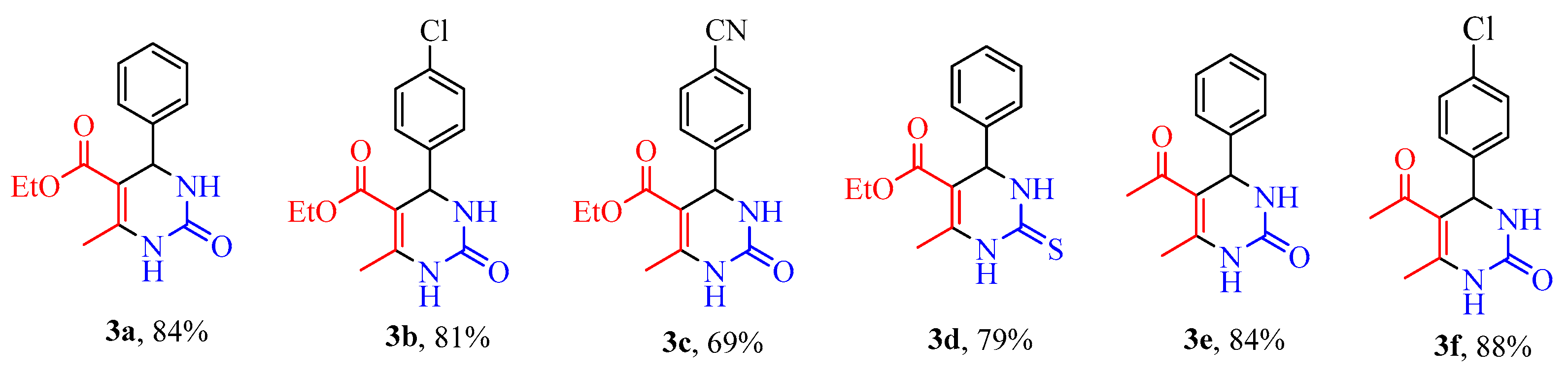


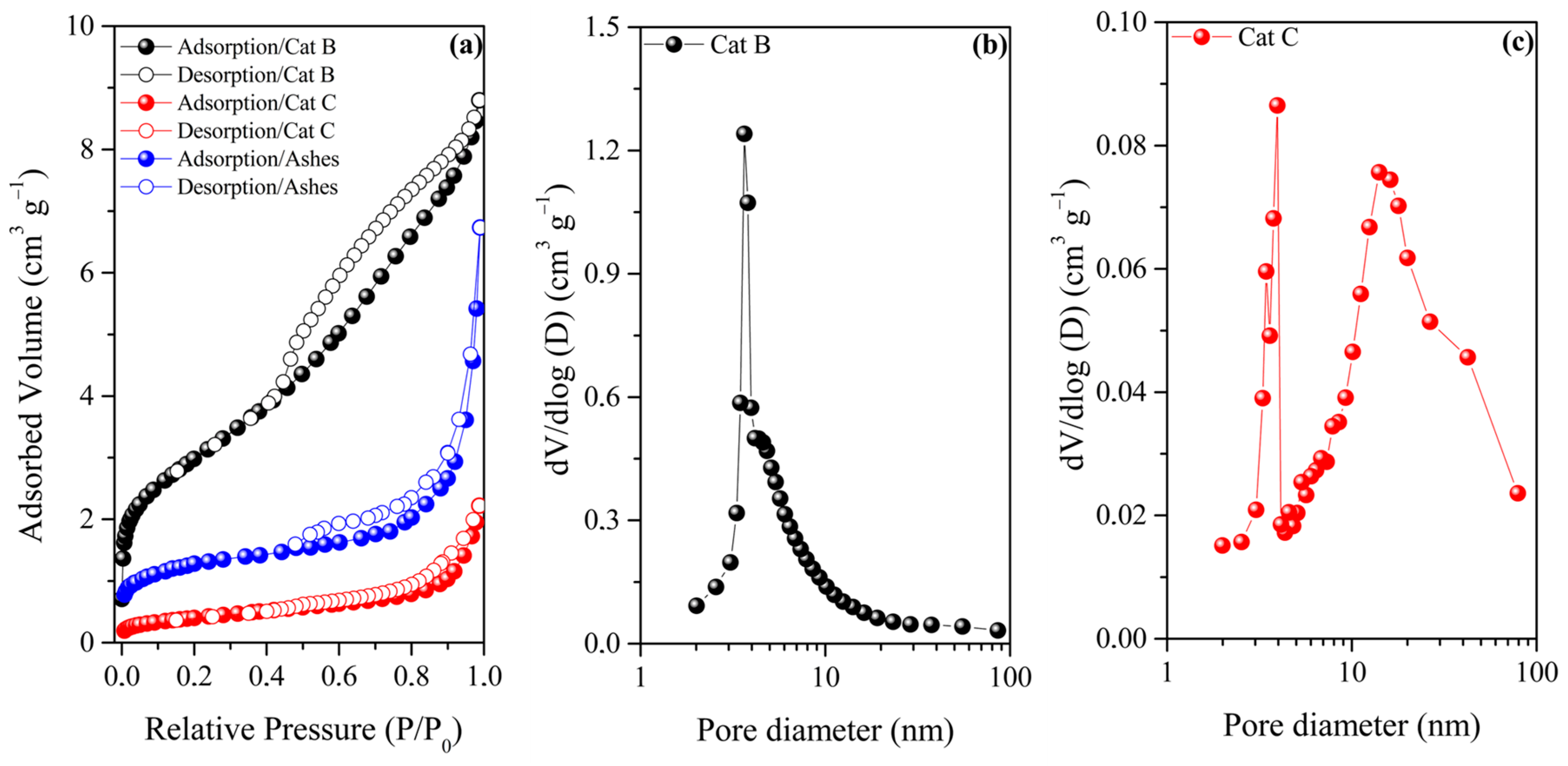
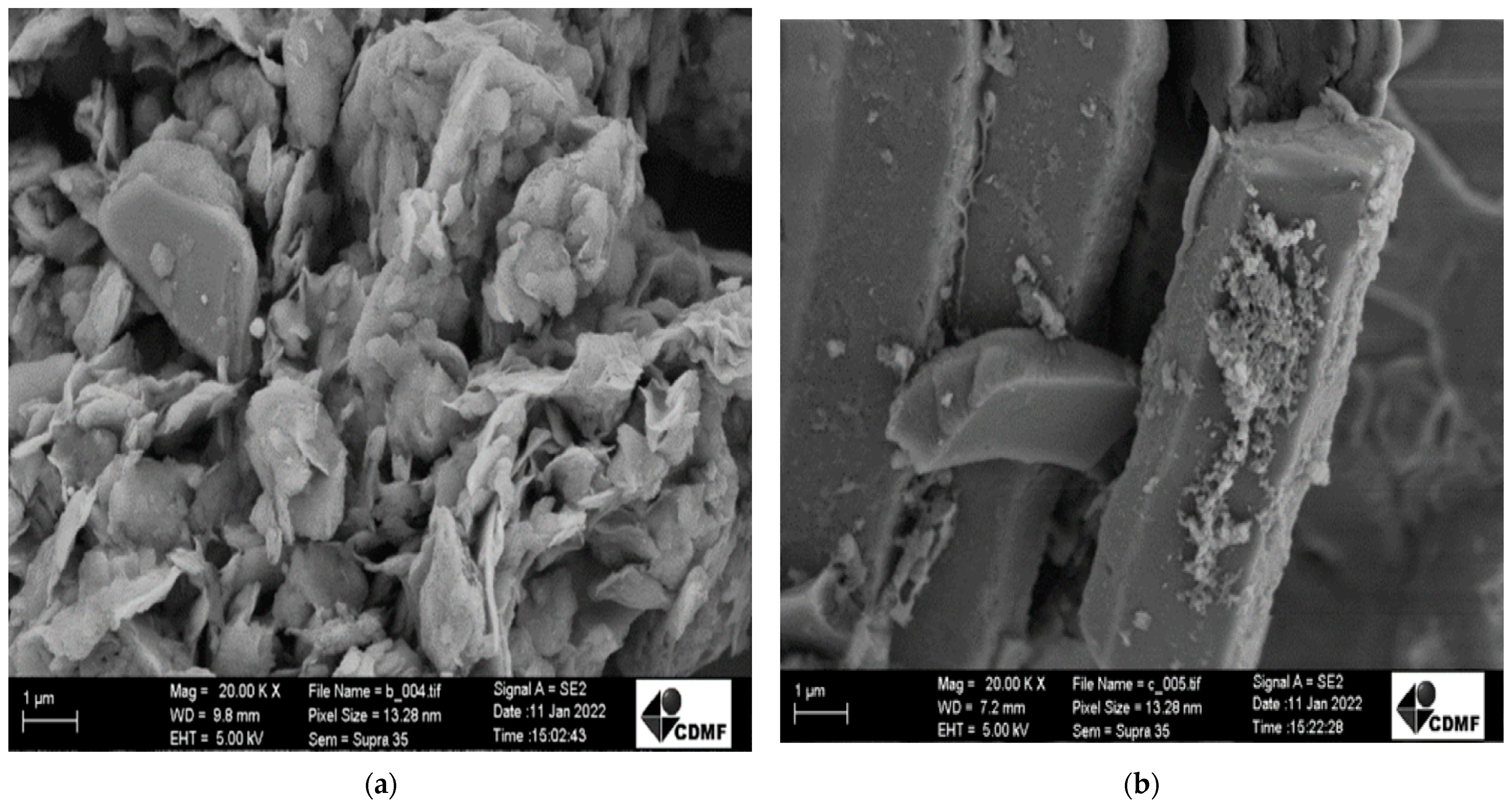
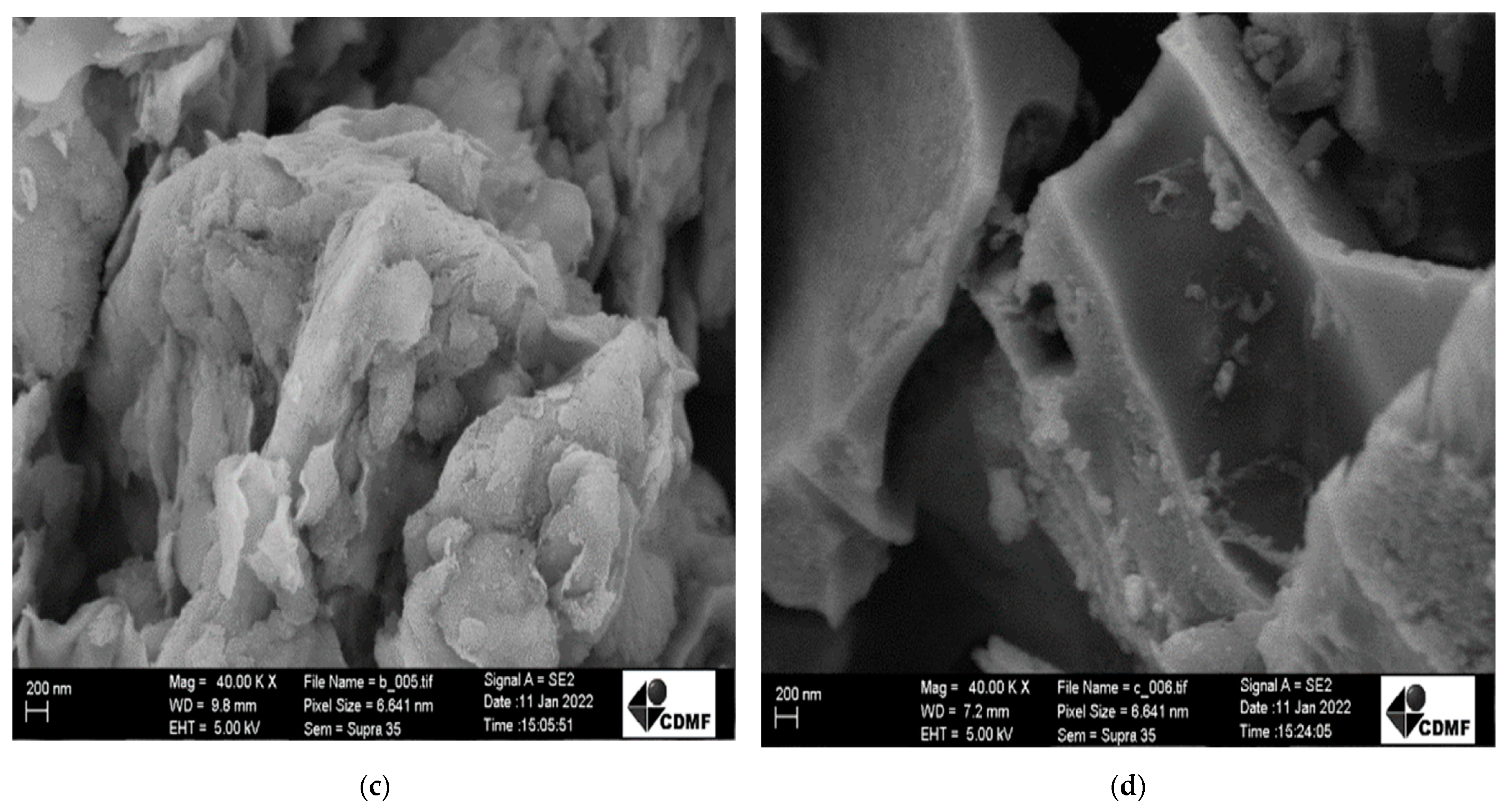
| Sample | Cu (mg kg−1) | Ni (mg kg−1) | Fe (mg kg−1) |
|---|---|---|---|
| Cecropia pachystachya leaves | 83.0 ± 5.2 | 3.6 ± 0.7 | 350 ± 2 |
| Cecropia pachystachya branches | 374 ± 8 | 52.5 ± 3 | 3900 ± 0.05 |
| Pluchea sagittalis leaves | 567 ± 16 | 31.4 ± 1.4 | 10,000 ± 0.1 |
| Pluchea sagittalis flowers | 174.1 ± 7.3 | 49.8 ± 3.2 | 3900 ± 0.02 |
| Typha domingensis leaves | 56.8 ± 4.1 | 23.7 ± 0.6 | 510 ± 1 |
 | ||||
|---|---|---|---|---|
| Entry a | Catalyst | Time (h) | 3a, Yield (%) b | MSMA f |
| 1 c | CuCl2.2H2O (1 mol%) | 24 | 20 | --- |
| 2 c | CuCl2.2H2O (5 mol%) | 12 | 62 | --- |
| 3 c | CuCl2.2H2O (5 mol%) | 24 | 96 | --- |
| 4 c | - | 24 | 21 | --- |
| 5 c | Cat A | 24 | 60 | 0.66 |
| 6 d | Cat B | 12 | 84 | 28.7 |
| 7 d | Cat C | 12 | 69 | 0.91 |
| 8 d | Ashes | 12 | 61 | 0.02 |
| 9 d | MK10 | 12 | 66 | --- |
| 10 e | Cat B | 1.5 | Traces | --- |
| 11 e | Ashes | 1.5 | Traces | --- |
 | ||
|---|---|---|
| Entry a | Catalyst | 5a, Yield (%) b |
| 1 | Ashes (200 mg) | 25 |
| 2 | Ashes (100 mg) | 75 |
| 3 | Ashes (50 mg) | 86 |
| 4 | Cat B | 98 |
| 5 | Cat C | 88 |
| 6 | MK10 | 84 |
| Entry | Catalyst and Reaction Conditions | Cat. Recycling (cycles) | 3a, Yield (%) | 5a, Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | BNPs@SiO2(CH2)3NHSO3H, solvent-free, 80 °C, 30 min or EtOH, 70 °C, 35 min | 5 | 97 | 97 | [67] |
| 2 | NiFe2O4@SiO2@KIT-6-SO3H, solvent-free, 100 °C, 10 min or EtOH, reflux, 80 °C, 55 min | 5 | 95 | 93 | [68] |
| 3 | Fe-C-O-Mo alloy, EtOH, reflux, 2.5 h | 8 | 92 | - | [69] |
| 4 | bentonite/H4[W12SiO40], EtOH, 80 °C, 5 h | - | 86 | - | [70] |
| 5 | NH4H2PO4/MCM-41, solvent-free, 100 °C, 6 h | 6 | 72 | - | [71] |
| 6 | H3PW12O40@MIL-101, solvent-free, 100 °C, 60 min | 4 | 90 | - | [72] |
| 7 | SiO2-CuCl2, MeCN, MW, 80 °C, 15 min | 5 | 90 | - | [73] |
| 8 | [ImSi][PF6]@xanthan, EtOH, 80 °C, 37 min | 5 | - | 93 | [74] |
| 9 | H2SO4-activated montmorillonite, solvent-free, MW, 110 °C, 14 min | 4 | - | 79 | [75] |
| 10 | Cat B, solvent-free, 80 °C, 12 h or EtOH, MW, 110 °C, 20 min | 6 | 84 | 98 | this work |
| Catalyst | BET Area (m2 g−1) | Total Pore Volume (cm3 g−1) |
|---|---|---|
| Cat B | 232 | 0.286 |
| Cat C | 33 | 0.061 |
| Ashes | 4 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alponti, L.H.R.; Picinini, M.; Urquieta-Gonzalez, E.A.; da Silva, C.S.; Silva, S.Y.S.; Silva, S.C.; de Oliveira, M.N.; Viera, J.; da Silva, M.F.d.G.F.; Corrêa, A.G. Multicomponent Reactions Promoted by Ecocatalyst from Metal Hyperaccumulating Plant Pluchea sagittalis. Reactions 2023, 4, 552-568. https://doi.org/10.3390/reactions4040033
Alponti LHR, Picinini M, Urquieta-Gonzalez EA, da Silva CS, Silva SYS, Silva SC, de Oliveira MN, Viera J, da Silva MFdGF, Corrêa AG. Multicomponent Reactions Promoted by Ecocatalyst from Metal Hyperaccumulating Plant Pluchea sagittalis. Reactions. 2023; 4(4):552-568. https://doi.org/10.3390/reactions4040033
Chicago/Turabian StyleAlponti, Leonardo H. R., Monize Picinini, Ernesto A. Urquieta-Gonzalez, Caroline S. da Silva, Simone Y. S. Silva, Sebastião C. Silva, Marilene N. de Oliveira, Juliana Viera, Maria Fatima das G. F. da Silva, and Arlene G. Corrêa. 2023. "Multicomponent Reactions Promoted by Ecocatalyst from Metal Hyperaccumulating Plant Pluchea sagittalis" Reactions 4, no. 4: 552-568. https://doi.org/10.3390/reactions4040033
APA StyleAlponti, L. H. R., Picinini, M., Urquieta-Gonzalez, E. A., da Silva, C. S., Silva, S. Y. S., Silva, S. C., de Oliveira, M. N., Viera, J., da Silva, M. F. d. G. F., & Corrêa, A. G. (2023). Multicomponent Reactions Promoted by Ecocatalyst from Metal Hyperaccumulating Plant Pluchea sagittalis. Reactions, 4(4), 552-568. https://doi.org/10.3390/reactions4040033








