One-Pot Synthesis of Stable Poly([c2]Daisy–chain Rotaxane) with Pseudo-Stopper via Metathesis Reaction and Thiol-Ene Reaction
Abstract
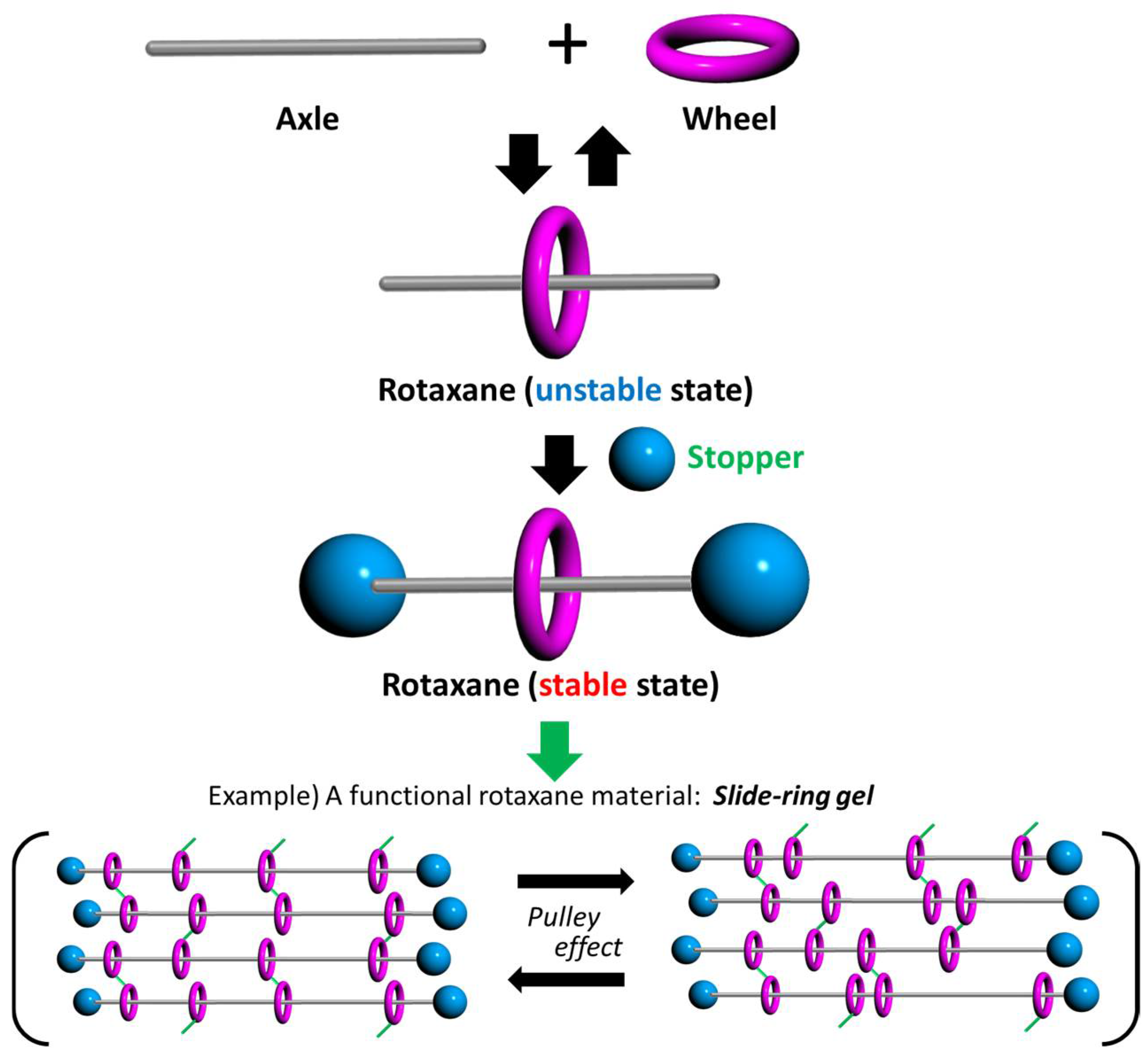
1. Introduction
2. Results and Discussion
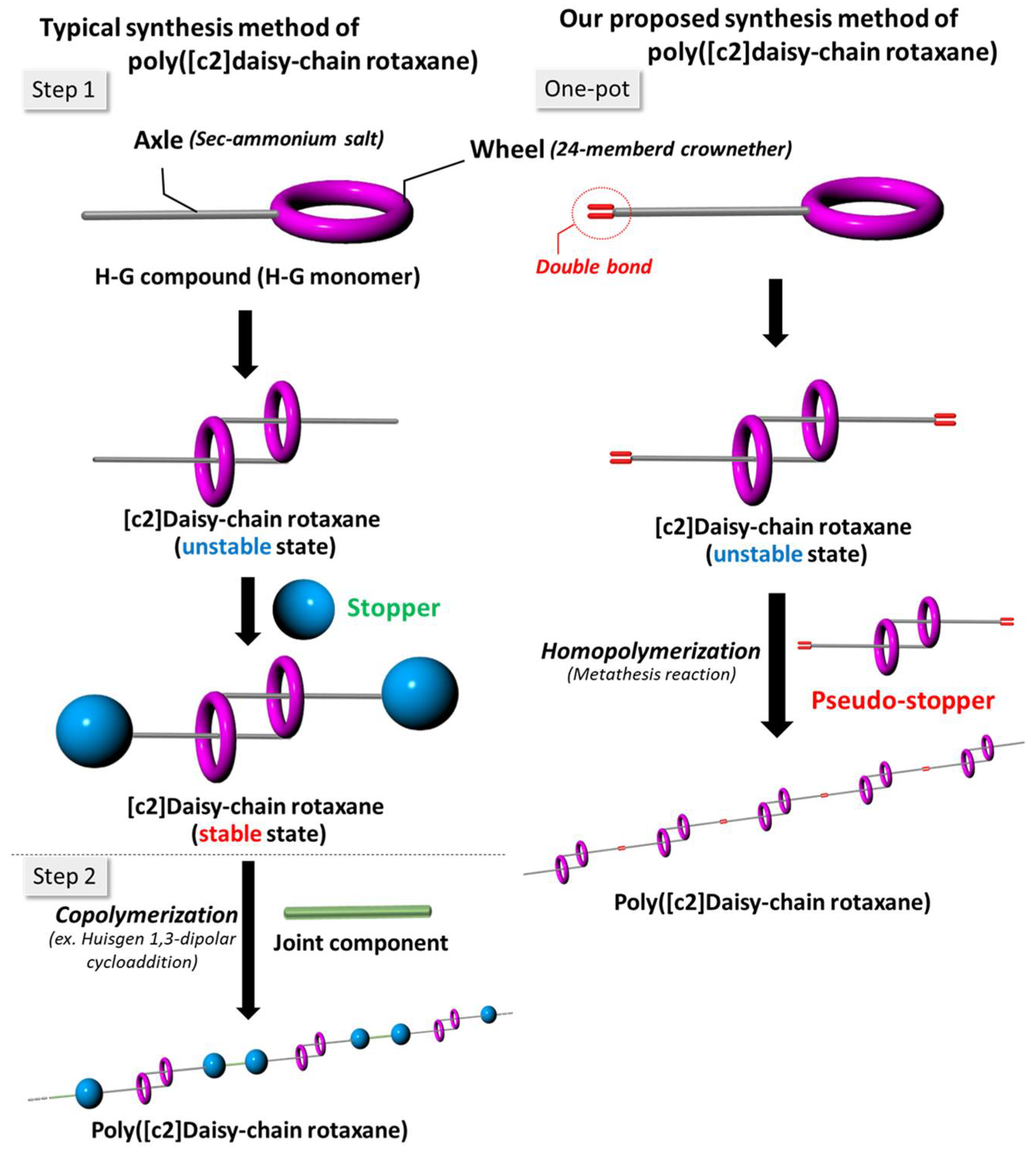
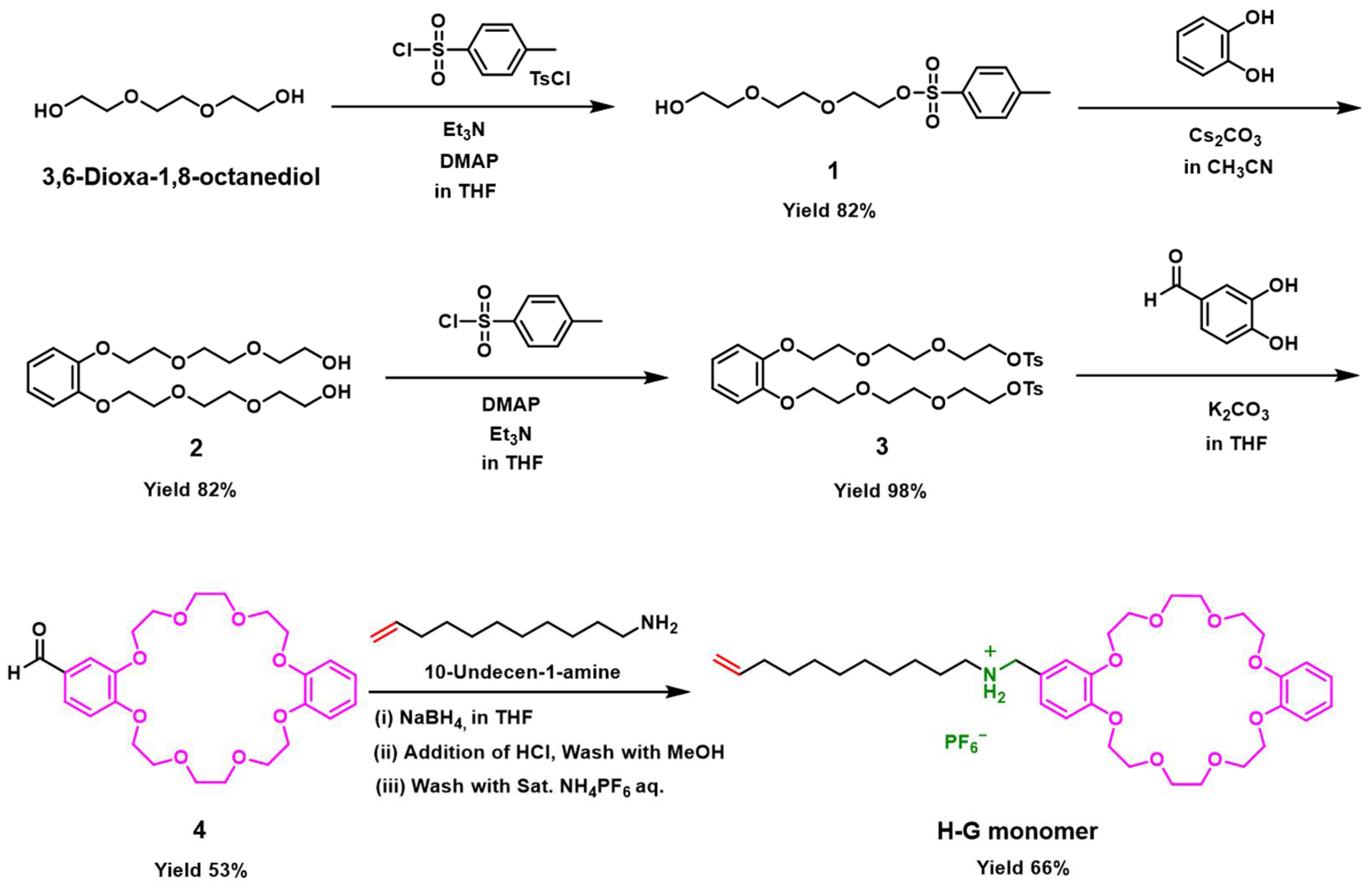
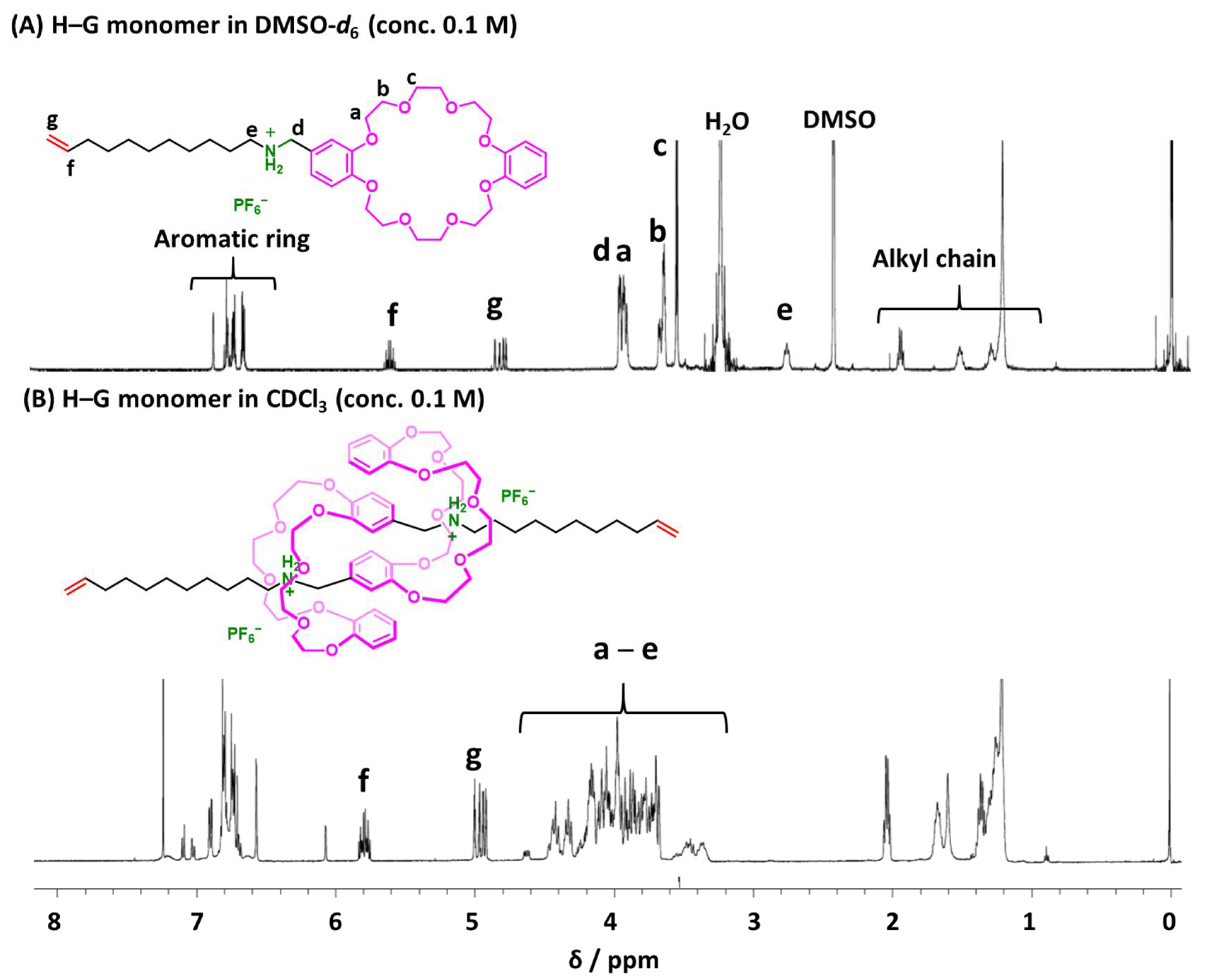
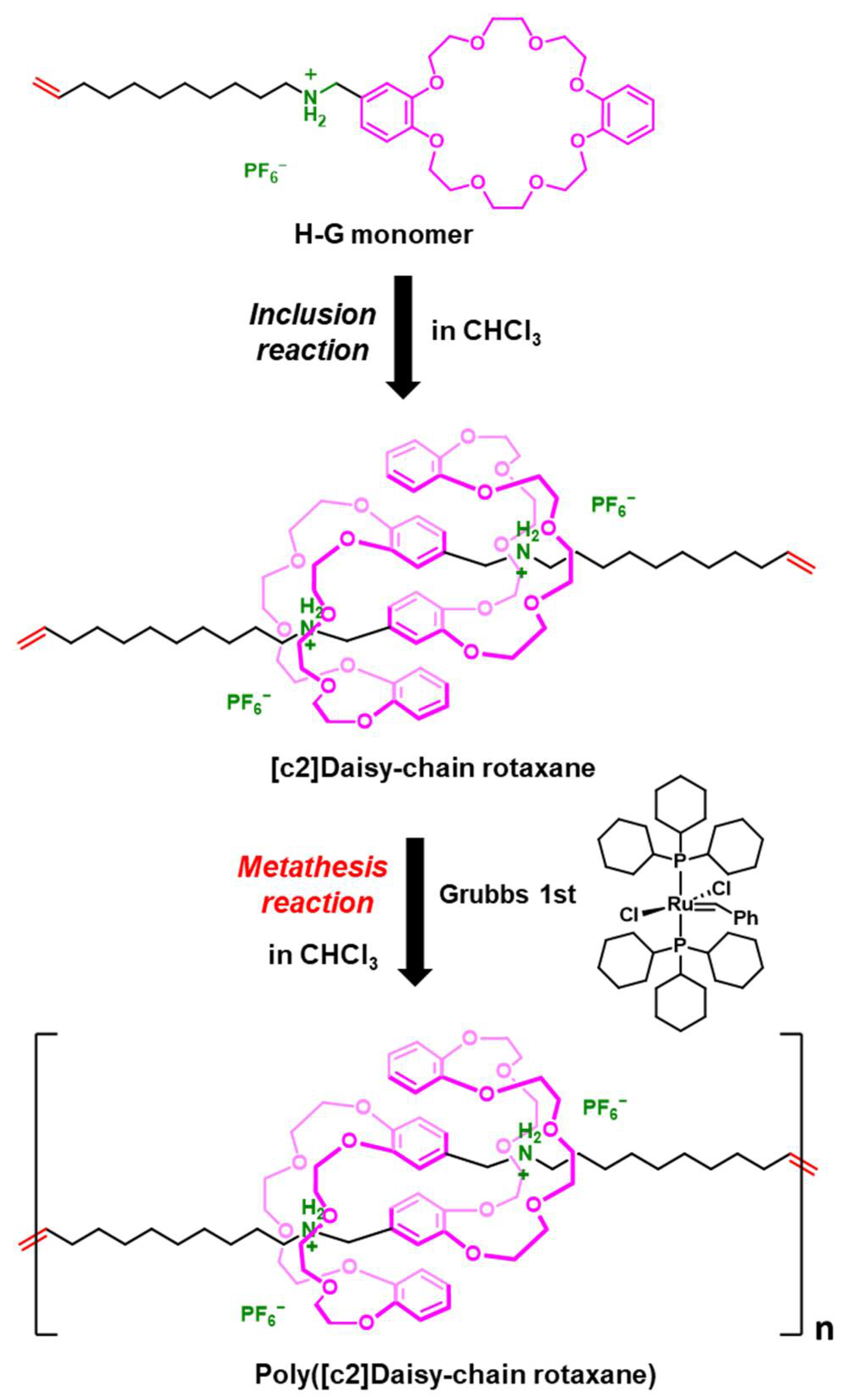
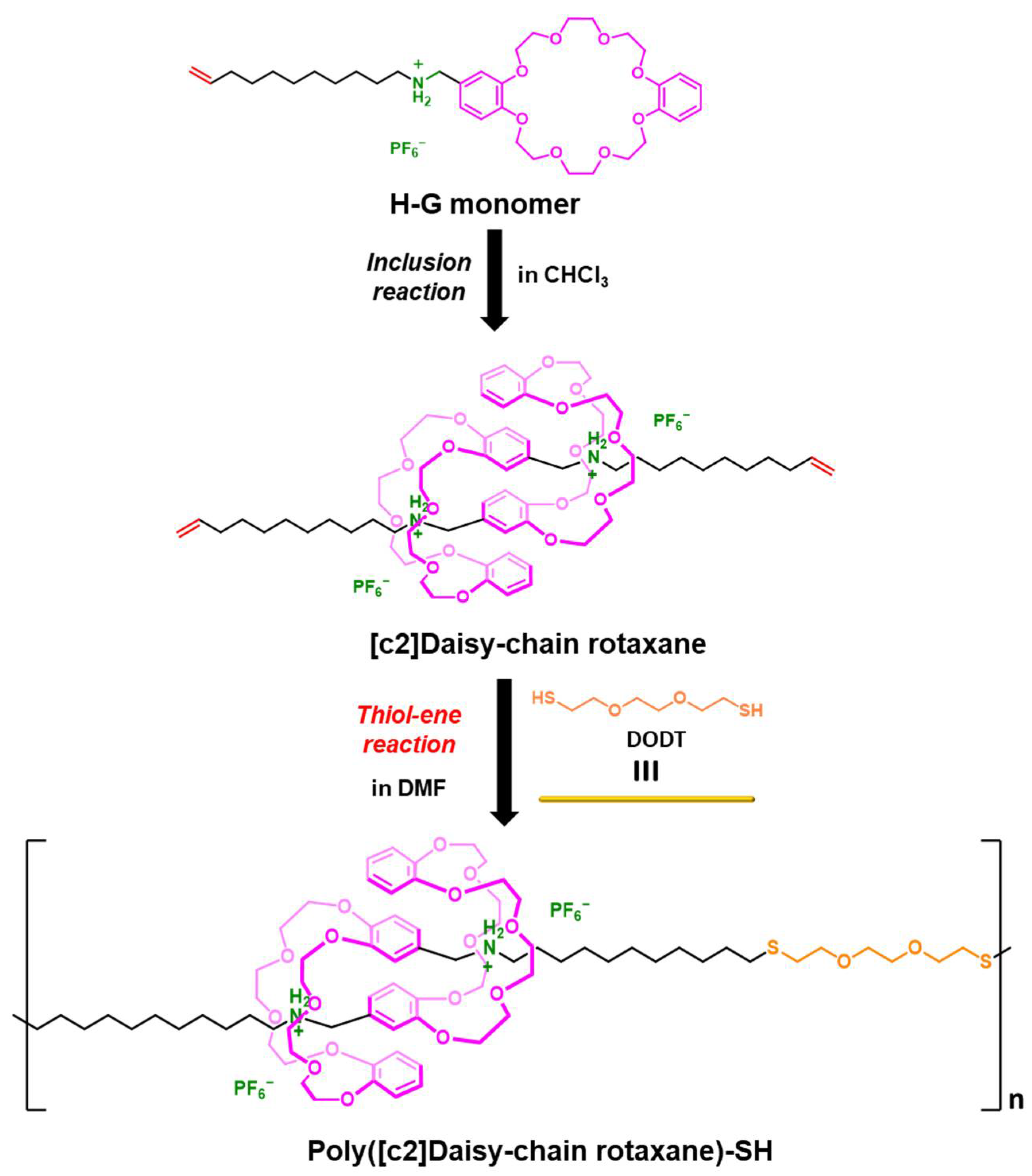
2.1. Design of H-G Monomer and Preparation of [c2]Daisy-chain Rotaxane
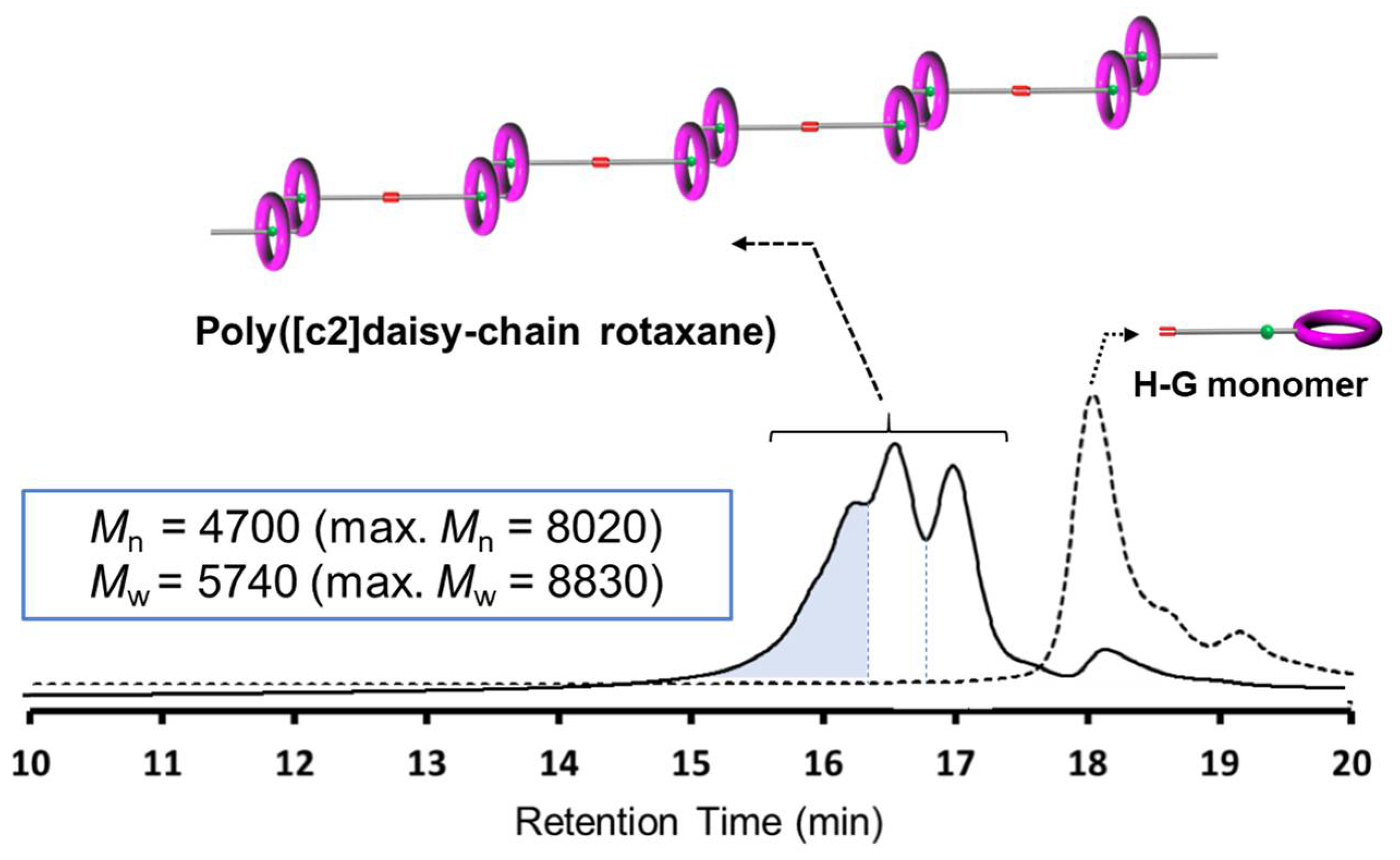
2.2. One-Pot Synthesis of Poly([c2]Daisy-chain Rotaxane) by Using Metathesis Reaction of H-G Monomer
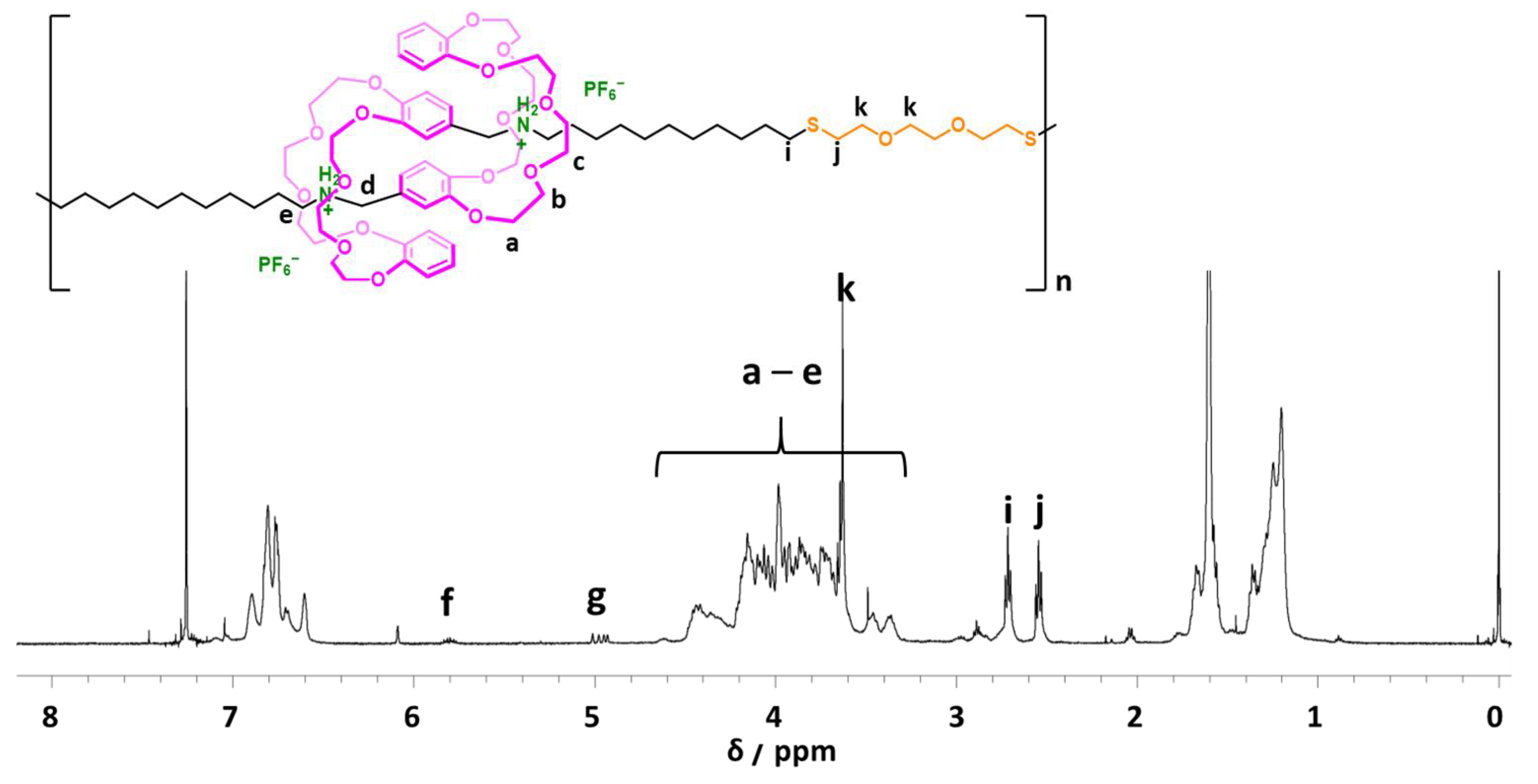
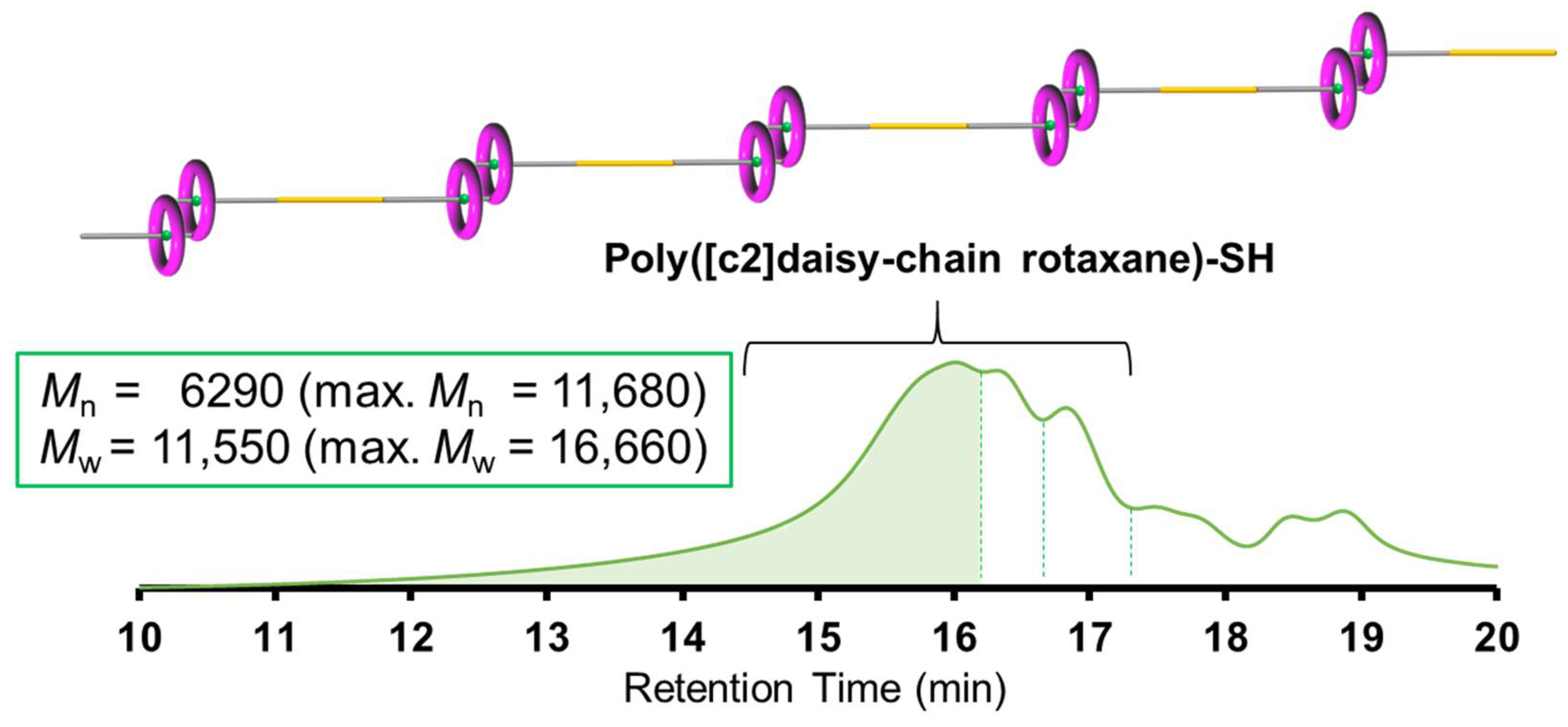
2.3. One-Pot Synthesis of Poly([c2]Daisy-chain Rotaxane) by Using Thiol-Ene Reaction of H-G Monomer
3. Conclusions
4. Experimental Section
4.1. Materials and Instruments
4.2. Synthesis of Compound 1
4.3. Synthesis of Compound 2
4.4. Synthesis of Compound 3
4.5. Synthesis of Compound 4
4.6. Synthesis of Compound 5
4.7. Synthesis of H-G Monomer
4.8. Synthesis of Poly([c2]Daisy-chain Rotaxane)
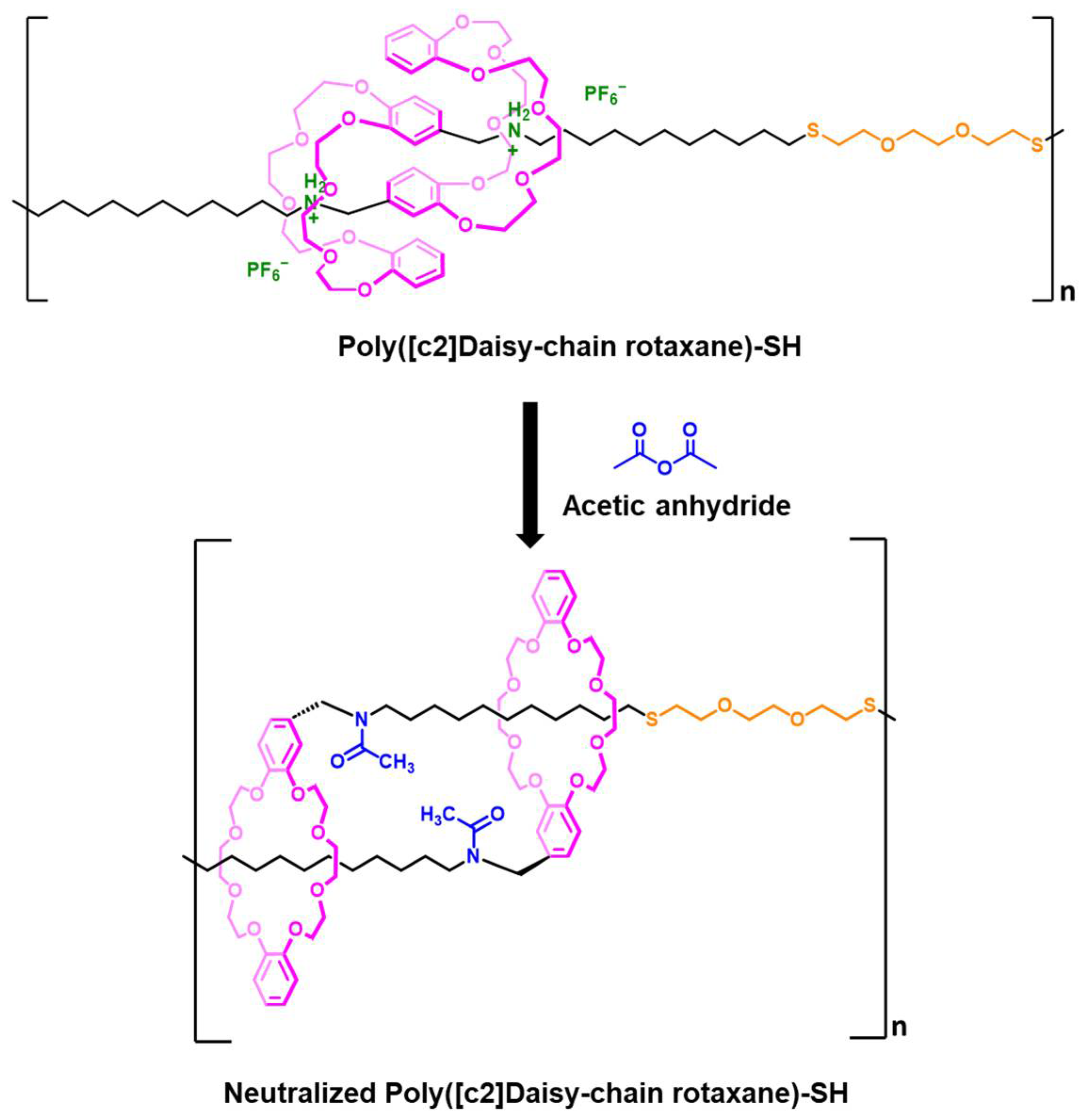
4.9. Synthesis of Poly([c2]Daisy-chain Rotaxane)-SH
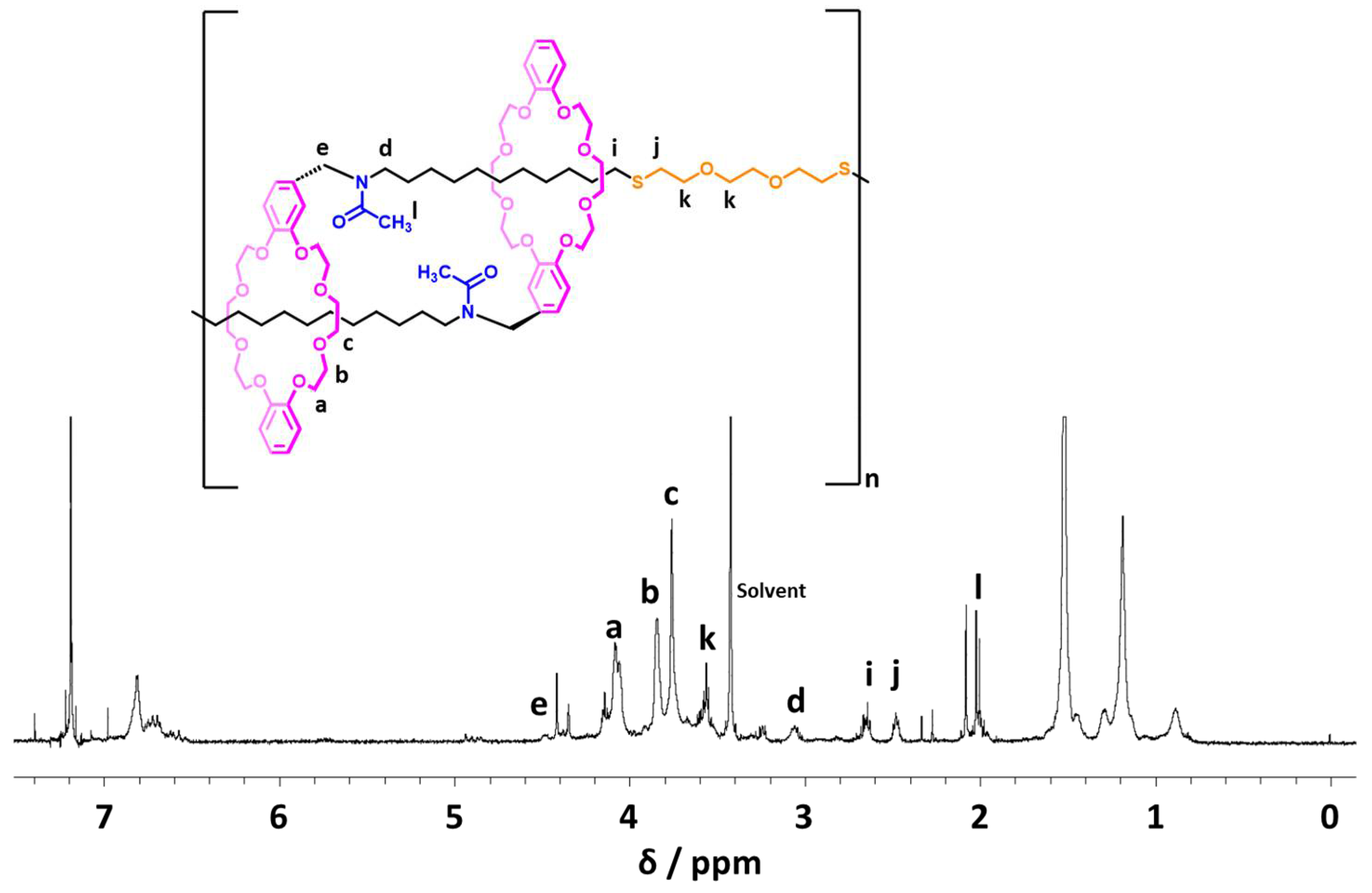
4.10. Synthesis of Neutralized Poly([c2]Daisy-chain Rotaxane)-SH
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harada, A.; Kamachi, M. Complex formation between cyclodextrin and poly(propylene glycol). J. Chem. Soc. Chem. Commun. 1990, 19, 1322–1323. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Synthesis of a tubular polymer from threaded cyclodextrins. Nature 1993, 364, 516–518. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Nakamitsu, T.; Kamachi, M. Preparation and characterization of polyrotaxanes containing many threaded α-cyclodextrins. J. Org. Chem. 1993, 58, 7524–7528. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Preparation and Characterization of a Polyrotaxane Consisting of Monodisperse Poly(ethylene glycol) and. alpha.-Cyclodextrins. J. Am. Chem. Soc. 1994, 116, 3192–3196. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Double-stranded inclusion complexes of cyclodextrin threaded on poly(ethylene glycol). Nature 1994, 370, 126–128. [Google Scholar] [CrossRef]
- Harada, A. Design and construction of supramolecular architectures consisting of cyclodextrins and polymers. Adv. Polym. Sci. 1997, 133, 141–191. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Molecular recognition: Preparation of polyrotaxane and tubular polymer from cyclodextrin. Polym. Adv. Technol. 1997, 8, 241–249. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Non-ionic [2]rotaxanes containing methylated α-cyclodextrins. Chem. Commun. 1997, 15, 1413–1414. [Google Scholar] [CrossRef]
- Harada, A. Construction of supramolecular structures from cyclodextrins and polymers. Carbohydr. Polym. 1997, 34, 183–188. [Google Scholar] [CrossRef]
- Okada, M.; Kamachi, M.; Harada, A. Preparation and Characterization of Inclusion Complexes between Methylated Cyclodextrins and Poly(tetrahydrofuran). Macromolecules 1999, 32, 7202–7207. [Google Scholar] [CrossRef]
- Ashton, P.R.; Grognuz, M.; Slawin, A.M.Z.; Stoddart, J.F.; Williams, D.J. The template-directed synthesis of a [2]rotaxane. Tetrahedron Lett. 1991, 32, 6235–6238. [Google Scholar] [CrossRef]
- Ashton, P.R.; Philp, D.; Spencer, N.; Stoddart, J.F. The self-assembly of [n]pseudorotaxanes. J. Chem. Soc. 1991, 23, 1677–1679. [Google Scholar] [CrossRef]
- Shen, Y.X.; Gibson, H.W. Synthesis and some properties of polyrotaxanes comprised of polyurethane backbone and crown ethers. Macromolecules 1992, 25, 2058–2059. [Google Scholar] [CrossRef]
- Ashton, P.R.; Philp, D.; Spencer, N.; Stoddart, J.F. A new design strategy for the self-assembly of molecular shuttles. J. Chem. Soc. 1992, 16, 1124–1128. [Google Scholar] [CrossRef]
- Gibson, H.W.; Marand, H. Polyrotaxanes: Molecular composites derived by physical linkage of cyclic and linear species. Adv. Mater. 1993, 5, 11–21. [Google Scholar] [CrossRef]
- Bissell, R.A.; Cordova, E.; Kaifer, A.E.; Stoddart, J.F. A chemically and electrochemically switchable molecular shuttle. Nature 1994, 369, 133–137. [Google Scholar] [CrossRef]
- Diederich, F.; Dietrich-Buchecker, C.; Nierengarten, J.; Sauvage, J.P. A copper(I)-complexed rotaxane with two fullerene stoppers. J. Chem. Soc. 1995, 7, 781–782. [Google Scholar] [CrossRef]
- Gibson, H.W.; Liu, S.; Lecavalier, P.; Wu, C.; Shen, Y.X. Synthesis and Preliminary Characterization of Some Polyester Rotaxanes. J. Am. Chem. Soc. 1995, 117, 852–874. [Google Scholar] [CrossRef]
- Ashton, P.R.; Glink, P.T.; Martinez-Diaz, M.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Thermodynamically controlled self-assembly of pseudorotaxanes and pseudopolyrotaxanes with different recognition motifs operating self-selectively. Angew. Chem. Int. Ed. 1996, 35, 1930–1933. [Google Scholar] [CrossRef]
- Gong, C.; Gibson, H.W. Synthesis and Characterization of a Polyester/Crown Ether Rotaxane Derived from a Difunctional Blocking Group. Macromolecules 1996, 29, 7029–7033. [Google Scholar] [CrossRef]
- Cao, J.; Fyfe, M.C.T.; Stoddart, J.F.; Cousins, G.R.L.; Glink, P.T. Molecular Shuttles by the Protecting Group Approach. J. Chem. Soc. 2000, 65, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Budesinksy, M.; Cvacka, J.; Sauvage, J.P. Copper(I)-directed formation of a cyclic pseudorotaxane tetramer and its trimeric homologue. Angew. Chem. Int. Ed. 2006, 45, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Nakazono, K.; Kuwata, S.; Takata, T. Crown ether–tert-ammonium salt complex fixed as rotaxane and its derivation to nonionic rotaxane. Tetrahedron Lett. 2008, 49, 2397–2401. [Google Scholar] [CrossRef]
- Zhu, N.; Nakazono, K.; Takata, T. Solid-state Rotaxane Switch: Synthesis of Thermoresponsive Rotaxane Shuttle Utilizing a Thermally Decomposable Acid. Chem. Lett. 2016, 45, 445–447. [Google Scholar] [CrossRef]
- Takata, T.; Kawasaki, H.; Asai, S.; Furusho, Y.; Kihar, N. Conjugate addition-approach to end-capping of pseudorotaxanes for rotaxane synthesis. Chem. Lett. 1999, 3, 223–224. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Oku, T.; Furusho, Y.; Takata, T. A concept for recyclable cross-linked polymers: Topologically networked polyrotaxane capable of undergoing reversible assembly and disassembly. Angew. Chem. Int. Ed. 2004, 43, 966–969. [Google Scholar] [CrossRef]
- Takata, T. Polyrotaxane and Polyrotaxane Network: Supramolecular Architectures Based on the Concept of Dynamic Covalent Bond Chemistry. Polym. J. 2006, 38, 1–20. [Google Scholar] [CrossRef]
- Yamabuki, K.; Isobe, Y.; Onimura, K.; Oishi, T. Pseudorotaxane-coupled Gel: A New Concept of Interlocked Gel Synthesis by Using Metathesis Reaction. Polym. J. 2008, 40, 205–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Jia, S.; Lin, K.; Fan, G.; Yuan, J.; Yu, S.; Shi, J. Specific Modification with TPGS and Drug Loading of Cyclodextrin Polyrotaxanes and the Enhanced Antitumor Activity Study In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2019, 11, 46427–46436. [Google Scholar] [CrossRef] [PubMed]
- Takata, T. Switchable Polymer Materials Controlled by Rotaxane Macromolecular Switches. ACS Cent. Sci. 2020, 6, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Uenuma, S.; Maeda, R.; Yokoyama, H.; Ito, K. Molecular Recognition of Fluorescent Probe Molecules with a Pseudopolyrotaxane Nanosheet. ACS Macro Lett. 2021, 10, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.F.A.; Othman, M.H.; Taharabaru, T.; Elamin, K.M.; Ito, K.; Inoue, M.; El-Badry, M.; Saleh, K.I.; Onodera, R.; Motoyama, K. Stabilization and Movable Ligand-Modification by Folate-Appended Polyrotaxanes for Systemic Delivery of siRNA Polyplex. ACS Macro Lett. 2022, 11, 1225–1229. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Xu, H.; Wang, Q.; Peng, W.; Chi, S.; Wang, C.; Wang, J.; Deng, Y. Polymer-in-Salt Electrolyte from Poly(caprolactone)-graft-polyrotaxane for Ni-Rich Lithium Metal Batteries at Room Temperature. ACS Appl. Mater. Interfaces 2023, 15, 31552–31560. [Google Scholar] [CrossRef]
- Mayumi, K.; Ito, K. Structure and dynamics of polyrotaxane and slide-ring materials. Polymer 2010, 51, 959–967. [Google Scholar] [CrossRef]
- Hoshino, T.; Miyauchi, M.; Kawaguchi, Y.; Yamaguchi, H.; Harada, A. Daisy Chain Necklace: Tri[2]rotaxane Containing Cyclodextrins. J. Am. Chem. Soc. 2000, 122, 9876–9877. [Google Scholar] [CrossRef]
- Jiménez, M.C.; Dietrich-Buchecker, C.; Sauvage, J.P. Towards Synthetic Molecular Muscles: Contraction and Stretching of a Linear Rotaxane Dimer. Angew. Chem. Int. Ed. 2000, 39, 3284–3287. [Google Scholar] [CrossRef]
- Amirsakis, D.G.; Elizarov, A.M.; Garcia-Garibay, M.A.; Glink, P.T.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Diastereospecific Photochemical Dimerization of a Stilbene-Containing Daisy Chain Monomer in Solution as well as in the Solid State. Angew. Chem. Int. Ed. 2003, 42, 1126–1132. [Google Scholar] [CrossRef]
- Tsukagoshi, S.; Miyawaki, A.; Takashima, Y.; Yamaguchi, Y.; Harada, A. Contraction of Supramolecular Double-Threaded Dimer Formed by α-Cyclodextrin with a Long Alkyl Chain. Org. Lett. 2007, 9, 1053–1055. [Google Scholar] [CrossRef]
- Coutrot, F.; Romuald, C.; Busseron, E. A New pH-Switchable Dimannosyl[c2]Daisy Chain Molecular Machine. Org. Lett. 2008, 10, 3741–3744. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Leung, K.C.F.; Bnitez, D.; Han, J.Y.; Cantrill, S.J.; Fang, L.; Stoddart, J.F. An Acid–Base-Controllable [c2]Daisy Chain. Angew. Chem. Int. Ed. 2008, 47, 7470–7474. [Google Scholar] [CrossRef] [PubMed]
- Romuald, C.; Busseron, E.; Coutrot, F. Very Contracted to Extended co-Conformations with or without Oscillations in Two- and Three-Station [c2]Daisy Chains. J. Org. Chem. 2010, 75, 6516–6531. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Klautzsch, F.; Xue, M.; Huang, F.; Schalley, C.A. Self-sorting of crown ether/secondary ammonium ion hetero-[c2]daisy chain pseudorotaxanes. Org. Chem. Front. 2014, 1, 532–540. [Google Scholar] [CrossRef][Green Version]
- Bruns, C.J.; Stoddart, J.F. Rotaxane-Based Molecular Muscles. Acc. Chem. Res. 2014, 47, 2186–2199. [Google Scholar] [CrossRef]
- Wolf, A.; Moulin, E.; Cid, J.J.; Goujon, A.; Du, G.; Busseron, E.; Fuks, G.; Giuseppone, N. pH and light-controlled self-assembly of bistable [c2] daisy chain rotaxanes. Chem. Commun. 2015, 51, 4212–4215. [Google Scholar] [CrossRef]
- Goujon, A.; Du, G.; Moulin, E.; Fuks, G.; Maaloum, M.; Buhler, E.; Giuseppone, N. Hierarchical Self-Assembly of Supramolecular Muscle-Like Fibers. Angew. Chem. 2015, 55, 703–707. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, Q.; Rao, S.J.; Qu, D.H.; Tian, H. One-pot synthesis of a [c2]daisy-chain-containing hetero[4]rotaxane via a self-sorting strategy. Chem. Sci. 2016, 7, 1696–1701. [Google Scholar] [CrossRef]
- Wang, P.; Gao, Z.; Yuan, M.; Zhu, J.; Wang, F. Mechanically linked poly[2]rotaxanes constructed from the benzo-21-crown-7/secondary ammonium salt recognition motif. Polym. Chem. 2016, 7, 3664–3668. [Google Scholar] [CrossRef]
- Aoki, D.; Aibara, G.; Uchida, S.; Takata, T. A Rational Entry to Cyclic Polymers via Selective Cyclization by Self-Assembly and Topology Transformation of Linear Polymers. J. Am. Chem. Soc. 2017, 139, 6791–6794. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, S.; Xie, T.; Li, X.; Xu, T.; Li, D.; Qu, D.; Long, Y.; Tian, H. Muscle-like Artificial Molecular Actuators for Nanoparticles. Chem 2018, 4, 2670–2684. [Google Scholar] [CrossRef]
- Rao, S.J.; Ye, X.H.; Zhang, Q.; Gao, C.; Wang, W.Z.; Qu, D.H. Light-Induced Cyclization of A [c2]Daisy-Chain Rotaxane to Form a Shrinkable Double-Lasso Macrocycle. Asian J. Org. Chem. 2018, 7, 902–905. [Google Scholar] [CrossRef]
- Wolf, A.; Cid, J.-J.; Moulin, E.; Niess, F.; Du, G.; Goujon, A.; Busseron, E.; Ruff, A.; Ludwigs, S.; Giuseppone, N. Unsymmetric Bistable [c2]Daisy Chain Rotaxanes which Combine Two Types of Electroactive Stoppers. Eur. J. Org. Chem. 2019, 2019, 3421–3432. [Google Scholar] [CrossRef]
- Van Raden, J.M.; Jarenwattananon, N.N.; Zakharov, L.N.; Jasti, R. Active Metal Template Synthesis and Characterization of a Nanohoop [c2]Daisy Chain Rotaxane. Chem. Eur. J. 2020, 26, 10205–10209. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Shi, Y.; Zhuang, G.; Zhang, L.; Qiu, Y.; Shen, D.; Chen, H.; Jiao, Y.; Wu, H.; Cheng, C.; et al. Molecular-Pump-Enabled Synthesis of a Daisy Chain Polymer. J. Am. Chem. Soc. 2020, 142, 10308–10313. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.V.; Zhang, Y.; Link, A.J. Dynamic covalent self-assembly of mechanically interlocked molecules solely made from peptides. Nat. Chem. 2021, 13, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Komai, Y.; Fujiwara, S.; Nishiyama, Y. Cyclodextrin-Based [c2]Daisy Chain Rotaxane Insulating Two Diarylacetylene Cores. Chem. Eur. J. 2021, 27, 1966–1969. [Google Scholar] [CrossRef]
- Chu, C.; Stares, D.L.; Schalley, C.A. Light-controlled interconversion between a [c2]daisy chain and a lasso-type pseudo[1]rotaxane. Chem. Commun. 2021, 57, 12317–12320. [Google Scholar] [CrossRef]
- Nhien, P.Q.; Tien, J.H.; Cuc, T.T.K.; Khang, T.M.; Trung, N.T.; Wu, C.H.; Hue, B.T.B.; Judy, I.W.; Lin, H.C. Reversible fluorescence and Forster resonance energy transfer switching behaviours of bistable photo-switchable [c2] daisy chain rotaxanes and photo-patterning applications. J. Mater. Chem. C 2022, 10, 18241–18257. [Google Scholar] [CrossRef]
- Saura-Sanmartin, A.; Pastor, A.; Martinez-Cuezva, A.; Berna, J. Maximizing the [c2]daisy chain to lasso ratio through competitive self-templating clipping reactions. Chem. Commun. 2022, 58, 290–293. [Google Scholar] [CrossRef]
- Trung, N.T.; Nhien, P.Q.; Kim Cuc, T.T.; Wu, C.; Buu Hue, B.T.; Wu, J.I.; Li, Y.; Lin, H. Controllable Aggregation-Induced Emission and Förster Resonance Energy Transfer Behaviors of Bistable [c2]Daisy Chain Rotaxanes for White-Light Emission and Temperature-Sensing Applications. ACS Appl. Mater. Interfaces 2023, 15, 15353–15366. [Google Scholar] [CrossRef] [PubMed]
- Saura-Sanmartin, A.; Schalley, C.A. The Mobility of Homomeric Lasso- and Daisy Chain-Like Rotaxanes in Solution and in the Gas Phase as a means to Study Structure and Switching Behaviour. Isr. J. Chem. 2023, 63, e202300022. [Google Scholar] [CrossRef]
- Clark, P.G.; Day, M.W.; Grubbs, R.H. Switching and Extension of a [c2]Daisy-Chain Dimer Polymer. J. Am. Chem. Soc. 2009, 131, 13631–13633. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Moulin, E.; Jouault, N.; Buhler, E.; Giuseppone, N. Muscle-like Supramolecular Polymers: Integrated Motion from Thousands of Molecular Machines. Angew. Chem. 2012, 51, 12504–12508. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Z.; Zheng, B.; Huang, F. Construction of muscle-like metallo-supramolecular polymers from a pillar[5]arene-based [c2]daisy chain. Polym. Chem. 2014, 5, 5734–5739. [Google Scholar] [CrossRef]
- Fu, X.; Gu, R.R.; Zhang, Q.; Rao, S.J.; Zheng, X.L.; Qu, D.H.; Tian, H. Phototriggered supramolecular polymerization of a [c2]daisy chain rotaxane. Polym. Chem. 2016, 7, 2166–2170. [Google Scholar] [CrossRef]
- Goujon, A.; Mariani, G.; Lang, T.; Moulin, E.; Rawiso, M.; Buhler, E.; Giuseppone, E. Controlled Sol–Gel Transitions by Actuating Molecular Machine Based Supramolecular Polymers. J. Am. Chem. Soc. 2017, 139, 4923–4928. [Google Scholar] [CrossRef]
- Goujon, A.; Lang, T.; Mariani, G.; Moulin, E.; Fuks, G.; Raya, J.; Buhler, E.; Giuseppone, N. Bistable [c2] Daisy Chain Rotaxanes as Reversible Muscle-like Actuators in Mechanically Active Gel. J. Am. Chem. Soc. 2017, 139, 14825–14828. [Google Scholar] [CrossRef]
- Goujon, A.; Moulin, E.; Fuks, G.; Giuseppone, N. [c2]Daisy chain rotaxanes as molecular muscles. CCS Chem. 2019, 1, 83–96. [Google Scholar] [CrossRef]
- Zhang, Z.; You, W.; Li, P.; Zhao, J.; Guo, Z.; Xu, T.; Chen, J.; Yu, W.; Yan, X. Insights into the Correlation of Microscopic Motions of [c2]Daisy Chains with Macroscopic Mechanical Performance for Mechanically Interlocked Networks. J. Am. Chem. Soc. 2023, 145, 567–578. [Google Scholar] [CrossRef]
- Hmadeh, M.; Fang, L.; Trabolsi, A.; Elhabiri, M.; Albrecht-Gary, A.M.; Stoddart, J.F. On the thermodynamic and kinetic investigations of a [c2]daisy chain polymer. J. Mater. Chem. 2010, 20, 3422–3430. [Google Scholar] [CrossRef]
- Fu, G.C.; Nguyen, S.T.; Grubbs, R.H. Catalytic ring-closing metathesis of functionalized dienes by a ruthenium carbene complex. J. Am. Chem. Soc. 1993, 115, 9856–9857. [Google Scholar] [CrossRef]
- Fox, H.H.; Schrock, R.R.; O’Dell, R. Coupling of Terminal Olefins by Molybdenum(VI) Imido Alkylidene Complexes. Organometallics 1994, 13, 635–639. [Google Scholar] [CrossRef]
- Miller, S.J.; Grubbs, R.H. Synthesis of Conformationally Restricted Amino Acids and Peptides Employing Olefin Metathesis. J. Am. Chem. Soc. 1995, 117, 5855–5856. [Google Scholar] [CrossRef]
- Hoveyda, A.H.; Zhugralin, A.R. The remarkable metal-catalysed olefin metathesis reaction. Nature 2007, 450, 243–251. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Sadaiah, K.; Ramakrishna, D.S.; Police, N.; Sridhar, B.; Bharatam, J. Highly enantioselective carbon-carbon bond formation by Cu-catalyzed asymmetric [2,3]-sigmatropic rearrangement: Application to the syntheses of seven-membered oxacycles and six-membered carbocycles. Chem. Commun. 2008, 42, 5324–5326. [Google Scholar] [CrossRef][Green Version]
- Reddy, K.K.S.; Rao, B.V.; Raju, S.S. A common approach to pyrrolizidine and indolizidine alkaloids; formal synthesis of (-)-isoretronecanol, (-)-trachelanthamidine and an approach to the synthesis of (-)-5-epitashiromine and (-)-tashiromine. Tetrahedron Asymmetry 2011, 22, 662–668. [Google Scholar] [CrossRef]
- Rao, G.B.D.; Anjaneyulu, B.; Kaushik, M.P. Greener and expeditious one-pot synthesis of dihydropyrimidinone derivatives using non-commercial β-ketoesters via the Biginelli reaction. RSC Adv. 2014, 4, 43321–43325. [Google Scholar] [CrossRef]
- Rao, G.B.D.; Anjaneyulu, B.; Kaushik, M.P. A facile one-pot five-component synthesis of glycoside annulated dihydropyrimidinone derivatives with 1,2,3-triazol linkage via transesterification/Biginelli/click reactions in aqueous medium. Tetrahedron Lett. 2014, 55, 19–22. [Google Scholar] [CrossRef]
- Bendi, A.; Rao, G.B.D. Strategic One-pot Synthesis of Glycosyl Annulated Phosphorylated/Thiophosphorylated 1,2,3-Triazole Derivatives Using CuFe2O4 Nanoparticles as Heterogeneous Catalyst, their DFT and Molecular Docking Studies as Triazole Fungicides. Lett. Org. Chem. 2023, 20, 568–578. [Google Scholar] [CrossRef]
- Okay, O.; Reddy, S.K.; Bowman, C.N. Molecular Weight Development during Thiol-Ene Photopolymerizations. Macromolecules 2005, 38, 4501–4511. [Google Scholar] [CrossRef]
- Sumerlin, B.S.; Vogt, A.P. Macromolecular Engineering through Click Chemistry and Other Efficient Transformations. Macromolecules 2010, 43, 1–13. [Google Scholar] [CrossRef]
- Mazzolini, J.; Boyron, O.; Monteil, V.; Gigmes, D.; Bertin, D.; D’Agosto, F.; Boisson, C. Polyethylene End Functionalization Using Radical-Mediated Thiol-Ene Chemistry: Use of Polyethylenes Containing Alkene End Functionality. Macromolecules 2011, 44, 3381–3387. [Google Scholar] [CrossRef]
- Yokochi, H.; Ohira, M.; Oka, M.; Honda, S.; Li, X.; Aoki, D.; Otsuka, H. Topology Transformation toward Cyclic, Figure-Eight-Shaped, and Cross-Linked Polymers Based on the Dynamic Behavior of a Bis(hindered amino)disulfide Linker. Macromolecules 2021, 54, 9992–10000. [Google Scholar] [CrossRef]
- Nomura, T.; Onimura, K.; Yamabuki, K. Synthesis and Polymerization of Acid-degradable Rotaxane Using Boc Protecting Group. Chem. Lett. 2022, 51, 16–19. [Google Scholar] [CrossRef]
- Moeller, N.; Ruehling, A.; Lamping, S.; Hellwig, T.; Fallnich, C.; Ravoo, B.J.; Glorius, F. Stabilization of High Oxidation State Upconversion Nanoparticles by N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2017, 56, 4356–4360. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Wang, M.; Zheng, Y.; Koynov, K.; Auernhammer, G.K.; Butt, H.; Ikeda, T. Supramolecular Organogel Based on Crown Ether and Secondary Ammoniumion Functionalized Glycidyl Triazole Polymers. Macromolecules 2013, 46, 4617–4625. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamoto, R.; Onimura, K.; Yamabuki, K. One-Pot Synthesis of Stable Poly([c2]Daisy–chain Rotaxane) with Pseudo-Stopper via Metathesis Reaction and Thiol-Ene Reaction. Reactions 2023, 4, 448-464. https://doi.org/10.3390/reactions4030027
Kamoto R, Onimura K, Yamabuki K. One-Pot Synthesis of Stable Poly([c2]Daisy–chain Rotaxane) with Pseudo-Stopper via Metathesis Reaction and Thiol-Ene Reaction. Reactions. 2023; 4(3):448-464. https://doi.org/10.3390/reactions4030027
Chicago/Turabian StyleKamoto, Risako, Kenjiro Onimura, and Kazuhiro Yamabuki. 2023. "One-Pot Synthesis of Stable Poly([c2]Daisy–chain Rotaxane) with Pseudo-Stopper via Metathesis Reaction and Thiol-Ene Reaction" Reactions 4, no. 3: 448-464. https://doi.org/10.3390/reactions4030027
APA StyleKamoto, R., Onimura, K., & Yamabuki, K. (2023). One-Pot Synthesis of Stable Poly([c2]Daisy–chain Rotaxane) with Pseudo-Stopper via Metathesis Reaction and Thiol-Ene Reaction. Reactions, 4(3), 448-464. https://doi.org/10.3390/reactions4030027





