Catalytic Valorisation of Biomass-Derived Levulinic Acid to Biofuel Additive γ-Valerolactone: Influence of Copper Loading on Silica Support
Abstract
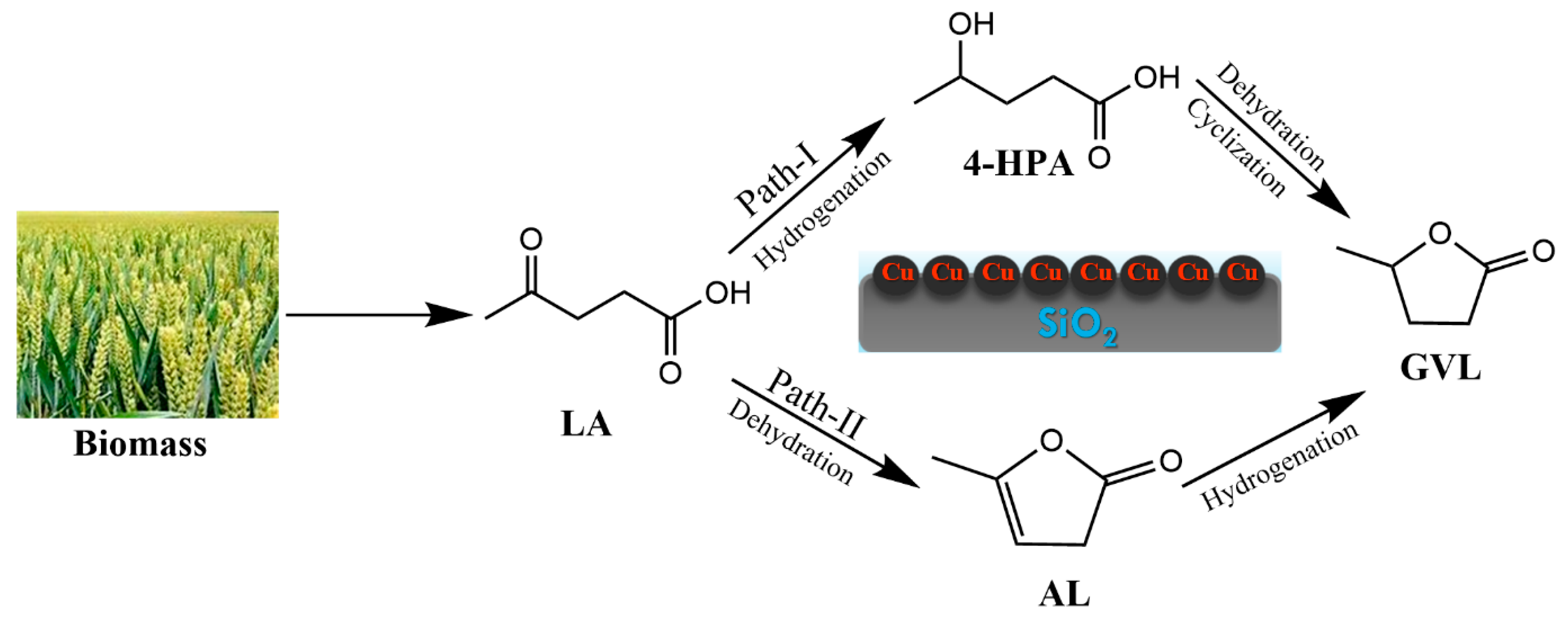
:1. Introduction
2. Materials and Methods
3. Results and Discussion
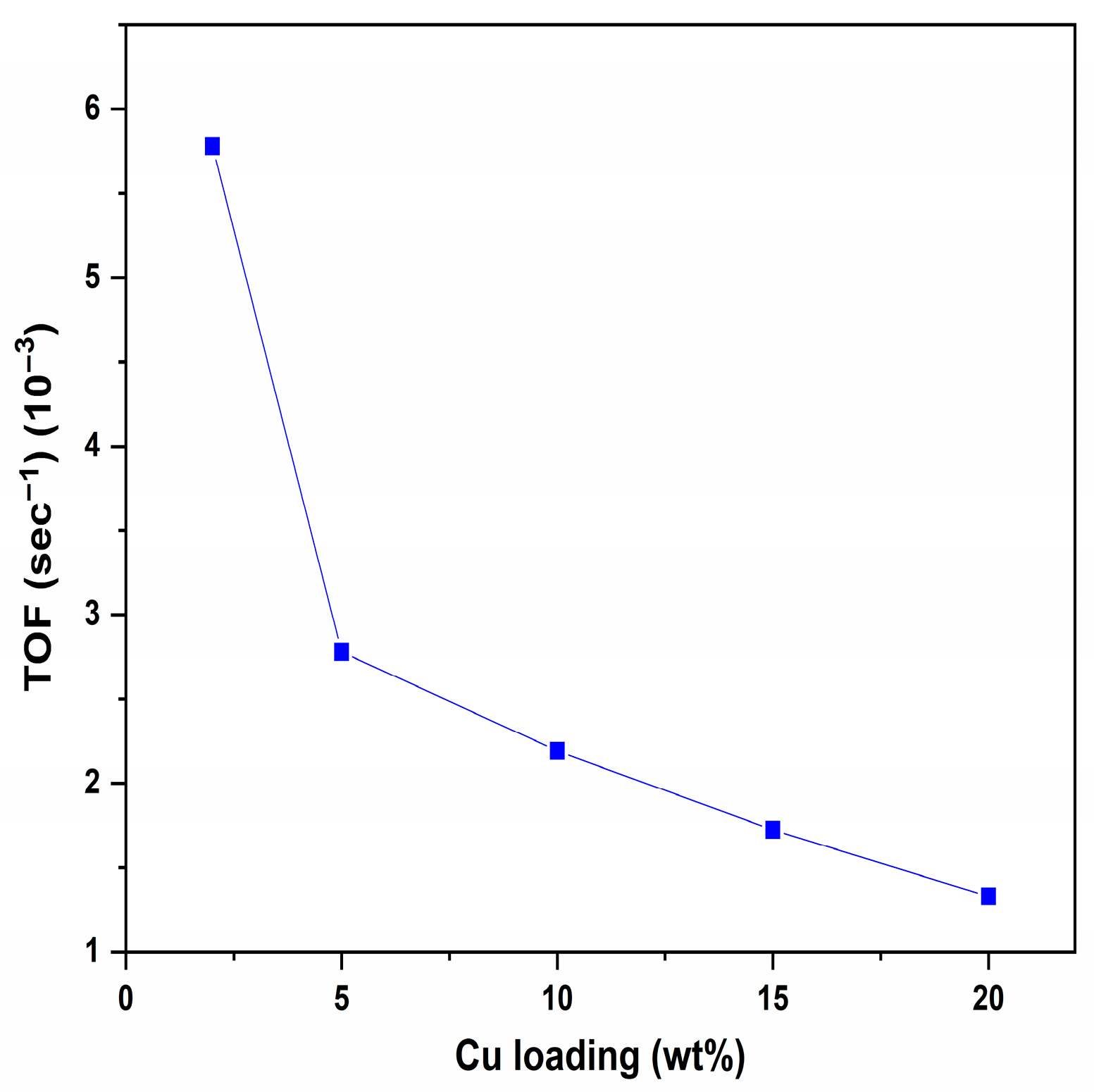
3.1. Effect of Copper Loadings on Catalytic Activity
3.2. Effect of TOF
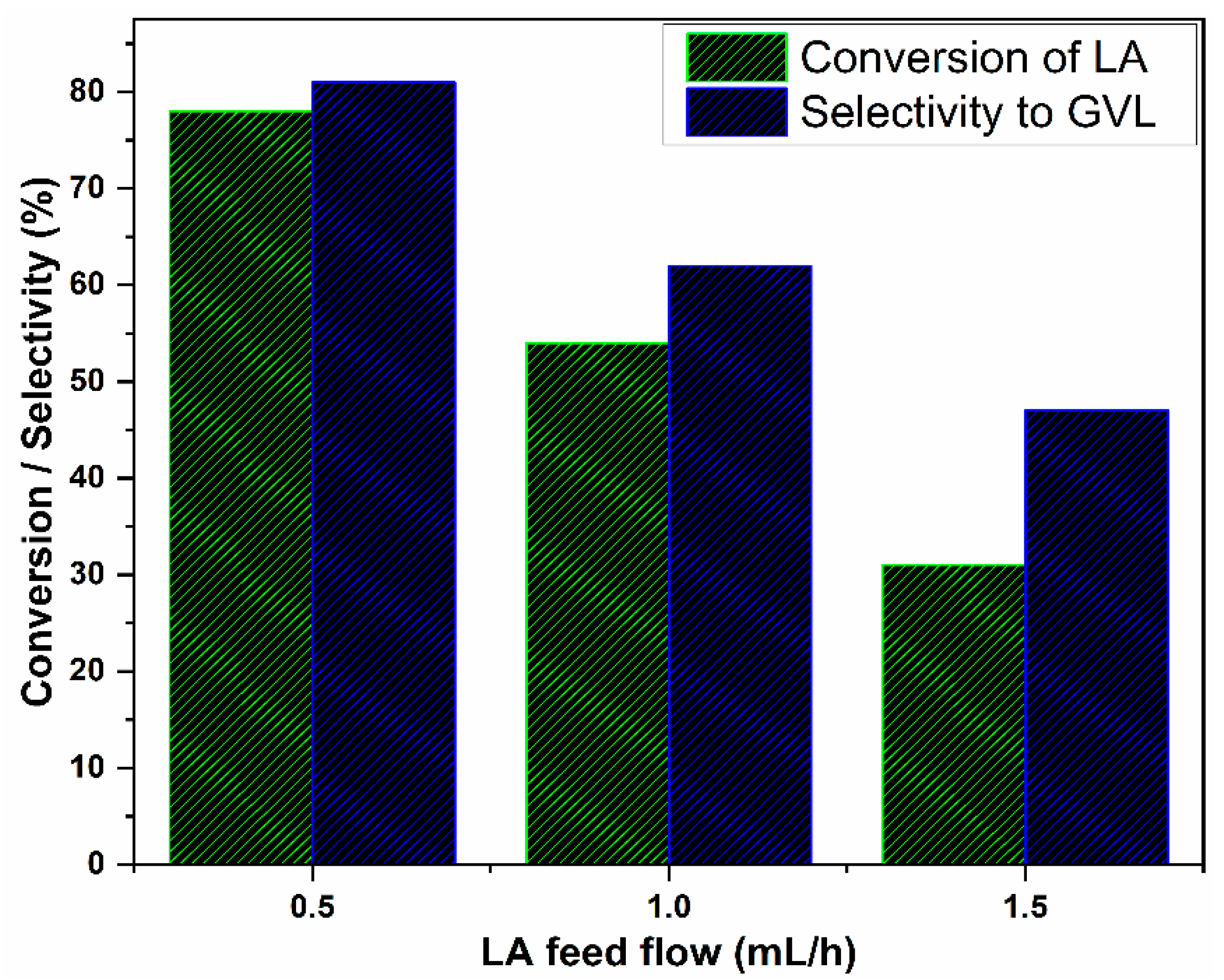
3.3. Effect of LA Flow Rates on Catalytic Activity
3.4. Effect of Reaction Temperatures on Catalytic Activity
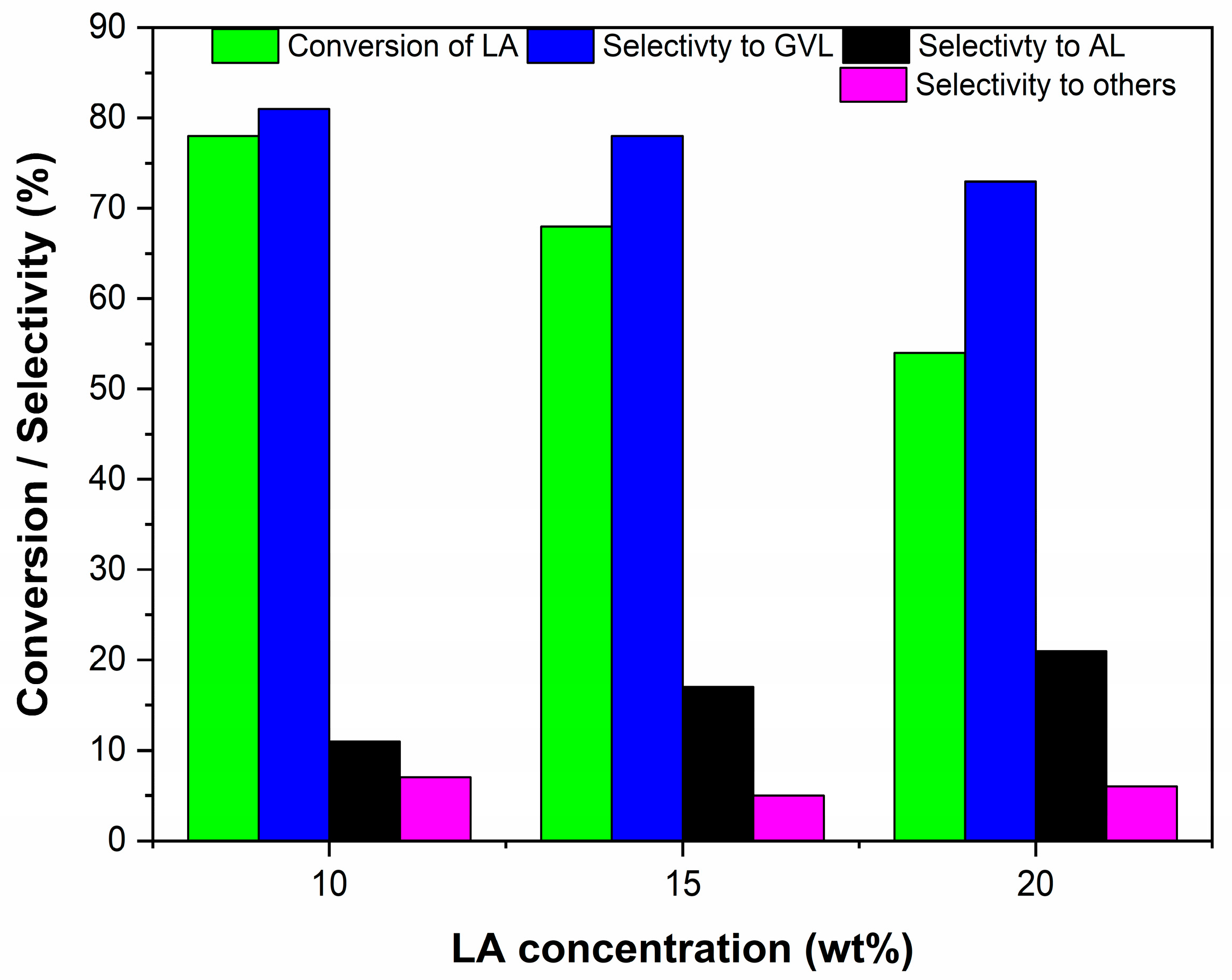
3.5. Effect of LA Concentrations on Catalytic Activity
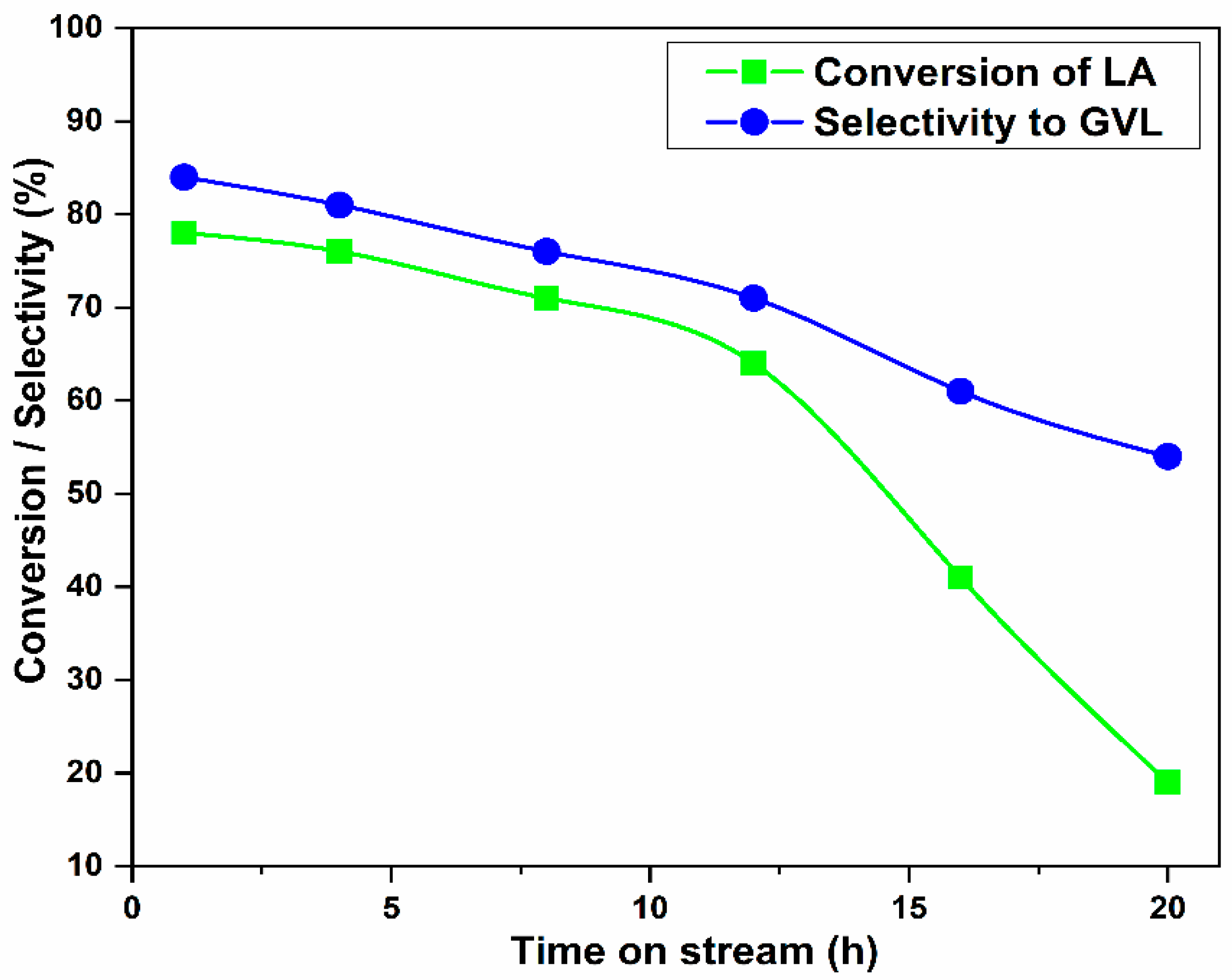
3.6. Effect of Time-on-Stream on Catalytic Activity
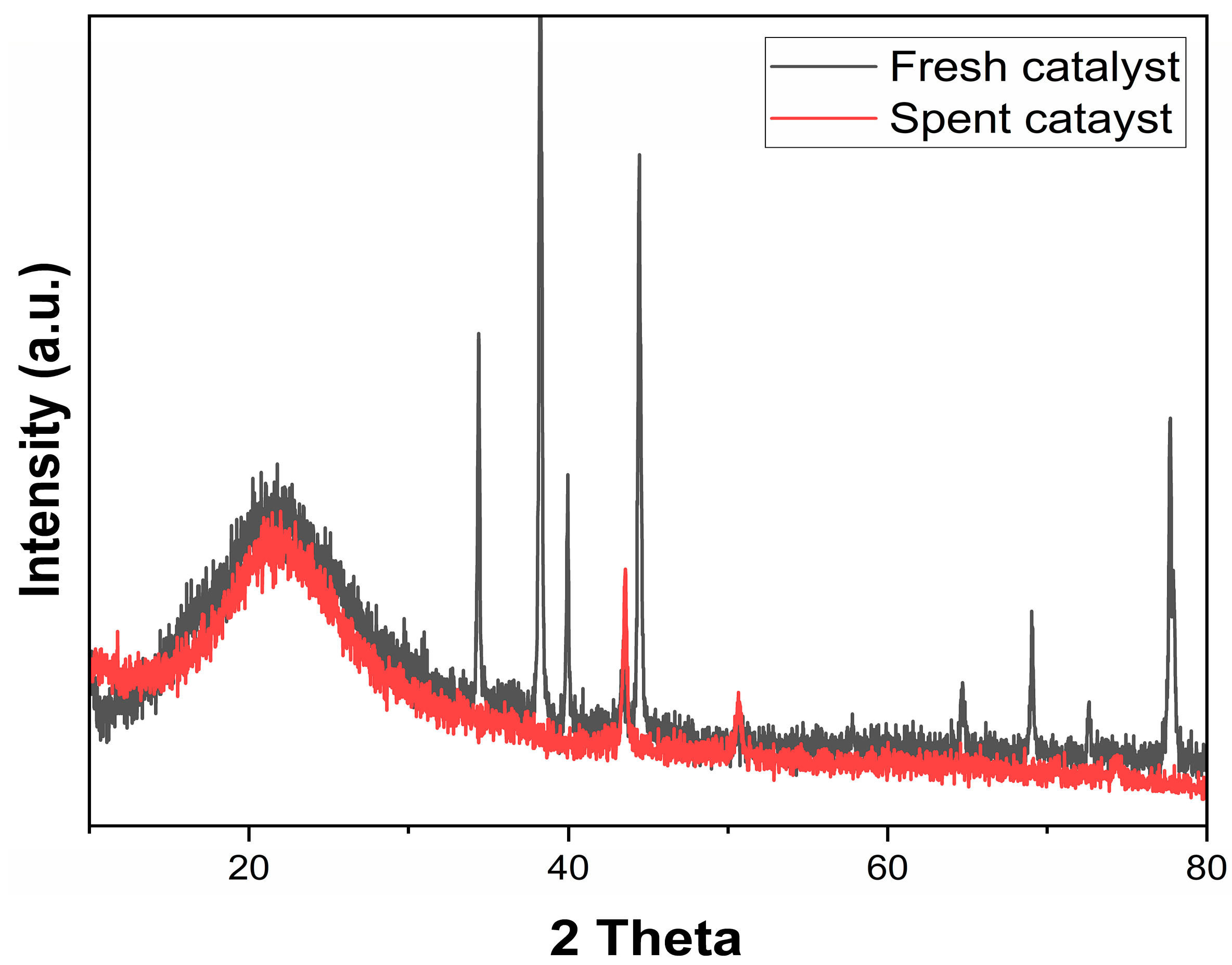
3.7. Deactivation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Kwon, E.E.; Nadagouda, M.N.; Aminabhavi, T.M. Biomass Utilization and Production of Biofuels from Carbon Neutral Materials. Environ. Pollut. 2021, 276, 116731. [Google Scholar] [CrossRef]
- Sharifi, M.; Pothu, R.; Boddula, R. Inamuddin Lignin to Value-Added Chemical Synthesis. Curr. Anal. Chem. 2021, 17, 936–946. [Google Scholar] [CrossRef]
- Alonso, D.M.; Hakim, S.H.; Zhou, S.; Won, W.; Hosseinaei, O.; Tao, J.; Garcia-Negron, V.; Motagamwala, A.H.; Mellmer, M.A.; Huang, K.; et al. Increasing the Revenue from Lignocellulosic Biomass: Maximizing Feedstock Utilization. Sci. Adv. 2017, 3, 1603301. [Google Scholar] [CrossRef]
- Pothu, R.; Mameda, N.; Boddula, R.; Mitta, H.; Perugopu, V.; Al-Qahtani, N. Sustainable Conversion of Biodiesel-Waste Glycerol to Acrolein over Pd-Modified Mesoporous Catalysts. Mater. Sci. Energy Technol. 2023, 6, 226–236. [Google Scholar] [CrossRef]
- Tabassum, N.; Pothu, R.; Pattnaik, A.; Boddula, R.; Balla, P.; Gundeboyina, R.; Challa, P.; Rajesh, R.; Perugopu, V.; Mameda, N.; et al. Heterogeneous Catalysts for Conversion of Biodiesel-Waste Glycerol into High-Added-Value Chemicals. Catalysts 2022, 12, 767. [Google Scholar] [CrossRef]
- Victor, A.; Sharma, P.; Pulidindi, I.N.; Gedanken, A. Levulinic Acid Is a Key Strategic Chemical from Biomass. Catalysts 2022, 12, 909. [Google Scholar] [CrossRef]
- Pothu, R.; Mitta, H.; Boddula, R.; Balla, P.; Gundeboyina, R.; Perugopu, V.; Ma, J. Direct Cascade Hydrogenation of Biorenewable Levulinic Acid to Valeric Acid Biofuel Additives over Metal (M = Nb, Ti, and Zr) Supported SBA-15 Catalysts. Mater. Sci. Energy Technol. 2022, 5, 391–398. [Google Scholar] [CrossRef]
- Pothu, R.; Mameda, N.; Mitta, H.; Boddula, R.; Gundeboyina, R.; Perugopu, V.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. High Dispersion of Platinum Nanoparticles over Functionalized Zirconia for Effective Transformation of Levulinic Acid to Alkyl Levulinate Biofuel Additives in the Vapor Phase. J. Compos. Sci. 2022, 6, 300. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Luo, X. The Relationship between Structure and Catalytic Activity-Stability of Non-Precious Metal-Based Catalysts towards Levulinic Acid Hydrogenation to γ-Valerolactone: A Review. Energies 2022, 15, 8093. [Google Scholar] [CrossRef]
- Pothu, R.; Challa, P.; Rajesh, R.; Boddula, R.; Balaga, R.; Balla, P.; Perugopu, V.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. Vapour-Phase Selective Hydrogenation of γ-Valerolactone to 2-Methyltetrahydrofuran Biofuel over Silica-Supported Copper Catalysts. Nanomaterials 2022, 12, 3414. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green Synthesis of Gamma-Valerolactone (GVL) through Hydrogenation of Biomass-Derived Levulinic Acid Using Non-Noble Metal Catalysts: A Critical Review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Kovács, H.; Orosz, K.; Papp, G.; Joó, F.; Horváth, H. Immobilization of an Iridium(I)-NHC-Phosphine Catalyst for Hydrogenation Reactions under Batch and Flow Conditions. Catalysts 2021, 11, 656. [Google Scholar] [CrossRef]
- Cao, W.; Lin, L.; Qi, H.; He, Q.; Wu, Z.; Wang, A.; Luo, W.; Zhang, T. In-Situ Synthesis of Single-Atom Ir by Utilizing Metal-Organic Frameworks: An Acid-Resistant Catalyst for Hydrogenation of Levulinic Acid to γ-Valerolactone. J. Catal. 2019, 373, 161–172. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Dorcet, V.; Roisnel, T.; Bruneau, C.; Fischmeister, C. Efficient Iridium Catalysts for Base-Free Hydrogenation of Levulinic Acid. Organometallics 2017, 36, 3152–3162. [Google Scholar] [CrossRef]
- Feng, J.; Li, M.; Zhong, Y.; Xu, Y.; Meng, X.; Zhao, Z.; Feng, C. Hydrogenation of Levulinic Acid to γ-Valerolactone over Pd@UiO-66-NH2 with High Metal Dispersion and Excellent Reusability. Microporous Mesoporous Mater. 2020, 294, 109858. [Google Scholar] [CrossRef]
- Siddiqui, N.; Pendem, C.; Goyal, R.; Khatun, R.; Khan, T.S.; Samanta, C.; Chiang, K.; Shah, K.; Ali Haider, M.; Bal, R. Study of γ-Valerolactone Production from Hydrogenation of Levulinic Acid over Nanostructured Pt-Hydrotalcite Catalysts at Low Temperature. Fuel 2022, 323, 124272. [Google Scholar] [CrossRef]
- Esposito, S.; Silvestri, B.; Rossano, C.; Vermile, V.; Imparato, C.; Manzoli, M.; Bonelli, B.; Russo, V.; Gaigneaux, E.M.; Aronne, A.; et al. The Role of Metallic and Acid Sites of Ru-Nb-Si Catalysts in the Transformation of Levulinic Acid to γ-Valerolactone. Appl. Catal. B 2022, 310, 121340. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Mikhailov, S.P.; Kuchkina, N.V.; Bykov, A.V.; Vasiliev, A.L.; Ezernitskaya, M.G.; Golovin, A.L.; Nikoshvili, L.Z.; Sulman, M.G.; Shifrina, Z.B. Ru@hyperbranched Polymer for Hydrogenation of Levulinic Acid to Gamma-Valerolactone: The Role of the Catalyst Support. Int. J. Mol. Sci. 2022, 23, 799. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megías-Sayago, C.; Navarro, J.C.; Ammari, F.; Ivanova, S.; Centeno, M.Á.; Odriozola, J.A. Functionalized Biochars as Supports for Ru/C Catalysts: Tunable and Efficient Materials for γ-Valerolactone Production. Nanomaterials 2023, 13, 1129. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Maroney Lawrence, Y.; Fernandez, A.D.; Grady, T.L.; Wiredu, B. Conversion of Levulinic Acid and Cellulose to γ-Valerolactone over Raney-Ni Catalyst Using Formic Acid as a Hydrogen Donor. Biofuels 2021, 12, 423–427. [Google Scholar] [CrossRef]
- Balla, P.; Perupogu, V.; Vanama, P.K.; Komandur, V.C. Hydrogenation of Biomass-Derived Levulinic Acid to γ-Valerolactone over Copper Catalysts Supported on ZrO2. J. Chem. Technol. Biotechnol. 2016, 91, 769–776. [Google Scholar] [CrossRef]
- Putrakumar, B.; Seelam, P.K.; Srinivasarao, G.; Rajan, K.; Harishekar, M.; Riitta, K.; Liang, T.X. A Comparison of Structure–Activity of Cu-Modified Over Different Mesoporous Silica Supports for Catalytic Conversion of Levulinic Acid. Waste Biomass Valorization 2022, 13, 67–79. [Google Scholar] [CrossRef]
- Putrakumar, B.; Nagaraju, N.; Kumar, V.P.; Chary, K.V.R. Hydrogenation of Levulinic Acid to γ-Valerolactone over Copper Catalysts Supported on γ-Al2O3. Catal. Today 2015, 250, 209–217. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, Q.; Wang, J.; Mu, T. Valorization of Levulinic Acid over Non-Noble Metal Catalysts: Challenges and Opportunities. Green Chem. 2018, 20, 4391–4408. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Yu, X.; Zhang, W.; Zhang, G.; Liu, M.; Shen, J.; Yang, C.; Jin, X. Non-Noble Metal Catalysts for Transfer Hydrogenation of Levulinic Acid: The Role of Surface Morphology and Acid-Base Pairs. Mater. Today Energy 2020, 18, 100501. [Google Scholar] [CrossRef]
- Sudhakar, M.; Kumar, V.V.; Naresh, G.; Kantam, M.L.; Bhargava, S.K.; Venugopal, A. Vapor Phase Hydrogenation of Aqueous Levulinic Acid over Hydroxyapatite Supported Metal (M = Pd, Pt, Ru, Cu, Ni) Catalysts. Appl. Catal. B 2016, 180, 113–120. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Y.; Pan, T.; Xu, Q.; Guo, Q.-X.; Fu, Y. Conversion of Carbohydrate Biomass to γ-Valerolactone by Using Water-Soluble and Reusable Iridium Complexes in Acidic Aqueous Media. ChemSusChem 2013, 6, 1163–1167. [Google Scholar] [CrossRef]
- Li, W.; Xie, J.-H.; Lin, H.; Zhou, Q.-L. Highly Efficient Hydrogenation of Biomass-Derived Levulinic Acid to γ-Valerolactone Catalyzed by Iridium Pincer Complexes. Green Chem. 2012, 14, 2388–2390. [Google Scholar] [CrossRef]
- Dutta Chowdhury, A.; Jackstell, R.; Beller, M. Towards the Efficient Development of Homogeneous Catalytic Transformation to γ-Valerolactone from Biomass-Derived Platform Chemicals. ChemCatChem 2014, 6, 3360–3365. [Google Scholar] [CrossRef]
- Delhomme, C.; Schaper, L.-A.; Zhang-Preße, M.; Raudaschl-Sieber, G.; Weuster-Botz, D.; Kühn, F.E. Catalytic Hydrogenation of Levulinic Acid in Aqueous Phase. J. Organomet. Chem. 2013, 724, 297–299. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Novák, M.; Dibó, G.; Mika, L.T. An Improved Catalytic System for the Reduction of Levulinic Acid to γ-Valerolactone. Catal. Sci. Technol. 2014, 4, 2908–2912. [Google Scholar] [CrossRef]
- Al-Naji, M.; Popova, M.; Chen, Z.; Wilde, N.; Gläser, R. Aqueous-Phase Hydrogenation of Levulinic Acid Using Formic Acid as a Sustainable Reducing Agent over Pt Catalysts Supported on Mesoporous Zirconia. ACS Sustain. Chem. Eng. 2020, 8, 393–402. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Jędrzejczyk, M.; Sneka-Płatek, O.; Keller, N.; Dumon, A.S.; Michel, C.; Sautet, P.; Grams, J. Ru Catalysts for Levulinic Acid Hydrogenation with Formic Acid as a Hydrogen Source. Green Chem. 2016, 18, 2014–2028. [Google Scholar] [CrossRef]
- Son, P.A.; Nishimura, S.; Ebitani, K. Production of γ-Valerolactone from Biomass-Derived Compounds Using Formic Acid as a Hydrogen Source over Supported Metal Catalysts in Water Solvent. RSC Adv. 2014, 4, 10525. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Martinelli, M. A Sustainable Process for the Production of γ-Valerolactone by Hydrogenation of Biomass-Derived Levulinic Acid. Green Chem. 2012, 14, 688–694. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Han, A.; Wang, J.; Tan, H.R.; Tok, E.S.; Jaenicke, S.; Chuah, G.K. Ru/ZrO2 Catalysts for Transfer Hydrogenation of Levulinic Acid with Formic Acid/Formate Mixtures: Importance of Support Stability. ChemistrySelect 2018, 3, 1343–1351. [Google Scholar] [CrossRef]
- Sudhakar, M.; Lakshmi Kantam, M.; Swarna Jaya, V.; Kishore, R.; Ramanujachary, K.V.; Venugopal, A. Hydroxyapatite as a Novel Support for Ru in the Hydrogenation of Levulinic Acid to γ-Valerolactone. Catal. Commun. 2014, 50, 101–104. [Google Scholar] [CrossRef]
- Du, X.-L.; He, L.; Zhao, S.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Hydrogen-Independent Reductive Transformation of Carbohydrate Biomass into γ-Valerolactone and Pyrrolidone Derivatives with Supported Gold Catalysts. Angew. Chem. Int. Ed. 2011, 50, 7815–7819. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.K.; Chauhan, A.; Srivastava, R. A CoPd Nanoalloy Embedded N-Doped Porous Carbon Catalyst for the Selective Reduction and Reductive Amination of Levulinic Acid Using Formic Acid in Water. Sustain. Energy Fuels 2023, 7, 1855–1869. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Song, G. Synergy of Ultra-Low-Loaded Ruthenium with Alumina Stimulating the Catalytic Hydrogenation of Levulinic Acid into γ-Valerolactone. Chem. Eng. J. 2023, 470, 143869. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, J.; Wang, Y.; Guo, H.; Qi, X. Bimetallic Ni-Zn@OMC Catalyst for Selective Hydrogenation of Levulinic Acid to γ-Valerolactone in Water. Fuel Process. Technol. 2023, 240, 107559. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, Z.; Wang, L.; Lv, J.; Wang, Y.; Wang, Y. Efficient Conversion of Levulinic Acid into γ-Valerolactone over Raney Ni Catalyst Prepared from Melt-Quenching Alloy. Catal Lett. 2014, 144, 1766–1771. [Google Scholar] [CrossRef]
- Murugesan, K.; Alshammari, A.S.; Sohail, M.; Jagadeesh, R.V. Levulinic Acid Derived Reusable Cobalt-Nanoparticles-Catalyzed Sustainable Synthesis of γ-Valerolactone. ACS Sustain. Chem. Eng. 2019, 7, 14756–14764. [Google Scholar] [CrossRef]
- Wang, D.; Luo, M.; Yue, L.; Wei, J.; Zhang, X.; Cai, J. Co-Embedded N-Doped Hierarchical Porous Biocarbons: Facile Synthesis and Used as Highly Efficient Catalysts for Levulinic Acid Hydrogenation. Fuel 2022, 329, 125364. [Google Scholar] [CrossRef]
- Long, X.; Sun, P.; Li, Z.; Lang, R.; Xia, C.; Li, F. Magnetic Co/Al2O3 Catalyst Derived from Hydrotalcite for Hydrogenation of Levulinic Acid to γ-Valerolactone. Chin. J. Catal. 2015, 36, 1512–1518. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thiripuranthagan, S.; Durai, M.; Erusappan, E.; Vembuli, T. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid to γ-Valerolactone over Sn/Al-SBA-15 Catalysts. New J. Chem. 2020, 44, 8209–8222. [Google Scholar] [CrossRef]
- Gupta, S.S.R.; Kantam, M.L. Selective Hydrogenation of Levulinic Acid into γ-Valerolactone over Cu/Ni Hydrotalcite-Derived Catalyst. Catal. Today 2018, 309, 189–194. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, K.; Li, Q.; Liu, Q.; Zhang, S.; Liu, Q.; Hu, G.; Hu, X. Copper-Based Catalysts with Tunable Acidic and Basic Sites for the Selective Conversion of Levulinic Acid/Ester to γ-Valerolactone or 1,4-Pentanediol. Green Chem. 2019, 21, 4499–4511. [Google Scholar] [CrossRef]
- Orlowski, I.; Douthwaite, M.; Iqbal, S.; Hayward, J.S.; Davies, T.E.; Bartley, J.K.; Miedziak, P.J.; Hirayama, J.; Morgan, D.J.; Willock, D.J.; et al. The Hydrogenation of Levulinic Acid to Γ-Valerolactone over Cu–ZrO2 Catalysts Prepared by a PH-Gradient Methodology. J. Energy Chem. 2019, 36, 15–24. [Google Scholar] [CrossRef]
- Lomate, S.; Sultana, A.; Fujitani, T. Effect of SiO2 Support Properties on the Performance of Cu–SiO2 Catalysts for the Hydrogenation of Levulinic Acid to Gamma Valerolactone Using Formic Acid as a Hydrogen Source. Catal. Sci. Technol. 2017, 7, 3073–3083. [Google Scholar] [CrossRef]
- Mitta, H.; Perupogu, V.; Boddula, R.; Ginjupalli, S.R.; Asiri, A.M. Enhanced Production of γ-Valerolactone from Levulinic Acid Hydrogenation-Cyclization over ZrxCe1-XO2 Based Cu Catalysts. Int. J. Hydrogen Energy 2020, 45, 26445–26457. [Google Scholar] [CrossRef]
- Mitta, H.; Seelam, P.K.; Chary, K.V.R.; Mutyala, S.; Boddula, R.; Inamuddin; Asiri, A. M. Efficient Vapor-Phase Selective Hydrogenolysis of Bio-Levulinic Acid to γ-Valerolactone Using Cu Supported on Hydrotalcite Catalysts. Glob. Chall. 2018, 2, 1800028. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zhang, C.; Liu, C.L.; Yang, R.Z.; Dong, W.S. Construction of Mesoporous Cu/ZrO2-Al2O3 as a Ternary Catalyst for Efficient Synthesis of γ-Valerolactone from Levulinic Acid at Low Temperature. J. Catal. 2020, 381, 163–174. [Google Scholar] [CrossRef]
- He, D.; He, Q.; Jiang, P.; Zhou, G.; Hu, R.; Fu, W. Novel Cu/Al2O3-ZrO2 Composite for Selective Hydrogenation of Levulinic Acid to Γ-Valerolactone. Catal. Commun. 2019, 125, 82–86. [Google Scholar] [CrossRef]
- Sun, D.; Ohkubo, A.; Asami, K.; Katori, T.; Yamada, Y.; Sato, S. Vapor-Phase Hydrogenation of Levulinic Acid and Methyl Levulinate to Γ-Valerolactone over Non-Noble Metal-Based Catalysts. Mol. Catal. 2017, 437, 105–113. [Google Scholar] [CrossRef]
- Yanase, D.; Yoshida, R.; Kanazawa, S.; Yamada, Y.; Sato, S. Efficient Formation of γ-Valerolactone in the Vapor-Phase Hydrogenation of Levulinic Acid over Cu-Co/Alumina Catalyst. Catal. Commun. 2020, 139, 105967. [Google Scholar] [CrossRef]
- Shan, J.; Liu, H.; Lu, K.; Zhu, S.; Li, J.; Wang, J.; Fan, W. Identification of the Dehydration Active Sites in Glycerol Hydrogenolysis to 1,2-Propanediol over Cu/SiO2 Catalysts. J. Catal. 2020, 383, 13–23. [Google Scholar] [CrossRef]
- Naikwadi, D.R.; Bankar, B.D.; Kachgunde, H.G.; Biradar, A.V. Highly Active and Efficient Cu@SiO2 Catalyst: Enabled Nucleophilic and Electrophilic Activation of Active Methylene Compounds. Asian J. Org. Chem. 2022, 11, e202200473. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Sot, P.; Nachtegaal, M.; Ranocchiari, M.; van Bokhoven, J.A.; Mesters, C. Direct Stepwise Oxidation of Methane to Methanol over Cu–SiO2. ACS Catal. 2018, 8, 5721–5731. [Google Scholar] [CrossRef]
- Kurniawan, E.; Hosaka, S.; Kobata, M.; Yamada, Y.; Sato, S. Vapor-Phase Oxidant-Free Dehydrogenation of 2,3- and 1,4-Butanediol over Cu/SiO2 Catalyst Prepared by Crown-Ether-Assisted Impregnation. Chemistry 2023, 5, 406–421. [Google Scholar] [CrossRef]
| S. No. | Catalyst | Reaction Conditions | LA Conversion (%) | GVL Selectivity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (MPa) | Time (h) | |||||
| 1 | [Cp*Ir(dpa)(OSO3)] a,b | 100 | 0.5 | 72 | 92 | - | [14] |
| 2 | Ir complex (6) a,b,c | 120 | 1.01 | 36 | - | 78 | [27] |
| 3 | [Ir(COE)2Cl]2 a,b | 100 | 5 | 17 | 100 | 99 | [28] |
| 4 | [Ru(acac)3]/TPP a,b | 140 | 8 | 168 | >99 | 95 | [29] |
| 5 | [Ru(acac)3] + TPPTS a,b | 140 | 5 | 5 | 99 | 97 | [30] |
| 6 | [Ru(acac)3]/DPPB a,b | 140 | 10 | 1.8 | 99.9 | 99.9 | [31] |
| 7 | Pt/ZrO2 b,c | 240 | 4 | 24 | 97 | 90 | [32] |
| 8 | Ru/C b,c | 190 | 1 | 5 | 81 | 57 | [33] |
| 9 | Ru/C b,c | 150 | - | 5 | 100 | 97 | [34] |
| 10 | Ru/C + A70 b | 70 | 3 | 3 | 100 | 99 | [35] |
| 11 | Pd/C b,c | 150 | - | 5 | 9 | 17 | [34] |
| 12 | Pt/C b,c | 150 | - | 5 | 13 | 13 | [34] |
| 13 | Ru/ZrO2 b,c | 150 | - | 5 | 11 | 18 | [34] |
| 14 | Ru/ZrO2 b,c | 150 | 1 | 12 | 73 | >99 | [36] |
| 15 | Ru/SBA-15 b,c | 150 | - | 5 | 31 | 71 | [34] |
| 16 | Ru/HAP b | 70 | 0.5 | 4 | 99 | 99 | [37] |
| 17 | Au/ZrO2-VS b,c | 150 | 0.5 | 6 | 95 | 99 | [38] |
| 18 | Co8Pd2@N-C b,c | 150 | - | 9 | ~100 | 99.3 | [39] |
| 19 | Ru0.18/Al2O3/NC b | 150 | 4 | 3 | - | 99.7 | [40] |
| 20 | Ni1-Zn1@OMC b | 180 | 2 | 1.5 | 100 | 93 | [41] |
| 21 | Raney Ni b | 100 | 1.5 | 4 | 99.3 | 98.1 | [42] |
| 22 | Co-LA@SiO2-800 b,d | 120 | 3 | 24 | >99 | 97 | [43] |
| 23 | Co@NC-700 b,d | 190 | 1.9 | 2 | 100 | 100 | [44] |
| 24 | 4Co/Al2O3 b,d | 180 | 5 | 3 | 100 | ˃99 | [45] |
| 25 | Sn/Al-SBA-15 b,e | 200 | 0.1 | 3 | 99 | 100 | [46] |
| 26 | Cu/Ni hydrotalcite b,d | 140 | 3 | 3 | 100 | 100 | [47] |
| 27 | CuAl b,e | 110 | 3 | 2 | 100 | 95.3 | [48] |
| 28 | Cu/MCM-41 f | 265 | 0.1 | 5 | 85 | 77 | [22] |
| 29 | Cu/MCM-48 f | 265 | 0.1 | 5 | 92 | 92 | [22] |
| 30 | Cu/KIT-6 f | 265 | 0.1 | 5 | 78 | 69 | [22] |
| 31 | Cu/SBA-15 f | 265 | 0.1 | 5 | 100 | 98 | [22] |
| 32 | Cu/Al2O3 f | 265 | 0.1 | 1 | 98 | 87 | [23] |
| 33 | Cu/ZrO2 f | 200 | 2.7 | 4 | - | 75 | [49] |
| 34 | Cu/ZrO2 f | 265 | 0.1 | - | 81 | 83 | [21] |
| 35 | Cu/SiO2-Q6 f,c | 250 | 66 | 81 | [50] | ||
| 36 | Cu/Zr0.8-Ce0.2 f,c | 260 | 0.5 | 2 | 88.5 | 94.2 | [51] |
| 37 | Cu-hydrotalcite f | 265 | 0.1 | 2–3 | 87.5 | 95 | [52] |
| 38 | Cu/ZrO2-Al2O3 b | 130 | 3 | 5 | 100 | 100 | [53] |
| 39 | Cu/Al2O3-ZrO2 b | 200 | 3 | 2 | 100 | 100 | [54] |
| 40 | Cu/Al2O3 f | 240 | 3 | 1–5 | 93.7 | 91.5 | [55] |
| 41 | CuCo-Al2O3 f | 250 | 0.1 | 24 | 100 | 99 | [56] |
| 42 | Cu/SiO2 f | 265 | 0.1 | 1 | 78 | 81 | This work |
| Catalyst (% Cu Loading) | Specific Copper Surface Area (m2 g−1Cu) a | Average Particle Size of Cu (nm) b | Conversion of LA (%) a | Selectivity (%) c | |||
|---|---|---|---|---|---|---|---|
| GVL | AL | VA | Others | ||||
| 2 | 120 | 5.55 | 56 | 61 | 27 | 1 | 11 |
| 5 | 132 | 5.11 | 78 | 81 | 11 | 1 | 7 |
| 10 | 76 | 8.97 | 61 | 57 | 29 | - | 14 |
| 15 | 52 | 13.2 | 49 | 41 | 48 | - | 11 |
| 20 | 34 | 20.7 | 33 | 29 | 59 | - | 12 |
| Reaction Temperature (°C) | Conversion of LA (%) a | Selectivity (%) b | |||
|---|---|---|---|---|---|
| GVL | AL | VA | Others | ||
| 250 | 55 | 74 | 20 | 0 | 6 |
| 265 | 78 | 81 | 11 | 1 | 7 |
| 280 | 89 | 69 | 21 | 1 | 9 |
| 295 | 100 | 61 | 27 | 2 | 10 |
| 310 | 100 | 54 | 31 | 5 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boddula, R.; Shanmugam, P.; Srivatsava, R.K.; Tabassum, N.; Pothu, R.; Naik, R.; Saran, A.; Viswanadham, B.; Radwan, A.B.; Al-Qahtani, N. Catalytic Valorisation of Biomass-Derived Levulinic Acid to Biofuel Additive γ-Valerolactone: Influence of Copper Loading on Silica Support. Reactions 2023, 4, 465-477. https://doi.org/10.3390/reactions4030028
Boddula R, Shanmugam P, Srivatsava RK, Tabassum N, Pothu R, Naik R, Saran A, Viswanadham B, Radwan AB, Al-Qahtani N. Catalytic Valorisation of Biomass-Derived Levulinic Acid to Biofuel Additive γ-Valerolactone: Influence of Copper Loading on Silica Support. Reactions. 2023; 4(3):465-477. https://doi.org/10.3390/reactions4030028
Chicago/Turabian StyleBoddula, Rajender, Paramasivam Shanmugam, Rajesh K. Srivatsava, Nabila Tabassum, Ramyakrishna Pothu, Ramachandra Naik, Aditya Saran, Balaga Viswanadham, Ahmed Bahgat Radwan, and Noora Al-Qahtani. 2023. "Catalytic Valorisation of Biomass-Derived Levulinic Acid to Biofuel Additive γ-Valerolactone: Influence of Copper Loading on Silica Support" Reactions 4, no. 3: 465-477. https://doi.org/10.3390/reactions4030028
APA StyleBoddula, R., Shanmugam, P., Srivatsava, R. K., Tabassum, N., Pothu, R., Naik, R., Saran, A., Viswanadham, B., Radwan, A. B., & Al-Qahtani, N. (2023). Catalytic Valorisation of Biomass-Derived Levulinic Acid to Biofuel Additive γ-Valerolactone: Influence of Copper Loading on Silica Support. Reactions, 4(3), 465-477. https://doi.org/10.3390/reactions4030028








