2.1. Immobilization of Commercial Celluclast® 1.5 L in Accurel® MP1000
We started this work by determining the number of proteins and the activities of cellulolytic enzymes present in both the enzymatic cocktail
Celluclast® 1.5 L and in the enzymatic extract obtained from the
Trichoderma harzianum I14-12 (
Th I14-12) strain as the initial parameters for the immobilization steps in Accurel
® MP1000, which results are summarized in
Table 1.
As observed, in the enzymatic extract
Th I14-12, both similar distribution and activities of the enzymes CMCase, FPase, and β-glucosidase could be found when compared to
Celluclast® 1.5 L, even at 140× lower protein concentrations (45 m·L
−1 for
Celluclast® 1.5 L versus 0.32 mg of protein per L for the extract Th I14-12). These proteins of the cellulolytic complex are crucial to perform the total hydrolysis of the cellulose fraction of the lignocellulosic biomass [
20]. These data are important since
Celluclast® 1.5 L is an enzymatic preparation of Novozymes
® rich in cellulases from a genetically modified strain of
Trichoderma reezei, which is a known producer of CMCase, FPase, and β-glycosidase [
20,
21]. However, through several genetic engineering maneuvers, besides producing modified and modeled enzymes, the engineered microorganism can secrete high concentrations of proteins in the medium, and probably for this reason, the amount of protein in the commercial preparation is much higher than the home-made extract obtained from the wild-type
Trichoderma harzianum I14-12 strain [
20,
22]. However, our preparation was obtained under simple fermentation conditions and using residual renewable sources of carbon and nitrogen, making it a cheaper, more ecological, and interesting alternative for future industrial applications, which is also proven to be composed of highly active proteins.
In this work, because there was a similar composition in enzymatic activities of the cellulolytic complex in both extracts, and with the aim of performing a comparative study of the immobilization of the commercial and home-made cellulolytic extracts on the Accurel
® MP1000 support, the best immobilization conditions on the support were outlined for the commercial preparation and then applied to the extract
Th I14-12. For this purpose, we started a
Celluclast® 1.5 L concentration of 45 m·L
−1 in the immobilization media. To determine the best immobilization conditions, two different initial temperatures (20 and 40 °C) and two different ionic strengths (25 and 50 mM of sodium citrate buffer) were investigated for 24 h. The results of the immobilization yield and recovered activities are shown in
Table 2.
It was observed that applying a protein concentration of 45 m·L
−1, the highest temperature (40 °C), and the highest ionic strength (50 mM) resulted in the highest immobilization efficiency, in terms of quantity of proteins in the supernatant, when evaluated during the initial 24 h of the process (kinetic curves shown in
Figure S1 of
Supplementary Materials).
In an immobilization process via adsorption, the success of the technique used depends on several factors, including the hydrophobicity of the support, the pH of the medium, and the ionic strength and temperature, so that the enzyme is in its most non-ionized form as much as possible, thus maintaining solubility [
23]. Temperature is a factor of great importance since, besides contributing to the solubility of a protein in an immobilization system, it contributes to an enzyme reaching various conformations that may be more compatible with the support, thereby contributing to the better adsorption of the protein on the support [
24]. Moreover, temperature can also cause greater movement of the protein content in the immobilization system, thus contributing to better adsorption. It is also important to note that the Accurel
® MP1000 support applied in this work is porous. Several authors have reported that in these materials, the increase in temperature may favor the migration of proteins into the pores since this phenomenon is endothermic [
24,
25,
26,
27,
28]. Ionic strength is directly linked to the solvation of a protein in an immobilization medium, where at low concentrations, the protein is found with a small solvation layer, thus generating larger contact surfaces between the enzyme and the support [
25]. However, when the ionic strength becomes higher, more significant amounts of ions are added to the immobilization solution, causing the protein macromolecule to be less solvated and reducing its solubility in the medium, which means it may also interact with the support [
25,
29]. Therefore, in immobilization studies of extracts containing different enzymes, as in our work, it is of great importance to study this variable in the system.
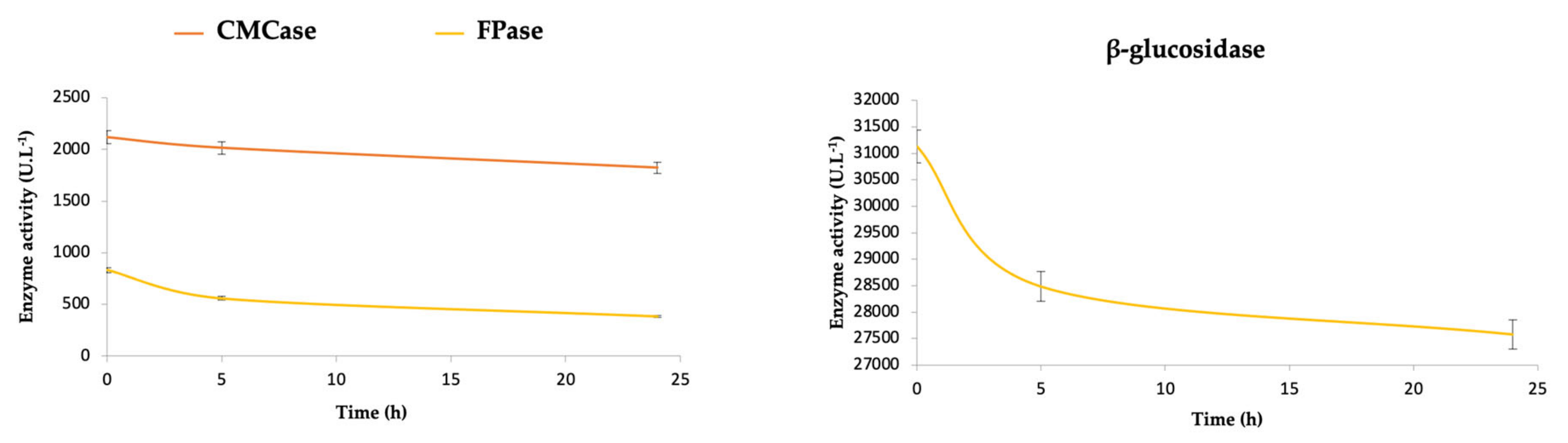
In
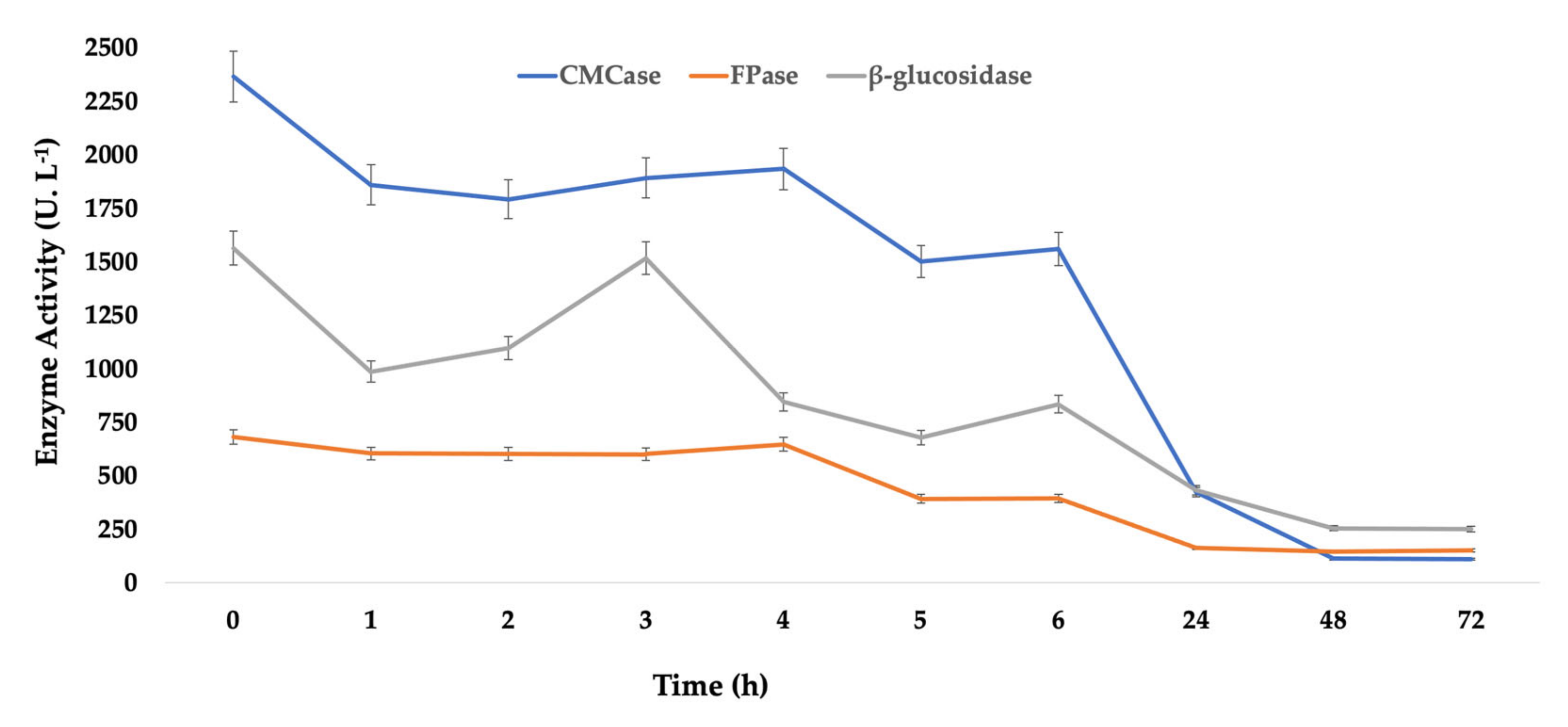
Table 1, it is also possible to observe that during the 24 h of immobilization, relatively low values of recovered activity are obtained for CMCase (1.45%), FPase (1.04%), and β-glucosidase (0.03%). These values can be better understood by observing the curves of enzymatic activity of the supernatant over time (
Figure 1). Although these curves show a tendency to decrease during the 24 h at 50 mM and 40 °C, it is still possible to detect high residual enzymatic activity, demonstrating that more time of immobilization would be necessary to observe the stabilization of enzymatic activities.
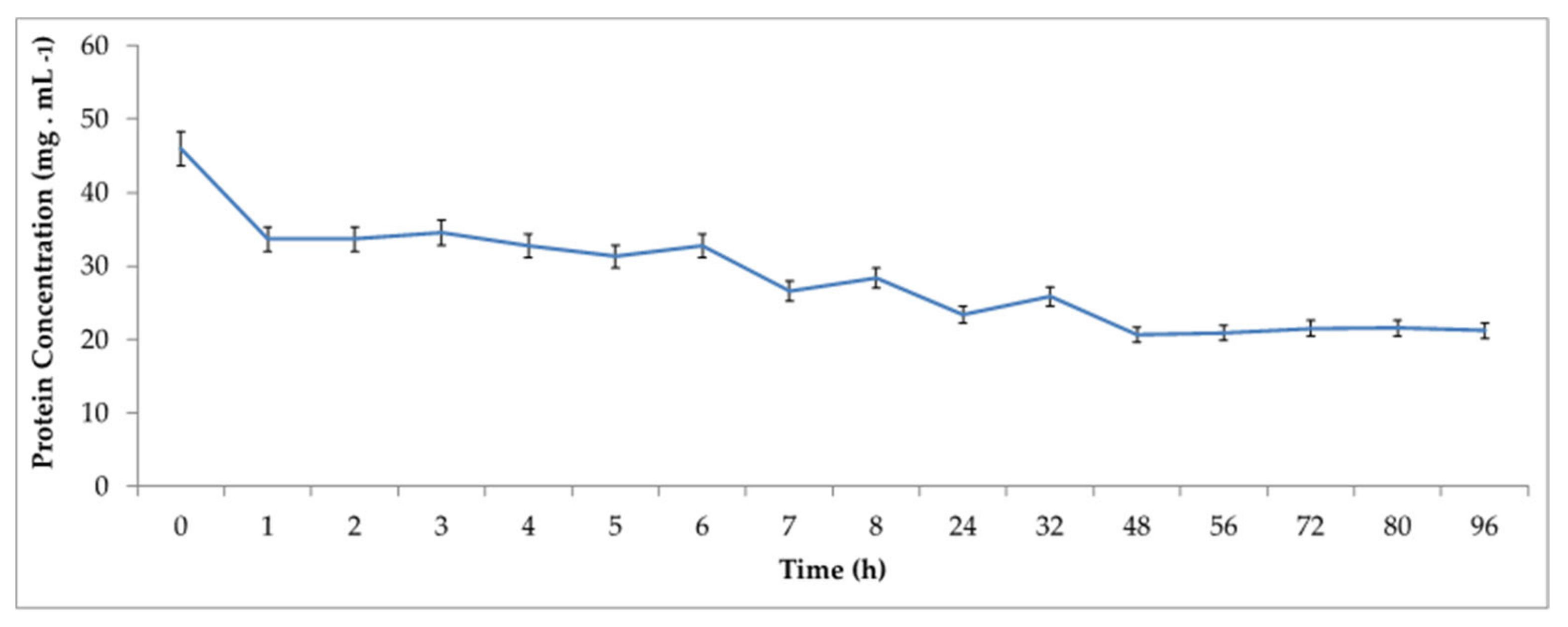
Therefore, in order to verify the influence of time on the immobilization process, the adsorption kinetics of
Celluclast® 1.5 L in Accurel
® MP1000 was performed at various times up to 96 h. The profile of residual protein concentration in the supernatant is shown in
Figure 2.
As observed, at 48 h of immobilization, more than 50% of all the proteins present in the enzymatic extract has been adsorbed on the support, according to the applied method. In order to understand the characteristics of the enzymes immobilized on the support, the due immobilization efficiencies and recovered activities were also calculated over time, which results are shown in
Table 3.
Compared to the 24 h immobilization time, at 72 h, 55% of immobilization yield was achieved, with the percentage of proteins adsorbed on the support stabilized at higher times. However, although with slightly higher values than those found at 24 h of immobilization, the recovered activities of CMCase (4%), FPase (1%), and β-glucosidase (1.2%) are still low. The enzyme activity profiles over time present the same profile (
Figure S2 of
Supplementary Materials). The commercial
Celluclast® 1.5 L cocktail is known to offer a different amount among these three enzymes, where it is more enriched with endoglucanases, such as CMCase and FPase, which are enzymes vital for the access of cellulose fibers that are highly recalcitrant, crystalline, and branched biopolymers [
21]. Therefore, in order to achieve successful hydrolysis and to maximize the obtainment of oligosaccharides and cellobiose, endoglucanases are key proteins in this process, in addition to the various accessory proteins already reported [
30,
31]. In this sense, β-glucosidase is, in fact, activated in high concentrations of cellobiose, generating glucose as the final product. However, even in lower protein concentrations, as it is a highly specific enzyme, its activity is higher compared to endoglucanases present in the extract.
Another important factor to consider at this point in the experiments is the initial protein concentration fed to the support. Because at this point of the experiments, a protein concentration of 45 m·L
−1 was applied in order to verify if there was a possible saturation point of the support, and immobilizations of the enzyme extract were performed at lower concentrations in 48 h of incubation. The results are shown in
Table 4.
As observed, the immobilization efficiencies showed an increase proportional to the reduction in the amount of protein offered to the support, demonstrating that, in fact, the support could present a protein loading capacity lower than 45 m·L
−1, using the initial amount of our experiments with
Celluclast® 1.5 L. From 15 m·L
−1 onward, immobilization efficiencies close to 100% were found, demonstrating that practically all the proteins present in the enzymatic extract were associated with the support Accurel
® MP1000. In an immobilization process via adsorption, not only the amount of adsorbed proteins is essential, but so is the last activity expressed in the biocatalyst [
32]. Therefore, an excess of adsorbed proteins on the support can generate several layers of enzyme deposition, where part of the proteins may not be completely accessible to interact with the substrate, resulting in low values of recovered activity [
32,
33]. Thus, the study of the optimal ratio between enzyme and support is of great importance when expressing the maximum catalytic activity of an immobilized biocatalyst [
34]. Moreover, the characteristics of the support used, such as surface area, size, porosity, and hydrophobicity, can influence the immobilization protocols and are of great importance in predicting the interactions to be performed with enzymes [
33,
35].
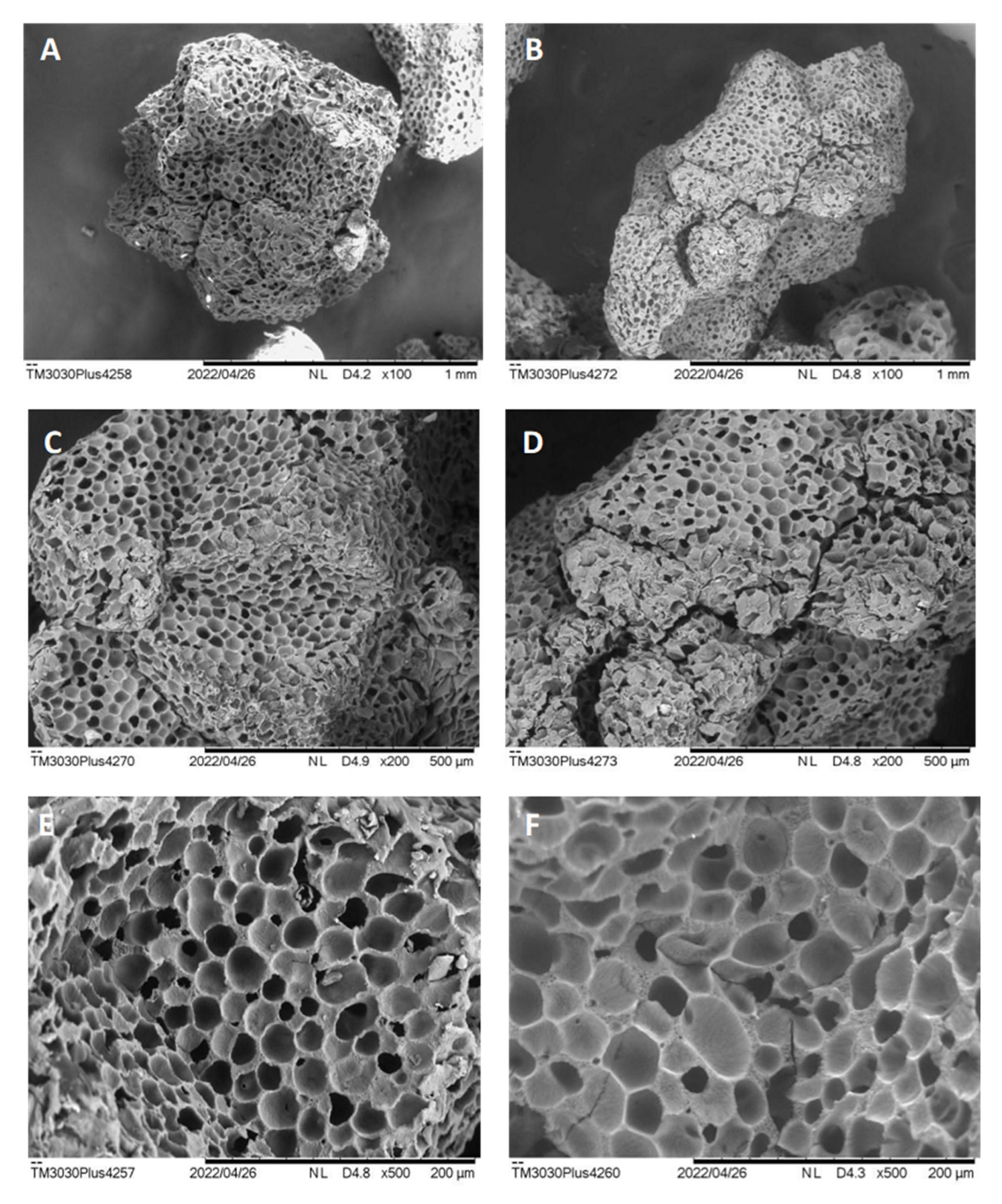
Aiming to contextualize the characteristics of the support and the enzyme with the results found, SEM analysis of Accurel
® MP1000 was performed before and after
Celluclast® 1.5 L immobilization at 15 mg·mL
−1 (
Figure 3).
According to the microscopic results, it is possible to observe that Accurel
® MP1000 is an amorphous and multiporous resin, which is regularly distributed throughout the extension of the polymer (
Figure 3A,C,E). According to all the magnifications performed, it could also be better observed that the presence of several filled pores after the immobilization process (
Figure 3B,D,F) demonstrates a possible lodging effect of the enzymes inside the pores. Our research group demonstrated in previous work that the surface hydrophobicity of Accurel
® MP1000, in addition to the distribution and pore size, was important for multiple adsorption interactions and accommodation of lipases, thus generating a more active biocatalyst and a higher thermal stability, with a low percentage of desorption or leaching into the reaction medium [
13,
14].
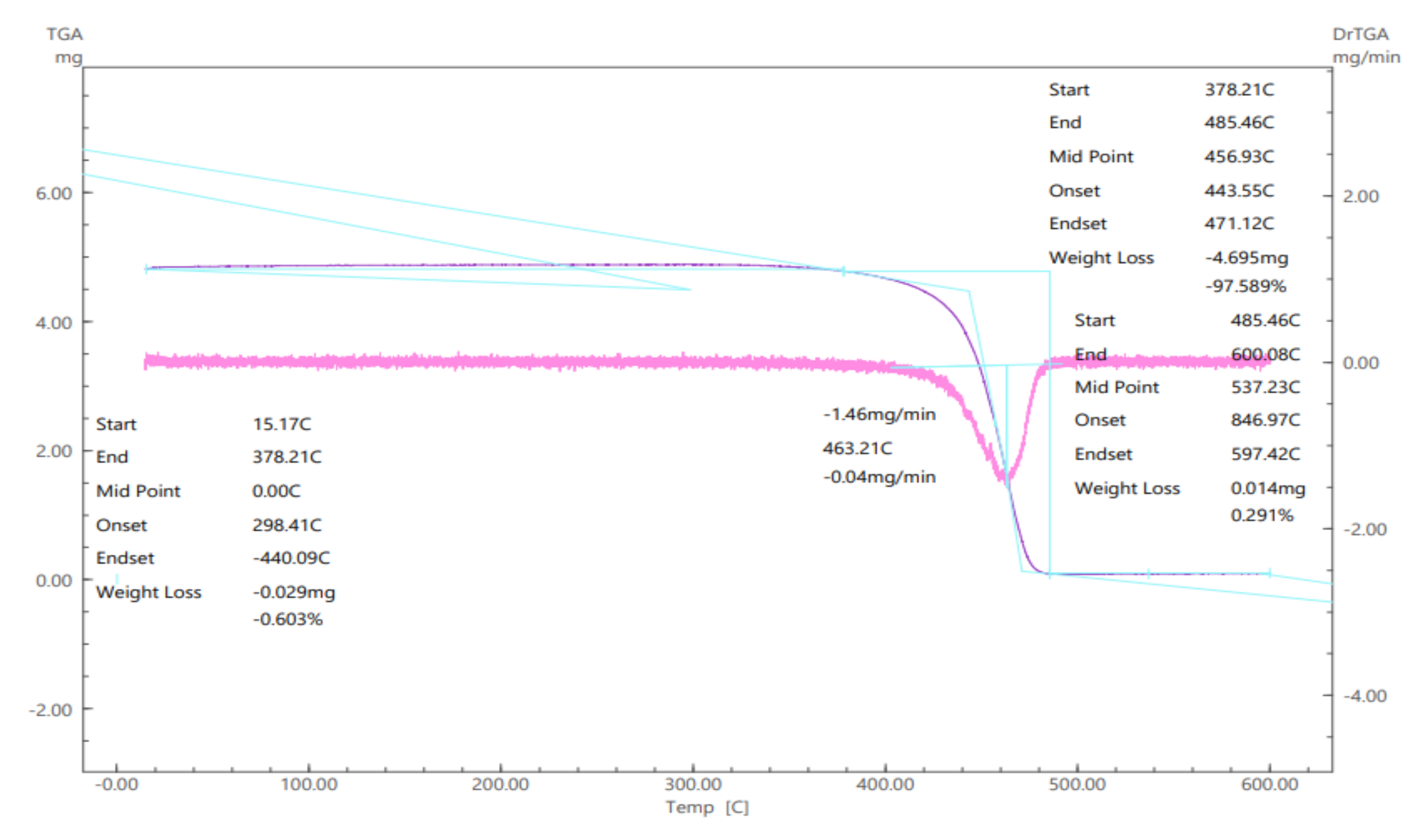
In order for these variables to be actually evaluated in the immobilization process, Accurel
® MP1000 was submitted to analysis using thermogravimetry (TGA), which results are demonstrated in
Figure 4.
Moreover, the determination of surface area, porous volume, and porous particle size was also performed using the BET method, as well as via hydrophobicity calculation using the Bengal rose method. The results are shown in
Table 5.
According to the data obtained, Accurel
® MP1000 presents a hydrophobicity of 22.7 μg.g
−1, which characterizes it as a highly hydrophobic support, which is favorable for the realization of hydrophobic adsorption methodologies since some enzymes, when interacting with hydrophobic supports, can be associated with the support with distorted or more stable conformations, where the catalytic site is more exposed, or can be in a conformation more favorable to recognition by the substrate [
35,
36].
Besides the hydrophobicity of the support, the surface area, pore size, volume and distribution, and the size of the support granules can influence the immobilization process [
34,
37]. The size of the pores, for example, needs to be compatible with the size and volume of the enzyme so that the enzyme is correctly accommodated and remains stable. Otherwise, there is a risk of leaching and loss of activity [
38,
39].
The surface area results obtained via the BET method in this work (5.6 m
2.g
−1) are lower than those found by Singh and collaborators (30 m
2.g
−1) in 2011 [
4], which may be due to the batch used, the manufacturer, or even a lower sensitivity of the applied method, given the time of execution of the experiments by the authors. However, even though the value is lower, our results still demonstrate a high surface area of the support, which, combined with the high hydrophobicity found, makes it suitable for enzyme immobilization via surface adsorption methodology. According to Zdravkov and collaborators in 2007 [
37], the pore size found on the support would classify it as mesoporous because, for enzyme immobilization, the pore size needs to be between 2 and 50 nm. This porosity found can be correlated with the results presented in
Table 5: when the immobilization of
Celluclast® 1.5 L is at a concentration of 15 mg. mL
−1, the recovered activity of β-glucosidases is lower than that found for CMCase and FPase. Such a result may be related to the difference in the molecular weight of these enzymes and a possible limitation regarding the pore size of Accurel
® MP1000, which can generate selective immobilization of a specific protein species to the detriment of another present in the extract. Proteins of small molecular weights tend to spread faster through the support and can lodge in the pores faster than those of higher molecular weights, thus being dominant at the beginning of the adsorption immobilization processes [
40,
41]. However, larger proteins may interact more intensely with the support due to their larger contact area and may be excluded from the pores and stick to the surface, and consequently, be more prone to deleterious actions of the microenvironment [
40,
42,
43].
2.3. Immobilization of Enzymatic Extract ThI14-12 in Accurel® MP1000
Aiming to investigate the interference of these characteristics in the final biocatalyst, the extract
ThI14-12 was also submitted to immobilization in Accurel
® MP1000 under the same conditions determined for
Celluclast® 1.5 L (
Figure 6).
As it could be observed, for this enzyme extract, all the activities detected were reduced in the supernatant during the immobilization process by adsorption, where at 48 h, in the same way as occurred with the commercial preparation Celluclast® 1.5 L, it reached the equilibrium, expressing around 112 U.L−1 of CMCase, 146 U.L−1 of FPase, and 255 U.L−1 of b-glucose. This last enzyme, unlike the others, showed a behavior of fluctuating activity during the immobilization kinetics, demonstrating that, in fact, this enzyme, by being larger than the others present in the extract, was probably adsorbed to the surface of the support, with this efficiency being more evident during the last hours of the process at the time during which the other enzymes were perhaps housed in the pores, thus making it possible for a better interaction of B-glucosidase with the surface of Accurel® MP1000.
Table 6 shows the results of immobilization efficiency, recovered activity, and support activity for the immobilized biocatalysts obtained in this work.
As observed, both immobilized biocatalysts showed similar profiles of enzyme distribution on the support in the same concentration of applied protein, obtaining, at the end of the process, higher specific activities in CMCase, followed by β-glucosidase and FPase. However, the ACC-
Th I14-12 biocatalyst presented a higher immobilization efficiency (95%) and higher retention of activities, thus generating higher final activities on the support. Such data are pretty interesting since this extract was obtained using a wild strain and simple culture media, and thus, an immobilized biocatalyst was generated with the same enzymatic characteristics obtained in comparison with a commercial cellulolytic preparation, which could become a competitive option for various processes of hydrolysis of residual lignocellulosic biomass and its applications in bioprocesses. Moreover, the higher immobilization efficiencies found in this biocatalyst can possibly be a consequence of the difference between the molecular weights of the enzymes that compose it since the same concentration of proteins was applied in both immobilization processes. Thus, the enzymes present in the enzymatic extract
ThI14-12 probably interacted more efficiently with the support, where more active conformations were generated for all enzymes, besides having been immobilized in a protein concentration that perhaps did not generate saturation of the support to the point of causing impediment or restriction of access of the enzymes to the substrate. These results are in line with several works reported in the literature for the immobilization of cellulases, where various immobilization techniques, such as covalent bonds, entrapment, and CLEA, are important to improve activity retention and enzyme activity [
31,
35,
37,
40,
41,
42]. However, this is the first work where complex extracts are immobilized using a hydrophobic adsorption methodology on Accurel
® MP1000, a versatile support that is able to promote different hosting and adsorption interactions on its surface. However, most of the works demonstrate the results in global activity, thus not taking into account the isolated activity of the three main enzyme components of the extract, making this work important and unprecedented.
2.4. Characterization of the New Biocatalysts
In order to explore the catalytic properties of the new immobilized biocatalysts obtained in this work, thermal stability at temperatures ranging from 30 °C to 70 °C was evaluated in comparison with the free
Celluclast® 1.5 L, which results are compiled in
Table 7.
As a result, it could be observed that the immobilized enzymes showed greater thermal stabilities at higher temperatures when compared to the commercial Celluclast® 1.5 L. At 40 °C, the free commercial extract presented the maximum catalytic activities of endoglucanases and exoglucanases, besides β-glucosidase which suffered decreases with an increase in temperature, losing more than 50% of enzymatic activities at 60 °C and more than 90% at 70 °C. Comparing these data with the ACC-ThI14-12 biocatalyst, immobilization promoted the maintenance of the catalytic activities of the enzymes at temperatures up to 60 °C, where more than 95% of the activities of endoglucanases and exoglucanases, as well as β -glucosidase, were still detected under the conditions studied. At 65 °C, 88% of FPase, 90% of CMCase, and 79% of β-glucosidase activities were still found, demonstrating relative thermal stability at this temperature and showing its high potential for application in bioprocesses requiring this temperature. At 70 °C, the enzyme that suffered the greatest decrease in relative activity was β-glucosidase, probably due to the fact that it is supposedly spread over the surface of the support, thus being more exposed to the deleterious actions of the environment. Similar profiles of thermal stability and maintenance of residual activity were obtained for the ACC-Celluclast biocatalyst, demonstrating that the immobilized home-made extract presented competitive characteristics when compared to a commercial preparation, with high retention of activity, thermal stability, and efficiency of immobilization, thus being a potential candidate as a biocatalyst in new bioprocesses.
Operational stability and reusability potential are crucial factors to reduce long-term costs in processes involving immobilized enzymes. In this way, aiming to glimpse the potential application for the new biocatalyst ACC-
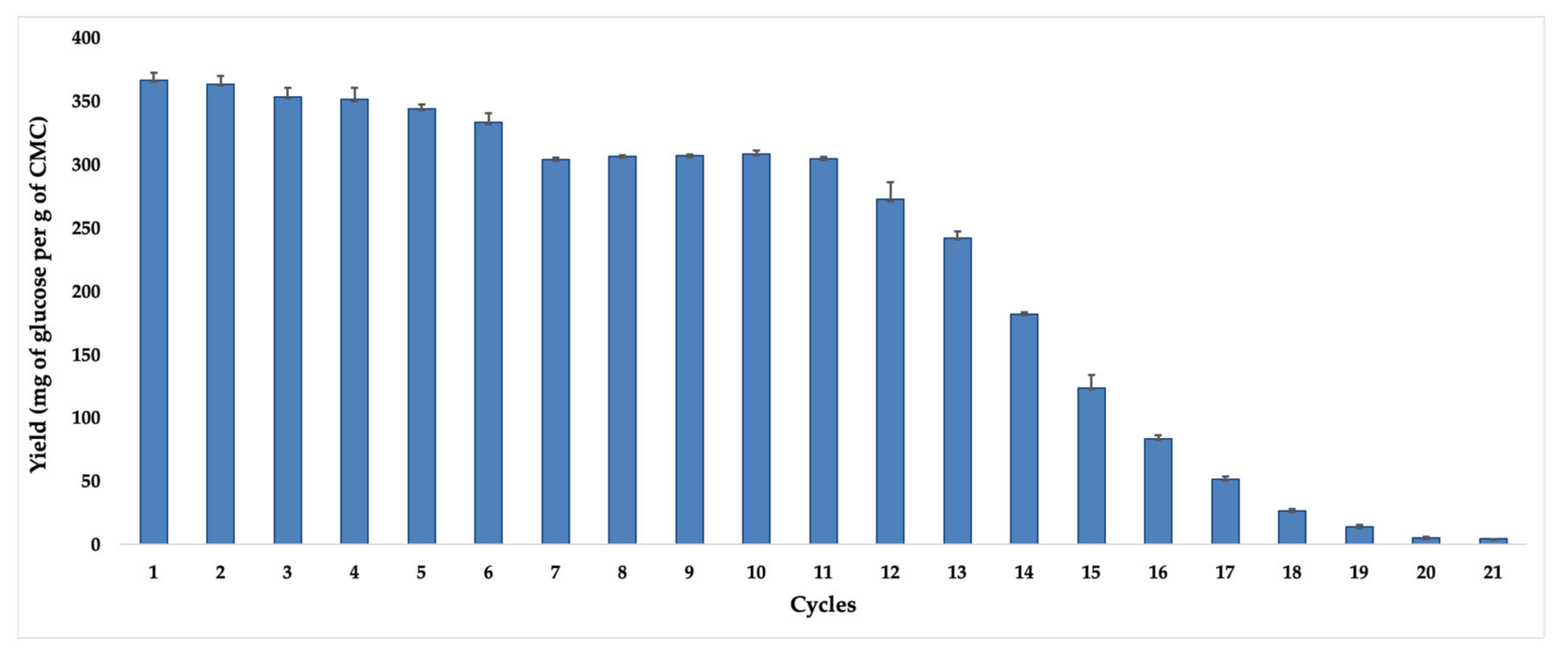
ThI14-12, it was submitted to the standard test of CMC hydrolysis for eight hours and 60 °C temperature, when the biocatalyst still kept a high recovered activity in the support. The results, expressed as glucose yield released at each reaction cycle, are shown in
Figure 7.
As observed, in the first hydrolysis cycle, a maximum glucose concentration of 366 mg per g of CMC was obtained, which remained without significant reduction in efficiencies until the sixth cycle. Until the 11th recycling, the ACC-
ThI14-12 biocatalyst was still able to generate glucose concentrations of around 300 mg per g of CMC. From the 12th recycle onward, significant losses in hydrolysis efficiency were detected, probably due to denaturation of the immobilized enzymes or desorption and leaching effects since the methodology applied was surface adsorption [
53]. Such results are superior to the work by Mo et al. [
54], who immobilized cellulases in a preparation of chitosan/magnetic porous biochar as a support. As for the recycle results, a maximum of 330.9 mg of glucose per g of CMC was obtained in the reaction cycles at 24 h and 40 °C, where the biocatalyst, obtained by covalent bonding, was able to maintain its operational stability for ten cycles. Therefore, our biocatalyst is competitive and presents operational stability for hydrolytic processes.