Zeolite-Containing Co Catalysts for Fischer–Tropsch Synthesis with Tailor-Made Molecular-Weight Distribution of Hydrocarbons
Abstract
1. Introduction
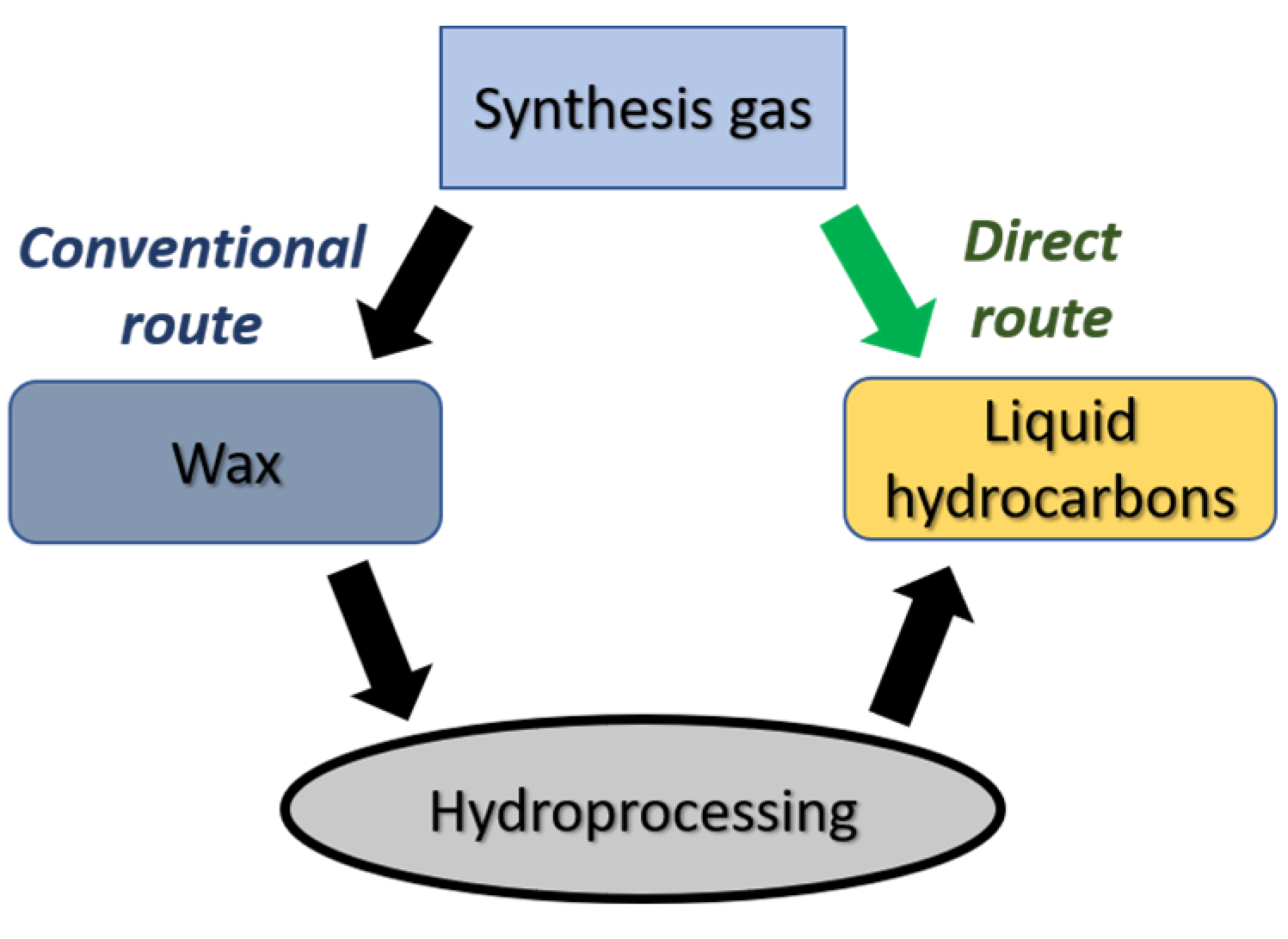
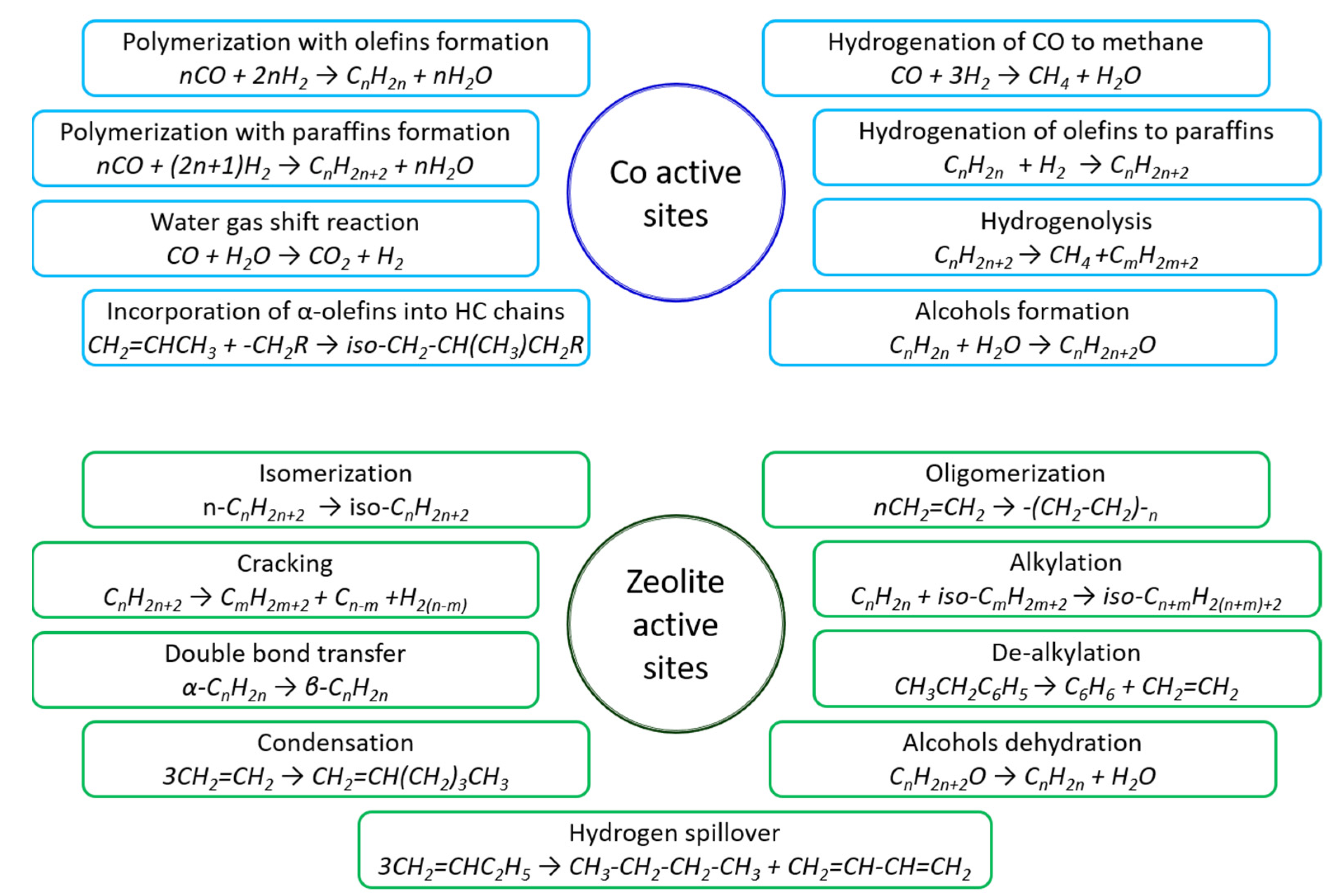
2. The Role of Cobalt in the Formation of Fischer–Tropsch Synthesis Products
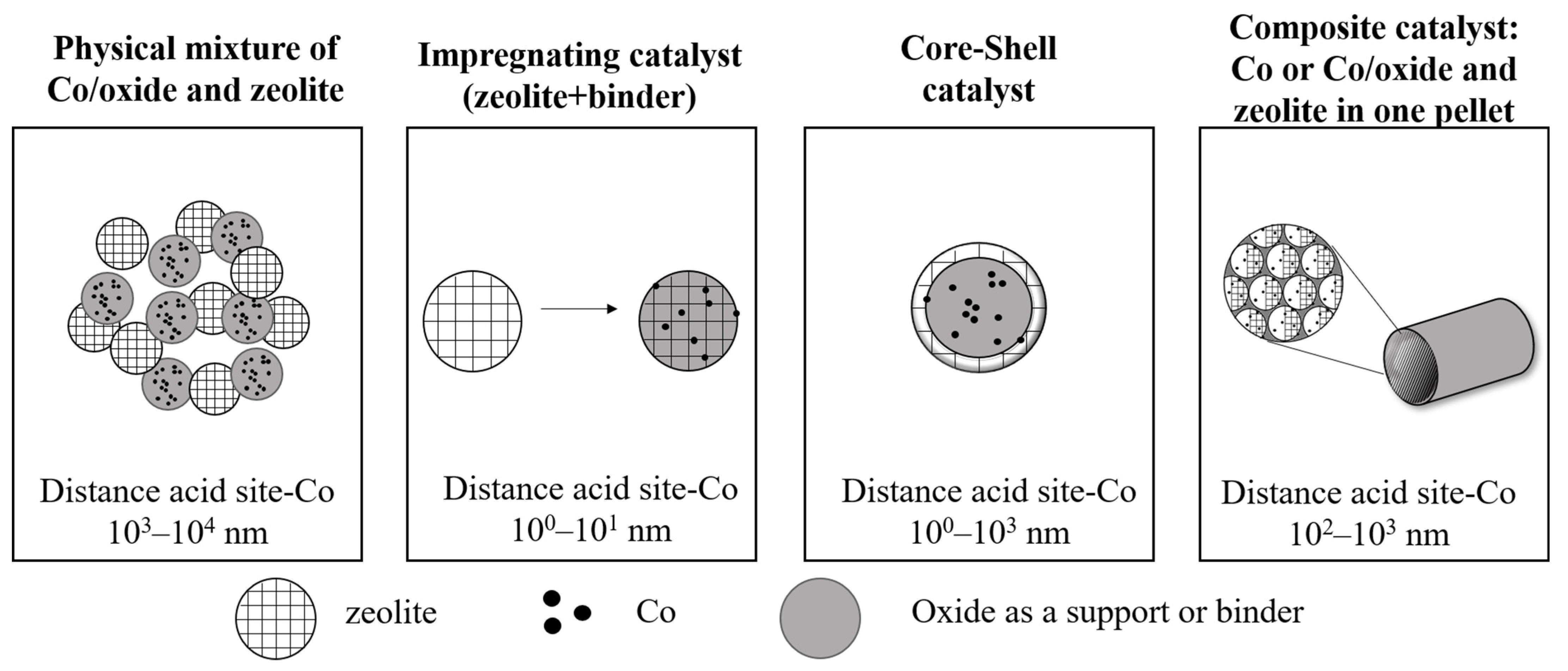
3. Zeolites Properties Responsible for Their Role in Bifunctional Catalysts
4. The Effect of Zeolite in FTS Co Catalysts on the Composition of Products
4.1. One-Step Production of Hydrocarbons Gasoline Fraction
4.2. Selective Production of Isoparaffins
4.3. Single-Stage Production of Diesel Fuel Components
4.4. Direct Production of Synthetic Oil
4.5. Direct Production of Olefins
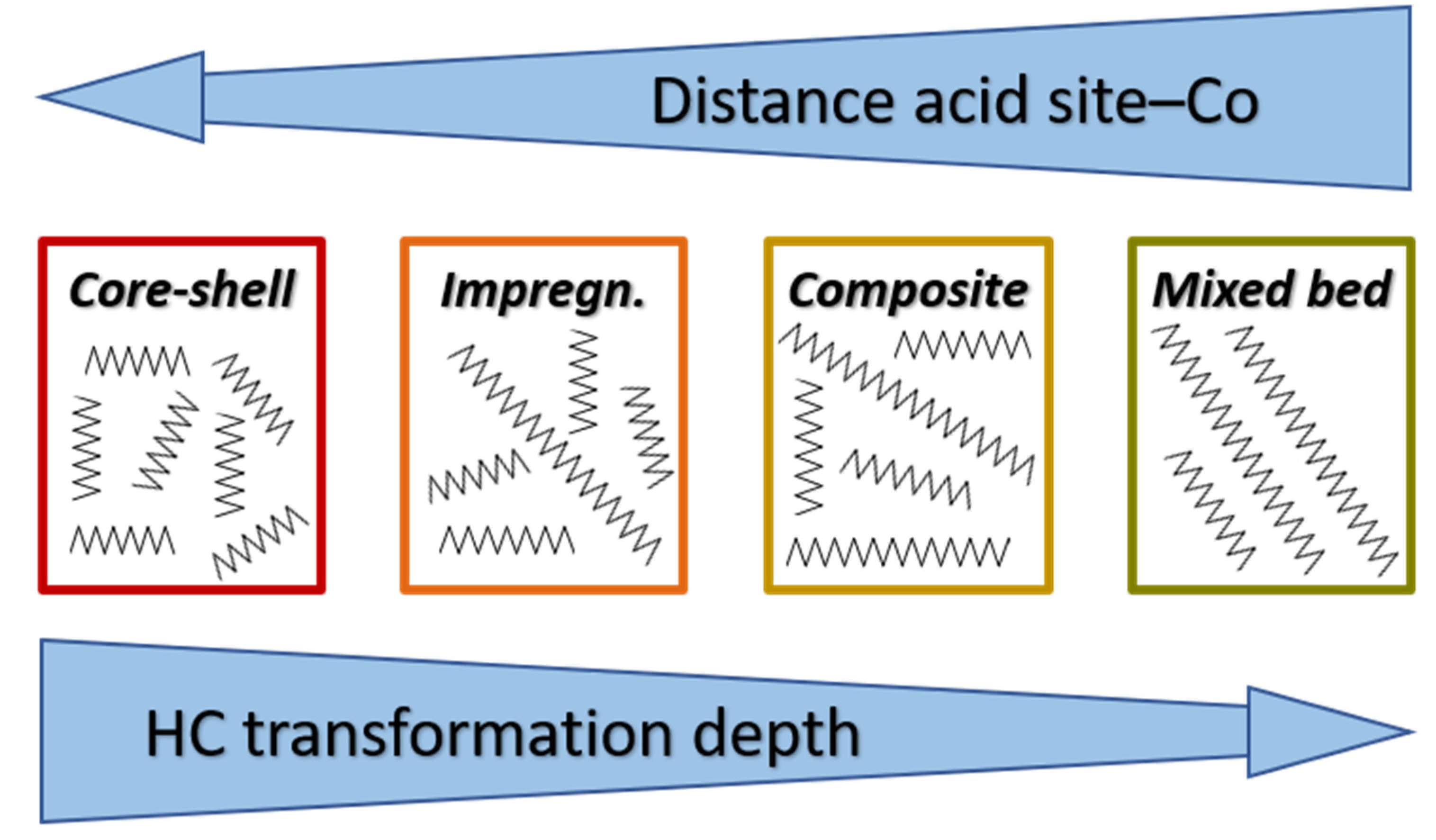
5. Possibilities for the Product Composition Control
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronald, F.; Probstein, R.; Edwin, H. (Eds.) Synthetic Fuels; Courier Corporation: New York, NY, USA, 2006; 490p. [Google Scholar]
- Maitlis, P.M.; Klerk, A. (Eds.) Greener Fischer–Tropsch Processes; Wiley-VCH: Weinheim, Germany, 2013; 372p. [Google Scholar]
- Sineva, L.V.; Mordkovich, V.Z. Trends in gas chemistry catalysis: Cobalt catalysts for Fischer–Topsch synthesis. Part 1. Sci. J. Russ. Gas Soc. 2019, 20, 42–57. [Google Scholar]
- Sineva, L.V.; Mordkovich, V.Z. Trends in gas chemistry catalysis: Cobalt catalysts for Fischer–Topsch synthesis. Part 2. Sci. J. Russ. Gas Soc. 2019, 21, 56–68. [Google Scholar]
- Steynberg, A.P.; Dry, M.E. (Eds.) Fisher–Tropsch Technology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2004; p. 722. [Google Scholar]
- Henrici-Olive, G.; Olive, S. The Chemistry of the Catalyzed Hydrogenation of Carbon Monoxide; Springer: Berlin/Heidelberg, Germany, 1984; 232p. [Google Scholar]
- Lapidus, A.L.; Krylova, A.Y. Catalytic synthesis of isoalkanes and aromatic hydrocarbons from CO and H2. Russ. Chem. Rev. 1998, 67, 941. [Google Scholar] [CrossRef]
- Sartipi, S.; Parashar, K.; Valero-Romero, M.J.; Santos, V.P.; Linden, B.; Makkee, M.; Kapteijn, F.; Gascon, J. Hierarchical H-ZSM-5-supported cobalt for the direct synthesis of gasoline-range hydrocarbons from syngas: Advantages, limitations, and mechanistic insight. J. Catal. 2013, 305, 179–190. [Google Scholar] [CrossRef]
- Sineva, L.V.; Asalieva, E.Y.; Mordkovich, V.Z. The role of zeolite in the Fischer–Tropsch synthesis over cobalt–zeolite catalysts. Russ. Chem. Rev. 2015, 84, 1176. [Google Scholar] [CrossRef]
- Murzin, D.Y. Mesolevel Bifunctional Catalysis. Kinet. Catal. 2020, 61, 80–92. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Wu, C.; Li, H.; Qin, X.; Tsubaki, N. Gasoline-range hydrocarbon synthesis over Co/SiO2/HZSM-5 catalyst with CO2-containing syngas. Fuel Process. Technol. 2010, 91, 388–393. [Google Scholar] [CrossRef]
- Chang, C.D.; Lang, W.H.; Silvestri, A.J. Synthesis gas conversion to aromatic hydrocarbons. J. Catal. 1979, 56, 268–273. [Google Scholar] [CrossRef]
- Oukaci, R.; Wu, J.C.S.; Goodwin, J.R., Jr. Effect of SiAl ratio on secondary reactions during CO hydrogenation on zeolite-supported metal catalysts. J. Catal. 1988, 110, 47–57. [Google Scholar] [CrossRef]
- Jong, S.-J.; Cheng, S. Reduction behavior and catalytic properties of cobalt containing ZSM-5 zeolites. Appl. Catal. A Gen. 1995, 126, 51–66. [Google Scholar] [CrossRef]
- Botes, F.G.; Böhringer, W. The addition of HZSM-5 to the Fischer–Tropsch process for improved gasoline production. Appl. Catal. A Gen. 2004, 267, 217–225. [Google Scholar] [CrossRef]
- Martinez, A.; Prieto, G. The Application of Zeolites and Periodic Mesoporous Silicas in the Catalytic Conversion of Synthesis Gas. Top. Catal. 2009, 52, 75–90. [Google Scholar] [CrossRef]
- Sineva, L.V.; Gorokhova, E.O.; Gryaznov, K.O.; Ermolaev, I.S.; Mordkovich, V.Z. Zeolites as a tool for intensification of mass transfer on the surface of a cobalt Fischer–Tropsch synthesis catalyst. Catal. Today 2021, 378, 140–148. [Google Scholar] [CrossRef]
- Falbe, J. Chemical Feedstocks from Coal, Reprint ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1982; 662p. [Google Scholar]
- Shi, B.; Keogh, R.A.; Davis, B.H. Fischer–Tropsch synthesis: The formation of branched hydrocarbons in the Fe and Co catalyzed reaction. J. Mol. Catal. A Chem. 2005, 234, 85–97. [Google Scholar] [CrossRef]
- Cheng, K.; Kang, J.; King, D.L.; Subramanian, V.; Zhou, C.; Zhang, Q.; Wang, Y. Chapter Three—Advances in Catalysis for Syngas Conversion to Hydrocarbons. Adv. Catal. 2017, 60, 125–208. [Google Scholar] [CrossRef]
- Kuipers, E.W.; Scheper, C.; Wilson, J.H.; Vinkenburg, I.H.; Oosterbeek, H. Non-ASF Product Distributions Due to Secondary Reactions during Fischer–Tropsch Synthesis. J. Catal. 1996, 158, 288–300. [Google Scholar] [CrossRef]
- Jam, S.; Ahangary, M.; Tavasoli, A.; Sadaghiani, K.; Pour, A.N. Enhancement of distillate selectivity in Fischer-Tropsch synthesis by using iron and cobalt catalysts in a novel dual-bed reactor. React. Kinet. Catal. Lett. 2006, 89, 71–79. [Google Scholar] [CrossRef]
- Shi, B.; Wu, L.; Liao, Y.; Jin, C.; Montavon, A. Explanations of the Formation of Branched Hydrocarbons during Fischer–Tropsch Synthesis by Alkylidene Mechanism. Top. Catal. 2014, 57, 451–459. [Google Scholar] [CrossRef]
- Shi, B.; Liao, Y.; Naumovitz, J.L. Formation of 2-alkenes as secondary products during Fischer–Tropsch synthesis. Appl. Catal. A: Gen. 2015, 490, 201–206. [Google Scholar] [CrossRef]
- Busca, G. Heterogeneous Catalytic Materials; Elsevier, B.V.: Amsterdam, The Netherlands, 2014; p. 478. [Google Scholar]
- Chen, N.Y.; Garwood, W.E.; Dwyer, F.G. Shape Selective Catalysis in Industrial Applications, 2nd ed.; Dekker: New York, NY, USA, 1996; 303p. [Google Scholar]
- Guisnet, M.; Gilson, J.P. Zeolites for Cleaner Technologies; Imperial College Press: London, UK, 2002; 378p. [Google Scholar]
- Cejka, J.; Corma, A.; Zones, S. Zeolites and Catalysis; Wiley-VCH: Weinheim, Germany, 2010; 881p. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; John Wiley: New York, NY, USA, 1973; 771p. [Google Scholar]
- Rabo, J.A. Zeolite Chemistry and Catalysis; American Chemical Society: Washington, DC, USA, 1976; 796p. [Google Scholar]
- Sievers, C.; Onda, A.; Olindo, R.; Lercher, J.A. Low-Temperature Activation of Branched Octane Isomers over Lanthanum-Exchanged Zeolite X Catalysts. J. Phys. Chem. 2007, 111, 5454–5464. [Google Scholar] [CrossRef]
- Eder, F.; Lercher, J.A. Alkane sorption in molecular sieves: The contribution of ordering, intermolecular interactions, and sorption on Brønsted acid sites. Zeolites 1997, 18, 75–81. [Google Scholar] [CrossRef]
- Pieterse, J.A.Z.; Veefkind-Reyes, S.; Seshan, K.; Lercher, J.A. Sorption and Ordering of Dibranched Alkanes on Medium-Pore Zeolites Ferrierite and TON. J. Phys. Chem. B 2000, 104, 5715–5723. [Google Scholar] [CrossRef]
- Denayer, J.F.; Souverijns, W.; Jacobs, P.A.; Martens, J.A.; Baron, G.V. High-Temperature Low-Pressure Adsorption of Branched C5−C8 Alkanes on Zeolite Beta, ZSM-5, ZSM-22, Zeolite Y, and Mordenite. J. Phys. Chem. B 1998, 102, 4588–4597. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Pidko, E.A. Intensities of IR Stretching Bands as a Criterion of Polarization and Initial Chemical Activation of Adsorbed Molecules in Acid Catalysis. Ethane Adsorption and Dehydrogenation by Zinc Ions in ZnZSM-5 Zeolite. J. Phys. Chem. B 2005, 109, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, M.; Busca, G.; Lenarda, M.; Storaro, L.; Pavan, M. An investigation of the surface acidity of mesoporous Al-containing MCM-41 and of the external surface of ferrierite through pivalonitrile adsorption. Appl. Catal. A 1999, 182, 225–235. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Danilova, I.G.; Arzumanov, S.S.; Toktarev, A.V.; Freude, D.; Stepanov, A.G. Strong acidity of silanol groups of zeolite beta: Evidence from the studies by IR spectroscopy of adsorbed CO and 1H MAS NMR. Microporous Mesoporous Mater. 2010, 131, 210–216. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Zhou, D.; Liu, X.; Han, X.; Bao, X. Density functional theory calculations on various M/ZSM-5 zeolites: Interaction with probe molecule H2O and relative hydrothermal stability predicted by binding energies. J. Mol. Catal. A Chem. 2005, 237, 36–44. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Ugliengo, P. Thermodynamic study of water adsorption in high-silica zeolites. J. Phys. Chem. B 2006, 110, 14849–14859. [Google Scholar] [CrossRef]
- Subbotin, A.N.; Zhidomirov, G.M.; Subbotina, I.R.; Kazansky, V.B. Molecular and dissociative adsorption of H2O on zeolite Zn/ZSM-5 studied by diffuse-reflectance IR spectroscopy and quantum chemical calculations. Kinet. Catal. 2013, 54, 744–748. [Google Scholar] [CrossRef]
- Ryder, J.A.; Chakraborty, A.K.; Bell, A.T. Density Functional Theory Study of Proton Mobility in Zeolites: Proton Migration and Hydrogen Exchange in ZSM-5. J. Phys. Chem. B 2000, 104, 6998–7011. [Google Scholar] [CrossRef]
- Chen, K.; Gumidyala, A.; Abdolrhamani, M.; Villines, C.; Crossley, S.; White, J.L. Trace water amounts can increase benzene H/D exchange rates in an acidic zeolite. J. Catal. 2017, 351, 130–135. [Google Scholar] [CrossRef]
- Whitmore, F.C. Mechanism of the Polymerization of Olefins by Acid Catalysts. Ind. Eng. Chem. 1934, 26, 94–95. [Google Scholar] [CrossRef]
- Stepanov, A.G.; Luzgin, M.V.; Romannikov, V.N.; Zamaraev, K.I. Carbenium ion properties of octene-1 adsorbed on zeolite H-ZSM-5. Catal. Lett. 1994, 24, 271–284. [Google Scholar] [CrossRef]
- Jentoft, F.C.; Gates, B.C. Solid-acid-catalyzed alkane cracking mechanisms: Evidence from reactions of small probe molecules. Top. Catal. 1997, 4, 1–13. [Google Scholar] [CrossRef]
- Corma, A.; Orchilles, A.V. Current Views on the Mechanism of Catalytic Cracking. Microporous Mesoporous Mater. 2000, 35–36, 21–30. [Google Scholar] [CrossRef]
- Haag, W.O.; Dessau, R.M. Duality of Mechanism for Acid-Catlayzed Paraffin Cracking. In Proceedings of the 8th International Congress on Catalysis, Berlin, Germany, 2–6 July 1984; Varlag-Chemie: Weinheim, Germany, 1984; Volume 2. [Google Scholar]
- Krannila, H.; Haag, W.O.; Gates, B.C. Monomolecular and bimolecular mechanisms of paraffin cracking: N-butane cracking catalyzed by HZSM-5. J. Catal. 1992, 135, 115–124. [Google Scholar] [CrossRef]
- Anderson, B.G.; Schumacher, R.R.; van Duren, R.; Singh, A.P.; van Santen, R.A. An Attempt to Predict the Optimum Zeolite-based Catalyst for Selective Cracking of Naphtha-range Hydrocarbons to Light Olefins. J. Mol. Catal. A Chem. 2002, 181, 291–301. [Google Scholar] [CrossRef]
- Williams, B.A.; Ji, W.; Miller, J.T.; Snurr, R.Q.; Kung, H.H. Evidence of different reaction mechanisms during the cracking of n-hexane on H-USY zeolite. Appl. Catal. A Gen. 2000, 203, 179–190. [Google Scholar] [CrossRef]
- Patarin, J.; Gies, H. Crystalline and Organized Porous Solids (thematic issue). Comptes Rendus. Chim. 2005, 8, 243. [Google Scholar] [CrossRef]
- Titiloye, J.O.; Parker, S.C.; Stone, F.S.; Catlow, C.R.A. Simulation studies of the structure and energetics of sorbed molecules in high-silica zeolites. 1 Hydrocarbons. J. Phys. Chem. 1991, 95, 4038–4044. [Google Scholar] [CrossRef]
- Bessel, S. Investigation of bifunctional zeolite supported cobalt Fischer–Tropsch catalysts. Appl. Catal. A Gen. 1995, 126, 235–244. [Google Scholar] [CrossRef]
- Wang, H.; Pei, Y.; Qia, M. Design of Bifunctional Solid Catalysts for Conversion of Biomass-Derived Syngas into Biofuels. In Production of Biofuels and Chemicals with Bifunctional Catalysts; Fang, Z., Smith, R.L., Jr., Li, H., Eds.; Springer: Singapore, 2017; pp. 137–158. [Google Scholar]
- Bao, J.; Tsubaki, N. Core-shell catalysts and bimodal catalysts for Fischer–Tropsch synthesis. In Catalysis; Spivey, J.J., Han, Y.-F., Dooley, K.M., Eds.; RSC Publishing: Cambridge, UK, 2013; Volume 25, pp. 216–245. [Google Scholar]
- Sun, B.; Qiao, M.; Fan, K.; Ulrich, J.; Tao, F. Fischer–Tropsch Synthesis over Molecular Sieve Supported Catalysts. ChemCatChem 2011, 3, 542–550. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Liu, X.; Lu, X.; Moyo, M.; Hildebrandt, D. Cobalt hybrid catalysts in Fischer–Tropsch synthesis. Rev. Chem. Eng. 2020, 36, 437–457. [Google Scholar] [CrossRef]
- Yang, G.; Xing, C.; Hirohama, W.; Jin, Y.; Zeng, C.; Suehiro, Y.; Wang, T.; Yoneyama, Y.; Tsubaki, N. Tandem catalytic synthesis of light isoparaffin from syngas via Fischer–Tropsch synthesis by newly developed core–shell-like zeolite capsule catalysts. Catal. Today 2013, 215, 29–35. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Chen, J.-F.; Zhang, Y. Cobalt nanoparticles imbedded into zeolite crystals: A tailor-made catalyst for one-step synthesis of gasoline from syngas. Int. J. Hydrogen Energy 2016, 41, 21965–21978. [Google Scholar] [CrossRef]
- Kruse, N.; Machoke, A.G.; Schwieger, W.; Güttel, R. Nanostructured Encapsulated Catalysts for Combination of Fischer–Tropsch Synthesis and Hydroprocessing. ChemCatChem 2015, 7, 1018–1022. [Google Scholar] [CrossRef]
- Huang, X.; Hou, B.; Wang, J.; Li, D.; Jia, L.; Chen, J.; Sun, Y. CoZr/H-ZSM-5 hybrid catalysts for synthesis of gasoline-range isoparaffins from syngas. Appl. Catal. A Gen. 2011, 408, 38–46. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, Q.; Yang, G.; Chen, Q.; Li, J.; Wei, Q.; Tan, Y.; Wan, H.; Tsubaki, N. Insights into the promotional roles of palladium in structure and performance of cobalt-based zeolite capsule catalyst for direct synthesis of C5–C11 iso-paraffins from syngas. J. Catal. 2016, 344, 378–388. [Google Scholar] [CrossRef]
- Xu, K.; Cheng, Y.; Sun, B.; Pei, Y.; Yan, S.-R. Fischer–Tropsch synthesis over skeletal Co/HZSM-5 core-shell catalysts. Acta Phys.—Chim. Sin. 2015, 31, 1137–1144. [Google Scholar] [CrossRef]
- Zhu, C.; Bollas, G.M. Gasoline selective Fischer–Tropsch synthesis in structured bifunctional catalysts. Appl. Catal. B Environ. 2018, 235, 92–102. [Google Scholar] [CrossRef]
- Cheng, S.; Mazonde, B.; Zhang, G.; Javed, M.; Dai, P.; Cao, Y.; Tu, S.; Wu, J.; Lu, C.; Xing, C.; et al. Co-based MOR/ZSM-5 composite zeolites over a solvent-free synthesis strategy for improving gasoline selectivity. Fuel 2018, 223, 354–359. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Meng, M.; Yoneyama, Y.; Tsubaki, N. One-step Synthesis of H–β Zeolite-Enwrapped Co/Al2O3 Fischer–Tropsch Catalyst with High Spatial Selectivity. J. Catal. 2009, 265, 26–34. [Google Scholar] [CrossRef]
- Sartipi, S.; Dijk, J.E.; Gascon, J.; Kapteijn, F. Toward bifunctional catalysts for the direct conversion of syngas to gasoline range hydrocarbons: H-ZSM-5 coated Co versus HZSM-5 supported Co. Appl. Catal. A Gen. 2013, 456, 11–22. [Google Scholar] [CrossRef]
- Lapidus, A.L.; Krylova, A.Y.; Potapova, S.N. Isoparaffin synthesis from CO and H2 on Co-MgO/Zeolite catalysts. Solid Fuel Chem. 2008, 42, 86–92. [Google Scholar] [CrossRef]
- Zola, A.S.; Bidart, A.M.F.; Fraga, A.C.; Hori, C.E.; Sousa-Aguiar, E.F.; Arroyo, P.A. Cobalt supported on different zeolites for Fischer–Tropsch synthesis. Stud. Surf. Sci. Catal. 2007, 167, 129–134. [Google Scholar] [CrossRef]
- Baranak, M.; Gürünlü, B.; Sarıoğlan, A.; Ataç, Ö.; Atakül, H. Low acidity ZSM-5 supported iron catalysts for Fischer–Tropsch synthesis. Catal. Today 2013, 207, 57–64. [Google Scholar] [CrossRef]
- Wang, S.; Yin, Q.; Guo, J.; Ru, B.; Zhu, L. Improved Fischer–Tropsch synthesis for gasoline over Ru, Ni promoted Co/HZSM-5 catalysts. Fuel 2013, 108, 597–603. [Google Scholar] [CrossRef]
- Kang, S.; Ryu, J.; Kim, J.; Kim, H.; Lee, C.; Lee, Y.; Jun, K. Fischer–Tropsch Synthesis of the Promoted Co/ZSM-5 Hybrid Catalysts for the Production of Gasoline Range Hydrocarbons. Mod. Res. Catal. 2014, 3, 99–106. [Google Scholar] [CrossRef]
- Yao, M.; Yao, N.; Shao, Y.; Han, Q.; Ma, C.; Yuan, C.; Li, C.; Li, X. New insight into the activity of ZSM-5 supported Co and CoRu bifunctional Fischer–Tropsch synthesis catalyst. Chem. Eng. J. 2014, 239, 408–415. [Google Scholar] [CrossRef]
- Yao, M.; Yao, N.; Liu, B.; Li, S.; Xu, L.; Li, X. Effect of SiO2/Al2O3 ratio on the activities of CoRu/ZSM-5 Fischer–Tropsch synthesis catalysts. Catal. Sci. Technol. 2015, 5, 2821–2828. [Google Scholar] [CrossRef]
- Subramanian, V.; Zholobenko, V.L.; Cheng, K.; Lancelot, C.; Heyte, S.; Thuriot, J.; Paul, S.; Ordomsky, V.V.; Khodakov, A.Y. The role of steric effects and acidity in the direct synthesis of iso-paraffins from syngas on cobalt zeolite catalysts. ChemCatChem 2015, 8, 380–389. [Google Scholar] [CrossRef]
- Espinosa, G.; Domínguez, J.M.; Morales-Pacheco, P.; Tobon, A.; Aguilar, M.; Beníteza, J. Catalytic behavior of Co/(Nanoβ-Zeolite) bifunctional catalysts for Fischer–Tropsch reactions. Catal. Today 2011, 166, 47–52. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, G.; Javed, M.; Gao, W.; Mazonde, B.; Zhang, Y.; Lu, C.; Yang, R.; Xing, C. Solvent-Free Synthesis of 1D Cancrinite Zeolite for Unexpectedly Improved Gasoline Selectivity. ChemistrySelect 2018, 3, 2115–2119. [Google Scholar] [CrossRef]
- Xing, C.; Sun, J.; Yang, G.; Shen, W.; Tan, L.; Zhu, P.; Wei, Q.; Li, J.; Kyodo, M.; Yang, R.; et al. Tunable isoparaffin and olefin synthesis in Fischer–Tropsch synthesis achieved by composite catalyst. Fuel Process. Technol. 2015, 136, 68–72. [Google Scholar] [CrossRef]
- Kim, J.-C.; Lee, S.; Cho, K.; Na, K.; Lee, C.; Ryoo, R. Mesoporous MFI zeolite nanosponge supporting cobalt nanoparticles as a Fischer–Tropsch catalyst with high yield of branched hydrocarbons in the gasoline range. ACS Catal. 2014, 4, 3919–3927. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Sartipi, S.; Sun, X.; Rodríguez-Mirasol, J.; Cordero, T.; Kapteijn, F.; Gascon, J. Carbon/H-ZSM-5 composites as supports for bi-functional Fischer–Tropsch synthesis catalysts. Catal. Sci. Technol. 2016, 6, 2633–2646. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, L.; Kang, J.; Peng, X.; Zhang, Q.; Wang, Y. Selective Transformation of Syngas into Gasoline-Range Hydrocarbons over Mesoporous H-ZSM-5-Supported Cobalt Nanoparticles. Chem. Eur. J. 2015, 21, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Yang, G.; Wu, M.; Yang, R.; Tan, L.; Zhu, P.; Wei, Q.; Li, J.; Mao, J.; Yoneyama, Y.; et al. Hierarchical zeolite Y supported cobalt bifunctional catalyst for facilely tuning the product distribution of Fischer–Tropsch synthesis. Fuel 2015, 148, 48–57. [Google Scholar] [CrossRef]
- Li, H.; Hou, B.; Wang, J.; Qin, C.; Zhong, M.; Huang, X.; Jia, L.; Li, D. Direct conversion of syngas to isoparaffins over hierarchical beta zeolite supported cobalt catalyst for Fischer–Tropsch synthesis. Mol. Catal. 2018, 459, 106–112. [Google Scholar] [CrossRef]
- Flores, C.; Batalha, N.; Ordomsky, V.V.; Zholobenko, V.L.; Baaziz, W.; Marcilio, N.R.; Khodakov, A.Y. Direct Production of Iso-Paraffins from Syngas over Hierarchical Cobalt-ZSM-5 Nanocomposites Synthetized by using Carbon Nanotubes as Sacrificial Templates. ChemCatChem 2018, 10, 2291–2299. [Google Scholar] [CrossRef]
- Flores, C.; Batalha, N.; Marcilio, N.R.; Ordomsky, V.V.; Khodakov, A.Y. Influence of Impregnation and Ion Exchange Sequence on Metal Localization, Acidity and Catalytic Performance of Cobalt BEA Zeolite Catalysts in Fischer–Tropsch Synthesis. ChemCatChem 2019, 11, 568–574. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, K.; Kang, J.; Gu, B.; Yu, X.; Zhang, Q.; Wang, Y. Impact of hydrogenolysis onthe selectivity of the Fischer–Tropsch synthesis: Diesel fuel production over mesoporouszeolite-Y supported cobalt nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 4553–4556. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, X.; Peng, X.; Yang, Y.; Cheng, K.; Zhang, Q.; Wang, Y. Mesoporous zeolite Y-supported Co nanoparticles as efficient Fischer–Tropsch catalysts for selective synthesis of diesel fuel. Ind. Eng. Chem. Res. 2016, 55, 13008–13019. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Ma, H.; Zhang, H.; Jiang, Y.; Wang, H.; Li, Z.; Wu, J. Effect of the desilication of H-ZSM-5 by alkali treatment on the catalytic performance in Fischer–Tropsch synthesis. React. Kinet. Mech. Catal. 2016, 120, 775–790. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Jiang, Y.; Zhang, H.; Liang, J.; Wang, H.; Li, Z.; Wu, J. The Influence of Alkali Treatment for Synthesizing Hierarchical Zeolite on Behavior of Cobalt Fischer–Tropsch Synthesis Catalysts. Catal. Surv. Asia 2017, 21, 28–36. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Zubkov, I.N.; Bakun, V.G.; Agliullin, M.R.; Saliev, A.N.; Savost’yanov, A.P. Bifunctional cobalt catalyst for the Fischer—Tropsch synthesis of low-freezing diesel fuel—From development to implementation: Part 1 Selection of a commercial sample of the HZSM-5 zeolite component. Katal. V Promyshlennosti 2021, 1, 30–40. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Zubkov, I.N.; Bakun, V.G.; Papeta, O.P.; Savostyanov, A.P. Effects of SiO2/Al2O3 ratio in ZSM-5 zeolite on the activity and selectivity of a bifunctional cobalt catalyst for synthesis of low-pour-point diesel fuels from CO and H2. Pet. Chem. 2022, 62, 101–111. [Google Scholar] [CrossRef]
- Mordkovich, V.; Sineva, L.; Kulchakovskaya, E.; Asalieva, E. Four Generations of Technology for Production of Synthetic Liquid Fuel Bbased on Fischer—Tropsch Synthesis. Historical Overvie. Katal. V Promyshlennosti 2015, 15, 23–45. [Google Scholar] [CrossRef]
- Ermolaev, V.S.; Gryaznov, K.O.; Mitberg, E.B.; Mordkovich, V.Z.; Tretyakov, V.F. Laboratory and pilot plant fixed-bed reactors for Fischer–Tropsch synthesis: Mathematical modeling and experimental investigation. Chem. Eng. Sci. 2015, 138, 1–8. [Google Scholar] [CrossRef]
- Kibby, C.; Jothimurugesan, K.; Das, T.; Lacheen, H.S.; Rea, T.; Saxton, R.J. Chevron’s gas conversion catalysis-hybrid catalysts for wax-free Fischer–Tropsch synthesis. Catal. Today 2013, 215, 131–141. [Google Scholar] [CrossRef]
- Sineva, L.V.; Nalivaiko, E.O.; Gryaznov, K.O.; Mordkovich, V.Z. Role of Zeolites in Heat and Mass Transfer in Pelletized Multifunctional Cobalt-Based Fischer–Tropsch Catalysts. Kinet. Catal. 2022, 63, 321–329. [Google Scholar] [CrossRef]
- Gryaznov, K.O.; Sineva, L.V.; Asalieva, E.Y.; Mordkovich, V.Z. Comprehensive comparison of high productivity cobalt catalysts for Fischer—Tropsch synthesis with different types of heat-conductive frames. Katal. V Promyshlennosti 2022, 22, 6–21. [Google Scholar] [CrossRef]
- Liu, C.; Chena, Y.; Zhao, Y.; Lyu, S.; Wei, L.; Li, X.; Zhang, Y.; Li, J. Nano-ZSM-5-supported cobalt for the production of liquid fuel in Fischer–Tropsch synthesis: Effect of preparation method and reaction temperature. Fuel 2020, 263, 116619. [Google Scholar] [CrossRef]
- Min, J.-E.; Kim, S.; Kwak, G.; Kim, Y.T.; Han, S.J.; Lee, Y.; Jun, K.-W.; Kim, S.K. Role of mesopores in Co/ZSM-5 for the direct synthesis of liquid fuel by Fischer–Tropsch synthesis. Catal. Sci. Technol. 2018, 8, 6346–6359. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Dalai, A.K.; Ma, W.; Zhang, L. Fischer–Tropsch Synthesis for Light Olefins from Syngas: A Review of Catalyst Development. Reactions 2021, 2, 227–257. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Hubáček, J.; Tomas, M.; Bačiak, M.; Vakili, M. Production of Light Olefins via Fischer–Tropsch Process Using Iron-Based Catalysts: A Review. Catalysts 2022, 12, 174. [Google Scholar] [CrossRef]
- Khodakov, A.Y. Fischer–Tropsch synthesis: Relations between structure of cobalt catalysts and their catalytic performance. Catal. Today 2009, 144, 251–257. [Google Scholar] [CrossRef]
- Liu, Y.; Florea, I.; Ersen, O.; Pham-Huu, C.; Meny, C. Silicon carbide coated with TiO2 with enhanced cobalt active phase dispersion for Fischer–Tropsch synthesis. Chem. Commun. 2015, 51, 145–148. [Google Scholar] [CrossRef]
- Qi, H.; Xing, C.; Huang, W.; Li, M.; Jiang, Y.; Suna, X.; Liu, H.; Lu, P.; Chen, J.; Chen, S. Design of a hierarchical Co@ZSM-5/SiC capsule catalyst for direct conversion of syngas to middle olefin. Microporous Mesoporous Mater. 2022, 343, 112134. [Google Scholar] [CrossRef]
- Zecevic, J.; Vanbutsele, G.; de Jong, K.P.; Martents, J.A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 2015, 528, 245–248. [Google Scholar] [CrossRef]
- Sineva, L.V.; Khatkova, E.Y.; Kriventceva, E.V.; Mordkovich, V.Z. Effect of introduced zeolite on the Fischer–Tropsch synthesis over a cobalt catalyst. Mendeleev Commun. 2014, 24, 316–318. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Han, D.; Wu, J. Characterizations and product distribution of Co-based Fischer–Tropsch catalysts: A comparison of the incorporation manner. Fuel 2018, 220, 257–263. [Google Scholar] [CrossRef]
- Daisley, A.; Hargreaves, J.S.J.; Hermann, R.; Poya, Y.; Wang, Y.A. Comparison of the Activities of Various Supported Catalysts for Ammonia Synthesis. Catal. Today 2020, 357, 534–540. [Google Scholar] [CrossRef]

| Product | Catalytic Bed Organization | Zeolite | Synthesis Conditions | Ref. |
| Hydrocarbons gasoline fraction | Core-shell catalyst | HZSM-5 | 1 MPa, 280 °C, H2/CO = 2, 2.24 L/(g·h) | [58] |
| 1 MPa, 260 °C, H2/CO = 2, Wcat/Fsyngas = 5 g·h·mol−1` | [59] | |||
| 2.1 MPa, 200–250 °C, H2/CO = 2, 0.5 L/h | [60] | |||
| 2 MPa, 210–260 °C, H2/CO = 2, GHSV = 1000 h−1 | [61] | |||
| 1 MPa, 260 °C, H2/CO = 2 | [62] | |||
| 2 MPa, 250 °C, H2/CO = 2 | [63] | |||
| 1.2 MPa, 230 °C | [64] | |||
| ZSM-5 + MOR | 1 MPa, 260 °C, H2/CO = 2 | [65] | ||
| HBeta | 1 MPa, 265 °C | [66] | ||
| Impregnation of zeolite | H-USY, H-Beta, H-Mordenite or H-ZSM-5 | 10 bar, 240 °C, H2/CO = 2, GHSV = 1287 h−1 | [69] | |
| ZSM-5 | 1.9 MPa, 280 °C, H2/CO = 2, GHSV = 750 h−1 | [70] | ||
| HZSM-5 | 2 MPa, 235–300 °C, H2/CO = 2, GHSV = 1500 h−1 | [71] | ||
| ZSM-5 | 2 MPa, 240–260 °C, H2/CO = 2, 3000 mL/gcat/h | [72] | ||
| ZSM-5 | 2 MPa, 210 or 250 °C, H2/CO = 2, 15 or 45 cm3/(gcat·min) | [73] | ||
| Impregnation of zeolite or mixed bed | ZSM-5, MOR or Beta | 2 MPa, 250 °C, H2/CO = 2, 34 L/(gCo·h) | [75] | |
| Impregnation of zeolite | Beta | 1 MPa, 220 °C, H2/CO = 2 | [76] | |
| Cancrinite | 1 MPa, 260 °C, H2/CO = 2, 6 g·h·mol−1` | [77] | ||
| HZSM-5 | 1.5 MPa, 220–240 °C, H2/CO = 2 | [8] | ||
| HZSM-5 | 1 MPa, 240 °C | [78] | ||
| HZSM-5 | 2 MPa, 220 °C, H2/CO = 2, 2.4 L/(g·h) | [79] | ||
| H-ZSM-5 | 2 MPa, 240 °C, H2/CO = 2 | [80] | ||
| H-ZSM-5 | 2 MPa, 240 °C, H2/CO = 1, 20 mL/min | [81] | ||
| Isoparaffins | Impregnation of zeolite | Y | 1 MPa, 260 °C, H2/CO = 2 | [82] |
| Beta | 2 MPa, 225 °C, H2/CO = 2, 4.8 L/(g·h) | [83] | ||
| ZSM-5 | 2 MPa, 250 °C, H2/CO = 2, 20–70 L/(gCo·h) | [84] | ||
| HBeta | 2 MPa, 250 °C, H2/CO = 2, 66 L/(gcat·h) | [85] | ||
| Impregnation of zeolite or mixed bed | ZSM-5, MOR or BEA | 2 MPa, 250 °C, H2/CO = 2, 34 L/(gCo·h) | [75] | |
| Diesel fuel components | Impregnation or melt infiltration | HY or NaY | 2 MPa, 230 °C, H2/CO = 1, 20 mL/min | [86,87] |
| Impregnation of zeolite | HZSM-5 | 2 MPa, 210 °C, H2/CO = 2, GHSV = 1200 h−1 | [88,89] | |
| Composite | HZSM-5 | 2 MPa, 180–250 °C, H2/CO = 2, GHSV = 1000 h−1 | [90,91] | |
| Synthetic oil | Composite | ZSM-5 or ZSM-12 | 0.5–3 MPa, 205–235 °C, H2/CO = 1–2 | [94] |
| Impregnation of composite or composite | HBeta | 2 MPa, 170–260 °C, H2/CO = 2, GHSV = 1000–5000 h−1 | [93,95,96] | |
| Impregnation of zeolite | ZSM-5 | 1 MPa, 230 °C, | [97] | |
| ZSM-5 | 2 MPa, 240 °C, H2/CO = 2, 4 and 10 L/(gcat·h) | [98] | ||
| Olefins | Core-shell catalyst | NaZSM-5 | 1 MPa, 300 °C, H2/CO = 2 | [104] |
| Impregnation of zeolite | HZSM-5 | 1 MPa, 240 °C, H2/CO = 2 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sineva, L.; Mordkovich, V.; Asalieva, E.; Smirnova, V. Zeolite-Containing Co Catalysts for Fischer–Tropsch Synthesis with Tailor-Made Molecular-Weight Distribution of Hydrocarbons. Reactions 2023, 4, 359-380. https://doi.org/10.3390/reactions4030022
Sineva L, Mordkovich V, Asalieva E, Smirnova V. Zeolite-Containing Co Catalysts for Fischer–Tropsch Synthesis with Tailor-Made Molecular-Weight Distribution of Hydrocarbons. Reactions. 2023; 4(3):359-380. https://doi.org/10.3390/reactions4030022
Chicago/Turabian StyleSineva, Lilia, Vladimir Mordkovich, Ekaterina Asalieva, and Valeria Smirnova. 2023. "Zeolite-Containing Co Catalysts for Fischer–Tropsch Synthesis with Tailor-Made Molecular-Weight Distribution of Hydrocarbons" Reactions 4, no. 3: 359-380. https://doi.org/10.3390/reactions4030022
APA StyleSineva, L., Mordkovich, V., Asalieva, E., & Smirnova, V. (2023). Zeolite-Containing Co Catalysts for Fischer–Tropsch Synthesis with Tailor-Made Molecular-Weight Distribution of Hydrocarbons. Reactions, 4(3), 359-380. https://doi.org/10.3390/reactions4030022








